Abstract
Fibroblast growth factors (FGFs) play important roles in many aspects of embryonic development. During eye development, the lens and corneal epithelium are derived from the same surface ectodermal tissue. FGF receptor (FGFR)-signaling is essential for lens cell differentiation and survival, but its role in corneal development has not been fully investigated. In this study, we examined the corneal defects in Fgfr2 conditional knockout mice in which Cre expression is activated at lens induction stage by Pax6 P0 promoter. The cornea in LeCre, Fgfr2loxP/loxP mice (referred as Fgfr2CKO) was analyzed to assess changes in cell proliferation, differentiation and survival. We found that Fgfr2CKO cornea was much thinner in epithelial and stromal layer when compared to WT cornea. At embryonic day 12.5–13.5 (E12.5–13.5) shortly after the lens vesicle detaches from the overlying surface ectoderm, cell proliferation (judged by labeling indices of Ki-67, BrdU and phospho-histone H3) was significantly reduced in corneal epithelium in Fgfr2CKO mice. At later stage, cell differentiation markers for corneal epithelium and underlying stromal mesenchyme, keratin-12 and keratocan respectively, were not expressed in Fgfr2CKO cornea. Furthermore, Pax6, a transcription factor essential for eye development, was not present in the Fgfr2CKO mutant corneal epithelial at E16.5 but was expressed normally at E12.5, suggesting that FGFR2-signaling is required for maintaining Pax6 expression in this tissue. Interestingly, the role of FGFR2 in corneal epithelial development is independent of ERK1/2-signaling. In contrast to the lens, FGFR2 is not required for cell survival in cornea. This study demonstrates for the first time that FGFR2 plays an essential role in controlling cell proliferation and differentiation, and maintaining Pax6 levels in corneal epithelium via ERK-independent pathways during embryonic development.
Introduction
The cornea is a transparent tissue on the surface of the eye with refractive properties for bending light rays. The development of the vertebrate cornea involves inductive interactions between surface ectodermal and mesenchymal tissues [1]. At embryonic day 8.5 to 9.0 (E8.5–9.0), a thickened region of the head ectoderm, defined as the lens placode, gives rise to both the lens and the presumptive corneal epithelium. The primitive corneal epithelium forms after the lens vesicle detaches from the overlying surface ectoderm. At around E12.0–12.5, the perioptic mesenchyme (mostly neural crest cells) migrates into the space between the lens and the primitive corneal epithelium [1,2]. At E14.5–15.5 in the mouse eye, the posterior mesenchymal cells closest to the lens differentiate into a thin layer of corneal endothelium, and the anterior chamber subsequently forms between the lens and cornea. The mesenchymal cells between the corneal epithelium and endothelium begin to differentiate into keratocytes and form corneal stroma. The corneal epithelium continues to differentiate after birth and, upon eyelid opening at two weeks of age, the corneal epithelium expands from two cell layers to a self-renewing, stratified epithelium comprising eight to 10 cell layers [3,4]. The fully developed cornea is composed of three layers derived from two embryonic germ tissues: a stratified corneal epithelium with surface ectoderm origin on the outer surface, expressing the keratin 3 and 12 (K3/K12) pair [5]; the stromal layer underneath, sparsely populated by keratocytes composed of highly aligned collagen, and the inner surface of the cornea, covered by a single-layer endothelium.
Corneal injury and disease can lead to opacification, neovascularization, fibrosis and defective wound healing. These pathological conditions together constitute the second leading cause of blindness worldwide [6]. Understanding the inductive factors and signals that regulate corneal cell proliferation and differentiation has important implications for the development of therapeutic approaches for controlling corneal repair and homeostasis and preventing blindness. Several lines of evidence support the integral role of fibroblast growth factors (FGFs) in corneal cell proliferation and differentiation [7]. As many as 22 FGFs have been identified in vertebrates [8]. FGF signaling is activated through binding of the growth factor to its cell surface receptors to stimulate receptor dimerization and activation of receptor tyrosine kinases, ultimately leading to activation of various downstream signal transduction cascades [9]. Four fibroblast growth factor receptor (FGFR) genes (FGFR1 to FGFR4) have been cloned and identified in mammals. Additionally, multiple FGFR isoforms, differing in structure and ligand affinity, can be generated through alternative splicing of primary transcripts. For example, two FGFR2 variants, FGFR2IIIb and FGFR2IIIc, are generated by alternative splicing at the second half of Ig domain III of the FGFR2 locus [10,11]. During corneal development, FGF-7 and FGF-10 are secreted by corneal mesenchymal cells and both can bind with affinity to FGF receptor 2 (FGFR2-IIIb) isoform, which is expressed mainly in limbal and central corneal epithelium [12–14]. These expression patterns imply that FGFR2-signaling may promote limbal stem cell proliferation and participate in modulation of corneal epithelium renewal and homeostasis. In vitro functional studies have shown that FGF-7 enhances the growth and proliferation of cultured corneal epithelial cells but does not significantly affect motility [15] [16]. Topical application of FGF-7 was shown in vivo and in vitro to accelerate corneal epithelial wound healing [17–19]. In an investigation of the role of FGFR activation in corneal development, transgenic mice overexpressing FGF-7 or FGF-10 in the developing lens (starting as early as E11.5) exhibited hyperproliferative corneal epithelial cells that subsequently were induced to alter their cell fate from corneal epithelium to lacrimal gland epithelium [20–22]. In another study of transgenic mice, overexpression of FGF-3, another member in the FGF family also capable of activating FGFR2IIIb, was found to stimulate epithelial-to-glandular transformation in the developing cornea of the transgenic mice [23]. However, when excess FGF-7 was induced in the corneal epithelium of young mice, the main phenotype was hyperplasia in the epithelial layer, without alteration in cell fate [24]. The corneal epithelium increased in thickness from 6 or 7 cell layers to more than 20 cell layers, with extended K14 expression from the basal to suprabasal to superficial layers. Phenotypic variations caused by excessive FGF-7 were found in the eyes of embryos and young pups, which may be explained by the age-dependent differences of FGFR2-activated signaling network in developing corneal epithelium and the plasticity of progenitor cells. However, these gain-of-function studies have not defined the normal biological role of FGFR2 in corneal development.
The function of FGFR2 in the development of ocular surface ectodermal tissues, including the lens and the lacrimal glands, has been investigated using the Fgfr2 conditional knockout mice (referred as Fgfr2 CKO) driven by a surface ectodermal Cre line, the Le-Cre [25–27]. These studies revealed that the FGFR2-activated Ras-ERK signaling pathway is essential for cell survival and cell cycle exit during ocular lens development and for induction of the lacrimal glands. Although FGFR2 is known to be expressed in the corneal epithelium, the developmental changes in the cornea of Fgfr2 conditional knockout mice have not been investigated in detail. In this study, we demonstrate that FGFR2 is required for corneal epithelial cell proliferation at the stage shortly after the lens vesicle detaches from the surface ectoderm. In contrast to its role in the lens, FGFR2 is not essential for corneal epithelial cell survival. Furthermore, we demonstrate that FGFR2 plays an essential role in maintaining the Pax6 expression, independent of ERK-signaling, in corneal epithelium. In the absence of Pax6, differentiation and maturation of corneal epithelium is inhibited in Fgfr2 CKO mice. We also found that the abnormal development of corneal epithelium in Fgfr2 CKO mice does not affect the migration and proliferation of the corneal mesenchymal cells, but prevents these cells from differentiating into mature keratocytes, suggesting that loss of FGFR2 interferes with the signaling interactions between the corneal epithelium and underlying mesenchyme.
Materials and Methods
Mice
Mice carrying the Fgfr2 flox alleles and the Le-Cre transgenic mice were obtained from Dr. Michael Robinson (Miami University, Oxford, OH, USA), with permission from Drs. David Ornitz and Ruth Ashery-Padan, respectively [25,27,28]. The ERK1/2 double deletion mice (LeCre-Mapk1 fl/f l;Mapk3 -/-) were described previously [29]. To generate Fgfr2 conditional knockout mice, Le-Cre mice were bred to Fgfr2 flox/flox mice and the heterozygous offspring Le-Cre;Fgfr2 flox/+ mice were then crossed with Fgfr2 flox/flox to make Le-Cre;Fgfr2 flox/flox (referred as Fgfr2 CKO) mice. The Le-Cre mice in all experiments were heterozygous for the transgene. The Fgfr2 loxP/loxP mice are referred to as wild type (WT). Because Le-Cre hemizygous transgenic mice were shown to develop eye abnormalities on some genetic backgrounds [30], we recently crossed the Fgfr2 CKO mice with C57BL6J mice to examine potential defects in the cornea of Le-Cre;Fgfr2 flox/+ mice. Animal use was in accordance with the Association of Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and all experimental procedures were approved by the Animal Care and Use Committee of the University of Missouri-Columbia.
Histology, immunohistochemistry and immunofluorescence
Embryonic and newborn mouse heads were fixed in 4% paraformaldehyde for 2 hours or overnight and processed for histological analysis by hematoxylin and eosin (H&E) staining, as described previously [31]. For immunohistochemistry and immunofluorescence, the following primary antibodies were used: anti-Ki67 (M7249, Dako, Carpinteria, CA, USA); anti-keratin-14 (K14) (PBR-159P) and anti-Pax6 (PBR-278P), both from Covance Inc, Princeton, NJ, USA; anti-N-cadherin (33–3900, Zymed, Camarillo, CA, USA), and anti-phospho-histone H3 (sc-8656-R, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Anti-keratocan and anti-keratin-12 (K12) antibodies were gifts from Dr. Chia-yang Liu at University of Cincinnati (Cincinnati, OH, USA) [24]. For immunofluorescence, Alexa Fluor conjugated secondary antibodies were purchased from Invitrogen (Carlsbad, CA, USA) and cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). The signal-enhancement TSA kit (NEL741B001KT, PerkinElmer, Boston, MA, USA) was used for immunofluorescence against Ki67, K12 and N-cadherin. For immunohistochemistry, biotinylated secondary antibodies were from Vector Laboratories (Burlingame, CA, USA) and color was developed by using 3, 3′-diaminobenzidine as a substrate (D4293, Sigma, St Louis, MO, USA). Sections were counterstained for cell nuclei by hematoxylin.
Brdu incorporation and TUNEL assays
5-Bromo-2′-deoxyuridine (BrdU) was administered intraperitoneally into pregnant mice at a concentration of 0.1μg/gm body weight and labeled for 1 hour prior to embryo isolation. BrdU immunohistochemistry was performed as previously described [32]. Terminal deoxynucleotidyl transferse dUTP nick end labeling (TUNEL) assay was performed with in situ apoptosis detection kit (S7165, Millipore, Billerica, MA, USA), following the manufacturer’s instructions.
Statistical analysis
Quantification of cell proliferation was performed by determining the fraction of labeled nuclei over the total number of nuclei present in a given section. A minimum of 3 embryos for each genotype were analyzed at a given time point. Data are expressed as mean ± SEM and p-values were calculated using Mann-Whitney Test (p<0.05 was considered significant).
Results
Conditional deletion of Fgfr2 in ocular surface ectoderm affects corneal development
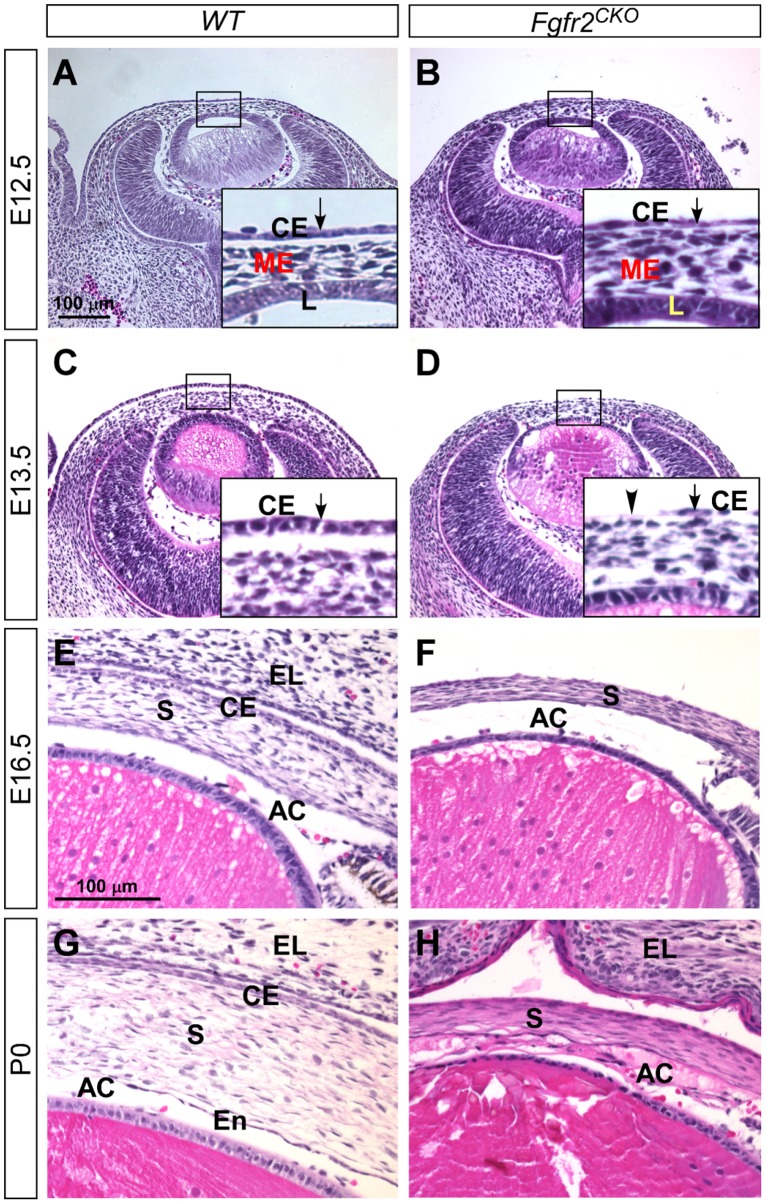
In eye development, the surface ectoderm gives rise to both corneal epithelium and the lens. The essential role of FGFR2 in lens development has been shown in the Fgfr2 conditional deletion mice by a surface ectoderm driver Le-Cre [25]. However, the function of FGFR2 in corneal epithelial development has not been assessed. We first examined corneal development in the eyes of Fgfr2 CKO mice by histology (H&E staining). We found that at E12.5 the presumptive corneal epithelium in Fgfr2 CKO eyes looked either similar to or slightly thinner than the corneal epithelium in WT control eyes (Fig. 1A and 1B). In both genotypes, the ocular mesenchymal cells had migrated into the space between the lens and the corneal epithelial layer. At E13.5, however, developmental abnormalities were noted in Fgfr2 CKO corneas (Fig. 1C and 1D). The corneal epithelial layer in Fgfr2 CKO eyes was significantly thinner than normal. The cell density in corneal epithelium was reduced and cells were absent in some areas (indicated by the arrowhead in Fig. 1D). The corneal defects in Fgfr2 CKO eyes progressed beyond the epithelial layer. The WT corneal epithelium consisted of basal cuboidal and superficial flattened cells, whereas the Fgfr2 CKO corneal epithelium consisted entirely of flattened cells. In E16.5 Fgfr2 CKO eyes, corneal stroma was thinner but cells were more densely packed, with intensified eosin staining, when compared to the age-matched WT corneal stroma (Fig. 1E and 1F). The anterior chamber was formed in both genotypes (Fig. 1E and 1F). As previously reported, eyelid fusion did not occur in FGFR2 CKO eyes [33,34]. Additional defects also occurred in anterior segments development of Fgfr2 CKO eyes, including abnormal accumulation of extra cells and tissues in the anterior chamber and a defective corneal endothelial layer (Fig. 1G and 1H). These data suggest that in addition to normal lens development [25], FGFR2 function is also required for normal corneal development.
Fig 1. Corneal development (H&E staining) in WT and Fgfr2 CKO eyes.
A, B) At E12.5, ocular mesenchymal cells migrated into the space between the lens (L) and the corneal epithelium (CE, arrow in enlarged inset) in both WT and Fgfr2 CKO eyes. The corneal epithelial layer in Fgfr2 CKO eyes was slightly thinner than that in WT eyes. C, D) At E13.5, the corneal epithelial layer was significantly thinner in Fgfr2 CKO eyes when compared to WT eyes. In some areas, the epithelial cells were absent (arrowhead in D, insert). E, F) At E16.5, corneal stroma (S) was thinner but cells were more densely packed and eosin-staining was intensified in Fgfr2 CKO cornea as compared to WT. Anterior chamber (AC) was formed in both genotypes. As previously reported, eyelid (EL) fusion did not occur in Fgfr2 CKO eyes. G, H) At P0, Fgfr2 CKO mice developed additional defects in the anterior segments, including abnormal accumulation of extra cells and tissues in the anterior chamber (AC), and loss of a distinctive corneal endothelial (En) layer.
FGFR2 is not required for corneal epithelial cell survival
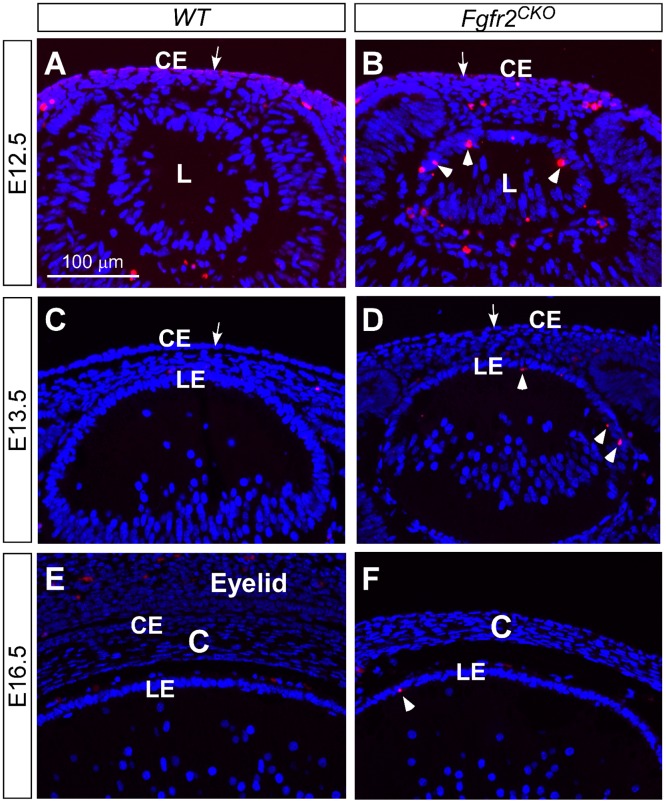
The lens has been shown to undergo the loss of FGFR2-induced apoptosis during development [25]. To investigate whether the reduced cell density in corneal epithelium of FGFR2CKO mice is caused by apoptosis, TUNEL assay was performed on eyes at different ages (Fig. 2A through 2F). We found a drastic increase in apoptotic cells in Fgfr2CKO lenses, a result consistent with the previous finding [25]. The corneal epithelial layer in both WT and Fgfr2CKO eyes, however, did not demonstrate an increase in apoptotic cells, suggesting that FGFR2 is not required for corneal epithelial cell survival. TUNEL-positive cells were also detected in other ocular tissues, including the corneal mesenchymal cells and the hyaloid vascular cells in both WT and Fgfr2CKO eyes, but no significant difference was noted between the two genotypes.
Fig 2. Apoptosis detected by TUNEL assay.
Loss of FGFR2-induced apoptosis in mouse lens (l) (arrowheads in B, D, and F), but not in cornea epithelium (CE) (arrows in A-D). In E12.5 WT and Fgfr2 CKO eyes, TUNEL-positive cells were found in the corneal mesenchymal cells and in the hyaloid cells of the primary vitreous. (LE, lens epithelium; C, cornea)
FGFR2 is essential for corneal epithelial cell proliferation during early-stage development
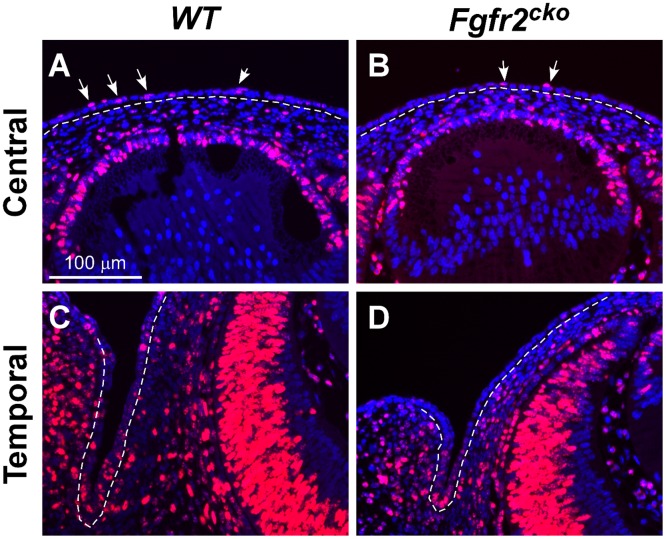
We investigated whether the reduced cell number in Fgfr2 CKO corneal epithelium was affected by defects in cell proliferation. Immunofluorescence of Ki67, a cell proliferation marker expressed in active cycling cells, was performed on E13.5 eyes, the age when the corneal epithelial defect becomes apparent. We found that Ki67-positive cells were markedly reduced in both the central and peripheral regions of the corneal epithelial layer in Fgfr2 CKO eyes when compared to the littermate WT eyes (Fig. 3A through 3D). In contrast to the differences between Fgfr2 CKO and WT Ki67-positive cells in corneal epithelium, no noticeable difference was found between the two genotypes in the number of Ki67-positive cells in the lens epithelium and corneal stroma.
Fig 3. Ki67 immunofluorescence in the central and temporal areas of E13.5 WT and Fgfr2 CKO eyes.
Compared to Ki67-expressing cells WT eyes (arrows in A, C), there was a significant reduction in Ki67-expressing cells in the surface ectodermal layer of Fgfr2 CKO eyes (arrows in B, D). The central epithelial layer later forms the corneal epithelium, and peripheral cells differentiate into limbal and conjuctival epithelium. There was no obvious difference between the WT and Ffgr2 CKO mice in the number of Ki67-positive cells in the stroma and lens.
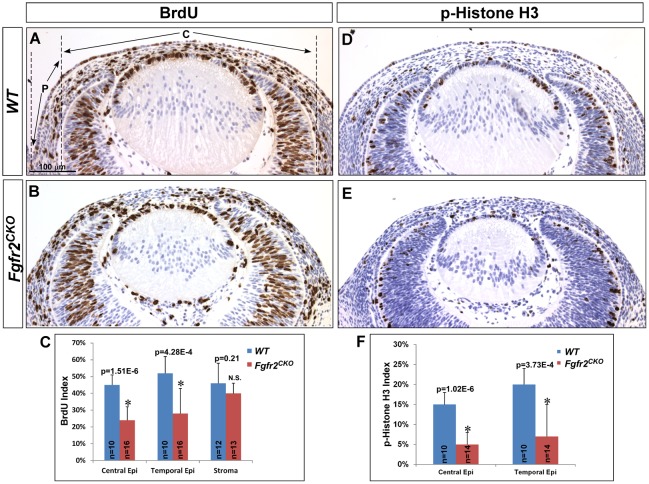
To further analyze the effect of FGFR2 deficiency on cell cycle progression, BrdU incorporation assay and expression of phospho-histone H3 (p-H3) were performed on E13.5 eyes (Fig. 4A through 4F). BrdU is a marker for cells in the S-phase, whereas p-H3 is a marker for G2-M phase cells. Consistent with the results of Ki67 expression, both BrdU and p-H3–labeled cells were significantly reduced in the corneal epithelial layer of E13.5 Fgfr2 CKO eyes when compared to WT eyes. To quantify the labeling index, the ocular surface ectodermal layer was divided into central and peripheral areas, as illustrated in Fig. 4A. In the central area, WT BrdU and p-H3 indices were 0.45±0.06 and 0.15±0.03, respectively, whereas they were 0.24±0.08 and 0.05±0.03, respectively, in Fgfr2 CKO cornea (p<0.05), a statistically significant decrease for both cell cycle markers when compared to control WT eyes. For the peripheral area, BrdU and p-H3 indices were obtained from the temporal side where lacrimal gland budding occurs at the fornix. Similar to the central area, indices were significantly decreased in Fgfr2 CKO eyes (0.28±0.15 for BrdU and 0.07±0.07 for p-H3 in Fgfr2 CKO eyes vs. 0.52±0.1 and 0.20±0.04, respectively, in WT eyes). BrdU index in the central area was calculated to determine whether Fgfr2 deletion in surface ectodermal tissues affects cell proliferation in corneal mesenchyme migrated (Fig. 4C). There was no statistical difference between WT and FGFR2 CKO mice (0.46±0.12 vs. 0.40±0.06), suggesting that cell proliferation in corneal stromal cells is not affected by deletion of Fgfr2 in corneal epithelium. Taken together, our data suggest that after lens vesicle detachment, FGFR2 is required for cell proliferation in the overlying surface ectoderm, which later differentiates into the corneal epithelium, lacrimal glands and conjunctival epithelium.
Fig 4. Cell proliferation analysis.
A, B, C) BrdU incorporation assay in E13.5 WT and Fgfr2 CKO mice demonstrated that BrdU-positive cells were significantly reduced in the corneal epithelium of Fgfr2 CKO mice as compared to WT mice. The surface epithelium is divided into central (C) and peripheral (P) area as illustrated in A. Quantitative analysis confirmed that BrdU-labeling index was decreased in both regions in FGFR2 CKO eyes. Note: only the data from temporal side is shown in C. In contrast to corneal epithelium, BrdU indices in corneal stroma were not significantly different between the two genotypes. D, E, F) Immunohistochemistry of phospho-histone H3 (p-H3) in E13.5 eyes. Similar to the BrdU results, p-H3-labeling indices were reduced in both the central and peripheral corneal epithelial layer in Fgfr2 CKO mice. (n = sections counted; *p<0.0005; N.S. = not significant)
FGFR2 is important for differentiation of corneal epithelial cells and for maintaining Pax6 expression in these cells
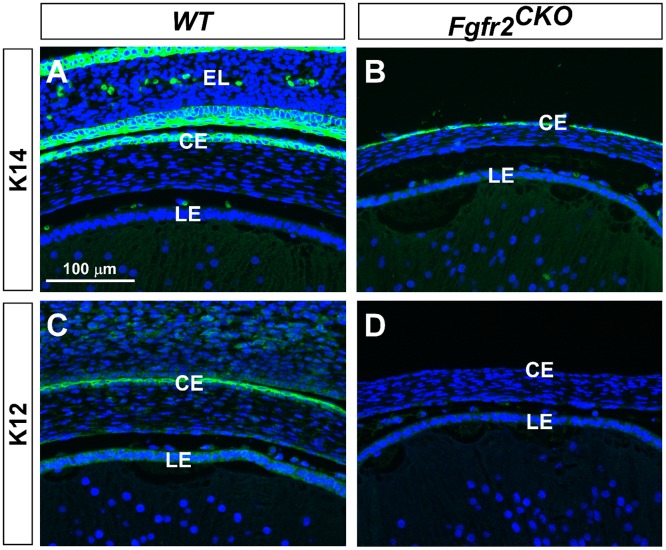
At the stages between E16.5 to P0 (Fig. 1 E–H), the WT corneal epithelium consists of two cell layer, so as in the Fgfr2 CKO cornea. However, the cell shape is changed from cuboidal to flattened in the mutant epithelium, suggesting differentiation could be affected by Fgfr2 deletion. The hallmark of corneal epithelial differentiation and maturation is the expression of keratin 12 (K12) by E14.5 [35]. K12 is not expressed in the limbal and conjunctival epithelium. In contrast to K12, K14 is expressed in all ocular surface epithelial tissues. To investigate whether loss of FGFR2 in the corneal epithelium affects cell differentiation, K12 and K14 immunofluorescence was examined in E16.5 WT and Fgfr2 CKO eyes. The results showed that although the corneal epithelial layer was significantly thinner in Fgfr2 CKO eyes, K14 was expressed in this cell layer in Fgfr2 CKO eyes, as well as in WT eyes (Fig. 5A and 5B). However, K12 expression was not detected in Fgfr2 CKOcorneal epithelium but was in WT corneal epithelium (Fig. 5C and 5D). This result suggests that loss of FGFR2 affects corneal epithelial cell differentiation and maturation.
Fig 5. Corneal epithelial cell differentiation.
A, B) K14 was expressed in corneal epithelium (CE) in both WT and Fgfr2 CKO eyes, and in eyelid (EL) epithelia in WT eyes. Eyelid fusion did not occur in Fgfr2 CKO eyes (B). C, D) K12 expression was found in corneal epithelium of WT eyes but not in Fgfr2 CKO eyes, suggesting that corneal epithelial cell differentiation was abnormal in mutant eyes. (LE = lens epithelium.)
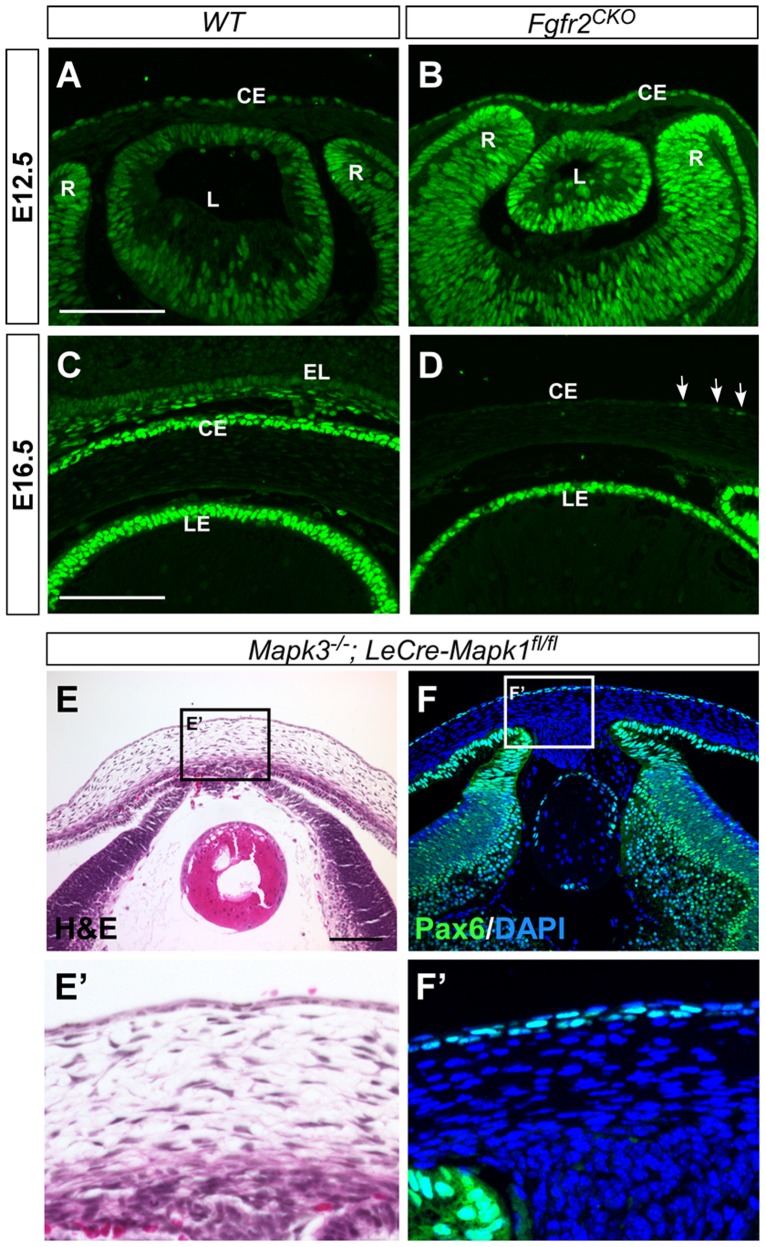
Transcription factor Pax6 is known to be essential for K12 expression and corneal development. Pax6 is expressed in several ocular cell types, including the corneal and limbal epithelia, where gene expression is maintained throughout life [36–39]. In E12.5 WT and Fgfr2 CKO eyes, Pax6 was expressed in developing corneal epithelium as well as in the lens and retina (6A and 6B). The expression patterns were similar in WT and Fgfr2 CKO eyes. In E16.5 WT cornea, Pax6 expression was also found in conjunctival epithelium of the eyelid (Fig. 6C). In contrast, Pax6 immunofluorescence was almost abolished in Fgfr2 CKO cornea and a low-level expression was detected in a few cells (labeled by arrows in Fig. 6D). This finding suggests that FGFR2 plays a critical role in maintaining Pax6 expression. Loss of Pax6 expression could result in the absence of K12 expression and account for the abnormal differentiation of corneal epithelium in Fgfr2 CKO mice.
Fig 6. Pax6 immunofluorescence.
A, B) In E12.5 WT and Fgfr2 CKO eyes, Pax6 was expressed in developing corneal epithelial (CE), lens (L) and retinal (R) cells. The expression patterns were similar between WT and Fgfr2 CKO eyes. C, D) At E16.5, Pax6 expression was found in corneal and conjunctival epithelium in WT eyes (C) but was significantly reduced in corneal epithelium of Fgfr2 CKO eye, with a weak signal detected in a few cells (arrows in D). LE, lens epithelium. E, F) Deletion of Mapk1 and Mapk3, encoding for ERK2 and ERK1 respectively, in the surface ectodermal-derived tissues severely affected lens and corneal development (H&E staining in E and E’). However, Pax6 expression appears to be normal in these tissues (F and F’).
ERK is one of the major downstream effector of FGFR activation. To investigate whether FGFR2′s role of maintaining Pax6 level requires ERK activity, we examined the Pax6 expression in the E16.5 cornea of ERK1/2 double conditional deletion mice [29]. We found that, in contrast to the Fgfr2 CKO cornea, Pax6 expression was maintained normally in the ERK1/2-deficient corneal epithelial cells (Fig. 6 F and F’), suggesting that FGFR2 controls the Pax6 level through an ERK-independent signaling pathway. In the ERK1/2-deficient cornea, the stromal layer looked abnormal and corneal endothelium was absent, probably due to the severe defective and degenerative lens in these mice [40].
Abnormal differentiation of corneal mesenchymal cells in Fgfr2 CKO mice
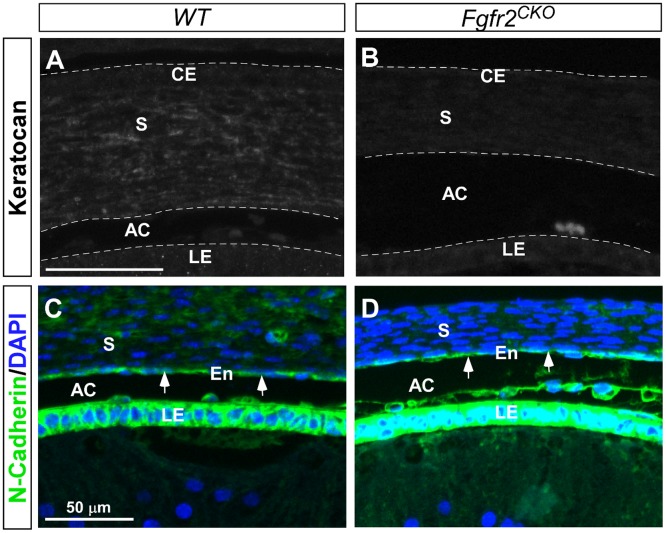
Cre expression in the Le-Cre mice is limited to the ocular surface ectodermal tissues [27,29], however, developmental defects were seen in the mesenchyme-derived tissues, such as the corneal stroma. We assessed whether differentiation of corneal mesenchymal cells was affected by defective corneal epithelium in Fgfr2 CKO mice. Keratocan is a cornea-specific keratan sulfate proteoglycan, and is considered a phenotypic marker for keratocytes [41,42]. Keratocan expression was found in the stroma of WT eyes but not in Fgfr2 CKO eyes (Fig. 7A and 7B). In contrast, expression of N-cadherin, a marker for corneal endothelial cells [43], was seen in eyes of both genotypes (Fig. 7C and 7D). Taken together, the data suggest that abnormal differentiation of the corneal epithelial cells affects the normal differentiation of corneal keratocytes but not endothelial cells in Fgfr2 CKO mice.
Fig 7. Corneal mesenchymal cell differentiation at E16.5 in WT and Fgfr2 CKO eyes.
A, B) Keratocan expression was found in the stroma (S) of WT cornea, indicating the formation of keratocytes. Keratocan was not detected in the stroma of Fgfr2 CKO cornea, suggesting that normal differentiation of corneal mesenchymal cells into keratocytes was disrupted. C, D) N-cadherin was expressed in corneal endothelium (En, arrows) in both WT and Fgfr CKO corneas. N-cadherin was also highly expressed in the lens epithelium (LE) in both genotypes. (AC = anterior chamber.)
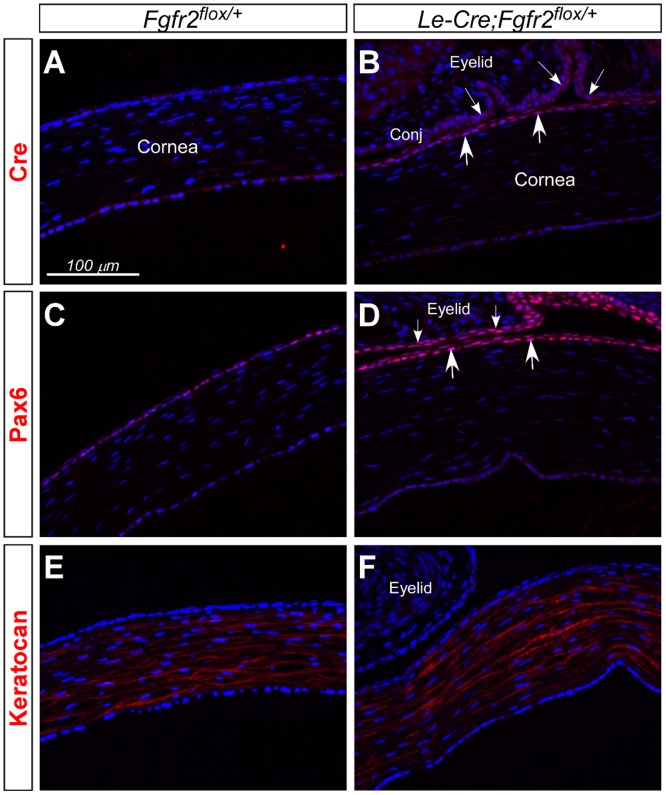
Throughout the study, we have used Fgfr2 flox/flox mice as a control (referred as WT) to compare with Le-Cre;Fgfr2 flox/flox mice (or Fgfr2 CKO). Recently it was reported that hemizygous Le-Cre transgenic mice can develop severe eye defects on some genetic background [30]. We examined the expression of Pax6 and keratocan in the corneas of Le-Cre;Fgfr2 flox/+ heterozygous mice at postnatal day 5 (P5) (Fig. 8, C-F). We found no significant changes in the expression of these proteins in corneas between Fgfr2 flox/+ and Le-Cre;Fgfr2 flox/+ mice. This result suggests that the changes we have demonstrated in this study are unlikely caused by the expression of Le-Cre transgene in corneal epithelium.
Fig 8. Cre, Pax6 and keratocan immunofluorescence in corneas of Fgfr2 flox/+ heterozygous mice with and without Le-Cre transgene at P5.
A, B) Cre was expressed in corneal (arrows) and conjunctival (small arrows) epithelium of Le-Cre;Fgfr2 flox/+ eyes (B), but not in Fgfr2 flox/+ eyes (A). C-F) Pax6 expression was found in corneal (arrows, D) and conjunctival (small arrows, D) epithelium, and keratocan expression in corneal stroma (E, F). The expression patterns of these proteins were similar in corneas between Fgfr2 flox/+ (C, E) and Le-Cre;Fgfr2 flox/+ eyes (D, F). Note that histological artifact caused the corneas in Fgfr2 flox/+ eyes (without eyelids) to appear slightly thinner than those in Le-Cre;Fgfr2 flox/+ eyes (with eyelids).
Discussion
FGFR2 is required for corneal epithelial cell proliferation during early eye development
In early steps of eye development, FGF-7 and FGF-10 are expressed in the perioptic mesenchyme, some of these cells migrate into the space between lens and corneal epithelial layer to form the presumptive corneal stroma [20,44]. Both FGF-7 and FGF-10 are ligands for FGFR2. In the previous “gain-of-function” studies, overexpression of FGF-7 and FGF-10 driven by the lens-specific αA-crystallin promoter resulted in suppression of corneal epithelial cell fate and induction of ectopic lacrimal gland formation at the corneal surface of transgenic mice [20–22]. These results suggest that tight regulation of FGFR-signaling in corneal epithelium plays a critical role in controlling cell fate determination and commitment. In this study, we used a surface ectodermal Cre driver (Le-Cre) and demonstrate that loss of FGFR2 in corneal epithelial cells causes significant decrease in cell proliferation without affecting the epithelial cell fate and cell survival. Combining the ligand expression patterns with our data from this “loss-of-function” study, we propose that cell proliferation in the prospective corneal epithelium is regulated by FGFR2-signaling through epithelial-mesenchymal interaction in a paracrine fashion.
The mature corneal epithelium is a stratified tissue that continuously renews itself. The mitogens for later stage and older corneas are likely secreted by the lacrimal gland and distributed via the tears over the ocular surface. For example, epidermal growth factor (EGF) and transforming growth factor α (TGFα) exist as a component of human tears. EGF and TGF-α share a common EGF receptor (EGFR). Both growth factors are capable to stimulate corneal epithelial cell proliferation in vitro and in vivo [3,43,45]. Other growth factors, including hepatocyte growth factor (HGF), FGF-7, insulin-like growth factor (IGF)-1 and IGF-2 had all been proved to stimulate corneal epithelia cells proliferation in a dose-dependent manner in vitro [15,46–48]. HGF and FGF-7 are expressed in stromal keratocytes and are highly upregulated following epithelial injury [48]. The levels of FGF-7 and FGFR2 transcripts were highest in limbal fibroblasts and epithelial cells respectively in the periphery [49], while the expression of HGF and its receptor is higher in central cornea [14], suggesting a regional specificity of these two growth factor-signaling in control of cell proliferation. In our study, we also demonstrate that the cell proliferation indices in the peripheral corneal epithelium are higher than that in the central region in both WT and Fgfr2 CKO (Fig. 4). The role of FGFR2 in later developmental stage and in mature cornea can be investigated using an inducible Cre line.
Corneal epithelial cell fate is specified but cannot commit for further differentiation and maturation in Fgfr2 CKO mice
We demonstrate that cytokeratin K12 (K12), a marker for corneal epithelial cell differentiation, is not expressed in Fgfr2 CKO eyes (Fig. 6) [50], suggesting the FGFR2-signaling is also required for corneal epithelial cell differentiation and maturation. However, K14 is expressed in both WT and Fgfr2 CKO corneal epithelium, suggesting that corneal epithelial cell fate is specified but remains in a less differentiated form. One potential mechanism for the differentiation defect in Fgfr2 CKO mice might be due to the loss of Pax6 expression in corneal epithelium. We show that Pax6 is expressed in E12.5 corneal epithelial layer of Fgfr2 CKO eyes but was almost absent at later stage (E16.5), suggesting that FGFR2-signaling activity plays an important role in maintaining Pax6 level in corneal epithelium. Similar observation was reported by Faber et al that, when a dominant negative FGFR1 is expressed in the lens, Pax6 expression levels were reduced [51]. However, the underlying mechanism of FGFR-signaling in maintaining Pax6 level might be different between these two surface ectodermal derived tissues. For example, FGFR-ERK signaling plays a major role in lens development, whereas ERK activity (judged by phosphorylated ERK level) was hardly detectable in the developing corneal epithelium (S1 Fig.) and loss of ERK1/2 did not affect the Pax6 levels in these cells (Fig. 6E–F’). The downstream signal transduction pathways of FGFR2 in corneal epithelial cells require further investigation.
Pax6 is known to be essential for normal corneal morphogenesis [52,53]. During development, Pax6 is expressed in the surface ectoderm prior to and during corneal epithelial differentiation, and is maintained in the adult corneal epithelium including the limbal region where the stem/progenitor cell population exists [38]. Pax6 functions as a co-activating factor for K12 expression [54]. K12 expression was reduced in Pax6+/- mouse cornea [55]. Thus, loss of Pax6 in Fgfr2 CKO cornea can directly affect activation of genes, such as K12, which are crucial for corneal epithelial cell differentiation and maturation. Additionally cellular adhesion was also compromised in the Pax6+/- mutant corneal epithelium [56]. In postnatal and adult Pax6+/- mouse cornea, the epithelial layer is thinner owing to a reduction in the number of cell layers, despite a tenfold increase in the proliferative index and no change in TUNEL labeling. Because proliferation of limbal and corneal epithelial cells in Pax6+/- mice was not reduced, it confirms that decrease of cell proliferation in corneal epithelium of Fgfr2 CKO mice is a direct result of FGFR2-deficiency not due to the loss of Pax6.
It is worthwhile to mention that during normal development FGFR-signaling level in corneal epithelial cells must be under tight control to ensure normal differentiation. As mentioned in the “Introduction”, FGF-3, FGF-7 and FGF-10 are the ligands of FGFR2. Overexpression of any of these FGFs from the lens at early developmental stage can alter the corneal epithelial cell fate, initially induce these cells to over-proliferate and then differentiate into secretory cell types (e.g. lacrimal and Harderian glands) [20–22]. Expressing an active form of Ras in corneal epithelium can also inhibit K12 expression [57]. The gain- and loss-of-function studies all indicate that proper corneal epithelium differentiation and maturation depend on the tightly controlled FGFR-signaling activity in these cells.
Interaction between corneal epithelium and underlying mesenchyme is critical for cell fate commitment and differentiation in both tissues
When the lens vesicle and primitive corneal epithelium are completely separated, the space between them is filled by invading corneal mesenchymal cells which are mostly of neural crest origin. In mouse, the posterior mesenchyme cells closest to the lens differentiate into the corneal endothelium and subsequently the anterior chamber is formed. The mesenchyme cells between the corneal epithelium and endothelium start to differentiate into stromal keratocytes [2,58]. One of the differentiation markers for keratocytes is keratocan, a type of keratan sulfate-containing proteoglycans (KSPGs) uniquely abundant in the corneal stroma [41]. In Fgfr2 CKO corneal stroma, keratocan was not expressed, even though the mesenchymal cell proliferation was not affected, suggesting that differentiation of keratocytes was disrupted by Fgfr2 deletion in the corneal epithelium. While more molecular markers for keratocyte differentiation need to be examined, we suspect that the stromal cell differentiation defect could result from loss of Pax6 expression in the corneal epithelial layer. In Pax6 +/- mutant embryos, corneal epithelium was abnormal in K12 expression and was thinner than WT littermates, and corneal stroma appeared irregular, hypercellular, and thickened [39,59]. However, the abnormalities seen in Fgfr2 CKO stroma did not completely resemble the defects in Pax6 +/- cornea, suggesting that in addition to lower level of Pax6, other factors as a result of FGFR2-signaling in corneal epithelium also contribute to stromal cell differentiation in normal corneal development.
FGFR2 plays a different role in corneal epithelium and lens development
During vertebrate eye development, lens and corneal epithelium arise from the same surface ectoderm and share several common features, for example, they are both transparent and require sustained expression of Pax6. At E11.5–12.0, the lens vesicle detaches from the overlying surface ectoderm which subsequently forms the prospective corneal epithelium (Fig. 1A). Our study demonstrates that FGFR2 plays a different role in these two ectodermal tissues with the same origin. In the lens, FGFR2 is required for cell survival and cell cycle withdrawal during fiber differentiation, but it is dispensable for cell proliferation [25]. In contrast, FGFR2 is essential for cell proliferation in corneal epithelium but is dispensable for cell survival. The different response is likely caused by different downstream signal transduction pathways activated by FGFR2. Previous study by Burgess et al showed that activation of Ras, a downstream effector of FGF-signaling, triggers different sets of downstream targets in lens and in corneal epithelium [57]. Constitutive activation of Ras initially increased cell proliferation in both lens and corneal epithelial cells, correlating with increased levels of cyclin D1 and D2 expression in both cell types. This initial increase was sustained in the corneal epithelium, but not in the lens. Instead, cell cycle inhibitors, p27kip1 and p57kip2, were upregulated in the lens followed by hyperproliferation, probably through upregulation of transcription factor Prox1. Furthermore, the downstream effectors of FGFR2-Ras signaling also differ between lens and corneal epithelial cells. For example, the phospho-ERK1/2 level was increased in the lens, but not in the corneal epithelial cells, in response to Ras activation. When we examined the pERK1/2 levels in E12.5 cornea by immunofluorescence, we found that the pERK1/2 level was hardly detectable in central corneal epithelium in either WT or Fgfr2 CKO eyes. Double deletion of Mapk1 and Mapk3 (encoding for ERK2 and ERK1 respectively) in the surface ectoderm did not cause any visible changes in early steps of central corneal development (Fig. 6, E-F’) although the cell numbers appeared to be reduced in the peripheral area near conjunctival fornix (data not shown), suggesting that role of FGFR2 in central corneal development is not controlled by ERK-signaling. This result is also consistent with the previous report in Ras-overexpression transgenic mice [57]. We conclude that FGFR2-signaling activates ERK-dependent and ERK-independent pathways in the lens and in central corneal epithelial cells respectively [29]. The signaling effectors and targets of FGFR2 in corneal epithelial cells have not been well defined and needs to be further investigated.
In summary, we demonstrate that FGFR2-activated ERK-independent signal is essential for corneal epithelial cell proliferation during early eye development. In later stage, FGFR2 is also critical for corneal epithelial cell differentiation and maturation, one potential mechanism is to maintain Pax6 expression in corneal epithelium. In contrast to the ocular lens, FGFR2 is dispensable for survival of corneal epithelial cells. Our study implies that keratocyte differentiation in corneal stroma depends on some inductive signals released from the overlying corneal epithelium. More studies are needed to investigate the downstream signaling events of FGFR2 in corneal epithelium and signals/factors from the corneal epithelial cells responsible for keratocyte differentiation.
Supporting Information
Frozen sections of E12.5 eyes (A, B) and paraffin-sections of E14.5 (C, D) and P0 (E, F) eyes were used for immunostaining using an anti-pERK antibody from Cell Signaling Technology Inc. (Cat#9101). TSA enhancement reagents (Life Technology, Cat#T20948) were used on E14.5 eye sections (C, D) to increase the immunofluorescence signals. We found that pERK proteins were localized in elongating fiber cells of E12.5 lenses and in the cortical region of E14.5 and P0 lenses (indicated by arrowheads). pERK was also found in the retina of E12.5 and E14.5 eyes. pERK signal in cornea epithelium was not detected under standard immunostaining condition (A, B, E, F), however, a low level of pERK was detected in E14.5 corneal epithelium when TSA was used (arrows in C, D).
(TIF)
Acknowledgments
We thank Drs Ruth Ashery-Padan and David Ornitz for permission to use the Le-Cre and Ffgr2-flox mice, respectively; Dr Winston Kao for anti-K12 and anti-keratocan antibodies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
JZ was supported by International Program of Project 985 (Sun Yat-Sen University, China). LWR was supported by NIH EY13146. This work was also supported by NIH minicore grant EY14795 and unrestricted funding from Research to Prevent Blindness (RPB) to the Department of Ophthalmology at University of Missouri. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hay ED, Linsenmayer TF, Trelstad RL, von der Mark K (1979) Origin and distribution of collagens in the developing avian cornea. Curr Top Eye Res 1: 1–35. [PubMed] [Google Scholar]
- 2. Graw J (2010) Eye development. Curr Top Dev Biol 90: 343–386. 10.1016/S0070-2153(10)90010-0 [DOI] [PubMed] [Google Scholar]
- 3. Zieske JD, Wasson M (1993) Regional variation in distribution of EGF receptor in developing and adult corneal epithelium. J Cell Sci 106 (Pt 1): 145–152. [DOI] [PubMed] [Google Scholar]
- 4. Zieske JD (2004) Corneal development associated with eyelid opening. Int J Dev Biol 48: 903–911. [DOI] [PubMed] [Google Scholar]
- 5. O’Guin WM, Galvin S, Schermer A, Sun TT (1987) Patterns of keratin expression define distinct pathways of epithelial development and differentiation. Curr Top Dev Biol 22: 97–125. [DOI] [PubMed] [Google Scholar]
- 6. Whitcher JP, Srinivasan M, Upadhyay MP (2001) Corneal blindness: a global perspective. Bull World Health Organ 79: 214–221. [PMC free article] [PubMed] [Google Scholar]
- 7. Kao WW, Xia Y, Liu CY, Saika S (2008) Signaling pathways in morphogenesis of cornea and eyelid. Ocul Surf 6: 9–23. [PubMed] [Google Scholar]
- 8. Ornitz DM, Itoh N (2001) Fibroblast growth factors. Genome Biol 2: REVIEWS3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Powers CJ, McLeskey SW, Wellstein A (2000) Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer 7: 165–197. [DOI] [PubMed] [Google Scholar]
- 10. Miki T, Bottaro DP, Fleming TP, Smith CL, Burgess WH, et al. (1992) Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci U S A 89: 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson DE, Williams LT (1993) Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res 60: 1–41. [DOI] [PubMed] [Google Scholar]
- 12. de Iongh RU, Lovicu FJ, Chamberlain CG, McAvoy JW (1997) Differential expression of fibroblast growth factor receptors during rat lens morphogenesis and growth. Invest Ophthalmol Vis Sci 38: 1688–1699. [PubMed] [Google Scholar]
- 13. Li DQ, Tseng SC (1996) Differential regulation of cytokine and receptor transcript expression in human corneal and limbal fibroblasts by epidermal growth factor, transforming growth factor-alpha, platelet-derived growth factor B, and interleukin-1 beta. Invest Ophthalmol Vis Sci 37: 2068–2080. [PubMed] [Google Scholar]
- 14. Li DQ, Tseng SC (1997) Differential regulation of keratinocyte growth factor and hepatocyte growth factor/scatter factor by different cytokines in human corneal and limbal fibroblasts. J Cell Physiol 172: 361–372. [DOI] [PubMed] [Google Scholar]
- 15. Wilson SE, Walker JW, Chwang EL, He YG (1993) Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Invest Ophthalmol Vis Sci 34: 2544–2561. [PubMed] [Google Scholar]
- 16. Andresen JL, Ehlers N (1998) Chemotaxis of human keratocytes is increased by platelet-derived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and transforming growth factor-beta. Curr Eye Res 17: 79–87. [DOI] [PubMed] [Google Scholar]
- 17. Carrington LM, Boulton M (2005) Hepatocyte growth factor and keratinocyte growth factor regulation of epithelial and stromal corneal wound healing. J Cataract Refract Surg 31: 412–423. [DOI] [PubMed] [Google Scholar]
- 18. Chandrasekher G, Kakazu AH, Bazan HE (2001) HGF- and KGF-induced activation of PI-3K/p70 s6 kinase pathway in corneal epithelial cells: its relevance in wound healing. Exp Eye Res 73: 191–202. [DOI] [PubMed] [Google Scholar]
- 19. Sotozono C, Inatomi T, Nakamura M, Kinoshita S (1995) Keratinocyte growth factor accelerates corneal epithelial wound healing in vivo. Invest Ophthalmol Vis Sci 36: 1524–1529. [PubMed] [Google Scholar]
- 20. Govindarajan V, Ito M, Makarenkova HP, Lang RA, Overbeek PA (2000) Endogenous and ectopic gland induction by FGF-10. Dev Biol 225: 188–200. [DOI] [PubMed] [Google Scholar]
- 21. Lovicu FJ, Kao WW, Overbeek PA (1999) Ectopic gland induction by lens-specific expression of keratinocyte growth factor (FGF-7) in transgenic mice. Mech Dev 88: 43–53. [DOI] [PubMed] [Google Scholar]
- 22. Makarenkova HP, Ito M, Govindarajan V, Faber SC, Sun L, et al. (2000) FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development 127: 2563–2572. [DOI] [PubMed] [Google Scholar]
- 23. Robinson ML, Ohtaka-Maruyama C, Chan CC, Jamieson S, Dickson C, et al. (1998) Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev Biol 198: 13–31. [DOI] [PubMed] [Google Scholar]
- 24. Chikama T, Liu CY, Meij JT, Hayashi Y, Wang IJ, et al. (2008) Excess FGF-7 in corneal epithelium causes corneal intraepithelial neoplasia in young mice and epithelium hyperplasia in adult mice. Am J Pathol 172: 638–649. 10.2353/ajpath.2008.070897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia CM, Yu K, Zhao H, Ashery-Padan R, Ornitz DM, et al. (2005) Signaling through FGF receptor-2 is required for lens cell survival and for withdrawal from the cell cycle during lens fiber cell differentiation. Dev Dyn 233: 516–527. [DOI] [PubMed] [Google Scholar]
- 26. Robinson ML (2006) An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol 17: 726–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ashery-Padan R, Marquardt T, Zhou X, Gruss P (2000) Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev 14: 2701–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu K, Xu J, Liu Z, Sosic D, Shao J, et al. (2003) Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130: 3063–3074. [DOI] [PubMed] [Google Scholar]
- 29. Upadhya D, Ogata M, Reneker LW (2013) MAPK1 is required for establishing the pattern of cell proliferation and for cell survival during lens development. Development 140: 1573–1582. 10.1242/dev.081042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dora NJ, Collinson JM, Hill RE, West JD (2014) Hemizygous Le-Cre Transgenic Mice Have Severe Eye Abnormalities on Some Genetic Backgrounds in the Absence of LoxP Sites. PLoS One 9: e109193 10.1371/journal.pone.0109193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xie L, Overbeek PA, Reneker LW (2006) Ras signaling is essential for lens cell proliferation and lens growth during development. Dev Biol 298: 403–414. [DOI] [PubMed] [Google Scholar]
- 32. Fromm L, Shawlot W, Gunning K, Butel JS, Overbeek PA (1994) The retinoblastoma protein-binding region of simian virus 40 large T antigen alters cell cycle regulation in lenses of transgenic mice. Mol Cell Biol 14: 6743–6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li C, Guo H, Xu X, Weinberg W, Deng CX (2001) Fibroblast growth factor receptor 2 (Fgfr2) plays an important role in eyelid and skin formation and patterning. Dev Dyn 222: 471–483. [DOI] [PubMed] [Google Scholar]
- 34. Huang J, Dattilo LK, Rajagopal R, Liu Y, Kaartinen V, et al. (2009) FGF-regulated BMP signaling is required for eyelid closure and to specify conjunctival epithelial cell fate. Development 136: 1741–1750. 10.1242/dev.034082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saika S, Liu CY, Azhar M, Sanford LP, Doetschman T, et al. (2001) TGFbeta2 in corneal morphogenesis during mouse embryonic development. Dev Biol 240: 419–432. [DOI] [PubMed] [Google Scholar]
- 36. Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, et al. (1991) Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature 354: 522–525. [DOI] [PubMed] [Google Scholar]
- 37. Grindley JC, Davidson DR, Hill RE (1995) The role of Pax-6 in eye and nasal development. Development 121: 1433–1442. [DOI] [PubMed] [Google Scholar]
- 38. Koroma BM, Yang JM, Sundin OH (1997) The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci 38: 108–120. [PubMed] [Google Scholar]
- 39. Ramaesh T, Collinson JM, Ramaesh K, Kaufman MH, West JD, et al. (2003) Corneal abnormalities in Pax6+/- small eye mice mimic human aniridia-related keratopathy. Invest Ophthalmol Vis Sci 44: 1871–1878. [DOI] [PubMed] [Google Scholar]
- 40. Zhang Y, Overbeek PA, Govindarajan V (2007) Perinatal ablation of the mouse lens causes multiple anterior chamber defects. Mol Vis 13: 2289–2300. [PubMed] [Google Scholar]
- 41. Carlson EC, Liu CY, Chikama T, Hayashi Y, Kao CW, et al. (2005) Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulated by lumican. J Biol Chem 280: 25541–25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu CY, Shiraishi A, Kao CW, Converse RL, Funderburgh JL, et al. (1998) The cloning of mouse keratocan cDNA and genomic DNA and the characterization of its expression during eye development. J Biol Chem 273: 22584–22588. [DOI] [PubMed] [Google Scholar]
- 43. Reneker LW, Silversides DW, Xu L, Overbeek PA (2000) Formation of corneal endothelium is essential for anterior segment development—a transgenic mouse model of anterior segment dysgenesis. Development 127: 533–542. [DOI] [PubMed] [Google Scholar]
- 44. Finch PW, Cunha GR, Rubin JS, Wong J, Ron D (1995) Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev Dyn 203: 223–240. [DOI] [PubMed] [Google Scholar]
- 45. Watanabe K, Nakagawa S, Nishida T (1987) Stimulatory effects of fibronectin and EGF on migration of corneal epithelial cells. Invest Ophthalmol Vis Sci 28: 205–211. [PubMed] [Google Scholar]
- 46. Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, et al. (2000) Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res 19: 113–129. [DOI] [PubMed] [Google Scholar]
- 47. Yanai R, Yamada N, Inui M, Nishida T (2006) Correlation of proliferative and anti-apoptotic effects of HGF, insulin, IGF-1, IGF-2, and EGF in SV40-transformed human corneal epithelial cells. Exp Eye Res 83: 76–83. [DOI] [PubMed] [Google Scholar]
- 48. Wilson SE, He YG, Weng J, Zieske JD, Jester JV, et al. (1994) Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp Eye Res 59: 665–678. [DOI] [PubMed] [Google Scholar]
- 49. Li DQ, Tseng SC (1995) Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol 163: 61–79. [DOI] [PubMed] [Google Scholar]
- 50. Tanifuji-Terai N, Terai K, Hayashi Y, Chikama T, Kao WW (2006) Expression of keratin 12 and maturation of corneal epithelium during development and postnatal growth. Invest Ophthalmol Vis Sci 47: 545–551. [DOI] [PubMed] [Google Scholar]
- 51. Faber SC, Dimanlig P, Makarenkova HP, Shirke S, Ko K, et al. (2001) Fgf receptor signaling plays a role in lens induction. Development 128: 4425–4438. [DOI] [PubMed] [Google Scholar]
- 52. Li S, Goldowitz D, Swanson DJ (2007) The requirement of pax6 for postnatal eye development: evidence from experimental mouse chimeras. Invest Ophthalmol Vis Sci 48: 3292–3300. [DOI] [PubMed] [Google Scholar]
- 53. Collinson JM, Quinn JC, Hill RE, West JD (2003) The roles of Pax6 in the cornea, retina, and olfactory epithelium of the developing mouse embryo. Dev Biol 255: 303–312. [DOI] [PubMed] [Google Scholar]
- 54. Liu JJ, Kao WW, Wilson SE (1999) Corneal epithelium-specific mouse keratin K12 promoter. Exp Eye Res 68: 295–301. [DOI] [PubMed] [Google Scholar]
- 55. Ou J, Lowes C, Collinson JM (2010) Cytoskeletal and cell adhesion defects in wounded and Pax6+/- corneal epithelia. Invest Ophthalmol Vis Sci 51: 1415–1423. 10.1167/iovs.09-4023 [DOI] [PubMed] [Google Scholar]
- 56. Davis J, Duncan MK, Robison WG Jr, Piatigorsky J (2003) Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J Cell Sci 116: 2157–2167. [DOI] [PubMed] [Google Scholar]
- 57. Burgess D, Zhang Y, Siefker E, Vaca R, Kuracha MR, et al. (2010) Activated Ras alters lens and corneal development through induction of distinct downstream targets. BMC Dev Biol 10: 13 10.1186/1471-213X-10-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cvekl A, Tamm ER (2004) Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays 26: 374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mort RL, Bentley AJ, Martin FL, Collinson JM, Douvaras P, et al. (2011) Effects of aberrant Pax6 gene dosage on mouse corneal pathophysiology and corneal epithelial homeostasis. PLoS One 6: e28895 10.1371/journal.pone.0028895 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Frozen sections of E12.5 eyes (A, B) and paraffin-sections of E14.5 (C, D) and P0 (E, F) eyes were used for immunostaining using an anti-pERK antibody from Cell Signaling Technology Inc. (Cat#9101). TSA enhancement reagents (Life Technology, Cat#T20948) were used on E14.5 eye sections (C, D) to increase the immunofluorescence signals. We found that pERK proteins were localized in elongating fiber cells of E12.5 lenses and in the cortical region of E14.5 and P0 lenses (indicated by arrowheads). pERK was also found in the retina of E12.5 and E14.5 eyes. pERK signal in cornea epithelium was not detected under standard immunostaining condition (A, B, E, F), however, a low level of pERK was detected in E14.5 corneal epithelium when TSA was used (arrows in C, D).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.