Abstract
Objective
Hyperglycemia has been recognized for decades to be an exacerbating factor in ischemic stroke, but the mechanism of this effect remains unresolved. Here we evaluated superoxide production by neuronal NADPH oxidase as a link between glucose metabolism and neuronal death in ischemia-reperfusion.
Methods
Superoxide production was measured by the ethidium method in cultured neurons treated with oxygen-glucose deprivation and in mice treated with forebrain ischemia-reperfusion. The role of NADPH oxidase was examined using genetic disruption of its p47phox subunit and with the pharmacological inhibitor, apocynin.
Results
In neuron cultures, post-ischemic superoxide production and cell death were completely prevented by removing glucose from the medium, by inactivating NADPH oxidase, or by inhibiting the hexose monophosphate shunt which generates NADPH from glucose. In murine stroke, neuronal superoxide production and death were decreased by the glucose anti-metabolite, 2-deoxyglucose, and increased by high blood glucose concentrations. Inactivating NADPH oxidase with either apocynin or deletion of the p47phox subunit blocked neuronal superoxide production and negated the deleterious effects of hyperglycemia.
Interpretation
These findings identify glucose as the requisite electron donor for reperfusion-induced neuronal superoxide production and establish a previously unrecognized mechanism by which hyperglycemia can exacerbate ischemic brain injury.
Keywords: ischemia, reperfusion, superoxide, NADPH oxidase, hexose monophosphate shunt
Ischemic brain injury is reduced by early reperfusion 1, 2, but reperfusion can itself contribute to brain injury 3, 4. It has long been recognized that ischemia/reperfusion injury is greatly exacerbated by hyperglycemia 5, 6, 7, despite the fact that glucose is required for normal brain function. The underlying mechanism of this “glucose paradox” remains unresolved. One widely accepted mechanism by which hyperglycemia can exacerbate injury brain is by fueling the accumulation of lactic acid in hypoxic tissues 8; however, elevated glucose concentrations also exacerbate injury in brain slice models of ischemia in which pH is tightly controlled 9, 10, and in animal models of brain ischemia in which tissue acidosis is prevented by reperfusion during hyperglycemia 11-13. Moreover, clinical studies suggest that hyperglycemia during reperfusion increases risk of hemorrhage and poor clinical outcome, independent of diabetes or pre-ischemic glucose concentrations 14, 15. These observations suggest that a mechanism other than lactic acidosis may underlie the effect of hyperglycemia on reperfusion injury.
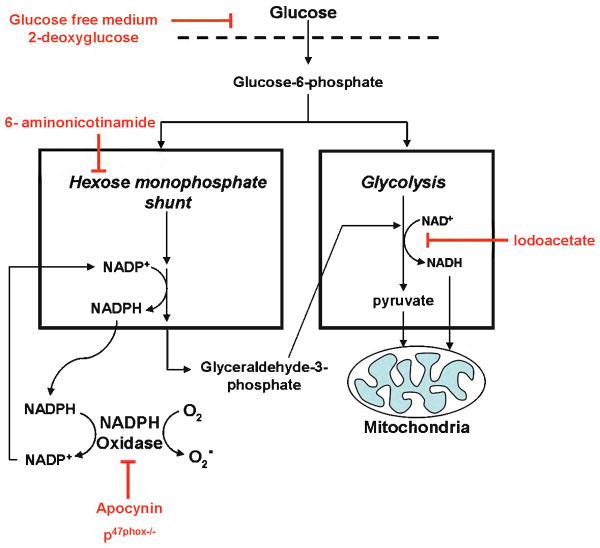
Brain reperfusion injury is mediated in part by the production of superoxide and other reactive oxygen species 16. Although neuronal superoxide production is widely attributed to mitochondria, neurons also express nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a superoxide-generating enzyme first identified in phagocytes 17-19. Superoxide produced by neuronal NADPH oxidase is thought to function in long-term potentiation and intercellular signaling 20, 21, but superoxide can also cause oxidative stress and neuronal death 22, 23. Importantly, the activity of NADPH oxidase requires the continuous metabolism of glucose by the hexose monophosphate shunt for supply of NADPH substrate 23-25, thus suggesting a mechanistic link between glucose and neuronal superoxide production in ischemia-reperfusion (Fig. 1).
Figure 1. Metabolic coupling between glucose and superoxide production.
Glucose can support superoxide production by supplying reducing equivalents to either NADPH oxidase or the mitochondria. Glucose transport and entry into cells is blocked by glucose-free medium or by 2-deoxyglucose. Flux of glucose carbon and glucose-derived NADPH to mitochondria is blocked by iodoacetate at the glyceraldehyde-3-phosphate dehydrogenase step of glycolysis. The production of NADPH in the hexose monophosphate shunt is blocked by 6-aminonicotinamide, and the activity of NADPH oxidase is blocked by apocynin or by p47phox deficiency.
Here we evaluated the contribution of NADPH oxidase to reperfusion - induced neuronal superoxide production and neuronal death, using pharmacological and genetic suppression of NADPH oxidase activity in cell culture and mouse stroke models. In the mouse model, hyperglycemia and functional hypoglycemia (produced with 2-deoxyglucose) were initiated at the time of reperfusion in order to specifically evaluate their effects on reperfusion injury. Results of these studies show that neuronal superoxide production is entirely dependent on glucose and is increased by hyperglycemia during the reperfusion period, and further show that NADPH oxidase is the primary source of neuronal superoxide production.
Materials and Methods
Studies were approved by the San Francisco Veterans Affairs Medical Center animal studies committee. The p47phox-/- mice and the wild-type (wt) mice used as their controls were of the C57/Bl6 background strain (Jackson Labs). Mice in the p47phox-/- colony are maintained as homozygotes, with use of fresh breeder stock, fully back-crossed to wt C57/Bl6 mice, after every 10 generations. All other mice were Swiss Webster (Simonsen, Gilroy, CA). Cell culture reagents were obtained from Mediatech (Herndon, VA), and all other reagents were obtained form Sigma-Aldrich except where noted.
Neuron cultures
Neuron cultures were prepared from the cortices of embryonic day 16 Swiss-Webster or C57/Bl6 mice as described previously 23. The dissociated cells were plated on poly-D-lysine coated plates or poly-D-lysine-coated glass coverslips and maintained with serum-free NeuroBasal medium containing 5 mM glucose. The neurons were used at day 9 - 10 in vitro, at which time they contained greater than 99% neurons, as assessed by immunostaining for the neuron marker MAP2 and the astrocyte marker GFAP (Supplementary Fig. 1).
Oxygen glucose deprivation (OGD)
Cultures were placed in a 37°C anaerobic chamber (Coy Labs) with O2 tension < 0.02%, washed thrice, and incubated with, glucose-free balanced salt solution (BSS) containing (in mM) KCl, 3.1; NaCl, 134; CaCl2, 1.2; MgSO4, 1.2; KH2PO4, 0.25; NaHCO3, 15.7; HEPES, 5 that had been de-oxygenated by 10-minute sparging with nitrogen. Control wells on each 24-well plate were washed and incubated with standard (non-deoxygenated) BSS containing 5 mM glucose. After 2 hours the culture plates were returned to a 5% CO2 / balance air atmosphere for passive re-oxygenation and, where indicated, 5 mM glucose was returned to the designated culture wells. pH was maintained at 7.2- 7.4 throughout.
Immunostaining
Cultures were fixed with 1:1 methanol:acetone at 4° C prior to immunostaining for microtubule-associated protein 2 (MAP2; Chemicon), glial fibrillary acidic protein (GFAP; Chemicon), Iba-1 (Waco), or 4-hydroxy-2-nonenal (4-HNE; Alpha Diagnostic) as described previously 23. Antibody binding was visualized with Alexa Fluor - conjugated anti-IgG (Molecular Probes). Immunostaining for p47 phox (Upstate), was performed with cultures fixed in ice-cold 4% paraformaldehyde, and images were obtained by confocal microscopy. Negative controls were prepared by omitting the primary antibodies (not shown).
Neuron viability
Twenty-four hours after OGD, cultures were incubated for 5 minutes in 0.5% Trypan Blue and the number of unstained (viable) neurons and stained (dead) neurons were counted in 3 randomly selected fields from each well by an observer blinded to the experimental conditions. The total number of neurons counted was > 60 in each well, and the results from 3 wells were pooled to generate a single value for each experiment.
Ischemia-reperfusion
Bilateral common carotid artery occlusions were performed with 3-month old male C57/Bl6 wild-type and p47phox-/- mice 26, 27. The mice were anesthetized with 2% isoflurane and a 75:25 mixture of nitrous oxide:oxygen, and core temperature was kept at 36.5 - 37.5 °C with a homoeothermic blanket control unit (Harvard apparatus, Holliston, MA). Both carotid arteries were exposed through a midline neck incision and the thymus tissue was separated by scissors. The common carotid arteries were encircled with a 4-O silk suture to facilitate artery clip placement and inspection of the vessels after clip removal. To reduce variability, EEG recordings were used to time the ischemic interval 26, 27. EEG was monitored as described previously 28 using needle electrodes placed in the cortical surface (BIOPAC, Santa Barbara, CA). Two burr holes were made in the skull bilaterally over parietal cortex (0.5 mm caudal from Bregma and 2.0 mm lateral to the midline) and monopolar electrodes were inserted beneath the dura. A reference needle was placed in neck muscle. Small aneurysm clips were applied to both common carotid arteries until 30 minutes of EEG isoelectricity had elapsed, with isoelectricity defined as fewer than 3 spikes of cortical activity per 60-second interval. Onset of isoelectricity generally occurred within 3 - 4 minutes of clip placement. At the end of the 30-minute isoelectric period, the aneurysm clips were removed and the common carotid arteries were inspected for normal recovery of blood flow. Mice were given 0.2 ml intraperitoneal injections of apocynin (15 mg / kg), glucose (25% solution), 2-deoxyglucose (100 mg / kg), or saline vehicle immediately before reperfusion. Blood samples (15 μl) were withdrawn from the tail vein at the designated time points (pre- and post- ischemia) for glucose determination with a BD Logic blood glucose monitor (Becton-Dickinson, NJ). Anesthetics were discontinued following closure of the skin incision and the mice were returned to a 37 °C recovery chamber until ambulatory. Sham operated animals received the same neck skin incision and thymus separation but without carotid occlusion. In both the wt and p47phox -/- genotype groups, approximately 15% of the mice failed to achieve isoelectricity during carotid artery occlusion, and approximately 5% showed no reflow in one or both carotid arteries. These mice were euthanized and not entered into any of the reperfusion treatment groups.
Superoxide detection
Superoxide was detected in cell culture by the ethidium fluorescence method as previously described 23, 29. In cell culture studies, 5 μM dihydroethidium (Molecular Probes) was added to the BSS at the beginning of OGD. Four 20x fields were photographed with a fluorescence microscope 1 hour after removal from the anaerobic chamber, using 510-550 nm excitation and > 580 nm emission for detection of oxidized ethidium species 30, 31. As a positive control, some wells were incubated with 100 μM H2O2 in BSS for 30 minutes prior to photography. An observer blinded to the experimental conditions counted the percent of neurons in each well with a fluorescent signal 50% higher than background. The total number of neurons counted was > 60 in each well, and the results from 3 wells were pooled to generate a single value for each experiment. The same method was used to evaluate superoxide production during H2O2 incubations, with the dihydroethidium present during the 30 minute H2O2 incubation interval. For studies in vivo, dihydroethidium was prepared as a 1 mg / ml solution in 1% dimethyl sulfoxide (DMSO) and administered 1 mg / kg by intraperitoneal injection at the onset of ischemia 23, 32. Three hours after carotid artery reperfusion, the mice were euthanized and perfusion fixed with 4 % paraformaldehyde. Brains were cryostat sectioned and photographs were prepared from 5 sections of each brain, taken at 40 μm intervals to span the hippocampus, using confocal fluorescent microscopy with 510-550 nm excitation and > 580 nm emission. The fluorescence of 10 representative CA1 hippocampal neuron cell bodies in each section was normalized to background fluorescence in the stratum radiatum 23. The normalized values for the 50 neurons evaluated were averaged to generate a single value for each brain.
Neuronal death
Mice were perfusion-fixed with 4% paraformaldehyde 3 days after ischemia-reperfusion. Five 20 μm thick coronal sections were collected from each brain, spaced 40 μm apart and spanning the hippocampus. The sections were stained by the Fluoro-Jade B method (Histo-Chem, Jefferson, AR) 33. An observer blinded to the experimental conditions counted the total number of Fluoro-Jade B positive neurons in the CA1 hippocampus of each hemisphere. The mean number of degenerating neurons (total number from both hemispheres / 2) was recorded for each brain and expressed as neurons / slice.
Statistical analyses
For cell culture studies the “n” denotes the number of independent experiments, each performed with an independent culture preparation and with triplicate wells for each data point. For in vivo studies, the ‘n” denotes the number of mice, with each mouse providing a single data point. All data are expressed as means ± standard error. Group differences were assessed by one-way ANOVA followed by either the Tukey-Kramer test for multiple comparisons between groups or the Dunnett's test for comparisons of multiple groups against a common control group.
Results
Glucose is required for neuronal ROS generation after oxygen - glucose deprivation (OGD)
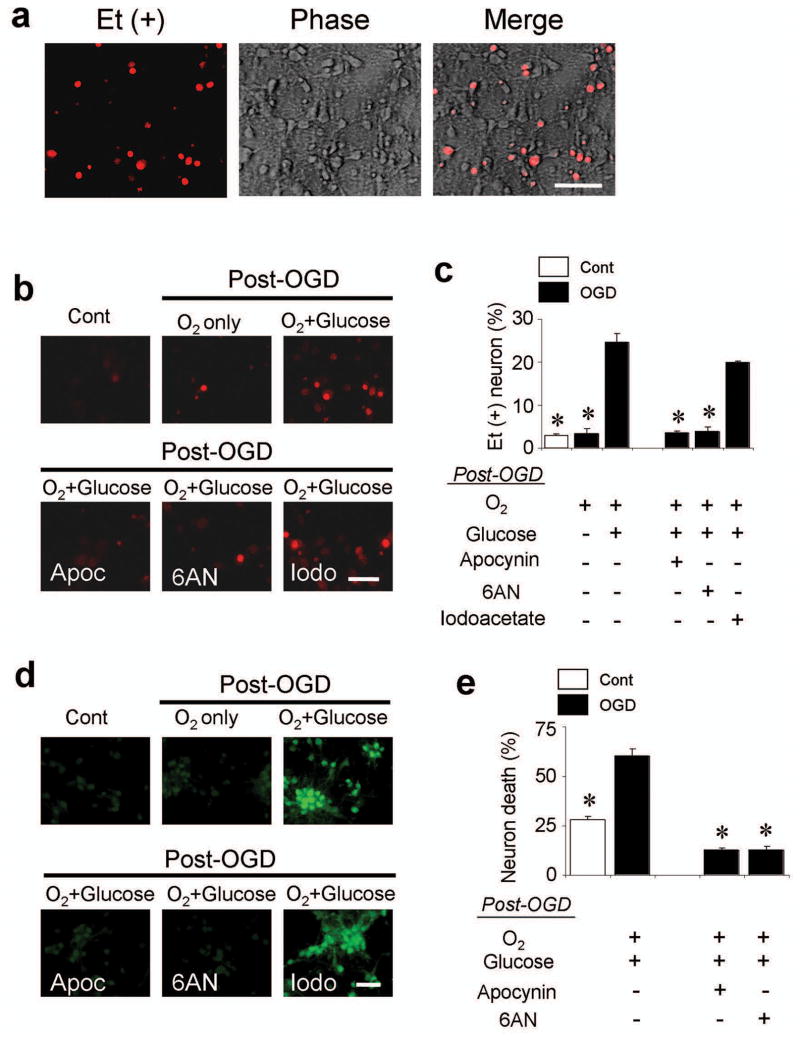
Neuron cultures were subjected to the oxygen-glucose deprivation (OGD) model of brain ischemia-reperfusion 34. These cultures contained less than 1% astrocytes and no detectable microglia (Supplementary Fig. 1). Cultures exposed to 2 hours of OGD (ischemia), followed by return to air-equilibrated medium containing 5 mM glucose (reperfusion), showed a roughly 10-fold increase in the percent of neurons with detectable Et signal (Fig. 2), as evaluated by the ethidium (Et) fluorescence method 30, 31. By contrast, there was no increased Et signal when glucose was omitted from the post-OGD medium. This glucose-dependent superoxide production was not attenuated by the glycolytic inhibitor iodoacetate 35, but 6-aminonicotinamide, an inhibitor of the hexose monophosphate shunt 25, reduced the Et signal as effectively as glucose-free medium (Fig. 2b,c). The NADPH oxidase inhibitor apocynin 36 also blocked superoxide production after OGD, suggesting NADPH oxidase as the major source of superoxide. The degree of superoxide production was not influenced by glucose concentration over the range of 1 - 20 mM in these cell culture studies (not shown).
Figure 2. Glucose is required for neuronal superoxide production after oxygen - glucose deprivation (OGD).
(a) Neuronal superoxide production imaged by ethidium (Et) fluorescence. Cultures underwent 2 hours of OGD followed by 1 hour incubation in air-equilibrated medium containing 5 mM glucose (O2 + glucose). Ethidium fluorescence is evident in a subset of the neuronal cell bodies. Scale bar = 100 μm. (b) Et fluorescence was markedly reduced in cultures maintained in glucose-free (O2 only) medium for the 1-hour interval after OGD. Control wells received medium exchanges only. Where indicated, cultures were also treated with apocynin (Apoc, 500 μM), 6-aminonocotinamide (6AN, 500 μM) or iodoacetate (Iodo, 500 μM). (c) Quantified ethidium fluorescence, conditions as in (b). The increased signal observed after OGD was prevented by 0-glucose medium, apocynin, and 6-aminonicotinamide, but not by iodoacetate. n = 7, * P < 0.05 vs. OGD, O2+glucose. (d) Immunostaining for 4-hydroxynonenal in cultured neurons showed the same pattern as observed with ethidium fluorescence. Conditions as in (b); representative of n = 3. Scale bar = 100 μm. (e) Neuronal death in cultures treated as in (b), assessed 24 hours after OGD. n = 3-5, * P < 0.05 vs. OGD, O2+glucose.
Neuronal cultures were immunostained for 4-hydroxynonenal, a product of lipid peroxidation, to confirm the findings obtained by the Et fluorescence method. The same pattern was observed: OGD induced formation of 4-hydroxynonenal, and this was blocked by apocynin, by 6-aminonicotinamide, and by omitting glucose from the post-OGD medium, but not blocked by iodoacetate (Fig. 2d). Neuronal survival was evaluated in cultures maintained for 24 hours after OGD to determine whether reduced superoxide production led to reduced neuronal death. The glucose-free medium and iodoacetate conditions were not testable because they produce extensive neuronal death when maintained for 24 hours, independent of OGD exposure. However, 24-hour incubations with apocynin and 6-aminonicotinamide were not toxic, and both of these agents significantly reduced OGD-induced neuronal death (Fig. 2e).
Effects of p47 phox gene deletion on OGD-induced ROS production and neuronal death
NADPH oxidase is comprised of several subunits, including the p47phox subunit, which coalesce at the plasma membrane to form the active enzyme complex 17. Immunostaining confirmed the presence of p47phox in neurons and showed its translocation to the neuronal plasma membrane after OGD (Fig. 3a). Translocation was blocked by glucose deprivation and by 6-aminonicotinamide following OGD, suggesting that the NADPH substrate is required to maintain enzyme complex assembly at the plasma membrane. To confirm the observations made with pharmacological inhibitors of NADPH oxidase, neuronal cultures were prepared from p47phox deficient mice, which are unable to assemble an active NADPH oxidase complex 37. The p47phox deficient neurons did not show an increase in superoxide production following OGD, in marked contrast to wild-type (wt) neurons of the same C57/Bl6 strain (Fig. 3b,c). Superoxide production by p47phox-/- neurons was also reduced under control (wash-only) conditions. These differences between p47phox-/- and wt neurons cannot be attributed to differences in uptake or compartmentalization or dihydroethidium because p47phox-/- and wt neurons exposed to H2O2 showed an equivalent Et signal. Neuronal survival evaluated 24 hours after OGD showed less cell death in the p47phox-/- neurons (Fig. 3d), consistent with the findings obtained with apocynin and 6-aminonicotinamide.
Figure 3. p47phox -/- neurons do not produce superoxide after OGD.
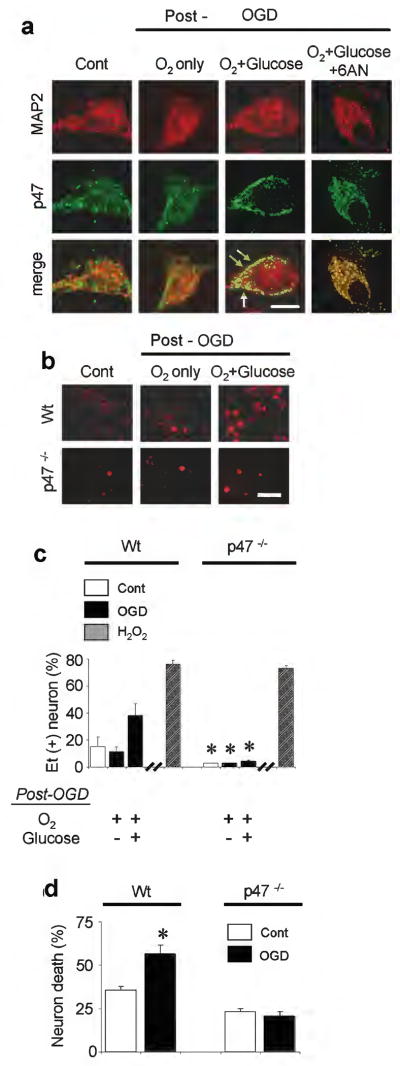
(a) In wild-type neurons, immunostaining for the p47phox subunit of NADPH oxidase shows migration to the neuronal plasma membrane area (arrows) in neurons placed in standard medium after OGD, but not in neurons placed in 0-glucose medium or treated with 6-aminonicotinamide (500 μM) after OGD. MAP2 immunostaining demarcates the neuronal cytoplasmic area. Scale bar = 10 μm. Representative of n = 3. (b) Ethidium fluorescence in wild-type (wt) and p47phox -/- neurons subjected to 2 hours OGD, followed by 1 hour incubation in standard medium (O2 + glucose) or glucose-free medium (O2 only). Scale bar = 100 μm. (c) Quantified ethidium fluorescence. H2O2, 30 minutes exposure to 100 μM hydrogen peroxide; other conditions as in (a). n = 3-8, * P < 0.05 vs. wt neurons treated identically. (d) OGD - reperfusion produced less death in p47phox -/- neurons than wt neurons. n = 5, *P < 0.05 vs. control.
NADPH oxidase inhibition reduces neuronal ROS production and cell death after ischemia-reperfusion in vivo
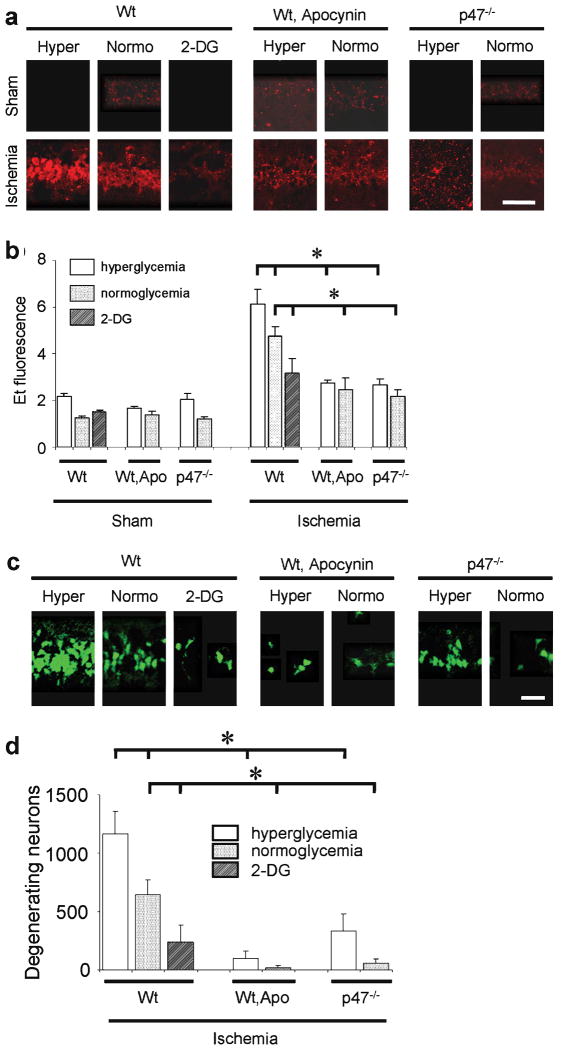
Superoxide production was also evaluated in C57/Bl6 mice following transient forebrain ischemia, which causes selective neuronal death primarily in CA1 hippocampus 38. Brains harvested 3 hours after ischemia-reperfusion showed a several-fold increase in Et signal in the CA1 neurons relative to mice undergoing sham surgery (Fig. 4a,b). This increase was almost completely blocked both in p47phox-/- mice and wt mice treated with apocynin. The number of degenerating neurons identified in CA1 hippocampus 3 days after ischemia-reperfusion was also markedly reduced in these mice (Fig. 4c,d).
Figure 4. Ischemia-induced neuronal superoxide production and cell death are influenced by blood glucose and NADPH oxidase activity.
(a) Ethidium fluorescence in CA1 hippocampal neurons 3 hours after ischemia-reperfusion. Sham mice received surgery without ischemia. Mice were wild-type (wt) or p47phox-/- genotype. Apo, 15 mg / kg apocynin; 2-DG, 100 mg / kg 2-deoxyglucose. Scale bar = 100 μm. (b) Quantification of ethidium fluorescence, conditions as in (a). n = 4- 5, * P < 0.05. (c) Fluoro-Jade-B staining of degenerating neurons in CA1 hippocampus 3 days after ischemia. (d) Quantified neuronal death; n = 7 - 10, * P < 0.05.
To determine whether the deleterious effect of hyperglycemia on ischemia-reperfusion brain injury was mediated by NADPH oxidase, mice were rendered hyperglycemic, normoglycemic, or functionally hypoglycemic with 2-deoxyglucose during the reperfusion period. Blood glucose concentrations for each treatment group are shown in Table 1. Relative to the normoglycemic group, superoxide production was increased in the hyperglycemic mice and reduced in the 2-deoxyglucose - treated mice (Fig. 4a,b). Importantly, p47phox-/- mice did not exhibit increased superoxide production when rendered hyperglycemic, and wt mice treated with apocynin also showed no increased superoxide production when rendered hyperglycemic. The effect of these treatment conditions on neuronal survival was assessed in brains harvested 3 days after ischemia. A similar pattern was observed: CA1 neuronal death was increased in the hyperglycemic mice and reduced in the 2-deoxyglucose-treated mice (Fig. 4c,d), and hyperglycemia did not increase neuronal death in the apocynin-treated or p47phox-/- mice (although this approached statistical significance, p = 0.056, in the latter group). These results may underestimate the effect of p47phox deficiency on neuronal survival, because the 3-day post-ischemic mortality in the hyperglycemic p47phox-/- mice was also less than in the hyperglycemic wt mice (Table 2).
Table 1. Mouse blood glucose concentrations.
| Wt | Wt, Apo | p47-/- | |
|---|---|---|---|
| Hyperglycemic | |||
| Pre-ischemia | 97± 2(n=12) | 99 ± 3 (n=12) | 99 ± 2(n=8) |
| Reperfusion | 435 ±11 (n=12) | 460 ±16 (n=12) | 411 ± 8 (n= 8) |
| Normoglycemic | |||
| Pre-ischemia | 98 ± 4 (n=10) | 99 ± 3 (n=7) | 101 ± 3 (n=10) |
| Reperfusion | 100 ± 4 (n=10) | 101 ± 2 (n=7) | 99 ± 2 (n=10) |
| 2-deoxyglucose | |||
| Pre-ischemia | 98 ± 3 (n=5) | n.d | n.d. |
| Reperfusion | 102 ± 3 (n=5) | n.d. | n.d. |
The treatment groups are as defined as in Fig. 4. Mice were given 0.2 ml of 25% glucose (hyperglycemia), 0.2 ml saline (normoglycemia), or 100 mg / kg 2-deoxyglucose by i.p. injection immediately prior to reperfusion. Values are mg / dl; n.d., not determined.
Table 2. Mortality (%) after ischemia-reperfusion.
| Wt | Wt, Apo | p47-/- | |
|---|---|---|---|
| Normoglycemia | 22.2 (n=11) | 11.1 (n=9) | 14.3 (n=7) |
| Hyperglycemia | 54.6 (n=22) | 44.4 (n=9) | 23.1 (n=13) |
| 2-Deoxyglucose | 22.2 (n= 9) | n.d. | n.d. |
The treatment groups are as defined in Fig. 4. “Hyperglycemia” mice were given 0.2 ml of 25% glucose, “normoglycemia” mice were given 0.2 ml saline, and “2-deoxyglucose” mice were given 100 mg / kg 2-deoxyglucose i.p., immediately prior to reperfusion. Values are mg / dl; n.d., not determined.
Discussion
The superoxide production that occurs during reperfusion of ischemic brain is widely attributed to the return of oxygen to damaged tissues, particularly mitochondria. However, the formation of superoxide requires an electron donor in addition to oxygen. In the cell culture studies presented here, superoxide production was rendered undetectable in the absence of glucose and when NADPH oxidase or the hexose monophosphate shunt were inactivated after OGD. These findings indicate that glucose is required as the electron donor during reperfusion-induced superoxide production in neurons, through its metabolism by the hexose monophosphate shunt to generate NADPH. In the brain ischemia studies, neuronal superoxide production and neuron death were both increased by hyperglycemia. The increase in superoxide production was eliminated in apocynin-treated and p47phox-/- mice, thus establishing NADPH oxidase as the primary source of hyperglycemia-induced superoxide production. The increase in neuronal death was also mitigated in apocynin-treated and p47phox-/- mice. Together with the cell culture studies, these results suggest that hyperglycemia exacerbates reperfusion injury by supplying substrate for superoxide production by NADPH oxidase.
The cell culture results show that superoxide is generated by neurons after ischemia-reperfusion, and the studies in vivo show localization of Et signal to the CA1 neuronal pyramidal layer. We cannot exclude the possibility that other cell types, notably microglia, might contribute to superoxide production in the in vivo studies, but microglia are distributed diffusely throughout the hippocampus and thus would be unlikely to contribute to the Et signal confined to the CA1 pyramidal layer. In addition, these brains were harvested at 3 hours post-ischemia to minimize any influence of the brain inflammatory response. The finding that elevated glucose concentrations influenced superoxide production in vivo, but not in the cell culture studies, may be attributable to an effect of hyperglycemia on glucose flux across the blood-brain barrier and other tissue barriers that are not present in cell culture preparations.
2-deoxyglucose, which slows glucose transport and impairs glucose utilization at the hexokinase step 39, had effects on superoxide production and cell death that were the opposite of those observed with hyperglycemia. Prior studies have reported a beneficial effect of 2-deoxyglucose on outcome from cerebral ischemia 40, and attributed this effect to an increased stress protein response. The present studies findings suppression of post-ischemic superoxide production as an alternative mechanism for the neuroprotective effect of 2-deoxyglucose.
Et fluorescence is widely used as a marker of superoxide formation, but the interactions between superoxide and dihydroethidium are complex. Cellular peroxidases enable oxidants other than superoxide to react with dihydroethidium, either alone or in sequence with superoxide, to generate multiple fluorescent products with overlapping spectra 30, 31. Evidence that Et fluorescence is attributable to superoxide in the present studies comes in part from prior work, using a similar ischemia-reperfusion model, that showed the Et signal was both attenuated by superoxide dismutase-1 (SOD-1) over-expression and increased by SOD-1 deletion 38, 41. The Et signal observed here was blocked by both pharmacological and genetic ablation of NADH oxidase activity, further indicating that the Et signal originates from superoxide or superoxide metabolites such as H2O2.
An extensive literature establishes hyperglycemia as a deleterious factor in stroke, and this effect is generally attributed to the increased accumulation of lactic acid under hyperglycemic conditions (reviewed in 42). However, a cause-effect relationship has not been formally established between lactic acid accumulation and deleterious outcome, and hyperglycemia also exacerbates ischemic injury in settings in which lactic acid is pH - buffered 9, 10 or does not accumulate 11-13. In the present study, hyperglycemia was initiated just prior to reperfusion in order to isolate the effects of hyperglycemia on reperfusion injury. The findings indicate that hyperglycemia can exacerbate ischemic injury by promoting superoxide production, independent of lactic acidosis. This does not refute the idea that hyperglycemia can exacerbate ischemic injury by fueling lactic acidosis, but it does provide an alternative possibility.
We emphasize that the findings obtained here may not necessarily be extrapolated to other ischemic settings or cell types. NADPH oxidase may not be the major source of superoxide in ischemia without reperfusion, or with delayed reperfusion. Particularly under acidosis conditions, iron stores in mitochondria and elsewhere may be liberated to catalyze superoxide production 43. Nitric oxide may be produced by neurons and other cell types, and xanthine oxidase can be a major source of superoxide in endothelial cells. Microglia and infiltrating leukocytes may become the major source of superoxide production after several hours post-ischemia. Nonetheless, the present findings indicate that NADPH oxidase is a primary source of neuronal superoxide in the immediate reperfusion phase after transient ischemia, and that the activity of NADPH oxidase is strongly influenced by circulating blood glucose concentrations during reperfusion. This suggests a mechanism by which control of blood glucose or inhibition of neuronal NADPH oxidase may improve stroke outcome or extend the therapeutic time window of thrombolytic therapy.
Supplementary Material
Acknowledgments
This work was supported by the Juvenile Diabetes Research Foundation (2-2006-113, to S.W.S.), the National Institutes of Health (NS051855 and P50 NS14543 to R.A.S., NS40516 to M.A.Y.), and the Department of Veterans Affairs.
Footnotes
Conflict of interest statement: None of the authors have any financial interest relevant to the work presented in this manuscript.
References
- 1.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Zivin JA, Lyden PD, DeGirolami U, et al. Tissue plasminogen activator. Reduction of neurologic damage after experimental embolic stroke. Arch Neurol. 1988;45:387–391. doi: 10.1001/archneur.1988.00520280033012. [DOI] [PubMed] [Google Scholar]
- 3.Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Hallenbeck JM, Dutka AJ. Background review and current concepts of reperfusion injury. Arch Neurol. 1990;47:1245–1254. doi: 10.1001/archneur.1990.00530110107027. [DOI] [PubMed] [Google Scholar]
- 5.Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab. 2007;27:435–451. doi: 10.1038/sj.jcbfm.9600355. [DOI] [PubMed] [Google Scholar]
- 6.Myers RE, Yamaguchi S. Nervous system effects of cardiac arrest in monkeys. Preservation of vision. Arch Neurol. 1977;34:65–74. doi: 10.1001/archneur.1977.00500140019003. [DOI] [PubMed] [Google Scholar]
- 7.Parsons MW, Barber PA, Desmond PM, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–28. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- 8.Plum F. What causes infarction in ischemic brain?: The Robert Wartenberg Lecture. Neurology. 1983;33:222–223. doi: 10.1212/wnl.33.2.222. [DOI] [PubMed] [Google Scholar]
- 9.Newell DW, Barth A, Papermaster V, Malouf AT. Glutamate and non-glutamate receptor mediated toxicity caused by oxygen and glucose deprivation in organotypic hippocampal cultures. J Neurosci. 1995;15:7702–7711. doi: 10.1523/JNEUROSCI.15-11-07702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rytter A, Cronberg T, Asztely F, et al. Mouse hippocampal organotypic tissue cultures exposed to in vitro “ischemia” show selective and delayed CA1 damage that is aggravated by glucose. J Cereb Blood Flow Metab. 2003;23:23–33. doi: 10.1097/01.WCB.0000034361.37277.1B. [DOI] [PubMed] [Google Scholar]
- 11.Park WS, Chang YS, Lee M. Effects of hyperglycemia or hypoglycemia on brain cell membrane function and energy metabolism during the immediate reoxygenation-reperfusion period after acute transient global hypoxia-ischemia in the newborn piglet. Brain Res. 2001;901:102–108. doi: 10.1016/s0006-8993(01)02295-8. [DOI] [PubMed] [Google Scholar]
- 12.D’Alecy LG, Lundy EF, Barton KJ, Zelenock GB. Dextrose containing intravenous fluid impairs outcome and increases death after eight minutes of cardiac arrest and resuscitation in dogs. Surgery. 1986;100:505–511. [PubMed] [Google Scholar]
- 13.Venables GS, Miller SA, Gibson G, et al. The effects of hyperglycaemia on changes during reperfusion following focal cerebral ischaemia in the cat. J Neurol Neurosurg Psychiatry. 1985;48:663–669. doi: 10.1136/jnnp.48.7.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribo M, Molina C, Montaner J, et al. Acute hyperglycemia state is associated with lower tPA-induced recanalization rates in stroke patients. Stroke. 2005;36:1705–1709. doi: 10.1161/01.STR.0000173161.05453.90.9f. [DOI] [PubMed] [Google Scholar]
- 15.Bruno A, Biller J, Adams HP, Jr, et al. Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology. 1999;52:280–284. doi: 10.1212/wnl.52.2.280. [DOI] [PubMed] [Google Scholar]
- 16.Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 17.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 18.Serrano F, Kolluri NS, Wientjes FB, et al. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- 19.Tejada-Simon MV, Serrano F, Villasana LE, et al. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 21.Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol. 1998;80:452–457. doi: 10.1152/jn.1998.80.1.452. [DOI] [PubMed] [Google Scholar]
- 22.Hwang JJ, Choi SY, Koh JY. The role of NADPH oxidase, neuronal nitric oxide synthase and poly(ADP ribose) polymerase in oxidative neuronal death induced in cortical cultures by brain-derived neurotrophic factor and neurotrophin-4/5. J Neurochem. 2002;82:894–902. doi: 10.1046/j.1471-4159.2002.01040.x. [DOI] [PubMed] [Google Scholar]
- 23.Suh SW, Gum ET, Hamby AM, et al. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decoursey TE, Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci. 2005;62:2173–2193. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupte SA, Levine RJ, Gupte RS, et al. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol. 2006;41:340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Wellons JC, 3rd, Sheng H, Laskowitz DT, et al. A comparison of strain-related susceptibility in two murine recovery models of global cerebral ischemia. Brain Res. 2000;868:14–21. doi: 10.1016/s0006-8993(00)02216-2. [DOI] [PubMed] [Google Scholar]
- 27.Jeffs GJ, Meloni BP, Sokolow S, et al. NCX3 knockout mice exhibit increased hippocampal CA1 and CA2 neuronal damage compared to wild-type mice following global cerebral ischemia. Exp Neurol. 2008;210:268–273. doi: 10.1016/j.expneurol.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Suh SW, Aoyama K, Chen Y, et al. Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) polymerase inhibitors administered after hypoglycemia. J Neurosci. 2003;23:10681–10690. doi: 10.1523/JNEUROSCI.23-33-10681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budd SL, Castilho RF, Nicholls DG. Mitochondrial membrane potential and hydroethidine-monitored superoxide generation in cultured cerebellar granule cells. FEBS Lett. 1997;415:21–24. doi: 10.1016/s0014-5793(97)01088-0. [DOI] [PubMed] [Google Scholar]
- 30.Robinson KM, Janes MS, Pehar M, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao H, Kalivendi S, Zhang H, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 32.Murakami K, Kondo T, Kawase M, et al. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmued LC, Hopkins KJ. Fluoro-jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikemoto A, Bole DG, Ueda T. Glycolysis and glutamate accumulation into synaptic vesicles. Role of glyceraldehyde phosphate dehydrogenase and 3-phosphoglycerate kinase. J Biol Chem. 2003;278:5929–5940. doi: 10.1074/jbc.M211617200. [DOI] [PubMed] [Google Scholar]
- 36.Simons JM, Hart BA, Ip Vai Ching TR, et al. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic Biol Med. 1990;8:251–258. doi: 10.1016/0891-5849(90)90070-y. [DOI] [PubMed] [Google Scholar]
- 37.He L, Dinger B, Sanders K, et al. Effect of p47phox gene deletion on ROS production and oxygen sensing in mouse carotid body chemoreceptor cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L916–924. doi: 10.1152/ajplung.00015.2005. [DOI] [PubMed] [Google Scholar]
- 38.Kawase M, Murakami K, Fujimura M, et al. Exacerbation of delayed cell injury after transient global ischemia in mutant mice with CuZn superoxide dismutase deficiency. Stroke. 1999;30:1962–1968. doi: 10.1161/01.str.30.9.1962. [DOI] [PubMed] [Google Scholar]
- 39.Bertoni JM. Competitive inhibition of rat brain hexokinase by 2-deoxyglucose, glucosamine, and metrizamide. J Neurochem. 1981;37:1523–1528. doi: 10.1111/j.1471-4159.1981.tb06322.x. [DOI] [PubMed] [Google Scholar]
- 40.Guo ZH, Mattson MP. In vivo 2-deoxyglucose administration preserves glucose and glutamate transport and mitochondrial function in cortical synaptic terminals after exposure to amyloid beta-peptide and iron: evidence for a stress response. Exp Neurol. 2000;166:173–179. doi: 10.1006/exnr.2000.7497. [DOI] [PubMed] [Google Scholar]
- 41.Chan PH, Kawase M, Murakami K, et al. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci. 1998;18:8292–8299. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wass CT, Lanier WL. Glucose modulation of ischemic brain injury: review and clinical recommendations. Mayo Clin Proc. 1996;71:801–812. doi: 10.1016/S0025-6196(11)64847-7. [DOI] [PubMed] [Google Scholar]
- 43.Ying W, Han SK, Miller JW, Swanson RA. Acidosis potentiates oxidative neuronal death by multiple mechanisms. J Neurochem. 1999;73:1549–1556. doi: 10.1046/j.1471-4159.1999.0731549.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.