Abstract
An effective immune response requires the engagement of host receptors by pathogen-derived molecules and the stimulation of an appropriate cellular response. Therefore, a crucial factor in our ability to control an infection is the accessibility of our immune cells to the foreign material. Exosomes—which are extracellular vesicles that function in intercellular communication—may play a key role in the dissemination of pathogen- as well as host-derived molecules during infection. In this review, we highlight the composition and function of exosomes and other extracellular vesicles produced during viral, parasitic, fungal and bacterial infections and describe how these vesicles could function to either promote or inhibit host immunity.
Keywords: exosomes, extracellular vesicles, immunity, pathogens
Introduction
The ability of the immune system to recognize and respond to pathogenic organisms is essential for the body's ability to control an infection. Divided into the innate and acquired branches, immune responses elicit protection against most pathogenic invaders. A number of mechanisms for how the immune system accomplishes this protection have been defined. Cytokines and chemokines, as well as other inflammatory mediators produced by infected or resident immune cells, clearly recruit and activate leukocytes and other cells, culminating in the elimination of the invading organism. Pathogen-derived products are major drivers of both the innate and acquired immune response. The microbial components that trigger the innate immune response do so either directly by inducing the production of immune effector molecules like reactive oxygen species or indirectly through stimulating the production of cytokines and chemokines. These microbial components that promote the innate immune response are generally defined as PAMPs (pathogen-associated molecular patterns). PAMPs are structurally diverse molecules found across many species of non-pathogenic and pathogenic organisms and include lipids, proteins, carbohydrates and genetic material. Pattern recognition receptors (PRRs), such as TLRs, present on leukocytes and various non-immune cells, can bind to PAMPs, thereby initiating cell-signaling cascades. This ultimately leads to the activation of an immune response against the pathogen. The importance of PRRs in the recognition and response to pathogens has been shown in both animal infection models and human studies 1. Given their role in immunity, it is not surprising that pathogenic organisms have evolved methods to modulate the binding and/or signaling through the PRRs as mechanisms to promote their virulence and evade surveillance by the immune system 2,3.
The mechanisms for exposure of PAMPs to PRRs differ between intracellular and extracellular pathogens. For extracellular pathogens, PAMPs are released through an active process or through shedding or death of the organism. The released factors can then directly bind PRRs on immune cells, stimulating or inhibiting host responses. In contrast, intracellular pathogens, such as Mycobacteria, Salmonella and Toxoplasma, produce PAMPs which—like the pathogens they are derived from—have limited exposure to the immune system. Nevertheless, the host does respond to these PAMPs, likely through multiple mechanisms. An obvious source of interaction is the cell invasion process 4,5,6. Moreover, a pathogen may be present in the extracellular milieu between host cell entries, allowing for release of PAMPs. This is likely a particularly important mechanism for viral proteins and viral RNA/DNA. The ability of HIV to invade host cells is known to be heterogeneous depending on the interactions with its cellular receptors; a slower invasion leads to increased phagocytosis of the virion by phagocytic cells 7. However, some intracellular pathogens like Listeria monocytogenes can go from cell to cell without being exposed to the extracellular environment 8. Debris from necrotic infected cells or release of apoptotic bodies from infected cells can also disseminate pathogenic components to surrounding cells/tissue 9. However, what has become increasingly clear is that exosomes and other extracellular vesicles released from infected cells, as well as from the pathogens, likely play an important role in this dissemination process 10,11,12. These components not only include PAMPs, but also T- and B-cell antigens, as well as pathogen-derived toxins.
In this review, we briefly introduce exosomes and how they are generated, as well as their role in non-infectious diseases, with an emphasis on their immune modulatory activity. We then focus in-depth on the production and activity of exosomes and other extracellular vesicles during infection, and how these vesicles could benefit the host immune response but also be used to promote pathogen survival. Finally, we discuss their therapeutic potential, including their use as vaccines and diagnostic tools.
Extracellular vesicles
Extracellular vesicles are broadly defined as membrane-bound vesicles released from cells. Those produced during an infection can be pathogen or host derived. The former include, for example, outer membrane vesicles from gram-negative bacteria and membrane vesicles from gram-positive bacteria. The content and function of these bacteria-generated vesicles has recently been under intensive investigation and excellently reviewed elsewhere 13,14,15. Although these vesicles likely play an important role during the course of an extracellular bacterial infection, their role in intracellular pathogen infections is less clear, as mechanisms to transport the vesicles outside the host cell are not known. Parasitic and fungal pathogens also release extracellular vesicles, which may function in modulating the immune response 16,17.
Host-derived vesicles are present during viral, bacterial, parasitic and fungal infections. These vesicles have different origins and composition and, based on their biogenesis, are divided into three main categories: apoptotic bodies, exosomes and microvesicles. All three of these cell-derived vesicles are enclosed by a lipid bilayer, but vary in size (from 30 to 2,000 nm in diameter), as well as in composition. In contrast to microvesicles, which are generated by budding from the plasma membrane 18, exosomes are derived from the endolysosomal pathway and have a unique lipid and protein makeup. Exosomes have been the most studied in the context of infection. An important note, however, is that exosome purity was not always analyzed in these studies, and therefore, the vesicle population may have consisted of both exosomes and microvesicles, which overlap in size and density. Nevertheless, we will use the terminology as defined in the original studies when discussing the results.
Exosomes
Exosomes are formed through the fusion of multivesicular bodies (MVBs) with the plasma membrane and subsequent release of intraluminal vesicles (ILVs) as exosomes (Fig1). Exosomes are 30–100 nm vesicles, surrounded by a lipid bilayer, that have a density of 1.13–1.19 g/ml. Biophysically, exosomes are equivalent to cytoplasm enclosed in a lipid bilayer with the external domains of transmembrane proteins exposed to the extracellular environment. EM studies have demonstrated the fusion of the limiting membrane of MVB with the plasma membrane, as well as the release of ILVs, in different cell types of hematopoietic origin, such as Epstein–Barr virus (EBV)-transformed B cells 19, mastocytes 20, DCs 21,22, platelets 23, macrophages 10 and cells of non-hematopoietic origin such as neurons and epithelial cells 24,25,26. Exosomes can act locally or circulate through various bodily fluids, including blood and lymph, resulting in a systemic response 27. Exosomes were first identified in the culture media of reticulocytes 28,29. However, over the past two decades, the study of exosomes has extended to most cell types, and they have been isolated from different organisms—including unicellular eukaryotes—suggesting that this is an evolutionarily conserved mechanism of cell–cell communication. The advantage of using exosomes for cell–cell communication stems from their complex composition, which allows more control over the communication process. Moreover, the presence of signaling lipids, proteins and various species of RNA within a single structure can lead to rapid and profound changes in the target cell, enabling a swift response to cellular perturbations, which can be local or systemic. These changes may be induced under physiological or pathological conditions. Although the complexity of exosomes has clear benefits to the organisms that produce them, it has made the study of their function exceedingly difficult, as the effect of an exosome or pool of exosomes is a result of all the different components within them, including lipids, proteins, carbohydrates and RNA. Moreover, the tools available to modulate exosome production and composition in vitro and in vivo are severely limited, hampering our ability to define exosome function in normal and diseased states. Nevertheless, we have gained important insights into exosome biogenesis, composition and function over the past decade, a decade that has seen a rapid expansion in publications on this type of extracellular vesicle.
Figure 1. Exosome biogenesis.
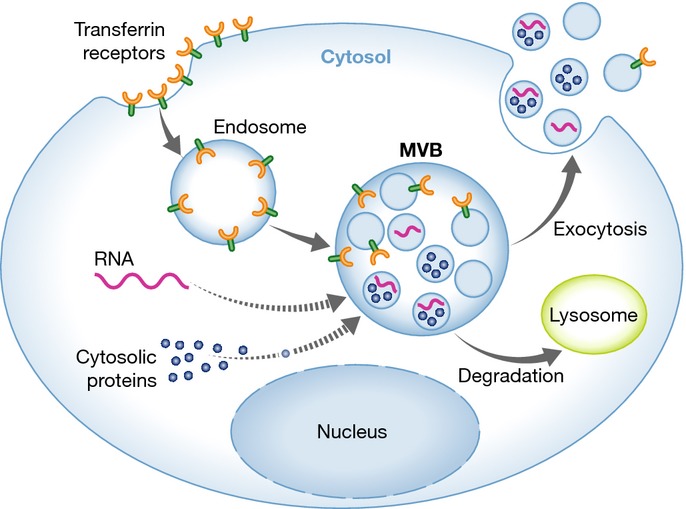
Lipids, proteins and nucleic acids are transported to MVBs and onto or into the intraluminal vesicles, which upon fusion of the MVB with the plasma membrane are released as exosomes. Originally identified as a way to release transferrin receptor from maturing reticulocytes, other plasma membrane proteins have been shown to be targeted to MVBs through various mechanisms and released on exosomes. RNA and cytoplasmic proteins are also transported to MVBs, although the mechanisms mediating this transport are less understood (indicated by dashed line). See Glossary for definitions.
Exosome biogenesis
A major mechanism for down-regulating and degrading plasma membrane receptors is through their endocytosis and trafficking to an MVB, which can subsequently fuse with the lysosome to mediate protein degradation 30. However, at least a subpopulation of MVBs can also fuse with the plasma membrane, resulting in the release of the intraluminal vesicles (ILV) as exosomes. Despite their discovery nearly three decades ago, the mechanism for MVB biogenesis and exosome release is still being defined. Several models have been suggested as a mechanism for ILV formation. Initial studies in yeast demonstrated a role for the ESCRT proteins 31. Although the ESCRT machinery has primarily been studied for its role in the endosomal sorting and degradation of ubiquitinated proteins, it has also been implicated in mediating membrane invagination 32,33. Through its ubiquitin-interacting domains, ESCRT-0 clusters ubiquitinated proteins for delivery into MVBs 34. ESCRT-0 subsequently recruits ESCRT-1 to the endosomal membrane, which in turn recruits the remaining members of the ESCRT machinery, ESCRT-II and ESCRT-III 35,36. Through the formation of polymeric filaments mediated by ESCRT-III, membrane invagination results in ILV formation 37 (for a recent review, see 38). Indeed, various studies support a role for the ESCRT machinery in exosome formation. Proteomic analysis of exosomes has demonstrated the presence of ESCRT machinery within exosomes, and knockdown of key components of ESCRT machinery can abrogate ILV formation and exosome release 39, although this is likely cell type-specific 40,41. While this general model for MVB biogenesis has been well characterized, it is unclear whether this constitutes the major mechanism of MVB formation. A number of studies suggest there are ESCRT-independent mechanisms for MVB biogenesis and exosome release. In oligodendroglial cell lines, exosome formation is driven by the production of ceramide, rather than the ESCRT machinery 41. Stuffers and colleagues found that depleting specific subunits from the four ESCRTs complexes did not completely inhibit MVB formation 40. Furthermore, a mechanism independent of both ESCRTs and ceramide has been proposed. Studies by van Niel and colleagues found that the tetraspanin CD63, which is present on exosomes in high abundance, mediates cargo sorting and ILV formation 42. Additionally, CD81 has been demonstrated to mediate cargo sorting of tetraspanin ligands, such as Rac GTPase, although knockdown of this tetraspanin does not appear to alter MVB morphology or exosome secretion 43. These different observations suggest that the mechanism for exosome biogenesis and protein sorting may be cell type-specific or specific to different subpopulations of MVBs within a cell. In support of the latter, Stoorvogel and colleagues have shown that within immature DCs, the MHC molecules are targeted to MVBs that are low in cholesterol but enriched for lysobisphosphatidic acid, which are destined for lysosomal degradation. However, in mature DCs, MHC molecules are sorted into MVBs that are enriched in CD9 and cholesterol, which are targeted for fusion with the plasma membrane 44.
Once MVBs are formed, their fusion with the plasma membrane is mediated by the cytoskeleton, fusion machinery—such as the SNARE proteins—and molecular switches (such as small molecular weight GTPases) 45. Rab GTPases are members of the Ras GTPase superfamily and are known to regulate four steps in membrane trafficking: vesicle formation, trafficking, tethering and fusion with target organelles. Almost 70 different Rab GTPases have been identified to date in mammalian cells 46. Several of these have been found on exosomes, including Rab5, Rab11, Rab27 and Rab35. Some of these Rab effectors have been experimentally shown to function in exosome release. Early studies suggested that Rab11 might function to promote MVB fusion with the plasma membrane in the K562 erythroleukemic cell line 47. More recent studies have implicated Rab35 in mediating MVB docking to the plasma membrane in neuralgia cells, where depletion of Rab35 resulted in a significant loss in exosome release 48. Rab27a and Rab27b were also shown to have different, but sometimes redundant, roles in MVB biogenesis, with Rab27a more implicated in mediating MVB docking to the plasma membrane 49. Although the Rab GTPases have been implicated in MVB trafficking and fusion, their role in the process is still under investigation and will likely be cell type-dependent, as well as dependent on the physiological/pathological state of the cell.
Exosome composition
Exosomes contain all types of biomolecules, including proteins, carbohydrates, lipids and nucleic acids. Their lipid and protein composition has been extensively analyzed by various techniques, including Western blotting, FACS, immuno-EM and mass spectrometry. Exosome composition will vary depending on the cell type of origin, its physiological/pathological state and even the cell site of origin, as seen in epithelial cells. Epithelial exosomes have different composition if they are released from the apical or basolateral surfaces 50. Cell type-specific markers can help define the exosome cellular origin; for example, the presence of T-cell or B-cell receptors is indicative of T-cell and B-cell origin, respectively. The exosome protein composition can also be informative of the existence of a pathology, as they can, for example, carry tumor antigens or inflammatory mediators. In addition, exosomes contain a number of common proteins, including Tsg101, Hsc70 and various tetraspanins 51, as well as proteins that participate in vesicle formation and trafficking, such as the LBPA-binding protein, Alix 52. Exosome lipid composition has also been well characterized. As for proteins, the ratios of the different lipids can vary between exosomes released from different cellular origins. In general, exosomes are enriched in lipids such as sphingomyelin, phosphatidylserine, gangliosides and cholesterol, as compared to plasma membranes and other intracellular membranes 53. A number of reviews have highlighted the protein and lipid content of exosomes 54,55, and various databases have cataloged the protein, lipid and RNA content of exosomes (ExoCarta, http://www.exocarta.org/, Vesiclepedia, http://microvesicles.org/).
Most recent studies have focused on exosomal RNA; the types of RNA and their nucleotide sequence, their ability to be transferred between cells, their function once transferred and the mechanism by which they are trafficked to MVBs and into exosomes. Pioneering studies by Valadi and colleagues showed that exosomes are enriched in mRNA and miRNA 56. More recent studies have identified other non-coding RNAs in exosomes, but limited amounts of DNA or ribosomal RNA 57. The exosomes derived from a human (HMC-1) and mouse (MC/9) mast cell lines were found to transport mRNA to neighboring mast cells. This mRNA was subsequently translated, indicating that it is biologically active 56. Exosomes released by immune cells have been shown to contain a selective repertoire of miRNAs that can be functionally transferred to recipient cells 58,59. The source of these exosomes/extracellular vesicles were cultured cells 60 and body fluids 61,62. Together, these data suggest that exosomes function as carriers of genetic information and that this genetic material plays a role in cell–cell communication. However, the exosomal RNA content differs both in quantity and in composition depending of the cellular origin and cellular environment. Eldh and colleagues found that the exosomes released by mast cells differ in their mRNA content after exposure to an oxidative stress, and oxidative stress resistance was induced in recipient cells 63. These results indicate that the incorporation of RNA into vesicles is a regulated event leading to selective packaging of RNA into exosomes and other extracellular vesicles 64,65. The mechanism(s) responsible for the targeted loading of RNA into exosomes is still being defined and remains an active area of investigation.
In addition to host components, a number of pathogen-derived components have been found on exosomes after cell or animal infection (Table1). Unfortunately, we know very little about how these diverse pathogen-derived proteins, glycolipids, etc. are sorted to MVBs and onto exosomes (see Sidebar A). Much of our current understanding stems from studies of viruses, where viral assembly and exosome biogenesis share many similarities. For example, HIV assembly and release from infected cells depend on both ESCRT machinery and tetraspanin-rich lipid domains 66,67. The presence of viral proteins in exosomes and the similarities in biogenesis and assembly suggest that a degree of ‘crosstalk’ or ‘hijacking’ could be responsible for sorting the viral proteins into exosomes. However, some viral proteins—such as the HIV protein Nef—may contain necessary signals to mediate their direct sorting into exosomes 68. For other types of pathogens, even less is understood. Some intracellular bacterial pathogens, such as Mycobacterium tuberculosis, are also known to interfere with host machinery implicated in exosome biogenesis, such as ESCRTs 69, although the extent to which this contributes to protein sorting during exosome biogenesis is unclear. Based on our observations, sorting of mycobacterial proteins seems to be independent of cell entry mechanisms, as mycobacterial proteins are found on exosomes whether added as free protein, and therefore taken in through an endocytic route, or expressed in mycobacteria, which enters by phagocytosis 11. This finding suggests that these mycobacterial proteins have the necessary ‘signal’ to be trafficked to the MVB during exosome biogenesis. However, further investigation is needed to shed light on potential sorting mechanisms.
Table 1.
Pathogen components present on exosomes/extracellular vesicles released from infected cells
| Pathogen | Vesicle contents | References |
|---|---|---|
| HIV | Gag proteins | 119 |
| Nef protein | 68,121,123,124 | |
| TAR transcripts | 120 | |
| EBV | Viral RNAs | 134 |
| LMP1 | 129 | |
| LMP2a | 130 | |
| CMV | Glycoprotein B | 138 |
| Hepatitis C virus | Viral RNAs | 70 |
| Viral RNA/proteins | 141,142 | |
| Envelope glycoprotein E2 | 139 | |
| HSV | Viral tegument proteins and various glycoproteins | 146 |
| Toxoplasma gondii | PAMPs | 10 |
| Leishmania mexicana | GP63 | 156 |
| Proteomic analysis | 156 | |
| Leishmania major | GP63 | 157 |
| Leishmania donovani | GP63 | 158 |
| Proteomic analysis | 155 | |
| Plasmodium yoelii | Proteomic analysis | 160 |
| Plasmodium falciparum | EBA-175, EBA-181 | 162 |
| Proteomic analysis | 162 | |
| PfPTP2 | 163 | |
| Mycobacterium tuberculosis | LAM, PIM | 10,180,181 |
| 19 kDa lipoprotein | 10 | |
| Proteomic analysis | 11 | |
| mRNA | J.S. Schorey, unpublished data | |
| Mycobacterium avium | GPLs | 182 |
| Salmonella typhimurium | LPS | 10 |
| Mycoplasma | Lethal factor | 195 |
| Proteomic analysis | 195 |
Sidebar A:In need of answers.
Through which mechanism(s) are microbial components transported to MVBs and onto exosomes?
Do pathogens modify the MVB/exosome biogenesis pathway to limit the exposure of their components to the extracellular environment? Do they have mechanisms to specifically target certain molecules to MVBs/exosomes?
How does the function and composition of exosomes vary with the type or virulence of a pathogen?
Do exosomes function as an important mechanism for antigen presentation during an infection? Under which circumstances does this occur?
Which cells, in addition to those infected, release exosomes during the course of an infection? Do these exosomes affect the immune or inflammatory response?
Viral RNAs have also been found within exosomes. HCV viral RNA transport to exosomes was found to be dependent on the ESCRT machinery and on Annexin A2, an RNA-binding protein involved in membrane vesicle trafficking 70. Similarly, EAP30—a subunit of ESCRT-II—controls HIV-1 RNA trafficking and gene expression through a complex formed by HIV-1 Gag, ESCRT-II and Staufen-1 71. The mechanism by which EAP30/ESCRT-II facilitates HIV-1 genomic RNA trafficking remains unclear, although—considering the roles for ESCRT-II in the nucleus 72—EAP30/ESCRT-II is likely part of the RNP complex that mediates the nuclear export of viral RNA. Other partners of EAP30, such as EAP45, have an RNA-binding domain that is likely conserved 73,74, and RILP—which associates with EAP30—can also have an effect on the localization of viral RNA in the cytoplasm. EAP30/ESCRT-II could also contribute to the stability of cellular factors that are required for viral RNA trafficking.
Exosomes as modulators of the immune response
Most studies of exosomes and their effect on the immune response are in the context of cancer and autoimmunity. These studies have: (i) defined the host molecules that facilitate exosome transfer; (ii) characterized the presence of tumor antigens on exosomes and mechanisms by which these antigens can promote T-cell activation; (iii) defined the mechanisms by which some exosomes can induce T-cell anergy and deletion; and (iv) defined the RNA content within the exosomes, as well as characterized their transcriptional and translational effect on recipient cells 75. Other studies have characterized exosomes as drivers of an innate immune response, although significantly less work has been done in this area 76.
Innate immunity
Dendritic cells produce exosomes constitutively and have been implicated in the activation of the innate immune response (Fig2). Exosomes from both immature and mature DCs contain multiple TNF superfamily members—such as TNF, FasL and Trail—on their surface, which directly bind to the surface receptors on NK cells to enhance their cytotoxic activity. However, the activation of NK cells is significantly stronger in response to exosomes released from activated DCs 77. Similarly, exosomes from DCs express BAT3 (HLA-B-associated transcript-3) and thus are recognized by the NK surface receptor, NKp30, leading to NK-cell activation 78. DCs activated by lipopolysaccharide (LPS) release extracellular vesicles that can stimulate epithelial cells to secrete chemokines—such as IL-8 and RANTES—which may be an important component in the pathogenesis of sepsis 79. DC-derived microvesicles have also been shown to induce NF-κB activation in microglia cells, which may play a role in the inflammatory response observed in the CNS during experimental autoimmune encephalomyelitis (EAE) 80. IL-1β, a major driver of the innate immune response, is produced as a pro-form and converted to its active form through cleavage by the inflammasome. The mature form is released by activated macrophages and DCs through a non-classical secretion pathway. Qu and colleagues propose that trafficking via exosomes may be one mechanism for IL-1β release, although this was not definitively proven because exosomes containing the mature IL-1β could not be isolated after ATP stimulation of bone marrow-derived macrophages 81,82. In the presence of oxidized low-density lipoprotein (oxLDL)-conjugated immune complexes, macrophages release exosomes containing IL-1β, as well as increased levels of acid sphingomyelinase and HSP70, and these exosomes may promote the propagation of atherosclerotic plaques 83. These and other studies clearly demonstrate a role for exosomes/extracellular vesicles in regulating inflammatory and innate immune responses. As described below, their role is likely more pronounced in the context of an infection, as these exosomes could carry both host and pathogen components.
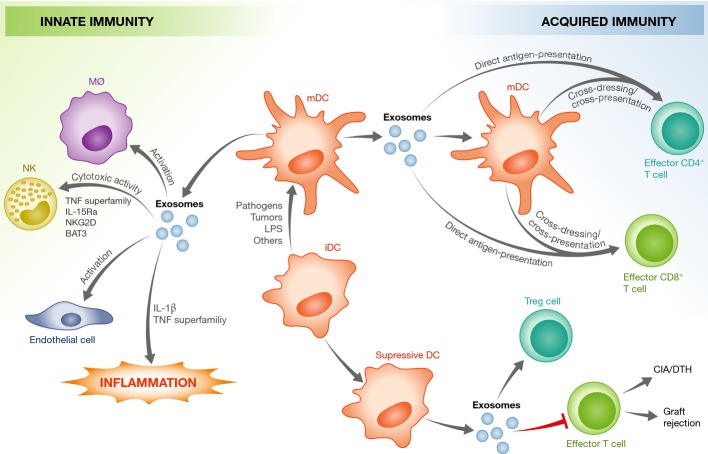
Figure 2. DC-derived exosomes modulate innate and acquired immune responses.
Exosomes from mature DCs (mDCs) can provide antigen to T cells, stimulate innate immune responses in various immune and non-immune cells and promote a pro-inflammatory response. This is mediated by host factors present within the exosomes, such as those indicated in the figure. In contrast, exosomes from immature DCs (iDCs) seem to be immuno-suppressive, and induce apoptosis in effector T cells or promote the activation of regulatory T cells. CIA, collagen-induced arthritis; DTH, delayed-type hypersensitivity. See Glossary for definitions.
Acquired immunity
The presence of MHC class I and II, as well as T-cell co-stimulatory molecules, on the surface of macrophage- and DC-derived exosomes has attracted the interest of immunologists, as exosomes may be an important mechanism of antigen presentation. Since exosomes can carry foreign antigens in the context of an infection, defining the mechanisms by which this antigen presentation occurs is important to understanding the immune response to the pathogen. There are currently three proposed models for exosome-mediated antigen delivery to T cells (Fig2).
Cross-dressing pattern. In this model, DCs capture extracellular exosomes and directly present antigenic peptide–MHC complex to CD4+ or CD8+ T cells, suggesting that exosomes shuttle peptide–MHC II complex between DC populations 84,85,86,87.
Cross-presentation pattern. In this scenario, bystander DCs capture the exosomes containing the proteins/peptides and then present these exosome-delivered antigens on their endogenous MHC class I and II molecules for subsequent activation of antigen-specific T cells 88,89. Both of these mechanisms require the capture of exosomes by recipient cells, and thus the receptors and ligands that facilitate this interaction have been the object of study. Several exosome surface ligands and adhesion molecules have been identified, including MFG-E8/lactadherin, tetraspanins, ICAM1 and phosphatidylserine 22,90,91. MFG-E8 binds to the integrins αvβ3 and αvβ5, which are constitutively expressed by human DCs and macrophages, but also recognizes phosphatidylserine on the cell surface through its phosphatidylserine-binding domain 22,90. Exosomal phosphatidylserine may also interact with DC surface protein Tim4 92,93. The uptake of DC-derived exosomes is not limited to bystander DCs, as exosomes can transfer peptide–MHC I complex and co-stimulatory molecule CD80 to non-specific CD4+ T cells, which then acquire the capacity to activate antigen-specific/naïve CD8+ CTLs in vivo and in vitro 94. Furthermore, upon the formation of an immunological synapse between mature DCs and T lymphocytes, exosomes released from DCs specifically bind to activated T cells in a LFA-1-dependent manner and could potentiate T-cell activation 95.
Direct exosome-induced T-cell activation. Since DC-derived exosomes contain MHC class I and II, as well as co-stimulatory molecules, they have the potential to directly activate CD4+ or CD8+ T cells 87,96,97,98. Although this mechanism has mostly been observed for memory or previously activated T cells 99,100, some studies have shown exosome-mediated activation of naïve T cells. Using transfected Drosophila APCs and mouse CD8+ T cells, Hwang and colleagues demonstrated that APC-derived extracellular vesicles directly activate naïve CD8+ T cells in vitro 101. This activation requires the presence of ICAM1 on the vesicles, which likely functions to promote adhesion between the exosomes and T cells. The presence of the co-stimulatory molecule B7 on the vesicles was also necessary. Direct stimulation of naïve PBMC-derived CD8+ T cells was also detected in the presence of viral MHC I-specific peptide-loaded exosomes 91. Although direct activation of naïve T cells has been observed, studies suggest that T-cell stimulatory activity by vesicles alone is 10- to 20-fold less efficient than when presented by an APC 19,102, suggesting that direct exosome–T-cell interaction may not be a major mechanism of naïve T-cell activation in vivo. Nevertheless, both in vitro and in vivo data suggest that DC-derived exosomes likely function in antigen presentation to T cells. This ability to stimulate T cells may depend on the activation state of the DC, as exosomes from activated DCs have more MHC class II, ICAM1 and costimulatory molecules than exosomes from immature DCs 91. Unfortunately, we do not yet have the tools to specifically block exosome-mediated antigen presentation in vivo and, therefore, the contribution of the exosome to T-cell activation in animal models is still unclear.
In contrast to exosomes from mature DCs, those derived from immature DCs have been shown to promote T-cell anergy/deletion as well as to promote the activation of regulatory T (Treg) cells. Thus, exosomes have been evaluated for the potential treatment of autoimmune diseases (Fig2), a topic that has recently been the subject of several excellent reviews 75,103,104. Exosomes are also released from lymphocytes, such as CD4+ and CD8+ T cells, which constitutively secret exosomes containing the TCR/CD3 complex. However, the production of these exosomes increases when they are activated through TCR ligand binding 105. The function of these exosomes will depend on the activation status and tissue microenvironment of the T cell and can broadly be classified into two main categories based on their immunological functions, either activating or suppressive.
Immuno-activation. Exosomes from activated T cells may potentiate an immune response through effects on resting autologous T cells. For example, when activated with anti-CD3 and IL-2, human peripheral CD3+ T cells release exosomes that stimulate cytokine secretion and proliferation of CD8+ T cells in vitro 106.
Immuno-suppression. CD4+CD25+FOXP3+ Treg cells release exosomes containing CD73, an ecto-5′-nucleotidase enzyme that converts extracellular adenosine-5-monophosphate to adenosine, which is an anti-inflammatory mediator, thereby inhibiting CD4+ T-cell proliferation 107. Exosomes from activated effector CD4+ T cells can interact with DCs through specific peptide–MHC I/TCR and CD54/LFA-1 interactions and inhibit the ability of the recipient DCs to stimulate CD4+ T-cell proliferation, as well as inhibit an in vivo CD8+ CTL response 108. Moreover, CD8+CD25+ Tregs release exosomes that could inhibit a CD8+ T-cell antitumor response 109. Exosomes released from CD4+ T cells containing FasL induce apoptosis in recipient T cells 110,111. Further, human B-cell-derived lymphoblastoid cell lines (LCL) constitutively produce FasL-positive exosomes that can induce apoptosis in CD4+ T cells 112.
The balance between exosomes that promote T- and B-cell activation and those that inhibit lymphocyte function is thought to be an important component in the pathogenesis of many diseases including cancers, cardiovascular diseases and autoimmune diseases. As reviewed below, during an infection, exosomes also function in immune modulation, including activation and inhibition of T cells, which likely has an important role in disease pathogenesis.
Exosomes and other extracellular vesicles in infectious disease
Exosomes and other extracellular vesicles, both pathogen- and host-derived, have been isolated and characterized in all known pathogen classes, including viruses, bacteria, fungi and parasites (Table2). As one would expect, the composition and activity of these exosomes vary significantly between the different taxa and even between pathogens in the same genus. Other factors such as the animal model used, the experimental design, the cell types chosen for the infection and which recipient cells are targeted can affect the observed immune response. We classify our current knowledge according to pathogen class and use specific examples to illustrate the major points.
Table 2.
Exosome production by host cells upon infection
| Pathogen | Host resources | References |
|---|---|---|
| Viruses | ||
| Human immunodeficiency virus (HIV) | DCs, T cells | 68,71,119,120,121,124,127 |
| Epstein–Barr virus (EBV) | B cells | 129,130,131,133,134,135 |
| Cytomegalovirus (CMV) | Endothelial cells | 137,138 |
| Herpes simplex virus (HSV) | Melanoma cells | 144,145,146 |
| Hepatitis C virus (HCV) | Hepatocytes, Serum | 70,139,140,141,142 |
| Bacteria | ||
| Chlamydia trachomatis | Fibroblast | 198 |
| Chlamydia pneumoniae | ECV304 cells | 197 |
| Mycobacterium tuberculosis | Macrophages, Plasma | 10,11,180,181,186,187,190,191 |
| Mycobacterium bovis BCG | Macrophages, Plasma, BALF | 186,189 |
| Mycobacterium smegmatis | Macrophages | 184 |
| Mycobacterium avium | Macrophages | 182,183,184 |
| Salmonella typhimurium | Macrophages | 10 |
| Mycoplasma spp. | Tumor cells | 195 |
| Bacillus anthracis | Retinal pigment epithelial cells | 196 |
| Protozoa | ||
| Leishmania donovani | Macrophages | 156 |
| Plasmodium vivax | Plasma | 161 |
| Plasmodium berghei | Plasma | 164 |
| Plasmodium falciparum | Erythrocytes | 162,163,164 |
| Plasmodium yoelii | Reticulocyte, Plasma | 160 |
| Toxoplasma gondii | Macrophages | 10 |
| Trypanosoma cruzi | Macrophages | 165 |
| Fungi | ||
| Malassezia sympodialis | DCs, Plasma | 176 |
Viruses
As mentioned above, a number of studies have recognized commonalities between the assembly and release of viruses and exosomes, and identified key host components that are used in both viral and exosome biogenesis. The readers are referred to recent reviews that have highlighted these interesting studies 113,114. During the course of a viral infection, host cells release exosomes and other extracellular vesicles carrying viral and host components, which can modulate the immune response.
Exosomes and other extracellular vesicles generated during HIV infection have been extensively studied. Most studies have addressed the origin of viral budding (plasma membrane or exosomes) and the role of host proteins such as the ESCRTs in this process 115,116, although a few have looked at the role of host-derived extracellular vesicles in the pathogenesis of HIV. Exosomes and other extracellular vesicles released from infected PBMC or megakaryocytes and platelets contain CCR5 and CXCR4, respectively. In both cases, the chemokine receptors could be transferred to target cells, enhancing their susceptibility to HIV infection 117,118. The HIV membrane protein Gag has been found on exosomes from Jurkat T cells, and this transport seems to be dependent on its oligomerization 119. Pathogen-associated RNAs, including viral TAR RNA, have been detected in exosomes isolated from the supernatants of HIV-1-infected T cells and from patient sera. TAR RNA can down-regulate apoptotic signals, and therefore, recipient cells might support enhanced HIV replication 120. Additionally, the HIV virulence factor Nef was detected in exosomes released from HIV-infected cells, the function of which has been extensively studied 121. Mortalin, a member of the HSP70 family, appears to interact specifically with Nef and may play a role in its targeting to MVBs 122. Work by Campbell and colleagues showed that Nef is present in exosomes secreted from transfected HEK 293 cells, which upon fusion with uninfected Jurkat T cells restores infectivity to Nef (-) HIV virions 121. Nef-containing exosomes released from transfected SupT1 and Jurkat T cells can induce CD4+ T-cell apoptosis in vitro, pointing to their possible role in the T-cell depletion inherent to HIV pathogenesis 68. The effect of Nef may not be limited to T cells, as endothelial cell exposure to Nef results in an increased production of ROS and MCP-1 and increased endothelial cell apoptosis 123. Nef expression also appears to affect the cellular miRNA content within exosomes, potentially limiting the effects of RNA interference in recipient cells 124. In contrast to the immune inhibitory effects, exosomes secreted from CD8+ T cells suppress HIV-1 transcription within infected cells in a protein-dependent but antigen-independent and MHC-unrestricted manner 125. Furthermore, host-derived exosomes containing APOBEC3G, a cytidine deaminase that functions in cellular anti-retroviral activity, can inhibit HIV replication in the recipient cells 126. These results suggest that exosomes and other extracellular vesicles can either promote or inhibit HIV replication, and the balance of these two functions will depend on the cells releasing the vesicles, the target cells and likely many other as yet undefined factors.
Human gamma herpesviruses, such as the Kaposi sarcoma-associated virus and EBV, have complex effects on the immune system, leading not only to viral infection but also, in some cases, to cancer, such as nasopharyngeal carcinoma (NPC). Most research on exosomes from herpesvirus-infected cells has focused on their role in cancer pathogenesis, more than on viral pathogenesis and immune responses 127,128. Nevertheless, a few key studies have shed light on the immunology of EBV infection in the context of extracellular vesicles. Dukers and colleagues discovered that LMP1, a signal transduction protein important in EBV infection, could block the proliferation of T cells and inhibit NK-cell cytotoxicity 129. LMP1 is present on exosomes from EBV-infected cells, suggesting that they could be a vehicle for the immunosuppressive effects of LMP1 during EBV infection. LMP2a was also found on exosomes, but its function within these extracellular vesicles has not been explored 130. Galectin-9, which is present in exosomes released from EBV-infected cells, induces apoptosis of EBV-specific CD4+ T cells through its interaction with the T-cell immunoglobulin mucin-3. This receptor is known to negatively regulate both macrophage and T-cell activation 131. However, exosomes from EBV-infected cells have also been shown to contain the dUTPase, which can induce NF-κB activation and cytokine release from primary DCs and PBMC, driving a pro-inflammatory/anti-viral response 132. In addition, EBV encodes for a surprising number of miRNAs—44 have been currently identified 133,134. These miRNAs not only modify the transcriptome of the infected cells, but also that of uninfected cells through the transfer of the viral miRNA through exosomes. Pegtel and colleagues demonstrated that EBV-infected B cells release exosomes containing EBV miRNAs that induce miRNA-mediated repression of confirmed EBV target genes, including CXCL11, an immunoregulatory gene involved in antiviral activity 135. The ability of EBV miRNAs to be transferred from infected B cells to non-infected T cells and monocytes suggests that exosomal transport of viral miRNAs could contribute to EBV persistence in humans. This hypothesis is supported by the observation that EBV miRNA present in exosomes can target the miR-223 binding site in the 3′-untranslated region of the NLRP3 inflammasome, inhibiting production of IL-1β 136.
Exosomes released from CMV-infected human endothelial cells can stimulate memory CD4+ T cells isolated from CMV-infected donors, likely through the transfer of antigen to allogenic DCs 137. In the case of the human CMV, microvesicles released by infected cells contain soluble DC-SIGN, a C-type lectin family molecule, in complex with the CMV glycoprotein B. The transport of this complex through microvesicles increases the susceptibility of recipient cells to CMV 138 Similarly, in HCV-infected patients, the association of cellular membrane protein CD81 with HCV envelope glycoprotein E2, and the subsequent release of this complex within microvesicles, increases the infectivity of previously naïve recipient cells 139. HCV structural proteins have been found in exosomes isolated from the sera of HCV-infected patients 139, and exosome-like vesicles have been purified from infected cells 140. Purified exosomes isolated from HCV-infected human hepatoma cells contain intact viral particles with full-length viral RNA and protein, and these exosomes can transfer infectivity to naïve hepatoma cells, resulting in a productive infection 141,142. Dreux and colleagues reported that exosomes released from HCV-infected cells can induce IFN-α release from uninfected plasmacytoid DCs due to the viral RNA present within the exosomes 70. These results suggest that export of viral RNA may serve both as a viral strategy to evade pathogen sensing within infected cells and as a host strategy to induce an innate response in bystander cells. Similar findings were reported by Li and colleagues, who showed that IFN-α-treated liver non-parenchymal cells release exosomes that contain a number of host molecules with antiviral activity, and this activity can be transferred to hepatocytes that were previously permissive to hepatitis B virus infection 143. HSV may divert transport of HLA-DR to MVBs and exosomes, thus limiting the amount of peptide–MHC complex on the cell surface of the HSV-infected cells, allowing the virus to evade detection by the immune system 144. HSV also releases exosome-like vesicles, previously known as L particles, which are of similar size to exosomes and originate on an internal membrane, but are not exosomes 145. These vesicles contain viral tegument proteins and glycoproteins, including transcription factors that can facilitate the replication of other viral particles in recipient cells 146. The generation of a recombinant Coxsackie virus expressing ‘fluorescence timer’ protein (Timer-CVB3) has recently allowed to detect increased shedding of microvesicles containing virus in partially differentiated infected progenitor cells in vivo, suggesting their role in virus dissemination 147.
Extracellular vesicles have thus been implicated in the pathogenesis of many different viruses (Fig3). Upon release, exosomes and other extracellular vesicles are ‘captured’ by cells and the transfer of host and viral proteins and/or RNA could enhance viral infection and replication in recipient cells, as observed for HIV and other viruses. Furthermore, as highlighted above, exosomes released from virus-infected cells can inhibit both CD4+ and CD8+ T-cell activation. Other studies further suggest that infected cell-derived extracellular vesicles can promote the innate and acquired immune response through cytokine production and antigen presentation 143,148. Additional studies are clearly needed to unravel how and when these extracellular vesicles promote or limit anti-viral immunity.
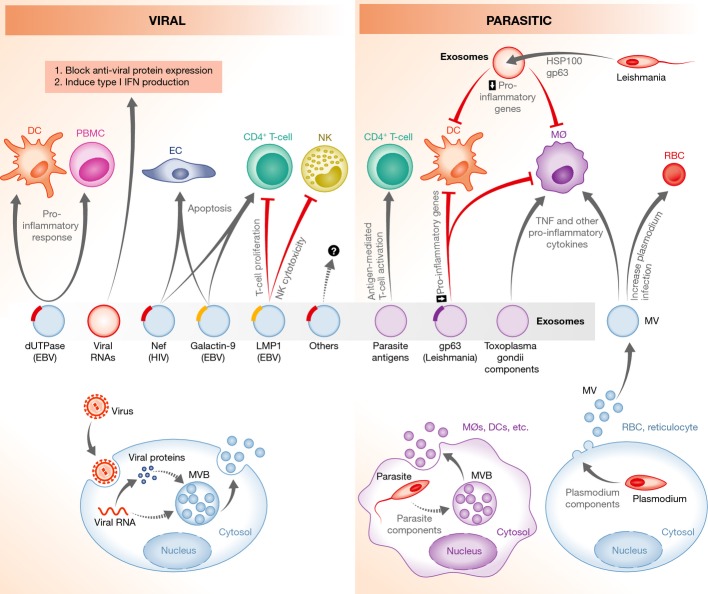
Figure 3. Modulation of host immunity by exosomes during a viral or parasitic infection.
Virus- or parasite-infected cells, or the parasites themselves, release exosomes or microvesicles that can stimulate T-cell activation by providing antigens to APCs. In contrast, exosomes containing microbial molecules, such as HIV Nef or Leishmania GP63, can block T-cell activation or induce the apoptosis of immune effector cells. Extracellular vesicles released from virus- or parasite-infected cells can modulate both the innate and acquired immune response. In some cases, this is to the benefit of the pathogen, whereas in others, this is to the benefit of the host. Dashed lines indicate unknown mechanisms. See Glossary for definitions.
Parasites
Toxoplasma gondii was the first non-viral pathogen to be studied in the context of exosomes. In studies by Dimier-Poisson and colleagues, DCs were pulsed with T. gondii proteins, and the exosomes released from the DCs could stimulate a protective immune response against acute and chronic T. gondii infection when adoptively transferred to mice. This response was antigen specific and included both cellular and humoral immunity 149. When used as a vaccine, these exosomes were also associated with the development of fewer brain cysts in T. gondii-infected CBA/1 mice 150. Exosomes isolated in a similar manner were shown to be an effective vaccine for preventing congenital toxoplasmosis when given to mice before pregnancy 151. We found that exosomes released from the human monocytic cell line THP-1 after infection with T. gondii could stimulate non-infected THP-1 cells to produce TNF-α and other pro-inflammatory mediators 10, although the exosomal component responsible for this activity remains undefined. T. gondii resides within a vacuole with only limited contact with the endocytic network 152, so how the parasite components are transported to the MVB and onto exosomes is unclear. However, T. gondii may be in contact with the endosomes, at least transiently, through a microtubule network 153.
Leishmania spp. have also been well studied in the context of exosomes 16. The initial studies by Reiner and colleagues established that pathogen-derived exosomes are a vehicle for Leishmania protein secretion and uptake by target macrophages 154. Exosomes were proposed to be a mechanism to deliver Leishmania molecules directly into macrophages. Leishmania exosomes were also found to suppress the immune response and the heat-shock protein 100 (HSP100) to have an important role in the packaging of the parasite's proteins into exosomes, as its absence from exosomes resulted in different cargo and a different (pro-inflammatory) effect on immune cells 155. A similar effect was seen in vivo, as mice treated with Leishmania major and Leishmania donovani-released exosomes prior to infection had higher parasite load compared to untreated mice, likely due to the suppression of the immune system by the exosomes 155. Moreover, proteomic analysis of exosomes from Leishmania mexicana-infected macrophages identified parasite and host proteins, which differed between exosomes from uninfected and infected cells 156. GP63—an important Leishmania virulence factor—is present in exosomes from infected cells, which can trigger the expression of genes related to the immune system in recipient cells. This includes signaling molecules such as MAP kinases (except JNK) and transcription factors like NF-κB. However, the overall effect is the down-regulation of pro-inflammatory genes and suppression of macrophage activation, which would promote parasite survival. This suppression may stem, at least in part, from the expression of GP63 within infected macrophages, as cells infected with a WT and GP63-deficient L. major released exosomes with significantly different host and microbial protein content, including proteins known to function in immune modulation 156,157. Moreover, exosomes containing GP63 released from L. donovani-infected macrophages target the pre-miRNA processor Dicer1 in hepatocytes to prevent miRNP formation and block production of the miR122, resulting in altered serum cholesterol concentration and higher parasite burden 158. In contrast, Schnitzer and colleagues showed that DC-derived exosomes containing Leishmania antigens, when used as a vaccine, provide protective immunity against cutaneous leishmaniasis 159.
The study of exosomes in the context of a Plasmodium infection is relatively new and has focused primarily on host-derived versus pathogen-produced vesicles. These studies suggest that host exosomes can modulate the host immune response or parasite survival (Table2). In work by Martin-Jaular and colleagues, exosomes isolated from the blood of P. yoelii-infected BALB/c mice were found to contain parasite proteins and, when used as a vaccine in naïve mice, provided protection against subsequent P. yoelii infection 160. These exosomes stimulated the production of IgG antibodies that recognized P. yoelii-infected red blood cells (RBC), decreased the level of parasitemia, allowed infected animals to survive longer and resulted in a preferential infection of reticulocytes over other RBC developmental stages. The production of exosomes during a P. falciparum infection may be more limited than during infections with the parasites described above, which replicate inside macrophages, as Plasmodium primarily infects RBCs, which lack MVBs. However, like P. vivax 161, P. falciparum can also infect reticulocytes and these infected cells could produce exosomes during a P. falciparum infection. In addition, P. falciparum-infected RBCs release exosome-like vesicles and microvesicles, which may enhance infectivity, as their number increases during an infection and they contain parasite components, including proteins that promote pathogen invasion of RBCs, such as EBA-175 and EBA-181 162. Interestingly, a recent study found exosome-like vesicles carrying the P. falciparum protein PfPTP2 released from infected RBCs, which promoted the sexual differentiation of a subset of parasites 163. Couper and colleagues demonstrated that microvesicles isolated from the plasma of malaria-infected, but not naïve, mice induce potent, TLR4-dependent activation of macrophages in vitro, as measured by CD40 up-regulation and TNF-α production 164. Conversely, host-derived microvesicles released from both Trypanosoma cruzi- and P. falciparum-infected cells limit host immune surveillance, leading to increased parasite production and transmission 165,166. These studies suggest that microvesicles released from Plasmodium-infected host cells may play a prominent role in modulating the immune response. Most data suggest that they enhance the pro-inflammatory response, thereby linking microvesicles to disease pathology 167. This conclusion is supported by the timing associated with microvesicle release, which peaks late during schizogony—a few hours prior to parasite egress—and therefore may partly drive the strong cytokine response associated with the 72-h P. falciparum infection cycle 166.
Exosomes have also been identified during other parasitic infections, including those by Eimeria spp., which are responsible for avian coccidiosis. Del Cacho and colleagues observed that DCs pulsed with Eimeria antigens or exosomes released from the antigen-pulsed DCs could be used as a vaccine against Eimeria infection in chickens 168. Infection with the gastrointestinal parasite Cryptosporidium parvum was shown to increase exosome release from intestinal and biliary epithelial cells into the lumen of the gastrointestinal tract 169. These exosomes also carried antimicrobial peptides from the epithelial cells, the export of which was increased through TLR4 activation by the parasite. The antimicrobial exosomes were shown to have a negative effect on the parasites in vitro and ex vivo, decreasing their ability to survive and infect host cells 169.
The study of exosomes and other extracellular vesicles in the context of a parasitic infection is complicated by the fact that both the pathogen and host make and release vesicles into the extracellular environment and both likely play a role in disease pathogenesis (Fig3). Despite significant interest in exosomes in the context of these infections, we have only begun to characterize their role in pathogenesis. Future studies should develop mechanisms to inhibit/modulate both pathogen- and host-derived vesicles.
Fungi
Most of the studies of extracellular vesicles in fungal infections have focused on exosome-like vesicles released directly by the fungi. Casadevall and colleagues demonstrated the export of exosome-like extracellular vesicles from the fungus Cryptococcus neoformans and found they could react with sera from patients infected with the fungus, indicating the presence of fungal antigens in/on vesicles 170,171,172,173. As foreign materials, these exosome-like vesicles activate macrophages, leading to increased production of cytokines, such as TNF-α, and other anti-microbial molecules, thereby restricting fungal infection 172. In contrast, other studies suggest that these vesicles may promote fungal virulence. Panepinto and colleagues showed that interfering with the export of exosomes from C. neoformans by knocking down Sec6 (a gene involved in the fusion of exocytic vesicles with the cell membrane) resulted in decreased virulence of the fungi in vivo 174. Knockdown of Sec6 completely inhibited extracellular vesicle production in C. neoformans, including that of fungal exosomes, and blocked the export of a major virulence factor, laccase. This enzyme is required for the synthesis of melanin, an important molecule for fungal virulence 171. The importance of melanin in fungal pathogenesis is suggested by the recent observation that fungal cell walls harboring melanin promote fungal infection by blocking macrophage phagocytosis 175. A number of studies have shown the release of extracellular vesicles from other fungal species, such as Malassezia sympodialis and Paracoccidioides brasiliensis, but they have provided limited insight into the influence of the vesicles in pathogenesis 176,177,178,179. The role of extracellular vesicles in fungi has been comprehensibly reviewed recently 17.
Bacteria
Exposure of the immune system to bacterial and other microbial components is key to both control of the pathogen and subversion of the immune system by the pathogen. Many of the bacterial components known to be involved in the activation/subversion of the immune response are secreted or released from the bacteria during an infection. Understanding how these bacterial components disseminate is important to our understanding of the disease and how our immune system responds to the infection. As highlighted above for viral and parasitic infections, exosomes could play a vital role in this dissemination process. Much of our information regarding exosome production and function during a bacterial infection stems from work on mycobacteria, which will be discussed first, followed an analysis of exosomes in other bacteria.
Russell and colleagues observed that M. tuberculosis PAMPs, such as LAM and PIM, are transported from the phagosome to the MVB during a macrophage infection. These PAMPs are also found in extracellular vesicles released by infected macrophages, and their content can be detected inside neighboring uninfected cells 180. These vesicles have markers of a late endosomal/lysosomal compartment and are released through calcium-dependent exocytosis 181, implying they are exosomes.
Our studies have expanded on these original observations (Fig4). We determined that Mycobacterium avium-infected macrophages release vesicles that can stimulate a pro-inflammatory response in non-infected or ‘bystander’ macrophages 182. Similar results were reported by Wang and colleagues 183. Anand and colleagues observed increased exosome production in macrophages infected with M. avium and M. smegmatis compared to uninfected cells, as well as increased levels of the host protein HSP70, a protein they found could activate macrophages in vitro 184. Exosomes released from M.tb- or M. bovis BCG-infected macrophages were also shown to be pro-inflammatory 10. The mycobacterial 19-kDa lipoprotein present on exosomes that are released from M. tuberculosis-infected cells was later shown to be a primary driver of this inflammatory response, which depends on the TLR/MyD88 pathway 185. Moreover, exosomes isolated from the bronchoalveolar lavage fluid (BALF) of M. bovis BCG-infected mice contained mycobacterial components, including the 19-kDa lipoprotein, and were pro-inflammatory ex vivo. In addition, exosomes from M. bovis BCG- or M.tb-infected macrophages could stimulate a pro-inflammatory response in vivo, as intranasal injection of mice induced TNF-α and IL-12 production, as well as recruitment of macrophages and neutrophils to the lung 10. Macrophages treated with exosomes from M.tb-infected macrophages secrete chemokines that induce naïve macrophage and T-cell migration in vitro 186. Together, these results suggest that exosomes from mycobacterial-infected cells can promote both recruitment and activation of immune cells in vitro and in vivo and may play a role in promoting the innate immune response upon a mycobacterial infection. However, the mycobacterial components present on/in exosomes could also function to suppress the immune response. In recent studies, we observed that exosomes from M.tb-infected cells could partially suppress the ability of recipient macrophages to respond to IFN-γ, inhibition that was dependent on macrophage expression of TLR2 and MyD88 187. Additional studies are needed to define the receptors and signaling responses induced upon exosome–macrophage interaction and how these interactions/responses change as the exosome composition is modified during an infection. In addition to their effect on macrophage activation, exosomes from M.tb-infected cells have also been shown to contain a member of the PE family (Rv1818c) that can induce apoptosis in Jurkat T cells 188, suggesting that the inhibitory effect of these exosomes may extend to T cells.
Figure 4. Bacteria-infected cells release exosomes that modify T-cell and macrophage function.
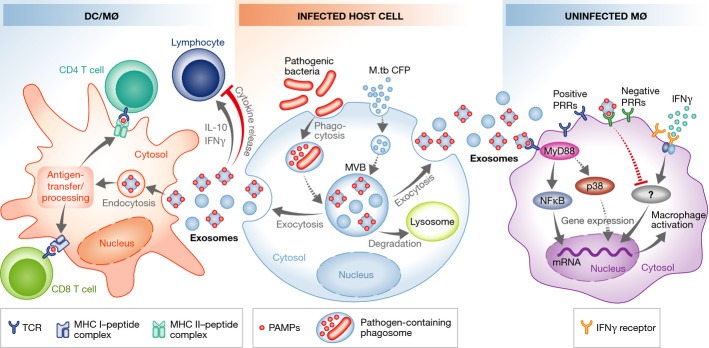
Exosomes from bacteria-infected macrophages release exosomes containing antigens that induce cross-priming to activate antigen-specific CD4+ and CD8+ T cells. In contrast, some exosomes released from infected cells inhibit cytokine production by T cells. Exosomes from infected cells also contain PAMPs that stimulate macrophage production of pro-inflammatory mediators like TNF-α, or limit the macrophage response to IFN-γ stimulation. Dashed lines indicate unknown mechanisms. See Glossary for definitions.
However, exosomes released from M.tb- or M. bovis BCG-infected cells, or from M.tb culture filtrate protein (CFP)-treated macrophages, can also activate antigen-specific CD4+ and CD8+ T cells in vivo and promote the activation and maturation of bone marrow-derived dendritic cells (BMDCs) 189. These exosomes induce a Th1 immune response, as defined by antigen-specific T-cell production of IFN-γ 187,189. Moreover, vaccination of mice with exosomes released from macrophages treated with CFP protects mice against a low-dose aerosolized M.tb inoculation, equivalent to BCG-vaccinated mice 190. The release of mycobacterial antigens from infected macrophages is not limited to exosomes. Ramachandra and colleagues observed that infection with M.tb or M. bovis BCG resulted in increase in both exosome and microvesicle release, and both vesicles could stimulate an antigen-specific T-cell response 191. Together, these results suggest that exosomes and perhaps other extracellular vesicles are a source of antigen to stimulate the acquired immune response. However, other mechanisms for antigen delivery during a mycobacterial infection have been proposed, including necrotic cells, apoptotic bodies and release of free antigen 192,193,194. Unfortunately, our ability to test the relative importance of exosomes in antigen delivery is limited, due to the lack of molecular tools to block exosome production in macrophages without affecting other aspects of vesicular transport and without blocking exosome production by other cell types.
In addition to mycobacteria, exosomes from Salmonella-infected macrophages are also pro-inflammatory, increasing TNF-α production by human monocytes (Fig4) 10. These exosomes contain LPS, a known PAMP present on Salmonella and other gram-negative bacteria. Exosomes from cells infected with Mycoplasma induce a mixed cytokine response, including production of both IFN-γ and IL-10 from B cells. However, these exosomes appear to be primarily inhibitory, at least in the context of T-cell activation 195. Exosomes can also be carriers of toxins, as shown by Abrami and colleagues, who found lethal factor—a well-characterized toxin produced by Bacillus anthracis—packaged into intraluminal vesicles and released on exosomes when expressed in a human epithelial cell line 196. Ettelaie and colleagues reported that ‘microparticles’ released from Chlamydia pneumoniae-infected cells contain TF, a blood coagulation protein, which has also been associated with cell proliferation, migration and apoptosis. The TF-positive microparticles activate NF-κB, the transcription factor that partially regulates TF expression in endothelial cells. C. pneumoniae elementary bodies were also proposed to be released in microparticles, pointing to a potential role in the dissemination of the infection through the bloodstream. These findings have implications not only for the control of the infection by the host, but also for the potential cardiovascular consequences in relation to inflammatory conditions such as atherosclerosis 197. Although the C. pneumoniae vesicles were referred to as microparticles, the isolation procedure used would enrich for exosomes. Several cytotoxic and secreted proteins were also associated with host vesicles released from Chlamydia trachomatis, which might function in the delivery of virulence factors 198.
Additional studies have analyzed the presence of bacterial antigens within host exosomes, with an eye toward developing cell-free vaccines against bacterial pathogens. Colino and colleagues treated BMDCs with diphtheria toxin (DT), isolated exosomes from the cells and injected them into mice. The exosomes stimulated an IgG response specific for DT 199. Similar results were obtained with Streptococcus pneumoniae. Exosomes from BMDCs pulsed with S. pneumoniae capsular polysaccharide 14 antigen (Cps14) were enriched in Cps14 and could stimulate a protective IgM and IgG response against S. pneumoniae when injected into naïve mice 200.
Summary
Together, the results discussed above suggest that microbial and host components can spread beyond the infected cell through exosomes to either activate or suppress immune responses. We hypothesize that exosomes will be involved in multiple steps during the infection process, including formation/modification of infection loci, discrimination of antigens during the initial stages of infection, source of antigens for activation of T cells and B cells and modulation of immune cell function. In addition, the exosomes released from infected cells could also interact with non-immune cells such as fibroblasts and endothelial cells, influencing matrix deposition, vascular permeability, etc., all of which could impact the outcome of an infection (see Sidebar A).
Exosomes: good or bad for the host?
Whether exosomes activate or suppress the immune response depends on multiple factors, as discussed above. One of them is the source of the exosomes, that is, the cell type from which the exosomes were derived and/or the bodily fluid from which they were isolated. For example, exosomes from Leishmania-infected DCs were found to be immune stimulatory, making them an effective vaccine against the parasite 159. In contrast, exosomes from infected macrophages were found to suppress the immune response to the parasite, promoting its survival in the host 155,156. These different responses stem from changes in exosome composition, including their internal cargo (proteins and RNA) and their surface markers. Differences in the composition of exosomes would, in turn, lead to different effects on the recipient cells. A proteomic analysis of exosomes released from M.tb-infected or CFP-treated macrophages identified 41 and 29 mycobacterial proteins present in the exosomes from infected and CFP-treated macrophages, respectively 11. Many of the proteins identified had been previously characterized as dominant antigens 201,202. There was also significant overlap between the mycobacterial proteins present in the two populations of exosomes. In contrast, a proteomic analysis of exosomes from Leishmania-infected macrophages revealed few parasite proteins and limited differences in host proteins between exosomes from infected compared to uninfected macrophages 156. One of the few differences was the presence of GP63 on exosomes from infected cells. GP63 is immune modulatory, but not pro-inflammatory, and contributes to parasite survival by down-regulating the immune response of the host. Another example of the significant effect that the presence or absence of a single protein can have on the immune response includes work by Miksa and colleagues in a rat septic model, where exosomes with or without MFG-E8 (milk fat globule epidermal growth factor 8) had a significantly different effect on removal of apoptotic bodies and survival of the rats. MFG-E8-containing exosomes down-regulated the inflammatory response, reducing TNF-α and IL-6 production 203. In this setting, in which increased immune response to bacteria could be detrimental (by leading to sepsis), the exosomes were able to dampen the immune response due to the presence of MFG-E8 within the vesicles.
Understanding the beneficial or harmful effects of exosomes in the context of an in vivo infection is a key goal that will require additional insight into exosome biogenesis, and new tools to specifically block exosome release in a cell-specific manner. At present, targeting Rab GTPases or the various ESCRT proteins does not afford the control and specificity we need to answer these questions. Other factors, such as adaptor proteins that are specific for the tethering and fusion of MVBs with the plasma membrane, would be logical choices. Unfortunately, although SNAREs such as VAMP7 have been implicated in the MVB–plasma membrane fusion 204, this SNARE is also involved in other intracellular membrane fusion processes and therefore not a useful target for blocking exosome biogenesis.
Exosomes as vaccines
The concept of using exosomes as vaccines has its origin in the cancer field. Exosomes released from dendritic cells pulsed with tumor antigens have been tested in clinical trials as tumor ‘vaccines’: a treatment that aims to mobilize a patient's immune system to recognize and destroy the tumor cells. More recently, the potential use of exosomes as vaccines against infectious diseases has been assessed. Specific examples have been discussed in the different pathogen sections above. There are a number of potential advantages to using exosomes as vaccines against pathogens. These include: (i) more stable conformational conditions for the proteins; (ii) improved molecular distribution due to the ability of exosomes to circulate in bodily fluids and reach distal organs; (iii) more efficient association with the antigen-presenting cells, due to the expression of adhesion molecules on exosomes; and (iv) the fact that exosomes are one of the body's ‘natural’ mechanisms for transporting antigens between cells and one that likely plays a role in cross-priming.
However, although the use of exosomes allows for a cell-free-based vaccine, there are both conceptual and practical issues that need to be addressed before this potential application can become a reality. These include obtaining exosomes with the correct mix of antigens that provide protection, being able to reproducibly generate exosomes with this correct antigen composition, the risks of introducing ‘non-self’ human molecules into a vaccinated individual, among other issues. This latter point is particularly important, as unlike the use of exosomes as vaccines in cancer patients—which uses exosomes obtained from autologous cells—a vaccine against a particular pathogen will likely be derived from a human cell line and therefore will have proteins and other molecules specific to this cell line. The effect of these ‘foreign’ antigens on the recipient's immune response is unknown, and additional experiments will be required before performing any clinical studies. Nevertheless, the available data indicate that exosomes may provide a unique approach to vaccine generation against various pathogens, and this area will likely grow significantly in the coming years.
Exosomes as a source of diagnostic markers
Exosomes have been isolated from many different body fluids, including serum, bronchoalveolar lavage fluid, urine, saliva and several others 205,206,207. A number of studies have shown quantitative and qualitative differences in exosome composition between healthy individuals and those with underlying diseases, including cancers and renal diseases 208. These differences, combined with their easy accessibility, make exosomes excellent biomarker candidates. The use of exosomes to diagnose infectious diseases has been less studied, but shows great promise, as the markers could be both host and pathogen derived. Some support for this idea comes from the fact that serum exosome levels are significantly elevated in M. bovis BCG-infected mice compared to uninfected controls, and exosome concentration correlates with bacterial load 186. Moreover, the exosomes isolated from the bronchoalveolar lavage fluid of M.tb-infected mice contain mycobacterial proteins, some of which are present in exosomes throughout a 112-day infection 209. Finally, we have isolated exosomes from TB patient serum and identified a number of mycobacterial proteins, some of which were consistently found in the patient population under study, suggesting that they could be viable biomarkers for disease 210. Surprisingly, we have also found that macrophages infected with M.tb release exosomes containing mycobacterial RNA (J.S. Schorey, unpublished data), which suggests that exosomal RNA may be a useful marker for active TB. Further experiments are warranted to determine whether RNA from other intracellular pathogens is also present in exosomes.
From the perspective of host markers, Welker and colleagues found the exosomal protein CD81 to be elevated in the serum of patients with chronic hepatitis C infection compared to healthy controls. There was a correlation between serum CD81 elevation, higher ALT levels (a measure of liver inflammation) and more severe liver fibrosis. This suggests that measuring CD81 in the exosomal fraction of patient serum could be useful in the diagnosis or in following the course of chronic hepatitis C infection 211. These examples illustrate the excellent potential for exosomes and other extracellular vesicles in the diagnosis and prognosis of infectious diseases.
Conclusion and future directions
Our understanding of the relevance of exosomes in host–pathogen interactions is still at an early stage relative to other fields of study, particularly cancer, on which most studies on exosomes have focused. Nevertheless, there are interesting and compelling results regarding the importance of exosomes and other extracellular vesicles during infection, and continued growth in this area will allow for a better understanding of virulence mechanisms and immune responses, as well as the development of new diagnostics and vaccines. However, more work is clearly needed on defining exosome composition and function during the course of an infection. This should include defining the cell types that produce the exosomes, the exosome recipient cells, the intracellular signaling pathways affected by exosomes, etc. Critical to this work will be the development of methods to specifically block exosome production and evaluate disease outcome, as a way to determine whether exosome production benefits the host or is used by the pathogen to subvert the immune response.
Acknowledgments
The unpublished studies highlighted in the review were supported through the grant RO1AI052439 from the National Institute of Allergy and Infectious Diseases.
Glossary
- APC
antigen-presenting cell
- APOBEC3G
apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3G
- BALF
bronchoalveolar lavage fluid
- BAT3
HLA-B-associated transcript 3
- BCG
bacillus Calmette–Guérin
- BMDCs
bone marrow-derived dendritic cells
- CCR5
C-C chemokine receptor type 5
- CD
cluster of differentiation
- CFP
culture filtrate protein
- CIA
collagen-induced arthritis
- CMV
cytomegalovirus
- CTL
cytotoxic T lymphocyte
- CXCR4
C-X-C chemokine receptor type 4
- DC
dendritic cell
- DT
diphtheria toxin
- DTH
delayed-type hypersensitivity
- EBA-175
erythrocyte binding antigen 175
- EBV
Epstein–Barr virus
- EM
electron microscopy
- ESCRT
endosomal sorting complexes required for transport
- FACS
fluorescence-activated cell sorting
- Fas/FasL
Fas and Fas ligand
- HCV
hepatitis C virus
- HEK
human embryonic kidney
- HIV
human immunodeficiency virus
- HLA-DR
major histocompatibility complex, class II, DR beta 1
- HMC-1
human mast cell line-1
- HSP
heat-shock protein
- HSV
herpes simplex virus
- ICAM1
intercellular adhesion molecule 1
- iDC
immature DC
- IFN
interferon
- IL
interleukin
- ILVs
intraluminal vesicles
- JNK
c-Jun N-terminal kinase
- LAM
lipoarabinomannan
- LBPA
lysobisphosphatidic acid
- LFA-1
lymphocyte function-associated antigen 1
- LMP1
latent membrane protein 1
- LPS
lipopolysaccharide
- M.tb
Mycobacterium tuberculosis
- MAP kinase
mitogen-activated protein kinase
- MC/9
Mus musculus mast cell line
- MCP-1
monocyte chemotactic protein 1
- mDC
mature DC
- MFG-E8
milk fat globule-EGF factor 8 protein
- MHC
major histocompatibility complex
- miRNA
microRNA
- MVB
multivesicular bodies
- MyD88
myeloid differentiation primary response gene (88)
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
natural killer
- NLRP3
NACHT, LRR and PYD domains-containing protein 3
- NPC
nasopharyngeal carcinoma
- PAMP
pathogen-associated molecular pattern
- PBMC
peripheral blood mononucleated cells
- PIM
phosphatidylinositol mannoside
- PRR
pattern recognition receptor
- RANTES
regulated on activation, normal T cell expressed and secreted
- RBC
red blood cell
- RILP
rab-interacting lysosomal protein
- RNP
ribonucleoprotein
- ROS
reactive oxygen species
- SNARE
SNAP (soluble NSF attachment protein) REceptor
- TAR
trans-activation response
- TB
tuberculosis
- TCR
T-cell receptor
- TF
tissue factor
- Tim4
T-cell immunoglobulin mucin protein 4
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- VAMP7
vesicle-associated membrane protein 7
- WT
wild-type
Author contributions
JSS, YC, PPC and VLS all contributed to researching and writing the review paper.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- Bliska JB, Wang X, Viboud GI, Brodsky IE. Modulation of innate immune responses by Yersinia type III secretion system translocators and effectors. Cell Microbiol. 2013;15:1622–1631. doi: 10.1111/cmi.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana RR, Simpson P, Zhang M, Jennions M, Ukegbu C, Spear AM, Alguel Y, Matthews SJ, Atkins HS, Byrne B. Yersinia pestis TIR-domain protein forms dimers that interact with the human adaptor protein MyD88. Microb Pathog. 2011;51:89–95. doi: 10.1016/j.micpath.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Schmolke M, Patel JR, de Castro E, Sánchez-Aparicio MT, Uccellini MB, Miller JC, Manicassamy B, Satoh T, Kawai T, Akira S, et al. RIG-I detects mRNA of intracellular Salmonella enterica serovar Typhimurium during bacterial infection. MBio. 2014;5:e01006–e01014. doi: 10.1128/mBio.01006-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Roach SK, Schorey JS. Increased mitogen-activated protein kinase activity and TNF-alpha production associated with Mycobacterium smegmatis- but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/protein kinase A pathways. J Immunol. 2004;172:5588–5597. doi: 10.4049/jimmunol.172.9.5588. [DOI] [PubMed] [Google Scholar]
- Yadav M, Schorey JS. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikere K, Chou T, Gorry PR, Lee B. Affinofile profiling: how efficiency of CD4/CCR5 usage impacts the biological and pathogenic phenotype of HIV. Virology. 2013;435:81–91. doi: 10.1016/j.virol.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerda J, Cossart P. Subversion of cellular functions by Listeria monocytogenes. J Pathol. 2006;208:215–223. doi: 10.1002/path.1888. [DOI] [PubMed] [Google Scholar]
- Ashida H, Mimuro H, Ogawa M, Kobayashi T, Sanada T, Kim M, Sasakawa C. Cell death and infection: a double-edged sword for host and pathogen survival. J Cell Biol. 2011;195:931–942. doi: 10.1083/jcb.201108081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri PK, Kruh NA, Dobos KM, Schorey JS. Proteomic analysis identifies highly antigenic proteins in exosomes from M. tuberculosis-infected and culture filtrate protein-treated macrophages. Proteomics. 2010;10:3190–3202. doi: 10.1002/pmic.200900840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Letley D, Rhead J, Atherton J, Robinson K. Helicobacter pylori membrane vesicles stimulate innate pro- and anti-inflammatory responses and induce apoptosis in Jurkat T cells. Infect Immun. 2014;82:1372–1381. doi: 10.1128/IAI.01443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. 2012;80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo R, Fernández S, Zayas C, Acosta A, Sarmiento ME, Ferro VA, Rosenqvist E, Campa C, Cardoso D, Garcia L, et al. Bacterial outer membrane vesicles and vaccine applications. Front Immunol. 2014;5:121. doi: 10.3389/fimmu.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JM, Reiner NE. Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell Microbiol. 2011;13:1–9. doi: 10.1111/j.1462-5822.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- Oliveira DL, Rizzo J, Joffe LS, Godinho RM, Rodrigues ML. Where do they come from and where do they go: candidates for regulating extracellular vesicle formation in fungi. Int J Mol Sci. 2013;14:9581–9603. doi: 10.3390/ijms14059581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287–1299. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8:2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- Marzesco AM, Janich P, Wilsch-Brauninger M, Dubreuil V, Langenfeld K, Corbeil D, Huttner WB. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005;118:2849–2858. doi: 10.1242/jcs.02439. [DOI] [PubMed] [Google Scholar]
- Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. 2008;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- Zhu M, Li Y, Shi J, Feng W, Nie G, Zhao Y. Exosomes as extrapulmonary signaling conveyors for nanoparticle-induced systemic immune activation. Small. 2012;8:404–412. doi: 10.1002/smll.201101708. [DOI] [PubMed] [Google Scholar]
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984;35:256–263. [PubMed] [Google Scholar]
- Woodman PG, Futter CE. Multivesicular bodies: co-ordinated progression to maturity. Curr Opin Cell Biol. 2008;20:408–414. doi: 10.1016/j.ceb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH. The ESCRT complexes. Crit Rev Biochem Mol Biol. 2010;45:463–487. doi: 10.3109/10409238.2010.502516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BA, Lee JR, Oestreich AJ, Katzmann DJ. Membrane protein targeting to the MVB/lysosome. Chem Rev. 2009;109:1575–1586. doi: 10.1021/cr800473s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D, Isaacs AM. The role of ESCRT proteins in fusion events involving lysosomes, endosomes and autophagosomes. Biochem Soc Trans. 2010;38:1469–1473. doi: 10.1042/BST0381469. [DOI] [PubMed] [Google Scholar]
- Raiborg C, Stenmark H. Hrs and endocytic sorting of ubiquitinated membrane proteins. Cell Struct Funct. 2002;27:403–408. doi: 10.1247/csf.27.403. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell. 2002;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- Tamai K, Tanaka N, Nakano T, Kakazu E, Kondo Y, Inoue J, Shiina M, Fukushima K, Hoshino T, Sano K, et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun. 2010;399:384–390. doi: 10.1016/j.bbrc.2010.07.083. [DOI] [PubMed] [Google Scholar]
- Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10:925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Hernandez D, Gutierrez-Vazquez C, Jorge I, Lopez-Martin S, Ursa A, Sanchez-Madrid F, Vazquez J, Yanez-Mo M. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem. 2013;288:11649–11661. doi: 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschow SI, Nolte-'t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, Ossendorp F, Melief CJ, Raposo G, Wubbolts R, Wauben MH, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10:1528–1542. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002;115:2505–2515. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2011;12:19–30. doi: 10.1038/ncb2000. ;sup pp 11–13. [DOI] [PubMed] [Google Scholar]
- Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One. 2010;5:e12578. doi: 10.1371/journal.pone.0012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom Rev. 2014 doi: 10.1002/mas.21420. doi: 10.1002/mas.21420. [DOI] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:205–212. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–1571. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- Wood SL, Knowles MA, Thompson D, Selby PJ, Banks RE. Proteomic studies of urinary biomarkers for prostate, bladder and kidney cancers. Nat Rev Urol. 2013;10:206–218. doi: 10.1038/nrurol.2013.24. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Nolte-'t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH, t Hoen PA. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40:9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. Jr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, Xiao T, Schafer J, Lee ML, Schmittgen TD, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M, Lotvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5:e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr. 2007;1:156–158. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CC, Eaton SA, Young PE, Lee M, Shuttleworth R, Humphreys DT, Grau GE, Combes V, Bebawy M, Gong J, et al. Glioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in recipient cells. RNA Biol. 2013;10:1333–1344. doi: 10.4161/rna.25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E, Sundquist WI. Retrovirus budding. Annu Rev Cell Dev Biol. 2004;20:395–425. doi: 10.1146/annurev.cellbio.20.010403.102350. [DOI] [PubMed] [Google Scholar]
- Garcia E, Pion M, Pelchen-Matthews A, Collinson L, Arrighi JF, Blot G, Leuba F, Escola JM, Demaurex N, Marsh M, et al. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic. 2005;6:488–501. doi: 10.1111/j.1600-0854.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, Krogan NJ, Plemenitas A, Peterlin BM. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra A, Zahra A, Thompson V, Sirisaengtaksin N, Wells A, Porto M, Köster S, Penberthy K, Kubota Y, Dricot A, et al. Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog. 2013;9:e1003734. doi: 10.1371/journal.ppat.1003734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreux M, Garaigorta U, Boyd B, Decembre E, Chung J, Whitten-Bauer C, Wieland S, Chisari FV. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12:558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoujal B, Milev MP, Ajamian L, Abel K, Mouland AJ. ESCRT-II's involvement in HIV-1 genomic RNA trafficking and assembly. Biol Cell. 2012;104:706–721. doi: 10.1111/boc.201200021. [DOI] [PubMed] [Google Scholar]
- Mathews A, Holland L, Yankulov K. The interaction between EAP30 and ELL is modulated by MCM2. FEBS Lett. 2009;583:3431–3436. doi: 10.1016/j.febslet.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Irion U, St Johnston D. bicoid RNA localization requires specific binding of an endosomal sorting complex. Nature. 2007;445:554–558. doi: 10.1038/nature05503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136:719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- Beninson LA, Fleshner M. Exosomes: an emerging factor in stress-induced immunomodulation. Semin Immunol. 2014;26:394–401. doi: 10.1016/j.smim.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2012;1:1074–1083. doi: 10.4161/onci.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, Kufer TA, Engert A, Pogge von Strandmann E. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One. 2008;3:e3377. doi: 10.1371/journal.pone.0003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obregon C, Rothen-Rutishauser B, Gerber P, Gehr P, Nicod LP. Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-alpha-mediated pathway. Am J Pathol. 2009;175:696–705. doi: 10.2353/ajpath.2009.080716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo BH, Wong SH. MHC class II-associated invariant chain (Ii) modulates dendritic cells-derived microvesicles (DCMV)-mediated activation of microglia. Biochem Biophys Res Commun. 2010;400:673–678. doi: 10.1016/j.bbrc.2010.08.126. [DOI] [PubMed] [Google Scholar]
- Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- Qu Y, Ramachandra L, Mohr S, Franchi L, Harding CV, Nunez G, Dubyak GR. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J Immunol. 2009;182:5052–5062. doi: 10.4049/jimmunol.0802968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman JP, Al Gadban MM, Smith KJ, Jenkins RW, Mayroo N, Virella G, Lopes-Virella MF, Bielawska A, Hannun YA, Hammad SM. Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized low-density lipoprotein (LDL) and oxidized LDL immune complexes: role in phagocytosis and cytokine release. Immunology. 2012;136:30–45. doi: 10.1111/j.1365-2567.2012.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- André F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- Chaput N, Schartz NE, André F, Taïeb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M, et al. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol. 2004;172:2137–2146. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- Segura E, Amigorena S, Thery C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35:89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJ, Papworth GD, Watkins SC, Robbins PD, Larregina AT, et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol. 2008;180:3081–3090. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- Qazi KR, Gehrmann U, Domange Jordo E, Karlsson MC, Gabrielsson S. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood. 2009;113:2673–2683. doi: 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]
- Veron P, Segura E, Sugano G, Amigorena S, Thery C. Accumulation of MFG-E8/lactadherin on exosomes from immature dendritic cells. Blood Cells Mol Dis. 2005;35:81–88. doi: 10.1016/j.bcmd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F, Amigorena S, Thery C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- Zakharova L, Svetlova M, Fomina AF. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J Cell Physiol. 2007;212:174–181. doi: 10.1002/jcp.21013. [DOI] [PubMed] [Google Scholar]
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology. 2007;120:90–102. doi: 10.1111/j.1365-2567.2006.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte-'t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, Minami M. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol Lett. 2003;89:125–131. doi: 10.1016/s0165-2478(03)00128-7. [DOI] [PubMed] [Google Scholar]
- Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur J Immunol. 2006;36:1772–1781. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- Luketic L, Delanghe J, Sobol PT, Yang P, Frotten E, Mossman KL, Gauldie J, Bramson J, Wan Y. Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL. J Immunol. 2007;179:5024–5032. doi: 10.4049/jimmunol.179.8.5024. [DOI] [PubMed] [Google Scholar]
- Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007;26:4263–4272. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, Gabrielsson S. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol. 2007;120:1418–1424. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Hwang I, Shen X, Sprent J. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc Natl Acad Sci USA. 2003;100:6670–6675. doi: 10.1073/pnas.1131852100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, Bonnerot C. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14:713–722. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- Yin W, Ouyang S, Li Y, Xiao B, Yang H. Immature dendritic cell-derived exosomes: a promise subcellular vaccine for autoimmunity. Inflammation. 2013;36:232–240. doi: 10.1007/s10753-012-9539-1. [DOI] [PubMed] [Google Scholar]
- Yang C, Robbins PD. Immunosuppressive exosomes: a new approach for treating arthritis. Int J Rheumatol. 2012;2012:573528. doi: 10.1155/2012/573528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- Wahlgren J, Karlson Tde L, Glader P, Telemo E, Valadi H. Activated human T cells secrete exosomes that participate in IL-2 mediated immune response signaling. PLoS One. 2012;7:e49723. doi: 10.1371/journal.pone.0049723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth LA, Ratnasothy K, Tsang JY, Boardman D, Warley A, Lechler R, Lombardi G. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013;43:2430–2440. doi: 10.1002/eji.201242909. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xie Y, Li W, Chibbar R, Xiong S, Xiang J. CD4(+) T cell-released exosomes inhibit CD8(+) cytotoxic T-lymphocyte responses and antitumor immunity. Cell Mol Immunol. 2011;8:23–30. doi: 10.1038/cmi.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zhang X, Zhao T, Li W, Xiang J. Natural CD8(+)25(+) regulatory T cell-secreted exosomes capable of suppressing cytotoxic T lymphocyte-mediated immunity against B16 melanoma. Biochem Biophys Res Commun. 2013;438:152–155. doi: 10.1016/j.bbrc.2013.07.044. [DOI] [PubMed] [Google Scholar]
- Alonso R, Mazzeo C, Merida I, Izquierdo M. A new role of diacylglycerol kinase alpha on the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. Biochimie. 2007;89:213–221. doi: 10.1016/j.biochi.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, Yang Y, Wang L, Cao X, Wang J. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol. 2012;188:5954–5961. doi: 10.4049/jimmunol.1103466. [DOI] [PubMed] [Google Scholar]
- Klinker MW, Lizzio V, Reed TJ, Fox DA, Lundy SK. Human B cell-derived lymphoblastoid cell lines constitutively produce Fas ligand and secrete MHCII(+)FasL(+) killer exosomes. Front Immunol. 2014;5:144. doi: 10.3389/fimmu.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E. Differential requirements of mammalian ESCRTs in multivesicular body formation, virus budding and cell division. FEBS J. 2012;279:1399–1406. doi: 10.1111/j.1742-4658.2012.08534.x. [DOI] [PubMed] [Google Scholar]
- Wurdinger T, Gatson NN, Balaj L, Kaur B, Breakefield XO, Pegtel DM. Extracellular vesicles and their convergence with viral pathways. Adv Virol. 2012;2012:767694. doi: 10.1155/2012/767694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashikar AG, Shim S, Roth R, Maldazys MR, Heuser JE, Hanson PI. Structure of cellular ESCRT-III spirals and their relationship to HIV budding. Elife. 2014;3:e02184. doi: 10.7554/eLife.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartel V, Ivanchenko S, Dupont A, Sergeev M, Wiseman PW, Krausslich HG, Brauchle C, Muller B, Lamb DC. Live-cell visualization of dynamics of HIV budding site interactions with an ESCRT component. Nat Cell Biol. 2011;13:469–474. doi: 10.1038/ncb2215. [DOI] [PubMed] [Google Scholar]
- Mack M, Kleinschmidt A, Brühl H, Klier C, Nelson PJ, Cihak J, Plachý J, Stangassinger M, Erfle V, Schlöndorff D. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6:769–775. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M, Ratajczak J, Gaulton GN, Ratajczak MZ. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17:33–42. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007;5:e158. doi: 10.1371/journal.pbio.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A, Iordanskiy S, Das R, Van Duyne R, Santos S, Jaworski E, Guendel I, Sampey G, Dalby E, Iglesias-Ussel M, et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J Biol Chem. 2013;288:20014–20033. doi: 10.1074/jbc.M112.438895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell TD, Khan M, Huang MB, Bond VC, Powell MD. HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions. Ethn Dis. 2008;18:S2. -14-19. [PMC free article] [PubMed] [Google Scholar]
- Shelton MN, Huang MB, Ali SA, Powell MD, Bond VC. Secretion modification region-derived peptide disrupts HIV-1 Nef's interaction with mortalin and blocks virus and Nef exosome release. J Virol. 2012;86:406–419. doi: 10.1128/JVI.05720-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Green LA, Gupta SK, Kim C, Wang L, Almodovar S, Flores SC, Prudovsky IA, Jolicoeur P, Liu Z, et al. Transfer of intracellular HIV Nef to endothelium causes endothelial dysfunction. PLoS One. 2014;9:e91063. doi: 10.1371/journal.pone.0091063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqil M, Naqvi AR, Bano AS, Jameel S. The HIV-1 Nef protein binds argonaute-2 and functions as a viral suppressor of RNA interference. PLoS One. 2013;8:e74472. doi: 10.1371/journal.pone.0074472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumne A, Prasad VS, Chen Y, Stolz DB, Saha K, Ratner DM, Ding M, Watkins SC, Gupta P. Noncytotoxic suppression of human immunodeficiency virus type 1 transcription by exosomes secreted from CD8+ T cells. J Virol. 2009;83:4354–4364. doi: 10.1128/JVI.02629-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatua AK, Taylor HE, Hildreth JE, Popik W. Exosomes packaging APOBEC3G confer human immunodeficiency virus resistance to recipient cells. J Virol. 2009;83:512–521. doi: 10.1128/JVI.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckes DG, Jr, Gunawardena HP, Dekroon RM, Heaton PR, Edwards RH, Ozgur S, Griffith JD, Damania B, Raab-Traub N. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc Natl Acad Sci USA. 2013;110:E2925–E2933. doi: 10.1073/pnas.1303906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh PE, Sin SH, Ozgur S, Henry DH, Menezes P, Griffith J, Eron JJ, Damania B, Dittmer DP. Systemically circulating viral and tumor-derived microRNAs in KSHV-associated malignancies. PLoS Pathog. 2013;9:e1003484. doi: 10.1371/journal.ppat.1003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukers DF, Meij P, Vervoort MB, Vos W, Scheper RJ, Meijer CJ, Bloemena E, Middeldorp JM. Direct immunosuppressive effects of EBV-encoded latent membrane protein 1. J Immunol. 2000;165:663–670. doi: 10.4049/jimmunol.165.2.663. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Longnecker R. Cholesterol is critical for Epstein-Barr virus latent membrane protein 2A trafficking and protein stability. Virology. 2007;360:461–468. doi: 10.1016/j.virol.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, Le Moulec S, Guigay J, Hirashima M, Guemira F, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113:1957–1966. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- Ariza ME, Rivailler P, Glaser R, Chen M, Williams MV. Epstein-Barr virus encoded dUTPase containing exosomes modulate innate and adaptive immune responses in human dendritic cells and peripheral blood mononuclear cells. PLoS One. 2013;8:e69827. doi: 10.1371/journal.pone.0069827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HJ, Huang TJ, Yang CF, Peng LX, Liu RY, Yang GD, Chu QQ, Huang JL, Liu N, Huang HB, et al. Comprehensive profiling of Epstein-Barr virus-encoded miRNA species associated with specific latency types in tumor cells. Virol J. 2013;10:314. doi: 10.1186/1743-422X-10-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S, Zavolan M, Grässer FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, et al. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, O'Neill LA, Masters SL. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. J Immunol. 2012;189:3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- Walker JD, Maier CL, Pober JS. Cytomegalovirus-infected human endothelial cells can stimulate allogeneic CD4+ memory T cells by releasing antigenic exosomes. J Immunol. 2009;182:1548–1559. doi: 10.4049/jimmunol.182.3.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plazolles N, Humbert JM, Vachot L, Verrier B, Hocke C, Halary F. Pivotal advance: the promotion of soluble DC-SIGN release by inflammatory signals and its enhancement of cytomegalovirus-mediated cis-infection of myeloid dendritic cells. J Leukoc Biol. 2011;89:329–342. doi: 10.1189/jlb.0710386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masciopinto F, Giovani C, Campagnoli S, Galli-Stampino L, Colombatto P, Brunetto M, Yen TS, Houghton M, Pileri P, Abrignani S. Association of hepatitis C virus envelope proteins with exosomes. Eur J Immunol. 2004;34:2834–2842. doi: 10.1002/eji.200424887. [DOI] [PubMed] [Google Scholar]
- Gastaminza P, Dryden KA, Boyd B, Wood MR, Law M, Yeager M, Chisari FV. Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture. J Virol. 2010;84:10999–11009. doi: 10.1128/JVI.00526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, Demmers JA, Stalin RajV, Jenster G, et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci USA. 2013;110:13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosset FL, Dreux M. HCV transmission by hepatic exosomes establishes a productive infection. J Hepatol. 2014;60:674–675. doi: 10.1016/j.jhep.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H, Liu J, Pan T, Chen J, Wu M, et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat Immunol. 2013;14:793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- Temme S, Eis-Hubinger AM, McLellan AD, Koch N. The herpes simplex virus-1 encoded glycoprotein B diverts HLA-DR into the exosome pathway. J Immunol. 2010;184:236–243. doi: 10.4049/jimmunol.0902192. [DOI] [PubMed] [Google Scholar]
- Dargan DJ, Patel AH, Subak-Sharpe JH. PREPs: herpes simplex virus type 1-specific particles produced by infected cells when viral DNA replication is blocked. J Virol. 1995;69:4924–4932. doi: 10.1128/jvi.69.8.4924-4932.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh PC, Han J, Chadha P, Meckes DG, Jr, Ward MD, Semmes OJ, Wills JW. Direct and specific binding of the UL16 tegument protein of herpes simplex virus to the cytoplasmic tail of glycoprotein E. J Virol. 2011;85:9425–9436. doi: 10.1128/JVI.05178-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SM, Tsueng G, Sin J, Mangale V, Rahawi S, McIntyre LL, Williams W, Kha N, Cruz C, Hancock BM, et al. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog. 2014;10:e1004045. doi: 10.1371/journal.ppat.1004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–632. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun. 2004;72:4127–4137. doi: 10.1128/IAI.72.7.4127-4137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvillain C, Ruiz S, Guiton R, Bout D, Dimier-Poisson I. A vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic mice. Microbes Infect. 2007;9:1614–1622. doi: 10.1016/j.micinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Beauvillain C, Juste MO, Dion S, Pierre J, Dimier-Poisson I. Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine. 2009;27:1750–1757. doi: 10.1016/j.vaccine.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Mordue DG, Sibley LD. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- Coppens I, Dunn JD, Romano JD, Pypaert M, Zhang H, Boothroyd JC, Joiner KA. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell. 2006;125:261–274. doi: 10.1016/j.cell.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Clos J, de'Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ, Reiner NE. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci. 2010;123:842–852. doi: 10.1242/jcs.056465. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, Lynn MA, McMaster WR, Foster LJ, Levings MK, et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol. 2010;185:5011–5022. doi: 10.4049/jimmunol.1000541. [DOI] [PubMed] [Google Scholar]
- Hassani K, Olivier M. Immunomodulatory impact of Leishmania-induced macrophage exosomes: a comparative proteomic and functional analysis. PLoS Negl Trop Dis. 2013;7:e2185. doi: 10.1371/journal.pntd.0002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani K, Shio MT, Martel C, Faubert D, Olivier M. Absence of metalloprotease GP63 alters the protein content of Leishmania exosomes. PLoS One. 2014;9:e95007. doi: 10.1371/journal.pone.0095007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh J, Bose M, Roy S, Bhattacharyya SN. Leishmania donovani targets Dicer1 to downregulate miR-122, lower serum cholesterol, and facilitate murine liver infection. Cell Host Microbe. 2013;13:277–288. doi: 10.1016/j.chom.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JK, Berzel S, Fajardo-Moser M, Remer KA, Moll H. Fragments of antigen-loaded dendritic cells (DC) and DC-derived exosomes induce protective immunity against Leishmania major. Vaccine. 2010;28:5785–5793. doi: 10.1016/j.vaccine.2010.06.077. [DOI] [PubMed] [Google Scholar]
- Martin-Jaular L, Nakayasu ES, Ferrer M, Almeida IC, Del Portillo HA. Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PLoS One. 2011;6:e26588. doi: 10.1371/journal.pone.0026588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FM, Franklin BS, Teixeira-Carvalho A, Filho AL, de Paula SC, Fontes CJ, Brito CF, Carvalho LH. Augmented plasma microparticles during acute Plasmodium vivax infection. Malar J. 2010;9:327. doi: 10.1186/1475-2875-9-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel PY, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Vorobjev I, Ghiran I, Toner M, Irimia D, Ivanov AR, et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe. 2013;13:521–534. doi: 10.1016/j.chom.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 2013;153:1120–1133. doi: 10.1016/j.cell.2013.04.029. [DOI] [PubMed] [Google Scholar]
- Couper KN, Barnes T, Hafalla JC, Combes V, Ryffel B, Secher T, Grau GE, Riley EM, de Souza JB. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. 2010;6:e1000744. doi: 10.1371/journal.ppat.1000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestari I, Ansa-Addo E, Deolindo P, Inal JM, Ramirez MI. Trypanosoma cruzi immune evasion mediated by host cell-derived microvesicles. J Immunol. 2012;188:1942–1952. doi: 10.4049/jimmunol.1102053. [DOI] [PubMed] [Google Scholar]
- Mantel PY, Marti M. The role of extracellular vesicles in Plasmodium and other protozoan parasites. Cell Microbiol. 2014;16:344–354. doi: 10.1111/cmi.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barteneva NS, Maltsev N, Vorobjev IA. Microvesicles and intercellular communication in the context of parasitism. Front Cell Infect Microbiol. 2013;3:49. doi: 10.3389/fcimb.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cacho E, Gallego M, Lee SH, Lillehoj HS, Quilez J, Lillehoj EP, Sanchez-Acedo C. Induction of protective immunity against Eimeria tenella infection using antigen-loaded dendritic cells (DC) and DC-derived exosomes. Vaccine. 2011;29:3818–3825. doi: 10.1016/j.vaccine.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Hu G, Gong AY, Roth AL, Huang BQ, Ward HD, Zhu G, Larusso NF, Hanson ND, Chen XM. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog. 2013;9:e1003261. doi: 10.1371/journal.ppat.1003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman HC, Frases S, Nicola AM, Rodrigues ML, Casadevall A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology. 2009;155:3860–3867. doi: 10.1099/mic.0.032854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, Casadevall A, Rodrigues ML, Nimrichter L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect Immun. 2010;78:1601–1609. doi: 10.1128/IAI.01171-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AM, Frases S, Casadevall A. Lipophilic dye staining of Cryptococcus neoformans extracellular vesicles and capsule. Eukaryot Cell. 2009;8:1373–1380. doi: 10.1128/EC.00044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepinto J, Komperda K, Frases S, Park YD, Djordjevic JT, Casadevall A, Williamson PR. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol Microbiol. 2009;71:1165–1176. doi: 10.1111/j.1365-2958.2008.06588.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Wei L, Guo T, Tan W. Detection of DOPA-melanin in the dimorphic fungal pathogen Penicillium marneffei and its effect on macrophage phagocytosis in vitro. PLoS One. 2014;9:e92610. doi: 10.1371/journal.pone.0092610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg L, Gabrielsson S, Scheynius A. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses–novel mechanisms for host-microbe interactions in atopic eczema. PLoS One. 2011;6:e21480. doi: 10.1371/journal.pone.0021480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo MC, Matsuo AL, Ganiko L, Medeiros LC, Miranda K, Silva LS, Freymüller-Haapalainen E, Sinigaglia-Coimbra R, Almeida IC, Puccia R. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic alpha-Galactosyl epitopes. Eukaryot Cell. 2011;10:343–351. doi: 10.1128/EC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo MC, Nakayasu ES, Longo LV, Ganiko L, Lopes FG, Matsuo AL, Almeida IC, Puccia R. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS One. 2012;7:e39463. doi: 10.1371/journal.pone.0039463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo MC, Nakayasu ES, Matsuo AL, Sobreira TJ, Longo LV, Ganiko L, Almeida IC, Puccia R. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. J Proteome Res. 2012;11:1676–1685. doi: 10.1021/pr200872s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WL, Russell DG. Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages. Infect Immun. 2000;68:6997–7002. doi: 10.1128/iai.68.12.6997-7002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WL, Ullrich HJ, Russell DG. Mycobacterial surface moieties are released from infected macrophages by a constitutive exocytic event. Eur J Cell Biol. 2001;80:31–40. doi: 10.1078/0171-9335-00131. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem. 2007;282:25779–25789. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JJ, Chen C, Xie PF, Pan Y, Tan YH, Tang LJ. Proteomic analysis and immune properties of exosomes released by macrophages infected with Mycobacterium avium. Microbes Infect. 2014;16:283–291. doi: 10.1016/j.micinf.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Anand PK, Anand E, Bleck CK, Anes E, Griffiths G. Exosomal Hsp70 induces a pro-inflammatory response to foreign particles including mycobacteria. PLoS One. 2010;5:e10136. doi: 10.1371/journal.pone.0010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PP, Smith VL, Karakousis PC, Schorey JS. Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo. J Immunol. 2012;189:777–785. doi: 10.4049/jimmunol.1103638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PP, LeMaire C, Tan JC, Zeng E, Schorey JS. Exosomes released from M. tuberculosis infected cells can suppress IFN-gamma mediated activation of naive macrophages. PLoS One. 2011;6:e18564. doi: 10.1371/journal.pone.0018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji KN, Goyal G, Narayana Y, Srinivas M, Chaturvedi R, Mohammad S. Apoptosis triggered by Rv1818c, a PE family gene from Mycobacterium tuberculosis is regulated by mitochondrial intermediates in T cells. Microbes Infect. 2007;9:271–281. doi: 10.1016/j.micinf.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Giri PK, Schorey JS. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One. 2008;3:e2461. doi: 10.1371/journal.pone.0002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Schorey JS. Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur J Immunol. 2013;43:3279–3290. doi: 10.1002/eji.201343727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra L, Qu Y, Wang Y, Lewis CJ, Cobb BA, Takatsu K, Boom WH, Dubyak GR, Harding CV. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing major histocompatibility complex class II molecules capable of antigen presentation. Infect Immun. 2010;78:5116–5125. doi: 10.1128/IAI.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar SM, Martin CJ, Nunes-Alves C, Divangahi M, Remold HG. Lipids, apoptosis, and cross-presentation: links in the chain of host defense against Mycobacterium tuberculosis. Microbes Infect. 2011;13:749–756. doi: 10.1016/j.micinf.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan H, Upton JW. Programmed necrosis in microbial pathogenesis. Trends Microbiol. 2014;22:199–207. doi: 10.1016/j.tim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Ernst JD. Cell-to-cell transfer of M. tuberculosis antigens optimizes CD4 T cell priming. Cell Host Microbe. 2014;15:741–752. doi: 10.1016/j.chom.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Chalasani G, Ng YH, Robbins PD. Exosomes released from Mycoplasma infected tumor cells activate inhibitory B cells. PLoS One. 2012;7:e36138. doi: 10.1371/journal.pone.0036138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami L, Brandi L, Moayeri M, Brown MJ, Krantz BA, Leppla SH, van der Goot FG. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep. 2013;5:986–996. doi: 10.1016/j.celrep.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettelaie C, Collier ME, James NJ, Li C. Induction of tissue factor expression and release as microparticles in ECV304 cell line by Chlamydia pneumoniae infection. Atherosclerosis. 2007;190:343–351. doi: 10.1016/j.atherosclerosis.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Frohlich K, Hua Z, Wang J, Shen L. Isolation of Chlamydia trachomatis and membrane vesicles derived from host and bacteria. J Microbiol Methods. 2012;91:222–230. doi: 10.1016/j.mimet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colino J, Snapper CM. Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J Immunol. 2006;177:3757–3762. doi: 10.4049/jimmunol.177.6.3757. [DOI] [PubMed] [Google Scholar]
- Colino J, Snapper CM. Dendritic cell-derived exosomes express a Streptococcus pneumoniae capsular polysaccharide type 14 cross-reactive antigen that induces protective immunoglobulin responses against pneumococcal infection in mice. Infect Immun. 2007;75:220–230. doi: 10.1128/IAI.01217-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassey EO, Life PF, Catty D, Gaston JS, Kumararatne DS. T-cell response to mycobacterial proteins: a comparative study of tuberculous and control immunoblots of Mycobacterium tuberculosis and M. bovis BCG. Tuber Lung Dis. 1996;77:146–153. doi: 10.1016/s0962-8479(96)90029-5. [DOI] [PubMed] [Google Scholar]
- Demissie A, Ravn P, Olobo J, Doherty TM, Eguale T, Geletu M, Hailu W, Andersen P, Britton S. T-cell recognition of Mycobacterium tuberculosis culture filtrate fractions in tuberculosis patients and their household contacts. Infect Immun. 1999;67:5967–5971. doi: 10.1128/iai.67.11.5967-5971.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksa M, Wu R, Dong W, Das P, Yang D, Wang P. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock. 2006;25:586–593. doi: 10.1097/01.shk.0000209533.22941.d0. [DOI] [PubMed] [Google Scholar]
- Fader CM, Sanchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta. 2009;1793:1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- Admyre C, Grunewald J, Thyberg J, Gripenback S, Tornling G, Eklund A, Scheynius A, Gabrielsson S. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J. 2003;22:578–583. doi: 10.1183/09031936.03.00041703. [DOI] [PubMed] [Google Scholar]
- Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput N, Thery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33:419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- Dobos K, Schorey JS, Kruh-Garcia N. Tuberculosis Biomarkers: Prospects from the Bench to the Clinic. Rijeka, Croatia: InTech – Open Access Publisher; 2011. [Google Scholar]
- Kruh-Garcia NA, Wolfe WL, Chaisson LH, Worodria WO, Nahid P, Schorey JS, Davis JL, Dobos KM. Use of multiple reaction monitoring mass spectrometry to enhance discovery of tuberculosis biomarkers in exosomes. PLoS One. 2014;9:e103811. doi: 10.1371/journal.pone.0103811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker MW, Reichert D, Susser S, Sarrazin C, Martinez Y, Herrmann E, Zeuzem S, Piiper A, Kronenberger B. Soluble serum CD81 is elevated in patients with chronic hepatitis C and correlates with alanine aminotransferase serum activity. PLoS One. 2012;7:e30796. doi: 10.1371/journal.pone.0030796. [DOI] [PMC free article] [PubMed] [Google Scholar]