Abstract
Organisms experience dramatic fluctuations in demands/stresses over the course of the day. In order to maintain biological processes within physiologic boundaries, it is imperative that mechanisms have evolved for anticipation of, and adaptation to, these daily fluctuations. Endocrine factors undoubtedly play an integral role in homeostasis. Not only do circulating levels of various endocrine factors oscillate over the 24 period, but so too does responsiveness of target tissues to these signals/stimuli. Emerging evidence suggests that these daily oscillations do not occur solely in response to behavioral fluctuations associated with sleep/wake and feeding/fasting cycles, but are orchestrated in part by an intrinsic timekeeping mechanism known as the circadian clock. Disruption of circadian clocks, through genetic and/or environmental means, appears to precipitate numerous common disorders, including cardiometabolic diseases and cancer. Collectively, these observations, which are reviewed within the current article, have led to suggestion that strategies designed to realign normal circadian rhythmicities hold a therapeutic potential for the treatment of various endocrine-related disorders.
INTRODUCTION
Consideration of the temporal relationship between processes/events is critical for our understanding of the molecular-basis of physiology, as well as the pathogenesis of disease. It is imperative that biological processes occur in an appropriate order, thereby preventing concurrent activation of potentially incompatible mechanisms. Should this level of control become impaired, the outcome can be catastrophic, often resulting in pathology. Temporal regulation is observed at multiple time scales, ranging from seconds (e.g., ion homeostasis) to years (e.g., development). One time scale that has received increasing attention over the past several decades is the circadian (i.e., 24 hour) period. Rejuvenated enthusiasm in chronobiology has been fueled, in part, by identification of the molecular underpinnings of the mammalian circadian timekeeping mechanism, revealing that this mechanism orchestrates a plethora of critical biological functions, including those precipitating death.
Endocrine factors are undoubtedly at the heart of circadian biology. Not only is the generation, secretion, and abundance of various endocrine factors subjected to stringent and often predictable time-of-day-dependent control, but so too is the sensitivity of target organs to these signals. Emerging evidence strongly suggests that these rhythms are driven not only by behavior-associated factors, but also by an intrinsic timekeeping mechanism known as the circadian clock. The purpose of this article is to review the importance of circadian clock control of endocrinology. Following a general overview of known endocrine factor oscillation characteristics, we will discuss evidence regarding mediation of endocrine rhythms by circadian clocks, the pathologies associated with disruption of normal rhythmicity, and the tactics employed to restore orchestration of circadian processes.
CIRCADIAN RHYTHMS IN STIMULUS-RESPONSE COUPLING
Stimulus-response coupling overview
By definition, hormones and endocrine factors are substances produced in one organ or cell type that act at a site that is distinct from the origin. As such, these factors are critical in complex species, such as humans and other vertebrates, for inter-organ communication (e.g., between the central nervous system and peripheral tissues). Such communication is essential for maintenance of homeostasis and adaptation to environmental changes or stresses. A substantial body of evidence suggests that various components of the endocrine system exhibit time-of-day-dependent rhythms, facilitating anticipation of and/or adaptation to environmental/behavioral fluctuations. In principle, cellular hormone action is governed by: 1) the concentration of the hormone relative to its affinity for its specific receptor; 2) the abundance and/or availability of the receptor; and 3) the activity status of post-receptor signaling components, including mechanisms of desensitization/downregulation of hormone signaling. Time-of-day has been shown to exert a modulatory effect on all of these governing mechanisms. Here, we will provide a brief summary of what is known regarding 24-hour rhythms in the level of the stimulus (i.e., endocrine factors) and target tissue responsiveness.
Daily oscillations in endocrine factors
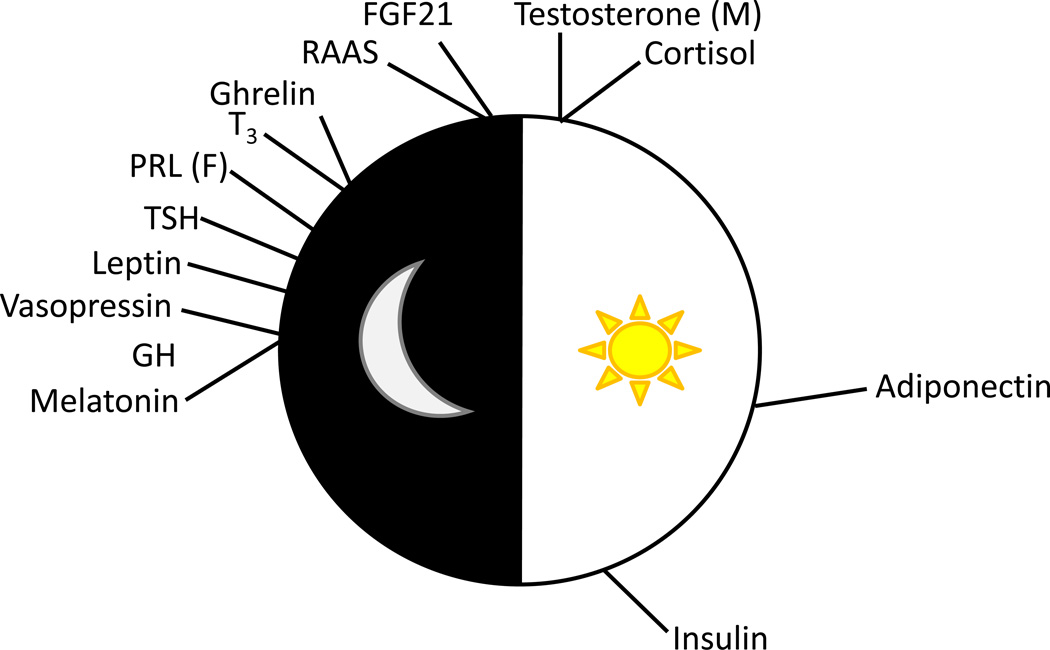
Among the best-studied examples of endocrine factors that fluctuate over the course of the day are those whose production is governed by hypothalamic-pituitary axes, such as cortisol, growth hormone (GH), prolactin (PRL), thyroid hormone, and gonadal steroids.1–5 Other factors, such as nutrient-sensitive hormones (such as insulin and adipokines) vary their circulating levels in part in response to the environment/behaviors, such as light/dark and feeding/fasting cycles, that typically occur in time-of-day-dependent patterns.6, 7 Figure 1 and Table 1 summarize what is known regarding time-of-day-dependent oscillations in these and other endocrine factors, while below we provide additional detail for several of these factors.
Figure 1.
Time-of-day at which circulating levels of key endocrine factors peak in humans. Abbreviations utilized include: GH, growth hormone; TSH, thyroid stimulating hormone; PRL, prolactin; T3, triiodothyronine; RAAS, renin-angiotensin-aldosterone system; FGF21, fibroblast growth factor 21; (F), females only; (M), males only.
Table 1.
Endocrine factors known to oscillate with a periodicity of 24 hours in humans.
| Hormone | Time-of-day- dependent Rhythm |
Time of Peak | Citation |
|---|---|---|---|
| Cortisol | Yes | 0700–0800h | Kalsbeek et al16; Carroll et al3 |
| Growth Hormone | Yes | Pulsatile secretion, with Increased amplitude at night | Avram et al1; Jaffe et al20; Villadolid et al21; Goji et al22 |
| Follicle-stimulating hormone (females) | No | NA | Klingman et al153 |
| Testosterone (males) | Yes | 0700h | Walton et al5 |
| Prolactin | Yes | 0200h (amplitude larger in females) | Freeman et al4 |
| Thyroid stimulating hormone | Yes | 0100–0200h | Russell et al2 |
| Triiodothyronine | Yes | 0230–0330h | Russell et al2 |
| Thyroxine | No | NA | Russell et al2 |
| Renin-angiotensin-aldosterone system | Yes | Early morning | White et al154 |
| Fibroblast growth factor 21 | Yes | 0500–0800h | Lee et al155; Yu et al156 |
| Ghrelin | Yes | 0200–0430h (fed state) 1300h (fasted state) | Koutkia et al157; Natalucci et al158 |
| Adiponectin | Yes | 1200–1400h | Scheer et al7; Gavrila et al23 |
| Leptin | Yes | 0100h | Gavrila et al23 |
| Vasopressin | Yes | Middle of night | Forsling et al159 |
| Insulin | Yes | 1700h | Goel et al6 |
| Melatonin | Yes | Middle of night | Van Cauter et al36 |
Melatonin
Circulating melatonin is produced in the pineal gland, under tight control by the central circadian pacemaker in the suprachiasmatic nucleus (SCN) region within the hypothalamus.8 A primary function of melatonin is to relay information regarding the environmental light-dark cycle, especially in response to changes in day length, triggering endocrine changes.9 Melatonin production is characteristically low in the presence of light and increases during the night, during which time it induces and supports sleep.10 It is important to note that additional functions of this hormone have been reported, including vasoconstrictor/vasodilator, anticonvulsant, and antioxidant properties.11 Interestingly, melatonin deficiency has been associated with increased incidence of colorectal and breast cancer, while polymorphisms in melatonin receptors have been associated with diabetes risk.10, 12 Melatonin supplementation has been used in the clinical setting, for the treatment of winter depression, numerous sleep disorders, and as an adjuvant therapy for epilepsy.13, 14
Cortisol and Hypothalamo-pituitary-adrenal axis
Release of cortisol from the adrenal cortex is under the regulation of the hypothalamo-pituitary-adrenal (HPA) axis.15 Corticotropin releasing hormone (CRH) is a component of this axis that is secreted by the hypothalamus and conveyed via the hypophyseal portal vasculature to the anterior pituitary gland. There, CRH triggers release of adrenocorticotropic hormone (ACTH) into the general circulation; upon binding to receptors in the adrenal gland, ACTH stimulates cortisol release. Cortisol levels exhibit a robust time-of-day-dependent rhythm in humans, normally peaking during the morning (0700–0800).16 In doing so, this catabolic hormone is believed to prepare the body for typical stresses associated with waking (e.g., energetic demand); the steady rise in cortisol levels during the sleep phase is consistent with its role in anticipation of wakefulness and increased activity.15, 16
Growth Hormone
Growth hormone (GH) is produced in the anterior pituitary and secreted in response to the integrated stimulatory and inhibitory effects of GH releasing hormone (GHRH) and somatostatin (SMS), respectively, both of which emanate from the hypothalamus.17, 18 GH has specific and powerful effects in metabolism, typically in opposition to insulin (e.g., decreasing glucose utilization, increasing lipolysis).19 Circulating GH levels exhibit both circadian and ultradian variation, as well as sexual dimorphism. In human females, GH is secreted in frequent non-discrete peaks of more uniform amplitude throughout the day with an increased mean secretion at night (during sleep). Males secrete GH in fewer more discrete pulses throughout the day, again with increased peak amplitude at night.1, 20 This dimorphism is also observed in children, with peaks in each case being greater than in adults.21, 22 Although the exact purpose of increased growth hormone availability at night is unknown, possibilities may include promotion of insulin resistance, mobilization of triglyceride, and/or protein synthesis/repair at this time.
Adiponectin
Adiponectin is one of several adipokines (i.e., adipose-derived endocrine factors) that exhibits a time-of-day-dependent rhythm in humans, peaking in the circulation between 1200 and 1400.7, 23 Adiponectin is best known as an insulin sensitizer, whose circulating levels vary inversely with body mass index (BMI).24 Interestingly, hypoadiponectinemia is associated with metabolic syndrome, but conversely, elevated levels are seen in chronic heart failure and chronic renal failure.24 The purpose of increased adiponectin secretion during the early afternoon is unclear, but this rhythm may provide anticipation of increased insulin levels later in the day (thereby promoting insulin action).
Insulin
Insulin is produced by beta cells of pancreatic islets, and is secreted in response to increased levels of circulating nutrients, particularly glucose.25 Its primary function is at the level of metabolism, stimulating glucose utilization and protein synthesis by peripheral tissues, such as liver, skeletal muscle, and fat.26 This anabolic hormone also inhibits fatty acid beta oxidation, and channels fatty acids into triglyceride.26 Insulin secretion displays a diurnal rhythm in humans, peaking at roughly 1700h (with a nadir at 0400h).6 Such a rhythm is consistent with nutrient storage during the awake/fed state, and subsequent mobilization during the sleep/fasted period.
Daily Oscillations in Sensitivity to Endocrine Factors
In contrast to information concerning circulating levels of hormones and endocrine factors, our understanding of the impact of time-of-day on end-organ hormonal sensitivity is less well developed. Standard techniques of evaluation make this variable more difficult to study, especially in humans. However, a few examples exist in which time-of-day-dependent rhythms in hormone sensitivity have been characterized, including responsiveness to ACTH and insulin. As mentioned in the previous sub-section, cortisol exhibits a robust diurnal variation in humans. However, this rhythm does not appear to be driven by fluctuations in ACTH levels; in contrast, the sensitivity of the adrenal cortex to ACTH exhibits a time-of-day-dependence, thereby mediating cortisol oscillations.15 Glucose homeostasis also exhibits interesting time-of-day-dependent changes, particularly in humans. The “dawn phenomenon”, for example, occurs in normal individuals and is exaggerated in type 1 and type 2 diabetes.27–31 This phenomenon is characterized by a spontaneous increase in plasma glucose and/or insulin requirement without nutrient intake prior to awakening (typically roughly 5–8 AM). This is believed to arise from increased hepatic glucose production that prompts (or requires) increased insulin levels to ensure glucose disposal and restrain hepatic glucose production. While the rise in hepatic glucose production corresponds temporally to rising cortisol, epinephrine, and norepinephrine (all counter-regulatory hormones), experiments in humans indicate that the dawn phenomenon is not accounted for by increases in these hormones, but rather to antecedent surges of GH earlier in the nocturnal period.27, 32
MEDIATORS OF CIRCADIAN RHYTHMS IN ENDOCRINE FACTORS
Role of intrinsic versus extrinsic influences
Daily rhythms in the abundance of many endocrine factors (as well as in the sensitivity of distinct tissues to these factors) are potentially mediated by a combination of influences. A classic view has often been that fluctuations in nutrients, hemodynamic stresses, body temperature, metabolism, sympathetic and autonomic tone, as well as various autocrine/paracrine factors are mediated by behavioral changes across the sleep/wake and feeding/fasting cycles.33–35 However, this ‘extrinsic’ concept has been challenged recently, as a wealth of evidence from both human and animal studies are consistent with a significant contribution of an intrinsic timekeeping mechanism towards these oscillations. For example, elegant studies by Van Cauter & colleagues were among the first to assess the contribution of sleep on oscillations a number of endocrine factors in humans, by enforcing a state of arousal during the night, followed by sleep during the subsequent day. These studies revealed that some (e.g., cortisol, TSH), but not all (e.g., growth hormone, prolactin), oscillations in endocrine factors occur independently of sleep/wake cycles.36–38 This raised the possibility that fluctuations in distinct endocrine factors over the course of the day may be driven, at least in part, by an endogenous mechanism (i.e., independent of behavioral rhythms). Similarly, Scheer & Shea have begun to dissociate the relative contribution of intrinsic versus extrinsic influences on biological processes in humans, through enforcement of multiple 20- or 28- hour contiguous days; these studies have successfully uncovered the contribution of the intrinsic circadian system on daily rhythms of multiple endocrine factors, including epinephrine and PAI-1.39–42
The endogenous circadian system in mammals is the circadian clock.43, 44 Circadian clocks are cell autonomous molecular mechanisms that enable a cell to perceive the time of day. In doing so, molecular clocks are able to alter biological processes over the course of the day, independent of extracellular stimuli/stresses. Circadian clocks are classically described as being transcriptional in nature, composed of a collection of transcriptional modulators that interact to form positive and negative regulatory feedback loops.43, 44 At the heart of the mechanism are two transcription factors, CLOCK (circadian locomotor output cycles kaput) and BMAL1 (brain and muscle ARNT-like 1), that, upon heterodimerization, bind to E-boxes within promoters of multiple target genes.45, 46 The translation products of a number of these target genes are themselves core clock components, which feedback upon CLOCK/BMAL1 activity, including the period (PER1/2/3) and cryptochrome (CRY1/2) proteins.47, 48 An additional well-established negative feedback loop involves REV-ERBα, which negatively influences BMAL1 at the transcriptional level.49 Those genes that are regulated by the circadian clock, yet do not directly influence the activity of core clock components, are termed clock controlled/output genes. It has been estimated that 7% to 13% of a cell’s transcriptome is under circadian control, including genes encoding for modulators of transcription, signal transduction, protein turnover, and metabolism; through fluctuations in clock controlled genes, the circadian clock influences cellular/organ function in a time-of-day-dependent manner.50–52 It should be noted that although the circadian clock is often described as a transcriptionally-based mechanism, a host of post-translational modifications (PTMs) are essential for its normal function, including phosphorylation, ubiquitination, sumoylation, acetylation, ADP-ribosylation, and O-GlcNAcylation.53–58 Many of these PTMs not only facilitate exquisite sensitivity of the clock mechanism to numerous extracellular factors (ensuring rapid entrainment to alterations in the environment), but also serve as an additional means by which the clock influences cellular processes. Interestingly, Reddy and colleagues have recently described a highly conserved circadian mechanism that is independent of transcription; redox biology is at the core of this ancestral clock mechanism, which can be monitored through oscillations in the oxidative state of peroxiredoxin.59
Influence of the SCN versus non-SCN clocks
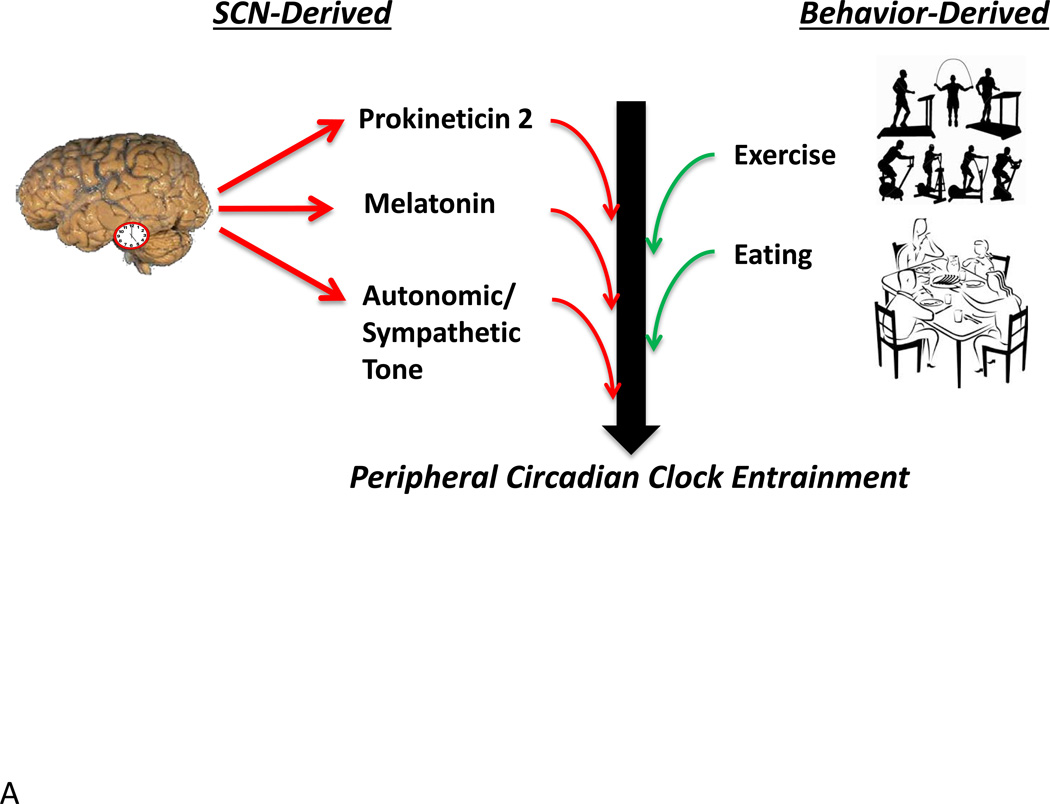
Circadian clocks can be broadly divided into two major classes: the central clock within the SCN and peripheral (or non-SCN) clocks.43 Through a classic series of lesion and transplant studies, the central circadian pacemaker (localized to the SCN of the hypothalamus) has been established as the orchestrator of the intrinsic circadian system in mammals. Specifically, lesions of the SCN result in severe arrhythmicity of multiple biological functions, including sleep/wake behavior, drinking/food intake, oxygen consumption, and stool output, as well as adrenal, pineal, and pituitary hormone release.60–62 Transplantation of fetal SCN tissue into the third ventricle of lesioned rodents re-instates locomotor rhythms with the rate of the donor clock tissue.63–65 Even when SCN grafts are encapsulated, behavioral rhythmicity is restored, suggesting that the SCN output signal involves, at least in part, a paracrine/endocrine signal.64 One releasable factor thought to be involved in signaling rhythmic locomotor behavior is prokineticin 2.66 Because transplantation does not restore rhythmic release of glucocorticoids, melatonin or luteinizing hormone, it is clear that many endocrine rhythms require a neural connection.
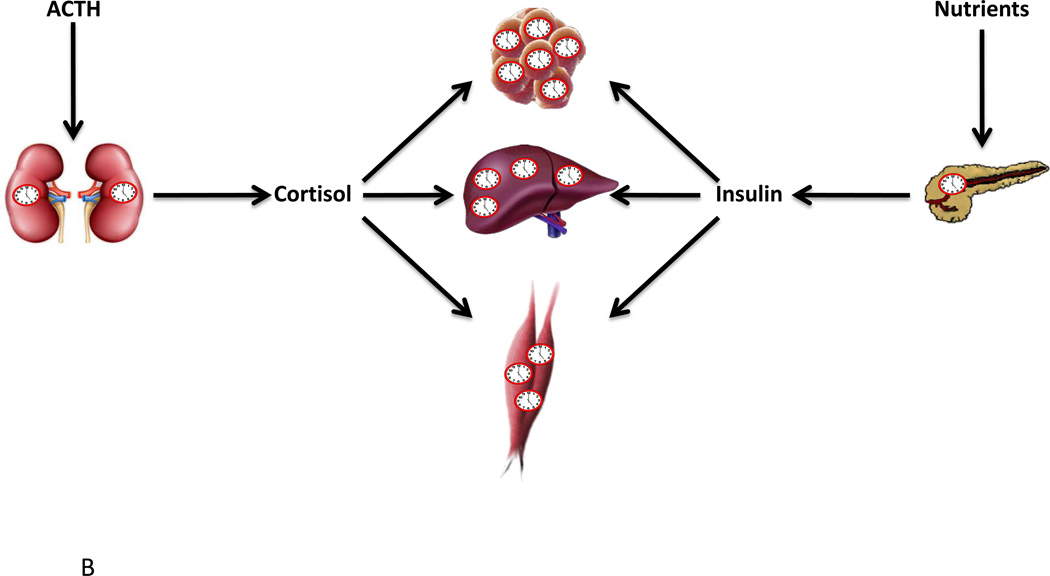
Advances over the last few decades have demonstrated that the circadian network is in reality a multiple oscillator system, in which different tissues/cells are capable of exhibiting self-sustained oscillations in isolation (although oscillations tend to dampen after several days for non-SCN tissue cultures).67–70 This realization has raised the possibility that circadian rhythms in various biological processes may be driven in part by local tissue oscillators. While melatonin rhythmicity is undoubtedly dependent upon central SCN clock control71, local clock control is evident in other peripheral endocrine tissues, such as the adrenal gland and pancreas (Figure 2). For example, transplantation of Per2/Cry1 mutant adrenal glands into wild-type animals results in a dampened rhythm of corticosterone release, despite normal ACTH levels.72 Similarly, adrenal-specific Bmal1 knockdown produces arrhythmic plasma corticosterone secretion.73 Taken together, these data suggest that the adrenal molecular clock locally regulates ACTH sensitivity (although it should be noted that the latter is also dependent on SCN signals transmitted by sympathetic fibers).15 Evidence also supports the concept that the pancreatic β-cell clock influences insulin secretion. Allaman-Pillet et al have previously reported that synchronized β-cells in culture secrete insulin in a rhythmic fashion, with a periodicity of 24-hours.74 More recently, β-cell specific Bmal1 null mice were shown to display impaired insulin secretion.90, 91 In summary, these results suggest that the local molecular clock plays a critical role in many endocrine tissues, and that disruption of this mechanism results in impaired function.
Figure 2.
SCN and non-SCN derived entrainment of peripheral circadian clocks (A) and the impact of cell autonomous circadian clocks on endocrine factor release and sensitivity (B). As discussed in the text, peripheral circadian clocks are entrained (re-set) by both SCN- (e.g., prokineticin 2, neural stimulation) and behavior- (e.g., feeding, physical activity) dependent influences (A). Circadian clocks within the adrenal cortex contribute to diurnal variations in cortisol release, through modulating sensitivity to ACTH, while the β-cell circadian clock is appears to be similarly critical for insulin secretion (B). In addition, circadian clocks within target tissues (e.g., adipose, liver, skeletal muscle) potentially modulate sensitivity to endocrine factors in a time-of-day-dependent manner (B).
PATHOLOGIC CONSEQUENCES OF CIRCADIAN DISRUPTION
Lessons learnt from animal models of impaired circadian biology
As highlighted above, and in Table 1, it is clear that a large number of endocrine factors exhibit time-of-day-dependent oscillations in both humans and animal models. Furthermore, cell autonomous circadian clocks appear to contribute towards these rhythms to varying degrees. Observations such as these raise a host of critical questions, for which answers are not clear. For example, what is the physiologic purpose of daily oscillations in endocrine factors, and what is the pathologic consequence of disruption of these oscillations? Impairment of normal circadian rhythmicity negatively impacts a host of biological processes in both humans and animal models.43, 75–77 Circadian disruption may occur secondarily to changes in the environment (e.g., fluctuations in the light/dark, feeding/fasting, sleep/wake cycles) and/or genetic abnormalities. The estrous cycle and conception success serves to illustrate this concept. Shift workers report decreased successful attempted conceptions during periods of night shift work, while in mouse models, modulation of the light/dark cycle, disruption of SCN and the pre-optic communication, or genetic manipulation of either CLOCK or BMAL1 all impede estrous cycling and successful pregnancies.78–82 The current sub-section will summarize key findings from experimental models with regards to the impact of disrupted circadian rhythms on endocrine factors and endocrine-regulated processes (particularly metabolism).
Manipulation of the light/dark cycle has proven to be a useful tool for the study of disrupted circadian rhythms on many biological processes, including endocrine- and metabolism- related parameters. Following an abrupt alteration in the light/dark cycle, intrinsic circadian clocks reset at varying rates, in an attempt to resynchronize to the new environment. The SCN resets relatively rapidly to a large shift in the light-dark cycle (at rate of ~1 day per hour of shift in the LD cycle) compared to peripheral organs which invariably reset at a slower rate, lagging behind the SCN in a tissue-specific manner.83–86 Accordingly, dyssynchrony occurs at both the organism-to-environment and inter-organ levels, until all clocks (and clock-regulated processes) reset fully to the new light/dark cycle. Should the light/dark cycle be altered multiple times in succession, with an interval shorter than that required for complete resynchronization (e.g., jet lag protocol, wherein the light/dark cycle is phase advanced 6 hours either once or twice a week), chronic dyssynchrony ensues. Prolonged periods of dyssynchrony are associated with altered immune function, increased tumor growth, reduced body temperature, and increased adiposity.87–90 Additional models of light/dark cycle manipulation induced circadian dyssynchrony include housing rodents in constant light or shortened daily cycles (e.g., 20 hour days).91 Invariably these manipulations negatively impact endocrine-regulated processes, including metabolic homeostasis.92–97
The unveiling of core components of the mammalian circadian clock mechanism over the past two decades has been followed closely by their genetic manipulation and characterization of the phenotypic consequence in rodents. One of the first clock components to be genetically modified was CLOCK; chemical mutagenesis resulted in a truncated dominant negative mutant CLOCK protein lacking the transactivation domain.98 Interestingly, although Clock mutant mice exhibit normal activity rhythms under light/dark cycles, feeding behavior appears to be altered, which is associated with increased adiposity and perturbations in endocrine factors (e.g., insulin levels).99 Both germline and tissue-restricted knockout mice have also been generated for distinct circadian clock components. Of these models, germline BMAL1 knockout mice exhibit one of the most striking phenotypes, being arrhythmic for most parameters under circadian conditions, developing markers of accelerated aging (e.g., sarcopenia, cataracts, lipoatrophy), concomitant with reduced lifespan.100 In terms of endocrine factors, Kennaway and colleagues have recently reported that circulating levels of insulin are lower, while adiponectin and leptin are higher, in Bmal1 null mice (relative to wild-type mice).101 Indeed, it has been suggested that BMAL1 null mice should be considered a model of diabetes, due in large part to impaired insulin secretion.102, 103 Genetic manipulation of circadian clock components invariably alters insulin sensitivity, in a manner that is dependent upon whether a positive or negative clock component is disrupted (leads to increased and decreased insulin sensitivity, respectively).104, 105
Endocrine and metabolic circadian abnormalities in humans
Misalignment of peripheral and central clocks with behavioral sleep-wake rhythms can be experimentally induced in the laboratory setting by use of a very short (20 h; 7 h sleep and 13 h wake) or long (28 h; 8 h sleep and 20 h wake) day that is outside the range of entrainment for a 24-h clock.106 For example, three cycles of a 28-h enforced sleep-wake schedule results in sleep-wake behavior that is ~180° out of phase with the intrinsic circadian clock and behavior. After three additional cycles, behavior and the circadian clock are back in alignment. Thus, comparison of cycles with full alignment versus misalignment provide the opportunity to determine which endocrine rhythms follow sleep/wake/eating behavior, and which rhythms are more directly coupled to the intrinsic circadian clock. Using this protocol, Scheer et al found that glucose, epinephrine, and cortisol faithfully follow a ~24-h rhythm during behavioral misalignment, while leptin, insulin, and norephinephrine did not (although it is important to note that all of these endocrine rhythms were influenced to varying degrees by the behavioral cycle).107 Overall leptin levels were lower during misaligned conditions, similar to when circadian misalignment is induced by a more subtle approach using a 24.6-h day. Volunteers in this condition for 25 days exhibited reduced leptin levels at physiologically significant levels,108 which is likely to have a metabolic outcome. In fact, after just 3 cycles of laboratory-induced circadian misalignment (i.e., a 28-h day), healthy volunteers show a dramatic loss of the number of rhythmic transcripts in the blood.109 Importantly, this same protocol also causes glucose intolerance and insulin resistance, suggesting that circadian misalignment may cause insufficient β-cell compensation.107 More recently, Leproult and colleagues have reported that then circadian misalignment is imposed on sleep deprived subjects, indices of insulin resistance worsen.110 Taken together, these studies are consistent with the concept that circadian misalignment impairs endocrine homeostasis, inducing a cardiometabolic state.
Aberrant metabolic homeostasis associated with experimental circadian misalignment is reminiscent of shift-work induced metabolic perturbations. It is clear that misalignment of melatonin and cortisol rhythms occur during shift work.111–113 In addition, shift workers are more likely to develop cardiometabolic syndrome,114 potentially due to increased postprandial levels of insulin, glucose, and triacylglycerol during the night shift,115 as well as increased energy intake and circulating triglycerides, as well as reduced insulin sensitivity and post-prandial ghrelin release.116 Shift workers also have an increased risk of developing cancer, gastrointestinal disorders, and cardiovascular diseases such as ischemic heart disease.107, 114, 117–120 Taken together, these observations underscore the importance of understanding synchronization of the endogenous circadian system with environmental/behavioral and endocrine factors.
In addition to circadian misalignment induced by environmental/lifestyle influences, evidence suggests that desynchrony among intrinsic oscillators occur during pathologic states, particularly those associated with endocrine dysfunction. For example, clock gene rhythms in white blood cells are dampened in patients with diabetes.121 Interestingly, patients with type 2 diabetes do not show a circadian rhythm in insulin secretion, although rhythmicity in insulin sensitivity appears to remain intact.122 Even first-degree relatives of patients with Type 2 diabetes show dampened and shortened rhythms in insulin secretion rates.123 These clinical observations are supported by findings in animal models of metabolic dysfunction (Zucker rats, diabetic db/db mice, and leptin knockout mice), revealing that circadian clock and metabolic perturbations occur in parallel.124–128 Recently, it has been reported that short term high fat diet disrupts alignment of circadian clock gene rhythms in multiple tissues prior to metabolic dysfunction, suggesting that circadian misalignment may precede (and potentially cause) metabolic and endocrine abnormalities.129, 130
FEASIBILITY OF NORMALIZING CIRCADIAN RHYTHMICITY
Lessons learnt from clock gene rescue studies in animal models
As mentioned above, disruption of the molecular clock in animal models often results in endocrine disruption and cardiometabolic impairment. Genetic rescue of clock-controlled gene transcription rhythms and associated metabolic/endocrine phenotypes may not only serve as proof-of-principal, but may shed light on feasibility of targeting this system for future treatment of disease states. For example, arrhythmic activity and metabolism as well as body weight and adiposity abnormalities observed in Bmal1 knockout mice can be rescued by germline over-expression of Bmal2.131 In addition, the low body weight and early death phenotypes of Bmal1 knockout mice can be rescued by muscle-specific, but not brain-specific, over expression of Bmal1.132 Rhythmicity of a number of clock controlled genes is also restored by these genetic rescue strategies. It should be noted that a sub-set of transcriptional rhythms normally display 12-h periodicity in peripheral tissues such as the liver, heart, kidney, and lungs from wild type mice.133 However, these 12-h rhythms become 24-h when CLOCK mutant mice are rescued only in the brain (using a Tet-OFF expression system).134 This finding is interesting in light of the fact that time-of-day-restricted feeding (during the inactive period, between 1 to 9 hours after lights on) in wild-type mice also converts these shorter, ultradian rhythms to 24-h rhythms.133 Taken together, these studies suggest that circadian desynchrony can be rescued, and that the central clock interacts with environmental/systemic signals to produce ultradian rhythms.
The utility of time-of-day-restricted feeding
Evidence to date undoubtedly suggests that the intrinsic circadian system plays an important role in daily rhythms of a host of endocrine factors. In several cases (e.g., cortisol, insulin, etc), non-SCN clocks appear to contribute in a significant manner. This raises the question whether strategies designed to selectively entrain peripheral circadian clocks would be beneficial for resynchronizing oscillations in endocrine factors during disease states (such as cardiometabolic disease states). Damiola & colleagues were among the first to report that restricting food access in rodents to a distinct period during the sleep phase causes a phase shift in circadian clocks within peripheral tissues (e.g., liver, kidney, pancreas, heart), while the SCN clock remains entrained to the light/dark cycle (i.e., relatively unresponsive to feeding entrainment).135 The strength of this intervention is exemplified by the demonstration that circadian clock gene oscillations are re-initiated in peripheral tissues of CLOCK mutant mice by time-of-day-restricted feeding.136 These initial studies led to various investigations into the negative impact of restricted feeding-induced circadian dyssynchrony on energy homeostasis. A consensus of these studies appears to be that consumption of an inappropriately large number of calories during the end of the active period and/or during the sleep phase, is associated with metabolic dyssynchrony and cardiometabolic disease development.93, 137–141 Importantly, evidence exists that observations in rodent models translate to humans. For example, night eating syndrome is associated with obesity.142 In addition, Qin et al have reported consumption of a large meal at night leads to an uncoupling of the relationship between plasma glucose and insulin levels, indicative of metabolic dysfunction.143 These findings have led several investigators to hypothesize that restriction of food intake to an appropriate time of the day may prove beneficial, via resetting peripheral clocks and reestablishment of circadian synchrony during cardiometabolic disease states. In support of such a concept, Kudo et al reported that restricting food access to the active phase normalized liver clock function in db/db mice, and concomitantly improved various cardiometabolic parameters (including lowering circulating insulin levels).144 Similarly, active phase-restricted feeding is able to normalize peripheral circadian clock gene oscillations concomitant with metabolic and endocrine abnormalities in both a rodent model of shift work, as well as during chronic high fat feeding.137, 145, 146 Clearly additional studies are required to elucidate further the potential utility of time-of-day-restricted feeding on normalization of circadian dyssynchrony and metabolic/endocrine factors.
The therapeutic potential of drugs that can alter circadian clock function
Treatment strategies involving genetic manipulation or alterations in a patient’s daily behavior are clearly not ideal, for a multitude of reasons. A more promising therapeutic approach would be to utilize a pharmaceutical agent that selectively targets the molecular clock.147 For example, one recently described small molecule compound is longdaysin, which has been shown to lengthen the circadian period by targeting multiple kinases simultaneously (CKIδ, CKIα, and ERK2), leading to PER1 degradation.148 Stabilization of CRY proteins (through prevention of ubiquitination and degradation) by another small molecule (KL001) also lengthens the period of the clock. Interestingly, KL001 is an attractive therapeutic prospect for diabetes since it has been shown to suppress hepatic glucose production induced by glucagon.149 Additional clock components are known to impact metabolism, including REV-ERBα. REV-ERBα agonists (SR9009 and SR9011) not only enhance Per2 rhythms, suppress Cry2 rhythms, and shift Bmal1 rhythms, but SR9009 also rescues increased fat, dyslipidaemia, and hyperglycaemia in obesity models.150 Targets of kinases such as glycogen synthase kinase 3 (GSK3) and c-Jun N-terminal kinases inhibitors (CHIR99021 and SP600125, respectively) are also effective at changing the period of molecular clock.151, 152 It is predicted that when coupled with time-of-day-specific delivery, many of these small molecule inhibitors may have therapeutic potential, through restoration of circadian synchrony of biologic processes, including secretion of, and sensitivity to, endocrine factors.
CONCLUSIONS
It has long been appreciated that circulating levels of various endocrine factors exhibit a time-of-day dependence. It is clear that these rhythms are not solely the result of environmentally/behaviorally derived influences, but are also largely mediated by the endogenous circadian system. With regards to the latter, it is becoming increasingly apparent that cell autonomous circadian clocks modulate endocrine factor levels, as well as tissue responsiveness to these stimuli, over the course of the day. Disrupted orchestration of these rhythms occurs readily through environmental and/or genetic means, resulting in a state of desynchrony known to promote a host of pathologies, including cardiometabolic diseases and cancer. Initial studies designed to reinstate synchrony through environmental (e.g., time-restricted feeding) and/or pharmacological means show promise for attenuated pathogenesis in animal models. With the recent advent of small molecule modulators of the circadian clock mechanism, the prospect of therapeutically targeting this mechanism in patients with endocrine disorders holds great potential.
Box 1. Definitions of commonly utilized terms in chronobiology.
Tau (period)
The length of time for one cycle of an oscillation to repeat. For example, the length of time for a distinct phase point (such as the peak) to re-occur during the subsequent cycle.
Amplitude
The size of an oscillation as measured by half the distance from the peak to the trough.
Phase
The timing of a consistent point in the cycle, such as the peak or trough.
Phase shift
A change in phase such that it occurs earlier or later, due to a displacement of the entire cycle. A stable phase shift will only produce a period change for one or a few “transient” cycle(s), after which the oscillation will continue with the same period as before the shift.
Zeitgeber
Literally means “time giver,” and refers to any resetting stimulus that serves as a time cue for the external environment. Light and food intake are classic examples of zeitgebers for the SCN and non-SCN clocks, respectively.
Circadian rhythm
“Circadian” literally means “about a day” and refers to a rhythm whose complete cycle repeats close to every 24 hours. In order to demonstrate that a circadian rhythm is endogenously generated, the rhythm must be measured under constant conditions (i.e., in the absence of a zeitgeber).
Diurnal rhythm
A physiological measurement (e.g., gene transcription, protein expression, body temperature, behavior, etc) that exhibits daily changes across the 24-h light dark cycle such that the peak(s) and nadir occur ~12-h apart or in a frequency that is a harmonic of 24 hours. In conditions in which a zeitgeber is present (e.g., a light-dark cycle), it is impossible to tell if a diurnal rhythm is endogenously generated or an acute response to the zeitgeber.
Entrainment
A stable state in which an external zeitgeber (e.g., light) induces small shifts in the phase of an endogenous oscillator on a daily basis so that the period of the physiological output of the oscillator (e.g., locomotor activity) is equal to the period of the external zeitgeber cycle with a specific phase relationship to one another.
Ultradian
A repeating rhythm that has a cycle period that is much less than 24 hours (e.g., 12-h gene expression rhythm, 1-h pulsatile GnRH release, etc.).
REVIEW CRITERIA.
PubMed and Google Scholar were utilized to search for original research and review articles published up to 2014; no abstracts from meeting reports have been cited. Search terms used included “circadian clock”, “diurnal”, “time of day”, “endocrine factors”, “insulin”, “growth hormone”, “cortisol”, “metabolism” and “desynchrony”. The references of the retrieved articles were also reviewed.
KEY POINTS.
Many endocrine factors oscillate in a time-of-day-dependent manner.
Endocrine factor rhythms are driven by in part by circadian clocks.
Circadian desynchrony is associated with pathologic states.
Re-instatement of circadian rhythms improves metabolic homeostasis.
ACKNOWLEDGEMENTS
DECLARATION OF INTEREST
This work was supported by the National Heart, Lung, and Blood Institute (HL-074259 [MEY], HL-106199 [MEY], HL-107709 [MEY]), the National Institute of Diabetes and Digestive and Kidney Diseases (DK-58259 [SJF]), the US Department of Veterans Affairs (Merit Review Award [SJF], and the Neurological Disease and Stroke Institute (NS082413 [KLG]).
Biographies
Karen L. Gamble, PhD is an Assistant Professor at UAB, who has studied circadian rhythms in hamsters, transgenic mice, and humans over the last 15 years. Her research includes investigation of the molecular clock gene regulation and neurophysiology of the SCN as well as circadian rhythm and sleep studies in humans, including sleep behavior strategies of shift workers, circadian dysfunction in adult ADHD patients with delayed sleep phase, and daytime sleepiness in adolescents. She holds memberships with the Society for Research in Biological Rhythms, Society for Neuroscience, Working Time Society, and the Society for Light Treatment and Biological Rhythms.
Ryan D. Berry, BS is currently in his third year of the National Institutes of Health Medical Scientist Training Program at the University of Alabama at Birmingham. Presently he is pursuing a PhD studying Growth Hormone Receptor signaling in the lab of Dr. Stuart J. Frank. He received his BS degree in Biochemistry and Spanish from Utah State University in 2011, during which time he presented posters at the Utah Conference on Undergraduate Research (2009) on the effects of mutations on the kinetics of PTP1B and YopH, and at the National Conference on Undergraduate Research (2011) on the promiscuous catalytic activity of PTP1B.
Stuart J. Frank, MD is Director of the Division of Endocrinology, Diabetes, and Metabolism in the Department of Medicine at the University of Alabama at Birmingham and Chief of the Endocrinology Section of the Birmingham VAMC Medical Service. Dr. Frank is also Professor of Medicine and Cell, Developmental, and Integrative Biology at UAB. He earned the M.D. at Harvard Medical School and was an Internal Medicine resident at Washington University, prior to Endocrinology fellowship and research training at the National Institutes of Health. Dr. Frank’s laboratory studies molecular mechanisms of growth hormone and prolactin action, determinants of GH and prolactin sensitivity, and the roles of these hormones in health and disease.
Martin E. Young, DPhil is an Associate Professor of Medicine in the Division of Cardiovascular Diseases at the University of Alabama at Birmingham. Dr. Young earned his PhD at the Oxford University (Biochemistry) before completing post-doctoral fellowships at Boston University (Diabetes/Metabolism) and the University of Texas at Houston (Cardiac Metabolism). Dr. Young’s current research interests include understanding the role of cell autonomous circadian clocks in metabolic regulation, and how desynchrony of these molecular mechanisms contributes towards cardiometabolic diseases.
Footnotes
DISCLOSURES
None declared.
REFERENCES
- 1.Avram AM, Jaffe CA, Symons KV, Barkan AL. Endogenous circulating ghrelin does not mediate growth hormone rhythmicity or response to fasting. J Clin Endocrinol Metab. 2005;90:2982–2987. doi: 10.1210/jc.2004-1785. [DOI] [PubMed] [Google Scholar]
- 2.Russell W, Harrison RF, Smith N, Darzy K, Shalet S, Weetman AP, Ross RJ. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab. 2008;93:2300–2306. doi: 10.1210/jc.2007-2674. [DOI] [PubMed] [Google Scholar]
- 3.Carroll T, Raff H, Findling JW. Late-night salivary cortisol measurement in the diagnosis of cushing's syndrome. Nature clinical practice. Endocrinology & metabolism. 2008;4:344–350. doi: 10.1038/ncpendmet0837. [DOI] [PubMed] [Google Scholar]
- 4.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: Structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 5.Walton MJ, Anderson RA, Kicman AT, Elton RA, Ossowska K, Baird DT. A diurnal variation in testicular hormone production is maintained following gonadotrophin suppression in normal men. Clinical endocrinology. 2007;66:123–129. doi: 10.1111/j.1365-2265.2006.02696.x. [DOI] [PubMed] [Google Scholar]
- 6.Goel N, Stunkard AJ, Rogers NL, Van Dongen HP, Allison KC, O'Reardon JP, Ahima RS, Cummings DE, Heo M, Dinges DF. Circadian rhythm profiles in women with night eating syndrome. J Biol Rhythms. 2009;24:85–94. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheer FA, Chan JL, Fargnoli J, Chamberland J, Arampatzi K, Shea SA, Blackburn GL, Mantzoros CS. Day/night variations of high-molecular-weight adiponectin and lipocalin-2 in healthy men studied under fed and fasted conditions. Diabetologia. 2010;53:2401–2405. doi: 10.1007/s00125-010-1869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borjigin J, Zhang LS, Calinescu AA. Circadian regulation of pineal gland rhythmicity. Mol Cell Endocrinol. 2012;349:13–19. doi: 10.1016/j.mce.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dardente H. Melatonin-dependent timing of seasonal reproduction by the pars tuberalis: Pivotal roles for long daylengths and thyroid hormones. J Neuroendocrinol. 2012;24:249–266. doi: 10.1111/j.1365-2826.2011.02250.x. [DOI] [PubMed] [Google Scholar]
- 10.Rondanelli M, Faliva MA, Perna S, Antoniello N. Update on the role of melatonin in the prevention of cancer tumorigenesis and in the management of cancer correlates, such as sleep-wake and mood disturbances: Review and remarks. Aging clinical and experimental research. 2013;25:499–510. doi: 10.1007/s40520-013-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol Cell Endocrinol. 2012;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chevre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, Weill J, Heude B, Tauber M, Lemaire K, Schuit F, Elliott P, Jorgensen T, Charpentier G, Hadjadj S, Cauchi S, Vaxillaire M, Sladek R, Visvikis-Siest S, Balkau B, Levy-Marchal C, Pattou F, Meyre D, Blakemore AI, Jarvelin MR, Walley AJ, Hansen T, Dina C, Pedersen O, Froguel P. A variant near mtnr1b is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nature genetics. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Barcelo EJ, Mediavilla MD, Reiter RJ. Clinical uses of melatonin in pediatrics. International journal of pediatrics. 2011;2011:892624. doi: 10.1155/2011/892624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A. 2006;103:7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200:3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- 16.Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin v1 antagonist. J Neurosci. 1996;16:5555–5565. doi: 10.1523/JNEUROSCI.16-17-05555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardi J, Obal F, Jr, Fang J, Zhang J, Krueger JM. Diurnal variations and sleep deprivation-induced changes in rat hypothalamic ghrh and somatostatin contents. Am J Physiol. 1999;277:R1339–R1344. doi: 10.1152/ajpregu.1999.277.5.R1339. [DOI] [PubMed] [Google Scholar]
- 18.Dimaraki EV, Jaffe CA, Bowers CY, Marbach P, Barkan AL. Pulsatile and nocturnal growth hormone secretions in men do not require periodic declines of somatostatin. Am J Physiol Endocrinol Metab. 2003;285:E163–E170. doi: 10.1152/ajpendo.00334.2002. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y. Essential roles of growth hormone (gh) and insulin-like growth factor-i (igf-i) in the liver. Endocrine journal. 2012;59:955–962. doi: 10.1507/endocrj.ej12-0322. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe CA, Ocampo-Lim B, Guo W, Krueger K, Sugahara I, DeMott-Friberg R, Bermann M, Barkan AL. Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J Clin Invest. 1998;102:153–164. doi: 10.1172/JCI2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villadolid MC, Takano K, Hizuka N, Asakawa K, Sukegawa I, Horikawa R, Shizume K. Twenty-four hour plasma gh, fsh and lh profiles in patients with turner's syndrome. Endocrinologia japonica. 1988;35:71–81. doi: 10.1507/endocrj1954.35.71. [DOI] [PubMed] [Google Scholar]
- 22.Goji K. Pulsatile characteristics of spontaneous growth hormone (gh) concentration profiles in boys evaluated by an ultrasensitive immunoradiometric assay: Evidence for ultradian periodicity of gh secretion. J Clin Endocrinol Metab. 1993;76:667–670. doi: 10.1210/jcem.76.3.8445023. [DOI] [PubMed] [Google Scholar]
- 23.Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: Comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–2843. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 24.Maeda N, Funahashi T, Shimomura I. Cardiovascular-metabolic impact of adiponectin and aquaporin. Endocrine journal. 2013;60:251–259. doi: 10.1507/endocrj.ej13-0016. [DOI] [PubMed] [Google Scholar]
- 25.Begg DP, Woods SC. Interactions between the central nervous system and pancreatic islet secretions: A historical perspective. Advances in physiology education. 2013;37:53–60. doi: 10.1152/advan.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 27.Bolli GB, De Feo P, De Cosmo S, Perriello G, Ventura MM, Calcinaro F, Lolli C, Campbell P, Brunetti P, Gerich JE. Demonstration of a dawn phenomenon in normal human volunteers. Diabetes. 1984;33:1150–1153. doi: 10.2337/diab.33.12.1150. [DOI] [PubMed] [Google Scholar]
- 28.Bolli GB, Gerich JE. The “dawn phenomenon”--a common occurrence in both non-insulin-dependent and insulin-dependent diabetes mellitus. N Engl J Med. 1984;310:746–750. doi: 10.1056/NEJM198403223101203. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt MI, Lin QX, Gwynne JT, Jacobs S. Fasting early morning rise in peripheral insulin: Evidence of the dawn phenomenon in nondiabetes. Diabetes care. 1984;7:32–35. doi: 10.2337/diacare.7.1.32. [DOI] [PubMed] [Google Scholar]
- 30.Campbell PJ, Bolli GB, Cryer PE, Gerich JE. Pathogenesis of the dawn phenomenon in patients with insulin-dependent diabetes mellitus. Accelerated glucose production and impaired glucose utilization due to nocturnal surges in growth hormone secretion. N Engl J Med. 1985;312:1473–1479. doi: 10.1056/NEJM198506063122302. [DOI] [PubMed] [Google Scholar]
- 31.Monnier L, Colette C, Dejager S, Owens D. Magnitude of the dawn phenomenon and its impact on the overall glucose exposure in type 2 diabetes: Is this of concern? Diabetes care. 2013;36:4057–4062. doi: 10.2337/dc12-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell PJ, Bolli GB, Cryer PE, Gerich JE. Sequence of events during development of the dawn phenomenon in insulin-dependent diabetes mellitus. Metabolism: clinical and experimental. 1985;34:1100–1104. doi: 10.1016/0026-0495(85)90153-2. [DOI] [PubMed] [Google Scholar]
- 33.Clark LA, Denby L, Pregibon D, Harshfield GA, Pickering TG, Blank S, Laragh JH. A quantitative analysis of the effects of activity and time of day on the diurnal variations of blood pressure. Journal of chronic diseases. 1987;40:671–681. doi: 10.1016/0021-9681(87)90103-2. [DOI] [PubMed] [Google Scholar]
- 34.Linsell CR, Lightman SL, Mullen PE, Brown MJ, Causon RC. Circadian rhythms of epinephrine and norepinephrine in man. J Clin Endocrinol Metab. 1985;60:1210–1215. doi: 10.1210/jcem-60-6-1210. [DOI] [PubMed] [Google Scholar]
- 35.Krauchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267:R819–R829. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- 36.Van Cauter E, Spiegel K. Neurobiology of Sleep and Circadian Rhythms. New York: Marcel Dekker; 1999. Circadian and sleep control of endocrine secretions. [Google Scholar]
- 37.Van Cauter E, Copinschi G. Human growth hormone secretion: basic and clinical research. Totowa, NJ: Humana Press, Inc; 1999. Interactions between growth hormone secretion and sleep. [Google Scholar]
- 38.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88:934–942. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A. 2010;107:20541–20546. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheer FA, Michelson AD, Frelinger AL, 3rd, Evoniuk H, Kelly EE, McCarthy M, Doamekpor LA, Barnard MR, Shea SA. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS One. 2011;6:e24549. doi: 10.1371/journal.pone.0024549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108:980–984. doi: 10.1161/CIRCRESAHA.110.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheer FA, Shea SA. Human circadian system causes morning peak in pro-thrombotic plasminogen activator inhibitor-1 (pai-1) independent of sleep/wake cycle. Blood. 2013 doi: 10.1182/blood-2013-07-517060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edery I. Circadian rhythms in a nutshell. Physiol Genomics. 2000;3:59–74. doi: 10.1152/physiolgenomics.2000.3.2.59. [DOI] [PubMed] [Google Scholar]
- 45.Gekakis N, Staknis D, Nguyen H, Davis F, Wilsbacher L, King D, Takahashi J, Weitz C. Role of the clock protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 46.Hogenesch J, Gu Y, Jain S, Bradfield C. The basic-helix-loop-helix-pas orphan mop3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Aad Sci U.S.A. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zylka M, Shearman L, Weaver D, Reppert S. Three period homologs in mammals: Differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 48.Miyamoto Y, Sancar A. Vitamin b2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Aad Sci U.S.A. 1998;95:6097–6102. doi: 10.1073/pnas.95.11.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor rev-erbalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 50.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 51.McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics. 2007;31:86–95. doi: 10.1152/physiolgenomics.00066.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martino T, Arab S, Straume M, Belsham DD, Tata N, Cai F, Liu P, Trivieri M, Ralph M, Sole MJ. Day/night rhythms in gene expression of the normal murine heart. J Mol Med. 2004;82:256–264. doi: 10.1007/s00109-003-0520-1. [DOI] [PubMed] [Google Scholar]
- 53.Cardone L, Hirayama J, Giordano F, Tamaru T, Palvimo JJ, Sassone-Corsi P. Circadian clock control by sumoylation of bmal1. Science. 2005;309:1390–1394. doi: 10.1126/science.1110689. [DOI] [PubMed] [Google Scholar]
- 54.Hardin PE, Yu W. Circadian transcription: Passing the hat to clock. Cell. 2006;125:424–426. doi: 10.1016/j.cell.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Kloss B, Price J, Saez L, Blau J, Rothenfluh A, Wesley C, Young M. The drosophila clock gene double-time encodes a protein closely related to human casein kinase iepsilon. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- 56.Millar AJ. Clock proteins: Turned over after hours? Curr Biol. 2000;10:R529–R531. doi: 10.1016/s0960-9822(00)00586-8. [DOI] [PubMed] [Google Scholar]
- 57.Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Durgan DJ, Pat BM, Laczy B, Bradley JA, Tsai JY, Grenett MH, Ratcliffe WF, Brewer RA, Nagendran J, Villegas-Montoya C, Zou C, Zou L, Johnson RL, Jr, Dyck JR, Bray MS, Gamble KL, Chatham JC, Young ME. O-glcnacylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J Biol Chem. 2011;286:44606–44619. doi: 10.1074/jbc.M111.278903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Neill JS, van Ooijen G, Dixon LE, Troein C, Corellou F, Bouget FY, Reddy AB, Millar AJ. Circadian rhythms persist without transcription in a eukaryote. Nature. 2011;469:554–558. doi: 10.1038/nature09654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140:207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- 61.Coomans CP, van den Berg SA, Lucassen EA, Houben T, Pronk AC, van der Spek RD, Kalsbeek A, Biermasz NR, Willems van Dijk K, Romijn JA, Meijer JH. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes. 2013;62:1102–1108. doi: 10.2337/db12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malloy JN, Paulose JK, Li Y, Cassone VM. Circadian rhythms of gastrointestinal function are regulated by both central and peripheral oscillators. Am J Physiol Gastrointest Liver Physiol. 2012;303:G461–G473. doi: 10.1152/ajpgi.00369.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LeSauter J, Romero P, Cascio M, Silver R. Attachment site of grafted scn influences precision of restored circadian rhythm. J Biol Rhythms. 1997;12:327–338. doi: 10.1177/074873049701200405. [DOI] [PubMed] [Google Scholar]
- 64.Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- 65.Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou QY, Cheng MY. Prokineticin 2 and circadian clock output. The FEBS journal. 2005;272:5703–5709. doi: 10.1111/j.1742-4658.2005.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. Period2::Luciferase real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 69.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 70.Leise TL, Wang CW, Gitis PJ, Welsh DK. Persistent cell-autonomous circadian oscillations in fibroblasts revealed by six-week single-cell imaging of per2::Luc bioluminescence. PLoS One. 2012;7:e33334. doi: 10.1371/journal.pone.0033334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klein DC, Moore RY. Pineal n-acetyltransferase and hydroxyindole-o-methyltransferase: Control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain research. 1979;174:245–262. doi: 10.1016/0006-8993(79)90848-5. [DOI] [PubMed] [Google Scholar]
- 72.Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, Hoffmann MW, Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, Lee HW, Choi S, Sun W, Kim H, Cho S, Lee KH, Kim K. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A. 2008;105:20970–20975. doi: 10.1073/pnas.0806962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allaman-Pillet N, Roduit R, Oberson A, Abdelli S, Ruiz J, Beckmann JS, Schorderet DF, Bonny C. Circadian regulation of islet genes involved in insulin production and secretion. Mol Cell Endocrinol. 2004;226:59–66. doi: 10.1016/j.mce.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Knutsson A, Akerstedt T, Jonsson B, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;12:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 76.Koller M. Health risks related to shift work: An example of time-contingent effects of long-term stress. Int Arch Occup Environ Health. 1983;53:59–75. doi: 10.1007/BF00406178. [DOI] [PubMed] [Google Scholar]
- 77.Arble DM, Ramsey KM, Bass J, Turek FW. Circadian disruption and metabolic disease: Findings from animal models. Best practice & research. Clinical endocrinology & metabolism. 2010;24:785–800. doi: 10.1016/j.beem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Labyak S, Lava S, Turek F, Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health care for women international. 2002;23:703–714. doi: 10.1080/07399330290107449. [DOI] [PubMed] [Google Scholar]
- 79.Summa KC, Vitaterna MH, Turek FW. Environmental perturbation of the circadian clock disrupts pregnancy in the mouse. PLoS One. 2012;7:e37668. doi: 10.1371/journal.pone.0037668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kennaway DJ. The role of circadian rhythmicity in reproduction. Human reproduction update. 2005;11:91–101. doi: 10.1093/humupd/dmh054. [DOI] [PubMed] [Google Scholar]
- 81.Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14:1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gamble KL, Resuehr D, Johnson CH. Shift work and circadian dysregulation of reproduction. Frontiers in endocrinology. 2013;4:92. doi: 10.3389/fendo.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van den Buuse M. Circadian rhythms of blood pressure and heart rate in conscious rats: Effects of light cycle shift and timed feeding. Physiol Behav. 1999;68:9–15. doi: 10.1016/s0031-9384(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 84.Durgan DJ, Trexler NA, Egbejimi O, McElfresh TA, Suk HY, Petterson LE, Shaw CA, Hardin PE, Bray MS, Chandler MP, Chow CW, Young ME. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281:24254–24269. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- 85.Davidson AJ, Castanon-Cervantes O, Leise TL, Molyneux PC, Harrington ME. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. The European journal of neuroscience. 2009;29:171–180. doi: 10.1111/j.1460-9568.2008.06534.x. [DOI] [PubMed] [Google Scholar]
- 86.Sellix MT, Evans JA, Leise TL, Castanon-Cervantes O, Hill DD, DeLisser P, Block GD, Menaker M, Davidson AJ. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J Neurosci. 2012;32:16193–16202. doi: 10.1523/JNEUROSCI.3559-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsai LL, Tsai YC, Hwang K, Huang YW, Tzeng JE. Repeated light-dark shifts speed up body weight gain in male f344 rats. Am J Physiol Endocrinol Metab. 2005;289:E212–E217. doi: 10.1152/ajpendo.00603.2004. [DOI] [PubMed] [Google Scholar]
- 88.Filipski E, Subramanian P, Carriere J, Guettier C, Barbason H, Levi F. Circadian disruption accelerates liver carcinogenesis in mice. Mutation research. 2009;680:95–105. doi: 10.1016/j.mrgentox.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Cambras T, Weller JR, Angles-Pujoras M, Lee ML, Christopher A, Diez-Noguera A, Krueger JM, de la Iglesia HO. Circadian desynchronization of core body temperature and sleep stages in the rat. Proc Natl Acad Sci U S A. 2007;104:7634–7639. doi: 10.1073/pnas.0702424104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. Journal of immunology. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de la Iglesia HO, Cambras T, Schwartz WJ, Diez-Noguera A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr Biol. 2004;14:796–800. doi: 10.1016/j.cub.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 92.Escobar C, Salgado-Delgado R, Gonzalez-Guerra E, Tapia Osorio A, Angeles-Castellanos M, Buijs RM. Circadian disruption leads to loss of homeostasis and disease. Sleep disorders. 2011;2011:964510. doi: 10.1155/2011/964510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bartol-Munier I, Gourmelen S, Pevet P, Challet E. Combined effects of high-fat feeding and circadian desynchronization. Int J Obes (Lond) 2006;30:60–67. doi: 10.1038/sj.ijo.0803048. [DOI] [PubMed] [Google Scholar]
- 94.Salgado-Delgado RC, Saderi N, Basualdo Mdel C, Guerrero-Vargas NN, Escobar C, Buijs RM. Shift work or food intake during the rest phase promotes metabolic disruption and desynchrony of liver genes in male rats. PLoS One. 2013;8:e60052. doi: 10.1371/journal.pone.0060052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bellastella A, Pisano G, Iorio S, Pasquali D, Orio F, Venditto T, Sinisi AA. Endocrine secretions under abnormal light-dark cycles and in the blind. Hormone research. 1998;49:153–157. doi: 10.1159/000023163. [DOI] [PubMed] [Google Scholar]
- 96.Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. Experimental 'jet lag' inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One. 2010;5:e15267. doi: 10.1371/journal.pone.0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest. 2010;120:2600–2609. doi: 10.1172/JCI41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vitaterna M, King D, Chang A, Kornhauser J, Lowrey P, McDonald J, Dove W, Pinto L, Turek F, Takahashi J. Mutagenesis and mapping of a mouse gene; clock; essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Turek F, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen D, Eckel R, Takahashi J, Bass J. Obesity and metabolic syndrome in clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in bmal1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kennaway DJ, Varcoe TJ, Voultsios A, Boden MJ. Global loss of bmal1 expression alters adipose tissue hormones, gene expression and glucose metabolism. PLoS One. 2013;8:e65255. doi: 10.1371/journal.pone.0065255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components clock and bmal1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. Bmal1 and clock, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carvas JM, Vukolic A, Yepuri G, Xiong Y, Popp K, Schmutz I, Chappuis S, Albrecht U, Ming XF, Montani JP, Yang Z. Period2 gene mutant mice show compromised insulin-mediated endothelial nitric oxide release and altered glucose homeostasis. Frontiers in physiology. 2012;3:337. doi: 10.3389/fphys.2012.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012;349:91–104. doi: 10.1016/j.mce.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nguyen J, Wright KP., Jr Influence of weeks of circadian misalignment on leptin levels. Nature and science of sleep. 2010;2:9–18. doi: 10.2147/NSS.S7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Archer SN, Laing EE, Moller-Levet CS, van der Veen DR, Bucca G, Lazar AS, Santhi N, Slak A, Kabiljo R, von Schantz M, Smith CP, Dijk DJ. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1316335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014 doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weibel L, Spiegel K, Gronfier C, Follenius M, Brandenberger G. Twenty-four-hour melatonin and core body temperature rhythms: Their adaptation in night workers. Am J Physiol. 1997;272:R948–R954. doi: 10.1152/ajpregu.1997.272.3.R948. [DOI] [PubMed] [Google Scholar]
- 112.Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms. 2002;17:556–567. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- 113.Hennig J, Kieferdorf P, Moritz C, Huwe S, Netter P. Changes in cortisol secretion during shiftwork: Implications for tolerance to shiftwork? Ergonomics. 1998;41:610–621. doi: 10.1080/001401398186784. [DOI] [PubMed] [Google Scholar]
- 114.Pietroiusti A, Neri A, Somma G, Coppeta L, Iavicoli I, Bergamaschi A, Magrini A. Incidence of metabolic syndrome among night-shift healthcare workers. Occup Environ Med. 2010;67:54–57. doi: 10.1136/oem.2009.046797. [DOI] [PubMed] [Google Scholar]
- 115.Lund J, Arendt J, Hampton SM, English J, Morgan LM. Postprandial hormone and metabolic responses amongst shift workers in antarctica. J Endocrinol. 2001;171:557–564. doi: 10.1677/joe.0.1710557. [DOI] [PubMed] [Google Scholar]
- 116.Schiavo-Cardozo D, Lima MM, Pareja JC, Geloneze B. Appetite-regulating hormones from the upper gut: Disrupted control of xenin and ghrelin in night workers. Clin Endocrinol (Oxf) 2012 doi: 10.1111/cen.12114. [DOI] [PubMed] [Google Scholar]
- 117.Boivin DB, Tremblay GM, James FO. Working on atypical schedules. Sleep Med. 2007;8:578–589. doi: 10.1016/j.sleep.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 118.Foster RG, Wulff K. The rhythm of rest and excess. Nat Rev Neurosci. 2005;6:407–414. doi: 10.1038/nrn1670. [DOI] [PubMed] [Google Scholar]
- 119.Knutsson A, Akerstedt T, Jonsson BG, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;2:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 120.Kroenke CH, Spiegelman D, Manson J, Schernhammer ES, Colditz GA, Kawachi I. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol. 2007;165:175–183. doi: 10.1093/aje/kwj355. [DOI] [PubMed] [Google Scholar]
- 121.Ando H, Takamura T, Matsuzawa-Nagata N, Shima KR, Eto T, Misu H, Shiramoto M, Tsuru T, Irie S, Fujimura A, Kaneko S. Clock gene expression in peripheral leucocytes of patients with type 2 diabetes. Diabetologia. 2009;52:329–335. doi: 10.1007/s00125-008-1194-6. [DOI] [PubMed] [Google Scholar]
- 122.Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with niddm caused by cyclic changes in hepatic glucose production. Diabetes. 1996;45:1044–1050. doi: 10.2337/diab.45.8.1044. [DOI] [PubMed] [Google Scholar]
- 123.Boden G, Chen X, Polansky M. Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes. 1999;48:2182–2188. doi: 10.2337/diabetes.48.11.2182. [DOI] [PubMed] [Google Scholar]
- 124.Sans-Fuentes MA, Diez-Noguera A, Cambras T. Light responses of the circadian system in leptin deficient mice. Physiol Behav. 2010;99:487–494. doi: 10.1016/j.physbeh.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 125.Danguir J. Sleep patterns in the genetically obese zucker rat: Effect of acarbose treatment. Am J Physiol. 1989;256:R281–R283. doi: 10.1152/ajpregu.1989.256.1.R281. [DOI] [PubMed] [Google Scholar]
- 126.Megirian D, Dmochowski J, Farkas GA. Mechanism controlling sleep organization of the obese zucker rats. J Appl Physiol. 1998;84:253–256. doi: 10.1152/jappl.1998.84.1.253. [DOI] [PubMed] [Google Scholar]
- 127.Kudo T, Akiyama M, Kuriyama K, Sudo M, Moriya T, Shibata S. Night-time restricted feeding normalises clock genes and pai-1 gene expression in the db/db mouse liver. Diabetologia. 2004;47:1425–1436. doi: 10.1007/s00125-004-1461-0. [DOI] [PubMed] [Google Scholar]
- 128.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R894–R903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- 129.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 130.Pendergast JS, Branecky KL, Yang W, Ellacott KL, Niswender KD, Yamazaki S. High-fat diet acutely affects circadian organisation and eating behavior. Eur J Neurosci. 2013 doi: 10.1111/ejn.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi S, Hida A, McGuinness OP, Wasserman DH, Yamazaki S, Johnson CH. Circadian clock gene bmal1 is not essential; functional replacement with its paralog, bmal2. Curr Biol. 2010;20:316–321. doi: 10.1016/j.cub.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McDearmon EL, Patel KN, Ko CH, Walisser JA, Schook AC, Chong JL, Wilsbacher LD, Song EJ, Hong HK, Bradfield CA, Takahashi JS. Dissecting the functions of the mammalian clock protein bmal1 by tissue-specific rescue in mice. Science. 2006;314:1304–1308. doi: 10.1126/science.1132430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, Baggs JE, Panda S, Hogenesch JB. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hughes ME, Hong HK, Chong JL, Indacochea AA, Lee SS, Han M, Takahashi JS, Hogenesch JB. Brain-specific rescue of clock reveals system-driven transcriptional rhythms in peripheral tissue. PLoS Genet. 2012;8:e1002835. doi: 10.1371/journal.pgen.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Damiola F, Le MN, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Minami Y, Horikawa K, Akiyama M, Shibata S. Restricted feeding induces daily expression of clock genes and pai-1 mrna in the heart of clock mutant mice. FEBS Lett. 2002;526:115–118. doi: 10.1016/s0014-5793(02)03159-9. [DOI] [PubMed] [Google Scholar]
- 137.Bray MS, Tsai JY, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O, Kueht M, Young ME. Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int J Obes (Lond) 2010;34:1589–1598. doi: 10.1038/ijo.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obes (Lond) 2013;37:843–852. doi: 10.1038/ijo.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hatori M, Vollmers C, Zarrinpar A, Ditacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012 doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stunkard AJ, Allison KC. Two forms of disordered eating in obesity: Binge eating and night eating. Int J Obes Relat Metab Disord. 2003;27:1–12. doi: 10.1038/sj.ijo.0802186. [DOI] [PubMed] [Google Scholar]
- 143.Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci. 2003;73:2467–2475. doi: 10.1016/s0024-3205(03)00628-3. [DOI] [PubMed] [Google Scholar]
- 144.Kudo T, Akiyama M, Kuriyama K, Sudo M, Moriya T, Shibata S. Night-time restricted feeding normalises clock genes and pai-1 gene expression in the db/db mouse liver. Diabetologia. 2004;47:1425–1436. doi: 10.1007/s00125-004-1461-0. [DOI] [PubMed] [Google Scholar]
- 145.Barclay JL, Husse J, Bode B, Naujokat N, Meyer-Kovac J, Schmid SM, Lehnert H, Oster H. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS One. 2012;7:e37150. doi: 10.1371/journal.pone.0037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schroeder AM, Colwell CS. How to fix a broken clock. Trends in pharmacological sciences. 2013;34:605–619. doi: 10.1016/j.tips.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hirota T, Lee JW, Lewis WG, Zhang EE, Breton G, Liu X, Garcia M, Peters EC, Etchegaray JP, Traver D, Schultz PG, Kay SA. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals ckialpha as a clock regulatory kinase. PLoS Biol. 2010;8:e1000559. doi: 10.1371/journal.pbio.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner DA, Doyle FJ, 3rd, Schultz PG, Kay SA. Identification of small molecule activators of cryptochrome. Science. 2012;337:1094–1097. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM, Burris TP. Regulation of circadian behaviour and metabolism by synthetic rev-erb agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hirota T, Lewis WG, Liu AC, Lee JW, Schultz PG, Kay SA. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of gsk-3beta. Proc Natl Acad Sci U S A. 2008;105:20746–20751. doi: 10.1073/pnas.0811410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vougogiannopoulou K, Ferandin Y, Bettayeb K, Myrianthopoulos V, Lozach O, Fan Y, Johnson CH, Magiatis P, Skaltsounis AL, Mikros E, Meijer L. Soluble 3',6-substituted indirubins with enhanced selectivity toward glycogen synthase kinase −3 alter circadian period. J Med Chem. 2008;51:6421–6431. doi: 10.1021/jm800648y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Klingman KM, Marsh EE, Klerman EB, Anderson EJ, Hall JE. Absence of circadian rhythms of gonadotropin secretion in women. J Clin Endocrinol Metab. 2011;96:1456–1461. doi: 10.1210/jc.2010-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.White WB. Importance of blood pressure control over a 24-hour period. Journal of managed care pharmacy : JMCP. 2007;13:34–39. doi: 10.18553/jmcp.2007.13.s8-b.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee P, Brychta RJ, Linderman J, Smith S, Chen KY, Celi FS. Mild cold exposure modulates fibroblast growth factor 21 (fgf21) diurnal rhythm in humans: Relationship between fgf21 levels, lipolysis, and cold-induced thermogenesis. J Clin Endocrinol Metab. 2013;98:E98–E102. doi: 10.1210/jc.2012-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yu H, Xia F, Lam KS, Wang Y, Bao Y, Zhang J, Gu Y, Zhou P, Lu J, Jia W, Xu A. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clinical chemistry. 2011;57:691–700. doi: 10.1373/clinchem.2010.155184. [DOI] [PubMed] [Google Scholar]
- 157.Koutkia P, Schurgin S, Berry J, Breu J, Lee BS, Klibanski A, Grinspoon S. Reciprocal changes in endogenous ghrelin and growth hormone during fasting in healthy women. Am J Physiol Endocrinol Metab. 2005;289:E814–E822. doi: 10.1152/ajpendo.00093.2005. [DOI] [PubMed] [Google Scholar]
- 158.Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: Maintenance of a meal-related pattern. European journal of endocrinology / European Federation of Endocrine Societies. 2005;152:845–850. doi: 10.1530/eje.1.01919. [DOI] [PubMed] [Google Scholar]
- 159.Forsling ML. Diurnal rhythms in neurohypophysial function. Experimental physiology. 2000:85. doi: 10.1111/j.1469-445x.2000.tb00022.x. Spec No:179S-186S. [DOI] [PubMed] [Google Scholar]