A periplasmic sensory domain of the C. jejuni chemoreceptor for aspartate A (CcaA) has been purified and crystallized by the hanging-drop vapour-diffusion method. A diffraction data set has been collected to 1.4 Å resolution.
Keywords: Campylobacter jejuni, chemotaxis, transducer-like proteins, methyl-accepting proteins
Abstract
In Campylobacter jejuni, chemotaxis and motility have been identified as important virulence factors that are required for host colonization and invasion. Chemotactic recognition of extracellular signals is mediated by the periplasmic sensory domains of its transducer-like proteins (Tlps). In this study, the sensory domain of the C. jejuni chemoreceptor for aspartate A (CcaA) has been expressed in Escherichia coli and purified from inclusion bodies. The urea-denatured protein was refolded and then crystallized by the hanging-drop vapour-diffusion method using PEG 3350 as a precipitating agent. A complete data set has been collected to 1.4 Å resolution using cryocooling conditions and synchrotron radiation. The crystals belonged to space group P1, with unit-cell parameters a = 39.3, b = 43.3, c = 50.9 Å, α = 92.5, β = 111.4, γ = 114.7°.
1. Introduction
Campylobacter jejuni is a leading cause of bacterial foodborne enterocolitis in humans (Zilbauer et al., 2008 ▶). C. jejuni colonizes many wild and domestic animals, and is also found in untreated water (Boes et al., 2005 ▶; Oporto et al., 2007 ▶). In humans, infection occurs after the ingestion of contaminated food, especially poultry products (Hepworth et al., 2011 ▶). Symptoms include fever, abdominal pain and diarrhoea (Acheson & Allos, 2001 ▶). Notably, Campylobacter enteritis has been associated with several post-infection complications, including Guillain–Barré syndrome, reactive arthritis and idiopathic peripheral neuropathy (Schmidt-Ott et al., 2006 ▶; Friedman et al., 2000 ▶).
The molecular mechanisms of C. jejuni pathogenesis are still poorly understood. However, flagellar motility and chemotaxis are known to play an important role during the colonization and infection of both avian and mammalian hosts (Josenhans & Suerbaum, 2002 ▶; Dasti et al., 2010 ▶). In chemotaxis, swimming of motile bacteria may be controlled by various extracellular chemical gradients. These external stimuli are detected by the sensory domains of methyl-accepting chemotactic proteins (MCPs) or transducer-like proteins (Tlps), which transfer information through their signalling domains, activating signalling cascades that control the direction of rotation of flagella (Zhulin, 2001 ▶; Fernando et al., 2007 ▶; Zautner et al., 2012 ▶).
The C. jejuni genome encodes at least ten different Tlps, which have been classified into three groups according to their domain organization: A (Tlp1, Tlp2, Tlp3, Tlp4, Tlp7mc, Tlp7m and Tlp10), B (Tlp9) and C (Tlp5, Tlp6, Tlp7c and Tlp8) (Parkhill et al., 2000 ▶; Zautner et al., 2012 ▶). Sequence-homology analysis has shown that the signalling domain is highly conserved in Tlps from different bacterial species; in contrast, the sensory domain, which is involved in the recognition of external ligands, is highly varied (Marchant et al., 2002 ▶; Parkhill et al., 2000 ▶), which reflects evolution to detect a broad spectrum of environmental cues.
To date, the ligand specificity of four C. jejuni Tlps has been characterized. Tlp1 has been identified as an aspartate receptor and termed the Campylobacter chemoreceptor for aspartate A (CcaA; Hartley-Tassell et al., 2010 ▶), Tlp3 as a multiligand-binding receptor (Rahman et al., 2014 ▶; Li et al., 2014 ▶), Tlp4 as a sodium deoxycholate receptor and Tlp7 as a formic acid receptor (Tareen et al., 2010 ▶). The periplasmic sensory domain of Tlp1 (CcaA) has no homology to that of Tar, the well characterized aspartate receptor of Escherichia coli (Hartley-Tassell et al., 2010 ▶), or any of the chemoreceptors described to date. Therefore, the mechanism involved in aspartate recognition by C. jejuni CcaA may be unique to this bacterial genus. In order to understand the molecular basis of ligand recognition by this novel sensory domain, we have expressed, refolded from inclusion bodies, purified and crystallized the periplasmic domain of CcaA (CcaAperi).
2. Materials and methods
2.1. Gene cloning, protein expression, refolding and purification
CcaAperi (amino-acid residues 31–327; UniProtKB Q0P8B2; also termed Tlp1 or cj1506c) was cloned and overexpressed in E. coli. Briefly, the DNA fragment encoding CcaAperi was amplified from the pGU0605 plasmid (Hartley-Tassell et al., 2010 ▶) using the primers tlp1-F (5′-CACCCACAAAACAAGTAAGTCAAAATATTACC-3′) and tlp1-R (5′-CTCGAGTTATTTTTTTAAGGGTTTAAATACTGAATA-3′) (Table 1 ▶). The PCR product was ligated into the pET151/D-TOPO vector using the TOPO cloning kit (Invitrogen) to generate an expression plasmid that contained an N-terminal His6 tag followed by a TEV protease cleavage site. The correct insertion of the fragment was verified by DNA sequencing.
Table 1. Macromolecule-production information.
| Source organism | C. jejuni serotype O:2 (strain NCTC 11168) |
| DNA source | Plasmid pGU0605 (Hartley-Tassell et al., 2010 ▶) |
| Forward primer | CACCACAAAACAAGTAAGTCAAAATATTACC |
| Reverse primer | CTCGAGTTATTTTTTTAAGGGTTTAAATACTGAATA |
| Cloning vector | pET151/D-TOPO |
| Expression vector | pET151/D-TOPO |
| Expression host | E. coli strain BL21-CodonPlus (DE3)-RIPL |
| Complete amino-acid sequence of the construct produced | GIDPFTKQVSQNITKNTEDILASITKEYATQTQGIFGEMIALNKSISGTLTEMFRSTSKEDLDIDNITNIITNTFDNSAYSNFTYLYLIDPPEYFKEESKFFNTQSGKFVMLYADEEKDNKGGIKAIQASDEIANLQVVQDILKKAKYGENKVYIGRPIKMNLEGQDFDAVNVAIPIFDRKNQVVGVIGMTLDFSDIATYLLDPKGQKYDGELRVLLNSDGFMAIHPNKNLVLKNLKDINPNKGAQETYKAISEGKNGVFNYIASDGDDSYAAINSFKVQDSSWAVLVTAPKYSVFKPLKK |
E. coli BL21(DE3)-RIPL cells (Stratagene) were transformed with the expression vector and cultured to exponential phase at 37°C in LB medium containing 50 µg ml−1 ampicillin. Expression was induced with 1 mM isopropyl β-d-1-thiogalactopyranoside at an OD600 nm of 0.6. The cells were grown for a further 4 h at 37°C and then harvested by centrifugation at 6000g for 15 min at 4°C. The cell pellet was resuspended in 100 ml buffer A (10 mM Tris–HCl pH 8.0) and lysed using an Avestin EmulsiFlex-C5 high-pressure homogenizer. The cell lysate was centrifuged at 10 000g for 15 min at 4°C. Analysis of the clarified supernatant and pellet by SDS–PAGE showed that the recombinant CcaAperi was predominantly deposited in inclusion bodies. We have recently reported a procedure for the production of a soluble, crystallizable form of the periplasmic sensory domain of the bacterial chemoreceptor TlpC by extraction from inclusion bodies and refolding (Liu & Roujeinikova, 2014 ▶). To purify CcaAperi for crystallization, we used a similar protocol with minor variations. The inclusion-bodies pellet was washed three times with buffer B [10 mM Tris–HCl pH 8.0, 0.2 mM phenylmethanesulfonyl fluoride (PMSF), 1% Triton X-100] and once with buffer C (10 mM Tris–HCl pH 8.0, 0.2 mM PMSF). The inclusion bodies were then solubilized in buffer D [10 mM Tris–HCl pH 8.0, 8 M urea, 10 mM dithiothreitol (DTT), 0.2 mM PMSF] for 30 min at 4°C with mixing by axial rotation. The mixture was then clarified by centrifugation at 30 000g for 30 min at 4°C and the concentration of solubilized protein was determined using the Bradford assay (Bradford, 1976 ▶).
CcaAperi refolding was performed by diluting 60 mg denatured protein (typical volume 1–2 ml) into 250 ml buffer E (3 M urea, 100 mM Tris–HCl pH 8.0, 0.4 M l-arginine monohydrochloride) followed by 48 h incubation at 4°C with continuous mixing. The protein sample was then dialyzed against 5 l buffer A for 16 h at 4°C. NaCl, Tris–HCl pH 8.0 and imidazole were added to the protein solution to final concentrations of 500, 20 and 20 mM, respectively, and the sample was loaded onto a 5 ml HiTrap Chelating HP column (GE Healthcare) equilibrated with buffer F (500 mM NaCl, 20 mM Tris–HCl pH 8.0, 20 mM imidazole). The column was washed with 30 column volumes of the same buffer and the protein was eluted with buffer G (500 mM NaCl, 20 mM Tris–HCl pH 8.0, 500 mM imidazole).
The N-terminal hexahistidine tag was cleaved off with His6-TEV protease (Invitrogen) whilst dialyzing the sample overnight at 4°C against buffer H [150 mM NaCl, 10 mM Tris–HCl pH 8.0, 2 mM DTT, 1%(v/v) glycerol]. NaCl and imidazole were then added to the sample to final concentrations of 500 and 20 mM, respectively, and the TEV protease and the uncleaved protein were removed on a HiTrap Chelating HP column. The flowthrough fractions containing protein were pooled, concentrated to 400 µl in an AmiconUltra Ultracel 10 kDa cutoff concentrator and passed through a Superdex 200 HiLoad 26/60 gel-filtration column (GE Healthcare) equilibrated with buffer I (10 mM Tris–HCl pH 8.0, 150 mM NaCl) at a flow rate of 0.4 ml min−1. The protein purity was estimated by SDS–PAGE to be greater than 95% (Fig. 1 ▶).
Figure 1.
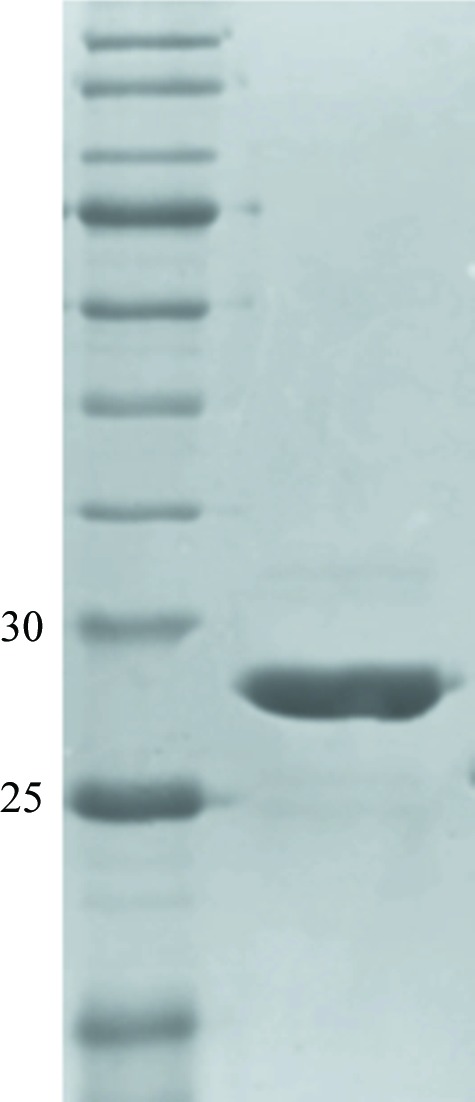
Reduced 12% SDS–PAGE Coomassie Blue-stained gel of purified recombinant CcaAperi. The left lane contains molecular-weight marker (labelled in kDa).
2.2. Crystallization
Prior to crystallization, the protein was concentrated to 10 mg ml−1, mixed with 10 mM aspartic acid and centrifuged for 20 min at 13 000g to clarify the solution. Crystallization screening was performed by the hanging-drop vapour-diffusion method using an automated Phoenix crystallization robot (Art Robbins Instruments) and JBScreen HTS I and II (Jena Bioscience), The JCSG+ Suite (Qiagen), Crystal Screen HT and PEG/Ion HT (Hampton Research). 200 nl protein solution was mixed with 200 nl reservoir solution and equilibrated against 50 µl reservoir solution in a 96-well Art Robbins CrystalMation Intelli-Plate (Hampton Research). Crystals appeared in many different conditions. The best-looking crystals grew in 200 mM ammonium acetate, 25%(w/v) PEG 3350, 100 mM bis-tris pH 5.5 (The JCSG+ Suite condition H10). Upon refinement of this condition to improve the quality of the crystals, the concentration of PEG was reduced to 23%(w/v); crystals appeared in the manually set up trays (drop size 2 µl) after two weeks. Crystallization information is summarized in Table 2 ▶.
Table 2. Crystallization.
| Method | Hanging-drop vapour diffusion |
| Plate type | 96-well Art Robbins CrystalMation Intelli-Plate (Hampton Research) |
| Temperature (K) | 293 |
| Protein concentration (mgml1) | 10 |
| Buffer composition of protein solution | 100mM ammonium acetate, 12%(w/v) PEG 3350, 50mM bis-tris pH 5.5 |
| Composition of reservoir solution | 200mM ammonium acetate, 23%(w/v) PEG 3350, 100mM bis-tris pH 5.5 |
2.3. Data collection and processing
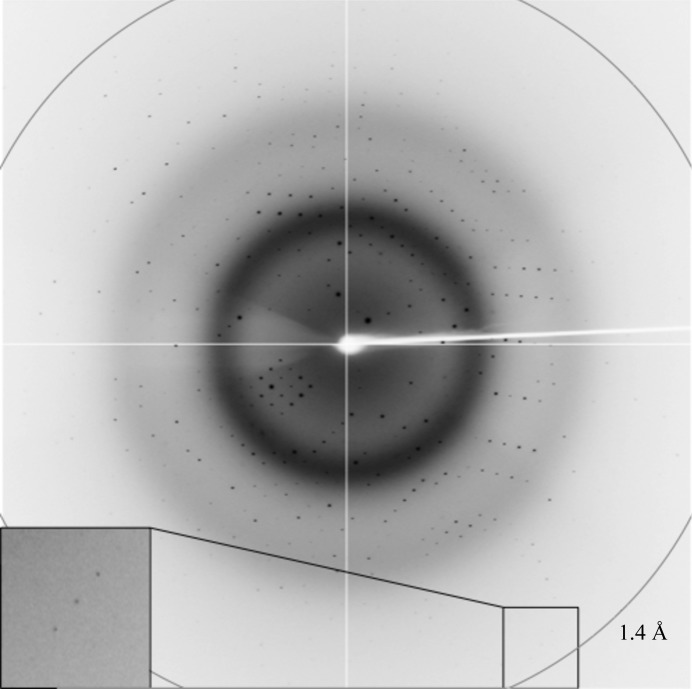
The crystals were briefly soaked in a cryoprotectant solution consisting of 220 mM ammonium acetate, 26%(w/v) PEG 3350, 100 mM bis-tris pH 5.5, 10 mM aspartic acid, 10%(v/v) glycerol and were flash-cooled by plunging them into liquid nitrogen. X-ray diffraction data were collected to 1.4 Å resolution on the MX1 beamline of the Australian Synchrotron (Fig. 2 ▶). A total of 360 images were collected using 0.5° oscillations. The data were processed and scaled using iMosflm (Battye et al., 2011 ▶) and AIMLESS (Evans & Murshudov, 2013 ▶) from the CCP4 suite (Winn et al., 2011 ▶). Calculation of the self-rotation function was performed using POLARRFN (Winn et al., 2011 ▶). The statistics of data collection are summarized in Table 3 ▶.
Figure 2.
A representative 0.5° oscillation image obtained from a CcaAperi crystal using a CCD ADSC Quantum 315r detector on beamline MX1 of the Australian Synchrotron. The magnified rectangle shows diffraction spots at 1.4 Å resolution.
Table 3. Data collection and processing.
Values in parentheses are for the outer shell.
| Diffraction source | MX1 beamline, Australian Synchrotron |
| Wavelength () | 1.0 |
| Temperature (K) | 100 |
| Detector | ADSC Quantum 315r CCD |
| Rotation range per image () | 0.5 |
| Total rotation range () | 180 |
| Exposure time per image (s) | 1 |
| Space group | P1 |
| a, b, c () | 39.3, 43.3, 50.9 |
| , , () | 92.5, 111.4, 114.7 |
| Mosaicity () | 0.6 |
| Resolution range () | 38.31.4 (1.421.40) |
| Total No. of reflections | 97980 (3448) |
| No. of unique reflections | 49677 (1741) |
| Completeness (%) | 92 (66)† |
| Multiplicity | 2 (2) |
| I/(I) | 11.9 (3.9) |
| R r.i.m. | 0.046 (0.150) |
| Overall B factor from Wilson plot (2) | 8.6 |
The low completeness in P1 resulted from the use of a single-axis goniometer.
3. Results and discussion
Recombinant CcaAperi from C. jejuni was overexpressed in E. coli BL21(DE3)-RIPL cells. The purified protein fragment contained CcaA residues 31–327 plus six additional residues at the N-terminus (a cloning artifact; GIDPFT). When subjected to gel filtration, the protein eluted as a single peak with a retention volume corresponding to an approximate molecular weight of 30 kDa, which is close to the theoretical molecular weight (33.6 kDa), suggesting that recombinant CcaAperi is a monomer in solution under the tested conditions.
Crystals of CcaAperi were obtained in the presence of its ligand (aspartic acid) using a sparse-matrix crystallization approach. Data collected from a cryocooled crystal at the Australian Synchrotron showed diffraction to 1.4 Å resolution (Fig. 2 ▶). Autoindexing of the diffraction data using iMosflm showed that it belonged to space group P1, with unit-cell parameters a = 39.3, b = 43.3, c = 50.9 Å, α = 92.5, β = 111.4, γ = 114.7°. The average I/σ(I) value was 11.9 for all reflections (resolution range 38.3–1.4 Å) and 3.9 in the highest resolution shell (1.42–1.40 Å). A total of 50 732 measurements were made of 49 677 independent reflections. Data processing gave an R r.i.m. of 0.046 for intensities (0.15 in the 1.42–1.40 Å resolution shell) and these data were 92% complete (66% completeness in the highest resolution shell).
Calculations of the Matthews coefficient (Matthews, 1977 ▶) and solvent content, assuming the presence of one molecule in the unit cell, gave values of 2.2 Å3 Da−1 and 43%, respectively. Analysis of the self-rotation function calculated using data in the resolution range 10–5 Å with an integration radius of 25 Å revealed no dominant features that could be confidently assigned to noncrystallographic axes. Together, this analysis suggests that the CcaAperi crystals contain one molecule per unit cell. Structure determination by molecular replacement was not possible, as a sequence-similarity search against the Protein Data Bank did not identify any homologues of known structure. A search for heavy-atom derivatives suitable for structure determination by the multiple isomorphous replacement and/or multiwavelength anomalous dispersion methods is under way.
Acknowledgments
Plasmid pGU0605 was kindly provided by Professor Victoria Korolik (Griffith University, Australia). We thank the staff at the Australian Synchrotron for their assistance with data collection. We also thank Dr Danuta Maksel and Dr Robyn Gray at the Monash Crystallography Unit for assistance in setting up robotic crystallization trials. AR is an Australian Research Council Research Fellow.
References
- Acheson, D. & Allos, B. M. (2001). Clin. Infect. Dis. 32, 1201–1206. [DOI] [PubMed]
- Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. (2011). Acta Cryst. D67, 271–281. [DOI] [PMC free article] [PubMed]
- Boes, J., Nersting, L., Nielsen, E. M., Kranker, S., Enøe, C., Wachmann, H. C. & Baggesen, D. L. (2005). J. Food Prot. 68, 722–727. [DOI] [PubMed]
- Bradford, M. M. (1976). Anal. Biochem. 72, 248–254. [DOI] [PubMed]
- Dasti, J. I., Tareen, A. M., Lugert, R., Zautner, A. E. & Gross, U. (2010). Int. J. Med. Microbiol. 300, 205–211. [DOI] [PubMed]
- Evans, P. R. & Murshudov, G. N. (2013). Acta Cryst. D69, 1204–1214. [DOI] [PMC free article] [PubMed]
- Fernando, U., Biswas, D., Allan, B., Willson, P. & Potter, A. A. (2007). Int. J. Food Microbiol. 118, 194–200. [DOI] [PubMed]
- Friedman, C., Neimann, J., Wegener, H. & Tauxe, R. (2000). Campylobacter, edited by I. Nachamkin & M. J. Blaser, pp. 121–138. Washington: ASM Press.
- Hartley-Tassell, L. E., Shewell, L. K., Day, C. J., Wilson, J. C., Sandhu, R., Ketley, J. M. & Korolik, V. (2010). Mol. Microbiol. 75, 710–730. [DOI] [PubMed]
- Hepworth, P. J., Ashelford, K. E., Hinds, J., Gould, K. A., Witney, A. A., Williams, N. J., Leatherbarrow, H., French, N. P., Birtles, R. J., Mendonca, C., Dorrell, N., Wren, B. W., Wigley, P., Hall, N. & Winstanley, C. (2011). Environ. Microbiol. 13, 1549–1560. [DOI] [PMC free article] [PubMed]
- Josenhans, C. & Suerbaum, S. (2002). Int. J. Med. Microbiol. 291, 605–614. [DOI] [PubMed]
- Li, Z., Lou, H., Ojcius, D. M., Sun, A., Sun, D., Zhao, J., Lin, X. & Yan, J. (2014). J. Med. Microbiol. 63, 343–354. [DOI] [PubMed]
- Liu, Y. C. & Roujeinikova, A. R. (2014). Protein Expr. Purif. 107, 29–34. [DOI] [PubMed]
- Marchant, J., Wren, B. & Ketley, J. (2002). Trends Microbiol. 10, 155–159. [DOI] [PubMed]
- Matthews, B. W. (1977). X-ray Structure of Proteins, 3rd ed., edited by H. Neurath & R. L. Hill, Vol. 3, pp. 468–477. New York: Academic Press.
- Oporto, B., Esteban, J. I., Aduriz, G., Juste, R. A. & Hurtado, A. (2007). J. Appl. Microbiol. 103, 977–984. [DOI] [PubMed]
- Parkhill, J. et al. (2000). Nature (London), 403, 665–668.
- Rahman, H., King, R. M., Shewell, L. K., Semchenko, E. A., Hartley-Tassell, L. E., Wilson, J. C., Day, C. J. & Korolik, V. (2014). PLoS Pathog. 10, e1003822. [DOI] [PMC free article] [PubMed]
- Schmidt-Ott, R., Schmidt, H., Feldmann, S., Brass, F., Krone, B. & Gross, U. (2006). Clin. Vaccine Immunol. 13, 779–783. [DOI] [PMC free article] [PubMed]
- Tareen, A. M., Dasti, J. I., Zautner, A. E., Gross, U. & Lugert, R. (2010). Microbiology, 156, 3123–3135. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Zautner, A. E., Tareen, A. M., Gross, U. & Lugert, R. (2012). Eur. J. Microbiol. Immunol. 2, 24–31. [DOI] [PMC free article] [PubMed]
- Zhulin, I. B. (2001). Adv. Microb. Physiol. 45, 157–198. [DOI] [PubMed]
- Zilbauer, M., Dorrell, N., Wren, B. W. & Bajaj-Elliott, M. (2008). Trans. R. Soc. Trop. Med. Hyg. 102, 123–129. [DOI] [PubMed]