Abstract
In the accompanying paper, we described behavioral incubation of heroin-seeking behavior in rats following 1 or 14 d of abstinence. To gain an understanding of genomic changes that accompany this behavioral observation, we measured the expression of genes previously reported to respond to drugs of abuse. Specifically, after 1 or 14 days of abstinence, mRNA expression was measured for 11 genes in the medial prefrontal cortex (mPFC) and nucleus accumbens (NAc) immediately following a single 90 min extinction session. Additionally, the role of contingency was examined in control rats that received yoked, response-independent heroin administration. Gene expression was quantified by real-time quantitative PCR. Expression of five genes (Arc, EGR1, EGR2, Fos, and Homer1b/c) was changed in the mPFC. EGR1 and EGR2 expression was increased following the 90 min of extinction session in a contingency-specific manner and this increase persisted through the 14 days of abstinence. Fos expression was also increased after 1 and 14 d of abstinence, but at 14 d this increase was response-independent (i.e., it occurred in both the rats with a history of heroin self-administration and in the yoked controls). Arc expression increased following the extinction session only in rats with a history of heroin self-administration and only when tested following 1, but not 14, d of abstinence. Homer 1 b/c decreased after 14 days of enforced abstinence in rats that received non-contingent heroin. Expression of only a single gene (EGR2) was increased in the NAc. These data demonstrate that behavioral incubation is coincident with altered levels of specific transcripts and that this response is contingently-specific. Moreover, EGR1 and EGR2 are specifically upregulated in self-administering rats following extinction and this finding persists through 14 days of abstinence, suggesting that these genes are particularly associated with the incubation phenomenon. These latter observations of persistent changes in gene expression following abstinence may reflect molecular correlates of relapse liability.
Keywords: heroin self-administration, incubation, genes, contingency
Introduction
Drug abuse (specifically heroin addiction) is a chronic relapsing disease (O'Brien 1997). Clinically, the intent of treatment is to initiate and sustain drug abstinence. However, heroin withdrawal is characterized by intense drug craving that can trigger drug relapse. Relapse liability persists well beyond the initial withdrawal period (Krupitsky et al. 2002;O'Brien 2005;Krupitsky et al. 2006), and changes in gene expression may accompany this persistent liability. The present study examined known drug-responsive genes in rats that experienced a 90-minute extinction session following either 1 day or 14 days of abstinence from heroin self-administration. The extinction session was crucial to mimic context-induced relapse and to provide insight into the genes whose expression is changed upon exposure to an environment previously paired with receipt of heroin.
Incubation of drug-seeking behavior is a phenomenon where drug-seeking increases as a function of increasing periods of abstinence. Incubation has been reported with both heroin (Shalev et al. 2001) and cocaine (Neisewander et al. 2000;Grimm et al. 2001) self-administration. This elevation in drug-seeking has been reported to persist during 25 days of abstinence from heroin self-administration (Shalev et al. 2001) and up to 90 days of abstinence from cocaine self-administration (Grimm et al. 2003). We have also observed incubation after 14 days of abstinence from heroin self-administration (Kuntz et al., accompanying manuscript). Tissues from the rats from that report (supplemented with tissues from response-independent, investigator-administered heroin control rats) were used in the present study of gene expression.
The second element addressed by this study is the role of contingency in long-lasting molecular changes that accompany heroin administration. Prior studies have demonstrated that the contingency under which a drug is administered can determine physiological responses. Differential increases in extracellular dopamine have been observed in the NAc between animals self-administering drug (heroin or cocaine) versus those receiving infusions administered by an experimenter (Hemby et al. 1995;Hemby et al. 1997). Distinctive patterns of brain activity resulting from passive- versus self-administration of µ-opioid agonists have been observed in human heroin abusers (Greenwald and Roehrs 2005). Heroin self-administration induces a greater increase in both brain and body temperature of rats when compared with rats that receive passively administered heroin (Kiyatkin and Wise 2002). Recently, non-contingently administered heroin has been shown to induce greater levels of stereotyped behavior than contingently administered heroin (Lecca et al. 2007). Gene expression responses to drug exposure have also been demonstrated to be contingency-dependent (Jacobs et al. 2004;Jacobs et al. 2005).
Genes examined in this study were selected for analysis because they have been previously reported by either our laboratory or other laboratories to exhibit changed expression following heroin (Jacobs et al. 2004;Jacobs et al., 2005;Koya et al. 2006), morphine (Ammon et al. 2003;Ammon-Treiber and Hollt 2005) or cocaine administration (Freeman et al. 2001;Freeman et al. 2002a;Freeman et al. 2002b). Many of the genes are immediate early genes that have the potential to participate in the well-documented phenomenon of drug-induced neuroplasticity (Robinson and Kolb 2004;Szumlinski et al. 2006;McClung and Nestler 2008;Szumlinski et al. 2008). By examining genes previously described to be responsive to heroin, morphine or cocaine, we sought to determine the relevance of these genes to the overall phenomenon of addiction; that is, we sought to identify genes that are potentially universally-responsive to drugs of abuse. Naturally, this is not a comprehensive list of genes whose expression is altered following administration of drugs of abuse. Other reports (e.g., Ahmed et al. 2005) have described a number of additional changes that are associated with synaptic plasticity.
In the present study, we examined heroin-induced changes that persist beyond the period of drug use and a 90-minute extinction session in rats either 1 day or 14 days following their final session of drug exposure. Specifically, the experiments were designed to examine gene expression under conditions in which heroin was not present in the system and rats were re-exposed to contextual cues. To gain an increased understanding of the pharmacological versus behavioral components of drug abuse in altering gene transcripts, we have included both a contingent and a dose-matched non-contingent (i.e., yoked heroin) group in this study. Gene expression in these groups was compared to each other and to that of a group of saline controls.
Methods
Heroin self-administration and non-contingent drug delivery
Subjects
Male, Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC) weighing 250–300g at the start of the experiment, were individually housed in standard wire mesh cages and were maintained on a 12 hr light/dark cycle (lights on at 0700 and lights off at 1900) and were provided food and water ad libitum except where otherwise noted. All studies were conducted in accordance with The Pennsylvania State University Institutional Animal Care and Use Committee, strictly adhering to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
Surgery
Surgeries were performed one week after rats were introduced to their home cages. Intravenous catheters were implanted as previously described (Grigson and Twining 2002). The catheter was coupled to a cannula that exited between the rat’s shoulder blades. Rats recovered from surgery in their home cages with food and water ad libitum for one week.
Apparatus
Behavioral procedures were performed in 12 self-administration chambers (MED Associates, St. Albans, VT) constructed as previously described (Grigson and Twining 2002). Each chamber was equipped with two retractable sipper tubes and a stimulus light was located above each tube. A lickometer circuit was used to monitor licking. Heroin reinforcement was controlled by an electronic circuit that operated a syringe pump and programs written in the Medstate notation language (MED Associates). See Kuntz et al. (this issue) for a more complete description of the apparatus.
Habituation
During habituation, all rats were placed on a water deprivation regimen that consisted of 5 min access to water in the self-administration chamber each morning and 1 h water access in the home cage each afternoon. This habituation training occurred daily for nine days. Water deprivation continued through the remainder of the study, with 1 h of water access in the home cage each afternoon.
Drug-administration
Forty-eight rats were used in the study. Sixteen rats self-administered heroin using licking of an empty spout as the operant behavior. The behavior of these rats is described in the accompanying manuscript (see Kuntz et al., this issue). The thirty-two additional rats were yoked to the self-administering rats, 16 received yoked heroin infusions and 16 received yoked saline infusions. Under this paradigm, the yoked animals received infusions on the same schedule (and for the yoked heroin animals, at the same dose) as the self-administering “executive” rats. At the start of each session, two empty spouts extended into the chamber and a stimulus light was illuminated above the right (active) spout. For the self-administering rats, every 10 licks on the rightmost spout resulted in a heroin infusion (fixed ratio 10; FR10). Licks on the left (inactive) spout had no consequence. For yoked rats, licks on either spout had no consequence. Rats were trained in triads, with the yoked heroin and yoked saline group receiving infusions simultaneously each time the infusion requirement was met by the executive rat. Identical consequences occurred in all three chambers in each triad - including extinguishment of the light above the active spout, retraction of the spouts for 20s, and onset of a 20s tone. During this 20s period, responses on the “active” spout had no consequence. There were 14 such daily acquisition trials.
Forced Abstinence and Extinction session
Following 14 days of heroin (or saline) administration, half of the rats in each group (8 animals) received 1 d of abstinence, while the other half (8 per group) received an 2-week period of forced abstinence (i.e., the rats remained in their home cages). All rats then experienced a 90-minute extinction session during which completion of 10 licks on the active spout by the executive rat resulted in identical consequences as described during the acquisition trials, except saline, rather than heroin, was infused across the triad. During this time, rats were handled daily, food was available ad libitum, and one hour of water was available in the afternoon. Catheters in all but three of the 2 week abstinence animals (one from each treatment group) remained patent throughout the extinction session.
Behavioral Measures
Rats were weighed daily. The number of licks made on both spouts were recorded during the self-administration and extinction sessions, as was the total number of infusions self-administered. In addition, the latency(s) to make the initial lick on each spout was recorded for each training session.
Drugs
Heroin HCl was generously provided by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, N.C., USA). Drug was dissolved in sterile physiological saline at a concentration of 0.3mg/ml. Each IV-injection was a 0.06mg/0.2ml infusion dose delivered over 6s.
Dissection and RNA isolation
Sacrifice and tissue dissection
Immediately following the 90-minute extinction session, all rats were sedated using Propofol (10mg/kg) and decapitated. The brain was rapidly removed from the skull, placed in pre-chilled phosphate buffered saline (PBS) and then sectioned in an ice-chilled ASI brain slicer (ASI Instruments, Warren MI). The section from Bregma +4.2 to 2.2mm was cut along the forceps minor and the cortex medial of this cut was collected (medial prefrontal cortex, mPFC). This includes the cingulate, prelimbic cortex, and medial orbital cortex. The section from +2.2 to 0.2mm was cut 0.5mm on each side of the midline along a line connecting the tip of the external capsule and previous cut from the tip of the external capsule and lateral ventricle, and between the ventricles. The nucleus accumbens (NAc) dissection includes core and shell. Following dissection, the tissue was placed in prechilled tubes, immediately frozen on dry ice, and then stored at −80°C.
RNA isolation
Total cellular RNA was isolated using Tri Reagent (Molecular Research Center Inc., Cincinnati, OH) (Chomczynski and Mackey 1995). Isolated RNA was further purified using an RNeasy Mini Kit for RNA clean-up (QIAGEN Sciences, Maryland). RNA quantity and quality were assessed using the RNA 6000 Nano Assay with an Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA).
QRT-PCR analysis of gene expression
cDNA synthesis was performed on total RNA using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). 1µg RNA, 500ng Oligo (dT), and 10mM each dNTP, were incubated for 5 minutes at 65° C and then chilled on ice for 2 minutes. 5X First Strand Buffer (250mM Tris-HCL (pH8.3), 375mM KCL, and 15mM MgCl2), 5mM DTT (final concentration), 40 U RNaseOut, and 200 U Superscript III RT were then added. The 20µl reaction was incubated for 60 minutes at 50° C followed by a final incubation at 70° C for 15 minutes for termination.
Quantitative PCR was carried out on a real-time detection instrument (ABI 7900HT Sequence Detection System) in 384-well optical plates using TaqMan Universal PCR Master Mix and Assay on Demand primers and probes (Applied Biosystems, Foster City, CA) as described previously (Bowyer et al. 2007). Primer/probe sets used are listed in Table 1. All of these studies were conducted in the Functional Genomics Core Facility of the PSU Division of Shared Research Resources.
Table 1.
TaqMan® Gene Expression Assays were used to quantify gene expression. The gene abbreviation, full name, and assay ID for each gene are listed in this table.
| Abbreviation | Name | Assay Number |
|---|---|---|
| Arc | Activity-regulated cytoskeletal-associated protein | Rn00571208_g1 |
| Beta-actin | Beta-actin | Rn00667869_m1 |
| cdk5 | Cyclin-dependent kinase 5 | Rn00590045_m1 |
| Cryab | Crystalline, alpha B | Rn00564026_m1 |
| EGR1 | Early growth response 1, a.k.a. krox 24, NGFI, zif268 | Rn000561138_m1 |
| EGR2 | Early growth response 2, a.k.a. krox 20 | Rn00586224_m1 |
| Fos | c-fos | Rn02396759_m1 |
| Homer 1 b/c | Homer homolog 1, a.k.a. ania-3, HOMER1F, Vesl-1 | Rn00581785_m1 |
| Homer 1 pan | Homer homolog 1, a.k.a ania-3, HOMER1F, Vesl-1 | Rn00668905_m1 |
| Nr4a1 | Nuclear receptor subfamily 4, group A, member 1, a.k.a. NGFI-B, Nur77 | Rn0057776_m1 |
| Per2 | Period homolog 2 | Rn00581577_m1 |
Statistical Analysis
Behavioral data were analyzed using 3 × 2 × 2 mixed factorial ANOVAs, varying group (self-administering, yoked heroin, yoked saline), spout (active, inactive), and abstinence period (1 or 14 d). Goal-directed behavior was determined by subtracting inactive spout responses from active spout responses. Gene expression values were evaluated using 3 × 2 mixed factorial ANOVAs varying group (self-administering, yoked heroin, yoked saline) and abstinence period (1 or 14 d). Gene expression data was then analyzed using one-way ANOVAs with a separate ANOVA at each time point (1 and 14 days) comparing gene expression across the three treatment groups (self-administering, yoked heroin, yoked saline). For both behavioral and gene expression data, post hoc tests were conducted using Student-Newman-Keuls tests with α set at 0.05.
Analyses were performed to determine whether gene expression values correlated with self-administration (specifically, the average of values from the last 2 days of self-administration) or with drug-seeking behavior during extinction testing. These analyses were only conducted for the 16 self-administering rats because yoked rats in this study could not express contingent behavior. The correlation between behavior and gene expression was analyzed separately for each abstinence period: 1d and 14d abstinence. This separation was necessary because, at the time of sacrifice, the groups had undergone different experiences, and gene expression levels have the potential to be affected by the length of the abstinence period. Pearson Correlation analyses examined the relationship between active responses, goal-directed behavior, and each of the genes studied during drug-taking (acquisition) and drug-seeking (extinction) behavior.
Results
Body weight and water intake
Rats in all treatment groups were weighed daily throughout the study, and all rats gained weight at equal rates. Self-administering rats increased from an average weight of 365.3 +/− 9.7g on the first day of self-administration to 416.0 +/− 10.4g on the final day of self-administration. Weight increases for rats yoked to heroin or saline were 380.6 +/− 6.4g to 429.5 +/− 7.5g and 372.3 +/− 7.2g to 421.3 +/− 9.7g, respectively. Rats were provided 20 ml of water daily during the self-administration and abstinence periods, and all 20 ml of water were consumed daily. Detailed behavioral data are presented in the accompanying article.
Acquisition: Heroin self-administration and non-contingent administration
The 16 self-administering rats increased their heroin intake from an average of 7.3 +/− 0.37 infusions on day 1 to 12.6 +/− 2.4 infusions on day 14. During the terminal acquisition period, the self-administering (executive) rats, but not the yoked heroin or yoked saline controls, exhibited clear heroin self-administration behavior by making more responses on the active than the inactive spout (see Kuntz et al., this issue).
Self-administration behavior was also demonstrated by executive rats initiating licking more quickly on the active spout than on the inactive spout (10.7 +/− 3.6s vs. 3043.0 +/− 953.2s, on the final day of self-administration). The latency to make initial contact with the active spout decreased on progressive days of heroin self-administration sessions and was significantly shorter for self-administering rats than the latency to contact the inactive spout (see Kuntz et al., this issue).
Extinction: Incubation of drug-seeking behavior
Behavioral data
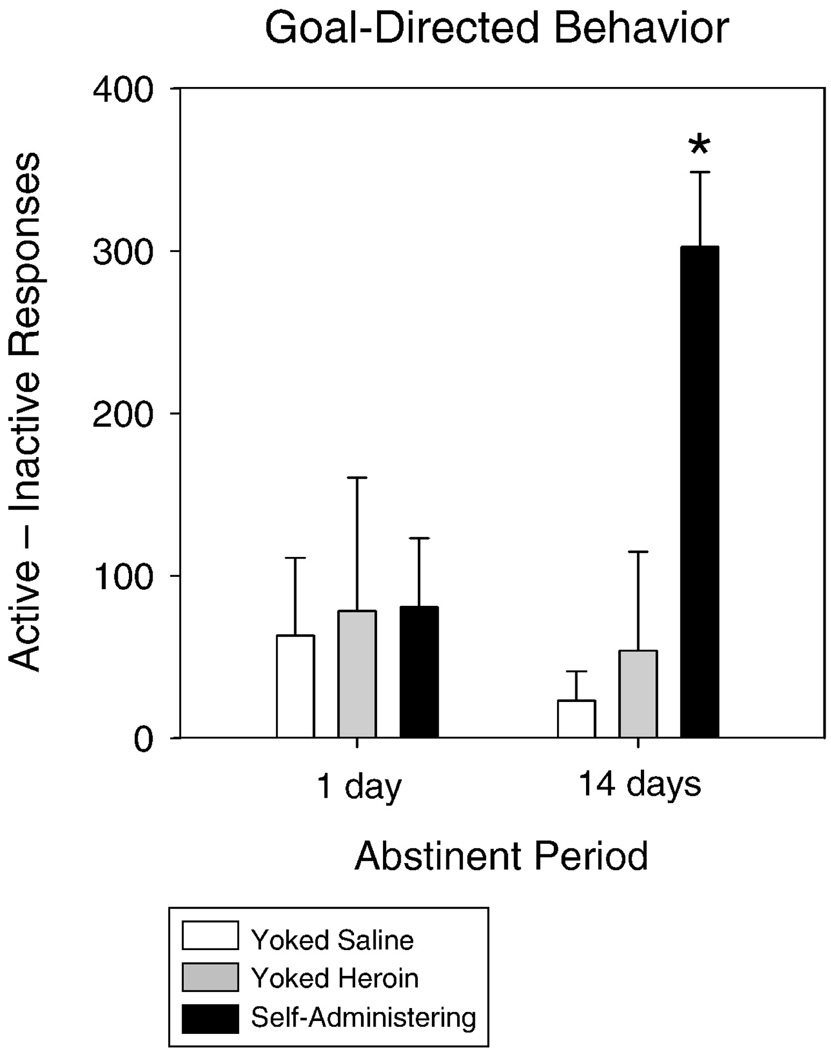
Responses on both the active and inactive spouts were monitored during a 90-minute extinction session that took place either 1 day or 14 days following final access to drug. Rats that underwent a 14 day period of enforced abstinence responded more on the previously active spout than did rats that experienced only 1 day of abstinence (see Figure 2 in Kuntz et al. this issue). Moreover, while the executive rats with a history of heroin self-administration exhibited a similar number of licks on the active and the inactive spout after 1 d of abstinence, responding on the active spout was nearly 3 times that made on the inactive spout when the extinction test followed 14 d of abstinence. No significant differences in latency to lick were observed for the self-administering rats depending on the length of their enforced abstinence period. That is, all rats with a history of heroin self-administration initiated licking significantly more quickly on the active than the inactive spout (4.8 +/− 1.9s vs. 8.2 +/− 2.6s). Finally, active vs. inactive spout responding did not differ for either the yoked heroin or the yoked saline controls, whether tested following either 1 d or 14 d of abstinence, ps > 0.05.
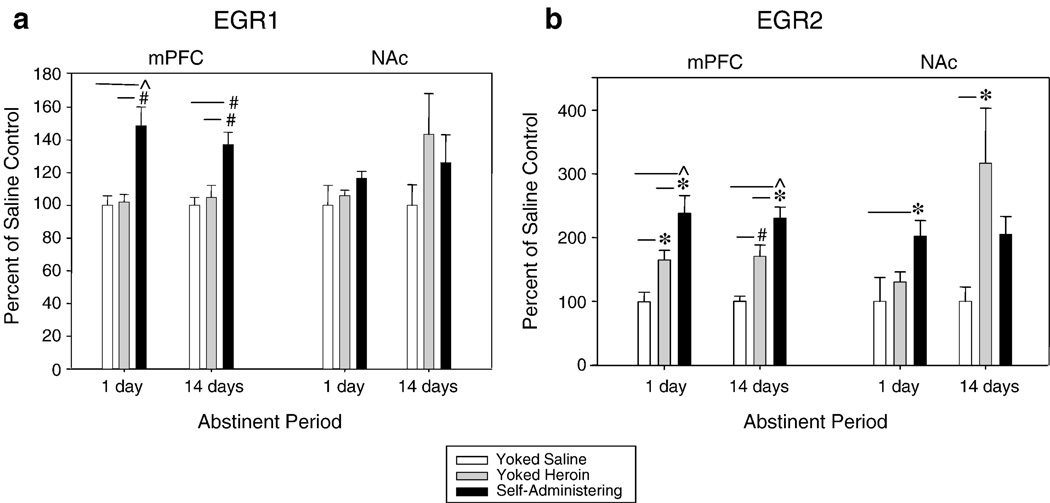
Figure 2.
(a) EGR1 gene expression was persistently elevated above saline in the mPFC in rats that either self-administered heroin or received yoked infusions of heroin. No significance was detected in the NAc. ^ p = 0.001; # p < 0.01 (b) – EGR2 gene expression was persistently elevated above saline in the mPFC in previously self-administering rats following both 1-day and 14-days of abstinence. Gene expression in these rats was higher than expression in rats that had previously received yoked heroin infusions, although EGR2 gene expression in the yoked heroin rats was significantly higher than in yoked saline rats. ^ p < 0.001; # p < 0.01; * p < 0.05
Calculations of goal-directed behavior (difference scores) indicate that goal-directed behavior increased concomitantly with incubation of heroin-seeking. No difference between responding on the two spouts was apparent when extinction occurred after only 1 day of abstinence, but executive rats responded significantly more on the active than the inactive spout when the extinction session was preceded by 14 days of abstinence (Figure 1).
Figure 1.
(a) Heroin infusions were administered in response to licks on an “active” spout. The number of responses on this spout, and subsequently the number of earned infusions during each daily training session, increased through the 14-day period of self-administration. (b) An incubation effect was observed by measuring the responses on the active spout during the 90-minute extinction session conducted 1 day or 14 days following the final session of self-administration. Self-administering rats responded significantly more after 14-days of abstinence when compared with 1 day of abstinence. * p < 0.05
Gene Expression Changes that Persist During Withdrawal
Gene expression analysis was performed in the mPFC and NAc for 11 genes previously described as responsive to morphine or cocaine administration. Gene expression was examined after the 90 min extinction session that occurred after 1 or 14 days of home cage abstinence.
One-way ANOVAs followed by Student-Newman-Keuls post hoc analysis determined that after 1 day of abstinence, EGR1 gene expression in the mPFC was significantly altered (F2,19 = 10.93, p < 0.001). Post-hoc analysis showed that expression was increased by 45% following extinction in rats with a history of heroin self-administration compared to yoked saline (p = 0.001) and yoked heroin (p < 0.01) rats. This differential expression persisted for the 14 day period of withdrawal (F2,18 = 10.007, p = 0.001, p < 0.005 for both comparisions). (Figure 2, panel a). No alteration in EGR1 gene expression occurred in the NAc.
EGR2 was significantly upregulated in the mPFC after the extinction session at both 1d (F2,19 = 10.76, p < 0.001) and 14d (F2,19 = 17.65, p < 0.001) of abstinence. Observed increases above yoked saline existed for self-administration following 1d abstinence (p < 0.001) and 14d abstinence (p < 0.001). Elevations above saline also existed for yoked heroin rats following 1d (p < 0.05) and 14d (p < 0.005) of abstinence. Importantly, levels of EGR2 gene expression were significantly higher in rats with a history of heroin self-administration compared with rats that received non-contingent administration of drug (p < 0.03) and this effect persisted 14 days into withdrawal (p < 0.03) (Figure 2, panel b). NAc EGR2 gene expression increased in self-administering, but not yoked rats, after 1 day of abstinence (F2,19 = 4.18, p < 0.05). A different expression pattern was observed in the NAc after 14 days of abstinence with rats that had received yoked infusions of heroin displaying a dramatic increase in EGR2 gene expression (300%; F2,16 = 5.37, p < 0.02) (Figure 2, panel b).
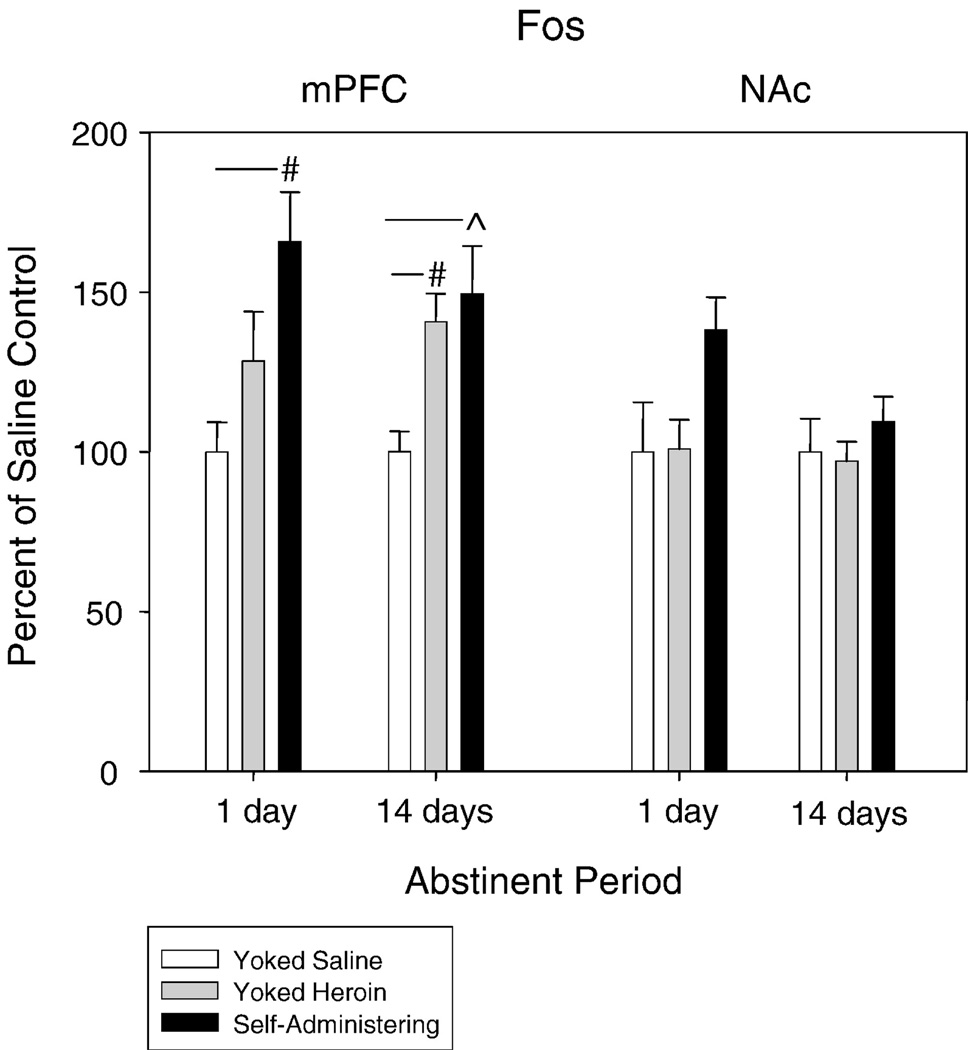
Fos gene expression was increased in the mPFC by a history of heroin self-administration when the extinction test occurred after 1 day of abstinence (F2,19 = 5.73, p < 0.01) and remained significantly elevated compared to yoked saline controls after 14 days of abstinence (F2,19 = 6.00, p < 0.02) (Figure 3). Fos gene expression was elevated in yoked heroin rats at both time points, although this elevation only reached significance after 14 days of abstinence (F2,19 = 6.00, p < 0.02). No significant differences in Fos expression were observed in the NAc although a trend for upregulation by heroin self-administration was evident when extinction testing occurred after 1 day of abstinence.
Figure 3.
Fos gene expression was persistently elevated above saline in the mPFC after 1-day of abstinence and 14-days of abstinence. Following 14-days of abstinence Fos gene expression in yoked heroin rats became significantly higher than in rats yoked to saline. No change was observed in the NAc at either time point. # p < 0.01; * p < 0.05
Changes Induced by Heroin that Do Not Persistent into Withdrawal
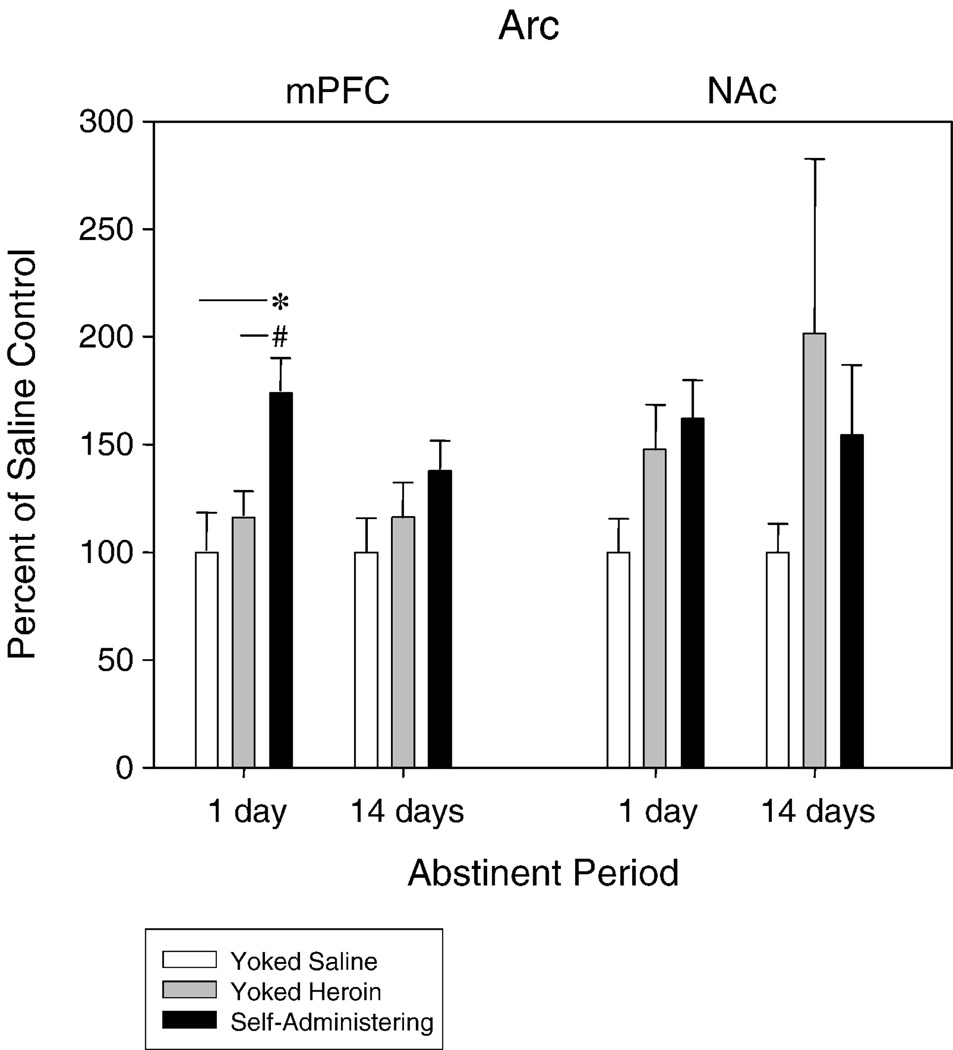
Arc was found to be upregulated in the mPFC following 1 day of abstinence (F2,19 = 7.11, p < 0.005). Gene expression in heroin self-administering rats (following extinction) was significantly higher than in rats receiving yoked heroin (p < 0.01) and yoked saline (p < 0.05) treatments. This change did not persist following 14 days of abstinence (Figure 4). No significant changes were detected in the NAc.
Figure 4.
Arc gene expression was elevated above saline in the mPFC in rats that had self-administered heroin. Self-administering rats experienced an increase in Arc gene expression that was significantly higher than Arc gene expression in rats that previously received yoked infusions of heroin. This elevation did not persist to 14-days of abstinence and no change in Arc gene expression was observed in the NAc. * p < 0.05; # p < 0.01
Changes Induced by Heroin Only Evident after 2 Weeks of Withdrawal
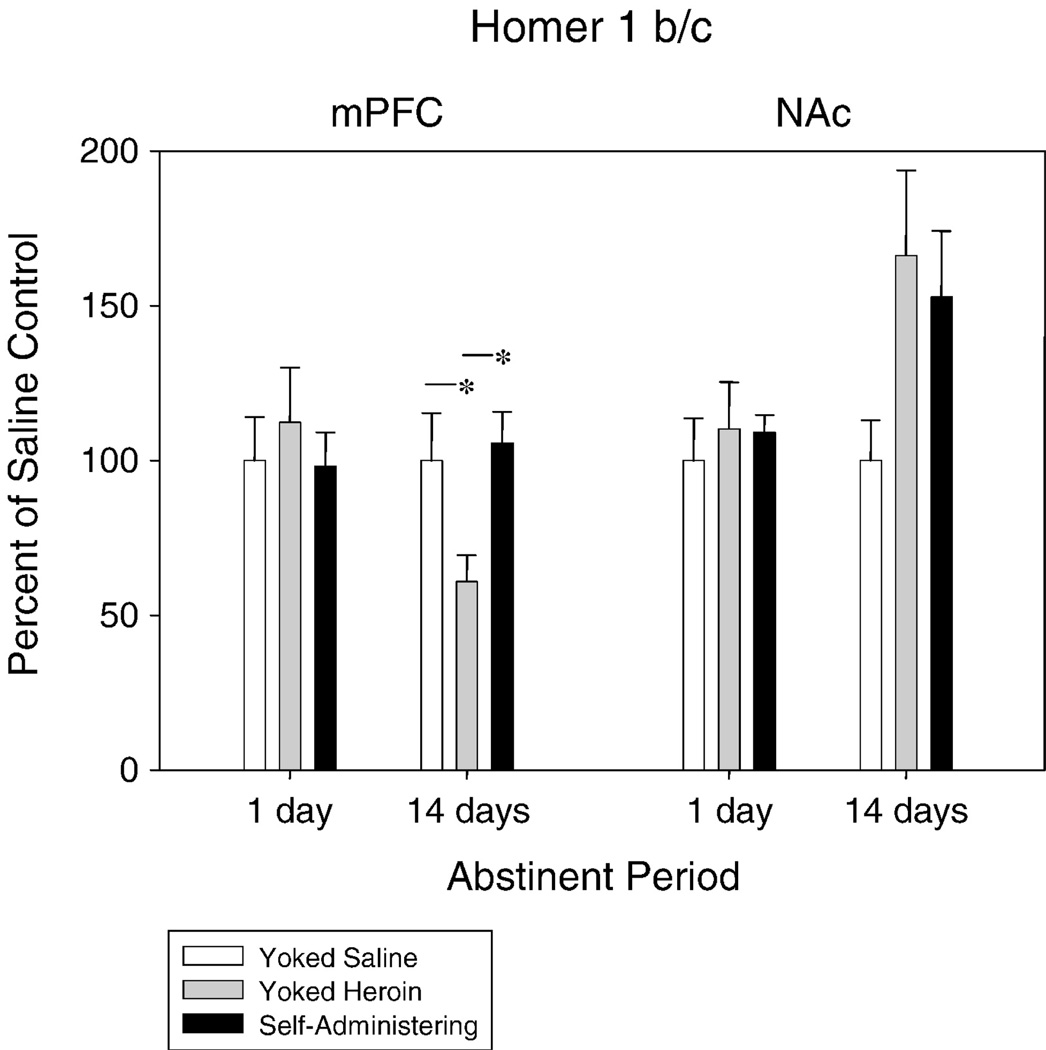
Homer1 b/c gene expression was not significantly changed in the mPFC following 1 day of abstinence but, after 14 days of abstinence, rats that had formerly received yoked infusions of heroin displayed a significant decrease in Homer1 b/c (F2,19 = 3.91, p < 0.04) when compared with previously self-administering (p < 0.05) and yoked saline (p < 0.05) rats (Figure 5). Homer1 b/c was not significantly changed in the NAc regardless of duration of abstinence (although a non-significant trend is evident).
Figure 5.
mPFC Homer 1 b/c gene expression was unchanged after 1-day of abstinence but was significantly decreased in yoked heroin rats below expression in yoked saline and self-administering rats following 14-days of abstinence. * p < 0.05
Genes Unaffected by Treatment
Six of the genes that were examined were not significantly changed by heroin treatment or abstinence period (Table 2). Despite the down regulation of Homer1 b/c observed in the mPFC, no significant changes were observed in either the mPFC or NAc for the pan isoform of Homer1 (the primer/probe set that recognizes the a, b, and c splice variants of Homer1) and Homer1a. Expression levels of Nr4a1, Per2, Cryab, and cdk5 were also unchanged. Importantly, beta-actin was confirmed to be unchanged in all samples (data not shown). Thus, this gene served as an endogenous control to which values were normalized for comparison. Similarly, levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were also confirmed to be unchanged (data not shown).
Table 2.
Six of the genes examined in this study (in addition to actin) were unchanged. The expression values for these genes are shown, standardized to the yoked saline expression value for that timepoint.
| A. mPFC | ||||||
|---|---|---|---|---|---|---|
| 1 day abstinence | 14 days abstinence | |||||
| Yoked Saline | Yoked Heroin | Self-Administering | Yoked Saline | Yoked Heroin | Self-Administering | |
| alpha B-crystallin | 1.00 +/− 0.10 | 1.42 +/− 0.19 | 1.12 +/− 0.084 | 1.00 +/− 0.093 | 1.17 +/− 0.095 | 0.923 +/− 0.18 |
| cdk5 | 1.00 +/− 0.069 | 1.07 +/− 0.10 | 1.03 +/− 0.019 | 1.00 +/− 0.061 | 0.96 +/− 0.038 | 0.96 +/− 0.051 |
| Homer1 a | 1.00 +/− 0.23 | 1.15 +/− 0.22 | 1.18 +/− 0.29 | 1.00+/− 0.44 | 0.69 +/− 0.22 | 1.14 +/− 0.29 |
| Homer1 pan | 1.00 +/− 0.15 | 1.19 +/− 0.18 | 1.06 +/− 0.10 | 1.00 +/− 0.18 | 0.69 +/− 0.08 | 1.16 +/− 0.18 |
| Nr4a1 | 1.00 +/− 0.19 | 0.67 +/− 0.12 | 1.14 +/− 0.12 | 1.00 +/− 0.14 | 1.07 +/− 0.18 | 1.50 +/− 0.26 |
| Per 2 | 1.00 +/− 0.17 | 1.37 +/− 0.32 | 1.71 +/− 0.094 | 1.00 +/− 0.17 | 1.03 +/− 0.19 | 1.04 +/− 0.16 |
| B. NAc | ||||||
|---|---|---|---|---|---|---|
| 1 day abstinence | 14 days abstinence | |||||
| Yoked Saline | Yoked Heroin | Self-Administering | Yoked Saline | Yoked Heroin | Self-Administering | |
| alpha B-crystallin | 1.00 +/− 0.13 | 1.14 +/− 0.064 | 1.13 +/− 0.060 | 1.00 +/− 0.069 | 1.26 +/− 0.16 | 1.09 +/− 0.12 |
| cdk5 | 1.00 +/− 0.040 | 1.08 +/− 0.056 | 1.04 +/− 0.045 | 1.00 +/− 0.019 | 1.15 +/− 0.052 | 0.97 +/− 0.068 |
| Homer1 a | 1.00 +/− 0.18 | 1.27 +/− 0.28 | 1.13 +/− 0.19 | 1.00 +/− 0.42 | 1.30 +/− 0.22 | 1.10 +/− 0.44 |
| Homer1 pan | 1.00 +/− 0.053 | 1.17 +/− 0.093 | 1.20 +/− 0.091 | 1.00+/− 0.17 | 1.34 +/− 0.16 | 1.21 +/− 0.15 |
| Nr4a1 | 1.00 +/− .25 | 1.10 +/− 0.12 | 1.25 +/− 0.11 | 1.00 +/− 0.19 | 1.92 +/− 0.44 | 1.46 +/− 0.32 |
| Per 2 | 1.00 +/− 0.092 | 1.02 +/− 0.019 | 1.13 +/− 0.048 | 1.00 +/− 0.073 | 1.20 +/− 0.12 | 1.13 +/− 0.037 |
Correlational Analyses
Pearson’s correlation analyses detected significant correlations between gene expression values and behavior for selected genes. The two behaviors included in the analysis were average responding on the final 2 days of self-administration (drug-taking) and responding during extinction (drug-seeking). In the mPFC, gene expression that correlated with drug taking occurred for EGR1 (r = 0.76; p < 0.04), Fos (r = 0.84; p < 0.02), and Nr4a1 (r = 0.87; p < 0.01), and these correlations only existed when evaluated after 14d of abstinence. At this time point, Fos gene expression was negatively correlated with the goal-directed behavior (r = −0.83; p < 0.02). Importantly, these correlations were strongly influenced by a single high-responding rat and therefore caution must be taken when noting these results. Homer 1 b/c and homer 1 pan gene expression in the mPFC was highly correlated (negative relationship) with drug-seeking behavior (r = −0.82; p < 0.03 and r = −0.77; p < 0.05, respectively). Homer 1 pan gene expression in the nucleus accumbens also was negatively correlated with goal-directed responses in SA rats after 1 day of abstinence (r = −0.94; p < 0.001). After 1 day of abstinence, Cryab was correlated with drug-seeking (r = 0.76; p < 0.03). No significant correlations were detected for Arc gene expression.
Discussion
Drug relapse is a major obstacle in overcoming addiction, and understanding the genomic contributors to incubation may assist in developing more effective therapies. Experimentally, incubation is described as a motivational process, inferred from a time-dependent increase in cue-induced drug seeking after withdrawal from drug self-administration in rats (Gawin and Kleber 1986;Grimm et al. 2001). In rats self-administering heroin, this increased drug-seeking behavior peaks at about 6 days of abstinence, but has been reported up to 25 days (Shalev et al. 2001). Similarly, rats that self-administer cocaine demonstrate increases in drug-seeking behavior for up to 90 days of abstinence (Grimm et al. 2003). In this study, following 14 days of abstinence from heroin self-administration, significant elevations in heroin-seeking behavior were observed when compared with rats experiencing only 1 day of abstinence (Figure 1 and Kuntz et al., accompanying manuscript).
Spout-licking represents an operant behavior that, in this study, engendered robust responding, reliable behavior, and demonstrable incubation. Because rats were maintained on a schedule of water deprivation throughout the study, saline controls were used to verify that responding by the rats with a drug history was not due to a history of having licked for water in the apparatus during habituation. Saline controls made significantly fewer spout licks than self-administering rats and distributed their behavior across both spouts equally. This finding indicates that the goal-directed responding of self-administering rats was focused on heroin. Furthermore, inclusion of yoked heroin rats in the present study allowed for the comparison of genomic changes resulting from the pharmacological action of heroin vs. those changes associated with contingent drug delivery. Together, analysis of gene expression of these three groups (self-administering, yoked saline control, and non-contingent heroin control) highlighted important changes associated specifically with behavioral contingency and abstinence-induced incubation.
Of the eleven genes examined (in two brain regions), several were changed following the 90 min extinction session in rats with a history of heroin self-administration and these changes remained evident even when tested following 14 days of abstinence. Three genes, all encoding transcription factors, fit this profile: EGR1, EGR2 and Fos. EGR1 is important in transcription of genes related to learning and memory. EGR1 mRNA has also been reported to increase upon exposure to cocaine-related stimuli (Thomas et al. 2003) and antisense inhibition of EGR1 has been demonstrated to decrease cue-induced cocaine seeking and relapse (Lee et al. 2006). In the present study, this cue-induced responsiveness persists following 14 days of abstinence, but is only manifested in the mPFC. EGR2 is highly homologous to EGR1 in its zinc finger DNA-binding domain, and recognizes the same DNA binding consensus sequence (Joseph et al. 1988;Lemaire et al. 1988;Chavrier et al. 1989;Chavrier et al. 1990). We found extinction-induced induction of EGR2 in the mPFC in both heroin self-administering and yoked, non-contingent animals. However, the effect was greater in rats with a history of heroin self-administration. This represents a case of dynamic interactions of pharmacology and behavior. Naltrexone-induced morphine withdrawal has been reported to elicit an increase in mRNA levels of EGR2 in the NAc (Bhat et al. 1992), and the present study found EGR2 expression to be increased in the NAc following 14 days of withdrawal, but only in rats that received yoked, non-contingent, heroin infusions. Greater withdrawal (as measured by ultrasonic volcalizations) has been observed in rats that previously received non-contingent cocaine infusions compared to previously self-administering rats (Mutschler and Miczek 1998). These distinctive contingency-dependent effects further highlight the need to discriminate behavior from pharmacology.
c-Fos is a well-characterized immediate early gene transcription factor. Fos is affected by nicotine, morphine, and cocaine treatment (Marttila et al. 2006), and multiple signaling pathways induce c-fos (Sadoshima and Izumo 1993). Morphine either increases or decreases c-fos gene expression in striatofugal circuits, and its expression appears to be dose-dependent and directly related to the contextual novelty in which morphine is administered (Ferguson et al. 2004). c-fos mRNA expression is greater when cocaine is administered in a novel environment (Day et al. 2001;Uslaner et al. 2001a;Uslaner et al. 2001b). Recently, Fos gene expression in both the mPFC and NAc was reported to be significantly increased in previously heroin self-administering rats only when re-exposed to the cues associated with self-administration (Koya et al. 2006). All of the rats in the present study were re-exposed during the 90-minute extinction session to the cues associated with the previous receipt of heroin. An induction in Fos expression was observed in the mPFC for self-administering animals both before and after enforced abstinence, suggesting that the response is quite persistent. An elevation in c-fos gene expression with yoked, non-contingent heroin was only present in the mPFC, and this elevation only emerged following 14 days of abstinence.
Homer1 b/c responsiveness was not immediately changed by drug self-administration or non-contingent exposure, but exhibited a decrease after 14 days of enforced abstinence. Interestingly, this change only occurred in yoked, non-contingent heroin rats. Homer 1 gene expression and protein levels are reportedly decreased following chronic cocaine administration (Swanson et al. 2001;Ghasemzadeh et al. 2003), so this gene is apparently affected by multiple drugs of abuse.
Heroin exposure induces immediate behavioral changes that are accompanied by transient changes in gene expression. mPFC extinction-induced Arc mRNA expression was dramatically upregulated following one day of abstinence from drug administration, but returned to yoked saline levels after 14 days of abstinence. Contingency appears to play an important role in the induction of this gene because the level of Arc in the mPFC is significantly higher in self-administering rats than rats yoked to heroin. Arc is a protein involved in neuronal plasticity and memory consolidation (Guzowski et al. 2006) and therefore may be playing a role in establishing an imprint (or memory) of self-administration behavior.
The genes for which no change in expression levels were detected in this study are homer1 a, homer1 pan, Nr4a1, per2, Cryab, and cdk5. We evaluated rPer2 (rat Per2) and Cryab because they have been reported to be induced by chronic morphine administration (Ammon et al. 2003). The lack of concordance between the present findings and the previous report may be due to differences in contingency or pharmacology. While heroin is deacetylated to form morphine, it crosses the BBB more rapidly than morphine and this increased speed of action may induce unique genomic changes. Homer1 a gene expression is reportedly increased in both the heroin self-administering rats and their saline controls upon re-exposure to cues, compared with rats not exposed to cues (Koya et al, 2006). This finding suggests that Homer1 a also may have been elevated in our rats following exposure to the test chamber, but this elevation could not be detected because all rats (those with a saline or heroin history) were exposed to the test chamber and there were no home cage (i.e., no cue) controls.
The combination of behavioral and gene expression data collected by this study provides an opportunity to examine correlations between behavior and gene expression that may reveal more subtle relationships than group means. Given limited statistical power (n = 8), several observations were made. EGR1, Fos, and Nr4a1 gene expression is correlated with drug-taking (self-administration responding) but not drug-seeking (extinction responding), but only when evaluated after 14d of abstinence. Thus, a history of greater heroin self-administration behavior is associated with an elevation in the expression of EGR1, Fos, and Nr4a1 in the mPFC, but only after an extended period of withdrawal (i.e., 14 days). The expression of Cryab, Homer1 b/c, and Homer1 pan in the mPFC, on the other hand, was significantly correlated with drug-seeking behavior during extinction (i.e., with responding on active spout) whether tested following either 1d or 14d of abstinence. These findings are important in several respects. First, these data suggest that this set of genes is linked to drug-seeking, but not to drug-taking. Second, several of these genes (Cryab, Nr4a1, and Homer1 pan) did not display differential responsiveness in the aggregate, consistent with the conclusion that there are important inter-individual differences in responsiveness. Future experiments, involving no extinction session, will be required to determine the impact of abstinence period alone on gene expression. Finally, for all these studies, it is important to note that we are measuring mRNA levels and that these need not, a priori, reflect protein levels.
In sum, EGR1, EGR2, and Fos are elevated in the mPFC of rats when extinction testing occurs following either 1 or 14 d of abstinence and these effects are most robust in rats with a history of heroin self-administration. Moreover, the results of correlational analyses show that higher drug self-administration behavior is associated with higher levels of EGR1, Fos, and Nr4a1 gene expression in the mPFC when measured following 14 d of abstinence. Homer 1 b/c was decreased in the mPFC, but only when examined after 14 d of abstinence, and only in the yoked, noncontingently administered rats. The mean expression of Homer 1 pan was not altered in the mPFC, but the level of expression of Homer 1 b/c and Homer 1 pan varied as a function of the magnitude of the drug-seeking behavior when tested after 14 d of abstinence in rats with a history of drug self-administration. Cryab mean gene expression was also not altered in the mPFC, but varied as a function of drug self-administration when tested after 1 d of abstinence. Collectively, then, gene expression (particularly in the mPFC) tracks the contingent nature of heroin administration, the vigor of the drug-taking behavior, withdrawal period, drug-seeking behavior during extinction, as well as the intensity of this drug-seeking behavior across individual subjects.
In conclusion, the ability to model the persistent neurobiological and behavioral changes following exposure to drugs is critical to our understanding of addiction and relapse. This study combined prior knowledge of the genomic effects of drug exposure with a model of heroin self-administration and incubation. The findings of altered gene expression with heightened drug-seeking behavior mark these gene targets for future studies into their role(s) in long-term relapse liability. By addressing addiction using a molecular to behavioral approach, progress will be made towards identifying pharmacotherapeutic targets to facilitate drug abstinence and prevent relapse.
Table 3.
Significant correlations between behavior and gene expression were detected. Most correlations occurred between behavior and mPFC gene expression. Italics indicate the correlation was detected between behavior and NAc gene expression.
| Gene | Behavior | Session | Abs. Pd. | Correlation Coefficient | P-value |
|---|---|---|---|---|---|
| Crystallin ab | active | extinction | 1 d | 0.76 | 0.03 |
| EGR1 | active | acquisition | 14 d | 0.79 | 0.03 |
| Fos | active | acquisition | 14 d | 0.84 | 0.02 |
| Fos | Goal-dir | acquisition | 14 d | −0.83 | 0.02 |
| Homer 1 b/c | active | extinction | 14 d | −0.82 | 0.03 |
| Homer 1 pan | active | extinction | 14 d | −0.77 | 0.04 |
| Homer 1 pan | Goal-dir | extinction | 1 d | −0.94 | 0.001 |
| Nr4a1 | active | acquisition | 14 d | 0.87 | 0.01 |
Acknowledgments
Supported by DA021450 (KLK), DA13770 (KEV) and DA09815 and DA12473 (PSG)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M, Koob GF, Repunte-Canonigo V, Sanna PP. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11533–11538. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammon S, Mayer P, Riechert U, Tischmeyer H, Hollt V. Microarray analysis of genes expressed in the frontal cortex of rats chronically treated with morphine and after naloxone precipitated withdrawal. Brain Res. Mol. Brain Res. 2003;112:113–125. doi: 10.1016/s0169-328x(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Ammon-Treiber S, Hollt V. Morphine-induced changes of gene expression in the brain. Addict. Biol. 2005;10:81–89. doi: 10.1080/13556210412331308994. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Worley PF, Cole AJ, Baraban JM. Activation of the zinc finger encoding gene krox-20 in adult rat brain: comparison with zif268. Brain Res. Mol. Brain Res. 1992;13:263–266. doi: 10.1016/0169-328x(92)90034-9. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Pogge AR, Delongchamp RR, O'callaghan JP, Patel KM, Vrana KE, Freeman WM. A threshold neurotoxic amphetamine exposure inhibits parietal cortex expression of synaptic plasticity-related genes. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2006.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Janssen-Timmen U, Mattei MG, Zerial M, Bravo R, Charnay P. Structure, chromosome location, and expression of the mouse zinc finger gene Krox-20: multiple gene products and coregulation with the proto-oncogene c-fos. Mol. Cell Biol. 1989;9:787–797. doi: 10.1128/mcb.9.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Vesque C, Galliot B, Vigneron M, Dolle P, Duboule D, Charnay P. The segment-specific gene Krox-20 encodes a transcription factor with binding sites in the promoter region of the Hox-1.4 gene. EMBO J. 1990;9:1209–1218. doi: 10.1002/j.1460-2075.1990.tb08228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Mackey K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques. 1995;19:942–945. [PubMed] [Google Scholar]
- Day HE, Badiani A, Uslaner JM, Oates MM, Vittoz NM, Robinson TE, Watson SJ, Jr, Akil H. Environmental novelty differentially affects c-fos mRNA expression induced by amphetamine or cocaine in subregions of the bed nucleus of the stria terminalis and amygdala. J. Neurosci. 2001;21:732–740. doi: 10.1523/JNEUROSCI.21-02-00732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Thomas MJ, Robinson TE. Morphine-induced c-fos mRNA expression in striatofugal circuits: modulation by dose, environmental context, and drug history. Neuropsychopharmacology. 2004;29:1664–1674. doi: 10.1038/sj.npp.1300465. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Nader MA, Nader SH, Robertson DJ, Gioia L, Mitchell SM, Daunais JB, Porrino LJ, Friedman DP, Vrana KE. Chronic cocaine-mediated changes in non-human primate nucleus accumbens gene expression. J. Neurochem. 2001;77:542–549. doi: 10.1046/j.1471-4159.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Brebner K, Lynch WJ, Patel KM, Robertson DJ, Roberts DC, Vrana KE. Changes in rat frontal cortex gene expression following chronic cocaine. Brain Res. Mol. Brain Res. 2002a;104:11–20. doi: 10.1016/s0169-328x(02)00197-3. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Brebner K, Patel KM, Lynch WJ, Roberts DC, Vrana KE. Repeated cocaine self-administration causes multiple changes in rat frontal cortex gene expression. Neurochem. Res. 2002b;27:1181–1192. doi: 10.1023/a:1020929526688. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch. Gen. Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Permenter LK, Lake R, Worley PF, Kalivas PW. Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur. J. Neurosci. 2003;18:1645–1651. doi: 10.1046/j.1460-9568.2003.02880.x. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Roehrs TA. Mu-opioid self-administration vs passive administration in heroin abusers produces differential EEG activation. Neuropsychopharmacology. 2005;30:212–221. doi: 10.1038/sj.npp.1300596. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav. Neurosci. 2002;116:321–333. [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J. Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Miyashita T, Chawla MK, Sanderson J, Maes LI, Houston FP, Lipa P, McNaughton BL, Worley PF, Barnes CA. Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1077–1082. doi: 10.1073/pnas.0505519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Martin TJ, Co C, Dworkin SI, Smith JE. The effects of intravenous heroin administration on extracellular nucleus accumbens dopamine concentrations as determined by in vivo microdialysis. J. Pharmacol. Exp. Ther. 1995;273:591–598. [PubMed] [Google Scholar]
- Jacobs EH, De Vries TJ, Smit AB, Schoffelmeer AN. Gene transcripts selectively down-regulated in the shell of the nucleus accumbens long after heroin self-administration are up-regulated in the core independent of response contingency. FASEB J. 2004;18:200–202. doi: 10.1096/fj.03-0317fje. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, De Vries TJ, Schoffelmeer AN. Long-term gene expression in the nucleus accumbens following heroin administration is subregion-specific and depends on the nature of drug administration. Addict. Biol. 2005;10:91–100. doi: 10.1080/13556210412331284748. [DOI] [PubMed] [Google Scholar]
- Joseph LJ, Le Beau MM, Jamieson GA, Jr, Acharya S, Shows TB, Rowley JD, Sukhatme VP. Molecular cloning, sequencing, and mapping of EGR2, a human early growth response gene encoding a protein with "zinc-binding finger" structure. Proc. Natl. Acad. Sci. U. S. A. 1988;85:7164–7168. doi: 10.1073/pnas.85.19.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Wise RA. Brain and body hyperthermia associated with heroin self-administration in rats. J. Neurosci. 2002;22:1072–1080. doi: 10.1523/JNEUROSCI.22-03-01072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Spijker S, Voorn P, Binnekade R, Schmidt ED, Schoffelmeer AN, De Vries TJ, Smit AB. Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J. Neurochem. 2006;98:905–915. doi: 10.1111/j.1471-4159.2006.03917.x. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Burakov A, Romanova T, Dunaevsky I, Strassman R, Grinenko A. Ketamine psychotherapy for heroin addiction: immediate effects and two-year follow-up. J. Subst. Abuse Treat. 2002;23:273–283. doi: 10.1016/s0740-5472(02)00275-1. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Zvartau EE, Masalov DV, Tsoy MV, Burakov AM, Egorova VY, Didenko TY, Romanova TN, Ivanova EB, Bespalov AY, Verbitskaya EV, Neznanov NG, Grinenko AY, O'Brien CP, Woody GE. Naltrexone with or without fluoxetine for preventing relapse to heroin addiction in St. Petersburg, Russia. J. Subst. Abuse Treat. 2006;31:319–328. doi: 10.1016/j.jsat.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Lecca D, Valentini V, Cacciapaglia F, Acquas E, Di CG. Reciprocal effects of response contingent and noncontingent intravenous heroin on in vivo nucleus accumbens shell versus core dopamine in the rat: a repeated sampling microdialysis study. Psychopharmacology (Berl) 2007;194:103–116. doi: 10.1007/s00213-007-0815-y. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J. Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P, Revelant O, Bravo R, Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc. Natl. Acad. Sci. U. S. A. 1988;85:4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marttila K, Raattamaa H, Ahtee L. Effects of chronic nicotine administration and its withdrawal on striatal FosB/DeltaFosB and c-Fos expression in rats and mice. Neuropharmacology. 2006;51:44–51. doi: 10.1016/j.neuropharm.2006.02.014. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology (Berl) 1998;136:402–408. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J. Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am. J. Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. A Range of Research-Based Phamacotherapies for Addiction. Science. 1997 Mar 10;:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993;12:1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J. Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J. Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Abernathy KE, Oleson EB, Klugmann M, Lominac KD, He DY, Ron D, During M, Kalivas PW. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacology. 2006;31:768–777. doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: Implications for addiction. Biochem Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KL, Arroyo M, Everitt BJ. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. Eur. J. Neurosci. 2003;17:1964–1972. doi: 10.1046/j.1460-9568.2003.02617.x. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Day HE, Watson SJ, Akil H, Robinson TE. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus, and nucleus accumbens. Brain Res. 2001a;920:106–116. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Norton CS, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine and cocaine induce different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. Eur. J. Neurosci. 2001b;13:1977–1983. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]