Abstract
Objective
To provide an update on a research device to ultrasonically reposition kidney stones transcutaneously. This paper reports preclinical safety and effectiveness studies, survival data, modifications of the system, and testing in a stone-forming porcine model. These data formed the basis for regulatory approval to test the device in humans.
Materials and Methods
The ultrasound burst was shortened to 50ms from previous investigations with 1s bursts. Focused ultrasound was used to expel 2–5mm calcium oxalate monohydrate stones placed ureteroscopically in five pigs. Additionally, de novo stones were imaged and repositioned in a stone-forming porcine model. Acute safety studies were performed targeting two kidneys (6 sites) and three pancreases (8 sites). Survival studies followed 10 animals for one week after simulated treatment. Serum and urine analyses were performed and tissues were evaluated histologically.
Results
All ureteroscopically-implanted stones (6/6) were repositioned out of the kidney in 14 ± 8 minutes with 13 ± 6 bursts. On average, three bursts moved a stone more than 4mm and collectively accounted for the majority of relocation. Stones (3mm) were detected and repositioned in the 200-kg stone-forming model. No injury was detected in the acute or survival studies.
Conclusions
Ultrasonic propulsion is safe and effective in the porcine model. Stones were expelled from the kidney. De novo stones formed in a large porcine model were repositioned. No adverse effects were identified with the acute studies directly targeting kidney or pancreatic tissue or during the survival studies indicating no evidence of delayed tissue injury.
Keywords: kidney, ultrasonography, kidney calculi, lithotripsy
Introduction
The incidence of nephrolithiasis is increasing, and the costs are estimated to represent a $5 billion social and economic burden annually1,2. Current treatment options for nephrolithiasis are effective, but commonly leave behind residual stones3,4. These residual stones may grow and lead to acute symptomatic stone episodes, emergency room visits, additional computed tomography (CT) scans, and up to 20% require further treatment procedures5,6.
The use of non-invasive ultrasound based technology to reposition urinary calculi has been previously described by our group7–11. Some of the many applications of the novel technology may include relieving obstructing calculi, pre-positioning of stones for improved surgical outcomes, and imaging confirmation of stone number and size9. This paper reports an investigation of the application of repositioning small kidney stones or residual fragments to facilitate their passage. Previous studies have demonstrated safety in animal studies and effectiveness in expelling primarily metal beads and artificial stones inserted in porcine kidneys8–10. The purpose of this study was to report on device refinements and improved effectiveness, to test relocation of de novo stones, and to evaluate for acute and delayed tissue injury in the initial survival studies. These studies represent the final preclinical safety and effectiveness evaluation of this technology prior to human subjects studies.
Materials and Methods
Equipment
The system, referred to as Generation 3 (Gen-3) is an extension of the Gen-2 device, with the addition of touch screen targeting and a modified push output8,9. With the Gen-3 system, the user targets and activates the push simultaneously by touching the stone location on screen. This improved target accuracy over the two-step approach used in the Gen-2 system. The push burst duration was also reduced from 1 second (s) to 50 ms. When a stone moves, it often quickly exits the focal field, which renders the majority of the 1 s burst ineffective7. The reduction in burst duration was achieved by increasing the duty factor, (the ratio of on-time to total time) from 3.3% (Gen-2) to 73% (Gen-3). Thus the same energy is delivered, but over a shorter time. The 50 ms burst contains 450-μs, 2-MHz pulses interlaced with 165 μs of quiet time. Pressure amplitude, and therefore intensity, of each pulse were unchanged. Greater system detail is provided in the paper by Cunitz et al. (2013)12.
The system operates similarly to a diagnostic ultrasound imager; the operator images the kidney and stone in standard ultrasound brightness mode (B-mode). The operator then touches the stone location on the screen and observes stone motion in real-time. The system focuses the ultrasound push burst to the targeted location, which creates an acoustic force on the stone causing the stone to move. The direction of the force is in the direction of the ultrasound beam. The user may pre-select one of two output levels for the burst, 50 or 90 V, compared to a movable sliding scale implemented in Gen-2.
Procedures
The studies on effectiveness and safety were approved by the University of Washington Institutional Animal Care and Use Committee (IACUC). Although ultrasonic propulsion is expected to be painless and delivered without anesthesia in clinical use, all pigs were anesthetized with 4% isoflurane as a method of restraint for all procedures. A veterinary pathologist blinded to the exposure conditions examined all harvested tissue sections for evidence of morphologic injury.
Effectiveness Study 1
Calcium oxalate monohydrate stones (2–5 mm) were ureteroscopically implanted into the right kidney lower pole of five 50–60 kg domestic female pigs. Figure 1 shows the first three stones that were implanted. Stone placement was confirmed via direct ureteroscopic visualization, fluoroscopy, and ultrasonography. The treatment goal was to use ultrasonic propulsion to reposition the lower pole stone into the ureteropelvic junction (UPJ) or proximal ureter. Stone displacement associated with each burst was graded on a scale of 0–4: 0 = no movement, 1 = stone vibration, 2 = movement and rollback to the original position, 3 = translation < 4 mm, and 4 = translation > 4 mm. The procedure was ceased once the operator felt the stone had been repositioned to the UPJ or proximal ureter, and ureteroscopy was then performed to confirm the final stone location and accuracy of the ultrasound-based assessment. Total number of push bursts and total procedure time were recorded from when the ultrasound probe was first placed on the animal.
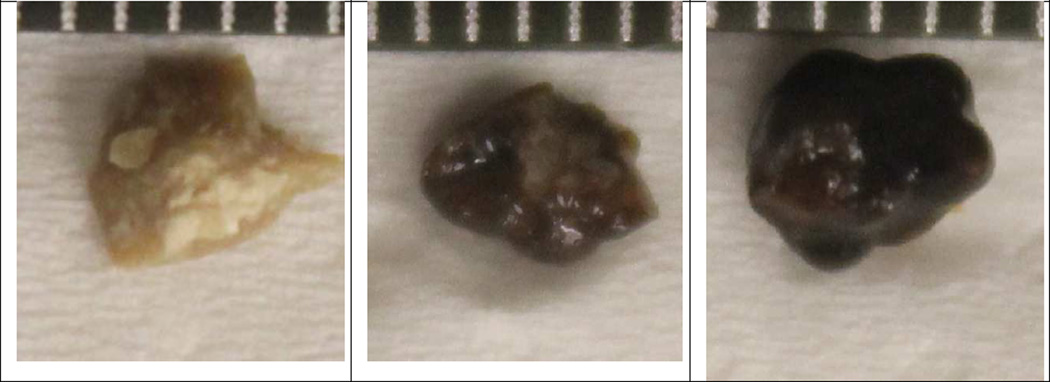
Figure 1.
Photographs of implanted wet stones next to mm scale. The center stone broke on implant, and both halves were treated.
Effectiveness Study 2
Ultrasonic propulsion of stones was performed in a diet-induced hyperoxaluria porcine model that formed de novo stones13. The objective was to test effectiveness at greater skin-to-stone distances and with stones potentially attached to tissue, as often seen with calcium oxalate stones. This study design eliminated complications related to surgical implantation, such as blood clots, the introduction of air, and iatrogenic hydronephrosis. Three sows (190–210 kg) were fed a hyperoxaluria diet for six weeks and were anesthetized and positioned prone. Our system was used to identify and reposition stones. Therapy was considered successful if displacement (grade 2, 3 or 4) was observed on ultrasound. The excised kidneys of the euthanized animals were surgically examined to obtain any stones.
Safety Study 1
Two 50–60 kg domestic female pigs were placed under general anesthesia but did not undergo surgical stone implantation. Kidneys were exposed to continuous B-mode imaging with a push burst every 30 s for a total of 120 bursts. B-mode and push output were at the maximum output level (90 V). Exposure was confined to one kidney and total delivery was divided into three pre-determined locations in the parenchyma (40 bursts each) starting in the lower pole, progressing to the mid-pole, and ending in the renal pelvis in simulation of lower-pole stone expulsion.
After treatment, the animals were euthanized. Kidneys were perfusion-fixed in situ with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, and processed for paraffin embedding. Five-micron sections were stained with hematoxylin and eosin (H&E). Six sections were taken from each treated kidney (two per treatment site) and one section was taken from each unexposed contralateral kidney for injury analysis.
Safety Study 2
Three female pigs (50–60 kg) were used to test potential injury from direct exposure to the pancreas, based on concern of sensitivity of pancreas to potential injury during shock wave lithotripsy14. For accurate targeting, the pancreas for each pig was exposed surgically, and the body cavity was filled with warm degassed phosphate buffer saline (PBS) for coupling to the ultrasound transducer. A single location on each pancreas received the same total acoustic exposure as in Safety Study 1 by reducing the voltage to 25 V to compensate for the lack of tissue attenuation. Additional locations on each pancreas received 10 or 40 burst exposures to test potential dose response. Tissue from the treated regions were fixed and embedded in paraffin.
Safety Study 3
Survival safety studies were performed to assess for renal injury up to seven days after treatment. A total of ten (5 female, 5 male) Hanford Miniature Swine (26–42 kg) were randomized to three treatment groups: high dose (6 animals, 6 kidneys), moderate dose (2 animals, 2 kidneys), or control (2 animals, 2 kidneys). All pigs underwent anesthesia but no invasive procedures. Control animals were anesthetized but did not receive ultrasound imaging or therapy exposure. Those in the high dose group underwent the same exposure as in Safety Study 1. The moderate dose was shortened to 20 push bursts at two separate sites (lower pole and renal pelvis) for a total of 40 bursts.
Animals were observed for signs of treatment related distress and were euthanized seven days after treatment. Blood and urine were obtained from animals before treatment, after treatment, and immediately prior to euthanasia and analyzed by Phoenix Central Laboratory (Mukilteo, WA). In necropsy, both renal units, tissue from the liver, adrenal gland, pancreas, spleen, skin, skeletal muscle, and rib in the acoustic path were harvested, fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with H&E. A total of 88 treated kidney sections, 12 controls kidney sections, and 61 other tissue sections were prepared.
Results
Effectiveness Study 1
Results are summarized in Table 1. Overall, 6/6 (100%) stones were successfully repositioned from the lower pole to the UPJ (4) or proximal ureter (2). Displacement to the UPJ or ureter was confirmed ureteroscopically in all pigs. Average procedure time was 14 ± 8 minutes using an average of 13 ± 6 bursts.
TABLE 1. Stone Propulsion Characteristics.
Effectiveness of repositioning ureteroscopically implanted human COM (Calcium Oxalate Monohydrate) kidney stones.
| Animal Number |
Number of Stones |
Stone Size (mm) |
Push Output (V) |
Motion Grading | Total Push Bursts |
Total Time (min) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||||
| 1 | 1 | 4.5 | 50 | 0 | 0 | 5 | 1 | 1 | 7 | 11 |
| 2 | 2 | 2 each | 90 | 17 | 3 | 7 | 5 | 3 | 35 | 45 |
| 3 | 1 | 4.5 | 50 | 2 | 0 | 9 | 1 | 4 | 16 | 9 |
| 4 | 1 | 3.5 | 50 | 0 | 0 | 0 | 1 | 3 | 4 | 4 |
| 5 | 1 | 3 | 90/50 | 0 | 0 | 11 | 0 | 6 | 17 | 16 |
| Mean ± Standard Deviation | 14 ± 5 | 14 ± 8 | ||||||||
0 = no movement; 1 = vibration; 2 = movement with rollback; 3= movement < 4mm; 4 = movement >4mm
Throughout the study we identified and overcame several issues. In animal #2, the stone fractured into two smaller fragments (~ 2 mm each), during the most time-consuming placement, which resulted in a blood clot and air in the collecting system. Air, in particular, complicated stone detection and delivery of ultrasonic propulsion to the target resulting in a large number of bursts with no movement, but ultimately ureteroscopy confirmed movement of both stones to the ureteropelvic junction.
In one animal (animal #3), we paused the ventilator to simulate breath holding and mitigate large respiratory excursion. This facilitated propulsion of the stone to the proximal ureter. Also, in animal #5, despite initial movement, the stone became trapped in the same location for 11 bursts. Several different probe positions were attempted until the last position moved the stone to the UPJ in an approximately 3 cm hop, underscoring the importance of alignment of the acoustic force. One additional burst moved the stone into the ureter.
Overall, 22% of bursts (17/79) resulted in displacements > 4 mm (Grade 4); on average, only 3 effective bursts were required to reposition each stone from the lower pole to the UPJ or beyond. Many of the ineffective bursts were misdirected at air bubbles that were confused for a stone. The air bubbles could be seen to break apart, float upward, and coalesce when mistakenly targeted. When animal #2 is excluded from analysis, average treatment time reduces to 10 ± 5 minutes with 11 ± 6 bursts. Also excluding animal #2, 32% (14/44) of bursts resulted in large jumps; 57% (25/44) of the bursts pushed the stones in a direction they were trapped; and the two bursts (5%) that had null effect missed the stone because of respiratory motion.
Effectiveness Study 2
Using B-mode ultrasound, two stones < 3 mm were identified in 6 renal units of the stone-forming pigs. Both stones were found in the right kidney, as all left kidneys were obscured by the pig’s spiral colon. No hydronephrosis was visualized in any renal unit. Ultrasonic propulsion was performed at a depth of 10 ± 1 cm with 2/2 (100%) stones successfully repositioned into the collecting system. Average treatment time was 20 ± 13 minutes requiring an average of 10 ± 8 bursts. On necropsy, a single 3 mm stone was found in each of the 2 treated kidneys, and two smaller stones were found in the third right kidney.
Safety Studies 1, 2 and 3
Acute safety studies demonstrated no evidence of kidney or pancreas injury in comparison to untreated control tissue samples. In survival studies, no animals displayed adverse clinical signs; there were no deaths during the course of the study. All blood and urine values were within the range of control values. In all tissues examined that were within the acoustic path, the histologic features did not differ from those of controls. Figure 2 shows representative histology images of the tissue studied from unexposed (control) and exposed locations.
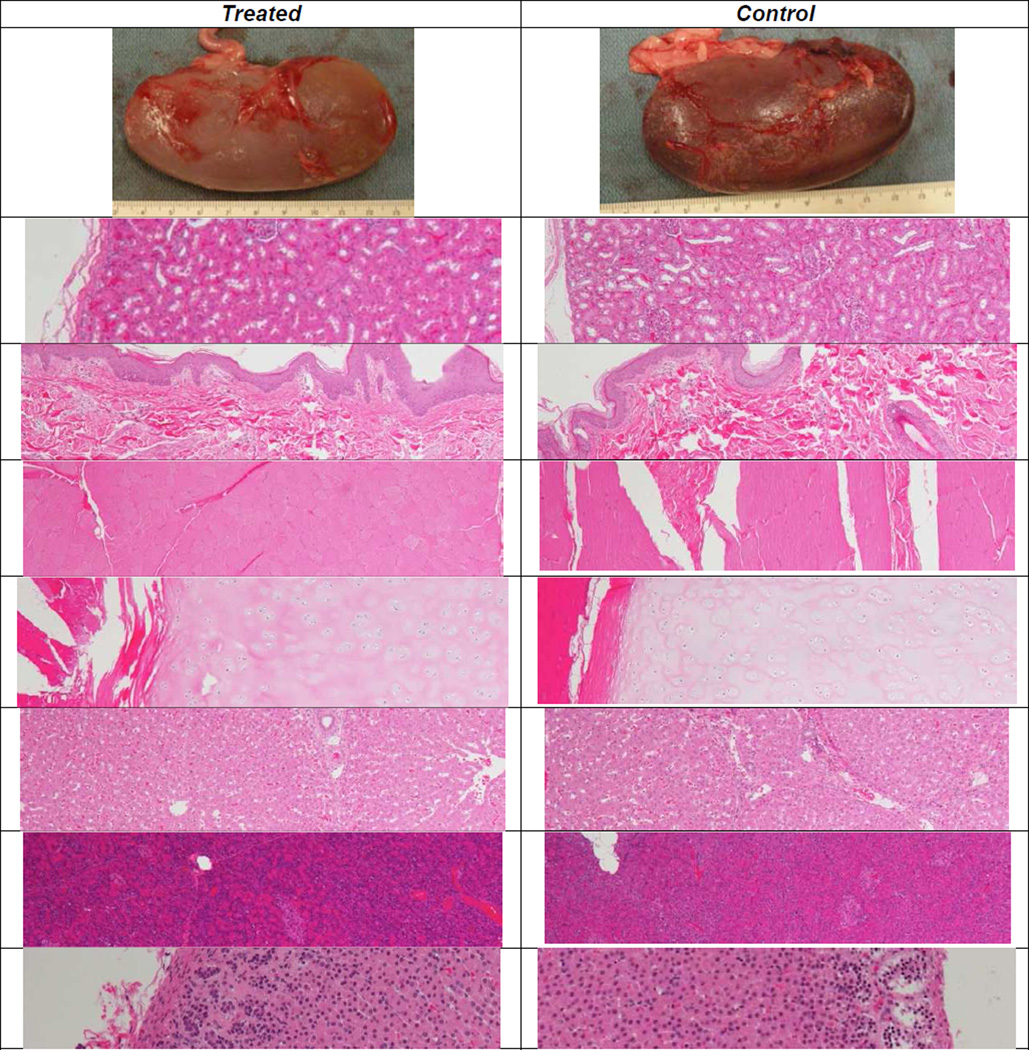
Figure 2.
Histology images (4× magnification except where noted) of treated (left) and control (right) tissues. From the top, tissues are: right kidney posterior (gross), kidney, skin, body wall muscle, rib cartilage, liver, pancreas, and adrenal gland (10×). The histologic features of the treated tissues did not differ from those of the controls.
Discussion
Our group has previously reported 65% success in using ultrasonic propulsion (Gen-2) to move primarily beads and artificial stones from the mid and lower poles of a porcine kidney model to the renal pelvis, UPJ or ureter8. Previously the procedure required an average 14 ± 8 minutes and 23 ± 16 ultrasound bursts, where the time was measured only while attempting to move the stone8. The current study reports significant improvement in effectiveness with all stones being successfully repositioned in less time with fewer bursts. This may be due to more efficient energy delivery, a user-friendlier targeting interface, and increased experience with the technology. In this study, our treatment time included the entire duration of the procedure from the time the probe contacted the skin to displacement of the stone to the UPJ. In all cases, the real-time imaging allowed the user to correctly determine that the stone had been expelled from the calyx, a finding confirmed ureteroscopically. Furthermore, de novo stones, 3 mm in size, were visualized in a large hyperoxaluric pig model and repositioned in both cases in which a stone was identified.
Overall, the majority of bursts induced some form of stone movement. But for most of those, it is believed that the direction of treatment force was not properly aligned with the stone’s exit path (infundibulum) through the collecting system. On average, only three well-aligned bursts were needed to move the stone out of the kidney. Thus, the potential exists for further improvement with training and practice on the system. A training curriculum was developed and tested on board certified urologists, who at the completion of training, moved a stone in a human torso phantom from the lower pole to the UPJ in 4.6 ± 2.2 minutes15. This reinforces the critical importance of the alignment of the acoustic force with the desired trajectory of the stone toward the renal pelvis and UPJ.
Acute animal studies demonstrated no evidence of injury in kidneys. Survival studies showed no evidence of injury on histopathologic analysis both within the renal unit as well as other tissues in the acoustic path. For this study we also obtained serum, blood, and urine analyses that demonstrated no abnormalities. These data are consistent with our prior studies demonstrating no injury with acoustic propulsion8,10. Injury is not expected as the energy intensity delivered to the tissue is similar to diagnostic ultrasound12 and well below the threshold for injury16.
Most exposures in the safety studies included a greater number of push bursts of higher intensity than the expected clinical exposures. In situ intensities were higher than expected in humans because penetration depth was lower in the smaller animals. Maximum exposure proposed for our human clinical trial is 40 bursts, delivered 1 minute apart at maximum power, 90 V. Most animals were exposed to an excessive dose of 120 bursts (1 maximum power burst every 30 s). The lack of injury at the excessive exposure did not justify completing additional studies at lesser exposures.
Furthermore, in Safety Studies 1 (acute kidney) and 3 (survival), the probe was positioned only 4 cm from the kidney and targeted on tissue not collecting space. The maximum acoustic pressure peaks 4 cm from the transducer. Beyond 4 cm, both poorer geometrical focusing and increased attenuating path length reduce the peak pressure. Because human skin to stone distance is expected to be greater than 7 cm in most, the local tissue will be exposed to lower intensities compared with the animal studies, and the in situ intensity for Safety Studies 1 and 3 was approximately twice the maximum clinical intensity. For the effectiveness studies and in clinical use, the acoustic outputs have been software limited to be no greater than the levels at 7 cm12.
The above findings represent a significant improvement in our ability to locate, target, and reposition renal calculi with ultrasonic propulsion, but have several limitations. First, the study was limited to stones <5 mm as our goal was expulsion of passable stones. Previously we have moved 10 and 11 mm surgically implanted stones within the porcine collecting system [8]. Larger stones require more force than smaller stones, but initial indication is our settings are sufficient. In addition, the use of retrograde ureteroscopy to place renal calculi introduced air and blood into the collecting system, decreasing our ability at times to detect and reposition stones. Animal numbers in each study are small, but sufficient, such that we were unable to justify additional studies that required animal sacrifice. Additionally, the porcine collecting system is a close but not exact replica of the human system. Lastly, an experienced ultrasonographer held the ultrasound probe on four of the animals; however, a urologist performed the ultrasonic propulsion on animal #5 and was comparably successful. The findings from our training study of ten urologists is reassuring that this technique can be successfully learned with training15.
Despite these limitations, our study demonstrates effectiveness and safety in the transcutaneous ultrasonic propulsion to expel small lower pole calculi from the kidney. These studies represent the final preclinical safety and effectiveness testing of ultrasonic propulsion of kidney stones. An investigational device exemption has been granted and a feasibility study of repositioning stones in human subjects is underway.
Conclusions
The use of ultrasonic propulsion to reposition small renal calculi is safe and effective in the porcine model. All stones were expelled from the lower pole in an average of 14 minutes. Ultrasonic propulsion may have a role in displacing stones adherent to the papilla, as expected in the hyperoxaluric model. Finally, acute and delayed injury survival studies demonstrated no evidence of injury.
Acknowledgements
This work supported by NIH DK43881, DK092197, NSBRI through NASA NCC 9–58, the Coulter Foundation, and the Institute of Translational Health Science. This material is the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, Washington. We thank the following for their help on the research: Thomas Crenshaw PhD and Stephen Nakada MD at University of Wisconsin for their help with the de novo stone experiments, James McAteer, PhD and Andrew Evan PhD at Indiana University and Martha Feldman RAC and Stephen Chernoff PhD at Drug and Device Development for help with study design. Thanks to Ziyue Liu PhD at Indiana University for statistical analysis.
Dr. Bailey reports grants from NIH, grants from National Space Biomedical Research Institute, grants from Institute of Translational Health Science, grants from Coulter Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Dr. Jonathan Harper has nothing to disclose. Ms. Dunmire has nothing to disclose. Dr. Wang has nothing to disclose. Dr. Simon has nothing to disclose. Dr. Liggit has nothing to disclose. Ms. Paun has nothing to disclose. Mr. Cunitz has nothing to disclose. Mr. Starr has nothing to disclose. Dr. Penniston has nothing to disclose. Dr. Lee has nothing to disclose. Dr. Hsi has nothing to disclose.
References
- 1.Pearle MS, Calhoun EA, Curhan GC. Urologic Diseases of America Project, Urologic diseases in America Project: Urolithiasis. J Urol. 2005;173:848–857. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 2.Scales CD, Jr, Smith AC, Hanley JM, et al. Urologic Diseases in America Project, Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearle MS, Lingeman JE, Leveillee R, et al. Prospective Randomized Trial Comparing Shock Wave Lithotripsy and Ureteroscopy for Lower Pole Caliceal Calculi 1 cm or Less. J Urol. 2008;179(5):S69–S73. doi: 10.1016/j.juro.2008.03.140. [DOI] [PubMed] [Google Scholar]
- 4.Tiselius HG. Urinary tract stone disease: Are all problems solved? Scand J Urol. 2013;47(1):4–9. doi: 10.3109/00365599.2012.680489. [DOI] [PubMed] [Google Scholar]
- 5.Osman M, Wendt-Nordahl G, Heger K, et al. Percutaneous nephrolithotomy with ultrasonography-guided renal access: experience from over 300 cases. BJU Int. 2005;96(6):875–878. doi: 10.1111/j.1464-410X.2005.05749.x. [DOI] [PubMed] [Google Scholar]
- 6.Osman MM, Alfano Y, Kamp S, et al. 5-year-follow-up of patients with clinically insignificant residual fragments after extracorporeal shockwave lithotripsy. Eur Urol. 2005;47(6):860–864. doi: 10.1016/j.eururo.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Shah A, Harper JD, Cunitz BW, et al. Focused ultrasound to expel calculi from the kidney. J Urol. 2012;187(2):739–743. doi: 10.1016/j.juro.2011.09.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper JD, Sorensen MD, Cunitz BW, et al. Focused ultrasound to expel calculi from the kidney: Safety and efficacy of a clinical prototype device. J Urol. 2013;190(3):1090–1095. doi: 10.1016/j.juro.2013.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorensen MD, Bailey MR, Hsi RS, et al. Focused ultrasound propulsion of kidney stones: Review and update of preclinical technology. J Endourol. 2013;27(10):1183–1186. doi: 10.1089/end.2013.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connors BA, Evan AP, Blomgren PM, et al. Comparison of tissue injury from focused ultrasonic propulsion of kidney stones versus extracorporeal shock wave lithotripsy. J Urol. 2014;191(1):235–241. doi: 10.1016/j.juro.2013.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorensen MD, Bailey MR, Hsi RS, et al. Focused Ultrasonic Propulsion of Kidney Stones. J Endourol, Part B: Videourology. 2013 Dec;27(6) doi: 10.1089/end.2013.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunitz BW, Dunmire B, Bailey MR. Characterization of an Ultrasound Propulsion System for Kidney Stones. IEEE UFFC. 2014 in review. [Google Scholar]
- 13.Patel SR, Penniston KL, Iwicki L, et al. Dietary Induction of Long-Term Hyperoxaluria in the Porcine Model. J Endourol. 2012;26(5):433–438. doi: 10.1089/end.2011.0182. [DOI] [PubMed] [Google Scholar]
- 14.Krambeck AE, Gettman MT, Rohlinger AL, et al. Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol. 2006;175(5):1742–1747. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 15.Hsi RS, Dunmire B, Cunitz BW, et al. Content and Face Validation of a Curriculum for Ultrasonic Propulsion of Renal Calculi in a Human Phantom. J Endourol. 2014;28(4):459–463. doi: 10.1089/end.2013.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y-N, Simon JC, Cunitz BW, et al. Focused ultrasound to displace renal calculi from the kidney: Threshold for tissue injury. J Ther Ultrasound. 2014;2:5. doi: 10.1186/2050-5736-2-5. http://www.jtultrasound.com/content/2/1/5. [DOI] [PMC free article] [PubMed] [Google Scholar]