Abstract
The ventromedial nucleus of the hypothalamus (VMH) influences a wide variety of physiological responses. Here, using two distinct but complementary genetic tracing approaches in mice, we describe the development of VMH efferent projections, as marked by steroidogenic factor-1 (SF-1; NR5A1). SF-1 neurons were visualized by Tau-green fluorescent protein (GFP) expressed from the endogenous Sf-1 locus (Sf-1TauGFP) or by crossing the transgenic Sf1:Cre driver to a GFP reporter strain (Z/EGSf1:Cre). Strikingly, VMH projections were visible early, at embryonic (E) 10.5, when few postmitotic SF1 neurons have been born, suggesting that formation of VMH circuitry begins at the onset of neurogenesis. At E14.5, comparison of these two reporter lines revealed that SF1-positive neurons in the ventrolateral VMH (VMHvl) persist in Z/EGSf1:Cre embryos but are virtually absent in Sf-1TauGFP. Therefore, although the entire VMH including the VMHvl shares a common lineage, the VMHvl further differentiates into a neuronal cluster devoid of SF-1. At birth, extensive VMH projections to broad regions of the brain were observed in both mouse reporter lines, matching well with those previously discovered by injection of axonal anterograde tracers in adult rats. In summary, our genetic tracing studies show that VMH efferent projections are highly conserved in rodents and are established far earlier than previously appreciated. Moreover, our results imply that neurons in the VMHvl adopt a distinct fate early in development, which might underlie the unique physiological functions associated with this VMH subregion.
INDEXING TERMS: VMH, SF-1, ventrolateral VMH, neurocircuitry, SF-1:Cre driver, GFP labeling, genetic tracing, ventral supraoptic commissure
The hypothalamus is the homeostatic center of the central nervous system and is composed of multiple nuclei. Among these, the ventromedial nucleus of the hypothalamus (VMH) is known to regulate many physiological processes, including reproduction, defensive responses, locomotor activity, glucose homeostasis, and appetite control (Canteras, 2002; King, 2006; Kow and Pfaff, 1998; Tong et al., 2007; Yokawa et al., 1989; Zhao et al., 2008). One of the earliest molecular markers expressed throughout the VMH is the nuclear receptor steroidogenic factor-1 (SF-1, NR5A1), which appears at embryonic (E) 9 (Ikeda et al., 2001). Although SF-1 is not required for the initial organization and migration of neurons to the developing VMH nucleus (Ikeda et al., 1995; Tran et al., 2003), this transcription factor is essential for terminal differentiation and maintenance of VMH neuronal populations (Davis et al., 2004; McClellan et al., 2006). Loss of SF-1 also results in diminished efferent projections to the amygdala (Tran et al., 2003) and altered afferent projections from the preoptic area to the VMH (Budefeld et al., 2011). Consistent with the essential role for SF-1 in the VMH, CNS-specific elimination of SF-1 in mice (Sf-1Nestin:Cre) disrupts the integrity of the VMH and leads to a variety of metabolic, thermogenic, reproductive, and anxiety deficits (Kim et al., 2009, 2010, 2011; Zhao et al., 2008).
To delineate further the cellular and functional complexity of the VMH, several groups have exploited two Sf1:Cre driver transgenic mouse lines (Bingham et al., 2008; Dhillon et al., 2006) to ablate general factors known or thought to be associated with energy homeostasis. Indeed, increased susceptibility to diet-induced obesity has been reported for conditional knockouts of the leptin receptor (Bingham et al., 2008; Dhillon et al., 2006), insulin receptor (Klockener et al., 2011), phosphoinositol 3-kinase (PI3K; Xu et al., 2010), or SIRT1 deactylase (Ramadori et al., 2011) with the use of these Sf1:Cre drivers. Moreover, removing the central mediator of leptin resistance, Socs3, with the same Sf1:Cre driver, protects against diet-induced hyperglycemia (Zhang et al., 2008). More recently, a mild disturbance in energy balance in females was noted after the Sf1:Cre driver was used to eliminate conditionally the estrogen receptor α (ERα) from all SF-1-expressing tissues, including the ventrolateral region of the VMH (VMHvl; Xu et al., 2011). Thus, these genetic lesions establish that disrupting cellular signaling in the VMH and its neuronal outputs (Klockener et al., 2011; Tong et al., 2007) alters normal energy homeostasis in mice.
Although these newer genetic approaches in mice have validated or refined the physiological roles of the VMH, the neuroanatomy of VMH projections in mice has yet to be described. Our current notion on how the VMH connects with other brain centers comes exclusively from stereotaxic injection studies with anterograde axonal tracers in adult rats (Canteras et al., 1994; Krieger et al., 1979; Saper et al., 1976). While results from these studies established projection patterns from different VMH subregions, the inherent limitations of this method make it difficult to assess development of VMH projections during embryonic stages. Based on our earlier observations suggesting that the VMH and its projections are already established in neonatal mice (Kurrasch et al., 2007), two approaches that exploit the global expression of SF-1 in the VMH were used to trace the major VMH axonal projections during embryonic and postnatal stages. The first relied on tandem reporters, wheat germ agglutinin (WGA; Braz et al., 2002) and Tau-green fluorescent protein (TauGFP), knocked into the 3′-untranslated region (UTR) of the Sf-1 (Nr5a1) locus. In this knock-in line, referred to as Sf-1TauGFP, WGA and GFP are under the control of intact regulatory elements and are thus coexpressed and coregulated with SF-1 expression. In the second, more standard approach, the transgenic Sf1:Cre mouse was crossed with a Z/EG reporter mouse, resulting in constitutive expression of eGFP (enhanced GFP) after Cre-mediated recombination (Dhillon et al., 2006). This second reporter line, referred to as Z/EGSf1:Cre, expresses eGFP in all neurons derived from the SF-1 lineage, including cells that might no longer express Sf-1. By using both Sf-1TauGFP and Z/EGSf1:Cre mice, we show the temporal and anatomical patterns of VMH projections and show a marked difference in GFP labeling in the VMHvl between these two lines.
MATERIALS AND METHODS
Generation of Sf-1TauGFP line
The IRES-WGA-IRES-tauGFP knock-in targeting vector was created using 3.5-kb 5′ (657,441–653,959) and 2-kb (653,937–651,900) 3′ targeting arms that align with the endogenous Sf-1 locus just after the stop codon in exon 7. The knock-in vector also included an ACN cassette (Bunting et al., 1999), which contains a loxP-flanked selectable marker Neor downstream of the testes-specific promoter from the angiotensin-converting enzyme gene used to drive expression of Cre. Thus, a self-induced deletion of the selectable marker occurred during passage through the male germline of mice. Southern blot analyses using a Neo-specific probe in Sf-1TauGFP offspring confirmed complete elimination of the Neor gene from the Sf-1 locus (data not shown). The gene encoding diphtheria toxin was also included outside the 3′ targeting arm for negative selection. Linearized targeting vector DNA was electroporated into E14Tg2A.4 (from 129/Ola mice) ES cells by the University of Michigan Transgenic Facility. ES clones were screened by using Southern blot analyses with both 5′ and 3′ probes. Positive clones were verified via PCR. Three positive clones were injected into 129-derived blastocysts and implanted into a pseudopregnant C57/Bl6 female. Offspring that were 70% chimeric or greater were then back-crossed to C57/Bl6 to confirm germline transmission
Animals
Mice were raised in our animal facility in strict accordance with a protocol approved by the UCSF Institutional Animal Care and Use Committee. All mice had ad libitum access to food and water. All Sf-1TauGFP mice used in this study were homozygous as confirmed by PCR genotyping. For Z/EGSf1:Cre mice, both the Sf1:Cre mice (line S7, generously provided by Dr. Elmquist) and the Z/EG line (provided by Dr. Pleasure, UCSF) were maintained as heterozygotes in a FVB/129 mixed background. Sf-1TauGFP mice were maintained on a mixed 129/C56Bl6 mixed background. Embryos were collected at E10.5, E12.5, E14.5, and E17.5 from timed-pregnant females (Tran et al., 2006) and from postnatal day (P) 0. Male mice were used for all studies.
Tissue collection and processing
Isolated embryos spanning stages E10.5–E14.5 were initially fixed in 4% paraformaldehyde (PFA) for 2 hours and equilibrated in 30% sucrose, followed by gross dissection of the upper body, which was equilibrated and mounted in OCT compound (Tissue-Tek, Torrance, CA). For E17.5 embryos, whole brains were removed, fixed in 4% PFA overnight, equilibrated in 30% sucrose, and mounted in OCT. P0 pups were perfused with phosphate-buffered saline (PBS) and 4% PFA as described previously (Kurrasch et al., 2007; Tran et al., 2003). Dissected brains were postfixed in 4% PFA for 2 hours, equilibrated in 30% sucrose, and mounted. All samples were sectioned at 20 μm.
Immunohistochemistry
Frozen brain sections (−80°C) were blown dry for 3 minutes and air dried at room temperature for 20 minutes. All steps described below were performed at room temperature, and washes were carried out for 3 × 5 minutes, unless otherwise noted. Sections were washed in a series of sequential buffers beginning with PBS (pH 7.5) and 0.3% Triton (PBST). This was followed by PBST and 0.3% hydrogen peroxide for 20 minutes and then PBST. Sections were transferred to a moistened incubation chamber (ThermoScientific, Fair Lawn, NJ), blocked with TNT blocking buffer (TNB; 1 M Tris-HCl pH 7.5, 0.15 M NaCl, 0.5% Blocking Reagent from PerkinElmer, Waltham, MA) for 1 hour, incubated with chicken anti-GFP antibody (1:2,500; Aves Labs, Tigard, OR) overnight at 4°C, and washed with TNT buffer (TNT; 0.1 M Tris-HCl, pH 7.5, 0.15 M NaCl, 0.05% Tween 20). Samples were then incubated with biotinylated anti-chicken antibody (1:500; Vector, Burlingame, CA) in TNT for 1 hour, washed in TNT, and incubated in streptavidin-HRP diluted in TNB (1:100; PerkinElmer) for 30 minutes. After TNT washes, samples were incubated with tyramide fluorescein diluted in amplification diluent (1:75; PerkinElmer) for 10 minutes and washed with TNT. Samples were equilibrated with a 5-minute wash (PBST), and incubated in 1× DAPI in PBST for 10 minutes. After the final washes in PBST, sections were mounted and cover-slipped with Fluoromount G (Southern Biotechnology, Birmingham, AL). Brains from the two mouse lines of the same developmental age were used in the same immunohistochemistry experiment. No-antibody controls were also performed.
For double-label experiments, perfused P0 tissues were used. Slides were washed in PBS and blocked for 1 hour (0.2% Triton and 10% donkey serum in 1× PBS). Tissues were incubated overnight with chicken anti-GFP (1:200; Aves Labs) and rabbit anti-SF-1 (1:100; Ingraham laboratory, UCSF) and washed with PBS and 0.3% Triton X. Samples were incubated with goat anti-chicken Alexa 488 (1:200; Invitrogen, Carlsbad, CA) and goat anti-rabbit Alexa 546 (1:200; Invitrogen) for 2 hours, washed in PBS with 0.3% Triton, and incubated with 1× DAPI for 10 minutes. Samples were washed in PBS for 10 minutes and then mounted in Fluoromount G. Sample size for all stages ranged between two and six per genotype.
Antibody characterization
See Table 1 for a list of all antibodies used in this study. The chicken anti-GFP antibody was generated against purified recombinant GFP emulsified in Freund’s adjuvant and was analyzed by Western blot analysis (1:5,000 dilution) and immunohistochemistry (1:500 dilution) using transgenic mice expressing GFP gene product. No staining was seen in mice that lacked GFP.
TABLE 1.
Primary Antibodies Used
| Antigen | Immunogen | Raised in | Manufacturer data | IHC dilution |
|---|---|---|---|---|
| GFP | Purified recombinant GFP | Chicken polyclonal | Aves Labs (GFP-1020) | 1:2,500 (TSA), 1:200 (non-TSA) |
| SF-1 | Purified recombinant SF-1 hinge LBD | Rabbit polyclonal | Bio.Synthesis (custom made) | 1:100 |
The rabbit anti-SF-1 antibody was generated against purified recombinant SF-1 hinge LBD that spans amino acids 178–462 and was analyzed by Western blotting (1:1,000 dilution) with SF-1-positive and SF-1-negative cell lines. Signals were seen only in the SF-1-positive cell lines and not SF-1-negative cell lines. Immunostaining showed a pattern of cellular morphology and distribution in the mouse brain identical to that described in previous reports (Tran et al., 2006).
Histology
Histology was carried out on E17.5 and P0 brain sections rinsed in 95% ethanol for 15 minutes, 70% ethanol for 1 minute, and 50% ethanol for 1 minute. Sections were then washed in purified water twice for 2 minutes each and stained in cresyl violet for 5 minutes, followed by a brief rinse in purified water for 1 minute. Sections were destained in 50% ethanol for 1 minute and 70% ethanol with 1% glacial acetic acid for 2 minutes, followed by rehydration in 95% ethanol for 2 minutes and 100% ethanol for 1 minute. Sections were rinsed in xylene twice for 5 minutes each prior to coverslipping with Permount (Fisher Scientific, Pittsburgh, PA).
Image acquisition
All images were collected at the UCSF Nikon Imaging Center on a Nikon Ti high-throughput microscope (Nikon Instruments, Melville, NY). Fluorescent photomicrographs were taken with a CoolSnap HQ2 CCD camera (Photometrics, Roper Scientific, Tucson, AZ), and brightfield images were captured with a Nikon DS-Fi1 color camera and NIS-Elements software (Nikon Instruments). Photomicrographs were adjusted for contrast and brightness in Photoshop. Best attempts were made to determine anatomical identification and nomenclature using multiple references (Jacobowitz and Abbott, 1998; Paxinos, 2007; Schambra, 2008).
RESULTS
Generation and validity of the Sf-1TauGFP reporter line
Sf-1TauGFP mice were generated as described in Materials and Methods using the construct shown in Figure 1A. Immunohistochemistry directed against the wheat germ agglutinin (WGA) and TauGFP proteins revealed signals in all organs known to express SF-1, including the adrenal gland (Fig. 1B), gonads, and pituitary (data not shown). Although plant lectins such as WGA have been used successfully for transsynaptic neuronal tracing in some circumstances (Braz et al., 2002), very few efferent fibers were marked by WGA immunostaining in the Sf-1TauGFP VMH. Expression of this plant lectin was limited to the soma and proximal neurites, with few if any signal observed in long axonal fibers, synaptic terminals, or postsynaptic neurons (data not shown). Therefore, staining for WGA to map VMH projections was not pursued further. By contrast, detection of TauGFP protein by immunohistochemistry revealed a strong signal in the soma, with GFP colocalized with SF-1 and in neurites of VMH neurons (Fig. 1C–E). Thus, GFP was used to assess systematically SF-1 neuronal projections in embryonic and postnatal Sf-1TauGFP mice. It should be noted that both Sf-1TauGFP male and female mice were viable and exhibited normal fertility.
Figure 1.
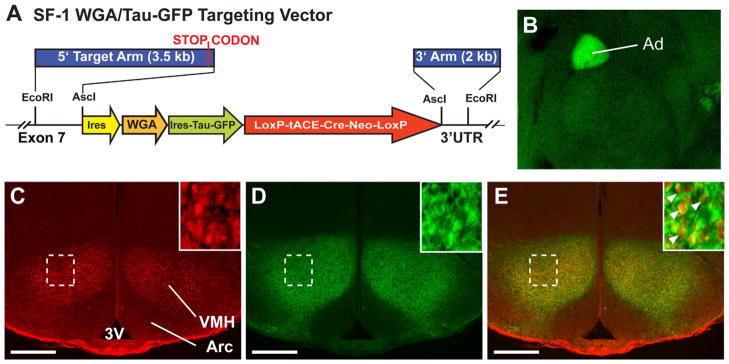
A: Schematic of the Sf-1TauGFP knock-in targeting vector positioned in the mouse nr5a1 (Sf-1) locus. B: Fluorescence image of the adrenal gland in the Sf-1TauGFP knock-in mouse. C–E: Photomicrographs of nuclear immunostaining of SF-1 (C), cytoplasmic immunostaining of GFP (D), and their colocalization in the VMH at P0 (E). Insets show higher magnifications of the boxed areas and with SF-1 and GFP colocalized in neurons (arrowheads). Scale bars = 0.25 mm.
Profile of GFP+ neurons in Sf-1TauGFP and Z/EGSf1:Cre mice from E10.5 to E14.5
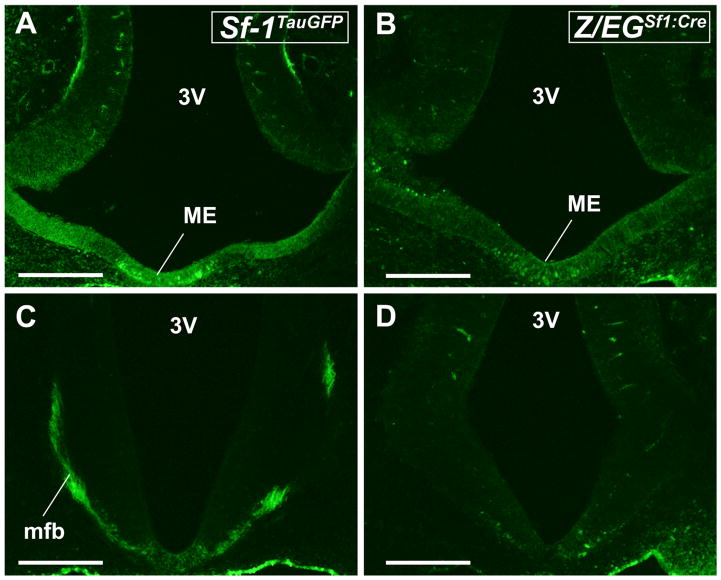
Beginning as early as E10.5, just prior to the onset of neurogenesis, GFP+ cells are observed in the median eminence (ME) of Sf-1TauGFP mice (Fig. 2A), with fibers extending into the medial forebrain bundle (MFB; Fig. 2C) and traveling toward the epithalamus. At this stage, expression in Z/EGSf1:Cre mice was nearly undetectable, with only sparse GFP+ cells present (Fig. 2B,D). However, in slightly older embryos, at E12.5, GFP+ cells were observed in the ME (Fig. 3A,B) and in the caudal, lateral postoptic area or presumptive VMH in both Sf-1TauGFP and Z/EGSf1:Cre mice (Fig. 3C–F), with GFP+ neurons occupying the lateral half of the hypothalamus and extending to the edge of the developing brain (Fig. 3E,F). Fiber strands that travel through the MFB are easily observed in Sf-1TauGFP (Fig. 3A,C) but are faintly visible in Z/EGSf1:Cre (Fig. 3B,D). The most dominant fiber tract visible in Sf-1TauGFP mice travels medial to the optic tract and lateral to the MFB, following a path similar to the presumptive ventral supraoptic commissure (vSOC; Fig. 3C) as previously reported for adult rats (Canteras et al., 1994).
Figure 2.
Coronal brain sections from Sf-1TauGFP mouse (A,C) and Z/EGSf1:Cre mouse (B,D) at E10.5. A,B: GFP-labeled neurons are present in the median eminence (ME) of the Sf-1TauGFP mouse. C,D: GFP-labeled fibers in the medial forebrain bundle (mfb) of the Sf-1TauGFP mouse but not the Z/EGSf1:Cre mouse. 3V, third ventricle. Scale bars = 0.5 mm.
Figure 3.
Anterior to posterior coronal brain sections from Sf-1TauGFP mouse (A,C,E) and Z/EGSf1:Cre reporter mouse (B,D,F) at E12.5. A,B: GFP-stained neurons in the median eminence (ME). C,D: GFP-labeled neurons in the ME with fibers traveling in the medial forebrain bundle (mfb) in the Sf-1TauGFP mouse but not in the Z/EGSf1:Cre mouse. E,F: GFP-stained neurons in the presumptive VMH and fibers in Sf-1TauGFP mice. Scale bars = 0.75 mm.
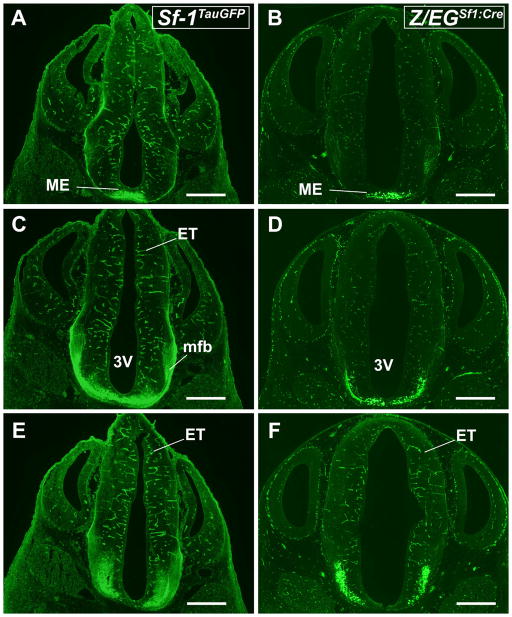
Later in neurogenesis (E14.5), Sf-1TauGFP and Z/EGSf1:Cre GFP+ cells begin to appear anterior to the ME (Fig. 4A,B) and in the coalescing VMH, which is bounded medially by the ventricular wall and laterally by the developing diencephalon (Fig. 4C,D). At this stage, striking differences between the Sf-1TauGFP and Z/EGSf1:Cre mice are observed in the most lateral aspect of the presumptive VMH (Fig. 4E–H). Notably, an absence of GFP+ cells is observed in this VMHvl region in Sf-1TauGFP mice (Fig. 4E,G). With respect to the vSOC tract, both reporter lines exhibit fibers that initially travel on a ventral anterior course, with some reaching the level of the suprachiasmatic nucleus (SCN), before moving laterally in a ventral and posterior direction. Most of these fibers travel along the lateral wall of the developing brain and branch toward the epithalamus (Fig. 4E,F) and the habenular nucleus. Few if any fibers reach the level of the periaqueductal gray (PAG; data not shown). A second, scattered group of fibers traverse through the MFB, ascending laterally and dorsolaterally in a fan-like pattern (Fig. 4C,D). These fibers likely originate from neurons located either in the anterior or in the anteriolateral aspect of the VMH. Although some of these fibers join the vSOC at the level of the external medullary lamina (EML), others spread into the thalamus extending beyond the EML (Fig. 4E,G). Few if any fibers travel medially in the periventricular zone (Fig. 4E–H).
Figure 4.
Anterior to posterior coronal brain sections from Sf-1TauGFP mouse (A,C,E,G) and Z/EGSf1:Cre reporter mouse (B,D,F,H) at E 14.5. A,B: Fibers in the ME and extending into the ventral supraoptic commissure (vsoc). C,D: GFP-stained neurons in the VMH and fibers tracts extending dorsally along the vsoc. E,F: Fibers traveling in the mfb angle toward and join the vsoc at the level of the external medullary lamina (eml). E–H: The ventrolateral aspect of the VMH in Sf-1TauGFP (E,G) and in Z/EGSf1:Cre mice (F,H) is indicated by arrows. Insets show expanded view of the VMH and its efferent fibers. Scale bars = 0.5 mm.
Collectively, data obtained with the Sf-1TauGFP reporter line establish that VMH projections begin as early as E10.5, just at the onset of the window of VMH neurogenesis (E10.5–E15.5). Despite the fact that GFP expression was observed to lag in Z/EGSf1:Cre brains, the intensity and pattern of labeling and the appearance of projections are roughly equivalent in both lines by E14.5. However, labeling in the VMHvl was markedly different. Whereas the number and pattern of GFP+ neurons is far greater and broader, respectively in Z/EGSf1:Cre mice (Fig. 4F), a much more restricted and medial pattern with fewer positive cells is observed in Sf-1TauGFP mice (Fig. 4E). Given that the SF-1 lineage is marked by Sf1:Cre-mediated recombination, we infer that SF-1 is only transiently expressed in neurons that migrate and populate the VMHvl.
Prenatal ascending and descending SF-1 tracts at E17.5
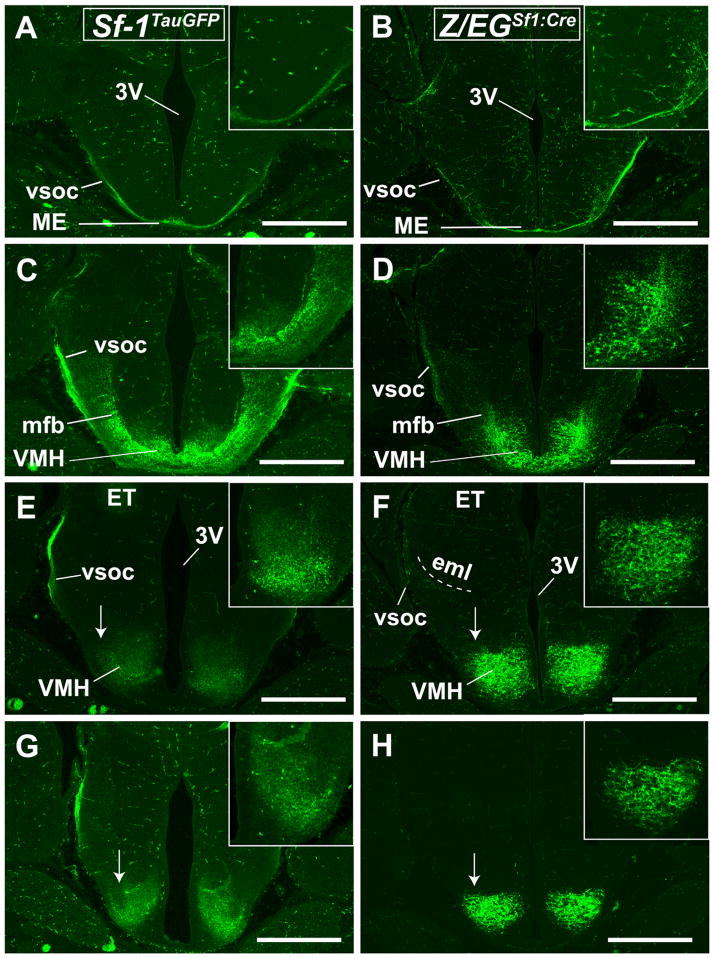
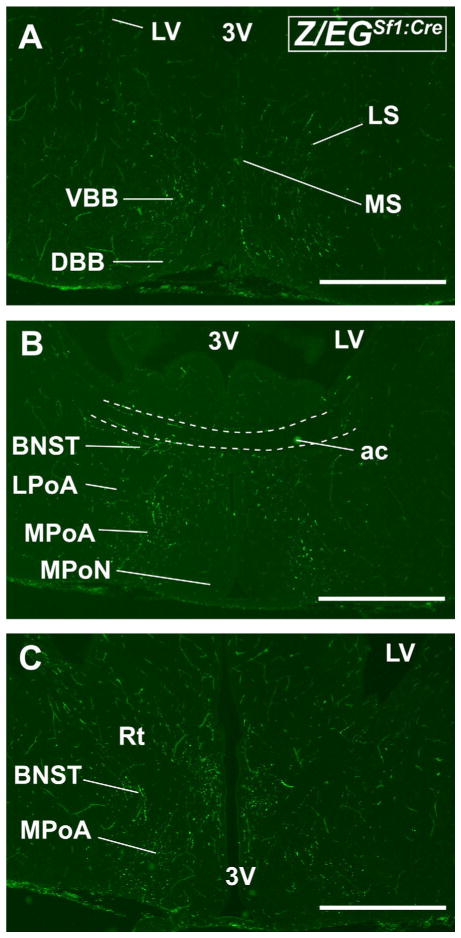
The intensities of VMH projections in Sf-1TauGFP and Z/EGSf1:Cre mice begin to differ at E17.5. Whereas the ascending projections are observed in Z/EGSf1:Cre mice, including those originating from the retrochiasmatic area (RCh) and traveling to the medial basal forebrain (Fig. 5A–C), these fiber tracts are less intense in Sf-1TauGFP mice (data not shown). The first ascending fiber tract, which is far brighter in Z/EGSf1:Cre mice, travels through the medial aspect of the hypothalamus and reaches the medial basal forebrain (Figs. 5A–C, 6A–D). Fibers were found in the RCh (Fig. 6C,D), the anterior hypothalamic area (Fig. 6A,B), and medial preoptic area (MPoA) but not in the lateral preoptic area (LPoA) or in the SCN (Fig. 6B,C). Other fibers were observed to turn ventrolaterally, traveling along the EML toward the bed nucleus of stria terminalis (BNST) or terminating in the BNST at the level of the anterior commissure (Fig. 5B,C). Moving anteriorly, positive fibers were detected in the diagonal and vertical band of Broca as well as in the medial septum (Fig. 5A).
Figure 5.
Anterior coronal brain sections from the Z/EGSf1:Cre mouse at E 17.5. A: Fibers are detected in the vertical band of Broca (VBB), the diagonal band of Broca (DBB), and the lateral septal nucleus (LS). Scant fibers are found in the medial septal nucleus (MS). B: Fibers can be seen in the bed nucleus of stria terminalis (BNST) and medial preoptic area (MPoA), sparing the lateral preoptic area (LPoA) and the medial preoptic nucleus (MPoN). C: Fibers are seen in the BNST, by the reticular nucleus of the thalamus (Rt), and in MPoA as well as in the periventricular zone of the third ventricle (3V). Scale bars = 0.5 mm.
Figure 6.
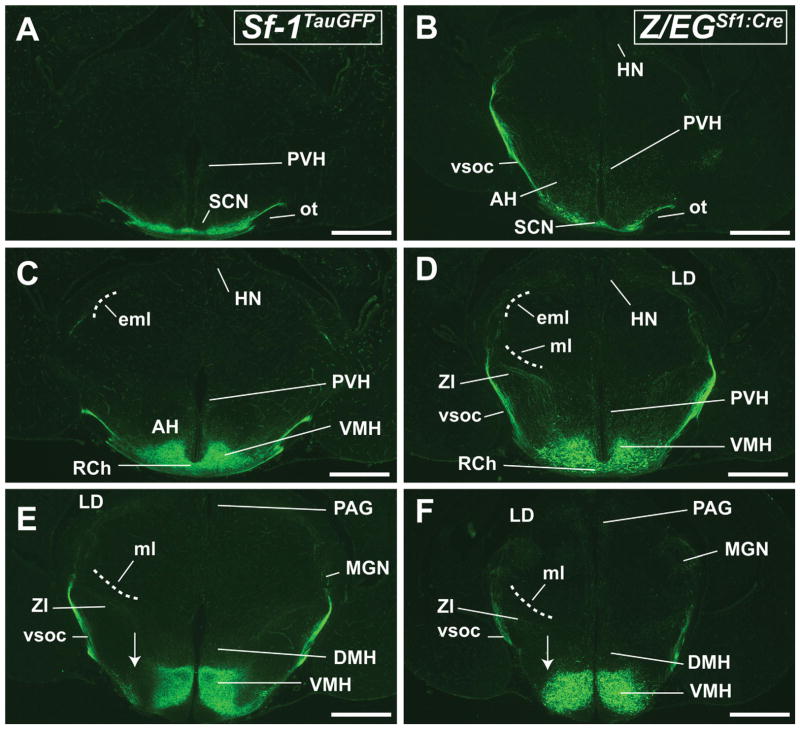
Anterior to posterior coronal brain sections from the Sf-1TauGFP mouse (A,C,E,G,I,K) and the Z/EGSf1:Cre reporter mouse (B,D,F,H,J,L) at E 17.5. A,B: Fibers are seen surrounding, but sparing, the suprachiasmatic nucleus (SCN) in both mouse models. Hypothalamic paraventricular nucleus (PVH) and the anterior hypothalamus (AH) also receive fiber projections. The vsoc is the major fiber tract and travels laterally and dorsally. C,D: Fine fiber tracts are seen projecting toward the HN and the PVH. E,F: Fibers projecting from the dorsolateral region of the VMH join the vsoc at the lateral aspect of the medial lemniscus (ml). Midline fibers travel through the dorsomedial hypothalamic nucleus (DMH) to terminate in the periaqueductal gray (PAG), whereas lateral fibers fanning out from the vsoc travel through the medial geniculate nucleus (MGN). Arrows point to the ventrolateral region of the VMH (VMHvl), where GFP+ neurons are present in the Z/EGSf1:Cre reporter mouse but not the Sf-1TauGFP mouse. G–J: GFP+ neurons in the VMHvl are absent in the Sf-1TauGFP mouse (G,I) but present in the Z/EGSf1:Cre mouse (H,J). Fibers travel through the posterior hypothalamus (PH) and the DMH medially and the MGN laterally. K,L: Fibers can be seen in the PAG and deeper layer of the superior colliculus (SC). For abbreviations see list. Scale bars = 0.5 mm.
The second group of fibers innervating targets anterior to the VMH travels along the base of the brain (Fig. 6A–D), follows the midline circumventing the RCh, and stays ventral to the SCN. At the posterior part of the SCN, some fibers travel medially along the ventricular wall to reach the paraventricular hypothalamic nucleus (PVH; Fig. 6A,B). Fibers in this tract show intense GFP labeling and are thus presumably tightly packed. At the level of the SCN, this tract begins to move laterally along the base of the brain, gradually turning caudally while continuing to ascend dorsally to form the brightly labeled vSOC tract (Fig. 6A–D). Throughout the anterior VMH, there are fibers that emerge from the ventral–lateral aspect of the nucleus to join directly with the vSOC (Fig. 6C,D).
Three major pathways constitute VMH projections to caudal regions of the brain. As with the ascending tracts, the intensity of these descending tracts appears brighter in Z/EGSf1:Cre mice. The first pathway begins by following the vSOC anterior along the base of the brain and then curves on a rostral–ventral path before arcing back in a caudal–ventral path (Fig. 6C,D). The C-shaped course is similar to that of efferent fibers that innervate the hypothalamus, such as the fornix and the stria medullaris. This tightly bundled tract disperses at the lateral aspect of the medial lemniscus, sending parallel branches through the thalamus along the EML (Fig. 6C,D). Many of these vSOC fibers traverse through the posterior thalamic nucleus, lateral posterior thalamic nucleus, and lateral dorsal nucleus and terminate in the habenular nucleus (Fig. 6C,D). More caudally, vSOC fibers course through the medial geniculate body (Fig. 6E–H).
The second descending pathway travels along the zona incerta (Fig. 6E–H). Many of these fibers emerge from the dorsal–lateral aspect of the nucleus and travel parallel to the vSOC at the lateral aspect of the medial lemniscus. Small numbers of fibers located more ventrally arc back across the internal capsule to join together with branches from the vSOC (Fig. 6E–H).
The third descending tract innervates midline structures in the posterior diencephalon and the midbrain (Fig. 6E–H). This tract originates from the posterior aspect of the VMH and follows the periventricular system extending along the midline of the brain to traverse through the dorsomedial nucleus and posterior hypothalamus (Fig. 6I,J) to reach the PAG region. Indeed, the entire PAG is decorated with intense GFP+ fibers (Fig. 6I–L).
At this late embryonic stage, the vSOC remains the major fiber tract in both mouse models, whereas other ascending and descending SF-1 tracts begin to mature and are generally brighter in Z/EGSf1:Cre mice. Furthermore, the aforementioned absence of GFP labeling in the Sf-1TauGFP VMHvl region becomes more evident at E17.5 (Fig. 6E–J), especially in the region lateral to the fornix. In stark contrast, GFP+ cells in the Z/EGSf1:Cre VMHvl extend beyond the VMH proper to the lateral border of the hypothalamus (Fig. 6E–J).
Neonatal ascending and descending SF-1 tracts in Sf-1TauGFP mouse at P0
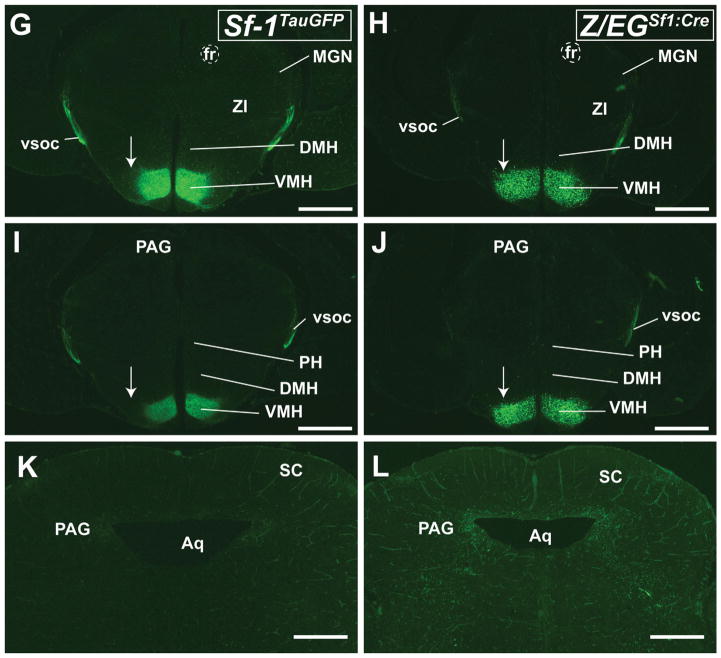
We next examined ascending and descending fibers in neonatal mice at P0. At this stage, Sf-1TauGFP mice show projection patterns similar to those of Z/EGSf1:Cre mice (Fig. 7A–D), except for the sparse GFP+ cells observed in the midbrain of Z/EGSf1:Cre mice (Fig. 7E,F). In addition, and similar to earlier developmental stages, GFP+ neurons are undetectable in the Sf-1TauGFP VMHvl region (Fig. 7G,H). To show that this absence truly reflects a loss of SF-1 in the VMHvl region, double labeling was carried out in both the Sf-1TauGFP and the Z/EGSf1:Cre lines. As expected, GFP+ cells in the Sf-1TauGFP line colabeled with SF-1-positive neurons, with a notable absence of both signals in the VMHvl region (Fig. 7I,K,L). In contrast, GFP+ cells that no longer express SF-1 continue to be present in the VMHvl region (Fig. 7J,M,N). Taken together, these data suggest that neurons in this VMH subregion adopt a different cell fate from the rest of the VMH.
Figure 7.
Comparisons between the Sf-1TauGFP mouse (A,C,E,G,I,K,L) and the Z/EGSf1:Cre mouse (B,D,F,H,J,M,N) at P0. A,B: Comparable projection patterns are seen in the vsoc and fibers traveling in the periventricular system. Cells are noticeably absent in the VMHvl of the Sf-1TauGFP mouse compared with the corresponding region in the Z/EGSf1:Cre mouse (arrow). C,D: Similar fiber projections are seen in the vsoc along the internal capsule (ic), across the medial geniculate nucleus (MGN), and into the PAG. E,F: Fiber projections are seen in the PAG. A solitary GFP+ cell can be seen in the tegmental area of the midbrain of the Z/EGSf1:Cre (arrow in F). G,H: Absence of GFP+ cells in the VMHvl of the Sf-1TauGFP mouse (G). Arrows indicate the presence of GFP+ cells in the presumptive tuberal region of both mouse models. I,J: Colabeling of cytoplasmic GFP+ staining (green) and nuclear SF-1+ staining (red) in the VMH. Absence of SF-1+ staining is noted in the VMHvl of both mouse lines. Boxed areas are shown as expanded views in K–N. K,L: Expanded views of the VMH in Sf-1TauGFP mice from Figure 7I showing colabeling (arrowheads) in the compact region (K) and the absence of any labeling in the VMHvl (L). M,N: Expanded views of the VMH in the Z/EGSf1:Cre mouse from Figure 7J showing colabeling (arrowheads) in the compact region (M) with only GFP+ labeling (arrows) in the VMHvl (N). For abbreviations see list. Scale bars = 0.5 mm.
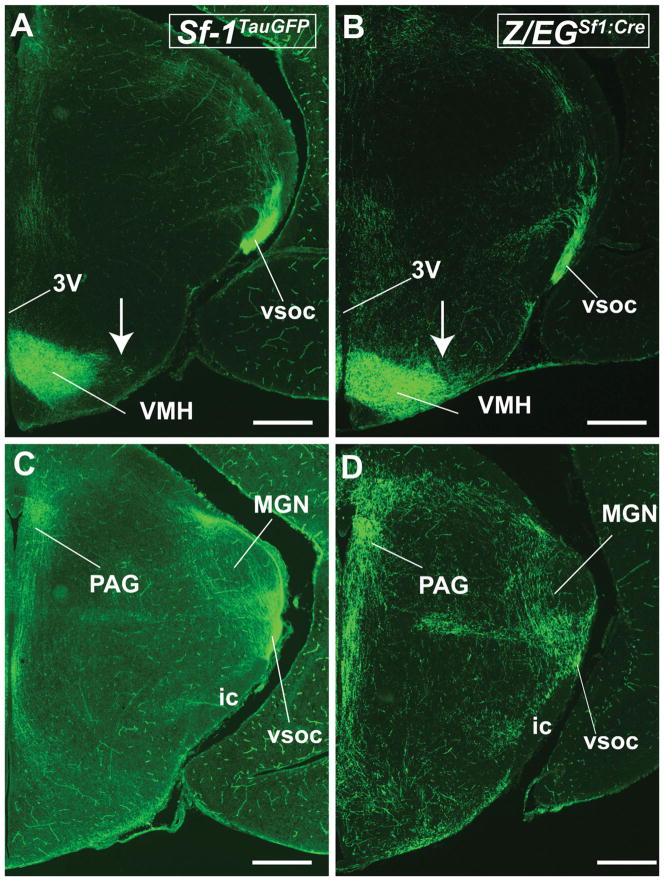
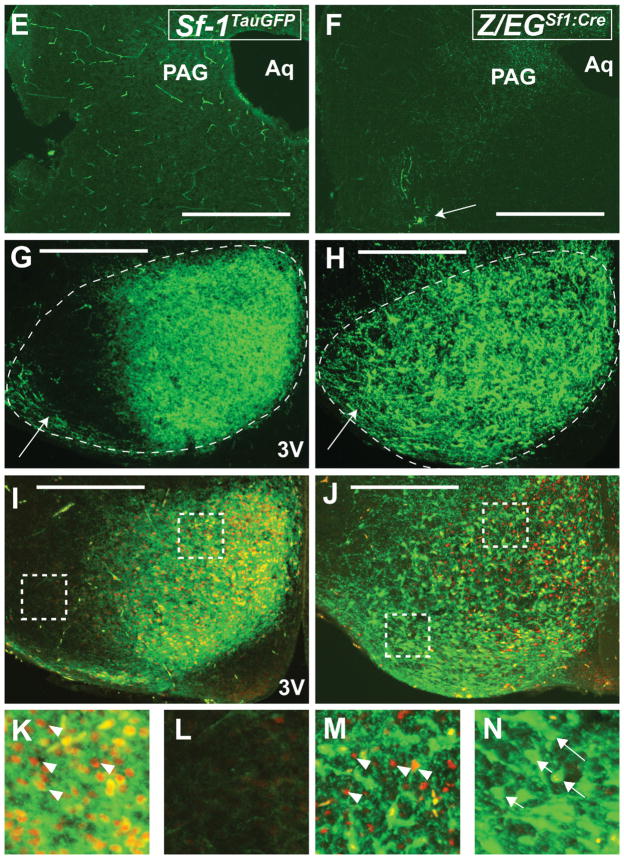
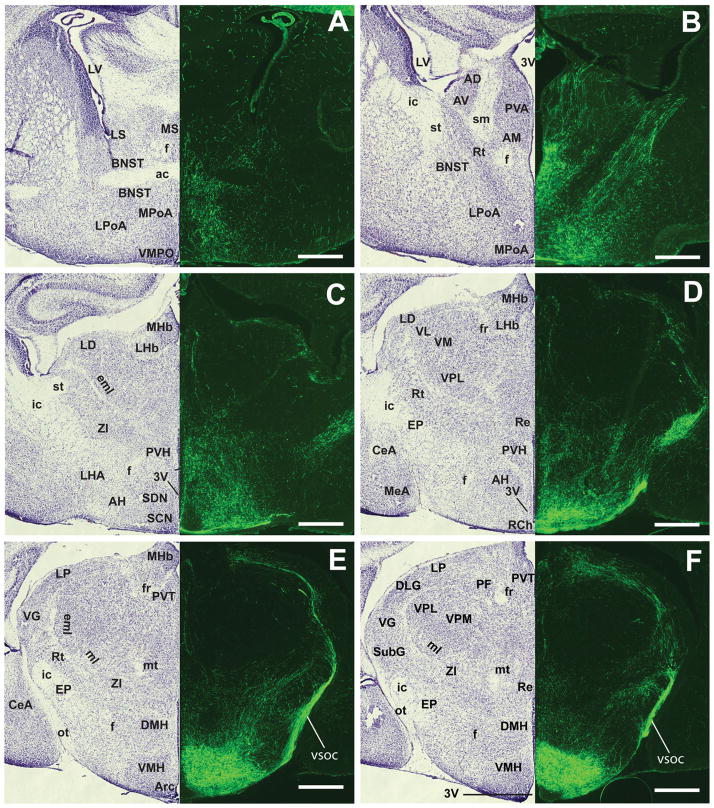
To describe projection patterns at a later stage, we used the Z/EGSf1:Cre line, which exhibited well-defined and visible VMH projections (Fig. 8A–R). Two ascending tracts innervate the anterior limbic system and the medial basal forebrain. Ascending fibers project anteriorly through the RCh and begin the lateral branching of the vSOC by traveling dorsally to the optic tract. Heavy innervation of the entopenducular nucleus by the vSOC is observed (Fig. 8D). Furthermore, multiple branches emerge from the vSOC to innervate the medial division of the central amygdaloid nucleus (Fig. 8D,E,S–U) and, to a lesser extent, the anterodorsal part of the medial amygdaloid nucleus. These fibers travel caudally to innervate the remaining divisions of the central amygdaloid nucleus, including the lateral division and the capsular part. The posterodorsal medial amygdaloid nucleus also receives some fiber projections. Few fibers if any were observed in other regions of the amygdala (data not shown).
Figure 8.
Anterior to posterior coronal brain sections from the Z/EGSf1:Cre reporter mouse at P0. A–R: Projection patterns of GFP-labeled fibers (right) and the corresponding contralateral Nissl-stained sections (left). S–U: High-power photomicrographs showing the corresponding amygdaloid nuclei in the field of GFP-labeled fibers. For abbreviations see list. Scale bars = 0.675 mm in A–R; 0.25 mm in S–U.
The anterior hypothalamus and the medial basal forebrain received the strongest ascending innervations from the VMH, with fibers traveling through the periventricular zone, the medial zone of the hypothalamus, and some possibly through the medial forebrain bundle. Dense fibers are found in the anterior hypothalamic area, the PVH, and the perifornical region (Fig. 8C,D). In contrast, the lateral hypothalamus is devoid of fibers. As noted at earlier stages, fibers surround, but do not directly innervate, the SCN (Fig. 8C). Rostrally, both the medial and the lateral parts of the medial preoptic nucleus, as well as the MPoA, are covered with fibers. Few fibers are found in the anteroventral periventricular nucleus and the LPoA (Fig. 8B). Fibers in the medial hypothalamus ascend to innervate heavily the BNST, spanning laterally to medially (Fig. 8A,B). Other fibers travel medially to innervate the thalamus, densely in the anteromedial nucleus and moderately in the paraventricular thalamic nucleus and anteroventral and anterodorsal thalamic nuclei. At the level where the anterior commissures join, intense fiber tracts are observed in the septohypothalamic nucleus, the BNST, and the lateral septal nucleus (Fig. 8A).
Three descending fiber tracts observed at E17.5 persist at P0: the vSOC, the zona incerta, and the periventricular system. These fibers begin as distinct tracts but join together distal to the VMH. The periventricular system emanates from the posterior aspect of the VMH and innervates the dorsal premammillary nucleus (Fig. 8H) and, to a lesser extent, the ventral premammillary nucleus (Fig. 8G–I). These fibers travel through the mammillary nucleus while staying medial to the mammilotegmental tract and the fasciculus retroflexus. They course through the posterior hypothalamic nucleus (Fig. 8H) and the precommissural nucleus (Fig. 8H) to reach the PAG. Upon reaching the PAG, these fibers merge with fibers from the dorsal arm of the vSOC (Fig. 8F–I).
The descending vSOC tract consists of fibers that emerge from the anterior part of the VMH, including cells in the RCh (Fig. 8D). The fiber bundle moves dorsally and gives off lateral branches that contribute to the amygdaloid complex (see above). The vSOC consists of mostly efferent fibers that emerge from the anterior half of the VMH and travel dorsally to the optic tract as a tightly bundled tract before turning medially at the lateral aspect of the medial leminiscus (Fig. 8D–F). After the turn, fibers in the vSOC follow the path of the EML through the thalamus to reach the habenular nucleus anteriorly (Fig. 8C,D) and the PAG posteriorly.
The zone incerta tract is a group of loosely bundled fibers emerging from the dorsal and dorsolateral aspects of the VMH (Fig. 8E,F). This fiber tract courses through the general area of the zona incerta and merges with the vSOC at approximately the lateral aspect of the medial lemniscus, medial to the ventral geniculate nucleus. Fiber density is extremely high at the level of the merger, which encompasses the subgeniculate nucleus, the epipenducular nucleus, the peripenducular nucleus, and the lateral terminal nucleus of the accessory optic tract (Fig. 8F,G). These fibers then fan out, with the majority traveling around it and coursing through the subbrachial nucleus, the posterior intralaminar thalamic nucleus, and the posterior thalamic nucleus; a few enter the medial geniculate nucleus (Fig. 8H–J). Some fibers enter the deep layer of the superior colliculus, but most travel toward the PAG (Fig. 8G–J). Notably, some fibers that travel in the zona incerta pathway appear to course ventrally in parallel to the periventricular system and generally proceed toward the dorsal end of the vSOC or directly to the PAG.
Although these three fiber tracts travel in distinct courses, they initially send branches that merge with each other, forming an intercalating network. Joining vSOC that courses through the thalamus are branches that emerge from the periventricular system (Fig. 8D–H). These fibers travel laterally, just ventral to the fasciculus retroflexus, to reach its lateral target of the ventral geniculate nucleus. At that point, they merge with the fibers from the vSOC as well as those from the zona incerta pathway. As these fibers course transversely across the brain, they intersect almost perpendicularly with fibers that come from the zone incerta that travel parallel to the periventricular system (Fig. 8H).
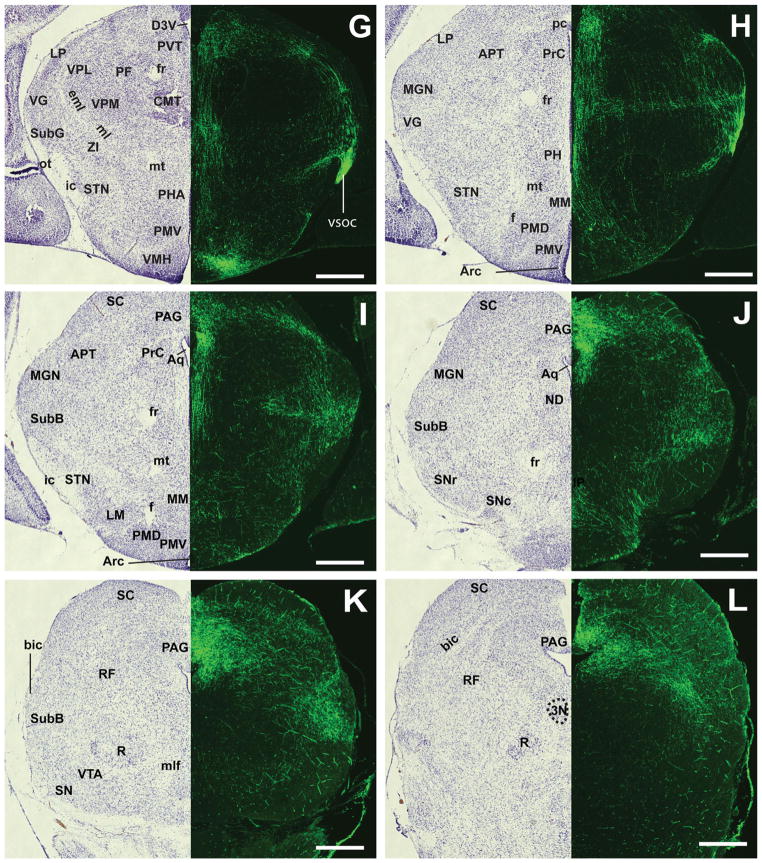
Fibers from the vSOC and the periventricular system meet at the PAG and travel along the aqueduct and innervate the rostrocaudal axis of the PAG (Fig. 8I–R). Dense fibers were also found in the deep layer of the superior colliculus (Fig. 8I–L) and the subbrachial nucleus (Fig. 8J–L). Moderate to dense fibers could also be seen in the mesencephalic reticular formation (Fig. 8K,L) and the ventral tegmental area (Fig. 8K) but not in the red nucleus or substantia nigra. Moving caudally, moderate fibers are seen throughout the tegmental nucleus, laterodorsal tegmental nucleus (Fig. 8M–O), and lateral parabrahchial nucleus (Fig. 8N,O). In the pons, the locus coeruleus receives fiber projections (Fig. 8P), but very few fibers are found in the pontine reticular area. The lateral tegmental nucleus and the dorsal raphe nucleus appear to be completely devoid of fibers. Taken together, the neonatal VMH projections at P0 extend to broad regions of the brain, including the anterior hypothalamus, the amygdala, discrete hypothalamic and thalamic nuclei, the rostrocaudal axis of the PAG, and sparse nuclei in the brainstem.
DISCUSSION
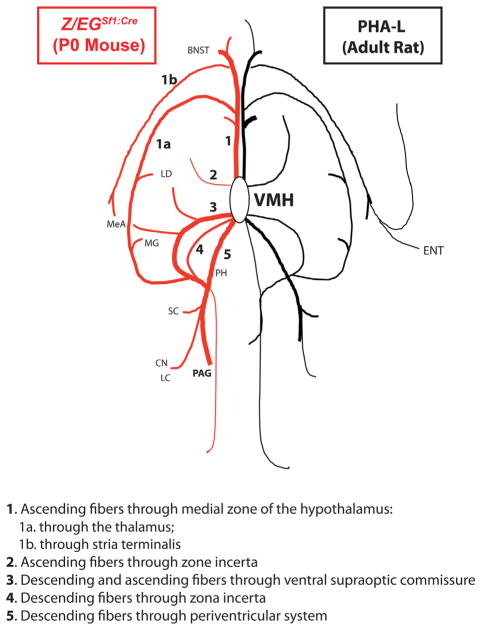
By using two distinct, but complementary, genetic tracing approaches, we have analyzed the development of VMH efferent projections, as marked by SF-1. The vSOC tract is the first of five major pathways to develop and can be observed prominently as early as E10.5, just 1 day after SF-1 is expressed in postmitotic neurons. Moreover, although VMH projections are conserved in mice and rats, as expected, we found that efferent projection patterns at early developmental stages are remarkably similar to adult patterns (Fig. 9). Although it has been generally assumed that SF-1 marks the entire VMH because of its early and broad expression, the striking differences observed in GFP+ labeling between the Sf-1TauGFP and the Z/EGSf1:Cre lines imply that SF-1 is transiently expressed at early embryonic stages and then silenced in neurons that populate the VMHvl. Thus, with the one exception of the VMHvl, the Sf1:Cre driver faithfully recapitulates SF-1 expression in the brain. These findings also suggest the female-specific traits classically associated with the VMHvl are mediated by distinct SF-1-negative neurons.
Figure 9.
Simplified graphic representation of the fiber projection patterns in the Z/EGSf1:Cre reporter mouse at P0 and the PHA-L-injected rat at the adult stage, modified from Canteras et al. (1994). For abbreviations see list. ENT, entorhinal area.
Developmental profiles of SF-1 neuronal projections
VMH neurons are born in the ventricular zone between E10 and E15 (Shimada and Nakamura, 1973; Tobet et al., 1999) and migrate radially to their final destination. SF-1 neurons share a similar developmental pattern in that they are born between E9.5 and E15.5 in mice, peaking at E13.5 (Ikeda et al., 1995, 2001; Tran et al., 2003). Formation of the VMH nuclei occurs later, at E15–E17 (Tobet et al., 1999). Both mouse models used in our study show SF-1 neurons in the presumptive VMH area by E10.5. However, these neurons begin to coalesce into the conventional oval nucleus only later in development at E14.5. Regardless of whether SF-1 neurons are marked by the endogenous SF-1 promoter or by Cre recombination, the overall projection patterns and final target sites appear invariable. The most prominent embryonic fiber tract is the presumptive vSOC, which travels through the dorsal thalamus and targets the PAG. Clearly, further work is needed to assess the functional significance, of these early embryonic VMH projections.
Comparison of the timing and expression patterns between the Sf-1TauGFP and the Z/EGSf1:Cre mice establish that anatomical divisions within the VMH begin early in development. One of the most striking differences noted between these two reporter mice was the presence or absence of GFP labeling in the VMHvl (Table 2). Indeed, although SF-1 staining has been used to define the VMH in rodents (Dellovade et al., 2000; Shinoda et al., 1995), our collective results show that the lateral boundary of the VMH, as defined by the Z/EGSf1:Cre line, extends well beyond SF-1 staining and the Nissl-stained VMH cluster. These data imply that, although the VMHvl shares a common SF-1 lineage with other VMH subregions, developmental signals must begin to silence SF-1 expression in the VMHvl prior to E14.5. That VMHvl has a unique molecular signature fits well with the specialized function of the VMHvl in female reproduction and energy expenditure. Indeed, expression of steroid nuclear receptors, ERα and progesterone receptors (Hagihara et al., 1992; Simerly et al., 1990), and the homeobox transcription factor Nkx2-1 is highly restricted to the VMHvl (Davis et al., 2004; Tran et al., 2003). Our findings also underscore the need for careful design when using an Sf1:Cre strategy to ablate genes in the VMH.
TABLE 2.
VMH Developmental Profiles
| Tract/region | Reporter line | E10.5 | E12.5 | E14.5 | E17.5 | P0 |
|---|---|---|---|---|---|---|
| MFB | Sf-1TauGFP | + | ++ | +++ | ++ | ++ |
| Z/EGSf1::Cre | − | + | + | ++ | ++ | |
| vSOC | Sf-1TauGFP | +/− | + | ++ | +++ | +++ |
| Z/EGSf1::Cre | − | +/− | + | ++ | +++ | |
| GFP+ neurons | Sf-1TauGFP | + | ++ | ++ | +++ | +++ |
| Z/EGSf1::Cre | +/− | + | ++ | +++ | +++ | |
| GFP+ in the VMHvl | Sf-1TauGFP | ND | ND | +/− | − | − |
| Z/EGSf1::Cre | ND | ND | + | + | + |
Timing of expression patterns was also different between Sf-1TauGFP and Z/EGSf1:Cre mice (Table 2). Both models show distinct GFP+ cells in the ME and the presumptive VMH between E10.5 and E12.5, but the numbers are generally higher in the Sf-1TauGFP model. Fiber tracts are poorly developed in Z/EGSf1:Cre mice at E10.5 but prominent in Sf-1TauGFP mice. By E14.5, Z/EGSf1:Cre mice “catch up” and exhibit identical fiber tracts with equivalent intensities as observed in Sf-1TauGFP mice. This lag in GFP expression is consistent with the well documented delay in Cre-recombinase activity observed in many Cre lines (Nagy, 2000). By contrast, the fidelity of GFP expression is maintained in the knock-in Sf-1TauGFP reporter and is easily observed at E10.5. At older stages, the situation is reversed, with the Z/EGSf1:Cre showing a much more intense pattern of GFP labeling than that observed in the Sf-1TauGFP knock-in reporter line. We attribute this weakening of postnatal GFP labeling to declining Sf-1 transcripts, as observed in neonatal mice (Kurrasch et al., 2007). This factor coupled with the positioning of the TauGFP gene downstream of the second IRES would exacerbate this postnatal drop in Sf-1 transcripts. Notably, other than the VMHvl, only a few GFP+ neurons were detected in extra-VMH sites in Z/EGSf1:Cre brain regions, specifically the brainstem. Thus, unlike the case in other Cre lines, including the hypothalamic Pomc:Cre line (Morrison and Munzberg, 2012; Padilla et al., 2010, 2012), appropriate regulatory elements of SF-1 transcription are present in the Sf1:Cre transgene, confirming the anatomical reliability of this line (Dhillon et al., 2006).
VMH ascending and descending projections
Efferent VMH projections described here in neonatal mice show remarkable similarity to those described previously for adult rats using stereotaxic injections (Canteras et al., 1994; Krieger et al., 1979; Saper et al., 1976). Efferent projections of SF-1 neurons can be divided into either 1) ascending tracts that innervate structures anterior to the nucleus in the hypothalamus, the basal forebrain, and the telencephalon or 2) descending tracts that innervate structures dorsal and posterior to the nucleus in the caudal hypothalamus, the thalamus, and the brainstem. Both the Sf-1TauGFP and the Z/EGSf1:Cre mice exhibit SF-1 efferent fibers that project to perifornical area and the PVH. Many of these fibers appear to originate from GFP+ cells in the RCh area as well as the very anterior aspect of the VMH. Although fibers from the medial zone of the hypothalamus projecting to the PVH, as proposed in an adult rat study (Canteras et al., 1994), are easily observed here by genetic tracing, nongenetic tracing methods have generally missed these tracts because of their near-midline location (Krieger et al., 1979; Saper et al., 1976).
In both Sf-1TauGFP and Z/EGSf1:Cre mice, the anterior hypothalamic area receives abundant projections from GFP+ neurons via the medial hypothalamic zone and the medial forebrain bundle, consistent with known VMH inputs into those areas (Canteras et al., 1994; Conrad and Pfaff, 1976; Krieger et al., 1979; Risold et al., 1994; Saper et al., 1976). Many GFP-labeled sites within the anterior hypothalamic area and forebrain are involved in the regulation of reproductive behavior and are known to be sexually dimorphic. Consistent with studies in rats (Canteras et al., 1994), the MPoA and the medial preoptic nucleus were both found to receive the majority of these innervations, whereas the LPoA was devoid of any GFP label. Most of these ascending fibers likely originate from the VMHvl (Canteras et al., 1994), supporting the prominent role of this VMH subregion in female reproductive physiology (Hagihara et al., 1992; Simerly et al., 1990). The BNST is another sexually dimorphic brain region that is innervated by the VMH. Indeed, our data show that fiber tracts course dorsally to terminate in a broad region within the BNST, which matches the pattern in adult mouse (Tong et al., 2007) as well as in the rat (Canteras et al., 1994; Krieger et al., 1979; Saper et al., 1976).
Several structures of the limbic system receive significant GFP+ innervation. The sexually dimorphic septal nuclei receive differential innervation with the lateral septal nucleus, receiving significantly more GFP+ fibers than the medial septal nucleus. Although the degrees of innervation to the lateral septal nucleus and to the BNST are comparable in mice, the lateral septal nuclei was found to receive significantly less innervation than the BNST in rats (Canteras et al., 1994). Another major limbic structure that receives VMH innervation is the amygdala. GFP+ fibers reach the amygdala in the mouse almost exclusively through the vSOC tract, specifically from branches of the vSOC that are traveling anterodorsally before arching back to course caudally toward the PAG. In contrast, VMH projections to the amygdala in the rat consist of ansa peduncularis fibers found in the vSOC and fibers that travel through the substantia innominata (Canteras et al., 1994). In addition, although many different regions in the amygdala receive significant input from the VMH in adult rats, our studies in mice show that the central amygdaloid nucleus receives the majority of the input.
Descending VMH efferents in the mouse share many similar targets with those in the rat, although mild differences are noted as well. Some periventricular fibers initially travel sparsely through other hypothalamic regions, but we were unable to detect prominent projections to the premammillary nucleus as previously reported (Canteras et al., 1994). Perhaps in the rat these fibers originate from the anterior hypothalamic nucleus, which is in close proximity to the anterior VMH (Risold et al., 1994). Both rats and mice harbor projections to the posterior hypothalamic nucleus with fibers that originate from the dorsal aspect of the VMH and travel through the zona incerta to the vSOC (Canteras et al., 1994). We also found that, as reported by others for the rat, the PAG receives the largest amount of fibers from the VMH, primarily through the midbrain periventricular system and vSOC (Canteras et al., 1994; Krieger et al., 1979; Saper et al., 1976). Furthermore, we confirm that brainstem structures such as the deep layer of the superior colliculus, the mesencehpalic reticular formation, the subbrachial nucleus, and the locus coeruleus all appear to receive VMH innervations in both the rat and the mouse (Canteras et al., 1994; Krieger et al., 1979).
Functional implications of SF-1 projections in VMH physiology in the mouse
Having mapped embryonic and postnatal VMH projections in mice, we can now ask how these different fiber tracts might be related to the known adult physiological functions of the VMH, including metabolism, defensive behavior, and female reproduction. From our data, we suggest that SF-1 neurons targeting the general vicinity of gonadotropin-releasing hormone (GnRH) neurons, including the medial POA and the diagonal band of Broca, have the potential to modulate expression and/or secretion of GnRH. Abnormal regulation of these neurons might underlie the abnormal estrous cycle and infertility observed in Sf-1Nestin:Cre female mice (Ikeda et al., 1995; Kim et al., 2010). In addition, intense projections to the PAG are also predicted to impact female reproductive behavior. In this regard, a recent study in female rats shows that the VMHvl is important for maternal care (Cameron et al., 2011) and that siRNA interference of ERa in the VMHvl results in increased aggression against juvenile rats (Musatov et al., 2006).
Significant inputs from the VMH to the septal nucleus, anterior hypothalamic nucleus, dorsal premammillary nucleus, and extrahypothalamic regions such as PAG are also predicted to mediate anxiety and defensive behaviors that have been linked to the VMH (Canteras, 2002). Indeed, Sf-1Nestin:Cre mice exhibit increased anxiety (Kim et al., 2009).
The VMH also plays an important role in metabolism (King, 2006; Lopez et al., 2010; Yi et al., 2011) that is likely mediated by SF-1 neurons. Although the Sf-1Nestin:Cre mice fail to exhibit an obvious metabolic phenotype, several mouse models show altered metabolism when components of the leptin signaling pathway are ablated in SF-1-expressing cells (Bingham et al., 2008; Dhillon et al., 2006; Xu et al., 2010; Zhang et al., 2008). With the assumption that these metabolic defects arise from central loss of SF-1 rather than peripheral ablation of these genes in gonads, adrenals, or the anterior pituitary, these collective metabolic phenotypes underscore the importance of SF-1 neurons in energy homeostasis. Consistent with these studies, SF-1 neurons project to areas implicated in body weight regulation, including the PVH. In contrast to an earlier study indicating that VMH neurons make synaptic contacts with ARC neurons (Sternson et al., 2005), we were unable to detect any GFP+ fibers within the ARC. It remains possible that synaptic connections between the VMH and the ARC occur on the shell of the VMH (Fu and van den Pol, 2008; Millhouse, 1973) or that SF-1-negative neurons innervate the ARC. The VMH also affects glucose homeostasis via direct or indirect innervation to the autonomic nervous system. A conditional knockout of vesicular glutamate transporter 2 (Vglut2Sf1:Cre) exhibited a blunted response to hypoglycemia, with a diminished release in glucagon. The dorsal motor nucleus of the vagus (DMV) is proposed to mediate this VMH function. (Tong et al., 2007). Although it has been suggested that DMV may receive neuronal projections from the VMH (Lopez et al., 2010), our study and other studies failed to detect any direct SF-1 or VMH projections to the DMV (Canteras et al., 1994; Tong et al., 2007).
Future molecular and genetic dissection of the VMH will surely provide insight into how this complex hypothalamic nucleus integrates emotional responses with metabolism and reproduction. We predict, based on the extensive VMH projections previously defined in rats, and now here in mice, that the VMH will influence other physiological functions. For instance, the strong VMH projections to areas surrounding the SCN could very well account for the diurnal rhythm of corticosterone release previously attributed to the VMH (Choi et al., 1998). In addition, efferent projections to the subthalamic locomotor region suggest that the VMH is involved in locomotor activity (Narita et al., 1998; Yokawa et al., 1989). Finally, the intense VMH projections to the central and medial amygdaloid nuclei are likely to mediate emotional processing.
In summary, our genetic tracing studies show that VMH efferent projections are established early in the embryo and are conserved in rodents. We also find that the VMHvl develops into a discrete region that extends well beyond the classical morphological boundary of the VMH. Thus, our results challenge the traditional anatomical definition of the VMH and underscore the regional complexity of a hypothalamic nucleus.
Acknowledgments
Grant sponsor: National Institute of Diabetes and Digestive and Kidney Diseases; Grant number: R01DK063592 (to H.A.I.); Grant sponsor: Lawson Wilkins Pediatric Endocrine Society (to C.C.C.); Grant sponsor: National Institute of Diabetes and Digestive and Kidney Diseases; Grant number: 1K08DK076721 (to C.C.C.); Grant sponsor: Giannini Postdoctoral Fellowship (to D.M.K.).
We thank Drs. A. Basbaum, N. Shah, and M. Capecchi for reagents used in building the construct for the Sf-1TauGFP mouse line. We especially thank Drs. J.L.R. Rubenstein, G. Lee, S. Correa, and E. Faivre for guidance on and discussion of this article. We also thank D. Newstrom for technical assistance. Data were acquired from the Nikon Imaging Center at UCSF/QB3.
Abbreviations
- 12N
hypoglossal nucleus
- 3N
oculomotor nucleus
- 3V
third ventricle
- 4N
trochlear nucleus
- 4V
fourth ventricle
- 5N
motor trigeminal nucleus
- ac
anterior commissure
- AD
anterodorsal nucleus of the thalamus
- AH
anterior hypothalamic area
- AM
anteromedial nucleus of the thalamus
- APT
anterior pretectal nucleus
- Aq
aqueduct
- Arc
arcuate nucleus
- AV
anteroventral nucleus of the thalamus
- bic
brachium of the inferior colliculus
- BM
basomedial amygdaloid nucleus
- BNST
bed nucleus of stria terminali
- CeA
central amygdaloid nucleus
- CG
central gray
- CN
cuneiform nucleus
- DLG
dorsal lateral geniculate nucleus
- DMH
dorsomedial nucleus of the hypothalamus
- DR
dorsal raphe nucleus
- eml
external medullary lamina
- EP
entopenduncular nucleus
- f
fornix
- fr
fasicuculus retroflexus
- ic
internal capsule
- IC
inferior colliculus
- IP
interpeduncular nucleus
- LC
locus coeruleus
- LD
lateral dorsal nucleus of the thalamus
- LDTg
lateral dorsal tegmental nucleus
- LHA
lateral hypothalamic area
- LHb
lateral habenular nucleus
- LM
lateral mammillary nucleus
- LP
lateral posterior nucleus of the thalamus
- LPB
lateral parabrachial nucleus
- LPoA
lateral preoptic area
- LS
lateral septal nucleus
- MeA
medial amygdaloid nucleus
- Me5N
mesencephalic trigeminal nucleus
- MG
medial geniculate nucleus
- MHb
medial habenular nucleus
- ml
medial lemniscus
- mlf
medial longitudinal fasciculus
- MM
medial mammillary nucleus
- MPoA
medial preoptic area
- MS
medial septal nucleus
- mt
mammillothalamic tract
- MVeN
medial vestibular nucleus
- ND
nucleus of Darkschewitsch
- ot
optic tract
- PAG
periaqueductal gray
- pc
posterior commissure
- PF
parafasicular nucleus (F)
- PH
posterior hypothalamic nucleus
- PMD
premammillary nucleus, dorsal part
- PMnR
paramedian raphe nucleus
- PMV
permammillary nucleus, ventral part
- PN
pontine nucleus
- PrC
precommissural nucleus
- PVA
paraventricular nucleus of the thalamus, anterior part
- PVH
paraventricular nucleus of the hypothalamus
- PVT
paraventricular nucleus of the thalamus
- R
red nucleus
- Re
nucleus reuniens
- RF
reticular formation, midbrain
- Rt
reticular nucleus of the thalamius
- SC
superior colliculus
- SCN
suprachiasmatic nucleus
- sm
stria medullaris
- SN
substantia nigra
- SNr
substantia nigra, reticular part
- Sol
neuclus of the solitary tract
- st
stria terminalis
- STN
subthalamic nucleus
- SubB
subbrachial nucleus
- SubG
subgeniculate nucleus
- TgN
tegmental nucleus
- VG
ventral geniculate nucleus
- VL
ventrolateral nucleus of the thalamus
- VM
ventromedial nucleus of the thalamus
- VMH
ventromedial nucleus of the hypothalamus
- VMPO
ventromedial preoptic nucleus
- VPL
ventral posterolateral nucleus
- VPM
ventral posteromedial nucleus
- vsoc
ventral supraoptic commissure
- VTA
ventral tegmental area
- ZI
zona incerta
Footnotes
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis. Study concept and design: CC, DK, and HI. Acquisition of data: CC, DK, and JL. Analysis and interpretation of data: CC, DK, and HI. Drafting of manuscript: CC and HI. Critical revision of the manuscript for important intellectual content: CC, DK, and HI.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest and nothing to disclose.
LITERATURE CITED
- Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventro-medial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149:2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Rico B, Basbaum AI. Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:15148–15153. doi: 10.1073/pnas.222546999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budefeld T, Tobet SA, Majdic G. Altered position of cell bodies and fibers in the ventromedial region in SF-1 knockout mice. Exp Neurol. 2011;232:176–184. doi: 10.1016/j.expneurol.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR. Targeting genes for self-excision in the germ line. Genes Dev. 1999;13:1524–1528. doi: 10.1101/gad.13.12.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Soehngen E, Meaney MJ. Variation in maternal care influences ventromedial hypothalamus activation in the rat. J Neuroendocrinol. 2011;23:393–400. doi: 10.1111/j.1365-2826.2011.02124.x. [DOI] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- Choi S, Wong LS, Yamat C, Dallman MF. Hypothalamic ventromedial nuclei amplify circadian rhythms: do they contain a food-entrained endogenous oscillator? J Neurosci. 1998;18:3843–3852. doi: 10.1523/JNEUROSCI.18-10-03843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad LC, Pfaff DW. Efferents from medial basal forebrain and hypothalamus in the rat. II. An autoradiographic study of the anterior hypothalamus. J Comp Neurol. 1976;169:221–261. doi: 10.1002/cne.901690206. [DOI] [PubMed] [Google Scholar]
- Davis AM, Seney ML, Stallings NR, Zhao L, Parker KL, Tobet SA. Loss of steroidogenic factor 1 alters cellular topography in the mouse ventromedial nucleus of the hypothalamus. J Neurobiol. 2004;60:424–436. doi: 10.1002/neu.20030. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Young M, Ross EP, Henderson R, Caron K, Parker K, Tobet SA. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J Comp Neurol. 2000;423:579–589. doi: 10.1002/1096-9861(20000807)423:4<579::aid-cne4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Fu LY, van den Pol AN. Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. J Neurosci. 2008;28:5433–5449. doi: 10.1523/JNEUROSCI.0749-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara K, Hirata S, Osada T, Hirai M, Kato J. Distribution of cells containing progesterone receptor mRNA in the female rat di- and telencephalon: an in situ hybridization study. Brain Res Mol Brain Res. 1992;14:239–249. doi: 10.1016/0169-328x(92)90179-f. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995;9:478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Takeda Y, Shikayama T, Mukai T, Hisano S, Morohashi KI. Comparative localization of Dax-1 and Ad4BP/SF-1 during development of the hypothalamic-pituitary-gonadal axis suggests their closely related and distinct functions. Dev Dyn. 2001;220:363–376. doi: 10.1002/dvdy.1116. [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, Abbott LC. Chemoarchitectonic atlas of the developing mouse brain. Boca Raton, FL: CRC Press; 1998. [Google Scholar]
- Kim KW, Zhao L, Parker KL. Central nervous system-specific knockout of steroidogenic factor 1. Mol Cell Endocrinol. 2009;300:132–136. doi: 10.1016/j.mce.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Kim KW, Li S, Zhao H, Peng B, Tobet SA, Elmquist JK, Parker KL, Zhao L. CNS-specific ablation of steroidogenic factor 1 results in impaired female reproductive function. Mol Endocrinol. 2010;24:1240–1250. doi: 10.1210/me.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Zhao L, Donato J, Jr, Kohno D, Xu Y, Elias CF, Lee C, Parker KL, Elmquist JK. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci U S A. 2011;108:10673–10678. doi: 10.1073/pnas.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Klockener T, Hess S, Belgardt BF, Paeger L, Verhagen LA, Husch A, Sohn JW, Hampel B, Dhillon H, Zigman JM, Lowell BB, Williams KW, Elmquist JK, Horvath TL, Kloppenburg P, Bruning JC. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci. 2011;14:911–918. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behav Brain Res. 1998;92:169–180. doi: 10.1016/s0166-4328(97)00189-7. [DOI] [PubMed] [Google Scholar]
- Krieger MS, Conrad LC, Pfaff DW. An autoradiographic study of the efferent connections of the ventromedial nucleus of the hypothalamus. J Comp Neurol. 1979;183:785–815. doi: 10.1002/cne.901830408. [DOI] [PubMed] [Google Scholar]
- Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J Neurosci. 2007;27:13624–13634. doi: 10.1523/JNEUROSCI.2858-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M, Varela L, Vazquez MJ, Rodriguez-Cuenca S, Gonzalez CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, Martinez de Morentin PB, Tovar S, Nogueiras R, Carling D, Lelliott C, Gallego R, Oresic M, Chatterjee K, Saha AK, Rahmouni K, Dieguez C, Vidal-Puig A. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan KM, Parker KL, Tobet S. Development of the ventromedial nucleus of the hypothalamus. Front Neuroendocrinol. 2006;27:193–209. doi: 10.1016/j.yfrne.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. The organization of the ventromedial hypothalamic nucleus. Brain Res. 1973;55:71–87. [PubMed] [Google Scholar]
- Morrison CD, Munzberg H. Capricious cre: the devil is in the details. Endocrinology. 2012;153:1005–1007. doi: 10.1210/en.2011-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci U S A. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- Narita K, Murata T, Honda K, Nishihara M, Higuchi T, Takahashi M. Efferent pathways involved in the running activity originate in the ventromedial hypothalamus of the rat. Ann N Y Acad Sci. 1998;860:556–559. doi: 10.1111/j.1749-6632.1998.tb09103.x. [DOI] [PubMed] [Google Scholar]
- Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, Reef D, Zeltser LM. Defining POMC neurons using transgenic reagents: impact of transient pomc expression in diverse immature neuronal populations. Endocrinology. 2012;153:1219–1231. doi: 10.1210/en.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. Atlas of the developing mouse brain at E17.5, P0 and P6. Amsterdam: Elsevier; 2007. [Google Scholar]
- Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michan S, Vianna CR, Sinclair DA, Elias CF, Coppari R. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 2011;14:301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Canteras NS, Swanson LW. Organization of projections from the anterior hypothalamic nucleus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:1–40. doi: 10.1002/cne.903480102. [DOI] [PubMed] [Google Scholar]
- Saper CB, Swanson LW, Cowan WM. The efferent connections of the ventromedial nucleus of the hypothalamus of the rat. J Comp Neurol. 1976;169:409–442. doi: 10.1002/cne.901690403. [DOI] [PubMed] [Google Scholar]
- Schambra UB. Prenatal mouse brain atlas. New York: Springer; 2008. [Google Scholar]
- Shimada M, Nakamura T. Time of neuron origin in mouse hypothalamic nuclei. Exp Neurol. 1973;41:163–173. doi: 10.1016/0014-4886(73)90187-8. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, Sasaki H, Osawa Y, Ninomiya Y, Niwa O, et al. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn. 1995;204:22–29. doi: 10.1002/aja.1002040104. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH→arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Henderson RG, Whiting PJ, Sieghart W. Special relationship of gamma-aminobutyric acid to the ventromedial nucleus of the hypothalamus during embryonic development. J Comp Neurol. 1999;405:88–98. doi: 10.1002/(sici)1096-9861(19990301)405:1<88::aid-cne7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Lee MB, Marin O, Xu B, Jones KR, Reichardt LF, Rubenstein JR, Ingraham HA. Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Mol Cell Neurosci. 2003;22:441–453. doi: 10.1016/S1044-7431(03)00027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Akana SF, Malkovska I, Dallman MF, Parada LF, Ingraham HA. Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J Comp Neurol. 2006;498:637–648. doi: 10.1002/cne.21070. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hill JW, Fukuda M, Gautron L, Sohn JW, Kim KW, Lee CE, Choi MJ, Lauzon DA, Dhillon H, Lowell BB, Zigman JM, Zhao JJ, Elmquist JK. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab. 2010;12:88–95. doi: 10.1016/j.cmet.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, Scherer T, Tschop MH. Cajal revisited: does the VMH make us fat? Nat Neurosci. 2011;14:806–808. doi: 10.1038/nn.2867. [DOI] [PubMed] [Google Scholar]
- Yokawa T, Mitsushima D, Itoh C, Konishi H, Shiota K, Takahashi M. The ventromedial nucleus of the hypothalamus outputs long-lasting running in rats. Physiol Behav. 1989;46:713–717. doi: 10.1016/0031-9384(89)90356-9. [DOI] [PubMed] [Google Scholar]
- Zhang R, Dhillon H, Yin H, Yoshimura A, Lowell BB, Maratos-Flier E, Flier JS. Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology. 2008;149:5654–5661. doi: 10.1210/en.2008-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Kim KW, Ikeda Y, Anderson KK, Beck L, Chase S, Tobet SA, Parker KL. Central nervous system-specific knockout of steroidogenic factor 1 results in increased anxiety-like behavior. Mol Endocrinol. 2008;22:1403–1415. doi: 10.1210/me.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]