Abstract
Dimethyl sulfide (DMS) is a climatically active gas released into the atmosphere from oceans. It is produced mainly by bacterial enzymatic cleavage of dimethylsulfoniopropionate (DMSP), and six DMSP lyases have been identified to date. To determine the biogeographical distribution of bacteria relevant to DMS production, we investigated the diversity of dddP—the most abundant DMS-producing gene—in the northwestern Pacific Ocean using newly developed primers and the pyrosequencing method. Consistent with previous studies, the major dddP-containing bacteria in coastal areas were those belonging to the Roseobacter clade. However, genotypes closely related to the SAR116 group were found to represent a large portion of dddP-containing bacteria in the surface waters of the oligotrophic ocean. The addition of DMSP to a culture of the SAR116 strain Candidatus Puniceispirillum marinum IMCC1322 resulted in the production of DMS and upregulated expression of the dddP gene. Considering the large area of oligotrophic water and the wide distribution of the SAR116 group in oceans worldwide, we propose that these bacteria may play an important role in oceanic DMS production and biogeochemical sulfur cycles, especially via bacteria-mediated DMSP degradation.
Introduction
Dimethylsulfoniopropionate (DMSP) is produced mainly by phytoplankton and macroalgae in the ocean [1,2], and may function as an osmolyte, antioxidant, predator deterrent, and cryoprotectant [3–7]. DMSP released into seawater by cell breakage processes, such as predation [5] and viral lysis [8], is catabolized via two enzymatic pathways: the demethylation and cleavage pathways [9,10]. The demethylation pathway is a major DMSP catabolic pathway and a source of reduced sulfur and carbon for microbial cells [11,12]. Alternatively, the cleavage pathway is mediated via various DMSP lyases [13] and produces dimethyl sulfide (DMS) gas, which can be released from oceans and photochemically oxidized, acting as a cloud condensing nucleus [14]. Although only a minor proportion (2–21%) of dissolved DMSP is cleaved into DMS [15], a great deal of attention has been paid to the cleavage pathway because of the relationship between DMS and climate change [14].
To date, six DMSP lyases have been identified from bacterial isolates: DddY from Alcaligenes faecalis [16], DddD from Marinomonas sp. and Ruegeria pomeroyi [10], DddL from Sulfitobacter sp. and Rhodobacter sphaeroides [17], DddP from Roseovarius nubinhibens and Ru. pomeroyi [18], DddQ from Ro. nubinhibens and Ru. pomeroyi [19], and DddW from Ru. pomeroyi [20]. Most lyases (DddY, DddP, DddQ, DddL, and DddW) cleave DMSP into DMS and acrylate, but DddD generates DMS and 3-hydroxypropionate from DMSP. Among the six DMSP lyases, dddP and dddQ genes were found to be most abundant in the Global Ocean Sampling (GOS) data set, indicating that they play important roles in ocean DMS production [19]. However, studies on the diversity and biogeography of these genes are rare [21,22].
To identify bacterial diversity related to DMS production, we established a transect from coast to tropical open ocean in the northwestern (NW) Pacific Ocean, and studied distribution of dddP gene diversity using a newly designed primer pair and amplicon pyrosequencing method.
Materials and Methods
Water samples were collected at nine stations during the NW Pacific Ocean study on the environment and interactions between deep ocean and marginal seas (POSEIDON) cruise in the NW Pacific Ocean from 26 May to 12 June 2010, aboard the R/V Onnuri (Fig. 1). Stations (Stns.) on lines F and P are located in a tropical area affected by the oligotrophic North Equatorial Current (NEC). Stations on line B are located in a subtropical area mainly affected by the oligotrophic Kuroshio Current (KC). Stations A3 and A5 are located in the eastern part of the East China Sea (ECS), through which a branch current of the KC passes. Finally, Stn. I is located in the central area of the ECS and mainly affected by coastal currents [23]. At each station, seawater was sampled at four to six depths between the surface and 150 m using Niskin bottles attached to a rosette sampler. As the sampling sites on the line B are located within the EEZ of Japan, we collected the samples with permission from the Ministry of Foreign Affairs of Japan. For the other stations, no specific permissions were required as samples were taken in domestic or international waters and did not involve endangered or protected species.
Figure 1. Map of sampling stations in the NW Pacific Ocean.
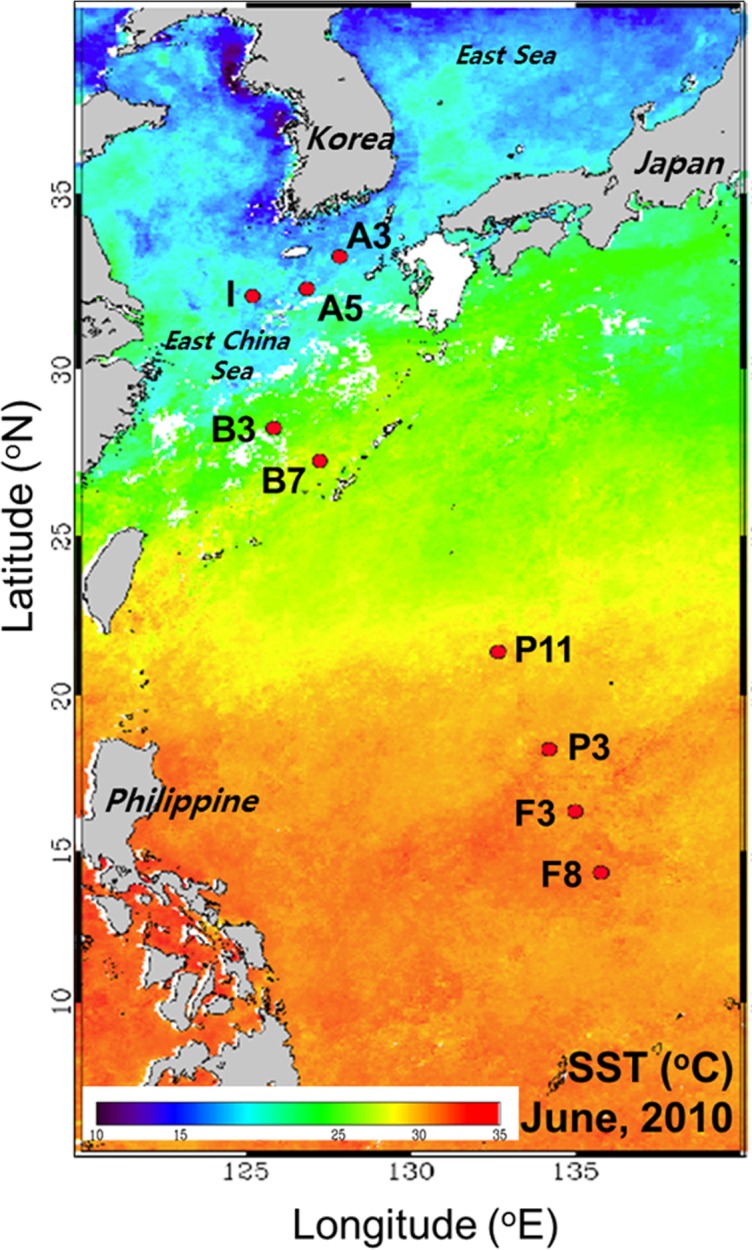
The base map is a composite image of sea surface temperature from 1 to 5 June 2010, obtained from SeaWiFS.
Primers for amplification of dddP genes
Amino acid sequences reported to be DddP-like polypeptides were obtained from known bacteria and fungi and from GOS data (refer to S1 Fig. in Todd et al. [18]), and used to design primers for dddP gene amplification. Using the amino acid sequences, partially degenerate CODEHOP (COnsensus-DEgenerate Hybrid Oligonuceotide Primer) PCR primers were designed by the iCODEHOP program [24]. For pyrosequencing using GS-FLX Titanium, a primer set that was expected to produce an amplicon size of ∼400 bp was selected from the entire set of degenerate primers designed by the program (Table 1). The degenerate core sequences of the forward and reverse primers were made from three (FYF; from 140th amino acid of DddP of Roseovarius nubinhibens ISM10994) and four (GEWI; from 264th amino acid) sequences conserved in DddP polypeptides from all known groups, respectively. In addition, the specificity of the designed primers was examined using the PCR–cloning–sequencing approach with selected samples; phylogenetic analysis showed that most clones (62 of 64) were clustered within the dddP clade and distributed among all known subclades (G1∼G3).
Table 1. Primers used in the pyrosequencing of dddP genes.
| Primer | Adapter | Key | MID | Specific oligonucleotide * (5’ → 3’) |
|---|---|---|---|---|
| B1F-fusion (forward) | CCATCTCATCCCTGCGTGTCTCCGAC | TCAG | Variable (10-mers) | CCGGCGCCGACHTNTTYTAYTT |
| D4R-fusion (reverse) | CCTATCCCCTGTGTGCCTTGGCAGTC | TCAG | None | CAGCCGGGTCTCGATCCAYTCNCC |
*Specific oligonucleotides consist of a degenerate ‘core’ (plain text) and non-degenerate ‘clamp’ (bold) region.
DNA extraction, PCR amplification, and pyrosequencing
Two-liter water samples were passed through a 0.2-μm Supor® filter (47 mm diameter, Gelman Sciences, Ann Arbor, MI, USA), and filters were frozen at −80°C after the addition of 1 ml of STE buffer (100 mM NaCl, 10 mM Tris–HCl, 1 mM EDTA, pH 8.0). For DNA extraction, filters were thawed, cut into small pieces with sterilized scissors, and placed in 50-ml sterile conical tubes. After adding 2 ml of STE buffer, microbial cells were lysed using lysozyme, sodium dodecyl sulfate, and proteinase K according to Somerville et al. [25]. The DNA was then purified from the lysates using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions.
To amplify dddP gene sequences, a two-round PCR method was adapted due to the low efficiency of the fusion primers. We added 1–10 ng of each template DNA to the PCR reaction (total 20 μl), which contained 1× GeneAmp PCR buffer I (Applied Biosystems, Foster City, CA, USA), 0.2 mM of each deoxyribonucleoside triphosphate, 0.5 μM of each primer with only a specific oligonucleotide (Table 1), and 2 units of AmpliTaq Gold DNA polymerase (Applied Biosystems). PCR amplification was conducted according to the following cycle parameters: an initial denaturation step (5 min, 94°C), followed by 30 cycles of denaturation (45 s, 94°C), annealing (45 s, 60°C), and extension (1 min, 72°C), and a final 10-min extension step at 72°C. After gel extraction of the target band using a gel extraction kit (Qiagen), a second round PCR was conducted using the fusion primers and the gel-purified first PCR products as templates (Table 1). Quantification of each final PCR product was performed on agarose gels using DNA QuantLadders (Lonza Rockland Inc., Rockland, ME, USA). Identical quantities of each PCR product were pooled and then purified using the AccuPrep PCR purification kit (Bioneer, Daejeon, Korea). After resolution on 2% agarose gel, the region between 450 and 550 bp was excised and DNA was extracted using a gel extraction kit (Qiagen). Pyrosequencing of PCR products was performed using GS-FLX Titanium (454 Life Sciences, Branford, CT, USA) at Macrogen Co. (Seoul, Korea). Sequence reads from this study were submitted to the National Center for Biotechnology Information (NCBI) sequence read archive (SRA; http://www.ncbi.nlm.nih.gov/Traces/sra; accession number SRX534398).
Pyrosequencing data analysis
Pyrosequencing data were analyzed using mainly the Qiime (v1.5; [26]) and Mothur softwares [27]. Raw reads were filtered to remove errors by allowing only perfect matches to the barcode and forward primer sequences. The allowed number of maximum homopolymers was 6. All reads <350 bp or >450 bp were removed. The flowgram data were then denoised [28]. Frameshift errors were corrected using the program HMM-FRAME [29]. Among the resulting reads, those without a reverse primer and those <330 bp were removed. In addition, reads were translated into amino acids, and any sequences possessing nonsense mutations were removed. Chimeric sequences were checked using the Perseus program implemented in the Mothur program. Operational taxonomic units (OTUs) were determined for the remaining DNA sequences using the Uclust at a similarity threshold of 90% [30].
Phylogenetic analysis
Phylogenetic analyses of DddP proteins were conducted using sequences from cultured α-proteobacteria and fungi, GOS metagenomic data [18], and the clone library [21]. In addition, we included genes annotated as dddP in the IMG-ER database [31]. After the amino acid sequences were aligned using the muscle program [32], a reference maximum-likelihood tree was constructed using PhyML 3.0 program [33] under JTT substitution model, incorporating a gamma distribution for among-site rate variation and an estimate of invariable sites (JTT + Γ + I). After translation, the phylogenetic positions of each representative sequence for the OTUs were placed onto the reference tree using the pplacer software [34].
DMS production by Candidatus Puniceispirillum marinum IMCC1322
To determine whether a strain belonging to the SAR116 group can produce DMS from DMSP, the IMCC1322 strain, which is one of two isolates belonging to SAR116 [35], was grown in the R2A broth. When the strain grew to an OD620 of 0.03, the culture was dispensed in two 30-ml sterile vials. Then, the DMSP substrate (final conc. of 500 μM) was injected into one of the vials, and DMS concentrations in the medium were quantified using DMS extraction, a trapping device, and a gas chromatograph equipped with a flame photometric detector (GF-FPD; [36]). Following the addition of DMSP, 50–100 μl of the samples was removed using a 250-μl gastight syringe (Hamilton 1725RN; Sigma-Aldrich, St. Louis, MO, USA). Sequential DMS measurements were carried out over periods of 15 min to 8 h. Abiotic control, containing the same concentration of DMSP substrate and filtered seawater, was prepared in parallel to correct for any background sources of DMS [37].
Reverse transcription (RT)-PCR was performed to examine expression of the dddP gene in IMCC1322 in the absence and presence of the DMSP substrate. Total RNA was extracted using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. cDNA was prepared from the RNA using the QuantiTech Reverse Transcription Kit (Qiagen). Subsequent PCR was conducted using the primer sets targeting 16S rRNA and dddP genes (S1 Table).
DMS and chlorophyll a concentrations
DMS analysis was conducted by gravitationally filtering 30 ml samples through a 47-mm glass-fiber filter (Whatman GF/F). Filtrates were stored in an amber glass vial with no headspace, and the vial was quickly sealed with a gas-tight cap, the inside of which was coated with Teflon. Within an hour of sampling, 10–20 ml of samples were delivered to the sparging chamber to measure DMS concentration by GC-FPD. The response of GC-FPD was calibrated against both gas standards with certified values of mole fractions of DMS (Scott Specialty Gases©, 522 ppbv DMS) and DMS solutions of known concentration prepared by alkaline hydrolysis of DMSP-Cl (Tokyo Kasei Inc.) in an amber vial (30 ml) with a gas tight Teflon cap. Chlorophyll a (chl a), extracted with 90% acetone, was determined using a Turner fluorometer (10 AU; Turner Designs, Sunnyvale, CA, USA; [38]).
Results and Discussion
Characteristics of the pyrosequencing run and OTUs
In this study, two-round of PCR was adapted. The extended PCR cycles might increase PCR artifacts, such as chimera and heteroduplex formations [39], and led to overestimation of species richness [40]. However, each clone library obtained by a normal 30 cycles PCR and two-round PCR with additional 20 cycles were similar (S1 Fig.), indicating that the PCR artifacts might not be significant. Further, we applied strict filtering and screening procedures including chimera removal and correction of frame-shift error,and moreover assigned OTUs at low sequence identity (90%) to minimize PCR artifacts. Thus, the genetic diversity of dddP genes, at least diversity of major groups, may not be seriously affected by the PCR artifacts in this study.
Through strict denoising and screening steps, we obtained a total of 54,548 reads. On the basis of 90% DNA sequence identity, sequences were clustered in 57 OTUs. As the dddP gene sequence differences among species belonging to the same genera were generally higher than 10% (S2 Table), the sequences clustered in each OTU likely belonged to the same genus.
Except for one sample (131 sequences of Stn. P3 at a depth of 108 m), the number of sequences among samples varied from 445 to 3,437 (mean ± 1SD, 1158 ± 575). In rarefaction curves, the observed OTU numbers tended to be saturated at each sampling depth (S2 Fig.).
Phylogenetic diversity of dddP genes
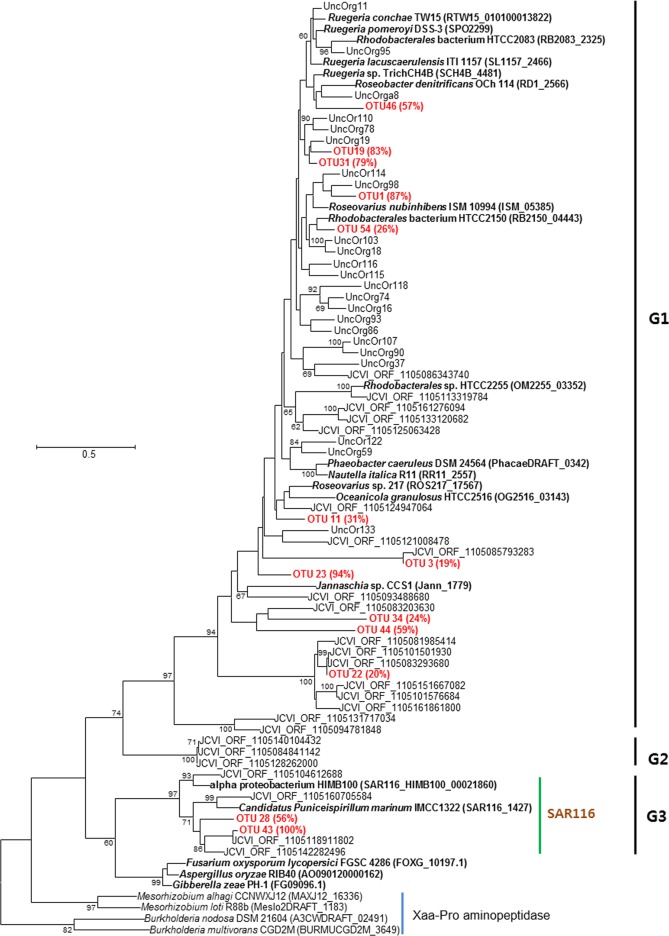
In the phylogenetic analyses (S3 Fig.), all 57 OTUs were distributed within the putative dddP clade, including dddP genes of α-proteobacteria and fungi, which are known to produce DMS from DMSP [18]. Among them, 42 were clustered with strains belonging to the Roseobacter clade (G1 group), indicating high diversity of dddP genes in the Roseobacter clade. Notably, 11 OTUs were clustered into a clade exclusively composed of two strains belonging to SAR116. DMS production by the SAR116 clade has not been well studied, despite the recognition that a dddP homolog exists in the SAR116 genome [13,22]. Although the SAR116 clade belonged to α-proteobacteria, the SAR116 cluster of dddP genes formed a sister clade with fungi (Fig. 2), confirming inter-domain horizontal gene transfer from α-proteobacteria to fungi [41].
Figure 2. Tree showing the phylogentic relationships of representative amino acid sequences of each OTU obtained in this study (red text).
Only OTUs constituting more than 10% of at least one sample are shown. The text in parentheses after bacterial names represent the locus tag for each genome in the IMG/ER database. The percentage in parentheses after OTU names represent maximum percentage of each OTU found in samples. Group names according to Todd et al. (2009) are shown on the right. A phylogentic tree for all OTUs can be found in S2 Fig. Only bootstrap values >60% are shown at the nodes.
Among the 57 OTUs, only 13 were dominant and occupied over 10% of at least one sample (Fig. 2). Conversely, 34 OTUs constituted less than 2% of all samples (S3 Fig.), suggesting that they are minor genotypes in the study area.
Distribution of dddP genes in the NW Pacific Ocean
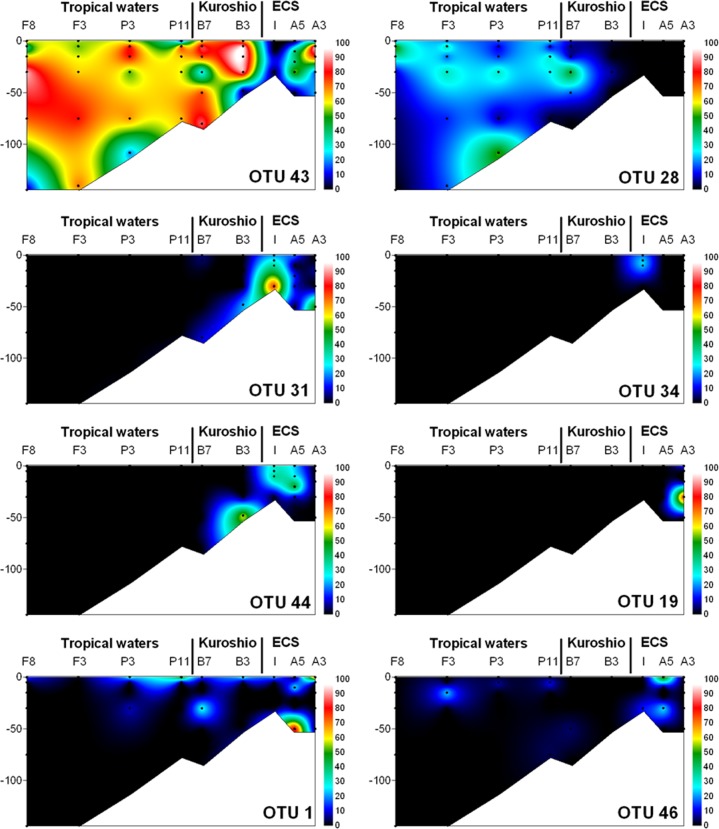
Two OTUs affiliated with the SAR116 clade dominated dddP genotypes in the euphotic depth of most stations, except Stn I (Fig. 3). Of them, OTU43 was the most dominant genotype in the oligotrophic open ocean and the coastal waters affected by the oligotrophic Kuroshio warm current. OTU28 was also found in the oligotrophic ocean but was less abundant than OTU43, and was not found in coastal waters. In tropical and subtropical waters, the two OTUs showed a complementary relationship, and thus the sum of two dddP genes, except the two lower euphotic samples, reached 82 ± 14% (mean ± 1SD). This suggests that bacteria related to SAR116 could play an important functional role in DMS production via DddP lyase.
Figure 3. Contour plots showing the percentage of each major OTU as a proportion of all sequences.
Black dots represent sampling depths.
Despite the fact that during the present study, dddP gene sequences in the SAR116 clade were dominant in the surface water of the oligotrophic NW Pacific Ocean with low chl a concentrations (Fig. 4A), Todd et al. [18] only retrieved five dddP gene sequences clustered in the SAR116 clade from the GOS data set. However, in a new BLAST search of the GOS data set using a DddP polypeptide of IMCC1322 as a query and a matching criterion of <e−80, 25 homologs clustered into group G3 were additionally retrieved from diverse samples (S4 Fig.). Although the previous study missed these due to the strict criterion for BLAST matching (<e−80), the dddP sequences in the SAR116 clade seemed to be abundant in the GOS samples.
Figure 4. Contour plots showing (A) chl a and (B) DMS concentration in the NW Pacific Ocean.
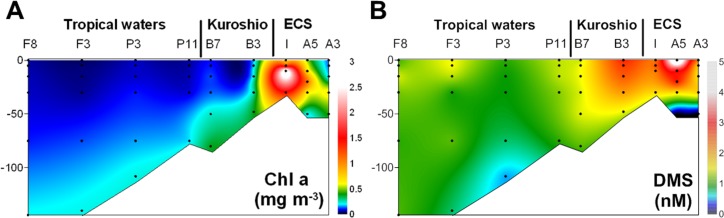
Black dots represent the sampling depths.
The composition of DMS-producing bacteria in coastal waters differed from that in oligotrophic open waters (Fig. 3). At Stn. I, having high chl a (3.2 μg l−1; Fig. 4A) due to influence of coastal water, three OTUs (OTU31, OTU34, and OTU44) clustered into the Roseobacter clade comprised of 68%–97% of the total sequences, suggesting that Roseobacter clade bacteria could contribute to DMS production via DddP lyase in coastal waters. However, the distribution of dddP genotypes in the ECS was largely variable among samples (Fig. 3). As seen in the spatial distribution of picocyanobacteria in the eastern ECS [42], this spatial variation seems to reflect complex physicochemical conditions resulting from the mixing of diverse water masses.
Despite maximal DMS concentration was found at surface of Stn A3 and tended to be higher in the coastal waters than in open waters, the difference of DMS concentrations in euphotic zone between open and coastal waters (mean ± 1SD of 2.4 ± 1.0 nM and 1.1 ± 0.2 nM, respectively) generally varied within 2-folds (Fig. 4B). Considering the vast area of the oligotrophic waters, thus, DMS production seems to be larger in oligotrophic open ocean than in coastal waters. The DddP lyases of SAR116 group were dominant from the oligotrophic tropical waters to the boundary waters of continental shelf. In this respect, the SAR116 group may play a significant role in global DMS production. On the contrary, Roseobacters seem to make a more contribution to DMS production in coastal area with relatively high chl a and DMS concentrations. Thus, SAR116 and Roseobacter groups seem to play a dominant role in DMS production via DddP lyases in the open and coastal waters, respectively.
DMS production by Candidatus Puniceispirillum marinum IMCC1322
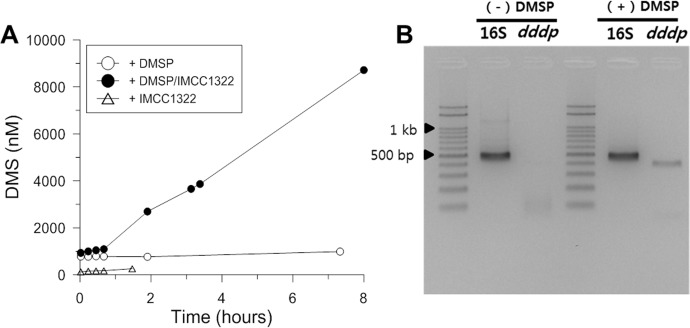
The production of DMS by strains in the SAR116 group has not yet been tested. When a culture of IMCC1322 was enriched with DMSP, DMS production greatly increased (Fig. 5A), confirming that this strain can produce DMS by utilizing DMSP. Furthermore, the transcription level of the dddP gene increased in a sample enriched with DMSP (Fig. 5B). As other kinds of putative DMSP lyases are not detected in the IMCC1322 genome yet [35], DMS production in the SAR116 strain most likely occurs via DddP lyase.
Figure 5. DMS production and expression of dddP mRNA of Candidatus Puniceispirillum marinum.
(A) Closed circles represent cells grown in R2A broth supplemented with 500 μM of DMSP. Open circles and triangles represent controls without cells and without DMSP, respectively. (B) RT-PCR of dddP mRNA and 16S rRNA of cells grown in media with DMSP and without DMSP, respectively.
In conclusion, although the Roseobacter clade was the dominant dddP-containing bacteria in coastal areas, the SAR116 group was found to be dominant dddP-containing bacteria in the oligotrophic NW Pacific Ocean. Furthermore, we experimentally confirmed DMS production in a SAR116 bacterium, a phenomenon that was neglected in previous studies. In the ocean, the “bacterial switch” between the competing demethylation and cleavage pathways could be regulated by diverse environmental factors such as UV radiation, carbon and sulfur demands, temperature, and DMSP availability [2,43–45]. But regulation mechanisms of the competing pathways have not yet been thoroughly understood. Strains belonging to SAR116 group also have the dmdA gene, and therefore participate in the demethylation of DMSP [35]. Furthermore, SAR116 group must be an important member in DMSP cycle in the ocean. Thus, further studies on DMSP metabolism of SAR116 groups would help our understanding of biogeochemical pathways of DMSP and environmental factors regulating these pathways in the ocean.
Supporting Information
Primer sequences used in the RT-PCR.
(DOC)
The number of base differences per site between dddP gene sequences of the bacterial isolates.
(DOC)
The DNA sample obtained at surface of Stn F8 in June, 2013 was used as a template and the primers used in the 1st and 2nd-PCR was shown in the Table 1 and Materials and Methods.
(DOC)
Rarefaction curves of surface sample of each sampling station.
(DOC)
Tree showing the phylogenetic relationships of representative amino acid sequences of each OTU obtained in this study.
(DOC)
Tree showing the phylogenetic positions of sequences (bold text) retrieved in this study from the GOS database.
(DOC)
Acknowledgments
We thank I-K Park for assisting in pyrosequencing and qPCR.
Data Availability
Sequence reads from this study were submitted to the National Center for Biotechnology Information (NCBI) sequence read archive (SRA; http://www.ncbi.nlm.nih.gov/Traces/sra; accession number SRX534398).
Funding Statement
This study was supported by in-house research programs (PE98704, PE99231, PE99212) of the Korea Institute of Ocean and Science Technology (KIOST). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Burgermeister S, Zimmermann RL, Georgii HW, Bingemer HG, Kirst GO, et al. (1990) On the biogenic origin of dimethylsulfide—relation between chlorophyll, ATP, organismic DMSP, phytoplankton species, and DMD distribution in Atlantic surface-water and atmosphere. Journal of Geophysical Research-Atmospheres 95: 20607–20615. [Google Scholar]
- 2. Kiene RP, Linn LJ, Bruton JA (2000) New and important roles for DMSP in marine microbial communities. Journal of Sea Research 43: 209–224. [Google Scholar]
- 3. Kirst GO (1990) Salinity tolerance of eukaryotic marine-algae. Annual Review of Plant Physiology and Plant Molecular Biology 41: 21–53. [Google Scholar]
- 4. Karsten U, Wiencke C, Kirst GO (1992) Dimethylsulphoniopropionate (DMSP) accumulation in green macroalgae from polar to temperate regions—interactive effects of light versus salinity and light versus temperature. Polar Biology 12: 603–607. [Google Scholar]
- 5. Wolfe GV, Steinke M, Kirst O (1997) Grazing-activated chemical defence in a unicellular marine alga. Nature 387: 894–897. [Google Scholar]
- 6. Sunda W, Kieber DJ, Kiene RP, Huntsman S (2002) An antioxidant function for DMSP and DMS in marine algae. Nature 418: 317–320. 10.1038/nature00851 [DOI] [PubMed] [Google Scholar]
- 7. Strom S, Wolfe G, Slajer A, Lambert S, Clough J (2003) Chemical defense in the microplankton II: Inhibition of protist feeding by beta-dimethylsulfoniopropionate (DMSP). Limnology and Oceanography 48: 230–237. [Google Scholar]
- 8. Hill RW, White BA, Cottrell MT, Dacey JWH (1998) Virus-mediated total release of dimethylsulfoniopropionate from marine phytoplankton: a potential climate process. Aquatic Microbial Ecology 14: 1–6. [Google Scholar]
- 9. Reisch CR, Moran MA, Whitman WB (2008) Dimethylsulfoniopropionate-dependent demethylase (DmdA) from Pelagibacter ubique and Silicibacter pomeroyi . Journal of Bacteriology 190: 8018–8024. 10.1128/JB.00770-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Todd JD, Rogers R, Li YG, Wexler M, Bond PL, et al. (2007) Structural and regulatory genes required to make the gas dimethyl sulfide in bacteria. Science 315: 666–669. 10.1126/science.1135370 [DOI] [PubMed] [Google Scholar]
- 11. Kiene RP, Linn LJ, Gonzalez J, Moran MA, Bruton JA (1999) Dimethylsulfoniopropionate and methanethiol are important precursors of methionine and protein-sulfur in marine bacterioplankton. Applied and Environmental Microbiology 65: 4549–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reisch CR, Moran MA, Whitman WB (2011) Bacterial catabolism of dimethylsulfoniopropionate. Frontiers in Microbiology 2: 172 10.3389/fmicb.2011.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curson ARJ, Todd JD, Sullivan MJ, Johnston AWB (2011) Catabolism of dimethylsulphoniopropionate: microorganisms, enzymes and genes. Nature Reviews Microbiology 9: 849–859. 10.1038/nrmicro2653 [DOI] [PubMed] [Google Scholar]
- 14. Charlson RJ, Lovelock JE, Andreae MO, Warren SG (1987) Oceanic phytoplankton, atmospheric sulfur, cloud albedo and climate. Nature 326: 655–661. [Google Scholar]
- 15. Kiene RP, Linn LJ (2000) Distribution and turnover of dissolved DMSP and its relationship with bacterial production and dimethylsulfide in the Gulf of Mexico. Limnology and Oceanography 45: 849–861. [Google Scholar]
- 16. Curson ARJ, Sullivan MJ, Todd JD, Johnston AWB (2011) DddY, a periplasmic dimethylsulfoniopropionate lyase found in taxonomically diverse species of Proteobacteria. ISME Journal 5: 1191–1200. 10.1038/ismej.2010.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Curson ARJ, Rogers R, Todd JD, Brearley CA, Johnston AWB (2008) Molecular genetic analysis of a dimethylsulfoniopropionate lyase that liberates the climate-changing gas dimethylsulfide in several marine alpha-proteobacteria and Rhodobacter sphaeroides . Environmental Microbiology 10: 757–767. 10.1111/j.1462-2920.2007.01499.x [DOI] [PubMed] [Google Scholar]
- 18. Todd JD, Curson ARJ, Dupont CL, Nicholson P, Johnston AWB (2009) The dddP gene, encoding a novel enzyme that converts dimethylsulfoniopropionate into dimethyl sulfide, is widespread in ocean metagenomes and marine bacteria and also occurs in some Ascomycete fungi. Environmental Microbiology 11: 1376–1385. 10.1111/j.1462-2920.2009.01864.x [DOI] [PubMed] [Google Scholar]
- 19. Todd JD, Curson ARJ, Kirkwood M, Sullivan MJ, Green RT, et al. (2011) DddQ, a novel, cupin-containing, dimethylsulfoniopropionate lyase in marine roseobacters and in uncultured marine bacteria. Environmental Microbiology 13: 427–438. 10.1111/j.1462-2920.2010.02348.x [DOI] [PubMed] [Google Scholar]
- 20. Todd JD, Kirkwood M, Newton-Payne S, Johnston AWB (2012) DddW, a third DMSP lyase in a model Roseobacter marine bacterium, Ruegeria pomeroyi DSS-3. ISME Journal 6: 223–226. 10.1038/ismej.2011.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peng MJ, Xie QY, Hu H, Hong K, Todd JD, et al. (2012) Phylogenetic diversity of the dddP gene for dimethylsulfoniopropionate-dependent dimethyl sulfide synthesis in mangrove soils. Canadian Journal of Microbiology 58: 523–530. 10.1139/w2012-019 [DOI] [PubMed] [Google Scholar]
- 22. Varaljay VA, Gifford SM, Wilson ST, Sharma S, Karl DM, et al. (2012) Bacterial dimethylsulfoniopropionate degradation genes in the oligotrophic north Pacific subtropical gyre. Applied and Environmental Microbiology 78: 2775–2782. 10.1128/AEM.07559-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ichikawa H, Beardsley RC (2002) The current system in the Yellow and East China Seas. Journal of Oceanography 58: 77–92. [Google Scholar]
- 24. Boyce R, Chilana P, Rose TM (2009) iCODEHOP: a new interactive program for designing COnsensus-DEgenerate Hybrid Oligonucleotide Primers from multiply aligned protein sequences. Nucleic Acids Research 37: W222–W228. 10.1093/nar/gkp379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Somerville CC, Knight IT, Straube WL, Colwell RR (1989) Simple, rapid method for direct isolation of nucleic acids from aquatic environments. Appl Environ Microbiol 55: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, et al. (2010) QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7: 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, et al. (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology 75: 7537–7541. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reeder J, Knight R (2010) Rapidly denoising pyrosequencing amplicon reads by exploiting rank-abundance distributions. Nature Methods 7: 668–669. 10.1038/nmeth0910-668b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y, Sun YN (2011) HMM-FRAME: accurate protein domain classification for metagenomic sequences containing frameshift errors. BMC Bioinformatics 12: 198 10.1186/1471-2105-12-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 31. Markowitz VM, Mavromatis K, Ivanova NN, Chen IMA, Chu K, et al. (2009) IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics 25: 2271–2278. 10.1093/bioinformatics/btp393 [DOI] [PubMed] [Google Scholar]
- 32. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, et al. (2010) New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Systematic Biology 59: 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 34. Matsen FA, Kodner RB, Armbrust EV (2010) pplacer: linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinformatics 11: 538 10.1186/1471-2105-11-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oh HM, Kwon KK, Kang I, Kang SG, Lee JH, et al. (2010) Complete genome Sequence of “Candidatus Puniceispirillum marinum” IMCC1322, a representative of the SAR116 clade in the Alphaproteobacteria . Journal of Bacteriology 192: 3240–3241. 10.1128/JB.00347-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park KT, Lee K (2008) High-frequency, accurate measurement of dimethylsulfide in surface marine environments using a microporous membrane contactor. Limnology and Oceanography-Methods 6: 548–557. [Google Scholar]
- 37. Steinke M, Malin G, Turner SM, Liss PS (2000) Determinations of dimethylsulphoniopropionate (DMSP) lyase activity using headspace analysis of dimethylsulphide (DMS). Journal of Sea Research 43: 233–244. [Google Scholar]
- 38. Parsons TR, Maita Y, Lalli CM (1984) A Manual of Chemical and Biological Methods for Seawater Analysis. Amsterdam: Pergamon. [Google Scholar]
- 39. Acinas SG, Sarma-Rupavtarm R, Klepac-Ceraj V, Polz MF (2005) PCR-induced sequence artifacts and bias: Insights from comparison of two 16S rRNA clone libraries constructed from the same sample. Applied and Environmental Microbiology 71: 8966–8969. 10.1128/AEM.71.12.8966-8969.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ahn JH, Kim BY, Song J, Weon HY (2012) Effects of PCR cycle number and DNA polymerase type on the 16S rRNA gene pyrosequencing analysis of bacterial communities. Journal of Microbiology 50: 1071–1074. 10.1007/s12275-012-2642-z [DOI] [PubMed] [Google Scholar]
- 41. Kirkwood M, Todd JD, Rypien KL, Johnston AWB (2010) The opportunistic coral pathogen Aspergillus sydowii contains dddP and makes dimethyl sulfide from dimethylsulfoniopropionate. ISME Journal 4: 147–150. 10.1038/ismej.2009.102 [DOI] [PubMed] [Google Scholar]
- 42. Choi DH, Noh JH, Shim J (2013) Seasonal changes in picocyanobacterial diversity as revealed by pyrosequencing in temperate waters of the East China Sea and the East Sea. Aquatic Microbial Ecology 71: 75–90. [Google Scholar]
- 43. Simo R (2001) Production of atmospheric sulfur by oceanic plankton: biogeochemical, ecological and evolutionary links. Trends in Ecology & Evolution 16: 287–294. [DOI] [PubMed] [Google Scholar]
- 44. Slezak D, Kiene RP, Toole DA, Simo R, Kieber DJ (2007) Effects of solar radiation on the fate of dissolved DMSP and conversion to DMS in seawater. Aquatic Sciences 69: 377–393. [Google Scholar]
- 45. Levine NM, Varaljay VA, Toole DA, Dacey JWH, Doney SC, et al. (2012) Environmental, biochemical and genetic drivers of DMSP degradation and DMS production in the Sargasso Sea. Environmental Microbiology 14: 1210–1223. 10.1111/j.1462-2920.2012.02700.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences used in the RT-PCR.
(DOC)
The number of base differences per site between dddP gene sequences of the bacterial isolates.
(DOC)
The DNA sample obtained at surface of Stn F8 in June, 2013 was used as a template and the primers used in the 1st and 2nd-PCR was shown in the Table 1 and Materials and Methods.
(DOC)
Rarefaction curves of surface sample of each sampling station.
(DOC)
Tree showing the phylogenetic relationships of representative amino acid sequences of each OTU obtained in this study.
(DOC)
Tree showing the phylogenetic positions of sequences (bold text) retrieved in this study from the GOS database.
(DOC)
Data Availability Statement
Sequence reads from this study were submitted to the National Center for Biotechnology Information (NCBI) sequence read archive (SRA; http://www.ncbi.nlm.nih.gov/Traces/sra; accession number SRX534398).