Crystal structures of Rab2 and Rab3 from D. melanogaster in complex with GMPPNP are presented.
Keywords: Rab, GTPase, GMPPNP, cysteine modification
Abstract
Rab GTPases belong to the large family of Ras proteins. They act as key regulators of membrane organization and intracellular trafficking. Functionally, they act as switches. In the active GTP-bound form they can bind to effector proteins to facilitate the delivery of transport vesicles. Upon stimulation, the GTP is hydrolyzed and the Rab proteins undergo conformational changes in their switch regions. This study focuses on Rab2 and Rab3 from Drosophila melanogaster. Whereas Rab2 is involved in vesicle transport between the Golgi and the endoplasmatic reticulum, Rab3 is a key player in exocytosis, and in the synapse it is involved in the assembly of the presynaptic active zone. Here, high-resolution crystal structures of Rab2 and Rab3 in complex with GMPPNP and Mg2+ are presented. In the structure of Rab3 a modified cysteine residue is observed with an enigmatic electron density attached to its thiol function.
1. Introduction
Rab proteins are small monomeric GTP-binding proteins (GTPases) which constitute the largest branch of the Ras superfamily (Pereira-Leal & Seabra, 2000 ▶). They are evolutionarily conserved, with 55–75% identity between orthologues from yeast to mammals. More than 70 different Rab proteins are encoded in the Homo sapiens genome (Zerial & McBride, 2001 ▶; Bhuin & Roy, 2014 ▶), 11 in Saccharomyces cerevisiae (Lazar et al., 1997 ▶), 29 in Caenorhabditis elegans (Pereira-Leal & Seabra, 2000 ▶), 57 in Arabidopsis thaliana (Vernoud et al., 2003 ▶) and about 33 in Drosophila melanogaster (Chan et al., 2011 ▶). Rab GTPases act as key regulators of membrane organization and intracellular trafficking in all eukaryotic cells (Pfeffer, 1994 ▶; Zerial & McBride, 2001 ▶; Stenmark, 2009 ▶; Bhuin & Roy, 2014 ▶), and as such take part in vesicle formation, motility, tethering and fusion of the vesicles with their target membrane (Zerial & McBride, 2001 ▶; Pfeffer, 2007 ▶). These functions are carried out by a diverse collection of effector molecules, which are recruited by specific Rab proteins, owing to their role as molecular switches. Thus, Rab proteins regulate their particular pathways by interacting with various effector proteins.
In their function as molecular switches, Rab proteins undergo two alternate conformational transitions upon binding to either GDP or GTP. Firstly, the protein is activated by a guanine-exchange factor (GEF), which exchanges GDP for GTP. In the GTP-bound active form, each Rab can interact with a different set of proteins (effectors) to facilitate the delivery of transport vesicles to different acceptor membranes (Molendijk et al., 2004 ▶). While in this conformation, Rabs can associate with their target membrane and interact with their effectors to recruit them to specific subcellular compartments or to activate them. Upon stimulation by GTPase-activating proteins (GAPs) the GTP is hydrolyzed, releasing an inorganic phosphate group, and the now inactive Rab returns to the cytosol. Owing to the identical mechanisms of effector binding and nucleotide exchange and hydrolysis, Rabs share a conserved and well characterized fold with most of the small GTPase family members.
The tertiary structure is composed of a six-stranded β-sheet surrounded by α-helices. Extensive analyses of other GTPases have defined two regions, termed switches I and II, located near the phosphate region of the bound guanine nucleotide (Dumas et al., 1999 ▶; Ostermeier & Brunger, 1999 ▶). These regions undergo dramatic conformational changes on nucleotide exchange and are involved in protein–protein interactions; hence, they account for the nucleotide-dependency of most GTPase interactions (Bhuin & Roy, 2014 ▶; Sprang, 1997 ▶). In the GDP-bound form these regions are highly disordered and thus inactive. They only become ordered upon GTP binding and then expose a triad of hydrophobic amino acids to the surface of the protein. This triad, together with other residues of the switch I and switch II regions, is crucial for the interaction of the Rabs with their respective effector proteins, and this is thought to define the specificity of Rabs for their different effector partners (Merithew et al., 2001 ▶; Eathiraj et al., 2005 ▶; Burguete et al., 2008 ▶).
Rab2 has been identified as a specific regulator of vesicle transport between the Golgi and the endoplasmic reticulum (Liu & Storrie, 2012 ▶; Stenmark, 2009 ▶) with several known effector proteins, including GM130 and golgin-45 (Short et al., 2001 ▶). For instance, Rab2 is able to promote the recruitment of COP I vesicles by binding to its effector PKC 1/λ (Tisdale, 2000 ▶). Furthermore, Tisdale and Balch showed that the amino-terminus of Rab2 might be involved in the maturation of pre-Golgi intermediates (Tisdale & Balch, 1996 ▶).
Rab3 is one of the most investigated Rab GTPases in the context of neuronal functions. It has been identified as a specific regulator in the exocytosis of secretory granules, including synaptic vesicles and vesicles, from the trans-Golgi-network to apico-lateral membranes (Stenmark, 2009 ▶; Bhuin & Roy, 2014 ▶). There are several known effector proteins of Rab3, including RIM, which plays a role in synaptic vesicle trafficking (Wang et al., 1997 ▶). In Drosophila Rab3 seems to have a different function at the synapse: not synaptic vesicle trafficking but rather the trafficking of membrane/cargo for assembly of the presynaptic active-zone cytomatrix (Graf et al., 2009 ▶).
2. Materials and methods
2.1. Macromolecule production
The cDNAs for full-length D. melanogaster Rab2 (dmRab2) and Rab3 (dmRab3) were purchased from the Drosophila Genomics Resource Center. The Rab2 and Rab3 genes were amplified by polymerase chain reaction and cloned into the pET-MBP vector using NcoI and SalI restriction sites (Table 1 ▶). The resulting constructs comprise an N-terminal MBP tag followed by a Tobacco etch virus (TEV) protease cleavage site followed by the N-terminal GTPase domain of dmRab2 (amino acids 1–172) and dmRab3 (amino acids 1–188), respectively. These construct boundaries were chosen based on bioinformatic analysis of deposited GTPase structures in the Protein Data Bank. Furthermore, the conserved glutamine (dmRab2 Gln65 and dmRab3 Gln80) located in the switch II region and involved in transition-state stabilization (Privé et al., 1992 ▶; Der et al., 1986 ▶) was mutated to a leucine. Rab mutants were prepared by site-directed mutagenesis according to the manufacturer’s protocol (EURx ‘Site-directed mutagenesis’). The correctness of the DNA sequences was confirmed by DNA sequencing.
Table 1. Macromolecule-production information.
| dmRab2Q65L | dmRab3Q80L | |
|---|---|---|
| Source organism | D. melanogaster | D. melanogaster |
| DNA source | cDNA | cDNA |
| Forward primer | ACCATGGGATGTCCTACGCGTACTTG | TATACCATGGGCATGGCGAGTGGCG |
| Reverse primer | TATAGTCGACTCACTGGATCTTCTCGTAAATC | TATAGTCGACTCACTCGGACATCTTATCG |
| Expression vector | pET-MBP | pET-MBP |
| Expression host | E. coli | E. coli |
| Complete amino-acid sequence of the construct produced† | GAMSYAYLFKYIIIGDTGVGKSCLLLQFTDKRFQPVHDLTIGVEFGARMITIDGKQIKLQIWDTAGLEAFRSITRSYYRGAAGALLVYDITRRETFNHLTTWLEDARQHSNSNMVIMLIGNKSDLDSRREVKKEEGEAFAREHGLVFMETSARTAANVEEAFINTAKEIYEKIQ | GAMASGGDPKWQKDAADQNFDYMFKLLIIGNSSVGKTSFLFRYADDSFTSAFVSTVGIDFKVKTVFRHDKRVKLQIWDTAGLERYRTITTAYYRGAMGFILMYDVTNEDSFNSVQDWVTQIKTYSWDNAQVILVGNKCDMEDQRVISFERGRQLADQLGVEFFETSAKENVNVKAVFERLVDIICDKMSE |
The remaining tag sequence after TEV cleavage is underlined.
2.2. Protein expression and purification
Protein expression was conducted using chemically competent Escherichia coli Rosetta cells. The cells were grown in autoinduction ZY medium (Studier, 2005 ▶) with kanamycin and chloramphenicol for 4 h at 37°C. The temperature was then decreased to 18°C and the cells were grown overnight. The cells were harvested by centrifugation and the cell pellet was resuspended in resuspension buffer [200 mM NaCl, 20 mM HEPES pH 7.5, 5 mM magnesium acetate, 2 mM DTT, 2%(v/v) glycerol, 10 mg l−1 lysozyme, 5 mg l−1 DNase I] and subsequently lysed by sonication for 15 min. The lysate was centrifuged at 56 000g for 45 min to pellet the cell debris. The supernatant was applied to affinity chromatography using a column packed with 20 ml amylose resin (NEB). The average incubation time was 1 h. Two washing steps were then performed using 50 ml washing buffer [200 mM NaCl, 20 mM HEPES pH 7.5, 5 mM magnesium acetate, 2 mM DTT, 2%(v/v) glycerol] for each step. For elution, the amylose resin was incubated with 20 ml washing buffer supplemented with 20 mM maltose for 15 min. The MBP tag of the truncated dmRab constructs was cleaved off using TEV protease (1 mg ml−1) in the presence of 100 µm guanosine 5′-(β,γ-imido)triphosphate (GMPPNP; Jena Bioscience). The protease was added to the eluted protein at a molar ratio of 1:25 and the reaction was incubated at 4°C overnight. TEV-cleaved constructs were purified using a Superdex 75 26/60 column (GE Healthcare). The protein-containing fractions were pooled and concentrated using a 10 kDa molecular-weight cutoff concentrator (Millipore). The progress of protein purification was monitored by SDS–PAGE. Protein concentrations were determined by UV absorption with extinction coefficients ∊(Rab2) = 21 430 l mol−1 cm−1 and ∊(Rab3) = 32 430 l mol−1 cm−1, respectively.
2.3. Crystallization
For crystallization experiments, dmRab2Q65L was concentrated to 50 mg ml−1 and dmRab3Q80L to 40 mg ml−1 and they were incubated with equimolar concentrations of GMPPNP (Jena Bioscience) prior to crystallization. Crystals were obtained by the sitting-drop vapour-diffusion method at 291 K with drops consisting of 1 µl reservoir solution and 1 µl protein solution (Table 2 ▶). No additional cryoprotection was necessary for flash-cooling the crystals in liquid nitrogen.
Table 2. Crystallization.
| dmRab2Q65L | dmRab3Q80L | |
|---|---|---|
| Method | Sitting-drop vapour diffusion | Sitting-drop vapour diffusion |
| Plate type | Cryschem plate | Cryschem plate |
| Temperature (K) | 291 | 291 |
| Protein concentration (mgml1) | 50 | 40 |
| Buffer composition of protein solution | 200mM NaCl, 20mM HEPES pH 7.5, 5mM magnesium acetate, 2mM DTT, 2%(v/v) glycerol | 200mM NaCl, 20mM HEPES pH 7.5, 5mM magnesium acetate, 2mM DTT, 2%(v/v) glycerol |
| Composition of reservoir solution | 34%(v/v) polyethylene glycol (PEG) 400, 200mM sodium acetate pH 4.6 | 28%(v/v) PEG 200, 5%(w/v) PEG 3000, 100mM MES buffer pH 6.0 |
| Volume and ratio of drop | 1:1 | 1:1 |
| Volume of reservoir (l) | 600 | 600 |
2.4. Data collection and indexing, structure determination and refinement
Synchrotron diffraction data were collected on beamline 14.3 of the MX Joint Berlin laboratory at BESSY, Berlin, Germany. X-ray data collection was performed at 100 K. Diffraction data were indexed and processed with XDS (Kabsch, 2010 ▶; Table 3 ▶).
Table 3. Data collection and processing.
Values in parentheses are for the highest resolution shell.
| dmRab2Q65LGMPPNP | dmRab3Q80LGMPPNP | |
|---|---|---|
| Diffraction source | BESSY 14.3 | BESSY 14.3 |
| Wavelength () | 0.895 | 0.895 |
| Temperature (K) | 100 | 100 |
| Detector | Rayonix MX-225 | Rayonix MX-225 |
| Crystal-to-detector distance (mm) | 230 | 145 |
| Rotation range per image () | 1.0 | 0.5 |
| Total rotation range () | 100 | 110 |
| Exposure time per image (s) | 5.2 | 12 |
| Space group | P3121 | P212121 |
| a, b, c () | 81.4, 81.4, 53.1 | 37.2, 80.5, 123.9 |
| , , () | 90.0, 90.0, 120.0 | 90.0, 90.0, 90.0 |
| Mosaicity () | 0.2 | 0.1 |
| Resolution () | 50.002.00 (2.122.00) | 50.001.50 (1.541.50) |
| Total No. of reflections | 70633 | 222056 |
| No. of unique reflections | 13981 (2209) | 60482 (4425) |
| Completeness (%) | 99.8 (99.2) | 99.9 (100.0) |
| Multiplicity | 5.0 (5.0) | 3.7 (3.7) |
| I/(I) | 7.9 (1.8) | 12.9 (2.6) |
| R meas † | 0.216 (0.989) | 0.082 (0.616) |
| CC1/2 ‡ | 99.0 (72.4) | 99.8 (75.0) |
| Wilson B (2) | 25.7 | 19.7 |
R
meas = 
 , where I(hkl) is the mean intensity of symmetry-equivalent reflections and N(hkl) is the redundancy.
, where I(hkl) is the mean intensity of symmetry-equivalent reflections and N(hkl) is the redundancy.
The high-resolution cutoff was estimated using CC1/2.
2.5. Structure solution and refinement
The structure of dmRab3Q80L was solved by molecular replacement using Phaser (McCoy et al., 2007 ▶) with the known structure of Rab3A from Rattus norvegicus (rnRab3A; PDB entry 3rab) as a search model (Dumas et al., 1999 ▶). The structure of dmRab2Q65L was solved by molecular replacement using our initially solved structure of dmRab3Q80L. For the calculation of the free R factor, a randomly generated set of 5% of the reflections from the diffraction data sets was used and was excluded from the refinement. The structures were initially refined by applying a simulated-annealing protocol and in later refinement cycles by maximum-likelihood restrained refinement using PHENIX (Adams et al., 2010 ▶; Afonine et al., 2012 ▶). Model building and water picking was performed with Coot (Emsley et al., 2010 ▶). The model quality was evaluated with MolProbity (Chen et al., 2010 ▶) and PROCHECK (Laskowski et al., 1993 ▶). Secondary-structure elements were assigned with DSSP (Kabsch & Sander, 1983 ▶). Final refinement statistics are given in Table 4 ▶. Figures were prepared using PyMOL (DeLano, 2002 ▶). The atomic coordinates and structure-factor amplitudes have been deposited in the Protein Data Bank under accession codes 4rke (dmRab2Q65L) and 4rkf (dmRab3Q80L).
Table 4. Structure solution and refinement.
Values in parentheses are for the highest resolution shell.
| dmRab2Q65LGMPPNP | dmRab3Q80LGMPPNP | |
|---|---|---|
| Resolution range () | 42.42.0 | 38.31.5 |
| Completeness (%) | 99.8 | 99.7 |
| No. of reflections, working set | 132267 | 57442 |
| No. of reflections, test set | 699 | 3025 |
| Final R work † | 0.167 (0.237) | 0.157 (0.206) |
| Final R free ‡ | 0.224 (0.313) | 0.194 (0.237) |
| No. of non H-atoms | ||
| Protein | 1412 | 2964 |
| Mg2+ | 1 | 2 |
| GMPPNP | 32 | 64 |
| PEG | 21 | 43 |
| Water | 113 | 378 |
| Total | 1579 | 3451 |
| R.m.s. deviation | ||
| Bond lengths () | 0.008 | 0.010 |
| Bond angles () | 1.10 | 1.26 |
| Average B factors (2) | ||
| Protein | 22.5 | 16.5 |
| Mg2+ | 19.6 | 9.1 |
| GMPPNP | 19.7 | 9.1 |
| PEG | 28.3 | 26.2 |
| Water | 28.4 | 28.7 |
| Ramachandran plot§ | ||
| Outliers (%) | 0 | 0 |
| Favoured (%) | 97.0 | 98.4 |
R
work = 
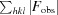 .
.
R free is the same as R cryst but calculated using 5% of the data, which were excluded from refinement.
As calculated by MolProbity.
2.6. Mass spectrometry
Protein masses were analyzed by matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF MS) using an Ultraflex-II TOF/TOF instrument (Bruker Daltonics, Bremen, Germany) equipped with a 200 Hz solid-state Smart beam laser. The mass spectrometer was operated in the positive linear mode. MS spectra were acquired over an m/z range of 5000−25 000 and data were analyzed using the FlexAnalysis 2.4 software provided with the instrument. The mass accuracy was estimated to be ±1% in the relevant mass range. Sinapinic acid was used as the matrix and samples were spotted using the dried-droplet technique undiluted and in a 1:5 dilution with 33% acetonitrile/0.1% trifluoroacetic acid.
3. Results
3.1. Expression, crystallization and structure determination
We prepared expression constructs of Rab2 and Rab3 from D. melanogaster comprising only the GTPase domain. In addition, we mutated the catalytically important glutamine to leucine, locking both dmRab2 and dmRab3 into the activated GTP-bound state (Der et al., 1986 ▶; Privé et al., 1992 ▶). To unravel the architecture of dmRab2Q65L and dmRab3Q80L, we overexpressed both proteins in E. coli and subsequently purified and crystallized them. We could collect high-resolution data sets to 2.0 Å resolution for dmRab2Q65L and to 1.5 Å resolution for dmRab3Q80L. Whereas the dmRab3Q80L crystals were indexed in space group P212121, the dmRab2Q65L crystals belonged to space group P3121 (Tables 3 ▶ and 4 ▶). Both structures were solved by molecular replacement, locating two dmRab3Q80L molecules and one dmRab2Q65L molecule in the asymmetric unit. The structure of dmRab2Q65L was refined to R work = 0.167 and R free = 0.224 and that of dmRab3Q80L to R work = 0.157 and R free = 0.194 with excellent geometry. Data collection and refinement statistics are given in Tables 3 ▶ and 4 ▶. The electron density maps for both proteins were well defined; therefore, the model of dmRab3Q80L could be built except for the 15 N-terminal and the two C-terminal amino acids of the construct. In addition, the structure of dmRab2Q65L could also be completely modelled, including four amino acids of the N-terminal linker region that remained after TEV cleavage. The initial 2mF o − DF c and mF o − DF c electron density maps clearly revealed the localization of GMPPNP and Mg2+ (Fig. 2). Moreover, we observed electron density in a horseshoe shape that we could interpret as PEG fragments originating from the crystallization cocktail.
3.2. Overall structure
Both proteins are monomeric based on an interface analysis with the PISA server (Krissinel & Henrick, 2007 ▶) and in agreement with our experimental size-exclusion chromatography. dmRab2Q65L and dmRab3Q80L adopt the classical fold of the Rab family of GTP-binding proteins, with one β-sheet that is composed of six β-strands surrounded by five α-helices (Fig. 1 ▶). dmRab2 and dmRab3 share 33% sequence identity and 52% similarity. The overall folds are related to the Ras superfamily (Tong et al., 1989 ▶; Pai et al., 1989 ▶). dmRab2Q65L and dmRab3Q80L are practically indistinguishable, with a root-mean-square deviation (r.m.s.d.) of 0.8 Å for 157 pairs of Cα atoms. dmRab2 shares 65% sequence identity and dmRab3 shares 73% sequence identity with rnRab3A. The reported structure of rnRab3A in a GMPPNP-bound form (PDB entry 3rab) superimposes with dmRab2Q65L and dmRab3Q80L with an r.m.s.d. of 0.6 Å for 169 pairs of Cα atoms. In both structures the switch I and II regions are very well defined (Sprang, 1997 ▶; Kjeldgaard et al., 1996 ▶) and are involved in a hydrogen-bonding network to stabilize the bound nucleotide and coordinate the Mg2+ cation (Fig. 1 ▶).
Figure 1.
Structures of dmRab2Q65L and dmRab3Q80L drawn in cartoon representation. α-Helices are coloured blue, β-strands salmon and connecting loop regions brown. The bound GMPPNP is shown in stick representation, as are the Mg2+-coordinating residues. The octahedrally coordinated Mg2+ is depicted as a black sphere and coordinating water molecules as red spheres. Grey dashed lines indicate the coordination sphere of Mg2+. (a) Structure of dmRab2Q65L; (b) structure of dmRab3Q80L.
3.3. Nucleotide-binding site of dmRab2Q65L
The guanine function of GMPPNP bound to dmRab2Q65L is involved in hydrogen bonding to Ala150, Asp122 and Asn119. The hydroxyl functions are in contact with Gln32 and Pro33 (Table 5 ▶). The α- and β-phosphate are stabilized by interactions with the Walker A motif or P-loop (Saraste et al., 1990 ▶; Walker et al., 1982 ▶). This motif in dmRab2Q65L is 13GDTDVDKS20, with the catalytic Walker A lysine at position Lys19. The switch II region of exocytic Rab GTPase is highly conserved and is found within the region 60WDTAGLEAFRSITRSYYRGA79 in dmRab2Q65L (Fig. 1 ▶ a). The γ-phosphate interacts with Thr15, His35 and Thr38 as well as Gly64 (Table 5 ▶). The Mg2+ ion is octahedrally coordinated by the hydroxyl functions of Ser20 and Thr38 of dmRab2Q65L, the β- and γ-phosphate groups of GMPPNP and two water molecules (Fig. 1 ▶ a and Table 5 ▶). The latter two water molecules are embedded in a dense hydrogen-bonding network including Asp36, Thr38, Asp61, Thr62 and the phosphate functions of GMPPNP.
Table 5. Hydrogen-bonding interactions of GMPPNP in complex with dmRab2Q65L and dmRab3Q80L .
Distances 3.2 are given. Canonical interactions of the - and -phosphates of GMPPNP with the protein backbone of the P-loops are not listed.
| GMPPNP | dmRab2Q65L | Distance () | dmRab3Q80L | Distance () | |
|---|---|---|---|---|---|
| Guanine base | O6 | Ala150N | 2.9 | Ala165N | 2.9 |
| N1 | Asp122OD1 | 2.8 | Asp137OD1 | 2.8 | |
| N2 | Asp122OD2 | 2.8 | Asp137OD2 | 2.9 | |
| N7 | Asn119ND2 | 3.1 | Asn134ND2 | 3.2 | |
| Ribose | O2 | Gln32O | 2.8 | Thr47O | 2.9 |
| O2 | Pro33O | 2.8 | |||
| O3 | Pro33O | 3.2 | Ser48O | 2.7 | |
| -Phosphate | O1G | Gly64N | 2.8 | Gly79N | 2.8 |
| O2G | Thr38N | 3.0 | Thr53N | 2.8 | |
| O2G | Thr38OG2 | 2.7 | Thr53OG2 | 2.9 | |
| O3G | Thr15OG2 | 2.6 | Ser30OG | 2.7 | |
| O3G | His35NE2 | 2.9 | Ser52OG | 2.6 | |
3.4. Nucleotide-binding site of dmRab3Q80L
The GMPPNP bound to dmRab3Q80L establishes similar interactions with the protein as described above for dmRab2Q65L. The guanine base is hydrogen-bonded to Ala165, Asp137 and Asn134, and the hydroxyls of the ribose moiety are hydrogen-bonded to Ser48 and Thr47 (Table 5 ▶). The Walker A motif is established by the motif 28GNSSVGKT35, with the conserved Walker A lysine being Lys34. The side chains of dmRab2 Lys19 and dmRab3 Lys34 point to and interact with the O atoms of the β- and γ-phosphates of the bound GMPPNP and hence adopt the so-called ‘conventional’ conformation (Dikfidan et al., 2014 ▶). The switch II region of dmRab3Q80L comprises the sequence 75WDTAGLERTITTAYYRGA94 (Fig. 1 ▶ b) and is shorter by two amino-acid residues compared with dmRab2Q65L. This difference is reflected in a α-helix within the switch II region in the structure of dmRab3Q80L. Switches I and II as well as the inter-switch regions are important in effector protein binding (Ostermeier & Brunger, 1999 ▶; Dumas et al., 1999 ▶). In the GTP-bound state these regions are well ordered and expose a hydrophobic aromatic triad of residues to the protein surface. In concert with other residues, these residues are believed to define the specificity of different Rabs for different effector molecules (Merithew et al., 2001 ▶). The triad is conserved in both dmRab2Q65L and dmRab3Q80L: Phe43, Trp60 and Tyr75 in dmRab2Q65L and Phe58, Trp75 and Tyr90 in dmRab3Q80L. All residues are solvent-exposed, with one exception, dmRab2Q65L Tyr75, the side chain of which points towards the protein. The γ-phosphate of GMPPNP is hydrogen-bonded to Gly79, Thr53, Ser30 and Ser32 (Table 5 ▶). In the dmRab3Q80L structure two threonine residues, Thr35 and Thr53, are involved in Mg2+ coordination as well as the γ-phosphate groups of GMPPNP and two water molecules (Figs. 1 ▶ b and 2 ▶). The latter two water molecules are hydrogen-bonded to Thr35, Asp76, Thr77, Val51 and Thr53.
Figure 2.

Nucleotide-binding site of dmRab3Q80L. 2mF o − DF c simulated-annealing OMIT map contoured at 1σ shown as a blue mesh for the omitted GMPPNP and in violet for the Mg2+ ion. The GMPPNP is shown in stick representation and the Mg2+ ion is shown as black sphere.
3.5. Modified cysteine in dmRab3Q80L
The free thiol function of cysteine allows a large variety of modifications. Many post-translational modifications such as phosphorylation, S-nitrosylation, S-glutathionylation, sulfhydration, sulfenylation, sulfinic acids, sulfonic acid polyprenylation and sulfenyl-amides are known (Walsh et al., 2005 ▶; Chung et al., 2013 ▶). On the other hand, the thiol function is extensively exploited in the context of in vitro protein modifications such as fluorescent tagging, paramagnetic spin labelling and many more diverse applications. In the initially calculated electron-density maps we could observe additional electron density attached to Cys183 that resides on the very C-terminal α-helix of dmRab3Q80L. The electron density has an approximately twofold rotational symmetry and has a planar shape (Fig. 3 ▶). The volume of the electron density is large enough to accommodate six atoms. The unknown electron density is located in a hydrophobic pocket on the surface of the protein formed by the side chains of Tyr20, Phe22, His66, Lys68, Val70 and Met186. The plane of the electron density is parallel to the phenolic ring of Tyr20 (Fig. 3 ▶), tentatively establishing a π-interaction with the unknown cysteine modification. Even though DTT was present in our purification buffers, the planarity and symmetry of the electron density rules out a mixed disulfide with DTT. To shed light on the modification, we performed MALDI-TOF MS of the dmRab3Q80L protein prior to crystallization. The experimental molecular weight of 21 925 Da is in reasonable agreement with the calculated theoretical mass of 21 939 Da. This raised the question of the point in time at which the modification is made. Hence, we washed and dissolved dmRab3Q80L crystals in water and subjected them to MALDI-TOF MS. We could now see a mass difference of 135 Da compared with the theoretical mass of the protein. Consequently, the thiol modification must take place during the crystallization process. Next, we intended to identify the atom establishing the thiol linkage. We therefore collected a highly redundant, anomalous diffraction data set at 1.7 Å wavelength. We could detect the positions of most of the sulfur atoms of cysteine and methionine amino-acid side chains in the anomalous difference electron density, but no anomalous difference electron density for the atom covalently attached to the thiol of Cys183. It is tempting to speculate about the origin of the modification. Since the modification seems to be nearly complete for Cys183, the only source could be the crystallization cocktail. We can merely speculate that a degradation product of the precipitant PEG 200 might have caused the modification.
Figure 3.
Protein surroundings of Cys183 in dmRab3Q80L. (a) mF o − DF c electron-density map contoured at 3σ shown as a green mesh. Difference electron density with a planar shape is attached to the S atom of Cys183. (b) The view in (a) rotated by 45°.
4. Discussion
We have determined the crystal structures of constitutively active dmRab2Q65L and dmRab3Q80L variants with bound Mg2+ and the nonhydrolysable GTP analogue GMPPNP to atomic resolution. Our structures provide information on the residues involved in Mg2+ coordination and interaction with GMPPNP. In the crystal structure of dmRab3Q80L we detected a covalently attached modification at Cys183. The latter modification remains enigmatic and hence has not been modelled in the crystal structure of dmRab3Q80L.
Our structure of dmRab2Q65L represents the first crystal structure of a Rab2 protein in the ‘GTP’-bound active state and allows comparison with the structure of rnRab2A (PDB entry 1z0a; Eathiraj et al., 2005 ▶) in the GDP-bound state. In the structure of rnRab2A–GDP the switch I region adopts different conformations in the four copies within the asymmetric unit. In three copies (chains A, B and D) the switch I region is not defined in the electron-density maps and hence is lacking from the model, whereas in one copy (chain C) the entire switch I region could be modelled but with truncated side chains, indicating increased flexibility. dmRab2Q65L and rnRab2A share 94% identity and the structures superimpose with an r.m.s.d. of 1.7 Å for 165 pairs of Cα atoms of chain C, whereas for chain A, which lacks residues Pro33–His35, the r.m.s.d. is 1.4 Å for 166 pairs of Cα atoms. The different conformations are likely to be influenced by crystal packing. Major structural differences between dmRab2Q65L and rnRab2A are observed in the switch I and II regions (Fig. 4 ▶ a). In the structure of dmRab2Q65L presented here the switch I region is well defined in the electron density and establishes the expected interactions with the hydroxyl functions of the ribose and the γ-phosphate of the GMPPNP nucleotide (Table 5 ▶). Even though the switch I region of dmRab2Q65L is involved in crystal packing, our structure strongly suggests that the switch I region adopts the conformation of the active state of dmRab2. The switch II region undergoes a more drastic conformational change (Fig. 4 ▶ a). By this conformational change it establishes contact with the γ-phosphate of the GMPPNP (Table 5 ▶). Again, the observed conformation of the switch II region could be potentially involved in crystal contacts.
Figure 4.
Superposition of different Rab structures shown in ribbon presentation. (a) Superposition of rnRab2A (PDB entry 1z0a, chain A) bound to GDP and dmRab2 bound to GMPPNP. The switch I and switch II regions of rnRab2A–GDP are coloured purple and orange, respectively, whereas the switch I and switch II regions of dmRab2A–GMPPNP are coloured light blue and red, respectively. (b) Superposition of dmRab2A (grey ribbon) and dmRab3A (black ribbon) both in the GMPPNP-bound state. The switch regions of dmRab2A are coloured as in (a). The switch I and switch II regions of dmRab3A are coloured green and pink, respectively.
dmRab2 and dmRab3 share 33% sequence identity and the structures of the proteins are nearly identical, with an r.m.s.d. of 0.8 Å for 157 pairs of Cα atoms (Fig. 4 ▶ b). Whereas the conformation of the switch I region of both proteins is very similar, the conformation of the switch II region is altered. In dmRab3Q80L we observe a α-helix of six residues in length from Arg84 to Ala89 (Fig. 1 ▶ b). In the structure of dmRab2Q65L the switch II region adopts a random-coil conformation. Since the switch region is involved in crystal contacts in both structures, we cannot fully exclude a possible influence on their conformation. These observed alterations, especially within the switch II regions, hint at the capability of dmRab2 and dmRab3 to bind to different effector proteins.
A potential interaction partner of Rab3A is Bruchpilot, since upon loss of Rab3 Bruchpilot is dramatically reduced (Graf et al., 2009 ▶). Bruchpilot acts as one of the main scaffolding proteins that decorate the intracellular face of the active zone in Drosophila where synaptic vesicles fuse with the membrane (Haucke et al., 2011 ▶; Kittel et al., 2006 ▶; Liu et al., 2011 ▶). Bruchpilot is critical for the structural integrity and functionality of the active zone. Graf and coworkers showed that the Rab3 GTPase is essential for correct assembly of the active zone in Drosophila (Graf et al., 2009 ▶). Owing to their described properties and function in vesicular transport, the question arose of whether Rab GTPases might interact with active zone proteins in synaptic vesicle tethering. In future experiments, we would like to shed light on these possible interactions by using our Rab GTPase constructs in pull-down experiments.
Supplementary Material
PDB reference: Rab3, 4rkf
PDB reference: Rab2, 4rke
Acknowledgments
All authors are grateful to the Deutsche Forschungsgemeinschaft for grants SFB958/A3, A6 and Z3. We accessed the beamlines of BESSY II (Berliner Elektronenspeicherring-Gesellschaft für Synchrotronstrahlung II storage ring, Berlin, Germany) via the Joint Berlin MX-Laboratory sponsored by the Helmholtz Zentrum Berlin für Materialien und Energie, the Freie Universität Berlin, the Humboldt-Universität zu Berlin, the Max-Delbrück Centrum and the Leibniz-Institut für Molekulare Pharmakologie.
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Afonine, P. V., Grosse-Kunstleve, R. W., Echols, N., Headd, J. J., Moriarty, N. W., Mustyakimov, M., Terwilliger, T. C., Urzhumtsev, A., Zwart, P. H. & Adams, P. D. (2012). Acta Cryst. D68, 352–367. [DOI] [PMC free article] [PubMed]
- Bhuin, T. & Roy, J. K. (2014). Exp. Cell Res. 328, 1–19. [DOI] [PubMed]
- Burguete, A. S., Fenn, T. D., Brunger, A. T. & Pfeffer, S. R. (2008). Cell, 132, 286–298. [DOI] [PMC free article] [PubMed]
- Chan, C.-C., Scoggin, S., Wang, D., Cherry, S., Dembo, T., Greenberg, B., Jin, E. J., Kuey, C., Lopez, A., Mehta, S. Q., Perkins, T. J., Brankatschk, M., Rothenfluh, A., Buszczak, M. & Hiesinger, P. R. (2011). Curr. Biol. 21, 1704–1715. [DOI] [PMC free article] [PubMed]
- Chen, V. B., Arendall, W. B., Headd, J. J., Keedy, D. A., Immormino, R. M., Kapral, G. J., Murray, L. W., Richardson, J. S. & Richardson, D. C. (2010). Acta Cryst. D66, 12–21. [DOI] [PMC free article] [PubMed]
- Chung, H. S., Wang, S.-B., Venkatraman, V., Murray, C. I. & Van Eyk, J. E. (2013). Circ. Res. 112, 382–392. [DOI] [PMC free article] [PubMed]
- DeLano, W. (2002). PyMOL. http://www.pymol.org.
- Der, C. J., Finkel, T. & Cooper, G. M. (1986). Cell, 44, 167–176. [DOI] [PubMed]
- Dikfidan, A., Loll, B., Zeymer, C., Magler, I., Clausen, T. & Meinhart, A. (2014). Mol. Cell, 54, 975–986. [DOI] [PubMed]
- Dumas, J. J., Zhu, Z., Connolly, J. L. & Lambright, D. G. (1999). Structure, 7, 413–423. [DOI] [PubMed]
- Eathiraj, S., Pan, X., Ritacco, C. & Lambright, D. G. (2005). Nature (London), 436, 415–419. [DOI] [PMC free article] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Graf, E. R., Daniels, R. W., Burgess, R. W., Schwarz, T. L. & DiAntonio, A. (2009). Neuron, 64, 663–677. [DOI] [PMC free article] [PubMed]
- Haucke, V., Neher, E. & Sigrist, S. J. (2011). Nature Rev. Neurosci. 12, 127–138. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kabsch, W. & Sander, C. (1983). Biopolymers, 22, 2577–2637. [DOI] [PubMed]
- Kittel, R. J., Wichmann, C., Rasse, T. M., Fouquet, W., Schmidt, M., Schmid, A., Wagh, D. A., Pawlu, C., Kellner, R. R., Willig, K. I., Hell, S. W., Buchner, E., Heckmann, M. & Sigrist, S. J. (2006). Science, 312, 1051–1054. [DOI] [PubMed]
- Kjeldgaard, M., Nyborg, J. & Clark, B. F. (1996). FASEB J. 10, 1347–1368. [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. (1993). J. Appl. Cryst. 26, 283–291.
- Lazar, T., Götte, M. & Gallwitz, D. (1997). Trends Biochem. Sci. 22, 468–472. [DOI] [PubMed]
- Liu, K. S. Y. et al. (2011). Science, 334, 1565–1569. [DOI] [PubMed]
- Liu, S. & Storrie, B. (2012). Cell. Mol. Life Sci. 69, 4093–4106. [DOI] [PMC free article] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Merithew, E., Hatherly, S., Dumas, J. J., Lawe, D. C., Heller-Harrison, R. & Lambright, D. G. (2001). J. Biol. Chem. 276, 13982–13988. [DOI] [PubMed]
- Molendijk, A. J., Ruperti, B. & Palme, K. (2004). Curr. Opin. Plant Biol. 7, 694–700. [DOI] [PubMed]
- Ostermeier, C. & Brunger, A. T. (1999). Cell, 96, 363–374. [DOI] [PubMed]
- Pai, E. F., Kabsch, W., Krengel, U., Holmes, K. C., John, J. & Wittinghofer, A. (1989). Nature (London), 341, 209–214. [DOI] [PubMed]
- Pereira-Leal, J. B. & Seabra, M. C. (2000). J. Mol. Biol. 301, 1077–1087. [DOI] [PubMed]
- Pfeffer, S. R. (1994). Curr. Opin. Cell Biol. 6, 522–526. [DOI] [PubMed]
- Pfeffer, S. R. (2007). Annu. Rev. Biochem. 76, 629–645. [DOI] [PubMed]
- Privé, G. G., Milburn, M. V., Tong, L., de Vos, A. M., Yamaizumi, Z., Nishimura, S. & Kim, S.-H. (1992). Proc. Natl Acad. Sci. USA, 89, 3649–3653. [DOI] [PMC free article] [PubMed]
- Saraste, M., Sibbald, P. R. & Wittinghofer, A. (1990). Trends Biochem. Sci. 15, 430–434. [DOI] [PubMed]
- Short, B., Preisinger, C., Körner, R., Kopajtich, R., Byron, O. & Barr, F. A. (2001). J. Cell Biol. 155, 877–883. [DOI] [PMC free article] [PubMed]
- Sprang, S. R. (1997). Curr. Opin. Struct. Biol. 7, 849–856. [DOI] [PubMed]
- Stenmark, H. (2009). Nature Rev. Mol. Cell Biol. 10, 513–525. [DOI] [PubMed]
- Studier, F. W. (2005). Protein Expr. Purif. 41, 207–234. [DOI] [PubMed]
- Tisdale, E. J. (2000). Traffic, 1, 702–712. [DOI] [PubMed]
- Tisdale, E. J. & Balch, W. E. (1996). J. Biol. Chem. 271, 29372–29379. [DOI] [PubMed]
- Tong, L., Milburn, M. V., de Vos, A. M. & Kim, S.-H. (1989). Science, 245, 244. [DOI] [PubMed]
- Vernoud, V., Horton, A. C., Yang, Z. & Nielsen, E. (2003). Plant Physiol. 131, 1191–1208. [DOI] [PMC free article] [PubMed]
- Walker, J. E., Saraste, M., Runswick, M. J. & Gay, N. J. (1982). EMBO J. 1, 945–951. [DOI] [PMC free article] [PubMed]
- Walsh, C. T., Garneau-Tsodikova, S. & Gatto, G. J. (2005). Angew. Chem. Int. Ed. 44, 7342–7372. [DOI] [PubMed]
- Wang, Y., Okamoto, M., Schmitz, F., Hofmann, K. & Südhof, T. C. (1997). Nature (London), 388, 593–598. [DOI] [PubMed]
- Zerial, M. & McBride, H. (2001). Nature Rev. Mol. Cell Biol. 2, 107–117. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: Rab3, 4rkf
PDB reference: Rab2, 4rke





