Abstract
Considerable insight has been gained into the comorbid, interactive effects of HIV and drug abuse in the brain using experimental models. This review, which considers opiates, methamphetamine, and cocaine, emphasizes the importance of host genetics and glial plasticity in driving the pathogenic neuron remodeling underlying neuro-acquired immunodeficiency syndrome (neuroAIDS) and drug abuse comorbidity. Clinical findings are less concordant than experimental work, and the response of individuals to HIV and to drug abuse can vary tremendously. Host-genetic variability is important in determining viral tropism, neuropathogenesis, drug responses, and addictive behavior. However, genetic differences alone cannot account for individual variability in the brain “connectome”. Environment and experience are critical determinants in the evolution of synaptic circuitry throughout life. Neurons and glia both exercise control over determinants of synaptic plasticity that are disrupted by HIV and drug abuse. Perivascular macrophages, microglia, and to a lesser extent astroglia can harbor the infection. Uninfected bystanders, especially astroglia, propagate and amplify inflammatory signals. Drug abuse by itself derails neuronal and glial function, and the outcome of chronic exposure is maladaptive plasticity. The negative consequences of coexposure to HIV and drug abuse are determined by numerous factors including genetics, sex, age, and multidrug exposure. Glia and some neurons are generated throughout life and their progenitors appear to be targets of HIV and opiates/psychostimulants. The chronic nature of HIV and drug abuse appears to result in sustained alterations in the maturation and fate of neural progenitors, which may affect the balance of glial populations within multiple brain regions.
Keywords: drug/substance abuse, gene polymorphisms, methamphetamine, cocaine, neurogenesis/gliogenesis, neuropathology, μ opioid receptor (OPRM1), chemokine (C-C motif) receptor 5 (CCR5), neuroimmunology, neuropharmacology
1. INTRODUCTION
For several decades drug abuse has been recognized as a significant risk factor for acquiring HIV infection and has been suggested to worsen some aspects of HIV infection within the brain. However, the enormity of the problem has been emphasized by a sustained nearly two-decade effort of the National Institute on Drug Abuse (NIDA) to study and treat this problem. As initially affirmed by Dr. Alan I. Leshner, former director of NIDA, “Drug abuse and HIV are truly interlinked epidemics” (Swan, 1997; Biber, Neumann, Inoue, & Boddeke, 2007; Andres et al., 2011). Despite widespread acceptance of this concept and impressive new gains in our understanding of this problem, there is a realization that the interplay between HIV and drug abuse is more complex than initially surmised. Many authoritative reviews describing the effects of drug abuse on neuroAIDS have been published and our goal here is not to repeat past dialogue. During the past decade, there has been considerable new information—especially with respect to increasing evidence that glia are fundamental sites of convergent drug abuse-HIV interactions and the emerging realization of the influence of host genetic factors on the severity of drug abuse and neuroAIDS comorbid interactions. This review will highlight recent findings emphasizing the role of glia and genetic factors in shaping the interactions of opiates and psychostimulants with neuroAIDS. Reviews on the effects of cannabinoids and ethanol on HIV neuropathogenesis are provided elsewhere within this issue, and not repeated here.
1.1. OPIATES AND HIV—PRECLINICAL AND CLINICAL FINDINGS
In this review, we will use the term “opiate” to refer to products or derivatives that can be found naturally in the opium poppy, Papaver somniferum. Opiates include heroin, morphine, and codeine; whereas, “opioids” refer to the endogenous system of related peptides and receptors initially revealed through the actions of opiate drugs.
Chronic opiate abuse alone is sufficient to promote neurodegenerative changes in the CNS. Clinical evidence from a cohort of preferential opiate abusers in Edinburgh, UK demonstrates that chronic abuse accelerates Alzheimer’s disease-like pathology in HIV-negative individuals (Ramage et al., 2005; Anthony et al., 2010). Chronic preferential opiate abuse caused an accumulation of hyperphosphorylated tau-positive neuropil threads in the frontal and temporal cortex, and in the locus coeruleus compared to similarly aged-control subjects (Anthony et al., 2010). Increases in hyperphosphorylated tau were accompanied by increases in glycogen synthase kinase 3β and cyclin-dependent kinase-5 levels, as well as microgliosis—indicative of accelerated aging, and signaling events associated with Alzheimer’s disease prematurely (Anthony et al., 2010).
There are compelling reasons to investigate opioid and HIV interactions and their role in a more severe and/or accelerated neuropathogenesis. In a seminal pre-combination antiretroviral (cART) era investigation, the presence of multinucleated giant cells and HIV p24 reactivity in the CNS was found more frequently in preferential opioid drug users (25 of 45; 56 percent) than in non-drug-abusing men (6 of 35; 17 percent) with AIDS (Bell, Brettle, Chiswick, & Simmonds, 1998). Chronic opiate exposure has been reported to increase the progression to HIV encephalitis (HIVE) in pre-cART era reports (Bell et al., 1998; Nath et al., 2000; Bell et al., 2002), and also worsens neuropathology in cART-treated patients (Smith, Simmonds, & Bell, 2014). Even in cART-treated patients, chronic opiates aggravate CNS inflammation (Anthony, Ramage, Carnie, Simmonds, & Bell, 2005; Anthony, Arango, Stephens, Simmonds, & Bell, 2008) and worsen HIV-associated neurocognitive disorders (HAND) symptomatology—including deficits in verbal and working memory and increased peripheral neuropathy (Cohen, 2009; Byrd et al., 2011; Byrd, Murray, Safdieh, & Morgello, 2012; Robinson-Papp et al., 2012; Meyer et al., 2013). A recent study of a cART naïve population of injecting drug users who preferentially abuse heroin in Indonesia showed consistent reductions in CD4 counts to non-injecting drug abusers (Meijerink et al., 2014). Another recent report suggests that concurrent exposure can selectively increase the severity some features of HIVE (Smith et al., 2014). Despite increasing clinical evidence that chronic opiate exposure can worsen neuroAIDS, it remains unknown how opioids interact with individual HIV expressed gene products to affect subclasses of neurons, astroglia, and microglia (Nath et al., 2002; Hauser, Fitting, Dever, Podhaizer, & Knapp, 2012).
Some clinical studies are inconsistent with the findings cited above, and report minimal or no neurocognitive differences between HIV ± opiate abuse (Royal, III et al., 1991; Donahoe & Vlahov, 1998), suggesting that there are critical genetic (Kreek, Bart, Lilly, LaForge, & Nielsen, 2005; Proudnikov et al., 2012), pharmacokinetic (Eap, Buclin, & Baumann, 2002), pharmacodynamic, sex (Zubieta et al., 2002; Becker & Hu, 2008; Hahn et al., 2014), and/or possible age-dependent differences among opiate abusers that can influence outcomes (discussed later). Moreover, the timing of opioid co-exposure in relation to the onset of HIV infection or vice versa may have a marked influence on outcome (Fitting et al., 2012). It is hoped that advances in the understanding of disease mechanisms, experimental models, and methodology will reveal opiate-HIV interactions with increasing clarity.
Preclinical studies more regularly suggest that chronic opiate exposure plays a fundamental role in the pathogenesis of HIV in the CNS (Hauser et al., 2012). Briefly, morphine can exacerbate HIV-1 toxicity through separate actions in neurons (Gurwell et al., 2001; Hu, Sheng, Lokensgard, & Peterson, 2005; Bruce-Keller et al., 2008), including human neurons (Hu et al., 2005; Turchan-Cholewo et al., 2006) and in glia. Many of the harmful neurotoxic effects are mediated through μ opioid receptors (MOR) (Zou et al., 2011; Hauser et al., 2012) and differ depending on the CNS cell type involved. Glial targets can include μ opioid receptor-expressing astroglia (Hauser et al., 2007; Zou et al., 2011; Hauser et al., 2012; El-Hage et al., 2013), microglia (Turchan-Cholewo et al., 2008; Turchan-Cholewo et al., 2009; Zou et al., 2011; Suzuki et al., 2011; El-Hage et al., 2013; Sorrell & Hauser, 2014), oligodendroglia (Hauser et al., 2009; Hauser et al., 2012; Hahn et al., 2014), and glial precursors (Khurdayan et al., 2004; Buch et al., 2007; Hahn et al., 2010; Hahn, Podhaizer, Hauser, & Knapp, 2012). Details of opiates and HIV interactions in neurons and glia have been reviewed previously (Hauser et al., 2005b; Hauser et al., 2006; Hauser et al., 2007; Banerjee et al., 2011; Abt & Meucci, 2011; Hauser et al., 2012; Reddy, Pilakka-Kanthikeel, Saxena, Saiyed, & Nair, 2012; Festa & Meucci, 2012; Dutta & Roy, 2012).
1.2. PSYCHOSTIMULANTS AND HIV
Numerous authoritative reviews exist on the generalized effects of chronic psychostimulant (such as methamphetamine, MDMA) abuse on brain pathology (Cadet & Krasnova, 2009; Buttner, 2011; Buch et al., 2012; Clark, Wiley, & Bradberry, 2013; Cadet, Bisagno, & Milroy, 2014; Halpin, Collins, & Yamamoto, 2014). Evidence suggests that psychostimulants can act directly on both neurons and glia to disrupt CNS function and promote the injury of vulnerable subpopulations of neurons in the CNS. The vulnerable subpopulations include dopaminergic, noradrenergic, and serotonergic neurons. Minimal attention has been given to stimulants besides methamphetamine, such as 3,4-methylenedioxymethamphetamine (MDMA or ecstasy), ketamine, or γ-hydroxybutyrate (GHB) (Colfax & Guzman, 2006), other than evidence that club drug use increases risky sexual behavior associated with increasing HIV transmission (Zuckerman & Boyer, 2012).
Chronic methamphetamine abuse and disruptions to dopaminergic function are especially deleterious with HIV-infected individuals (Nath et al., 2000; Theodore, Cass, Nath, & Maragos, 2007; Nath, 2010). Methamphetamine addiction results in marked structural pathology in the brain (Berman, O’Neill, Fears, Bartzokis, & London, 2008) and increases the probability of neuropsychological deficiencies in HIV-infected individuals (Rippeth et al., 2004). Many comprehensive reviews exist on the mechanisms underlying the neurotoxic effects of methamphetamine alone (Quinton & Yamamoto, 2006; Cadet, Krasnova, Jayanthi, & Lyles, 2007; Theodore et al., 2007; Ferris, Mactutus, & Booze, 2008; Berman et al., 2008; Krasnova & Cadet, 2009; Cadet & Krasnova, 2009; Nath, 2010; Kaushal & Matsumoto, 2011; Coller & Hutchinson, 2012; Cisneros & Ghorpade, 2012; Clark et al., 2013; Cadet et al., 2014).. Briefly, methamphetamine has both direct and indirect toxic effects on neurons. Indirect neurotoxicity is mediated by disrupting glia and/or targets such as the blood brain barrier (BBB) or the immune system. Chronic methamphetamine exposure can be accompanied by neurodegeneration. Dopaminergic neurons and presynaptic terminals are particularly vulnerable (Czub et al., 2001; Flora et al., 2003; Theodore, Cass, & Maragos, 2006a).
Methamphetamine selectively injures neurons in specific brain regions such as the basal ganglia (Seiden & Ricaurte, 1987), although the precise sequence of direct and indirect events leading to neuronal injury is not fully understood. Acute exposure to methamphetamine induces rapid release of dopamine from presynaptic terminals, while chronic dopamine use results in lasting decreases in striatal dopamine and serotonin and their metabolites (Kogan, Nichols, & Gibb, 1976; Wagner et al., 1980; Cass, 1997) that are accompanied by the destruction of dopaminergic presynaptic terminals in the caudate nucleus (Wagner et al., 1980; Brunswick, Benmansour, Tejani-Butt, & Hauptmann, 1992; Nakayama, Koyama, & Yamashita, 1993; Krasnova & Cadet, 2009; Pereira et al., 2012; Halpin et al., 2014; Nickell, Siripurapu, Vartak, Crooks, & Dwoskin, 2014). Methamphetamine inhibits the function of the vesicular monoamine transporter 2 (VMAT2) (Hanson, Sandoval, Riddle, & Fleckenstein, 2004; Hanson, Rau, & Fleckenstein, 2004) and the dopamine transporter (DAT) (Volkow et al., 2001; Theodore, Cass, & Maragos, 2006b; Krasnova & Cadet, 2009; Cadet & Krasnova, 2009; Halpin et al., 2014; Nickell et al., 2014). VMAT2 transports dopamine (and other monoamines) from the cytosol into presynaptic vesicles (Qi, Miller, & Voit, 2008; Cartier et al., 2010), while DAT reuptakes dopamine from the synaptic cleft into the presynaptic terminal. A transmembrane pH gradient is necessary for vesicular uptake (Sulzer & Rayport, 1990). Methamphetamine, a weak base, limits acidification of the presynaptic cytoplasm and therefore can disrupt vesicular transport. By targeting VMAT2, methamphetamine elevates levels of dopamine within the presynaptic terminal. Cumulative increases in dopamine and oxidative byproducts further reduce levels of VMAT2 creating a destructive positive feedback cycle. In humans, many (but not all) markers of dopamine nerve terminals, such as dopamine itself, tyrosine hydroxylase, and DAT, are decreased in brains of psychostimulant abusers (Wilson & Kish, 1996; Wilson et al., 1996). These effects may be enduring (McCann et al., 1998).
Methamphetamine-related disruption of intracellular redox potential, nitrogen metabolism, and pH exert the greatest burden on vesicular trafficking at presynaptic terminals, which include losses in DAT (Fleckenstein, Metzger, Wilkins, Gibb, & Hanson, 1997; Brown et al., 2002; Hanson et al., 2004) and VMAT2 function (Miller, Gainetdinov, Levey, & Caron, 1999; Larsen, Fon, Hastings, Edwards, & Sulzer, 2002). The selective presynaptic harm may be worsened by excessive peripheral ammonia caused by concurrent methamphetamine-induced hepatotoxicity (Halpin & Yamamoto, 2012).
Dopamine and the accumulation of other amines within the presynaptic cytoplasm can activate trace amine-associated receptor 1 (TAAR1) (Borowsky et al., 2001; Bunzow et al., 2001; Reese, Bunzow, Arttamangkul, Sonders, & Grandy, 2007). TAAR1 is localized within the presynaptic membranes of monoaminergic neurons and located intracellularly suggesting segregation to internalized vesicles (Xie & Miller, 2009; Revel et al., 2011). TAAR1 reportedly can dimerize with dopamine D2 receptors (Espinoza et al., 2011) and TAAR1 activation phosphorylates DAT, resulting in increased dopamine efflux and eventually DAT internalization (Miller, 2011). TAAR1 likely mediates key aspects of aberrant function following methamphetamine (Xie & Miller, 2009) and perhaps cocaine (Revel et al., 2011) exposure.
Methamphetamine and cocaine exposure can result in excessive glutamate within the extracellular space (ECS) at synaptic and extrasynaptic sites (Miyatake, Narita, Shibasaki, Nakamura, & Suzuki, 2005; Quinton & Yamamoto, 2006; Davidson et al., 2007; Cadet et al., 2007; Kaushal & Matsumoto, 2011; Pereira et al., 2012). Glutamate overflow, especially at extrasynaptic sites (Sattler, Xiong, Lu, MacDonald, & Tymianski, 2000; Hardingham, Fukunaga, & Bading, 2002), can induce excitotoxic neuronal injury and even death through overactivation of extrasynaptic GluN2B NMDA receptors (Ivanov et al., 2006; Liu et al., 2007). The NMDA receptor antagonists MK-801 or dextromethorphan can attenuate methamphetamine neurotoxicity (Thomas & Kuhn, 2005), suggesting that microglial activation and dopamine terminal losses may be intimately linked to excitotoxic glutamatergic transmission and imbalances in synaptic and extrasynaptic glutamate (Beardsley & Hauser, 2014).
Chronic HIV-1 or SIV infection deplete dopamine and reduce the number of dopamine terminals in the basal ganglia (Reyes, Faraldi, Senseng, Flowers, & Fariello, 1991; Nath et al., 2000; Czub et al., 2001; Maragos et al., 2002; Wang et al., 2004), which appears to be worsened by methamphetamine exposure (Nath et al., 2000; Czub et al., 2001; Maragos et al., 2002; Cass, Harned, Peters, Nath, & Maragos, 2003; Scheller et al., 2005) or dopamine agonists (Nath, Maragos, Avison, Schmitt, & Berger, 2001; Czub et al., 2001; Czub et al., 2004).
Dopaminergic neurons are especially vulnerable to Tat and/or gp120-induced insults (Hu, Sheng, Lokensgard, Peterson, & Rock, 2009). When methamphetamine is administered after intrastriatal HIV-1 Tat injection, it acts synergistically to diminish levels of dopamine and dopamine metabolites (Maragos et al., 2002). Dopamine D2 receptors levels are also decreased as indicated by reductions in raclopride binding (Maragos et al., 2002). Interestingly, HIV-1 Tat may directly inhibit VMAT2 function in the CNS (Theodore, Cass, Dwoskin, & Maragos, 2012). More recently, HIV-1 Tat has been demonstrated to interact directly with the DAT (Zhu, Mactutus, Wallace, & Booze, 2009; Zhu, Ananthan, Mactutus, & Booze, 2011; Midde, Gomez, & Zhu, 2012; Midde et al., 2013). Thus, HIV-1 Tat disrupts the same molecular targets as methamphetamine albeit through independent mechanisms, which is likely to contribute to the devastating neurological and psychiatric consequences of methamphetamine and HIV comorbidity.
Brain aging has been demonstrated to be accelerated in cocaine abusers (Ersche, Jones, Williams, Robbins, & Bullmore, 2013). Since psychostimulants alone cause marked pathogenesis, it is not surprising that in combination with HIV, the pathologic consequences to the brain can be severe (Cadet et al., 2014). Many authoritative reviews exist on cocaine neurotoxicity (Ferris et al., 2008; Nath, 2010; Bowers, Chen, & Bonci, 2010; Narayanan, Mesangeau, Poupaert, & McCurdy, 2011; Kousik, Napier, & Carvey, 2012; Pierce & Wolf, 2013; Clark et al., 2013; Cadet et al., 2014). Accordingly, only some of the more recent findings regarding the actions of cocaine will be discussed.
The dopamine transporter is thought to be a major site of cocaine action. By inhibiting DAT function, dopamine accumulates in the synaptic cleft, overactivating dopamine receptors postsynaptically and increasing its rewarding properties. Restricting DAT function alone as demonstrated in DAT knockout mice, however, is insufficient to block cocaine conditioned-place preference (Sora et al., 1998). When dopamine and serotonin transporters are both deleted, mice no longer self-administer or show cocaine place preference (Sora et al., 2001). These findings and others suggest that dopamine and serotonin, as well as norepinephrine, act in concert to contribute to cocaine’s addictive properties (Sora et al., 2001; Hall et al., 2004; Hall et al., 2009). Overall, cocaine appears to primarily act by inhibiting presynaptic dopamine transporters, but also hinders serotonin and norepinephrine transporters (Sora et al., 2001; Hall et al., 2004; Kreek et al., 2005; Hall et al., 2009), and may also secondarily dysregulate inhibitory GABAergic function (Cameron & Williams, 1994). Cocaine also modulates the endogenous opioid system, especially MOR, κ opioid receptors (KOR), and preprodynorphin (Kreek et al., 2005).
1.3. THE CENTRAL ROLE OF GLIA
Although neuronal interconnections form the synaptic circuitry that underlie behavior (Hebb, 1949; Yuste & Bonhoeffer, 2001), astroglia and microglia provide essential structural, trophic, and metabolic support necessary for maintaining synaptic integrity and function. Importantly, in HIV, glia are both targets and effectors in the progression of disease. Not only do glia harbor the virus and release inflammatory mediators, but they may also be functionally compromised and/or killed in the process. Unlike neurogenesis, which is restricted to limited brain regions and neuronal types, glia continue to be generated throughout ontogeny (discussed below). This includes microglia, and all classes of macroglia (including astroglia, oligodendroglia, and ependymal cells). The ability of glial populations to respond in a dynamic fashion to both HIV infection/exposure and to drugs of abuse prompts our glia-centric viewpoint, which provides extensive insight into the pathogenesis of neuroAIDS and into the molecular and cellular mechanisms by which drug abuse affect the progression the disease.
2. MICROGLIA
In the central nervous system (CNS), the principal cell types that are productively infected are perivascular macrophages and brain-resident microglia. Astrocytes can also become infected, and this is particularly evident in vitro; however, the production of new virions by astrocytes is greatly restricted compared to microglia (discussed below). Although the virus itself can infect and replicate in microglia, much of the subsequent spiraling inflammation, synaptodendritic injury, and neurotoxicity arise from the response of bystander microglia and astroglia that are not necessarily infected.
2.1. MICROGLIA AS INNATE IMMUNE EFFECTORS
A seminal study by Ginhoux et al., (2010) reports that microglia originate from a mesenchymal (incipient myeloid) precursor in the murine yolk sac on embryonic day 8.25–8.5, seed the incipient CNS in embryonic day 9.25–9.5, and remain in the CNS throughout life. Despite considerable overlap with myeloid-lineage cells (“myeloid” refers to the bone marrow) such as monocytes and macrophages, which also originate from a common progenitor in the yolk sac, microglia are an ontogenically distinct population—since they never inhabit the bone marrow (Saijo & Glass, 2011). This finding concurs with evidence that microglia and perivascular CNS macrophages can differ functionally and phenotypically—especially in their response to HIV (see below).
Akin to monocyte-derived macrophages (MDMs), microglia possess a wide variety of “pattern” or “pathogen” recognition receptors (PRRs) related to innate immune function. PRRs include Toll-like receptors (TLRs), nucleotide-binding oligomerization domain receptors (NOD-like receptors or NLRs), Mac-1, CD14 (Nadeau & Rivest, 2000), and a wide-variety of scavenger receptors, including those that recognize and remove low-density lipoproteins (Husemann, Loike, Kodama, & Silverstein, 2001; Coraci et al., 2002), and receptors for advanced glycation end-products (RAGE) (Farina, Aloisi, & Meinl, 2007). Microglia can express major histocompatibility complex-I (MHC-I) and MHC-II complexes, that allow them to contribute to adaptive immunity by processing both intracellular and extracellular foreign proteins for presentation as antigens to T-lymphocytes.
2.2. MICROGLIA AND HIV
Despite substantial overlap, microglia and perivascular CNS macrophages can differ functionally and phenotypically in their response to HIV (Fischer-Smith et al., 2001; Guillemin & Brew, 2004) or simian immunodeficiency virus (SIV) (Williams et al., 2001). Given that microglia reside and replicate within the brain throughout life (Ginhoux et al., 2010; Saijo & Glass, 2011), the virus must enter the brain before microglia can become infected. Perivascular macrophages are the major cell by which HIV (Fischer-Smith et al., 2001) or SIV (Williams et al., 2001) seeds the CNS. Perivascular macrophages originate from HIV infected and uninfected MDMs, which are thought to have some ability to traffic bidirectionally between the blood and perivascular sites within the CNS parenchyma (Gonzalez-Scarano & Martin-Garcia, 2005). Although infected MDMs are the major source of virions initially seeding CNS microglia, once the infection is established in microglia (Cosenza, Zhao, Si, & Lee, 2002)—current evidence suggests that resident microglia become a sustained site of both active and latent infection (Kaul, Garden, & Lipton, 2001; Garden, 2002; Persidsky & Gendelman, 2003; Fischer-Smith & Rappaport, 2005; Kramer-Hammerle, Rothenaigner, Wolff, Bell, & Brack-Werner, 2005; Gonzalez-Scarano & Martin-Garcia, 2005), and that HIV evolves independently in distinct CNS cell types (Schnell, Joseph, Spudich, Price, & Swanstrom, 2011). The exchange of HIV between MDMs and microglia makes the brain a reservoir of latent infection (Kaul et al., 2001; Persidsky & Gendelman, 2003; Fischer-Smith & Rappaport, 2005; Kramer-Hammerle et al., 2005; Gonzalez-Scarano & Martin-Garcia, 2005; Schnell et al., 2011; Joseph et al., 2014). This is thought to be particularly important in the post-cART era in which antiretroviral drugs have more limited access to the CNS parenchyma (where microglia reside) because of the BBB.
Infected macrophages and microglia produce “virotoxins” (Nath & Geiger, 1998), i.e., viral protein products such as Tat and gp120, Vpr and others, as well as “cellular toxins” including extracellular reactive oxygen species (ROS), reactive nitrogen species (RNS), and numerous cytokines, including TNF-α, IL-1β, IFN-γ, and IL-6, and chemokines (Persidsky, Buttini, Limoges, Bock, & Gendelman, 1997; Seilhean et al., 1997; Fiala et al., 1997; Kraft-Terry, Buch, Fox, & Gendelman, 2009). Both virotoxins and cellular toxins can independently interact in unique ways with opiates and psychostimulants, and will be the topic of the discussions that follow. In addition, cellular toxins [e.g., quinolinic acid (Guillemin, Kerr, & Brew, 2005) or the neurotoxic amine, Ntox (Giulian et al., 1996)], as well as excess glutamate (Gupta et al., 2010), released by macrophages/microglia also have toxic bystander effects on neighboring neurons (Mayne, Holden, Nath, & Geiger, 2000; Langford & Masliah, 2001; Saha & Pahan, 2003; Schuenke & Gelman, 2003; Yi, Lee, Liu, Freedman, & Collman, 2004; Eugenin et al., 2006; Alirezaei, Kiosses, Flynn, Brady, & Fox, 2008; Turchan-Cholewo et al., 2009; Kiebala & Maggirwar, 2011). The actions of macrophages and microglia in the context of HIV have been extensively reviewed (Masliah et al., 1997; Kaul et al., 2001; Gonzalez-Scarano & Martin-Garcia, 2005).
Microglia can express a wide-variety of neurotransmitter receptors, including AMPA and NMDA receptors, which presumably allows microglia to coordinate and synchronize their responses with neuronal function (Hanisch & Kettenmann, 2007; Pocock & Kettenmann, 2007; Gras et al., 2012; Eggen, Raj, Hanisch, & Boddeke, 2013; Prada, Furlan, Matteoli, & Verderio, 2013). Excitotoxic levels of glutamate can trigger inflammatory responses by microglia, including the release of proinflammatory cytokines and reactive oxygen species (ROS) (Noda, Nakanishi, Nabekura, & Akaike, 2000; Hagino et al., 2004). HIV-1 virions and gp120 have been shown to increase levels of extracellular glutamate by direct effects on uptake mechanisms in astroglia (Wang et al., 2003), and the increased glutamate likely drives further microglial reactivity.
2.2.1. Opioid and HIV actions in microglia
Microglia display more pronounced glial reactivity than astroglia in HIV-infected opiate abusers (Anderson et al., 2003). HIV-1 gp120 and/or Tat released from infected glia (microglia and astrocytes) trigger (1) cytokine release, (2) inflammatory lipid production by bystander glia (Bandaru, Patel, Ewaleifoh, & Haughey, 2011), (3) destabilize intracellular ion homeostasis, and (4) increase extracellular glutamate (Wang et al., 2003; Zou et al., 2011; Podhaizer et al., 2012) and extracellular ATP levels (Sorrell & Hauser, 2014) (figure 1). Bystander neurons are directly and indirectly damaged (Masliah et al., 1997; Kaul et al., 2001; Gonzalez-Scarano & Martin-Garcia, 2005; Mattson, Haughey, & Nath, 2005; Ellis, Langford, & Masliah, 2007). Opiates can affect all aspects of the above processes, including (1) MDM trafficking across the BBB, (2) viral replication in MDMs, microglia and astroglia (largely in in vitro studies), (3) the production of proinflammatory cytokines and chemokines, and (4) losses in extracellular ion homeostasis. The effects of opiates in MDMs and microglia have been comprehensively reviewed elsewhere (Chao, Hu, & Peterson, 1996; Rock & Peterson, 2006; Hauser et al., 2007; Banerjee et al., 2011; Hauser et al., 2012; Dutta & Roy, 2012; Regan, Dave, Datta, & Khalili, 2012; Reddy et al., 2012). Accordingly, only a few recent findings will be briefly considered in the present review.
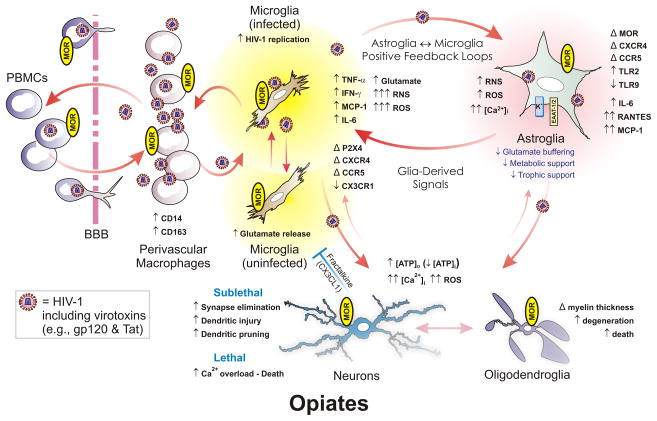
Figure 1.
Opiate drugs exacerbate HIV-1 neuropathogenesis through direct actions on glia—especially microglia and astroglia. Microglia are likely infected through interactions with infiltrating, perivascular macrophages, and propagate the bulk of HIV infection in the CNS. HIV-1 also infects astroglia, but to a far lesser extent, and perhaps without production of new virus., Infection results in the production of reactive oxygen and nitrogen species (ROS and RNS, respectively), pro-inflammatory cytokines, and the release of HIV-1 proteins such as gp120 and Tat. All of these promote inflammation and cytotoxicity in bystander neurons and glia. Opiate abuse alone can cause premature Alzheimer-like changes (Anthony et al., 2010) and morphine by itself can enhance neurotoxicity in vitro (Zou et al., 2011); however, opiates appear to potentiate many of the pathophysiological effects of HIV in the central nervous system of infected individuals. Multiple neuronal and glial types express μ-opioid receptors (MOR). Many of the neurodegenerative effects of opioid-HIV interactions are the result of direct actions on microglia and astroglia, which then lead to a positive feedback cycle of inflammatory/cytotoxic signaling between HIV-1-infected microglia and astroglia. Abbreviations: α-chemokine “C-X-C” receptor 4 (CXCR4); altered or changed (Δ); β-chemokine “C-C” receptor 5 (CCR5); blood-brain barrier (BBB); decreased (↓); fractalkine (CX3CL1); fractalkine receptor (CX3CR1); increased (↑); interferon-γ (IFN-γ); interleukin-6 (IL-6); intracellular Ca2+ concentration ([Ca2+]i); intracellular sodium concentration ([Na+]i); monocyte chemoattractant protein-1 (MCP-1 [or CCL2]); peripheral blood mononuclear cells (PBMCs); regulated upon activation, normal T-cell expressed, and secreted (RANTES [or CCL5]); Toll-like receptor (TLR). Fractalkine released by neurons (and astroglia) can be neuroprotective by limiting the neurotoxic actions of microglia (blue “┬”); red arrows suggest pro-inflammatory/cytotoxic interactions. Modified and reprinted from reference (Hauser et al., 2012) an “open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.5/), which permits unrestrictive use, distribution, and reproduction in any medium, provided the original work is properly cited.”
Acute exposure to opiate drugs such as morphine (El-Hage et al., 2013) or methadone (Li et al., 2002) tend to increase HIV replication by infected microglia. However, depending on the duration and timing of exposure, morphine can increase, act in a neutral manner, or inhibit HIV expression (Peterson, Gekker, Hu, Cabral, & Lokensgard, 2004). Moreover, selective MOR agonists such as endomorphin-1, but not DAMGO or morphine (Peterson et al., 1999) can increase HIV-1 replication in infected microglia—suggesting the involvement of a non-traditional MOR variant in HIV replication (Peterson et al., 1999) or suggesting that “biased agonism” (Hauser et al., 2012) may be operative. We have recently found that specific subsets of MOR splice variants, including MOR-1X and MOR-1K were differentially expressed by human astrocytes, but not expressed at detectable levels in microglia (Dever, Xu, Fitting, Knapp, & Hauser, 2012; Dever et al., 2014). Moreover, the expression of each MOR variant may be differentially regulated by HIV and in a cell specific manner (Dever et al., 2012; Dever et al., 2014). Thus, microglia express a subset of MOR variants each of which may respond uniquely to morphine and/or HIV (Dever et al., 2012; Dever et al., 2014). Collectively, the findings indicate that the effects of MOR activation on HIV replication and the response of microglia to HIV (discussed below) are complex and may differ significantly depending on context.
Acute exposure of microglia to HIV-1 Tat increases glutamate release via the xc− cystine-glutamate antiporter (Gupta et al., 2010). Tat-dependent increases in extracellular glutamate were attenuated by inhibitors of p38, p42/44 MAPK, or NADPH oxidase or by inhibiting the xc− cystine-glutamate antiporter directly (Gupta et al., 2010). Interestingly, morphine co-exposure with Tat can significantly increase glutamate release from microglia above maximal levels of secretion seen with Tat alone (Gupta et al., 2010). Although excitatory amino acid transporters-1 and 2 (EAAT1 or GLAST and EAAT2 or GLT-1, respectively) are minimally expressed by resting microglia and thought to be primarily expressed by astrocytes, recent evidence suggests both transporters are inducible in microglia with immune activation (Gras et al., 2011). Because the function of EAAT1/2 is reduced markedly by morphine by itself (Zou et al., 2011), or in combination with Tat (Zou et al., 2011) or gp120 (Podhaizer et al., 2012) in astrocytes, future studies examining the potential contributions of microglial EAAT1/2 to HIV protein ± morphine-induced increases in extracellular glutamate are warranted.
Chronic opiates disrupt glial function, which is especially problematic in microglia where normal cellular functions have been hijacked by the virus (Hauser et al., 2012). HIV-1 alone causes increases in extracellular glutamate, ROS, and reactive nitrogen species (RNS) by overactivating microglia (Gendelman et al., 1997; Nath, 1999; Kaul et al., 2001; Kaul & Lipton, 2005; Gonzalez-Scarano & Martin-Garcia, 2005; Li, Li, Steiner, & Nath, 2009). Opiates have been shown to modulate (typically, but not always, worsening) all of these neuroinflammatory events (Chao et al., 1996; Rock & Peterson, 2006; Hauser et al., 2007; Banerjee et al., 2011; Hauser et al., 2012; Dutta & Roy, 2012; Regan et al., 2012; Reddy et al., 2012). However, the nature of opiate-HIV interactions in microglia is complicated and depends on a variety of factors that are incompletely understood. For example, we find that isolated microglia display transient increases in cytokine and ROS production in response to acute morphine and HIV-1 Tat co-exposure that are quite robust (Turchan-Cholewo et al., 2009). However, after 24 h of sustained exposure to morphine and Tat the inflammatory response of isolated microglia has faded to levels below that seen with Tat alone and this is not due to increased microglial death (Turchan-Cholewo et al., 2009). Alternatively, when microglia are cocultured with astroglia (Zou et al., 2011; Podhaizer et al., 2012; Hauser et al., 2012), or when glial inflammation and/or neuronal injury is examined in vivo (Bruce-Keller et al., 2008; El-Hage, Bruce-Keller, Knapp, & Hauser, 2008; Fitting et al., 2010a), prolonged morphine and HIV-1 Tat co-exposure results in neuroinflammation and/or neuronal injury that is evident for as long as 10 days. Although the sustained microglial activation is presumed to be driven through reverberating inflammatory signaling between MOR-expressing astroglia and microglia (Suzuki et al., 2011; Zou et al., 2011; Podhaizer et al., 2012; Hauser et al., 2012), additional study is needed to confirm this notion and to identify the mechanisms involved.
MDMs can display phenotypic heterogeneity in their expression of PRRs and in response to regional differences in the extracellular milieu (Kigerl et al., 2009). CD163 and CD16 co-expressing MDMs appear to be preferentially involved in HIV (Ancuta, Wang, & Gabuzda, 2006; Fischer-Smith, Tedaldi, & Rappaport, 2008) or SIV (Borda et al., 2008) replication and AIDS progression. Akin to MDMs, microglia can also display considerable functional heterogeneity (Carson et al., 2007; Hanisch & Kettenmann, 2007; Saijo & Glass, 2011; Scheffel et al., 2012; Hanisch, 2013), and a variety of intermediate states of activation (Colton, 2009). The phenotypic heterogeneity extends to opioid receptors and endogenous opioid peptides, since both macrophages and microglia can variably express MOR, δ, and κ opioid receptors (Chao et al., 1996; Peterson, Molitor, & Chao, 1998; Sheng, Hu, Lokensgard, & Peterson, 2003; Gekker et al., 2004; Turchan-Cholewo et al., 2008).
Emerging evidence indicates that microglia contribute to synaptic plasticity and the stability of synaptodendritic structure during maturation and in response to CNS disorders in adults (Wake, Moorhouse, Jinno, Kohsaka, & Nabekura, 2009; Tremblay, Lowery, & Majewska, 2010; Paolicelli et al., 2011; Ransohoff & Stevens, 2011; Antonucci et al., 2012). Opiate drugs such as morphine and methadone can directly trigger the retraction of dendritic spines in cerebral cortical neurons (Liao, Lin, Law, & Loh, 2005; Liao et al., 2007; Liao, Grigoriants, Loh, & Law, 2007), and affect the plasticity of adult neurons (Robinson & Kolb, 1999; Robinson & Kolb, 2004; Liao et al., 2005; Liao et al., 2007; Liao et al., 2007). Morphine’s actions at MOR trigger decreases in NeuroD phosphorylation that impede glutamatergic signals originating from AMPA receptors (Liao et al., 2005). Subsequent increases in MOR-driven, dynamin-dependent receptor internalization retracts spines (Liao et al., 2005; Liao et al., 2007; Liao et al., 2007). In striatal medium spiny neurons, the relationship is less clear since only a subset of medium spiny neurons express MOR, despite evidence indicating that opiate-induced spine losses are consistent among all medium spiny neurons (Fitting et al., 2010a). While morphine may disrupt the excitotoxic response by decreasing NeuroD phosphorylation, and restricting glutamatergic transmission through neuroprotective AMPA and NMDA receptor subtypes (Liao et al., 2005; Liao et al., 2007; Liao et al., 2007), neurons in the striatum are less likely to be directly affected than in the cerebral cortex since only a subset of medium spiny neurons express MOR. MOR-expressing microglia and astroglia appear to contribute to the interactive neurotoxicity of morphine and Tat in the striatum (Zou et al., 2011; Sorrell & Hauser, 2014). In addition, e.g., GABA and fractalkine may serve as “off” signals—switching off overactive microglia (Beardsley & Hauser, 2014), and opiates can possibly modify these signals (Krebs, Gauchy, Desban, Glowinski, & Kemel, 1994; You et al., 1996; Steiner & Gerfen, 1998; McQuiston, 2007; Bagley et al., 2011; Suzuki et al., 2011). Thus, the innate immune and neurotransmitter signals disrupted by opiates and HIV strategically converge and are integrated into a unique “neuroimmune” logic by microglia.
2.2.2. Psychostimulant and HIV actions in microglia
2.2.2.1. Methamphetamine and HIV
Methamphetamine enhances HIV-1 replication in microglia (Liang et al., 2008). In addition to direct effects on viral replication, combined HIV Tat or gp120 and methamphetamine induce oxidative stress and free radical production in the CNS, which likely originates from reactive microglia (Banerjee, Zhang, Manda, Banks, & Ercal, 2010).
Psychostimulants including methamphetamine, cocaine, and ecstasy have all been suggested to activate the innate immune system (Clark, Wiley, & Bradberry, 2013). Immune activation may be an essential component of neurobiological adaption in alcohol and cocaine addiction (Crews, Zou, & Qin, 2011). Neuronal damage-associated molecular patterns (DAMPs), can directly activate, or under pathophysiological conditions, overactivate microglia (Block, Zecca, & Hong, 2007; Biber et al., 2007). DAMPs are released from stressed or injured cells (Bianchi, 2007; Srikrishna & Freeze, 2009) and trigger innate immune activation. Multiple classes of PRRs appear to be triggered through drug and alcohol abuse (Crews et al., 2011; Yakovleva, Bazov, Watanabe, Hauser, & Bakalkin, 2011; Beardsley & Hauser, 2014). Methamphetamine also reportedly affects subpopulations through the activation of trace aminoacid associated receptor-1 (TAAR1) (Bunzow et al., 2001; Reese et al., 2007; Xie & Miller, 2009). TAAR1 is co-expressed on dopamine D2 receptor and DAT-positive neurons in the striatum and TAAR1 activation results in increases in cAMP levels (Xie & Miller, 2009; Espinoza et al., 2011).
Alternatively, dopamine and norepinephrine, which accumulate in the synaptic cleft following psychostimulant exposure, are thought to be able to elicit responses in microglia (Farber, Pannasch, & Kettenmann, 2005), while GABAB receptor activation reduces lipopolysaccharide (LPS) –induced IL-6 and IL-12 p40 release (Kuhn et al., 2004). Minocycline preferentially blocks macrophage/microglial activation (IL-1β and IL-6 are attenuated, but not TNF-α), but fails to mitigate striatal dopaminergic neurotoxicity because minocycline does not attenuate methamphetamine-induced increases in TNF-α (Sriram, Miller, & O’Callaghan, 2006). Thus, as noted earlier in the context of opiate abuse, the innate immune and neurotransmitter signals disrupted by psychostimulants converge in microglia, which attempts to integrate the diverse input into a coordinated and measured response.
HIV-1 activates microglia directly causing increases in both viral and cellular toxins as outlined earlier. Tat alone mediates much of the glial proliferative and cytokine/chemokine-secreting effects of HIV-1. Methamphetamine exacerbates the neurotoxic effects of HIV-1 through enhanced cytokine production and microglial activation (Theodore et al., 2006a; Theodore et al., 2006b; Theodore et al., 2007) (Figure 2). Dopamine losses evident in the SIV model can be prevented by inhibiting macrophage/microglial activity (Scheller et al., 2005). As with methamphetamine, HIV-1 Tat causes a rapid increase in cortical neuronal excitability that is exacerbated by cocaine (Napier, Chen, Kashanchi, & Hu, 2014). However, in the case of cocaine, despite considerable overlap, some of the events triggering neuroinflammation and microglial activation differ. For example, TAAR1 is activated by methamphetamine and MDMA, but may play a less central role in cocaine’s actions (Bunzow et al., 2001).
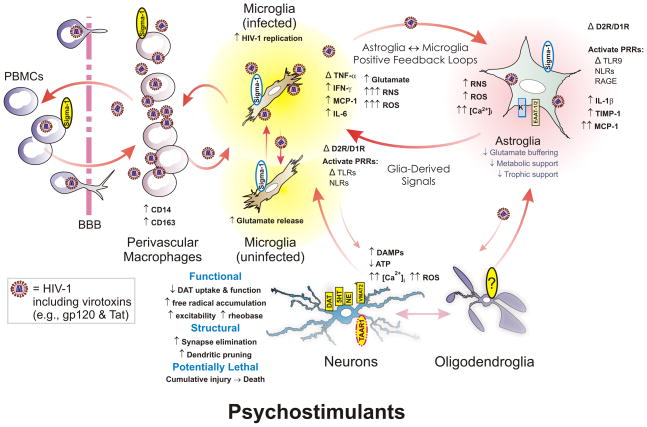
Figure 2.
Psychostimulants can increase synaptic damage through direct actions on neurons and glia, including both microglia and astroglia. Psychostimulants block dopamine, serotonin (5HT), and norepinephrine (NE) transport resulting in excessive accumulations of these neurotransmitters in the synaptic cleft. Dopaminergic neurons are particularly vulnerable to methamphetamine, which disrupts dopamine transporter (DAT) and vesicular monoamine transporter 1 (VMAT2) function and can damage presynaptic terminals of neurons. Synaptic injury is accompanied by the production of reactive oxygen (ROS) and nitrogen (RNS) species, and the production of damage-associated molecular patterns (DAMPs) that trigger activation of pattern recognition receptors (PRRs), including Toll-like receptor 9 (TLR9), nucleotide-binding oligomerization domain-like receptors (NLRs) and other PRRs (e.g., receptor for advanced glycation endproducts or RAGE) expressed by microglia and astroglia. Importantly, psychostimulants (especially methamphetamine) appears to activate neurons directly through the disruption of monoaminergic transporters and VMAT2 mentioned above and through the activation of trace amine-associated receptor 1 (TAAR1). Psychostimulants also disrupt glial function directly by increasing intracellular ROS and likely Ca2+ concentrations ([Ca2+]i), NF-κB transcriptional activity, and by activating sigma-1-receptors (sigma-1; red, dashed-line outline), especially in the case of cocaine, and enzyme systems driving oxidative and nitrosative stress especially in microglia (and other cell types). Increases in NF-κB transcriptional activity result in increased microglial, and to a lesser extent astroglial, production of tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1β (IL-1β), and various other cytokines, as well as tissue inhibitor of metalloproteinase-1 (TIMP-1). Psychostimulants also obstruct the buffering of extracellular glutamate by inhibiting excitatory amino acid transporters-1/2 (EAAT1/2) and the conversion of glutamate to glutamine by inhibiting glutamine synthetase, as well as by limiting glucose metabolism in astrocytes. Collectively, neuronal injury and intensified glial activation promotes positive microglial-astroglial, and neuronal-glial feedback that cause spiraling increases in neuroinflammation and neuronal injury. If unrestrained, the cumulative insults result in lasting neurodegenerative changes. Modified and reprinted from reference (Beardsley & Hauser, 2014). Reprinted from Advances in Pharmacology, Vol. 69, Patrick M. Beardsley and Kurt F. Hauser, Chapter One – Glial Modulators as Potential Treatments of Psychostimulant Abuse, 1–69, Copyright 2014, with permission from Elsevier.
Considerable evidence points toward sigma-1 receptors as a molecular target of cocaine actions (Su, Hayashi, Maurice, Buch, & Ruoho, 2010; Matsumoto, Nguyen, Kaushal, & Robson, 2014). Sigma-1 receptors are widely expressed throughout cells of the nervous system and elsewhere, and can contribute to a variety of pathological processes (Maurice & Su, 2009; Su et al., 2010). Cocaine increases chemokine (C-C motif) receptor 5 (CCR5) and CXCR4 HIV co-receptor expression, while transiently viral replication in human PBMCs in vitro (Roth, Whittaker, Choi, Tashkin, & Baldwin, 2005). Cocaine significantly increases the number of HIV-infected human peripheral blood mononuclear cells (PBMCs) and increases viral load in an infectious humanized SCID mouse model of HIV (Roth et al., 2005). In these studies, the sigma-1 receptor antagonist, BD1047, attenuated the effects of cocaine on HIV replication in HIV-infected humanized SCID mice, suggesting the sigma-1 receptor is a molecular target of cocaine’s actions (Roth et al., 2005). Peterson and colleagues similarly demonstrated using BD1047 that sigma-1 receptor blockade prevented cocaine-induced HIV replication in microglia (Gekker et al., 2006). These investigators subsequently showed the TGF-β inhibitor, SB-431542 (Inman et al., 2002), or immunoneutralizing TGF-β1 antibodies, to be effective in negating the cocaine-induced increases in HIV-1 expression (Gekker et al., 2006). Cocaine treatment accelerates monocyte extravasation across the endothelium of the BBB through an MCP-1-dependent mechanism that is initiated via sigma-1 receptors (Yao et al., 2010; Yao et al., 2011). Understanding the regulation of MCP-1 expression and functional role of sigma-1 receptors following cocaine exposure should provide novel insight into the basic mechanisms by which cocaine augments the severity of neuroAIDS (Yao et al., 2010).
3. ASTROGLIA
3.1. CRITICAL FUNCTIONS IN NEURONAL SUPPORT AND GLIOTRANSMISSION
Astrocytes form a close association with neurons and are strategically positioned to provide structural support, and to maintain metabolic, trophic, and functional processes including synaptic transmission that were previously thought to be regulated by neurons themselves (Parpura, Basarsky, Liu, Jeftinija, & Haydon, 1994; Araque, Parpura, Sanzgiri, & Haydon, 1999; Volterra & Meldolesi, 2005; Haydon & Carmignoto, 2006). Astrocytes also express nearly every class of neurotransmitter receptor, which permits them to coordinate their response precisely with neurons (Zhang & Barres, 2010; Beardsley & Hauser, 2014). The “tripartite synapse” refers to the intimate association between astrocytes and pre- and post-synaptic interconnections (Parpura et al., 1994; Araque et al., 1999; Perea, Navarrete, & Araque, 2009). Gliotransmission refers to the selective uptake and/or release of specific neurotransmitters by astroglia through vesicular (Montana, Malarkey, Verderio, Matteoli, & Parpura, 2006), extracellular membrane microvesicles (Verderio et al., 2012; Verderio, 2013), and/or nanotubes (Verderio, 2013). Gliotransmission significantly modulates neurotransmission (De Pittà et al., 2012; Tewari & Parpura, 2013).
In addition to modulating synaptic transmission, astrocytes regulate extrasynaptic transmission within the extracellular space (ECS) of the CNS (Sykova & Nicholson, 2008). This includes regulating “intersynaptic crosstalk” or the movement of excess neurotransmitters, including glutamate, between synapses, as well as the management of ion homeostasis (Vargova, Jendelova, Chvatal, & Sykova, 2001; Sykova, 2005) and of the movement of water within the ECS (Amiry-Moghaddam et al., 2003; King, Kozono, & Agre, 2004). The coordinated movement of ions and water within the ECS ultimately regulates tissue volume, including brain swelling during specific pathological conditions (Sykova, 2005; Anderova et al., 2011; Zamecnik et al., 2012). Aquaporin-4 (AQP-4) channels expressed largely by astrocytes are critical for regulating the volume of water within the ECS (Amiry-Moghaddam et al., 2003; King et al., 2004). Because HIV-induced deficits in astroglial glutamate and perhaps K+ buffering capacity, as well as AQP-4 function, appear to be independently disrupted by HIV and substance abuse (Wang et al., 2003; Li et al., 2006; Berman et al., 2006; Knackstedt, Melendez, & Kalivas, 2010; Kalivas & Volkow, 2011; Zou et al., 2011; Hauser et al., 2012; Cisneros & Ghorpade, 2012; Podhaizer et al., 2012), extrasynaptic transmission within the ECS is likely to be highly compromised in HIV-infected substance abusers.
3.2. INNATE IMMUNE EFFECTORS
Astroglia are particularly adept at sensing metabolic instability in neurons and in the surrounding microenvironment. They are critical for interpreting and translating intercellular communication between neurons and microglia—especially during pathologic situations (Maragakis & Rothstein, 2006; Molofsky et al., 2012; Verkhratsky, Rodriguez, & Parpura, 2013). Astrocytes can express a variety of PRRs categories against PAMPs and DAMPs, including TLRs, NODs, complement receptors [CR1, CR2, C3aR, C5aR (Gasque, Dean, McGreal, VanBeek, & Morgan, 2000)], mannose receptor (Liu et al., 2004), and RAGE (Husemann & Silverstein, 2001; Farina et al., 2007; Beardsley & Hauser, 2014). Thus, astroglia can act as transducers--both sensing neuronal injury and conveying information about neuron injury to microglia. Emerging evidence also suggests that astrocytes may express MHC-II following injury or stress (Jensen, Massie, & De Keyser, 2013).
3.3. ASTROGLIA AND HIV
Astroglia (Gorry, Purcell, Howard, & McPhee, 1998; Brack-Werner, 1999; Gorry et al., 2003; Kramer-Hammerle et al., 2005; Kramer-Hammerle, Hahn, Brack-Werner, & Werner, 2005) and perhaps also pericytes (Nakagawa, Castro, & Toborek, 2012), are the only resident CNS cells besides microglia that can become infected. Unlike microglia, astroglia tend not to display productive infection; rather, they harbor latent infection that can be reactivated from latency by specific proinflammatory cytokines such as TNF-α, GM-CSF or IFN-γ in SIVmac251-infected astrocytes (Guillemin et al., 2000; Carroll-Anzinger, Kumar, Adarichev, Kashanchi, & Al-Harthi, 2007; Narasipura et al., 2012) or specific, class I histone deacetylase (HDAC) inhibitors (Narasipura, Kim, & Al-Harthi, 2014). Latent HIV is dormant, meaning that viral DNA has been integrated into the host DNA, but that new virions are not being produced. A number of hypotheses have been put forth in an attempt to explain fundamental differences in the regulation of HIV infectivity by astroglia. The activation of NF-κB was proposed to play a less central role in driving viral production by astrocytes than in microglia (Conant, Atwood, Traub, Tornatore, & Major, 1994). Subsequent studies also suggested differences in Rev-astroglial RNA helicase DDX1 interactions (Fang et al., 2005). In addition, a specific class I HDACs and a lysine-specific histone methyltransferase, SU(VAR)3–9, demonstrated in an U87MG astroglial cell line (Narasipura et al., 2014), were proposed as uniquely restricting HIV transcription in astroglia. Overexpression of nef (an early, regulatory gene), but not gag (a late structural gene), is seen in approximately 20% of astrocytes in infected individuals (Saito et al., 1994). Accordingly, Nef serves as a marker and perhaps contributing factor of restricted HIV infection in astroglia (Saito et al., 1994).
Besides being cellular sites of latent infection (Canki et al., 1997; Bencheikh, Bentsman, Sarkissian, Canki, & Volsky, 1999; Brack-Werner, 1999; Kramer-Hammerle et al., 2005), astroglia respond robustly following exposure to HIV proteins (Tat and gp120) or intact virions (Wang et al., 2003; Li, Bentsman, Potash, & Volsky, 2007) by releasing proinflammatory cytokines. Following exposure to viral products, astroglia release toxic and inflammatory cellular products (e.g., glutamate, ROS or cytokines such as TNF-α, IFN-γ, and IL-6) creating pathophysiological conditions that are detrimental for neurons (Genis et al., 1992; Bell, 1998; Nath, Conant, Chen, Scott, & Major, 1999; Kaul et al., 2001; Garden, 2002; Persidsky & Gendelman, 2003).
The nature of the inflammatory response can differ among individual astrocytes (Zhang & Barres, 2010; Fitting et al., 2010b), as well as among microglia (Carson et al., 2007; Scheffel et al., 2012; Hanisch, 2013). Astrocytes are quite heterogeneous in the expression of a wide variety of phenotypic characteristics (Emsley & Macklis, 2006), including many receptor classes, and/or in their response to cues within the local microenvironment of the CNS (Shao & McCarthy, 1994; Shao, Porter, & McCarthy, 1994; Zhang & Barres, 2010; Fitting et al., 2010b). Astroglial heterogeneity has been historically attributed to unique environmental milieu imparted by neighboring neurons. Conversely, astrocytes generated along specific spatiotemporal domains within the ventricular zone (VZ) retain unique phenotypic characteristics throughout life (Tsai et al., 2012). Domain-specific astroglial variants have recently been shown to specify synaptic identity and regulate the ability of neurons to regenerate (Tsai et al., 2012).
Opiates and psychostimulants destabilize astroglial function directly. The destabilization usurps the ability of astrocytes to support neurons metabolically and trophically, while disrupting gliotransmission. Despite some attempts at neuroprotection, the net consequences of exposing astroglia to opiates or psychostimulants is they are less likely to aid neurons or to mitigate a reactive microglial response to HIV infection (Hauser et al., 2007).
3.4. EFFECTS OF OPIATES AND HIV IN ASTROGLIA
Histopathological studies demonstrate that astrocytes display fewer reactive changes than microglia in a chronic opiate abusing cohort of HIV-infected patients (Anderson et al., 2003). Nevertheless, astroglial function is markedly affected and astroglia are able to transduce and amplify signals from HIV-infected or uninfected perivascular macrophages and microglia—even in the absence of substance abuse co-exposure (Hauser et al., 2007). The release of proinflammatory cytokines and chemokines (e.g., MCP-1, MCP-5, and RANTES) can recruit macrophages/microglia, and these newly arriving cells likely contribute to neurotoxicity (El-Hage et al., 2005). The consequences of opiate abuse and HIV interactions in astroglia have been exhaustively reviewed previously (Peterson et al., 1998; Hauser et al., 2005a; Hauser et al., 2007; Banerjee et al., 2011; Hauser et al., 2012; Dutta & Roy, 2012; Reddy et al., 2012). Accordingly, we will only highlight key aspects of the interactions in the paragraphs that follow.
The high degree of phenotypic heterogeneity and plasticity that occurs among individual astrocytes in the expression of HIV co-receptors (Podhaizer et al., 2012) or PRRs such as TLRs (El-Hage, Podhaizer, Sturgill, & Hauser, 2011), as well as in the response to HIV proteins Tat and gp120 (Fitting et al., 2010b) are often-overlooked. Moreover, the prevalence of HIV infection appears to increase in immature astroglia (Tornatore, Nath, Amemiya, & Major, 1991; Tornatore, Meyers, Atwood, Conant, & Major, 1994; Tornatore, Chandra, Berger, & Major, 1994; Messam & Major, 2000; Lawrence et al., 2004), suggesting developmentally regulated differences in susceptibility to HIV-1 by astrocytes. With respect to opioids, astrocytes can express MOR, δ (DOR), and κ opioid receptors (KOR) (Stiene-Martin & Hauser, 1991; Eriksson, Hansson, & Rönnbäck, 1992; Eriksson, Nilsson, Wagberg, Hansson, & Rönnbäck, 1993; Ruzicka et al., 1995; Gurwell et al., 1996; Hauser et al., 1996; Hauser & Mangoura, 1998; Stiene-Martin, Zhou, & Hauser, 1998; Stiene-Martin et al., 2001; Curtis, Faull, & Eriksson, 2007; Turchan-Cholewo et al., 2008). Moreover, astrocytes can express endogenous opioid peptides associated with the preproenkephalin gene (Shinoda, Marini, Cosi, & Schwartz, 1989; Hauser, Osborne, Stiene-Martin, & Melner, 1990; Spruce, Curtis, Wilkin, & Glover, 1990) and preproenkephalin is upregulated by cytokines including IL-1β and interferon-γ (Low & Melner, 1990a; Low & Melner, 1990b; Ruzicka & Akil, 1997). Unlike neurons, which typically produce and release fully processed enkephalin pentapeptides with a high affinity for DOR, astroglia tend to release larger, intact or partially processed proenkephalin peptides that can have high affinity at DOR or KOR. Overall, the wide expression of MOR, DOR and KOR by astroglia makes them a significant target for both endogenous opioids and exogenous opiates.
By disrupting astrocyte function, opiate drug abuse likely subverts their ability to maintain a homeostatic balance of ions and neurochemicals within the ECS, which promotes neuronal injury and death. Morphine can modify cytokine and chemokine production by astroglia (Mahajan, Schwartz, Shanahan, Chawda, & Nair, 2002; El-Hage et al., 2005; Mahajan et al., 2005a; Mahajan et al., 2005b; El-Hage et al., 2008; Sawaya, Deshmane, Mukerjee, Fan, & Khalili, 2009; Avdoshina, Biggio, Palchik, Campbell, & Mocchetti, 2010). Opiates short-circuit the ability of astroglia to protect neurons from HIV (Hauser et al., 2005b; Hauser et al., 2007; reviewed in Hauser et al., 2012). Opiates can intrinsically affect the expression of the glutamate transporters GLAST (EAAT1) and GLT-1 (EAAT2) (Ozawa, Nakagawa, Shige, Minami, & Satoh, 2001; Mao, Sung, Ji, & Lim, 2002). In the presence of HIV-1 Tat, opiates exacerbate the deleterious effects of the disease on intracellular signaling and [Ca2+]i homeostasis (El-Hage et al., 2005; El-Hage et al., 2008), which results in further reducing the ability to buffer extracellular glutamate (Zou et al., 2011). The failure to buffer extracellular glutamate lowers the threshold for excitotoxicity in neurons (Zou et al., 2011; Podhaizer et al., 2012). Furthermore, opiate exposure alone can increase ROS (Zou et al., 2011; Podhaizer et al., 2012) and some proinflammatory cytokines (Mahajan et al., 2002; Mahajan et al., 2005b) in sufficient amounts to potentially be directly neurotoxic (Zou et al., 2011).
HIV-1 Tat is a potent activator of NF-κB (Conant, Ma, Nath, & Major, 1996; El-Hage et al., 2008) resulting in the release of a large number of cytokines and chemokines by astroglia (Conant et al., 1998; Kutsch, Oh, Nath, & Benveniste, 2000; El-Hage et al., 2005; El-Hage et al., 2006b; El-Hage et al., 2008). Besides potential actions destabilizing glutamate and triggering inflammation, Tat shares a Cys-Cys-Phe motif found in β-chemokine sequences such as CCL5 (Albini et al., 1998) that at least partly account for Tat’s chemotactic properties. HIV-1 Tat also destabilizes Ca2+ in astroglia (El-Hage et al., 2005) by mechanisms involving IP3-dependent release (Kumar, Manna, Dhawan, & Aggarwal, 1998). Increased [Ca2+]i dysregulates nuclear-cytoplasmic trafficking of NF-κB subunits (El-Hage et al., 2008), and leads to release of CCL2, CCL5, IL-6 and TNF-α. Morphine exacerbates this cycle (El-Hage et al., 2005; El-Hage et al., 2006a; El-Hage et al., 2006b; El-Hage et al., 2008), presumably by augmenting Tat-induced increases [Ca2+]i. Unlike other neural cell types, MOR can couple to Gβγ (Bonacci et al., 2006; Mathews, Smrcka, & Bidlack, 2008), Gq/11-α (Hauser et al., 1996), and/or Gsα via MOR-1K splice variants (Dever et al., 2014) in astroglia resulting in cellular excitation. Opiate and HIV-induced increases in astroglial-derived cytokines in turn enhance microglial recruitment and activation (El-Hage et al., 2006b). Morphine’s unique actions in HIV-1-exposed astrocytes, in particular, appear to drive escalating, intercellular feedback loops with microglia and perivascular macrophages that increase and sustain inflammation (El-Hage et al., 2006b; Hauser et al., 2007). We have proposed that, unlike other HIV-1-infected organs, which also can harbor MOR-expressing macrophages, the brain is unique because of the inflated response of astroglia to opioids (Hauser et al., 2007; Hauser et al., 2012). As a partial test this assertion, we recently tested whether drugs with selective anti-inflammatory activity in glia could attenuate the deleterious effects of HIV and opiate exposure. We found that ibudilast (also known as AV411 or MN-166) or an analogue lacking phosphodiesterase activity (AV1013), both of which preferentially suppress glial inflammation, attenuates HIV-1 ± morphine-dependent increases in HIV-1 replication and in HIV-1 Tat ± morphine-induced cytokine release and neurotoxicity in vitro (El-Hage et al., 2014).
This concept is supported by findings that HIV Tat ± morphine-induced death of medium spiny neurons is largely mediated via MOR-expressing glia (Zou et al., 2011), including astroglia (El-Hage et al., 2005; El-Hage et al., 2006b; El-Hage et al., 2008) and microglia (Turchan-Cholewo et al., 2008; Bokhari et al., 2009; Turchan-Cholewo et al., 2009; Gupta et al., 2010). Alternatively, the extent to which synaptodendritic culling is similarly driven by glia has not yet been established. Although glia undoubtedly play a significant role, as noted earlier, there is some evidence that morphine can converge with HIV Tat to cause spine retraction through direct actions on the dendrites of cerebral cortical neurons (Liao et al., 2005; Liao et al., 2007; Liao et al., 2007). Additionally, since morphine can excite dopaminergic neurons projecting from the ventral tegmental area (VTA) to striatal neurons by hyperpolarizing inhibitory GABAergic interneurons in the VTA (Johnson & North, 1992), it is likely that HIV-1 and opiate-related alterations in synaptic organization are affected by a complex interplay of events.
3.5. Effects of psychostimulants and HIV in astroglia
A number of reviews on the effects of psychomotor stimulants by themselves (Cadet & Krasnova, 2009; Clark et al., 2013; Beardsley & Hauser, 2014; Cadet et al., 2014) and in the context of HIV (Hauser et al., 2007; Nath, 2010; Cisneros & Ghorpade, 2012; Buch et al., 2012) on astrocytes are available. Briefly, methamphetamine and cocaine are thought to affect astrocyte function through a variety of indirect and direct actions. The profile of inflammatory cytokines released by astrocytes in response to methamphetamine notably include the release of TNF-α, IL-1β, IL-6, and the chemokine MCP-1, as well as intercellular adhesion molecule-1 (ICAM-1) (Flora et al., 2002; Nakajima et al., 2004; Theodore et al., 2006a; Goncalves et al., 2008; Clark et al., 2013). The astroglial response to cocaine is more limited than the response to methamphetamine. Cocaine can increase the expression of TNF-α, IL-1β, and IL-6 transcripts, while downregulating the anti-inflammatory cytokine IL-10 (Clark et al., 2013). These cytokines fuel inflammatory cascades and the release of chemokines such as MCP-1, which recruit macrophages and activate microglia within the CNS (Yao et al., 2010). Glial inflammation is proposed to be an essential step in the maladaptive neuroplasticity accompanying addiction (Crews et al., 2011; Frank, Watkins, & Maier, 2011; Clark et al., 2013). Excessive or sustained high levels of inflammation result in neuronal injury and potentially neuronal death (Jayanthi et al., 2005; Krasnova & Cadet, 2009; Buttner, 2011).
Gliotransmission has been reported to be necessary for reinstatement of cocaine-seeking behavior (Turner, Ecke, Briand, Haydon, & Blendy, 2013). Mice expressing a dominant negative soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) variant driven by a GFAP-dependent promoter were used to selectively disrupt gliotransmission (Turner et al., 2013). However, in these studies, the transmitter substance(s) released during gliotransmission are uncertain. Gliotransmission can involve the vesicular release of excitatory transmitters, including glutamate, serine, and adenosine triphosphate (ATP) (Pascual et al., 2005; D’Ascenzo et al., 2007; Parpura & Zorec, 2010; Santello, Cali, & Bezzi, 2012; Martineau, 2013; Van Horn, Sild, & Ruthazer, 2013). Of these, glutamate appears to contribute to drug-seeking behavior and other aspects of cocaine addiction (Beardsley & Hauser, 2014)
In combination with methamphetamine, HIV Tat exacerbates the disruption of EAAT-2 and perhaps EAAT-1 (aka, glutamate/aspartate transporter or GLAST) in astroglia (Cisneros & Ghorpade, 2012), and may additionally increase the release of glutamate from injured presynaptic terminals. Interestingly, ceftriaxone, which upregulates EAAT2 expression in astroglia, protects neurons against Tat or gp120-induced injury (Rumbaugh, Li, Rothstein, & Nath, 2007). Collectively, methamphetamine and HIV appear to dysregulate the buffering of extracellular glutamate by astrocytes, which contributes to excitotoxic injury in neurons. Propentofylline, which is thought to affect multiple molecular targets in both astroglia and microglia (Sweitzer, Schubert, & De Leo, 2001; Sweitzer & De Leo, 2011), impairs reinstatement to cocaine through an EAAT-2-related mechanism (Reissner et al., 2014). Furthermore, anti-inflammatory drugs with preferential actions in glia such as minocycline and/or ibudilast can limit key aspects of methamphetamine’s locomotor behaviors and/or reinforcing properties (Snider et al., 2012; Snider, Vunck, Hendrick, & Beardsley, 2012) or aspects of cocaine sensitization (Chen, Uz, & Manev, 2009; Chen & Manev, 2011).
Cocaine affects BBB permeability and increases MDM trafficking across the barrier (Fiala et al., 1998; Zhang et al., 1998; Gan et al., 1999). Key aspects of the higher rates of CNS infection (Fiala et al., 2005) and encephalitis (Clark et al., 2013) caused by cocaine are fueled by sigma 1 receptor-induced increases in MCP-1 derived from glia and especially astrocytes--recruiting new MDMs into the CNS (Yao et al., 2010). Moreover, sigma 1 receptor-dependent increases in the expression of activated-leukocyte cell adhesion molecule by endothelial cells (Yao et al., 2011), which increases diapedesis of MDMs and the recruitment of perivascular macrophages. Methamphetamine increases the shedding of matrix metalloproteinases (MMPs), especially MMP-1 and MMP-2 from astroglia, which can disrupt the blood brain barrier (BBB) (Conant et al., 2004). Besides, MMP-1 and MMP-2, HIV-1 Tat can increase MMP-5, which may reduce long-term potentiation (LTP) in the hippocampus (Conant et al., 2010). MMP-5 can cleave GluN1 NMDAR subunits (Szklarczyk et al., 2008) and aquaporin-4 (AQP-4).
AQP-4 expressed by astroglia (Rash, Yasumura, Hudson, Agre, & Nielsen, 1998) is essential for moving water from the ECS through astrocytes and across the BBB into the vasculature (King et al., 2004; Tait, Saadoun, Bell, & Papadopoulos, 2008). AQP-4 is intimately linked to astroglial function (King et al., 2004). AQP-4 levels are increased in HIV-associated dementia (HAD); however, it is uncertain whether this is a maladaptive or a compensatory response to counteract the effects of chronic inflammation (St Hillaire et al., 2005). Interestingly, the effects of cocaine are attenuated in AQP-4-null mice, leading to speculation that AQP-4 regulates cocaine reinforcement and dependence by alternating dopamine and glutamate release associated with drug reward (Li et al., 2006). Although AQP-4 expression per se appears to be unaffected by cocaine exposure (Narayana et al., 2014), AQP-4-null mice display attenuated locomotor and reward responses to cocaine suggesting its involvement in the neurobiological actions of cocaine (Li et al., 2006). By virtue of their critical role in regulating the volume of water within the ECS (Amiry-Moghaddam et al., 2003; King et al., 2004), including pathological brain swelling (Sykova, 2005; Anderova et al., 2011; Zamecnik et al., 2012), AQP-4 channels are likely to be important in regulating HIV and psychostimulant interactions.
4. GENETIC FACTORS THAT MODULATE HIV-1 INFECTIVITY AND NEUROPATHOGENESIS
4.1. INTRODUCTION
There are huge differences in the susceptibility of individuals to addiction or to acquiring HIV.. Emerging evidence indicates that different gene polymorphisms underlie the marked differences in HIV infectivity and/or in the response to combination antiretroviral therapy (cART) among individuals. Gene profiling differences have also suggested that HAND disorders of different severity may represent fundamentally different disease courses, and not a continuum of a single pathophysiological process (Gelman et al., 2012).
Polymorphisms in the genes associated with HIV-1 co-receptors and/or their endogenous ligands can markedly influence AIDS progression (Smith et al., 1997; Winkler et al., 1998; Carrington, Dean, Martin, & O’Brien, 1999). CCR5 in particular (Carrington et al., 1999), and mutations thereof, e.g., CCR5Δ32 (Huang et al., 1996; Boven, van der Bruggen, van Asbeck, Marx, & Nottet, 1999), as well as mutations in key cytokines, e.g., IL-10 (Shin et al., 2000) or TNF-α (Quasney et al., 2001), or other genes linked to specific neurodegenerative disorders such as apolipoprotein ε4 (ApoE4) (Verghese, Castellano, & Holtzman, 2011) may have a marked influence on neuroAIDS outcome measures (Shapshak et al., 2004a; Shapshak et al., 2011). Mutations in other human gene products, such as the specific apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G (APOBEC3G) (Kim et al., 2010), an innate viral restriction factor that inhibits the production of HIV (Mangeat et al., 2003; Shindo et al., 2003; Bishop, Holmes, Sheehy, & Malim, 2004), may have deleterious consequences. In this section, we will cite and discuss several examples in which human genetic variability is beginning to uncover essential sites of drug abuse and neuroAIDS interplay.
CCR5 plays a critical role in HIV infection as a co-receptor for entry of CCR5-preferring strains that appear largely responsible for initial infection (Berger, Murphy, & Farber, 1999; Moore, Kitchen, Pugach, & Zack, 2004). The importance of CCR5 in HIV infectivity is supported by evidence by a wide variety of approaches. CCR5 levels in general, and in brain MDMs and microglia, have been correlated with the severity of HIV neurologic disease (Vallat et al., 1998; An, Osuntokun, Groves, & Scaravilli, 2001). Individuals homozygous for the CCR5Δ32 mutation resist infection by HIV (Huang et al., 1996; Boven et al., 1999). CCR5 blockade employing the antagonist maraviroc to inhibit viral entry has marked clinic efficacy (MacArthur & Novak, 2008). Experimental reductions in CCR5 levels using gene silencing strategies have been successful in reducing HIV infectivity experimentally (Lee et al., 1999; Anderson & Akkina, 2007).
The importance of HIV co-receptors in viral infectivity and pathogenesis cannot be underestimated. Polymorphisms of CCR5, such as CCR5Δ32 (Huang et al., 1996; Boven et al., 1999) or CCR2 (Smith et al., 1997), can confer significant protection against HIV progression, while other mutations can worsen disease progression. Two studies have demonstrated that dual single nucleotide polymorphisms (SNPs) in the RANTES gene promoter (−471 and −96) reduce HIV disease advancement (McDermott et al., 2000; Gonzalez et al., 2001), while only one of these studies found an effect on transmission risk (McDermott et al., 2000). The RANTES-28G mutation increases RANTES transcript levels and is associated with increased protection against the clinical progression of HIV infection (Liu et al., 1999). The apparent eradication of HIV in a patient receiving a transplant of hematopoietic stem cells harboring a mutation (CCR5Δ32) in the chemokine (C-C Motif) receptor 5 (CCR5), a major HIV co-receptor that facilitates cell infection with HIV, has highlighted the insights that might be gained into mechanisms of HIV infectivity/pathogenesis by studying the role of genetic variability (Hutter et al., 2009; Allers et al., 2011).
Although polymorphisms of CXCR4 also exist, these are far less frequently identified than CCR5 polymorphisms. A likely reason may be that the deletion of CXCR4 or its cognate ligand SDF-1/CXCL12 is lethal (Ma et al., 1998; Zou, Kottmann, Kuroda, Taniuchi, & Littman, 1998). Combined mutations in multiple HIV co-receptors and/or in the cognate ligands of these co-receptors can interact to confer more or less protection against HIV infectivity or subsequent pathogenesis (Shapshak et al., 2004b; Gelman et al., 2012; Levine, Sinsheimer, Bilder, Shapshak, & Singer, 2012). Lastly, “elite suppressors” or “controllers” is the term given a subset of individuals who maintain plasma HIV copy numbers below 50 copies/mL (Han et al., 2008). SNPs in macrophage inflammatory protein 1α coincide with differences in disease progression (Gonzalez et al., 2001). In sum, gene variations in β-chemokines and their receptors can have marked influences on the clinical course HIV infection. There is considerable debate regarding the extent to which combinations of protective/non-protective allelic variants contribute to the subset of patients who are elite suppressors and are able to intrinsically suppress HIV replication (Miura et al., 2008; Baker, Block, Rothchild, & Walker, 2009).
Levels of monocyte chemoattractant protein (MCP-1 or CCL2) and its cognate receptor, CCR2, are increased with HIV infection and coincide with neurological impairment (Sozzani et al., 1997; Cinque et al., 1998). MCP-1 is released by HIV-exposed MDMs, microglia, and astrocytes (Conant et al., 1998; Nath et al., 1999). MCP-1 released by resident glia has been proposed as a key event in recruiting MDMs into the brain, an event that is exacerbated by opiates and psychostimulants (Zhang et al., 1998; Fiala et al., 2005; Eugenin, Dyer, Calderon, & Berman, 2005; Eugenin et al., 2006; Hauser et al., 2007; Berman et al., 2008; Yao et al., 2010). MCP-1 release from glia, especially astroglia, is a significant site of drug abuse and HIV interactions (Hauser et al., 2007). Morphine exacerbates the release of chemokines, especially RANTES and MCP-1, from HIV Tat-exposed astrocytes (El-Hage et al., 2005; El-Hage et al., 2006b) and microglia (Turchan-Cholewo et al., 2009) in a time- and concentration-dependent manner, while cocaine accelerates monocyte extravasation across the BBB endothelium through a MCP-1-dependent mechanism that is absent in CCR2 knockout mice (Yao et al., 2010). A number of mutations in the CCR2 and CCL2 genes have been demonstrated to affect aspects of HIV/AIDS. The CCR2 V64I allele is associated with a more rapid onset of neurocognitive impairment, but even after adjusting for estimated time of seroconversion, there is no correlation with increased viral loads in cerebrospinal fluid (CSF) or in plasma, of HIV-infected subjects (Singh et al., 2004). Interestingly, the CCR2 V64I allele affords some protection against progression to AIDS, especially during early phases of the disease process (Ioannidis et al., 2001; Mulherin et al., 2003), perhaps at the expense of CNS function. An MCP-1 (CCL2) -2578G/A promoter polymorphism was shown to enhance protein production and was associated with significantly reduced risk of acquiring HIV infection (Gonzalez et al., 2002). However, once infected, patients with this genotype showed faster disease progression and enhanced risk for HAD, presumably due to enhanced infiltration of infected monocytes (Gonzalez et al., 2002). CCR2 gene polymorphisms can act in a cooperative manner with other genes to affect HIV pathogenesis, including CCR5 (Rigato et al., 2008). By contrast, no connections were found between CCR2 V64I or CCR5Δ32 mutations and HIV infectivity in “preferential” opiate abusers in northeastern India (Sarkar et al., 2010). However, as is common with most drug abusing cohorts, complicated individual abuse patterns and polydrug use confound the interpretation of the findings: 59% of these subjects abused spasmo-proxyvon, which contains the synthetic opiates dextroprophoxyphene or prophoxyphene and acetaminophen (Mahanta, Borkakoty, Das, & Chelleng, 2009), 54% abused heroin, and 15% abused “brown sugar” (partially purified heroin) (Sarkar et al., 2010).
TNF-α is important in triggering subsequent proinflammatory cascades such that any abnormalities in the regulation of TNF-α responsiveness are likely to have resounding consequences for the CNS (Bradley, 2008; McCoy & Tansey, 2008). Accordingly, it is perhaps not surprising that polymorphisms in the TNF-α promoter are associated with higher incidence of HAND (Quasney et al., 2001). Notably, both classes of abused drugs, opiates and psychostimulants, can increase the release of TNF-α from HIV or virotoxin-exposed glia (Gendelman et al., 1997; Fiala et al., 1997; Zhang et al., 1998; Flora et al., 2002; Fiala et al., 2005; Sriram et al., 2006; Sawaya et al., 2009). Alternatively, it has been argued that while TNF-α appears to serve as a marker for HIV progression, interferon-γ may play a more central role as a causal factor in the development of the disease based on genetic analyses of polymorphisms of both genes in the same patient population (Shapshak et al., 2004a).
4.1.1 Mitochondrial genetics
Within the CHARTER cohort, several mtDNA SNP haplotypes are associated with marked protection from peripheral neuropathies (African haplogroup L1c and European haplogroup J) (Holzinger et al., 2012). mtDNA polymorphisms have also been linked to bipolar disorder, and may be associated with an increased risk for neurodegenerative/neurocognitive disorders (Chinnery et al., 2001; Lin & Beal, 2006), especially those associated with aging (Kato, 2001). Dopaminergic neurons may be particularly susceptible to mtDNA damage (Bender et al., 2008). Interestingly, methamphetamine increases mtDNA damage (Bachmann et al., 2009), and CNS damage caused by in utero methamphetamine exposure can be rescued by increasing DNA repair through the enhancement of oxoguanine glycosylase 1 activity (Wong, McCallum, Jeng, & Wells, 2008).
4.2. GENE VARIATION IN OPIATE DRUG ABUSE AND HIV INTERACTIONS
Given the importance of host genetic variability in determining and revealing fundamental mechanisms underlying HIV infectivity and pathogenesis, might host genetics also reveal basic processes underlying the interactions between substance abuse and HIV? An examination of specific human gene polymorphisms, especially genes for drug receptors (Bond et al., 1998; Kreek, Nielsen, Butelman, & LaForge, 2005; Kreek et al., 2005; Kreek et al., 2012), enzymes affecting drug metabolism (Meyer & Zanger, 1997), and/or neurochemical systems thought to underlie addiction (Lachman et al., 1996; Nebert, McKinnon, & Puga, 1996; Li et al., 2004; Kreek et al., 2005; Levine et al., 2012), has identified significant correlative relationships between gene polymorphisms and substance abuse (Kreek et al., 2005; Yuferov, Levran, Proudnikov, Nielsen, & Kreek, 2010; Crystal et al., 2012; Manini, Jacobs, Vlahov, & Hurd, 2013; Jacobs, Murray, Byrd, Hurd, & Morgello, 2013). Because addiction is principally a CNS disorder with neurobehavioral/neuropsychiatric underpinnings (Leshner, 1997; Volkow, Wang, Fowler, & Tomasi, 2012), examining substance abuse-HIV interactions in the brain seems a logical direction. The potential importance of studying human gene polymorphisms as an approach to identify novel factors and mechanisms underlying drug abuse and neuroAIDS pathogenesis cannot be underestimated.
In a sample population of 1,031 women, polymorphisms in OPRM1, the gene encoding MOR, were associated with the severity of HIV infection or the response to cART (Proudnikov et al., 2012). These investigators found both negative and positive correlations with HIV severity in a small subset of OPRM1 polymorphisms, while most variants displayed no association with HIV progression. Interestingly, although a subset of the patients sampled undoubtedly were substance abusers, many were not—suggesting that MOR receptor is inextricably linked to processes influencing HIV pathogenesis irrespective of opiate exposure. Although the mode of action is unclear, MOR activation can alter the expression of HIV-1 chemokine coreceptors involved in HIV entry, and MOR can undergo heterologous desensitization with CXCR4 (Szabo et al., 2002; Steele, Henderson, & Rogers, 2003; Patel et al., 2006; Finley et al., 2008; Burbassi, Aloyo, Simansky, & Meucci, 2008; Pitcher et al., 2014) or CCR5 (Chen et al., 2004; Happel, Steele, Finley, Kutzler, & Rogers, 2008; Song et al., 2011). Lastly, non-opioid genes may influence opiate drug and HIV interactions. For example, the presence of an apolipoprotein ε4 (ApoE4) allele increases the likelihood of neurotoxicity in response to combined morphine and HIV-1 Tat exposure in isolated human neurons in vitro (Turchan-Cholewo et al., 2006). The mechanisms responsible for opiate interactions with ApoE4 are unclear. Possessing an ApoE4 allele and increased ApoE4 levels in CSF was associated with an increased probability of neurocognitive impairment in HIV-infected patients in one study (Andres et al., 2011), while another failed to find a linkage between the ApoE4 gene frequency and cognitive deficits in HIV-infected subjects (Morgan et al., 2013).
A potentially important role of OPRM1 splice variants in neuroAIDS is indicated by our findings showing quantitative differences in specific human MOR splice variant expression levels with HIV encephalitis and/or neurocognitive status (Dever et al., 2012; Dever et al., 2014). In addition, MOR-1 (exon 1), MOR-1A, MOR-1X, and MOR-1K splice variants appear to differ across CNS cell types (Dever et al., 2012; Dever et al., 2014). Evolving evidence suggests that there are significant functional differences among MOR splice variants (Pan et al., 2005; Majumdar et al., 2011; Dever et al., 2012; Xu et al., 2014; Lu, Xu, Xu, Pasternak, & Pan, 2014). Thus far, the findings are limited to neural cells isolated from a relative small number of individuals, and from imprecisely defined brain regions. However; if our initial findings remain supported, this would be highly significant—indicating the existence of quantitative and functional differences in MOR subtypes among cell types.
A novel, truncated 6-transmembrane spanning (6TM) MOR-1K splice variant has been described, which unlike canonical 7-transmembrane spanning (7TM) MOR isoforms, couples into Gαs, increases [Ca2+]i and nitric oxide, and causes cellular excitation (Shabalina et al., 2009; Gris et al., 2010). Interestingly, HIVE and perhaps cognitive impairment correlates with increased MOR-1K (Dever et al., 2014), but not with MOR-1A or MOR-1X splice variants (Dever et al., 2012), in patient samples obtained through the National NeuroAIDS Tissue Consortium.
Global RANTES/CCL5 knockout reduced microgliosis and was neuroprotective in mice concurrently treated with morphine and HIV-1 Tat protein (El-Hage et al., 2008). This insinuates that CCL5-to-CCR5 signaling increases neuroinflammation even in non-infectious models, suggesting that in addition to viral entry, CCR5 blockade may be inherently neuroprotective (El-Hage et al., 2008). Preclinical studies in the SIV model demonstrating maraviroc protection against inflammatory markers in neuroAIDS lend support to this assertion (Kelly et al., 2013). Moreover, findings from other investigators suggest that CCR5 blockade may have advantages in other aspects of disease management besides preventing viral entry (Hunt et al., 2013). CCR5 blockade, either genetically or by maraviroc, eliminates synergistic morphine and Tat-related neurotoxicity in glial-neuronal co-cultures (unpublished results).
The above findings and work of others demonstrating heterologous, bidirectional cross-desensitization (Steele et al., 2003; Rogers & Peterson, 2003; Chen, Geller, Rogers, & Adler, 2007; Happel et al., 2008; Song et al., 2011) or direct molecular interactions (Suzuki, Chuang, Yau, Doi, & Chuang, 2002; Chen et al., 2004) between CCR5 and MOR, are guiding the development of novel therapeutics that selectively target CCR5-MOR and HIV interactions. Using translationally relevant, infectious models, we have started to screen novel bivalent ligands comprised of linked CCR5 and opioid receptor antagonists, which selectively target putative CCR5-MOR heterodimers (Yuan et al., 2012; Yuan et al., 2013; El-Hage et al., 2013) (Figure 3).
Figure 3.
Computer-generated model of a MOR-CCR5 dimer (A). The helical portions colored in blue and green represent CCR5, while the red and yellow helices represent MOR (A). Each ribbon was given an arbitrary color in order to distinguish individual helices from one another (A). Representation of the Poisson–Boltzmann electrostatic potentials at the surface of the heterodimer using the APBS plugin by PYMOL (El-Hage et al., 2013) (B). Acidic residues are shown in red (−2 kBT/e); basic residues are shown in blue (+2 kBT/e); white represents uncharged residues (B). The model predicts that a majority of the interactions between the two receptors are hydrophobic (B). Chemical structure of a bivalent ligand that binds both MOR and CCR5 receptors concurrently (C) (for complete description see, El-Hage et al., 2013). Reprinted with permission from Lippincott Williams and Wilkins/Wolters Kluwer Health: AIDS (El-Hage et al.), copyright 2013.
5. NEURAL/GLIAL PROGENITORS AND HIV
Neural progenitor cells (NPCs) are the undifferentiated precursors of both neurons and macroglia (astroglia and oligodendroglia); infection of NPCs might either lead to (occasional reports of) viral expression in more differentiated derivatives, or might directly influence their development and/or survival. NPCs may also be targets of HIV through interactions with HIV-1 proteins, or through the influence of extracellular changes that occur as a consequence of microglial or astroglial infection (e.g. enhanced levels of glutamate, upregulation of inflammatory signals) (Krathwohl & Kaiser, 2004; Okamoto et al., 2007; Buch et al., 2007; Peng et al., 2008; Mishra, Taneja, Malik, Khalique, & Seth, 2010; Hahn et al., 2010; Lee et al., 2011; Peng et al., 2011; Hahn et al., 2012). During development, both neurons and macroglia derive from NPCs in the subventricular zone (SVZ) lining the central canal of the developing CNS. While most neurons in both rodents and humans are formed prior to birth, production of astroglia and oligodendroglia continues postnatally (Skoff, 1990; Skoff & Knapp, 1991; Lee, Mayer-Proschel, & Rao, 2000; Chan, Lorke, Tiu, & Yew, 2002; Geha et al., 2010). In humans, for example, oligodendrocyte formation and myelination continue well into the late teenage years, to accommodate CNS growth and maturation. While NPCs are obviously critical for CNS development, they are also present and functional in the adult CNS, although their characteristics, localization, and cell-specific markers are somewhat different from NPCs in the developing brain. In adults, neurogenesis is normally quite limited, occurring only in specific regions. In humans and other primates, these regions include the subgranular zone of the hippocampal dentate gyrus (DG) and the SVZ of the lateral ventricles (aka, ependymal/subependymal zones) (Kaplan & Hinds, 1977; Doetsch, Garcia-Verdugo, & Alvarez-Buylla, 1997; Eriksson et al., 1998; Kornack & Rakic, 1999). In vertebrates, including rodents, who are heavily dependent upon olfactory cues, neurogenesis within the adult SVZ results in a pool of cells that continually enters the “rostral migratory stream”, a migratory route specialized for the delivery of newly formed neurons to the olfactory bulb (Doetsch et al., 1997). A structure analogous to the rostral migratory stream remains to be validated in adult humans, although the pathway exists during development (Pencea, Bingaman, Freedman, & Luskin, 2001; Bhardwaj et al., 2006; Sanai et al., 2011; Wang et al., 2011). Unlike neurons, glia are normally formed throughout the lifetime of an animal, and glial progenitors undergo a constant, slow turnover throughout the adult CNS parenchyma (MESSIER, LEBLOND, & Smart, 1958; Imamoto, Paterson, & LEBLOND, 1978; Sturrock, 1979; Kornack & Rakic, 1999; Kornack & Rakic, 2001). Whereas neonatal NPCs proliferate routinely and robustly, adult NPCs in the SVZ and DG are mostly quiescent. They display somewhat different cell markers, and often respond to milieu signals or interact with surrounding cells differently than NPCs in young tissues. In response to exercise, injury/perturbation, inflammation, or other stimuli, adult NPCs can become more active, generating a subset of highly proliferative progenitors that can form neurons and/or glia that integrate into surrounding tissue (Thored et al., 2006a; Kernie & Parent, 2010; Wang, Plane, Jiang, Zhou, & Deng, 2011). Whether the enhanced turnover of adult NPCs after injury involves “active” adult NPCs or transit amplifying cells that derive from “quiescent” NPCs, or both, it is a somewhat different process from formation of cell populations in development, with different effectors and results (Romanko et al., 2004; Rola et al., 2006; Burns, Murphy, Danzer, & Kuan, 2009). Deficits in NPC populations appear to contribute to diseases like Parkinson’s, Huntington’s, and Alzheimer’s diseases, which involve specific neuron types (Curtis et al., 2003; Curtis et al., 2007; Crews, Patrick, Adame, Rockenstein, & Masliah, 2011), and to more global injuries such as stroke/ischemia and epilepsy (Parent et al., 1997; Thored et al., 2006b; Curtis et al., 2007; Ohira et al., 2010). Increased NPC proliferation is often assumed to indicate beneficial plasticity. However, proliferation must renew NPCs while also producing new neurons or glia (asymmetric division), and cell production must be balanced to tissue requirements. In Huntington’s disease, for example, there is a loss of striatal neurons even though proliferation and SVZ size are significantly increased (Curtis et al., 2007; Kazanis, 2009), because the ratio of GFAP+ glia (type B cells) produced is too high (Curtis, Waldvogel, Synek, & Faull, 2005). Increased proliferation may also be offset by death or aberrant migration, as in some epilepsy models (Parent et al., 1997).
The effects of HIV and/or drugs of abuse on adult hippocampal neurogenesis have been recently reviewed (Eisch & Harburg, 2006; Venkatesan, Nath, Ming, & Song, 2007). Accordingly, the discussion here will include other brain regions and gliogenesis. It will also include developmental studies, because of relevance to HIV acquired in the perinatal and adolescent periods. Since NPCs are uniquely positioned as the forerunners of CNS neurons and macroglia, the potential effects of NPC infection or dysregulation by HIV are extensive. For example, the evidence that nestin+ or Sox-2+ human NPCs can be infected by HIV (Lawrence et al., 2004; Schwartz & Major, 2006; Rothenaigner et al., 2007; Hahn et al., 2012) under conditions or in numbers that are disease-relevant remains controversial. In numerous experimental paradigms, HIV or HIV proteins have been shown to alter the proliferative and survival characteristics of NPCs. These include rodent (Khurdayan et al., 2004; Buch et al., 2007; Hahn et al., 2012) and human NPCs (Krathwohl & Kaiser, 2004; Mishra et al., 2010; Hahn et al., 2012) exposed in culture or ex vivo settings, NPCs within the brains of gp120 and Tat transgenic rodent HIV models (Okamoto et al., 2007; Lee et al., 2011; Hahn et al., 2012; Avraham et al., 2014), and in HIV postmortem brains (Krathwohl & Kaiser, 2004). In general, whether the models have examined NPCs from/in adult or developing systems, HIV or HIV proteins have been reported to depress NPC proliferation. This was also the case in human hippocampal slice cultures exposed to either X4 HIV coat proteins or to the CSF from HIV patients with dementia (Krathwohl & Kaiser, 2004). Alternatively, NPCs showed enhanced proliferation when exposed to medium from infected macrophages co-stimulated with LPS (Peng et al., 2008). Survival is variably reported as being unchanged by Tat, gp120, or HIV (Mishra et al., 2010; Lee et al., 2011; Hahn et al., 2012; Malik, Saha, & Seth, 2014) or reduced by Tat or gp120 (Buch et al., 2007; Avraham et al., 2014), feline immunodeficiency virus (van Marle, Antony, Silva, Sullivan, & Power, 2005), or by infection with a viral vector expressing HIV envelope (van Marle et al., 2005). The different outcomes likely reflect varied susceptibility among stages of differentiation (Levison, Rothstein, Brazel, Young, & Albrecht, 2000; Brazel, Nunez, Yang, & Levison, 2005). Changes in these parameters have the potential to alter the balance of mature cells that derive from the NPCs.
Exposure to HIV, HIV proteins, or milieu changes that occur in the HIV-infected brain might also redirect the lineage of undifferentiated cells. Normally, NPCs undergo a series of self-renewing divisions prior to an asynchronous division that results in one daughter cell that is committed to a neuron or glial cell fate/lineage. HIV infection or viral protein exposure appears to skew NPC fate toward production of glia/astroglia at the expense of neurons and/or oligodendrocytes (Hahn et al., 2010; Peng et al., 2011; Hahn et al., 2012). Since viral production reportedly increases as infected NPCs differentiate into astroglia (Lawrence et al., 2004), cell lineage choices, i.e., the formation of astroglia at the expense of other NPC derivatives, may increase CNS consequences of NPC infection, including the potential for more latent virus in the CNS. This may be especially relevant in pediatric/adolescent patients given their larger and more mitotically active NPC population. Changes in the milieu can push more NPCs towards a particular lineage, for example, increasing glia or astroglia at the expense of neurons, which may explain the enhanced numbers of astroglia that we have seen in adult mice exposed to HIV-1 Tat for 3 months (Hahn et al., 2014). Increased astrogliogenesis also appears to be directly triggered by HIV-infected microglia both in vitro and in an infected SCID mouse model via a STAT3-dependent mechanism (Peng et al., 2011). While lineage redirection has not been directly demonstrated in the context of HIV, it has been well documented during development and in some diseases (Bithell, Finch, Hornby, & Williams, 2008; Sabo, Kilpatrick, & Cate, 2009; Lu et al., 2011; Ninkovic & Gotz, 2013).
HIV is a situation of reverberating inflammation. It is perhaps not surprising that HIV infection or viral protein exposure limits NPC proliferation, since brain inflammation is well known to impair neurogenesis (Monje, Toda, & Palmer, 2003; Ekdahl, Claasen, Bonde, Kokaia, & Lindvall, 2003; Moreno-Lopez et al., 2004; Whitney, Eidem, Peng, Huang, & Zheng, 2009; Lu et al., 2011). However, the relationship between inflammation and NPC function is nuanced. Endogenous ROS and nitric oxide may actually be necessary for NPC proliferation (Yoneyama, Kawada, Gotoh, Shiba, & Ogita, 2010), and the inflammatory milieu can inhibit, stimulate, and also influence the general direction of cell lineage (neuronal vs. glial), depending on the characteristics of the inflammation and the phenotype of the microglia involved in the inflammatory response (Butovsky et al., 2006).
5.1. Opiate and Opiate-HIV Interactions on Progenitors and Cell Populations
Morphine, which is a legal but regulated analgesic, is also an active metabolite of heroin (diacetylmorphine) and is used in many studies as a surrogate for heroin exposure/abuse. NPCs (nestin+, Sox2+; also GFAP+ in adult) and their early derivatives (e.g. DCX+/b-III tubulin+ neurons; CD44+/vimentin+ young astrocytes; Nkx2.2+/A2B5+/O4+/Olig1+/Olig2+ young oligodendrocytes) express MOR, DOR, and KOR (Buch et al., 2007; Tripathi, Khurshid, Kumar, & Iyengar, 2008; Hahn et al., 2010). Furthermore, diverse agonists and antagonists for MOR, KOR, and DOR (e.g., heroin, morphine, β-endorphin, naltrindole, [D-Ala2, D-Leu5]-enkephalin (DADLE), β-funaltrexamine (β-FNA), naltrexone) (Eisch, Barrot, Schad, Self, & Nestler, 2000; Holmes & Galea, 2002; Persson et al., 2003; Mandyam, Norris, & Eisch, 2004; Koehl et al., 2008; Tsai, Lee, Hayashi, Freed, & Su, 2010) affect their proliferation and other behaviors. To date, most opiate studies have focused on adult hippocampal neurogenesis, and a number have included physical exercise as a parameter, which promotes neurogenesis partly via β-endorphin effects (Persson, Thorlin, Bull, & Eriksson, 2003). Interestingly, the reduced NPCs and neurogenesis seen in transgenic HIV-1 gp120 mice are normalized by exercise, although the role of endogenous opiates in this effect was not assessed (Lee et al., 2013). When exercise is removed from consideration, morphine reduces adult neurogenesis (Eisch et al., 2000; Mandyam et al., 2004; Kolodziej, Stumm, Becker, & Hollt, 2008; Arguello et al., 2008), even after controlling for glucocorticoid levels (Eisch et al., 2000), and MOR-null mice show a transiently enhanced post-injury neurogenesis in hippocampus (Kolodziej et al., 2008). Important new evidence shows that opiates used in addiction therapy, such as buprenorphine, may also reduce NPC formation in either the perinatal (Wu et al., 2014) or adult (Pettit, Desroches, & Bennett, 2012) CNS, disturbing the normal balance of glial populations through actions at MOR and/or the nociceptin/orphanin FQ receptors (Eschenroeder, Vestal-Laborde, Sanchez, Robinson, & Sato-Bigbee, 2012).
NPC proliferation is reduced by exposure to either HIV/HIV proteins or opiates independently, suggesting the potential for interactive, co-morbid effects of combined HIV and opiate exposure. Morphine interactively increases CNS inflammation in most HIV models (Perez-Casanova, Husain, Noel, Jr., Rivera-Amill, & Kumar, 2008; Bruce-Keller et al., 2008; El-Hage et al., 2008; Turchan-Cholewo et al., 2009; Bokhari et al., 2011; Dever et al., 2014), so it might be expected to exacerbate the effects of HIV on NPCs through generalized inflammation. However, as NPCs and their progeny express opiate receptors, there may be interactive effects that are independent of inflammation. Several studies have now shown that morphine exacerbates acute effects of HIV and Tat in vitro and in the developing brain. Our in vitro work has shown that combined exposure to morphine and Tat, or morphine and HIV, further depressed proliferation of murine and human NPCs and decreased NPC pools (Hahn et al., 2012). Morphine exposure in perinatal HIV-1 Tat-transgenic mice had a remarkably similar effect in vivo (Hahn et al., 2012). In human NPCs, this effect was shown to reflect a delay at the G1 phase of the cell cycle involving increased extracellular-signal regulated kinase-1/2 (ERK1/2) activation and concomitant increases in p21 and p53 (Malik et al., 2014). The ramifications of reduced numbers of NPCs, or an increased astroglial population at the expense of neurons and/or oligodendroglia, are likely to significantly impact cognitive and motor function. It remains unclear whether the chronic exposure to opiates that occurs in patients or drug abusers, either during development (neonates, adolescents) or in adult brains, can permanently alter CNS cell populations. The very critical question of whether the nature of opiate exposure might yield fundamentally different outcomes has also not been addressed. For example, do opiates administered for pain yield a different outcome than opiates that have been self-administered (resulting in dependence and addiction)? Studies that compare outcomes of contemporaneous exposure to opiates and HIV/HIV proteins versus the more “real life” situation where drug exposure occurs prior to HIV infection are also lacking.
5.2. Psychostimulant-HIV Interactions on Progenitors and Cell Populations
Among the psychostimulants both cocaine and amphetamine/methamphetamine exposure can influence NPC behaviors. Similar to opiates, in most studies cocaine exposure appears to inhibit the proliferation of human (Hu et al., 2006) and rodent (Lee et al., 2008) NPCs in vitro as well as in various perinatal and adult rodent models in vivo (Dominguez-Escriba et al., 2006; Lee et al., 2008; Garcia-Fuster, Perez, Clinton, Watson, & Akil, 2010; Yao, Duan, Yang, & Buch, 2012). A number of mechanisms for these effects have been put forward, including down-regulation of cyclin A related to ER stress induced by cocaine metabolites (Lee et al., 2008), and changes in cytoskeletal-associated genes (Lee et al., 2009). In one study, exposure to cocaine during critical prenatal periods reduced normal numbers and disturbed radial migration of GABAergic and glutamatergic neurons in the neocortex of developing rats (Lee, Chen, Worden, & Freed, 2011), presumably in part through effects on NPCs. In the dentate gyrus of adult rats, both 8 d and 24 d exposure to cocaine decreased NPC proliferation, but did not affect survival or newly generated cells or their morphology, dendritic arborization, or localization in the areas examined (Dominguez-Escriba et al., 2006). These disparate findings are no doubt partly due to the inherently different properties of NPCs in developing and adult systems. Cocaine also reduced the motility of human NPCs in vitro through inhibition of CXCL12-to-CXCR4 signaling (Hu et al., 2006). Reduced motility is associated with downregulation of a transcription factor (SOX2) that supports progenitor phenotype, and the early differentiation of young neurons. Prenatal cocaine exposure also appears to have lasting, sex-specific effects on resident NPCs in adult brains (Patel, Booze, & Mactutus, 2012). In a genetic study with relevance to addicted individuals, cocaine had quite different effects on NPCs in two lines of rats bred to express different propensities for cocaine “abuse”. NPC proliferation was suppressed by chronic cocaine exposure in rats with low responses to novelty, but in rats with high novelty responses (enhanced sensitization/psychomotor response to cocaine) NPC proliferation was normal, as was the ratio of neurons-to-glia generated, although there was less survival of newly formed neurons (Garcia-Fuster et al., 2010).
The preponderance of studies show that methamphetamine or amphetamine exposure also reduces NPC proliferation and formation of new glia and/or neurons, although a few disparate outcomes may provide insight into important variables. The proliferation of both adult hippocampal and striatal progenitor cells in vivo is frequently assessed by 5-bromo-2′-deoxyuridine (BrdU) incorporation (a thymidine analogue incorporated during DNA synthesis) or Ki67 antigenicity (a nuclear protein associated with cell division) (Scholzen & Gerdes, 2000) in species including rats, mice, and gerbils (Teuchert-Noodt, Dawirs, & Hildebrandt, 2000; Mao & Wang, 2001; Mandyam, Wee, Eisch, Richardson, & Koob, 2007; Yuan, Quiocho, Kim, Wee, & Mandyam, 2011). Similar effects are seen in vitro, where survival is also variably reduced (Tian, Murrin, & Zheng, 2009; Venkatesan et al., 2011; Bento, Baptista, Malva, Silva, & Agasse, 2011). These effects have been correlated to enhanced nitration and modification of function of key metabolic proteins (Venkatesan et al., 2011). One report suggests that a single dose of methamphetamine at 14 d of age can result in reduced NPC proliferation in the hippocampus of adults (Hildebrandt, Teuchert-Noodt, & Dawirs, 1999). Another set of studies suggests that exposure to a neurotoxic dose of methamphetamine may temporarily increase activity of quiescent NPCs in the adult striatum, by effects mediated through dopamine D2 (but not D1) and neurokinin 1 receptors (Tulloch, Ghazaryan, Mexhitaj, Ordonez, & Angulo, 2011). These cells, most of which have glial phenotypes, contribute to an increase in the size of the striatum, but the increased glial population is not maintained in the absence of methamphetamine (Tulloch et al., 2011; Tulloch et al., 2014). The importance of the timing of drug exposure is highlighted in a study comparing self-administration in intermittent versus daily exposure (Mandyam et al., 2008). Increased NPC proliferation was reported with intermittent exposure to methamphetamine, while daily exposure had the opposite effect. Interestingly, intermittent exposure also resulted in formation of more immature neurons, although there was no net neuron gain due to offsetting effects on later stages of differentiation. Daily exposure decreased all aspects of cytogenesis. Other extended access self-administration models also showed reduced NPC proliferation and decreased neuron and/or glial formation (Mandyam et al., 2007; Yuan et al., 2011).
There has been very little exploration of psychostimulant-HIV interactions on NPCs. In both a rat hippocampal NPC cell line, and in transgenic mice, HIV-1 Tat and cocaine reduced NPC proliferation without affecting NPC survival. These individual effects and a modest tendency towards interaction were reversed by platelet-derived growth factor-BB (PDGF-BB) through a mechanism involving both the transient receptor potential cation channel-C1 (TRPC1), and ERK/CREB and mammalian target of rapamycin (mTOR) activation (Yao et al., 2012).
6. CONCLUSIONS
Although considerable progress has been made during the last decade toward identifying molecular and cellular sites of HIV and drug abuse convergence in the CNS, and several new therapeutic strategies have emerged that may prove beneficial, much work remains to be done. The high degree of variability in the response among individuals to HIV alone reveals a highly complex chemistry between host and viral genetics, a complexity that is undoubtedly more convoluted by drug abuse-neuroAIDS comorbidity. Moreover, many of the events underlying drug-abuse-HIV neuropathological interactions occur only within specific cell types and/or at specific times during ontogeny, which adds greatly to the intricacy of the problem. A full appreciation of the mechanisms underlying drug abuse and neuroAIDS interactions will likely require an understanding of the interrelationship of gene networks, rather than individual genes.
Table 1.
Examples of gene variants contributing to substance abuse that also correlate with the severity of HIV disease progression
| Neurochemical System | Gene | Variant (polymorphism/isoform | Outcome(s) measured | Finding/Interpretation | Selected Reference(s) |
|---|---|---|---|---|---|
| Dopamine | Drd1 | rs265975 | Drug dependence | Altered opiate/cocaine dependence | (Jacobs et al., 2013) |
| Drd2 | multiple | Drug dependence | Altered opiate/cocaine dependence | (Jacobs et al., 2013) | |
| Drd3 | rs6280TC | Cognition | Increased cognitive impairment | (Gupta et al., 2011) | |
| Drd4 | Impulsivity | Altered inhibitory control | (Congdon, Lesch, & Canli, 2008) | ||
| SLC6A3(DAT1) | Altered inhibitory control | (Congdon et al., 2008) | |||
| SLC6A3(DAT1) | 3′ UTR 40 bp | HAND | No relationship to cognitive impairment | (Levine et al., 2012) | |
| MAO1 | |||||
| MAO2 | |||||
| COMT | rs4680 (val158met) | HIV serostatus | Significant correlation | (Sundermann et al., 2013) | |
| COMT | rs4680 (val158met) | HAND | No relationship to cognitive impairment | (Levine et al., 2012) | |
| dopaminergic trophic factor | BDNF | rs6265 (val66met) | HAND | No relationship to cognitive impairment | (Levine et al., 2012) |
| Opioid | OPRM1 | HIV progression response to cART | Specific polymorphisms associated with HIV progression and response to treatment | (Proudnikov et al., 2012) | |
| OPRM1 |
MOR-1K MOR-1 |
HAND and HIVE | ↑MOR-1K with HAND & HIVE; MOR-1 unaltered | (Dever et al., 2014) | |
| OPRM1 | MOR-1 | Susceptibility to drug [opiate?] overdose | (Manini et al., 2013) | ||
| OPRM1 | A C17T | Drug use | (Crystal et al., 2012) | ||
| OPRM1 | MOR-1 | Drug use | MOR-1 isoform unaltered | (Crystal et al., 2012) | |
| OPRK1 | HIV risk | Specific polymorphisms associated with HIV progression & response to treatment | (Proudnikov et al., 2013) | ||
| OPKR1 | KOR-1 | ↑OPRK1 in anterior cingulate; homeostatic attempt to decrease inflammation in exchange for motor impairment | (Yuferov, Butelman, Ho, Morgello, & Kreek, 2014) | ||
| PDYN | ↓PDYN in anterior cingulate; homeostatic attempt to decrease inflammation in exchange for motor impairment | (Yuferov et al., 2014) |
Acknowledgments
This work was supported by grants K02 DA027374, R01 DA018633 and R01 DA024461 from the NIH, National Institute on Drug Abuse.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
References
- Abt AC, Meucci O. Regulation of neuronal ferritin heavy chain, a new player in opiate-induced chemokine dysfunction. J Neuroimmune Pharmacol. 2011;6(4):466–476. doi: 10.1007/s11481-011-9278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi MG, et al. HIV-1 Tat protein mimicry of chemokines. Proc Natl Acad Sci U S A. 1998;95(22):13153–8. doi: 10.1073/pnas.95.22.13153. Retrieved from http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer?http://www.pnas.org/cgi/content/full/95/22/13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS ONE. 2008;3(8):e2906. doi: 10.1371/journal.pone.0002906. Retrieved from PM:18682838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers K, Hutter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, et al. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011;117(10):2791–2799. doi: 10.1182/blood-2010-09-309591. blood-2010-09-309591 [pii] Retrieved from PM:21148083. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle NC, Nagelhus EA, et al. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc Natl Acad Sci USA. 2003;100(23):13615–13620. doi: 10.1073/pnas.2336064100. 2336064100 [pii]. Retrieved from PM:14597704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SF, Osuntokun O, Groves M, Scaravilli F. Expression of CCR-5/CXCR-4 in spinal cord of patients with AIDS. Acta Neuropathol. 2001;102(2):175–180. doi: 10.1007/s004010100383. Retrieved from PM:11563633. [DOI] [PubMed] [Google Scholar]
- Ancuta P, Wang J, Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol. 2006;80(5):1156–1164. doi: 10.1189/jlb.0206125. Retrieved from PM:17056766. [DOI] [PubMed] [Google Scholar]
- Anderova M, Vorisek I, Pivonkova H, Benesova J, Vargova L, Cicanic M, et al. Cell death/proliferation and alterations in glial morphology contribute to changes in diffusivity in the rat hippocampus after hypoxia-ischemia. J Cereb Blood Flow Metab. 2011;31(3):894–907. doi: 10.1038/jcbfm.2010.168. jcbfm2010168 [pii] Retrieved from PM:20877389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CE, Tomlinson GS, Pauly B, Brannan FW, Chiswick A, Brack-Werner R, et al. Relationship of Nef-positive and GFAP-reactive astrocytes to drug use in early and late HIV infection. Neuropathol Appl Neurobiol. 2003;29(4):378–388. doi: 10.1046/j.1365-2990.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- Anderson J, Akkina R. Complete knockdown of CCR5 by lentiviral vector-expressed siRNAs and protection of transgenic macrophages against HIV-1 infection. Gene Ther. 2007;14(17):1287–1297. doi: 10.1038/sj.gt.3302958. 3302958 [pii] Retrieved from PM:17597795. [DOI] [PubMed] [Google Scholar]
- Andres MA, Feger U, Nath A, Munsaka S, Jiang CS, Chang L. APOE epsilon 4 allele and CSF APOE on cognition in HIV-infected subjects. J Neuroimmune Pharmacol. 2011;6(3):389–398. doi: 10.1007/s11481-010-9254-3. Retrieved from PM:21184197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony IC, Arango JC, Stephens B, Simmonds P, Bell JE. The effects of illicit drugs on the HIV infected brain. Front Biosci. 2008;13:1294–1307. doi: 10.2741/2762. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Norrby KE, Dingwall T, Carnie FW, Millar T, Arango JC, et al. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain. 2010;133(Pt 12):3685–3698. doi: 10.1093/brain/awq263. Retrieved from PM:21126996. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Does drug abuse alter microglial phenotype and cell turnover in the context of advancing HIV infection? Neuropathol Appl Neurobiol. 2005;31(3):325–338. doi: 10.1111/j.1365-2990.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. Embo J. 2012;31(5):1231–1240. doi: 10.1038/emboj.2011.489. emboj2011489 [pii] Retrieved from PM:22246184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Arguello AA, Harburg GC, Schonborn JR, Mandyam CD, Yamaguchi M, Eisch AJ. Time course of morphine’s effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience. 2008;157(1):70–79. doi: 10.1016/j.neuroscience.2008.08.064. S0306-4522(08)01284-0 [pii] Retrieved from PM:18832014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdoshina V, Biggio F, Palchik G, Campbell LA, Mocchetti I. Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia. 2010;58(13):1630–1639. doi: 10.1002/glia.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham HK, Jiang S, Fu Y, Rockenstein E, Makriyannis A, Zvonok A, et al. The cannabinoid CB(2) receptor agonist AM1241 enhances neurogenesis in GFAP/Gp120 transgenic mice displaying deficits in neurogenesis. Br J Pharmacol. 2014;171(2):468–479. doi: 10.1111/bph.12478. Retrieved from PM:24148086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann RF, Wang Y, Yuan P, Zhou R, Li X, Alesci S, et al. Common effects of lithium and valproate on mitochondrial functions: protection against methamphetamine-induced mitochondrial damage. Int J Neuropsychopharmacol. 2009;12(6):805–822. doi: 10.1017/S1461145708009802. S1461145708009802 [pii] Retrieved from PM:19149911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley EE, Hacker J, Chefer VI, Mallet C, McNally GP, Chieng BC, et al. Drug-induced GABA transporter currents enhance GABA release to induce opioid withdrawal behaviors. Nat Neurosci. 2011;14(12):1548–1554. doi: 10.1038/nn.2940. Retrieved from PM:22037500. [DOI] [PubMed] [Google Scholar]
- Baker BM, Block BL, Rothchild AC, Walker BD. Elite control of HIV infection: implications for vaccine design. Expert Opin Biol Ther. 2009;9(1):55–69. doi: 10.1517/14712590802571928. Retrieved from PM:19063693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, Patel N, Ewaleifoh O, Haughey NJ. A failure to normalize biochemical and metabolic insults during morphine withdrawal disrupts synaptic repair in mice transgenic for HIV-gp120. J Neuroimmune Pharmacol. 2011;6(4):640–649. doi: 10.1007/s11481-011-9289-0. Retrieved from PM:21748284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Strazza M, Wigdahl B, Pirrone V, Meucci O, Nonnemacher MR. Role of mu-opioids as cofactors in human immunodeficiency virus type 1 disease progression and neuropathogenesis. J Neurovirol. 2011;17(4):291–302. doi: 10.1007/s13365-011-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Zhang X, Manda KR, Banks WA, Ercal N. HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: potential role of the thiol antioxidant N-acetylcysteine amide. Free Radic Biol Med. 2010;48(10):1388–1398. doi: 10.1016/j.freeradbiomed.2010.02.023. S0891-5849(10)00125-5 [pii] Retrieved from PM:20188164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Hauser KF. Glial modulators as potential treatments of psychostimulant abuse. Adv Pharmacol. 2014;69:1–69. doi: 10.1016/B978-0-12-420118-7.00001-9. B978-0-12-420118-7.00001-9 [pii] Retrieved from PM:24484974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. S0091-3022(07)00035-0 [pii] Retrieved from PM:17904621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JE. The neuropathology of adult HIV infection. Rev Neurol(Paris) 1998;154(12):816–829. [PubMed] [Google Scholar]
- Bell JE, Arango JC, Robertson R, Brettle RP, Leen C, Simmonds P. HIV and Drug Misuse in the Edinburgh Cohort. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S35–S42. doi: 10.1097/00126334-200210012-00003. [DOI] [PubMed] [Google Scholar]
- Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121(Pt 11):2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- Bencheikh M, Bentsman G, Sarkissian N, Canki M, Volsky DJ. Replication of different clones of human immunodeficiency virus type 1 in primary fetal human astrocytes: enhancement of viral gene expression by Nef [In Process Citation] J Neurovirol. 1999;5(2):115–124. doi: 10.3109/13550289909021993. [DOI] [PubMed] [Google Scholar]
- Bender A, Schwarzkopf RM, McMillan A, Krishnan KJ, Rieder G, Neumann M, et al. Dopaminergic midbrain neurons are the prime target for mitochondrial DNA deletions. J Neurol. 2008;255(8):1231–1235. doi: 10.1007/s00415-008-0892-9. [DOI] [PubMed] [Google Scholar]
- Bento AR, Baptista S, Malva JO, Silva AP, Agasse F. Methamphetamine exerts toxic effects on subventricular zone stem/progenitor cells and inhibits neuronal differentiation. Rejuvenation Res. 2011;14(2):205–214. doi: 10.1089/rej.2010.1109. Retrieved from PM:21453012. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Berman JW, Carson MJ, Chang L, Cox BM, Fox HS, Gonzalez RG, et al. NeuroAIDS, drug abuse, and inflammation: building collaborative research activities. J Neuroimmune Pharmacol. 2006;1(4):351–399. doi: 10.1007/s11481-006-9048-9. [DOI] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann NY Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. NYAS1141031 [pii] Retrieved from PM:18991959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci USA. 2006;103(33):12564–12568. doi: 10.1073/pnas.0605177103. 0605177103 [pii] Retrieved from PM:16901981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30(11):596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Bishop KN, Holmes RK, Sheehy AM, Malim MH. APOBEC-mediated editing of viral RNA. Science. 2004;305(5684):645. doi: 10.1126/science.1100658. 305/5684/645 [pii]. Retrieved from PM:15286366. [DOI] [PubMed] [Google Scholar]
- Bithell A, Finch SE, Hornby MF, Williams BP. Fibroblast growth factor 2 maintains the neurogenic capacity of embryonic neural progenitor cells in vitro but changes their neuronal subtype specification. Stem Cells. 2008;26(6):1565–1574. doi: 10.1634/stemcells.2007-0832. 2007-0832 [pii] Retrieved from PM:18339769. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. Retrieved from PM:17180163. [DOI] [PubMed] [Google Scholar]
- Bokhari SM, Hegde R, Callen S, Yao H, Adany I, Li Q, et al. Morphine potentiates neuropathogenesis of SIV infection in Rhesus macaques. J Neuroimmune Pharmacol. 2011;6(4):626–639. doi: 10.1007/s11481-011-9272-9. Retrieved from PM:21431470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokhari SM, Yao H, Bethel-Brown C, Fuwang P, Williams R, Dhillon NK, et al. Morphine enhances Tat-induced activation in murine microglia. J Neurovirol. 2009;15(3):219–228. doi: 10.1080/13550280902913628. 911592065 [pii] Retrieved from PM:19462331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, et al. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science. 2006;312(5772):443–446. doi: 10.1126/science.1120378. Retrieved from PM:16627746. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95(16):9608–9613. doi: 10.1073/pnas.95.16.9608. Retrieved from PM:9689128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda JT, Alvarez X, Mohan M, Hasegawa A, Bernardino A, Jean S, et al. CD163, a marker of perivascular macrophages, is up-regulated by microglia in simian immunodeficiency virus encephalitis after haptoglobin-hemoglobin complex stimulation and is suggestive of breakdown of the blood-brain barrier. Am J Pathol. 2008;172(3):725–737. doi: 10.2353/ajpath.2008.070848. Retrieved from PM:18276779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA. 2001;98(16):8966–8971. doi: 10.1073/pnas.151105198. 151105198 [pii]. Retrieved from PM:11459929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven LA, van der Bruggen T, van Asbeck BS, Marx JJ, Nottet HS. Potential role of CCR5 polymorphism in the development of AIDS dementia complex. FEMS Immunol Med Microbiol. 1999;26(3–4):243–247. doi: 10.1111/j.1574-695X.1999.tb01395.x. S0928824499001418 [pii]. Retrieved from PM:10575135. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67(1):11–24. doi: 10.1016/j.neuron.2010.06.004. Retrieved from PM:20624588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13(1):1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214(2):149–160. doi: 10.1002/path.2287. Retrieved from PM:18161752. [DOI] [PubMed] [Google Scholar]
- Brazel CY, Nunez JL, Yang Z, Levison SW. Glutamate enhances survival and proliferation of neural progenitors derived from the subventricular zone. Neuroscience. 2005;131(1):55–65. doi: 10.1016/j.neuroscience.2004.10.038. S0306-4522(04)01000-0 [pii] Retrieved from PM:15680691. [DOI] [PubMed] [Google Scholar]
- Brown JM, Riddle EL, Sandoval V, Weston RK, Hanson JE, Crosby MJ, et al. A single methamphetamine administration rapidly decreases vesicular dopamine uptake. J Pharmacol Exp Ther. 2002;302(2):497–501. doi: 10.1124/jpet.302.2.497. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Guerin T, Chauhan A, Reid R, et al. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswick DJ, Benmansour S, Tejani-Butt SM, Hauptmann M. Effects of high-dose methamphetamine on monoamine uptake sites in rat brain measured by quantitative autoradiography. Synapse. 1992;11(4):287–293. doi: 10.1002/syn.890110404. Retrieved from PM:1502685. [DOI] [PubMed] [Google Scholar]
- Buch S, Yao H, Guo M, Mori T, Mathias-Costa B, Singh V, et al. Cocaine and HIV-1 interplay in CNS: cellular and molecular mechanisms. Curr HIV Res. 2012;10(5):425–428. doi: 10.2174/157016212802138823. CHIVR-EPUB-20120511-4 [pii]. Retrieved from PM:22591366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch SK, Khurdayan VK, Lutz SE, Knapp PE, El-Hage N, Hauser KF. Glial-restricted precursors: Patterns of expression of opioid receptors and relationship to HIV-1 Tat and morphine susceptibility in vitro. Neuroscience. 2007;146:1546–1554. doi: 10.1016/j.neuroscience.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Molecular Pharmacology. 2001;60(6):1181–1188. doi: 10.1124/mol.60.6.1181. Retrieved from PM:11723224. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Aloyo VJ, Simansky KJ, Meucci O. GTPgammaS incorporation in the rat brain: a study on mu-opioid receptors and CXCR4. J Neuroimmune Pharmacol. 2008;3(1):26–34. doi: 10.1007/s11481-007-9083-1. Retrieved from PM:18247130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Murphy B, Danzer SC, Kuan CY. Developmental and post-injury cortical gliogenesis: a genetic fate-mapping study with Nestin-CreER mice. Glia. 2009;57(10):1115–1129. doi: 10.1002/glia.20835. Retrieved from PM:19115384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31(1):149–160. doi: 10.1016/j.mcn.2005.10.006. S1044-7431(05)00248-4 [pii] Retrieved from PM:16297637. [DOI] [PubMed] [Google Scholar]
- Buttner A. The neuropathology of drug abuse. Neuropathol Appl Neurobiol. 2011;37(2):118–134. doi: 10.1111/j.1365-2990.2010.01131.x. Retrieved from PM:20946118. [DOI] [PubMed] [Google Scholar]
- Byrd D, Murray J, Safdieh G, Morgello S. Impact of opiate addiction on neuroinflammation in HIV. J Neurovirol. 2012;18(5):364–373. doi: 10.1007/s13365-012-0118-x. Retrieved from PM:22797933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, et al. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 2011;58(2):154–162. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127(1):91–107. doi: 10.1007/s00401-013-1221-7. Retrieved from PM:24292887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol. 2009;88:101–119. doi: 10.1016/S0074-7742(09)88005-7. S0074-7742(09)88005-7 [pii] Retrieved from PM:19897076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11(3–4):183–202. doi: 10.1007/BF03033567. Retrieved from PM:17449459. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J Neurosci. 1994;14(11 Pt 1):6763–6767. doi: 10.1523/JNEUROSCI.14-11-06763.1994. Retrieved from PM:7965077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canki M, Potash MJ, Bentsman G, Chao W, Flynn T, Heinemann M, et al. Isolation and long-term culture of primary ocular human immunodeficiency virus type 1 isolates in primary astrocytes. J Neurovirol. 1997;3(1):10–15. doi: 10.3109/13550289709015788. [DOI] [PubMed] [Google Scholar]
- Carrington M, Dean M, Martin MP, O’Brien SJ. Genetics of HIV-1 infection: chemokine receptor CCR5 polymorphism and its consequences. Hum Mol Genet. 1999;8(10):1939–1945. doi: 10.1093/hmg/8.10.1939. [DOI] [PubMed] [Google Scholar]
- Carroll-Anzinger D, Kumar A, Adarichev V, Kashanchi F, Al-Harthi L. Human immunodeficiency virus-restricted replication in astrocytes and the ability of gamma interferon to modulate this restriction are regulated by a downstream effector of the Wnt signaling pathway. J Virol. 2007;81(11):5864–5871. doi: 10.1128/JVI.02234-06. Retrieved from PM:17392368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Bilousova TV, Puntambekar SS, Melchior B, Doose JM, Ethell IM. A rose by any other name? The potential consequences of microglial heterogeneity during CNS health and disease. Neurotherapeutics. 2007;4(4):571–579. doi: 10.1016/j.nurt.2007.07.002. Retrieved from PM:17920538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier EA, Parra LA, Baust TB, Quiroz M, Salazar G, Faundez V, et al. A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. J Biol Chem. 2010;285(3):1957–1966. doi: 10.1074/jbc.M109.054510. M109.054510 [pii] Retrieved from PM:19903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA. Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Pharmacol Exp Ther. 1997;280(1):105–113. Retrieved from PM:8996187. [PubMed] [Google Scholar]
- Cass WA, Harned ME, Peters LE, Nath A, Maragos WF. HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Research. 2003;984(1–2):133–142. doi: 10.1016/s0006-8993(03)03122-6. Retrieved from PM:12932847. [DOI] [PubMed] [Google Scholar]
- Chan WY, Lorke DE, Tiu SC, Yew DT. Proliferation and apoptosis in the developing human neocortex. Anat Rec. 2002;267(4):261–276. doi: 10.1002/ar.10100. Retrieved from PM:12124904. [DOI] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Hu S, Sheng WS, Shark KB, Bu DF, et al. kappa opioid receptors in human microglia downregulate human immunodeficiency virus 1 expression. Proc Natl Acad Sci USA. 1996;93(15):8051–8056. doi: 10.1073/pnas.93.15.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Hu S, Peterson PK. Glia: the not so innocent bystanders. J Neurovirol. 1996;2(4):234–239. doi: 10.3109/13550289609146886. Retrieved from PM:8799214. [DOI] [PubMed] [Google Scholar]
- Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483(2–3):175–186. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Chen H, Manev H. Effects of minocycline on cocaine sensitization and phosphorylation of GluR1 receptors in 5-lipoxygenase deficient mice. Neuropharmacology. 2011;60(7–8):1058–1063. doi: 10.1016/j.neuropharm.2010.09.006. S0028-3908(10)00227-3 [pii] Retrieved from PM:20868701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Uz T, Manev H. Minocycline affects cocaine sensitization in mice. Neuroscience Letters. 2009;452(3):258–261. doi: 10.1016/j.neulet.2009.01.078. S0304-3940(09)00150-5 [pii] Retrieved from PM:19348734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Geller EB, Rogers TJ, Adler MW. Rapid heterologous desensitization of antinociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend. 2007;88(1):36–41. doi: 10.1016/j.drugalcdep.2006.09.010. S0376-8716(06)00341-3 [pii] Retrieved from PM:17049756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery PF, Taylor GA, Howell N, Brown DT, Parsons TJ, Turnbull DM. Point mutations of the mtDNA control region in normal and neurodegenerative human brains. Am J Hum Genet. 2001;68(2):529–532. doi: 10.1086/318204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, et al. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication [In Process Citation] AIDS. 1998;12(11):1327–32. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Cisneros IE, Ghorpade A. HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Curr HIV Res. 2012;10(5):392–406. doi: 10.2174/157016212802138832. CHIVR-EPUB-20120511–1 [pii]. Retrieved from PM:22591363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotox Res. 2013;23(2):174–188. doi: 10.1007/s12640-012-9334-7. Retrieved from PM:22714667. [DOI] [PubMed] [Google Scholar]
- Cohen RA. The Changing Face of HIV-Associated Cognitive and Neuropsychiatric Disturbance. In: Paul RH, Sacktor NC, Valcour V, Tashima KT, editors. HIV and the Brain: New Challenges in the Modern Era. 1. New York: Humana Press Inc; 2009. pp. 133–186. Current Clinical Neurology. [Google Scholar]
- Colfax G, Guzman R. Club drugs and HIV infection: a review. Clin Infect Dis. 2006;42(10):1463–1469. doi: 10.1086/503259. CID38342 [pii] Retrieved from PM:16619161. [DOI] [PubMed] [Google Scholar]
- Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther. 2012;134(2):219–245. doi: 10.1016/j.pharmthera.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4(4):399–418. doi: 10.1007/s11481-009-9164-4. Retrieved from PM:19655259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Atwood WJ, Traub R, Tornatore C, Major EO. An increase in p50/p65 NF-kB binding to the HIV-1 LTR is not sufficient to increase viral expression in the primary human astrocyte. Virology. 1994;205:586–590. doi: 10.1006/viro.1994.1685. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, et al. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95(6):3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Ma M, Nath A, Major EO. Extracellular human immunodeficiency virus type 1 Tat protein is associated with an increase in both NF-kappa B binding and protein kinase C activity in primary human astrocytes. J Virol. 1996;70(3):1384–1389. doi: 10.1128/jvi.70.3.1384-1389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, St HC, Anderson C, Galey D, Wang J, Nath A. Human immunodeficiency virus type 1 Tat and methamphetamine affect the release and activation of matrix-degrading proteinases. J Neurovirol. 2004;10(1):21–28. doi: 10.1080/13550280490261699. Retrieved from PM:14982725. [DOI] [PubMed] [Google Scholar]
- Conant K, Wang Y, Szklarczyk A, Dudak A, Mattson MP, Lim ST. Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience. 2010;166(2):508–521. doi: 10.1016/j.neuroscience.2009.12.061. S0306-4522(09)02149-6 [pii] Retrieved from PM:20045450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(1):27–32. doi: 10.1002/ajmg.b.30557. Retrieved from PM:17525955. [DOI] [PubMed] [Google Scholar]
- Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, et al. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160(1):101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza MA, Zhao ML, Si Q, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002;12(4):442–455. doi: 10.1111/j.1750-3639.2002.tb00461.x. Retrieved from PM:12408230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. Epub@2011 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Patrick C, Adame A, Rockenstein E, Masliah E. Modulation of aberrant CDK5 signaling rescues impaired neurogenesis in models of Alzheimer’s disease. Cell Death Dis. 2011;2:e120. doi: 10.1038/cddis.2011.2. cddis20112 [pii] Retrieved from PM:21368891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal HA, Hamon S, Randesi M, Cook J, Anastos K, Lazar J, et al. A C17T polymorphism in the mu opiate receptor is associated with quantitative measures of drug use in African American women. Addict Biol. 2012;17(1):181–191. doi: 10.1111/j.1369-1600.2010.00265.x. Retrieved from PM:21070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Faull RL, Eriksson PS. The effect of neurodegenerative diseases on the subventricular zone. Nat Rev Neurosci. 2007;8(9):712–723. doi: 10.1038/nrn2216. nrn2216 [pii] Retrieved from PM:17704813. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, et al. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc Natl Acad Sci USA. 2003;100(15):9023–9027. doi: 10.1073/pnas.1532244100. 1532244100 [pii]. Retrieved from PM:12853570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Waldvogel HJ, Synek B, Faull RL. A histochemical and immunohistochemical analysis of the subependymal layer in the normal and Huntington’s disease brain. J Chem Neuroanat. 2005;30(1):55–66. doi: 10.1016/j.jchemneu.2005.05.001. Retrieved from PM:16108100. [DOI] [PubMed] [Google Scholar]
- Czub S, Czub M, Koutsilieri E, Sopper S, Villinger F, Muller JG, et al. Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta Neuropathol(Berl) 2004;107(3):216–226. doi: 10.1007/s00401-003-0801-3. Retrieved from PM:14712399. [DOI] [PubMed] [Google Scholar]
- Czub S, Koutsilieri E, Sopper S, Czub M, Stahl-Hennig C, Muller JG, et al. Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta Neuropathol (Berl) 2001;101(2):85–91. doi: 10.1007/s004010000313. [DOI] [PubMed] [Google Scholar]
- D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, et al. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA. 2007;104(6):1995–2000. doi: 10.1073/pnas.0609408104. Retrieved from PM:17259307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C, Chen Q, Zhang X, Xiong X, Lazarus C, Lee TH, et al. Deprenyl treatment attenuates long-term pre- and post-synaptic changes evoked by chronic methamphetamine. Eur J Pharmacol. 2007;573(1–3):100–110. doi: 10.1016/j.ejphar.2007.06.046. Retrieved from PM:17651730. [DOI] [PubMed] [Google Scholar]
- De Pittà M, Volman V, Berry H, Parpura V, Volterra A, Ben-Jacob E. Computational quest for understanding the role of astrocyte signaling in synaptic transmission and plasticity. Front Comput Neurosci. 2012;6:98. doi: 10.3389/fncom.2012.00098. Retrieved from PM:23267326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever SM, Costin BN, Xu R, El-Hage N, Balinang J, Samoshkin A, et al. Differential expression of the alternatively spliced OPRM1 isoform μ–opioid receptor-1K in HIV-infected individuals. AIDS. 2014;28(1):19–30. doi: 10.1097/QAD.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever SM, Xu R, Fitting S, Knapp PE, Hauser KF. Differential expression and HIV-1 regulation of μ-opioid receptor splice variants across human central nervous system cell types. J Neurovirol. 2012;18(3):181–190. doi: 10.1007/s13365-012-0096-z. Retrieved from http://www.springerlink.com/content/b540406322297676/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. Retrieved from PM:9185542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Escriba L, Hernandez-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, Garcia-Verdugo JM, et al. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci. 2006;24(2):586–594. doi: 10.1111/j.1460-9568.2006.04924.x. EJN4924 [pii] Retrieved from PM:16903860. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. Journal of Neuroimmunology. 1998;83(1–2):77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- Dutta R, Roy S. Mechanism(s) involved in opioid drug abuse modulation of HAND. Curr HIV Res. 2012;10(5):469–477. doi: 10.2174/157016212802138805. CHIVR-EPUB-20120511-9 [pii]. Retrieved from PM:22591371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41(14):1153–1193. doi: 10.2165/00003088-200241140-00003. 411403 [pii] Retrieved from PM:12405865. [DOI] [PubMed] [Google Scholar]
- Eggen BJ, Raj D, Hanisch UK, Boddeke HW. Microglial phenotype and adaptation. J Neuroimmune Pharmacol. 2013;8(4):807–823. doi: 10.1007/s11481-013-9490-4. Retrieved from PM:23881706. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA. 2000;97(13):7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16(3):271–286. doi: 10.1002/hipo.20161. Retrieved from PM:16411230. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. 2234031100 [pii]. Retrieved from PM:14581618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Yakovleva T, Bakalkin G, Knapp PE, Hauser KF. Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca2+]i, NF-κB trafficking and transcription. PLoS ONE. 2008;3(12):e4093. doi: 10.1371/journal.pone.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Knapp PE, Hauser KF. CCL5/RANTES gene deletion attenuates opioid-induced increases in glial CCL2/MCP-1 immunoreactivity and activation in HIV-1 Tat exposed mice. J Neuroimmune Pharmacol. 2008;3(4):275–285. doi: 10.1007/s11481-008-9127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Dever SM, Podhaizer EM, Arnatt CK, Zhang Y, Hauser KF. A novel bivalent HIV-1 entry inhibitor reveals fundamental differences in CCR5-μ-opioid receptor interactions between human astroglia and microglia. AIDS. 2013;27(14):2181–2190. doi: 10.1097/QAD.0b013e3283639804. Retrieved from PM:23751259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50(2):91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Podhaizer EM, Sturgill J, Hauser KF. Toll-like receptor expression and activation in astroglia: differential regulation by HIV-1 Tat, gp120, and morphine. Immunol Invest. 2011;40(5):498–522. doi: 10.3109/08820139.2011.561904. Retrieved from PM:21425908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Rodriguez M, Podhaizer EM, Zou S, Dever SM, Snider SE, et al. Ibudilast (AV411), and its AV1013 analog, reduce HIV-1 replication and neuronal death induced by HIV-1 and morphine. AIDS. 2014 doi: 10.1097/QAD.0000000000000291. Retrieved from PM:24732776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Ambati J, Bruce-Keller AJ, Knapp PE, Hauser KF. CCR2 mediates increases in glial activation caused by exposure to HIV-1 Tat and opiates. J Neuroimmunol. 2006a;178(1–2):9–16. doi: 10.1016/j.jneuroim.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, et al. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006b;53(2):132–146. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8(1):33–44. doi: 10.1038/nrn2040. Retrieved from PM:17180161. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Macklis JD. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2006;2(3):175–186. doi: 10.1017/S1740925X06000202. Retrieved from PM:17356684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Hansson E, Rönnbäck L. δ and kappa opiate receptors in primary astroglial cultures. Part II: Receptor sets in cultures from various brain regions and interactions with β-receptor activated cyclic AMP. Neurochemical Research. 1992;17:545–551. doi: 10.1007/BF00968781. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Nilsson M, Wagberg M, Hansson E, Rönnbäck L. Kappa-opioid receptors on astrocytes stimulate L-type Ca2+ channels. Neurosci. 1993;54(2):401–407. doi: 10.1016/0306-4522(93)90261-d. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Robbins TW, Bullmore ET. Cocaine dependence: a fast-track for brain ageing? Mol Psychiatry. 2013;18(2):134–135. doi: 10.1038/mp.2012.31. mp201231 [pii] Retrieved from PM:22525488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenroeder AC, Vestal-Laborde AA, Sanchez ES, Robinson SE, Sato-Bigbee C. Oligodendrocyte responses to buprenorphine uncover novel and opposing roles of mu-opioid- and nociceptin/orphanin FQ receptors in cell development: implications for drug addiction treatment during pregnancy. Glia. 2012;60(1):125–136. doi: 10.1002/glia.21253. Retrieved from PM:22002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza S, Salahpour A, Masri B, Sotnikova TD, Messa M, Barak LS, et al. Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Molecular Pharmacology. 2011;80(3):416–425. doi: 10.1124/mol.111.073304. mol.111.073304 [pii] Retrieved from PM:21670104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Dyer G, Calderon TM, Berman JW. HIV-1 tat protein induces a migratory phenotype in human fetal microglia by a CCL2 (MCP-1)-dependent mechanism: Possible role in NeuroAIDS. Glia. 2005;49(4):501–510. doi: 10.1002/glia.20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26(4):1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem. 2007;103(3):1219–1227. doi: 10.1111/j.1471-4159.2007.04837.x. Retrieved from PM:17683483. [DOI] [PubMed] [Google Scholar]
- Fang J, Acheampong E, Dave R, Wang F, Mukhtar M, Pomerantz RJ. The RNA helicase DDX1 is involved in restricted HIV-1 Rev function in human astrocytes. Virology. 2005;336(2):299–307. doi: 10.1016/j.virol.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Farber K, Pannasch U, Kettenmann H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol Cell Neurosci. 2005;29(1):128–138. doi: 10.1016/j.mcn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28(3):138–145. doi: 10.1016/j.it.2007.01.005. Retrieved from PM:17276138. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neuroscience and Biobehavioral Reviews. 2008;32(5):883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa L, Meucci O. Effects of opiates and HIV proteins on neurons: the role of ferritin heavy chain and a potential for synergism. Curr HIV Res. 2012;10(5):453–462. doi: 10.2174/157016212802138751. CHIVR-EPUB-20120511-7 [pii]. Retrieved from PM:22591369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Eshleman AJ, Cashman J, Lin J, Lossinsky AS, Suarez V, et al. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. J Neurovirol. 2005;11(3):281–291. doi: 10.1080/13550280590952835. Retrieved from PM:16036808. [DOI] [PubMed] [Google Scholar]
- Fiala M, Gan XH, Zhang L, House SD, Newton T, Graves MC, et al. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine’s connection to AIDS dementia and vasculitis? Adv Exp Med Biol. 1998;437:199–205. doi: 10.1007/978-1-4615-5347-2_22. [DOI] [PubMed] [Google Scholar]
- Fiala M, Looney DJ, Stins M, Way DD, Zhang L, Gan X, et al. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med. 1997;3(8):553–564. [PMC free article] [PubMed] [Google Scholar]
- Finley MJ, Chen X, Bardi G, Davey P, Geller EB, Zhang L, et al. Bi-directional heterologous desensitization between the major HIV-1 co-receptor CXCR4 and the kappa-opioid receptor. Journal of Neuroimmunology. 2008;197(2):114–123. doi: 10.1016/j.jneuroim.2008.04.021. Retrieved from PM:18533278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7(6):528–541. doi: 10.1080/135502801753248114. Retrieved from PM:11704885. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Rappaport J. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Rev Mol Med. 2005;7(27):1–26. doi: 10.1017/S1462399405010239. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Tedaldi EM, Rappaport J. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retroviruses. 2008;24(3):417–421. doi: 10.1089/aid.2007.0193. Retrieved from PM:18373432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Scoggins KL, Xu R, Dever SM, Knapp PE, Dewey WL, et al. Morphine efficacy is altered in conditional HIV-1 Tat transgenic mice. Eur J Pharmacol. 2012;689(1–3):96–103. doi: 10.1016/j.ejphar.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Xu R, Bull CM, Buch SK, El-Hage N, Nath A, et al. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: Chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010a;177(3):1397–1410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Zou S, Chen W, Vo P, Hauser KF, Knapp PE. Regional Heterogeneity and diversity in cytokine and chemokine production by astroglia: differential responses to HIV-1 Tat, gp120 and morphine revealed by multiplex analysis. J Proteome Res. 2010b;9(4):1795–1804. doi: 10.1021/pr900926n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR. Rapid and reversible effects of methamphetamine on dopamine transporters. J Pharmacol Exp Ther. 1997;282(2):834–838. Retrieved from PM:9262348. [PubMed] [Google Scholar]
- Flora G, Lee YW, Nath A, Hennig B, Maragos W, Toborek M. Methamphetamine potentiates HIV-1 Tat protein-mediated activation of redox-sensitive pathways in discrete regions of the brain. Exp Neurol. 2003;179(1):60–70. doi: 10.1006/exnr.2002.8048. [DOI] [PubMed] [Google Scholar]
- Flora G, Lee YW, Nath A, Maragos W, Hennig B, Toborek M. Methamphetamine-induced TNF-alpha gene expression and activation of AP-1 in discrete regions of mouse brain: potential role of reactive oxygen intermediates and lipid peroxidation. Neuromolecular Med. 2002;2(1):71–85. doi: 10.1385/NMM:2:1:71. [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF. Stress- and glucocorticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun. 2011;25(Suppl 1):S21–S28. doi: 10.1016/j.bbi.2011.01.005. S0889–1591(11)00010-9 [pii] Retrieved from PM:21256955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci. 1999;19(7):2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. Retrieved from PM:10087057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X, Zhang L, Berger O, Stins MF, Way D, Taub DD, et al. Cocaine enhances brain endothelial adhesion molecules and leukocyte migration. Clin Immunol. 1999;91(1):68–76. doi: 10.1006/clim.1998.4683. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Perez JA, Clinton SM, Watson SJ, Akil H. Impact of cocaine on adult hippocampal neurogenesis in an animal model of differential propensity to drug abuse. Eur J Neurosci. 2010;31(1):79–89. doi: 10.1111/j.1460-9568.2009.07045.x. Retrieved from PM:20104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA. Microglia in human immunodeficiency virus-associated neurodegeneration. Glia. 2002;40(2):240–251. doi: 10.1002/glia.10155. [DOI] [PubMed] [Google Scholar]
- Gasque P, Dean YD, McGreal EP, VanBeek J, Morgan BP. Complement components of the innate immune system in health and disease in the CNS. Immunopharmacology. 2000;49(1–2):171–186. doi: 10.1016/s0162-3109(00)80302-1. S0162310900803021 [pii]. Retrieved from PM:10904116. [DOI] [PubMed] [Google Scholar]
- Geha S, Pallud J, Junier MP, Devaux B, Leonard N, Chassoux F, et al. NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain. Brain Pathol. 2010;20(2):399–411. doi: 10.1111/j.1750-3639.2009.00295.x. BPA295 [pii] Retrieved from PM:19486010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekker G, Hu S, Sheng WS, Rock RB, Lokensgard JR, Peterson PK. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factor-beta1. Int Immunopharmacol. 2006;6(6):1029–1033. doi: 10.1016/j.intimp.2005.12.005. S1567-5769(05)00354-1 [pii] Retrieved from PM:16644490. [DOI] [PubMed] [Google Scholar]
- Gekker G, Hu S, Wentland MP, Bidlack JM, Lokensgard JR, Peterson PK. Kappa-opioid receptor ligands inhibit cocaine-induced HIV-1 expression in microglial cells. Journal of Pharmacology and Experimental Therapeutics. 2004;309(2):600–606. doi: 10.1124/jpet.103.060160. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS ONE. 2012;7(9):e46178. doi: 10.1371/journal.pone.0046178. PONE-D-11-14842 [pii]. Retrieved from PM:23049970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, et al. The neuropathogenesis of the AIDS dementia complex. AIDS. 1997;11(Suppl A):35–45. [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, et al. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: Implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176(6):1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Yu J, Li X, Tom D, Li J, Wendt E, et al. Study of receptor-mediated neurotoxins released by HIV-1-infected mononuclear phagocytes found in human brain. J Neurosci. 1996;16(10):3139–3153. doi: 10.1523/JNEUROSCI.16-10-03139.1996. Retrieved from PM:8627353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves J, Martins T, Ferreira R, Milhazes N, Borges F, Ribeiro CF, et al. Methamphetamine-induced early increase of IL-6 and TNF-alpha mRNA expression in the mouse brain. Ann NY Acad Sci. 2008;1139:103–111. doi: 10.1196/annals.1432.043. NYAS1139043 [pii] Retrieved from PM:18991854. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Dhanda R, Bamshad M, Mummidi S, Geevarghese R, Catano G, et al. Global survey of genetic variation in CCR5, RANTES, and MIP-1alpha: impact on the epidemiology of the HIV-1 pandemic. Proc Natl Acad Sci USA. 2001;98(9):5199–5204. doi: 10.1073/pnas.091056898. 98/9/5199 [pii]. Retrieved from PM:11320252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, et al. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci USA. 2002;99(21):13795–13800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gorry P, Purcell D, Howard J, McPhee D. Restricted HIV-1 infection of human astrocytes: potential role of nef in the regulation of virus replication. J Neurovirol. 1998;4(4):377–386. doi: 10.3109/13550289809114536. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, et al. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1(4):463–473. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- Gras G, Samah B, Hubert A, Leone C, Porcheray F, Rimaniol AC. EAAT expression by macrophages and microglia: still more questions than answers. Amino Acids. 2011 doi: 10.1007/s00726-011-0866-6. Retrieved from PM:21373769. [DOI] [PubMed] [Google Scholar]
- Gras G, Samah B, Hubert A, Leone C, Porcheray F, Rimaniol AC. EAAT expression by macrophages and microglia: still more questions than answers. Amino Acids. 2012;42(1):221–229. doi: 10.1007/s00726-011-0866-6. Retrieved from PM:21373769. [DOI] [PubMed] [Google Scholar]
- Gris P, Gauthier J, Cheng P, Gibson DG, Gris D, Laur O, et al. A novel alternatively spliced isoform of the mu-opioid receptor: functional antagonism. Mol Pain. 2010;6:33. doi: 10.1186/1744-8069-6-33. Retrieved from PM:20525224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin G, Croitoru J, Le Grand RL, Franck-Duchenne M, Dormont D, Boussin FD. Simian immunodeficiency virus mac251 infection of astrocytes. J Neurovirol. 2000;6(3):173–186. doi: 10.3109/13550280009015821. Retrieved from PM:10878708. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75(3):388–397. doi: 10.1189/jlb.0303114. jlb.0303114 [pii]. Retrieved from PM:14612429. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Brew BJ. Involvement of quinolinic acid in AIDS dementia complex. Neurotox Res. 2005;7(1–2):103–123. doi: 10.1007/BF03033781. [DOI] [PubMed] [Google Scholar]
- Gupta S, Bousman CA, Chana G, Cherner M, Heaton RK, Deutsch R, et al. Dopamine receptor D3 genetic polymorphism (rs6280TC) is associated with rates of cognitive impairment in methamphetamine-dependent men with HIV: preliminary findings. J Neurovirol. 2011;17(3):239–247. doi: 10.1007/s13365-011-0028-3. Retrieved from PM:21491142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Knight AG, Gupta S, Knapp PE, Hauser KF, Keller JN, et al. HIV-Tat elicits microglial glutamate release: role of NAPDH oxidase and the cystine-glutamate antiporter. Neuroscience Letters. 2010;485(3):233–236. doi: 10.1016/j.neulet.2010.09.019. Retrieved from PM:20849923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwell JA, Duncan MJ, Maderspach K, Stiene-Martin A, Elde RP, Hauser KF. κ-Opioid receptor expression defines a phenotypically distinct subpopulation of astroglia: relationship to Ca2+ mobilization, development, and the antiproliferative effect of opioids. Brain Research. 1996;737(1–2):175–187. doi: 10.1016/0006-8993(96)00728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, et al. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102(3):555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagino Y, Kariura Y, Manago Y, Amano T, Wang B, Sekiguchi M, et al. Heterogeneity and potentiation of AMPA type of glutamate receptors in rat cultured microglia. Glia. 2004;47(1):68–77. doi: 10.1002/glia.20034. [DOI] [PubMed] [Google Scholar]
- Hahn YK, Podhaizer EM, Farris SP, Miles MF, Hauser KF, Knapp PE. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct Funct, Epub. 2014 doi: 10.1007/s00429-013-0676-6. Retrieved from PM:24352707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn YK, Podhaizer EM, Hauser KF, Knapp PE. HIV-1 alters neural and glial progenitor cell dynamics in the central nervous system: Coordinated response to opiates during maturation. Glia. 2012;60(12):1871–1887. doi: 10.1002/glia.22403. Retrieved from PM:22865725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn YK, Vo P, Fitting S, Block ML, Hauser KF, Knapp PE. beta-Chemokine production by neural and glial progenitor cells is enhanced by HIV-1 Tat: effects on microglial migration. Journal of Neurochemistry. 2010;114(1):97–109. doi: 10.1111/j.1471-4159.2010.06744.x. Retrieved from PM:20403075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Li XF, Randall-Thompson J, Sora I, Murphy DL, Lesch KP, et al. Cocaine-conditioned locomotion in dopamine transporter, norepinephrine transporter and 5-HT transporter knockout mice. Neuroscience. 2009;162(4):870–880. doi: 10.1016/j.neuroscience.2009.05.058. S0306-4522(09)00968-3 [pii] Retrieved from PM:19482066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Sora I, Drgonova J, Li XF, Goeb M, Uhl GR. Molecular mechanisms underlying the rewarding effects of cocaine. Ann NY Acad Sci. 2004;1025:47–56. doi: 10.1196/annals.1316.006. 1025/1/47 [pii] Retrieved from PM:15542699. [DOI] [PubMed] [Google Scholar]
- Halpin LE, Collins SA, Yamamoto BK. Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci. 2014;97(1):37–44. doi: 10.1016/j.lfs.2013.07.014. S0024-3205(13)00401-3 [pii] Retrieved from PM:23892199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Yamamoto BK. Peripheral ammonia as a mediator of methamphetamine neurotoxicity. J Neurosci. 2012;32(38):13155–13163. doi: 10.1523/JNEUROSCI.2530-12.2012. 32/38/13155 [pii] Retrieved from PM:22993432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Lai J, Barditch-Crovo P, Gallant JE, Williams TM, Siliciano RF, et al. The role of protective HCP5 and HLA-C associated polymorphisms in the control of HIV-1 replication in a subset of elite suppressors. AIDS. 2008;22(4):541–544. doi: 10.1097/QAD.0b013e3282f470e4. 00002030-200802190-00016 [pii]. Retrieved from PM:18301071. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Functional diversity of microglia - how heterogeneous are they to begin with? Front Cell Neurosci. 2013;7:65. doi: 10.3389/fncel.2013.00065. Retrieved from PM:23717262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. Retrieved from PM:17965659. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Rau KS, Fleckenstein AE. The methamphetamine experience: a NIDA partnership. Neuropharmacology. 2004;47(Suppl 1):92–100. doi: 10.1016/j.neuropharm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Sandoval V, Riddle E, Fleckenstein AE. Psychostimulants and vesicle trafficking: a novel mechanism and therapeutic implications. Ann NY Acad Sci. 2004;1025:146–50. doi: 10.1196/annals.1316.019. [DOI] [PubMed] [Google Scholar]
- Happel C, Steele AD, Finley MJ, Kutzler MA, Rogers TJ. DAMGO-induced expression of chemokines and chemokine receptors: the role of TGF-beta1. J Leukoc Biol. 2008;83(4):956–963. doi: 10.1189/jlb.1007685. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5(5):405–414. doi: 10.1038/nn835. nn835 [pii]. Retrieved from PM:11953750. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Aldrich JV, Anderson KJ, Bakalkin G, Christie MJ, Hall ED, et al. Pathobiology of dynorphins in trauma and disease. Front Biosci. 2005a;10:216–235. doi: 10.2741/1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Buch S, Berger JR, Tyor WR, Nath A, et al. Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res. 2005b;8(1–2):63–80. doi: 10.1007/BF03033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Buch S, Tyor WR, Nath A, Bruce-Keller AJ, et al. Impact of opiate-HIV-1 interactions on neurotoxic signaling. J Neuroimmune Pharmacol. 2006;1:98–105. doi: 10.1007/s11481-005-9000-4. [DOI] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, et al. HIV-1 neuropathogenesis: Glial mechanisms revealed through substance abuse. J Neurochem. 2007;100(3):567–586. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE. Opiate drug use and the pathophysiology of neuroAIDS. Curr HIV Res. 2012;10(5):435–452. doi: 10.2174/157016212802138779. Retrieved from PM:22591368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Hahn YK, Adjan VV, Zou S, Buch SK, Nath A, et al. HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia. 2009;57(2):194–206. doi: 10.1002/glia.20746. Retrieved from PM:18756534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Mangoura D. Diversity of the endogenous opioid system in development: novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect Dev Neurobiol. 1998;5(4):437–449. [PubMed] [Google Scholar]
- Hauser KF, Osborne JG, Stiene-Martin A, Melner MH. Cellular localization of proenkephalin mRNA and enkephalin peptide products in cultured astrocytes. Brain Research. 1990;522:347–353. doi: 10.1016/0006-8993(90)91482-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC. μ-Opioid receptor-induced Ca2+ mobilization and astroglial development: Morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca2+-dependent mechanism. Brain Research. 1996;720(1–2):191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86(3):1009–1031. doi: 10.1152/physrev.00049.2005. Retrieved from PM:16816144. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- Hildebrandt K, Teuchert-Noodt G, Dawirs RR. A single neonatal dose of methamphetamine suppresses dentate granule cell proliferation in adult gerbils which is restored to control values by acute doses of haloperidol. Journal of Neural Transmission. 1999;106(5–6):549–558. doi: 10.1007/s007020050178. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Galea LA. Defensive behavior and hippocampal cell proliferation: differential modulation by naltrexone during stress. Behavioral Neuroscience. 2002;116(1):160–168. Retrieved from PM:11898802. [PubMed] [Google Scholar]
- Holzinger ER, Hulgan T, Ellis RJ, Samuels DC, Ritchie MD, Haas DW, et al. Mitochondrial DNA variation and HIV-associated sensory neuropathy in CHARTER. J Neurovirol. 2012;18(6):511–520. doi: 10.1007/s13365-012-0133-y. Retrieved from PM:23073667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Cheeran MC, Sheng WS, Ni HT, Lokensgard JR, Peterson PK. Cocaine alters proliferation, migration, and differentiation of human fetal brain-derived neural precursor cells. J Pharmacol Exp Ther. 2006;318(3):1280–1286. doi: 10.1124/jpet.106.103853. jpet.106.103853 [pii] Retrieved from PM:16766721. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine potentiates HIV-1 gp120-induced neuronal apoptosis. J Infect Dis. 2005;191(6):886–889. doi: 10.1086/427830. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK, Rock RB. Preferential sensitivity of human dopaminergic neurons to gp120-induced oxidative damage. J Neurovirol. 2009;15(5–6):401–410. doi: 10.3109/13550280903296346. Retrieved from PM:20175694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Paxton WA, Wolinsky SM, Neumann AU, Zhang L, He T, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2(11):1240–1243. doi: 10.1038/nm1196-1240. Retrieved from PM:8898752. [DOI] [PubMed] [Google Scholar]
- Hunt PW, Shulman N, Hayes TL, Dahl V, Somsouk M, Funderburg NT, et al. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T cell recovery: a randomized trial. Blood. 2013 doi: 10.1182/blood-2012-06-436345. Retrieved from PM:23589670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husemann J, Loike JD, Kodama T, Silverstein SC. Scavenger receptor class B type I (SR-BI) mediates adhesion of neonatal murine microglia to fibrillar beta-amyloid. J Neuroimmunol. 2001;114(1–2):142–150. doi: 10.1016/s0165-5728(01)00239-9. S0165572801002399 [pii]. Retrieved from PM:11240025. [DOI] [PubMed] [Google Scholar]
- Husemann J, Silverstein SC. Expression of scavenger receptor class B, type I, by astrocytes and vascular smooth muscle cells in normal adult mouse and human brain and in Alzheimer’s disease brain. Am J Pathol. 2001;158(3):825–832. doi: 10.1016/S0002-9440(10)64030-8. S0002-9440(10)64030-8 [pii] Retrieved from PM:11238031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–698. doi: 10.1056/NEJMoa0802905. 360/7/692 [pii] Retrieved from PM:19213682. [DOI] [PubMed] [Google Scholar]
- Imamoto K, Paterson JA, LEBLOND CP. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. I Sequential changes in oligodendrocytes. J Comp Neurol. 1978;180(1):115–117. doi: 10.1002/cne.901800108. Retrieved from PM:649784. [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Molecular Pharmacology. 2002;62(1):65–74. doi: 10.1124/mol.62.1.65. Retrieved from PM:12065756. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Rosenberg PS, Goedert JJ, Ashton LJ, Benfield TL, Buchbinder SP, et al. Effects of CCR5-Delta32, CCR2-64I, and SDF-1 3′A alleles on HIV-1 disease progression: An international meta-analysis of individual-patient data. Ann Intern Med. 2001;135(9):782–795. doi: 10.7326/0003-4819-135-9-200111060-00008. 200111060-00008 [pii]. Retrieved from PM:11694103. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, et al. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572(Pt 3):789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M, Murray J, Byrd DA, Hurd YL, Morgello S. HIV-related cognitive impairment shows bi-directional association with dopamine receptor DRD1 and DRD2 polymorphisms in substance-dependent and substance-independent populations. J Neurovirol. 2013;19(5):495–504. doi: 10.1007/s13365-013-0204-8. Retrieved from PM:24078558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, et al. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci USA. 2005;102(3):868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CJ, Massie A, De Keyser J. Immune Players in the CNS: The Astrocyte. J Neuroimmune Pharmacol. 2013;8(4):824–839. doi: 10.1007/s11481-013-9480-6. Retrieved from PM:23821340. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. Journal of Neuroscience. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SB, Arrildt KT, Swanstrom AE, Schnell G, Lee B, Hoxie JA, et al. Quantification of entry phenotypes of macrophage-tropic HIV-1 across a wide range of CD4 densities. J Virol. 2014;88(4):1858–1869. doi: 10.1128/JVI.02477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197(4308):1092–1094. doi: 10.1126/science.887941. Retrieved from PM:887941. [DOI] [PubMed] [Google Scholar]
- Kato T. The other, forgotten genome: mitochondrial DNA and mental disorders. Mol Psychiatry. 2001;6(6):625–633. doi: 10.1038/sj.mp.4000926. Retrieved from PM:11673790. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Experimental and potential future therapeutic approaches for HIV-1 associated dementia targeting receptors for chemokines, glutamate and erythropoietin. Neurotox Res. 2005;8(1–2):167–186. doi: 10.1007/BF03033828. [DOI] [PubMed] [Google Scholar]
- Kaushal N, Matsumoto RR. Role of sigma receptors in methamphetamine-induced neurotoxicity. Curr Neuropharmacol. 2011;9(1):54–57. doi: 10.2174/157015911795016930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanis I. The subependymal zone neurogenic niche: a beating heart in the centre of the brain: how plastic is adult neurogenesis? Opportunities for therapy and questions to be addressed. Brain. 2009;132(Pt 11):2909–2921. doi: 10.1093/brain/awp237. awp237 [pii] Retrieved from PM:19773354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM, Beck SE, Pate KA, Queen SE, Dorsey JL, Adams RJ, et al. Neuroprotective maraviroc monotherapy in simian immunodeficiency virus-infected macaques: reduced replicating and latent SIV in the brain. AIDS. 2013 doi: 10.1097/QAD.0000000000000074. Retrieved from PM:24051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010;37(2):267–274. doi: 10.1016/j.nbd.2009.11.002. S0969-9961(09)00313-1 [pii] Retrieved from PM:19909815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurdayan VK, Buch S, El-Hage N, Lutz SE, Goebel SM, Singh IN, et al. Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 Tat exposure in vitro. Eur J Neurosci. 2004;19(12):3171–3182. doi: 10.1111/j.0953-816X.2004.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebala M, Maggirwar SB. Ibudilast, a pharmacologic phosphodiesterase inhibitor, prevents human immunodeficiency virus-1 Tat-mediated activation of microglial cells. PLoS ONE. 2011;6(4):e18633. doi: 10.1371/journal.pone.0018633. Retrieved from PM:21494611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. 29/43/13435 [pii] Retrieved from PM:19864556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Bhattacharya T, Kunstman K, Swantek P, Koning FA, Malim MH, et al. Human APOBEC3G-mediated editing can promote HIV-1 sequence diversification and accelerate adaptation to selective pressure. J Virol. 2010;84(19):10402–10405. doi: 10.1128/JVI.01223-10. JVI.01223-10 [pii] Retrieved from PM:20660203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5(9):687–698. doi: 10.1038/nrm1469. nrm1469 [pii]. Retrieved from PM:15340377. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological Psychiatry. 2010;67(1):81–84. doi: 10.1016/j.biopsych.2009.07.018. S0006-3223(09)00892-0 [pii] Retrieved from PM:19717140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl M, Meerlo P, Gonzales D, Rontal A, Turek FW, Abrous DN. Exercise-induced promotion of hippocampal cell proliferation requires beta-endorphin. Faseb J. 2008;22(7):2253–2262. doi: 10.1096/fj.07-099101. fj.07-099101 [pii] Retrieved from PM:18263701. [DOI] [PubMed] [Google Scholar]
- Kogan FJ, Nichols WK, Gibb JW. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activity and on striatal dopamine levels. European Journal of Pharmacology. 1976;36(2):363–371. doi: 10.1016/0014-2999(76)90090-x. [DOI] [PubMed] [Google Scholar]
- Kolodziej A, Stumm R, Becker A, Hollt V. Endogenous opioids inhibit ischemia-induced generation of immature hippocampal neurons via the mu-opioid receptor. Eur J Neurosci. 2008;27(6):1311–1319. doi: 10.1111/j.1460-9568.2008.06111.x. EJN6111 [pii] Retrieved from PM:18331339. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA. 1999;96(10):5768–5773. doi: 10.1073/pnas.96.10.5768. Retrieved from PM:10318959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294(5549):2127–2130. doi: 10.1126/science.1065467. 294/5549/2127 [pii]. Retrieved from PM:11739948. [DOI] [PubMed] [Google Scholar]
- Kousik SM, Napier TC, Carvey PM. The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Front Pharmacol. 2012;3:121. doi: 10.3389/fphar.2012.00121. Retrieved from PM:22754527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE. A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron. 2009;64(1):133–145. doi: 10.1016/j.neuron.2009.09.042. S0896-6273(09)00753-3 [pii] Retrieved from PM:19840555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Hahn A, Brack-Werner R, Werner T. Elucidating effects of long-term expression of HIV-1 Nef on astrocytes by microarray, promoter, and literature analyses. Gene. 2005;358:31–38. doi: 10.1016/j.gene.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111(2):194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379–407. doi: 10.1016/j.brainresrev.2009.03.002. S0165-0173(09)00034-4 [pii] Retrieved from PM:19328213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004;190(2):216–226. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- Krebs MO, Gauchy C, Desban M, Glowinski J, Kemel ML. Role of dynorphin and GABA in the inhibitory regulation of NMDA-induced dopamine release in striosome- and matrix-enriched areas of the rat striatum. Journal of Neuroscience. 1994;14(4):2435–2443. doi: 10.1523/JNEUROSCI.14-04-02435.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacological Reviews. 2005;57(1):1–26. doi: 10.1124/pr.57.1.1. 57/1/1 [pii] Retrieved from PM:15734726. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Levran O, Reed B, Schlussman SD, Zhou Y, Butelman ER. Opiate addiction and cocaine addiction: underlying molecular neurobiology and genetics. J Clin Invest. 2012;122(10):3387–3393. doi: 10.1172/JCI60390. 60390 [pii] Retrieved from PM:23023708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8(11):1450–1457. doi: 10.1038/nn1583. Retrieved from PM:16251987. [DOI] [PubMed] [Google Scholar]
- Kuhn SA, van Landeghem FK, Zacharias R, Farber K, Rappert A, Pavlovic S, et al. Microglia express GABA(B) receptors to modulate interleukin release. Mol Cell Neurosci. 2004;25(2):312–322. doi: 10.1016/j.mcn.2003.10.023. S104474310300352X [pii]. Retrieved from PM:15019947. [DOI] [PubMed] [Google Scholar]
- Kumar A, Manna SK, Dhawan S, Aggarwal BB. HIV-Tat protein activates c-Jun N-terminal kinase and activator protein-1. J Immunol. 1998;161(2):776–81. [PubMed] [Google Scholar]
- Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000;74(19):9214–9221. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. Retrieved from PM:8807664. [DOI] [PubMed] [Google Scholar]
- Langford D, Masliah E. Crosstalk between components of the blood brain barrier and cells of the CNS in microglial activation in AIDS. Brain Pathol. 2001;11(3):306–312. doi: 10.1111/j.1750-3639.2001.tb00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci. 2002;22(20):8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. Retrieved from PM:12388602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci. 1999;19(4):1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. Retrieved from PM:9952424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78(14):7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Sharron M, Blanpain C, Doranz BJ, Vakili J, Setoh P, et al. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274(14):9617–9626. doi: 10.1074/jbc.274.14.9617. Retrieved from PM:10092648. [DOI] [PubMed] [Google Scholar]
- Lee CT, Chen J, Hayashi T, Tsai SY, Sanchez JF, Errico SL, et al. A mechanism for the inhibition of neural progenitor cell proliferation by cocaine. PLoS Med. 2008;5(6):e117. doi: 10.1371/journal.pmed.0050117. 07-PLME-RA-0879 [pii] Retrieved from PM:18593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Chen J, Worden LT, Freed WJ. Cocaine causes deficits in radial migration and alters the distribution of glutamate and GABA neurons in the developing rat cerebral cortex. Synapse. 2011;65(1):21–34. doi: 10.1002/syn.20814. Retrieved from PM:20506319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Lehrmann E, Hayashi T, Amable R, Tsai SY, Chen J, et al. Gene expression profiling reveals distinct cocaine-responsive genes in human fetal CNS cell types. J Addict Med. 2009;3(4):218–226. doi: 10.1097/ADM.0b013e318199d863. Retrieved from PM:20948987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000;30(2):105–121. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lee MH, Amin ND, Venkatesan A, Wang T, Tyagi R, Pant HC, et al. Impaired neurogenesis and neurite outgrowth in an HIV-gp120 transgenic model is reversed by exercise via BDNF production and Cdk5 regulation. J Neurovirol. 2013;19(5):418–431. doi: 10.1007/s13365-013-0194-6. Retrieved from PM:23982957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Wang T, Jang MH, Steiner J, Haughey N, Ming GL, et al. Rescue of adult hippocampal neurogenesis in a mouse model of HIV neurologic disease. Neurobiol Dis. 2011;41(3):678–687. doi: 10.1016/j.nbd.2010.12.002. S0969-9961(10)00395-5 [pii] Retrieved from PM:21146610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278(5335):45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Sinsheimer JS, Bilder R, Shapshak P, Singer EJ. Functional polymorphisms in dopamine-related genes: effect on neurocognitive functioning in HIV+ adults. J Clin Exp Neuropsychol. 2012;34(1):78–91. doi: 10.1080/13803395.2011.623118. Retrieved from PM:22082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, Rothstein RP, Brazel CY, Young GM, Albrecht PJ. Selective apoptosis within the rat subependymal zone: a plausible mechanism for determining which lineages develop from neural stem cells. Developmental Neuroscience. 2000;22(1–2):106–115. doi: 10.1159/000017432. 17432 [pii];17432. Retrieved from PM:10657703. [DOI] [PubMed] [Google Scholar]
- Li J, Bentsman G, Potash MJ, Volsky DJ. Human immunodeficiency virus type 1 efficiently binds to human fetal astrocytes and induces neuroinflammatory responses independent of infection. BMC Neurosci. 2007;8:31. doi: 10.1186/1471-2202-8-31. Retrieved from PM:17498309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chen CK, Hu X, Ball D, Lin SK, Chen W, et al. Association analysis of the DRD4 and COMT genes in methamphetamine abuse. Am J Med Genet B Neuropsychiatr Genet. 2004;129B(1):120–124. doi: 10.1002/ajmg.b.30024. Retrieved from PM:15274053. [DOI] [PubMed] [Google Scholar]
- Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res. 2009;16(3):205–220. doi: 10.1007/s12640-009-9047-8. Retrieved from PM:19526283. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang X, Tian S, Guo CJ, Douglas SD, Ho WZ. Methadone enhances human immunodeficiency virus infection of human immune cells. J Infect Dis. 2002;185(1):118–122. doi: 10.1086/338011. Retrieved from PM:11756991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Gao L, Liu Q, Cao C, Sun XL, Ding JH, et al. Aquaporin-4 knockout regulated cocaine-induced behavior and neurochemical changes in mice. Neuroscience Letters. 2006;403(3):294–298. doi: 10.1016/j.neulet.2006.05.004. S0304-3940(06)00473-3 [pii] Retrieved from PM:16797122. [DOI] [PubMed] [Google Scholar]
- Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, et al. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172(6):1617–1624. doi: 10.2353/ajpath.2008.070971. S0002-9440(10)61920-7 [pii] Retrieved from PM:18458095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Grigoriants OO, Loh HH, Law PY. Agonist-dependent postsynaptic effects of opioids on miniature excitatory postsynaptic currents in cultured hippocampal neurons. J Neurophysiol. 2007;97(2):1485–1494. doi: 10.1152/jn.00790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Grigoriants OO, Wang W, Wiens K, Loh HH, Law PY. Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol Cell Neurosci. 2007;35(3):456–469. doi: 10.1016/j.mcn.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Lin H, Law PY, Loh HH. Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci USA. 2005;102(5):1725–1730. doi: 10.1073/pnas.0406797102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. Retrieved from PM:17051205. [DOI] [PubMed] [Google Scholar]
- Little KY, Krolewski DM, Zhang L, Cassin BJ. Loss of Striatal Vesicular Monoamine Transporter Protein (VMAT2) in Human Cocaine Users. Am J Psychiatry. 2003;160(1):47–55. doi: 10.1176/appi.ajp.160.1.47. [DOI] [PubMed] [Google Scholar]
- Liu H, Chao D, Nakayama EE, Taguchi H, Goto M, Xin X, et al. Polymorphism in RANTES chemokine promoter affects HIV-1 disease progression. Proc Natl Acad Sci USA. 1999;96(8):4581–4585. doi: 10.1073/pnas.96.8.4581. Retrieved from PM:10200305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, et al. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78(8):4120–4133. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27(11):2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low KG, Melner MH. Expression of high levels of proenkephalin in an isolated glial cell type: Inhibition by gamma-interferon. Ann NY Acad Sci. 1990a;594:475–478. [Google Scholar]
- Low KG, Melner MH. Regulation of proenkephalin gene expression in astrocytes by cytokines. Soc Neurosci Abstr. 1990b:16. doi: 10.1210/endo.131.4.1396335. [DOI] [PubMed] [Google Scholar]
- Lu J, Esposito G, Scuderi C, Steardo L, Delli-Bovi LC, Hecht JL, et al. S100B and APP promote a gliocentric shift and impaired neurogenesis in Down syndrome neural progenitors. PLoS ONE. 2011;6(7):e22126. doi: 10.1371/journal.pone.0022126. PONE-D-11-08090 [pii]. Retrieved from PM:21779383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xu J, Xu M, Pasternak GW, Pan YX. Morphine regulates expression of mu-opioid receptor MOR-1A, an intron-retention carboxyl terminal splice variant of the mu-opioid receptor (OPRM1) gene via miR-103/miR-107. Molecular Pharmacology. 2014;85(2):368–380. doi: 10.1124/mol.113.089292. mol.113.089292 [pii] Retrieved from PM:24302561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95(16):9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur RD, Novak RM. Reviews of anti-infective agents: maraviroc: the first of a new class of antiretroviral agents. Clin Infect Dis. 2008;47(2):236–241. doi: 10.1086/589289. Retrieved from PM:18532888. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Reynolds JL, Nair BB, Fernandez SF, Schwartz SA, et al. Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells. Curr HIV Res. 2005a;3(3):277–288. doi: 10.2174/1570162054368048. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Aalinkeel R, Chawda RP, Sykes DE, Nair MP. Morphine modulates chemokine gene regulation in normal human astrocytes. Clin Immunol. 2005b;115(3):323–332. doi: 10.1016/j.clim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Shanahan TC, Chawda RP, Nair MP. Morphine regulates gene expression of alpha- and beta-chemokines and their receptors on astroglial cells via the opioid mu receptor. Journal of Immunology. 2002;169(7):3589–3599. doi: 10.4049/jimmunol.169.7.3589. [DOI] [PubMed] [Google Scholar]
- Mahanta J, Borkakoty B, Das HK, Chelleng PK. The risk of HIV and HCV infections among injection drug users in northeast India. AIDS Care. 2009;21(11):1420–1424. doi: 10.1080/09540120902862584. 916269548 [pii] Retrieved from PM:20024719. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Grinnell S, Le RV, Burgman M, Polikar L, Ansonoff M, et al. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci USA. 2011;108(49):19778–19783. doi: 10.1073/pnas.1115231108. Retrieved from PM:22106286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Saha R, Seth P. Involvement of Extracellular Signal-Regulated Kinase (ERK1/2)-p53-p21 Axis in Mediating Neural Stem/Progenitor Cell Cycle Arrest in Co-Morbid HIV-Drug Abuse Exposure. J Neuroimmune Pharmacol. 2014 doi: 10.1007/s11481-014-9523-7. Retrieved from PM:24469921. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Norris RD, Eisch AJ. Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. Journal of Neuroscience Research. 2004;76(6):783–794. doi: 10.1002/jnr.20090. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biological Psychiatry. 2008;64(11):958–965. doi: 10.1016/j.biopsych.2008.04.010. S0006-3223(08)00432-0 [pii] Retrieved from PM:18490002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci. 2007;27(42):11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. 27/42/11442 [pii] Retrieved from PM:17942739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424(6944):99–103. doi: 10.1038/nature01709. nature01709 [pii]. Retrieved from PM:12808466. [DOI] [PubMed] [Google Scholar]
- Manini AF, Jacobs MM, Vlahov D, Hurd YL. Opioid receptor polymorphism A118G associated with clinical severity in a drug overdose population. J Med Toxicol. 2013;9(2):148–154. doi: 10.1007/s13181-012-0286-3. Retrieved from PM:23318993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. Journal of Neuroscience. 2002;22(18):8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Gliogenesis in the striatum of the adult rat: alteration in neural progenitor population after psychostimulant exposure. Brain Res Dev Brain Res. 2001;130(1):41–51. doi: 10.1016/s0165-3806(01)00195-x. S016538060100195X [pii]. Retrieved from PM:11557092. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2(12):679–689. doi: 10.1038/ncpneuro0355. ncpneuro0355 [pii] Retrieved from PM:17117171. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, et al. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. Journal of Neurochemistry. 2002;83(4):955–963. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- Martineau M. Gliotransmission: focus on exocytotic release of L-glutamate and D-serine from astrocytes. Biochem Soc Trans. 2013;41(6):1557–1561. doi: 10.1042/BST20130195. BST20130195 [pii] Retrieved from PM:24256254. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42(6):963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Mathews JL, Smrcka AV, Bidlack JM. A novel Gbetagamma-subunit inhibitor selectively modulates mu-opioid-dependent antinociception and attenuates acute morphine-induced antinociceptive tolerance and dependence. Journal of Neuroscience. 2008;28(47):12183–12189. doi: 10.1523/JNEUROSCI.2326-08.2008. Retrieved from PM:19020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Nguyen L, Kaushal N, Robson MJ. Sigma (sigma) receptors as potential therapeutic targets to mitigate psychostimulant effects. Adv Pharmacol. 2014;69:323–386. doi: 10.1016/B978-0-12-420118-7.00009-3. B978-0-12-420118-7.00009-3 [pii] Retrieved from PM:24484982. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124(2):195–206. doi: 10.1016/j.pharmthera.2009.07.001. S0163-7258(09)00141-7 [pii] Retrieved from PM:19619582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne M, Holden CP, Nath A, Geiger JD. Release of calcium from inositol 1,4,5-trisphosphate receptor-regulated stores by HIV-1 Tat regulates TNF-alpha production in human macrophages. J Immunol. 2000;164(12):6538–6542. doi: 10.4049/jimmunol.164.12.6538. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18(20):8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. Retrieved from PM:9763484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. 1742-2094-5-45 [pii] Retrieved from PM:18925972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DH, Beecroft MJ, Kleeberger CA, Al-Sharif FM, Ollier WE, Zimmerman PA, et al. Chemokine RANTES promoter polymorphism affects risk of both HIV infection and disease progression in the Multicenter AIDS Cohort Study. AIDS. 2000;14(17):2671–2678. doi: 10.1097/00002030-200012010-00006. [DOI] [PubMed] [Google Scholar]
- McQuiston AR. Effects of mu-opioid receptor modulation on GABAB receptor synaptic function in hippocampal CA1. J Neurophysiol. 2007;97(3):2301–2311. doi: 10.1152/jn.01179.2006. Retrieved from PM:17215502. [DOI] [PubMed] [Google Scholar]
- Meijerink H, Wisaksana R, Iskandar S, den HM, van der Ven AJ, Alisjahbana B, et al. Injecting drug use is associated with a more rapid CD4 cell decline among treatment naive HIV-positive patients in Indonesia. J Int AIDS Soc. 2014;17(1):18844. doi: 10.7448/IAS.17.1.18844. 18844 [pii]. Retrieved from PM:24388495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messam CA, Major EO. Stages of restricted HIV-1 infection in astrocyte cultures derived from human fetal brain tissue. J Neurovirol. 2000;6(Suppl 1):S90–S94. Retrieved from PM:10871771. [PubMed] [Google Scholar]
- MESSIER B, LEBLOND CP, Smart I. Presence of DNA synthesis and mitosis in the brain of young adult mice. Exp Cell Res. 1958;14(1):224–226. doi: 10.1016/0014-4827(58)90235-0. 0014-4827(58)90235-0 [pii]. Retrieved from PM:13512326. [DOI] [PubMed] [Google Scholar]
- Meyer UA, Zanger UM. Molecular mechanisms of genetic polymorphisms of drug metabolism. Annual Review of Pharmacology and Toxicology. 1997;37:269–296. doi: 10.1146/annurev.pharmtox.37.1.269. Retrieved from PM:9131254. [DOI] [PubMed] [Google Scholar]
- Meyer VJ, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ET, et al. HIV and Recent Illicit Drug Use Interact to Affect Verbal Memory in Women. J Acquir Immune Defic Syndr. 2013;63(1):67–76. doi: 10.1097/QAI.0b013e318289565c. Retrieved from PM:23392462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Zhu J. HIV-1 Tat protein decreases dopamine transporter cell surface expression and vesicular monoamine transporter-2 function in rat striatal synaptosomes. J Neuroimmune Pharmacol. 2012;7(3):629–639. doi: 10.1007/s11481-012-9369-9. Retrieved from PM:22570010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Huang X, Gomez AM, Booze RM, Zhan CG, Zhu J. Mutation of tyrosine 470 of human dopamine transporter is critical for HIV-1 Tat-induced inhibition of dopamine transport and transporter conformational transitions. J Neuroimmune Pharmacol. 2013;8(4):975–987. doi: 10.1007/s11481-013-9464-6. Retrieved from PM:23645138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem. 2011;116(2):164–176. doi: 10.1111/j.1471-4159.2010.07109.x. Retrieved from PM:21073468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends in Pharmacological Sciences. 1999;20(10):424–429. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- Mishra M, Taneja M, Malik S, Khalique H, Seth P. Human immunodeficiency virus type 1 Tat modulates proliferation and differentiation of human neural precursor cells: implication in NeuroAIDS. J Neurovirol. 2010;16(5):355–367. doi: 10.3109/13550284.2010.513028. Retrieved from PM:20839920. [DOI] [PubMed] [Google Scholar]
- Miura T, Brockman MA, Brumme CJ, Brumme ZL, Carlson JM, Pereyra F, et al. Genetic characterization of human immunodeficiency virus type 1 in elite controllers: lack of gross genetic defects or common amino acid changes. J Virol. 2008;82(17):8422–8430. doi: 10.1128/JVI.00535-08. JVI.00535-08 [pii] Retrieved from PM:18562530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake M, Narita M, Shibasaki M, Nakamura A, Suzuki T. Glutamatergic neurotransmission and protein kinase C play a role in neuron-glia communication during the development of methamphetamine-induced psychological dependence. European Journal of Neuroscience. 2005;22(6):1476–1488. doi: 10.1111/j.1460-9568.2005.04325.x. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, et al. Astrocytes and disease: a neurodevelopmental perspective. Genes and Development. 2012;26(9):891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54(7):700–715. doi: 10.1002/glia.20367. Retrieved from PM:17006898. [DOI] [PubMed] [Google Scholar]
- Moore JP, Kitchen SG, Pugach P, Zack JA. The CCR5 and CXCR4 coreceptors--central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20(1):111–126. doi: 10.1089/088922204322749567. Retrieved from PM:15000703. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24(1):85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. 24/1/85 [pii]. Retrieved from PM:14715941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Letendre SL, Franklin DR, Bloss C, Goate A, et al. Apolipoprotein E4 genotype does not increase risk of HIV-associated neurocognitive disorders. J Neurovirol. 2013;19(2):150–156. doi: 10.1007/s13365-013-0152-3. Retrieved from PM:23408335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulherin SA, O’Brien TR, Ioannidis JP, Goedert JJ, Buchbinder SP, Coutinho RA, et al. Effects of CCR5-Delta32 and CCR2-64I alleles on HIV-1 disease progression: the protection varies with duration of infection. AIDS. 2003;17(3):377–387. doi: 10.1097/01.aids.0000050783.28043.3e. Retrieved from PM:12556692. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor kappa B activity in the brain during endotoxemia. J Neurosci. 2000;20(9):3456–3468. doi: 10.1523/JNEUROSCI.20-09-03456.2000. Retrieved from PM:10777809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Castro V, Toborek M. Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J Cell Mol Med. 2012;16(12):2950–2957. doi: 10.1111/j.1582-4934.2012.01622.x. Retrieved from PM:22947176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Yamada K, Nagai T, Uchiyama T, Miyamoto Y, Mamiya T, et al. Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J Neurosci. 2004;24(9):2212–2225. doi: 10.1523/JNEUROSCI.4847-03.2004. Retrieved from PM:14999072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Koyama T, Yamashita I. Long-lasting decrease in dopamine uptake sites following repeated administration of methamphetamine in the rat striatum. Brain Research. 1993;601(1–2):209–212. doi: 10.1016/0006-8993(93)91712-2. Retrieved from PM:8431767. [DOI] [PubMed] [Google Scholar]
- Napier TC, Chen L, Kashanchi F, Hu XT. Repeated Cocaine Treatment Enhances HIV-1 Tat-induced Cortical Excitability via Over-activation of L-type Calcium Channels. J Neuroimmune Pharmacol. 2014 doi: 10.1007/s11481-014-9524-6. Retrieved from PM:24567038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasipura SD, Henderson LJ, Fu SW, Chen L, Kashanchi F, Al-Harthi L. Role of beta-catenin and TCF/LEF family members in transcriptional activity of HIV in astrocytes. J Virol. 2012;86(4):1911–1921. doi: 10.1128/JVI.06266-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasipura SD, Kim S, Al-Harthi L. Epigenetic regulation of HIV-1 latency in astrocytes. J Virol. 2014;88(5):3031–3038. doi: 10.1128/JVI.03333-13. JVI.03333-13 [pii] Retrieved from PM:24352441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana PA, Herrera JJ, Bockhorst KH, Esparza-Coss E, Xia Y, Steinberg JL, et al. Chronic cocaine administration causes extensive white matter damage in brain: Diffusion tensor imaging and immunohistochemistry studies. Psychiatry Res. 2014;221(3):220–230. doi: 10.1016/j.pscychresns.2014.01.005. S0925-4927(14)00006-7 [pii] Retrieved from PM:24507117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S, Mesangeau C, Poupaert JH, McCurdy CR. Sigma receptors and cocaine abuse. Curr Top Med Chem. 2011;11(9):1128–1150. doi: 10.2174/156802611795371323. BSP/CTMC/E-Pub/-00016-11-3 [pii]. Retrieved from PM:21050176. [DOI] [PubMed] [Google Scholar]
- Nath A. Pathobiology of human immunodeficiency virus dementia. Semin Neurol. 1999;19(2):113–127. doi: 10.1055/s-2008-1040830. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann NY Acad Sci. 2010;1187:122–128. doi: 10.1111/j.1749-6632.2009.05277.x. Retrieved from PM:20201849. [DOI] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes : A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- Nath A, Geiger J. Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Prog Neurobiol. 1998;54(1):19–33. doi: 10.1016/s0301-0082(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nath A, Jones M, Maragos W, Booze R, Mactutus C, Bell J, et al. Neurotoxicity and dysfunction of dopamine systems associated with AIDS dementia. Psychopharmacol. 2000;14(3):222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol. 2001;7(1):66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- Nebert DW, McKinnon RA, Puga A. Human drug-metabolizing enzyme polymorphisms: effects on risk of toxicity and cancer. DNA and Cell Biology. 1996;15(4):273–280. doi: 10.1089/dna.1996.15.273. Retrieved from PM:8639263. [DOI] [PubMed] [Google Scholar]
- Nickell JR, Siripurapu KB, Vartak A, Crooks PA, Dwoskin LP. The vesicular monoamine transporter-2: an important pharmacological target for the discovery of novel therapeutics to treat methamphetamine abuse. Adv Pharmacol. 2014;69:71–106. doi: 10.1016/B978-0-12-420118-7.00002-0. B978-0-12-420118-7.00002-0 [pii] Retrieved from PM:24484975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Gotz M. Fate specification in the adult brain--lessons for eliciting neurogenesis from glial cells. Bioessays. 2013;35(3):242–252. doi: 10.1002/bies.201200108. Retrieved from PM:23335359. [DOI] [PubMed] [Google Scholar]
- Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci. 2000;20(1):251–258. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, et al. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci. 2010;13(2):173–179. doi: 10.1038/nn.2473. nn.2473 [pii] Retrieved from PM:20037576. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Kang YJ, Brechtel CW, Siviglia E, Russo R, Clemente A, et al. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1(2):230–236. doi: 10.1016/j.stem.2007.07.010. S1934-5909(07)00076-8 [pii] Retrieved from PM:18371353. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Nakagawa T, Shige K, Minami M, Satoh M. Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain Research. 2001;905(1–2):254–258. doi: 10.1016/s0006-8993(01)02536-7. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Moskowitz HS, Xu M, Pasternak GW. Identification of four novel exon 5 splice variants of the mouse mu-opioid receptor gene: functional consequences of C-terminal splicing. Molecular Pharmacology. 2005;68(3):866–875. doi: 10.1124/mol.105.011858. Retrieved from PM:15939800. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. Retrieved from PM:9133393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res Rev. 2010;63(1–2):83–92. doi: 10.1016/j.brainresrev.2009.11.008. S0165-0173(09)00128-3 [pii] Retrieved from PM:19948188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310(5745):113–116. doi: 10.1126/science.1116916. Retrieved from PM:16210541. [DOI] [PubMed] [Google Scholar]
- Patel DA, Booze RM, Mactutus CF. Prenatal cocaine exposure alters progenitor cell markers in the subventricular zone of the adult rat brain. Int J Dev Neurosci. 2012;30(1):1–9. doi: 10.1016/j.ijdevneu.2011.11.001. S0736-5748(11)00176-6 [pii] Retrieved from PM:22119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JP, Sengupta R, Bardi G, Khan MZ, Mullen-Przeworski A, Meucci O. Modulation of neuronal CXCR4 by the micro-opioid agonist DAMGO. J Neurovirol. 2006;12(6):492–500. doi: 10.1080/13550280601064798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol. 2001;172(1):1–16. doi: 10.1006/exnr.2001.7768. S0014-4886(01)97768-4 [pii]. Retrieved from PM:11681836. [DOI] [PubMed] [Google Scholar]
- Peng H, Sun L, Jia B, Lan X, Zhu B, Wu Y, et al. HIV-1-infected and immune-activated macrophages induce astrocytic differentiation of human cortical neural progenitor cells via the STAT3 pathway. PLoS ONE. 2011;6(5):e19439. doi: 10.1371/journal.pone.0019439. PONE-D-10-00609 [pii]. Retrieved from PM:21637744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Whitney N, Wu Y, Tian C, Dou H, Zhou Y, et al. HIV-1-infected and/or immune-activated macrophage-secreted TNF-alpha affects human fetal cortical neural progenitor cell proliferation and differentiation. Glia. 2008;56(8):903–916. doi: 10.1002/glia.20665. Retrieved from PM:18383342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32(8):421–431. doi: 10.1016/j.tins.2009.05.001. S0166-2236(09)00101-5 [pii] Retrieved from PM:19615761. [DOI] [PubMed] [Google Scholar]
- Pereira FC, Cunha-Oliveira T, Viana SD, Travassos AS, Nunes S, Silva C, et al. Disruption of striatal glutamatergic/GABAergic homeostasis following acute methamphetamine in mice. Neurotoxicol Teratol. 2012;34(5):522–529. doi: 10.1016/j.ntt.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Perez-Casanova A, Husain K, Noel RJ, Jr, Rivera-Amill V, Kumar A. Interaction of SIV/SHIV infection and morphine on plasma oxidant/antioxidant balance in macaque. Mol Cell Biochem. 2008;308(1–2):169–175. doi: 10.1007/s11010-007-9625-0. Retrieved from PM:17934700. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman HE. An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis. J Neurovirol. 1997;3(6):401–16. doi: 10.3109/13550289709031186. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74(5):691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Eriksson PS. Opioid-induced proliferation through the MAPK pathway in cultures of adult hippocampal progenitors. Mol Cell Neurosci. 2003;23(3):360–372. doi: 10.1016/s1044-7431(03)00061-7. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, et al. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci. 2003;17(6):1159–1172. doi: 10.1046/j.1460-9568.2003.02538.x. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Cabral G, Lokensgard JR. Cannabinoids and morphine differentially affect HIV-1 expression in CD4(+) lymphocyte and microglial cell cultures. Journal of Neuroimmunology. 2004;147(1–2):123–126. doi: 10.1016/j.jneuroim.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Lokensgard J, Portoghese PS, Chao CC. Endomorphin-1 potentiates HIV-1 expression in human brain cell cultures: implication of an atypical mu-opioid receptor. Neuropharmacology. 1999;38(2):273–278. doi: 10.1016/s0028-3908(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. Journal of Neuroimmunology. 1998;83(1–2):63–69. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- Pettit AS, Desroches R, Bennett SA. The opiate analgesic buprenorphine decreases proliferation of adult hippocampal neuroblasts and increases survival of their progeny. Neuroscience. 2012;200:211–222. doi: 10.1016/j.neuroscience.2011.10.039. S0306-4522(11)01232-2 [pii] Retrieved from PM:22079577. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Wolf ME. Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. Cold Spring Harb Perspect Med. 2013;3(2):a012021. doi: 10.1101/cshperspect.a012021. Retrieved from PM:23232118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher J, Abt A, Myers J, Han R, Snyder M, Graziano A, et al. Neuronal ferritin heavy chain and drug abuse affect HIV-associated cognitive dysfunction. J Clin Invest. 2014;124(2):656–669. doi: 10.1172/JCI70090. 70090 [pii] Retrieved from PM:24401274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30(10):527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Podhaizer EM, Zou S, Fitting S, Samano KL, El-Hage N, Knapp PE, et al. Morphine and gp120 toxic interactions in striatal neurons are dependent on HIV-1 strain. J Neuroimmune Pharmacol. 2012;7(4):877–891. doi: 10.1007/s11481-011-9326-z. Retrieved from http://www.springerlink.com/content/6020qx40u36uk8lw/export-citation/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada I, Furlan R, Matteoli M, Verderio C. Classical and unconventional pathways of vesicular release in microglia. Glia. 2013;61(7):1003–1017. doi: 10.1002/glia.22497. Retrieved from PM:23625857. [DOI] [PubMed] [Google Scholar]
- Proudnikov D, Randesi M, Levran O, Yuferov V, Crystal H, Ho A, et al. Polymorphisms of the kappa opioid receptor and prodynorphin genes: HIV risk and HIV natural history. J Acquir Immune Defic Syndr. 2013;63(1):17–26. doi: 10.1097/QAI.0b013e318285cd0c. Retrieved from PM:23392455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudnikov D, Randesi M, Orna L, Crystal H, Dorn M, Ott J, et al. Association of polymorphisms of the mu opioid receptor gene with the severity of HIV infection and response to HIV treatment. J Infect Dis. 2012;205(11):1745–1756. doi: 10.1093/infdis/jis264. Retrieved from PM:22457278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Miller GW, Voit EO. Computational systems analysis of dopamine metabolism. PLoS ONE. 2008;3(6):e2444. doi: 10.1371/journal.pone.0002444. Retrieved from PM:18568086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quasney MW, Zhang Q, Sargent S, Mynatt M, Glass J, McArthur J. Increased frequency of the tumor necrosis factor-alpha-308 A allele in adults with human immunodeficiency virus dementia. Annals of Neurology. 2001;50(2):157–162. Retrieved from PM:11506397. [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPSJ. 2006;8(2):E337–E347. doi: 10.1208/aapsj080238. Retrieved from PM:16796384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage SN, Anthony IC, Carnie FW, Busuttil A, Robertson R, Bell JE. Hyperphosphorylated tau and amyloid precursor protein deposition is increased in the brains of young drug abusers. Neuropathol Appl Neurobiol. 2005;31(4):439–448. doi: 10.1111/j.1365-2990.2005.00670.x. Retrieved from PM:16008828. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Stevens B. Neuroscience. How many cell types does it take to wire a brain? Science. 2011;333(6048):1391–1392. doi: 10.1126/science.1212112. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci USA. 1998;95(20):11981–11986. doi: 10.1073/pnas.95.20.11981. Retrieved from PM:9751776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PV, Pilakka-Kanthikeel S, Saxena SK, Saiyed Z, Nair MP. Interactive Effects of Morphine on HIV Infection: Role in HIV-Associated Neurocognitive Disorder. AIDS Res Treat. 2012;2012:953678. doi: 10.1155/2012/953678. Retrieved from PM:22666564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese EA, Bunzow JR, Arttamangkul S, Sonders MS, Grandy DK. Trace amine-associated receptor 1 displays species-dependent stereoselectivity for isomers of methamphetamine, amphetamine, and para-hydroxyamphetamine. J Pharmacol Exp Ther. 2007;321(1):178–186. doi: 10.1124/jpet.106.115402. jpet.106.115402 [pii] Retrieved from PM:17218486. [DOI] [PubMed] [Google Scholar]
- Regan PM, Dave RS, Datta PK, Khalili K. Epigenetics of micro-opioid receptors: Intersection with HIV-1 infection of the central nervous system. J Cell Physiol. 2012;227(7):2832–2841. doi: 10.1002/jcp.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Brown RM, Spencer S, Tran PK, Thomas CA, Kalivas PW. Chronic administration of the methylxanthine propentofylline impairs reinstatement to cocaine by a GLT-1-dependent mechanism. Neuropsychopharmacology. 2014;39(2):499–506. doi: 10.1038/npp.2013.223. npp2013223 [pii] Retrieved from PM:23985782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, et al. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci USA. 2011;108(20):8485–8490. doi: 10.1073/pnas.1103029108. 1103029108 [pii] Retrieved from PM:21525407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R. Nigral degeneration in acquired immune deficiency syndrome (AIDS) Acta Neuropathol(Berl) 1991;82(1):39–44. doi: 10.1007/BF00310921. [DOI] [PubMed] [Google Scholar]
- Rigato PO, Hong MA, Casseb J, Ueda M, de CI, Benard G, et al. Better CD4+ T cell recovery in Brazilian HIV-infected individuals under HAART due to cumulative carriage of SDF-1-3′A, CCR2-V64I, CCR5-D32 and CCR5-promoter 59029A/G polymorphisms. Curr HIV Res. 2008;6(5):466–473. doi: 10.2174/157016208785861131. Retrieved from PM:18855658. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. S1355617704101021 [pii]. Retrieved from PM:14751002. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33(2):160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. Retrieved from PM:15464124. [DOI] [PubMed] [Google Scholar]
- Robinson-Papp J, Gelman BB, Grant I, Singer E, Gensler G, Morgello S. Substance abuse increases the risk of neuropathy in an HIV-infected cohort. Muscle Nerve. 2012;45(4):471–476. doi: 10.1002/mus.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock RB, Peterson PK. Microglia as a pharmacological target in infectious and inflammatory diseases of the brain. J Neuroimmune Pharmacol. 2006;1(2):117–126. doi: 10.1007/s11481-006-9012-8. Retrieved from PM:18040778. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 2003;24(3):116–121. doi: 10.1016/s1471-4906(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, et al. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol. 2006;202(1):189–199. doi: 10.1016/j.expneurol.2006.05.034. S0014-4886(06)00334-7 [pii] Retrieved from PM:16876159. [DOI] [PubMed] [Google Scholar]
- Romanko MJ, Rola R, Fike JR, Szele FG, Dizon ML, Felling RJ, et al. Roles of the mammalian subventricular zone in cell replacement after brain injury. Prog Neurobiol. 2004;74(2):77–99. doi: 10.1016/j.pneurobio.2004.07.001. S0301-0082(04)00146-7 [pii] Retrieved from PM:15518954. [DOI] [PubMed] [Google Scholar]
- Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J Leukoc Biol. 2005;78(6):1198–1203. doi: 10.1189/jlb.0405219. Retrieved from PM:16204638. [DOI] [PubMed] [Google Scholar]
- Rothenaigner I, Kramer S, Ziegler M, Wolff H, Kleinschmidt A, Brack-Werner R. Long-term HIV-1 infection of neural progenitor populations. AIDS. 2007;21(17):2271–2281. doi: 10.1097/QAD.0b013e3282f12f27. 00002030-200711120-00003 [pii]. Retrieved from PM:18090275. [DOI] [PubMed] [Google Scholar]
- Royal W, III, Updike M, Selnes OA, Proctor TV, Nance-Sproson L, Solomon L, et al. HIV-1 infection and nervous system abnormalities among a cohort of intravenous drug users. Neurology. 1991;41(12):1905–1910. doi: 10.1212/wnl.41.12.1905. Retrieved from PM:1745346. [DOI] [PubMed] [Google Scholar]
- Rumbaugh JA, Li G, Rothstein J, Nath A. Ceftriaxone protects against the neurotoxicity of human immunodeficiency virus proteins. J Neurovirol. 2007;13(2):168–172. doi: 10.1080/13550280601178218. 778724764 [pii] Retrieved from PM:17505985. [DOI] [PubMed] [Google Scholar]
- Ruzicka BB, Akil H. The interleukin-1beta-mediated regulation of proenkephalin and opioid receptor messenger RNA in primary astrocyte-enriched cultures. Neuroscience. 1997;79(2):517–524. doi: 10.1016/s0306-4522(96)00669-0. [DOI] [PubMed] [Google Scholar]
- Ruzicka BB, Fox CA, Thompson RC, Meng F, Watson SJ, Akil H. Primary astroglial cultures derived from several rat brain regions differentially express μ, δ and kappa opioid receptor mRNA. Molecular Brain Research. 1995;34:209–220. doi: 10.1016/0169-328x(95)00165-o. [DOI] [PubMed] [Google Scholar]
- Sabo JK, Kilpatrick TJ, Cate HS. Effects of bone morphogenic proteins on neural precursor cells and regulation during central nervous system injury. Neurosignals. 2009;17(4):255–264. doi: 10.1159/000231892. 000231892 [pii] Retrieved from PM:19816062. [DOI] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Tumor necrosis factor-alpha at the crossroads of neuronal life and death during HIV-associated dementia. Journal of Neurochemistry. 2003;86(5):1057–1071. doi: 10.1046/j.1471-4159.2003.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11(11):775–787. doi: 10.1038/nri3086. nri3086 [pii] Retrieved from PM:22025055. [DOI] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, et al. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44(3 Pt 1):474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478(7369):382–386. doi: 10.1038/nature10487. nature10487 [pii] Retrieved from PM:21964341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Cali C, Bezzi P. Gliotransmission and the tripartite synapse. Adv Exp Med Biol. 2012;970:307–331. doi: 10.1007/978-3-7091-0932-8_14. Retrieved from PM:22351062. [DOI] [PubMed] [Google Scholar]
- Sarkar K, Das SS, Pal R, Bal B, Madhusudan P, Chakraborti S. HIV infection and host genetic mutation among injecting drug-users of northeastern states of India. J Health Popul Nutr. 2010;28(2):130–136. doi: 10.3329/jhpn.v28i2.4882. Retrieved from PM:20411675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, MacDonald JF, Tymianski M. Distinct roles of synaptic and extrasynaptic NMDA receptors in excitotoxicity. J Neurosci. 2000;20(1):22–33. doi: 10.1523/JNEUROSCI.20-01-00022.2000. Retrieved from PM:10627577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya BE, Deshmane SL, Mukerjee R, Fan S, Khalili K. TNF alpha production in morphine-treated human neural cells is NF-kappaB-dependent. J Neuroimmune Pharmacol. 2009;4(1):140–149. doi: 10.1007/s11481-008-9137-z. Retrieved from PM:19023660. [DOI] [PubMed] [Google Scholar]
- Scheffel J, Regen T, Van RD, Seifert S, Ribes S, Nau R, et al. Toll-like receptor activation reveals developmental reorganization and unmasks responder subsets of microglia. Glia. 2012;60(12):1930–1943. doi: 10.1002/glia.22409. Retrieved from PM:22911652. [DOI] [PubMed] [Google Scholar]
- Scheller C, Sopper S, Jenuwein M, Neuen-Jacob E, Tatschner T, Grunblatt E, et al. Early impairment in dopaminergic neurotransmission in brains of SIV-infected rhesus monkeys due to microglia activation. J Neurochem. 2005;95(2):377–387. doi: 10.1111/j.1471-4159.2005.03373.x. [DOI] [PubMed] [Google Scholar]
- Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 2011;7(10):e1002286. doi: 10.1371/journal.ppat.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [pii] Retrieved from PM:10653597. [DOI] [PubMed] [Google Scholar]
- Schuenke K, Gelman BB. Human microglial cell isolation from adult autopsy brain: brain pH, regional variation, and infection with human immunodeficiency virus type 1. J Neurovirol. 2003;9(3):346–357. doi: 10.1080/13550280390201056. Retrieved from PM:12775418. [DOI] [PubMed] [Google Scholar]
- Schwartz L, Major EO. Neural progenitors and HIV-1-associated central nervous system disease in adults and children. Curr HIV Res. 2006;4(3):319–327. doi: 10.2174/157016206777709438. Retrieved from PM:16842084. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Ricaurte GA. Neurotoxicity of methamphetamine and related drugs. In: Meltzer H, editor. Psychopharmacology: a third generation of progress. New York: Raven; 1987. pp. 359–366. [Google Scholar]
- Seilhean D, Kobayashi K, He Y, Uchihara T, Rosenblum O, Katlama C, et al. Tumor necrosis factor-alpha, microglia and astrocytes in AIDS dementia complex. Acta Neuropathol (Berl) 1997;93(5):508–17. doi: 10.1007/s004010050646. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, et al. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28(22):5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. 28/22/5756 [pii] Retrieved from PM:18509037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina SA, Zaykin DV, Gris P, Ogurtsov AY, Gauthier J, Shibata K, et al. Expansion of the human mu-opioid receptor gene architecture: novel functional variants. Hum Mol Genet. 2009;18(6):1037–1051. doi: 10.1093/hmg/ddn439. Retrieved from PM:19103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, McCarthy KD. Plasticity of astrocytes. Glia. 1994;11:147–155. doi: 10.1002/glia.440110209. [DOI] [PubMed] [Google Scholar]
- Shao Y, Porter JT, McCarthy KD. Neuroligand receptor heterogeneity among astroglia. Perspect Dev Neurobiol. 1994;2(3):205–215. Retrieved from PM:7850353. [PubMed] [Google Scholar]
- Shapshak P, Duncan R, Minagar A, Rodriguez dlV, Stewart RV, Goodkin K. Elevated expression of IFN-gamma in the HIV-1 infected brain. Front Biosci. 2004a;9:1073–1081. doi: 10.2741/1271. Retrieved from PM:14977530. [DOI] [PubMed] [Google Scholar]
- Shapshak P, Duncan R, Torres-Munoz JE, Duran EM, Minagar A, Petito CK. Analytic approaches to differential gene expression in AIDS versus control brains. Front Biosci. 2004b;9:2935–2946. doi: 10.2741/1449. 1449 [pii]. Retrieved from PM:15353327. [DOI] [PubMed] [Google Scholar]
- Shapshak P, Kangueane P, Fujimura RK, Commins D, Chiappelli F, Singer E, et al. Editorial neuroAIDS review. AIDS. 2011;25(2):123–141. doi: 10.1097/QAD.0b013e328340fd42. Retrieved from PM:21076277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Lokensgard JR, Peterson PK. U50,488 inhibits HIV-1 Tat-induced monocyte chemoattractant protein-1 (CCL2) production by human astrocytes. Biochem Pharmacol. 2003;65(1):9–14. doi: 10.1016/s0006-2952(02)01480-6. [DOI] [PubMed] [Google Scholar]
- Shin HD, Winkler C, Stephens JC, Bream J, Young H, Goedert JJ, et al. Genetic restriction of HIV-1 pathogenesis to AIDS by promoter alleles of IL10. Proc Natl Acad Sci USA. 2000;97(26):14467–14472. doi: 10.1073/pnas.97.26.14467. 97/26/14467 [pii]. Retrieved from PM:11121048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo K, Takaori-Kondo A, Kobayashi M, Abudu A, Fukunaga K, Uchiyama T. The enzymatic activity of CEM15/Apobec-3G is essential for the regulation of the infectivity of HIV-1 virion but not a sole determinant of its antiviral activity. J Biol Chem. 2003;278(45):44412–44416. doi: 10.1074/jbc.C300376200. C300376200 [pii]. Retrieved from PM:12970355. [DOI] [PubMed] [Google Scholar]
- Shinoda H, Marini AM, Cosi C, Schwartz JP. Brain region and gene specificity of neuropeptide gene expression in cultured astrocytes. Science. 1989;245:415–417. doi: 10.1126/science.2569236. [DOI] [PubMed] [Google Scholar]
- Singh KK, Ellis RJ, Marquie-Beck J, Letendre S, Heaton RK, Grant I, et al. CCR2 polymorphisms affect neuropsychological impairment in HIV-1-infected adults. Journal of Neuroimmunology. 2004;157(1–2):185–192. doi: 10.1016/j.jneuroim.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Skoff RP. Gliogenesis in the rat optic nerve: Astrocytes are generated in a single wave before oligodendrocytes. Dev Biol. 1990;139:149–168. doi: 10.1016/0012-1606(90)90285-q. [DOI] [PubMed] [Google Scholar]
- Skoff RP, Knapp PE. Division of Astroblasts and Oligodendroblasts in Postnatal Rodent Brain: Evidence for Separate Astrocyte and Oligodendrocyte Lineages. Glia. 1991;4:165–174. doi: 10.1002/glia.440040208. [DOI] [PubMed] [Google Scholar]
- Smith DB, Simmonds P, Bell JE. Brain viral burden, neuroinflammation and neurodegeneration in HAART-treated HIV positive injecting drug users. J Neurovirol. 2014 doi: 10.1007/s13365-013-0225-3. Retrieved from PM:24420447. [DOI] [PubMed] [Google Scholar]
- Smith MW, Dean M, Carrington M, Winkler C, Huttley GA, Lomb DA, et al. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science. 1997;277(5328):959–965. doi: 10.1126/science.277.5328.959. Retrieved from PM:9252328. [DOI] [PubMed] [Google Scholar]
- Snider SE, Vunck SA, Hendrick E, Beardsley PM. Ibudilast and AV1013 attenuate methamphetamine self-administration in rats. International Study Group Investigating Drugs as Reinforcers. 2012:12. [Google Scholar]
- Snider SE, Vunck SA, van den Oord EJ, Adkins DE, McClay JL, Beardsley PM. The glial cell modulators, ibudilast and its amino analog, AV1013, attenuate methamphetamine locomotor activity and its sensitization in mice. Eur J Pharmacol. 2012;679(1–3):75–80. doi: 10.1016/j.ejphar.2012.01.013. Retrieved from PM:22306241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Rahim RT, Davey PC, Bednar F, Bardi G, Zhang L, et al. Protein kinase Czeta mediates micro-opioid receptor-induced cross-desensitization of chemokine receptor CCR5. Journal of Biological Chemistry. 2011;286(23):20354–20365. doi: 10.1074/jbc.M110.177303. Retrieved from PM:21454526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, et al. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA. 2001;98(9):5300–5305. doi: 10.1073/pnas.091039298. 98/9/5300 [pii]. Retrieved from PM:11320258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, et al. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA. 1998;95(13):7699–7704. doi: 10.1073/pnas.95.13.7699. Retrieved from PM:9636213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell ME, Hauser KF. Ligand-gated purinergic receptors regulate HIV-1 Tat and morphine related neurotoxicity in primary mouse striatal neuron-glia co-cultures. J Neuroimmune Pharmacol. 2014;9(2):233–244. doi: 10.1007/s11481-013-9507-z. Retrieved from PM:24158495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani S, Introna M, Bernasconi S, Polentarutti N, Cinque P, Poli G, et al. MCP-1 and CCR2 in HIV infection: regulation of agonist and receptor expression. J Leukoc Biol. 1997;62(1):30–33. doi: 10.1002/jlb.62.1.30. [DOI] [PubMed] [Google Scholar]
- Spruce BA, Curtis R, Wilkin GP, Glover DM. A neuropeptide precursor in cerebellum: Proenkephalin exists in subpopulations of both neurons and astrocytes. EMBO J. 1990;9:1787–1795. doi: 10.1002/j.1460-2075.1990.tb08303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Miller DB, O’Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem. 2006;96(3):706–718. doi: 10.1111/j.1471-4159.2005.03566.x. [DOI] [PubMed] [Google Scholar]
- St Hillaire C, Vargas D, Pardo CA, Gincel D, Mann J, Rothstein JD, et al. Aquaporin 4 is increased in association with human immunodeficiency virus dementia: implications for disease pathogenesis. J Neurovirol. 2005;11(6):535–543. doi: 10.1080/13550280500385203. P44427R11L8828L1 [pii] Retrieved from PM:16338747. [DOI] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309(1):99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123(1–2):60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Hauser KF. Glial growth is regulated by agonists selective for multiple opioid receptor types in vitro. Journal of Neuroscience Research. 1991;29:538–548. doi: 10.1002/jnr.490290415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Knapp PE, Martin KM, Gurwell JA, Ryan S, Thornton SR, et al. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo. Glia. 2001;36(1):78–88. [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in μ, δ, and κ–opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22(3):249–259. [PMC free article] [PubMed] [Google Scholar]
- Sturrock RR. A quantitative lifespan study of changes in cell number, cell division and cell death in various regions of the mouse forebrain. Neuropathol Appl Neurobiol. 1979;5(6):433–456. doi: 10.1111/j.1365-2990.1979.tb00642.x. Retrieved from PM:537673. [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends in Pharmacological Sciences. 2010;31(12):557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990;5(6):797–808. doi: 10.1016/0896-6273(90)90339-h. 0896-6273(90)90339-H [pii]. Retrieved from PM:2268433. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: a review. Neurotox Res. 2000;1(3):181–195. doi: 10.1007/BF03033289. Retrieved from PM:12835101. [DOI] [PubMed] [Google Scholar]
- Sundermann EE, Bishop JR, Rubin LH, Aouizerat B, Wilson TE, Weber KM, et al. HIV serostatus differs by catechol-O-methyltransferase Val158Met genotype. AIDS. 2013;27(11):1779–1782. doi: 10.1097/QAD.0b013e328361c6a1. 00002030-201307170-00011 [pii]. Retrieved from PM:23807274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, El-Hage N, Zou S, Hahn YK, Sorrell ME, Sturgill JL, et al. Fractalkine/CX3CL1 protects striatal neurons from synergistic morphine and HIV-1 Tat-induced dendritic losses and death. Mol Neurodegener. 2011;6(1):78. doi: 10.1186/1750-1326-6-78. Retrieved from PM:22093090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY. Interactions of opioid and chemokine receptors: oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp Cell Res. 2002;280(2):192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]
- Swan N. CDC Report Highlights Link Between Drug Abuse and Spread of HIV. NIDA Notes (AIDS Research) 1997;12(2) Retrieved from http://archives.drugabuse.gov/NIDA_Notes/NNVol12N2/CDCReports.html. [Google Scholar]
- Sweitzer S, De Leo J. Propentofylline: glial modulation, neuroprotection, and alleviation of chronic pain. Handb Exp Pharmacol. 2011;(200):235–250. doi: 10.1007/978-3-642-13443-2_8. Retrieved from PM:20859798. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Schubert P, De Leo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297(3):1210–1217. Retrieved from PM:11356948. [PubMed] [Google Scholar]
- Sykova E. Glia and volume transmission during physiological and pathological states. J Neural Transm. 2005;112:137–147. doi: 10.1007/s00702-004-0120-4. [DOI] [PubMed] [Google Scholar]
- Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88(4):1277–1340. doi: 10.1152/physrev.00027.2007. 88/4/1277 [pii] Retrieved from PM:18923183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, et al. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci USA. 2002;99(16):10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk A, Ewaleifoh O, Beique JC, Wang Y, Knorr D, Haughey N, et al. MMP-7 cleaves the NR1 NMDA receptor subunit and modifies NMDA receptor function. Faseb J. 2008;22(11):3757–3767. doi: 10.1096/fj.07-101402. fj.07-101402 [pii] Retrieved from PM:18644839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait MJ, Saadoun S, Bell BA, Papadopoulos MC. Water movements in the brain: role of aquaporins. Trends Neurosci. 2008;31(1):37–43. doi: 10.1016/j.tins.2007.11.003. S0166-2236(07)00298-6 [pii] Retrieved from PM:18054802. [DOI] [PubMed] [Google Scholar]
- Teuchert-Noodt G, Dawirs RR, Hildebrandt K. Adult treatment with methamphetamine transiently decreases dentate granule cell proliferation in the gerbil hippocampus. Journal of Neural Transmission. 2000;107(2):133–143. doi: 10.1007/s007020050012. [DOI] [PubMed] [Google Scholar]
- Tewari S, Parpura V. A possible role of astrocytes in contextual memory retrieval: An analysis obtained using a quantitative framework. Front Comput Neurosci. 2013;7:145. doi: 10.3389/fncom.2013.00145. Retrieved from PM:24204341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Dwoskin LP, Maragos WF. HIV-1 protein Tat inhibits vesicular monoamine transporter-2 activity in rat striatum. Synapse. 2012;66(8):755–757. doi: 10.1002/syn.21564. Retrieved from PM:22517264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Maragos WF. Involvement of cytokines in human immunodeficiency virus-1 protein Tat and methamphetamine interactions in the striatum. Exp Neurol. 2006a;199(2):490–498. doi: 10.1016/j.expneurol.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Maragos WF. Methamphetamine and human immunodeficiency virus protein Tat synergize to destroy dopaminergic terminals in the rat striatum. Neuroscience. 2006b;137(3):925–935. doi: 10.1016/j.neuroscience.2005.10.056. Retrieved from PM:16338084. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Nath A, Maragos WF. Progress in understanding basal ganglia dysfunction as a common target for methamphetamine abuse and HIV-1 neurodegeneration. Curr HIV Res. 2007;5(3):301–313. doi: 10.2174/157016207780636515. Retrieved from PM:17504172. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. MK-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain Research. 2005;1050(1–2):190–198. doi: 10.1016/j.brainres.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. Journal of Pharmacology and Experimental Therapeutics. 2004;311(1):1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006a;24(3):739–747. doi: 10.1634/stemcells.2005-0281. 2005-0281 [pii] Retrieved from PM:16210404. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006b;24(3):739–747. doi: 10.1634/stemcells.2005-0281. 2005-0281 [pii] Retrieved from PM:16210404. [DOI] [PubMed] [Google Scholar]
- Tian C, Murrin LC, Zheng JC. Mitochondrial fragmentation is involved in methamphetamine-induced cell death in rat hippocampal neural progenitor cells. PLoS ONE. 2009;4(5):e5546. doi: 10.1371/journal.pone.0005546. Retrieved from PM:19436752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44(3 Pt 1):481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Meyers K, Atwood W, Conant K, Major E. Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. J Virol. 1994;68:93–102. doi: 10.1128/jvi.68.1.93-102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore C, Nath A, Amemiya K, Major EO. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991;65(11):6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8(11):e1000527. doi: 10.1371/journal.pbio.1000527. Retrieved from PM:21072242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Khurshid N, Kumar P, Iyengar S. Expression of delta- and mu-opioid receptors in the ventricular and subventricular zones of the developing human neocortex. Neuroscience Research. 2008;61(3):257–270. doi: 10.1016/j.neures.2008.03.002. Retrieved from PM:18455254. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337(6092):358–362. doi: 10.1126/science.1222381. science.1222381 [pii] Retrieved from PM:22745251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Lee CT, Hayashi T, Freed WJ, Su TP. Delta opioid peptide DADLE and naltrexone cause cell cycle arrest and differentiation in a CNS neural progenitor cell line. Synapse. 2010;64(4):267–273. doi: 10.1002/syn.20727. Retrieved from PM:19953654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch I, Ghazaryan N, Mexhitaj I, Ordonez D, Angulo JA. Role of neurokinin-1 and dopamine receptors on the striatal methamphetamine-induced proliferation of new cells in mice. Brain Res. 2011;1399:33–39. doi: 10.1016/j.brainres.2011.05.017. S0006-8993(11)00902-4 [pii] Retrieved from PM:21652034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch IK, Afanador L, Baker L, Ordonez D, Payne H, Mexhitaj I, et al. Methamphetamine Induces Low Levels of Neurogenesis in Striatal Neuron Subpopulations and Differential Motor Performance. Neurotox Res. 2014 doi: 10.1007/s12640-014-9456-1. Retrieved from PM:24549503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga FO, Ding Q, Keller JN, Hauser KF, Knapp PE, et al. Cell-specific actions of HIV-Tat and morphine on opioid receptor expression in glia. Journal of Neuroscience Research. 2008;86(9):2100–2110. doi: 10.1002/jnr.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga FO, Gupta S, Keller JN, Knapp PE, Hauser KF, et al. Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation. J Neurochem. 2009;108:202–215. doi: 10.1111/j.1471-4159.2008.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Liu Y, Gartner S, Reid R, Jie C, Peng X, et al. Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and L-deprenyl. Neurobiol Dis. 2006;23(1):109–119. doi: 10.1016/j.nbd.2006.02.005. Retrieved from PM:16697650. [DOI] [PubMed] [Google Scholar]
- Turner JR, Ecke LE, Briand LA, Haydon PG, Blendy JA. Cocaine-related behaviors in mice with deficient gliotransmission. Psychopharmacology(Berl) 2013;226(1):167–176. doi: 10.1007/s00213-012-2897-4. Retrieved from PM:23104263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallat AV, De Girolami U, He J, Mhashilkar A, Marasco W, Shi B, et al. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152(1):167–78. [PMC free article] [PubMed] [Google Scholar]
- Van Horn MR, Sild M, Ruthazer ES. D-serine as a gliotransmitter and its roles in brain development and disease. Front Cell Neurosci. 2013;7:39. doi: 10.3389/fncel.2013.00039. Retrieved from PM:23630460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle G, Antony JM, Silva C, Sullivan A, Power C. Aberrant cortical neurogenesis in a pediatric neuroAIDS model: neurotrophic effects of growth hormone. AIDS. 2005;19(16):1781–1791. doi: 10.1097/01.aids.0000189854.06194.87. [DOI] [PubMed] [Google Scholar]
- Vargova L, Jendelova P, Chvatal A, Sykova E. Glutamate, NMDA, and AMPA induced changes in extracellular space volume and tortuosity in the rat spinal cord. J Cereb Blood Flow Metab. 2001;21(9):1077–1089. doi: 10.1097/00004647-200109000-00005. [DOI] [PubMed] [Google Scholar]
- Venkatesan A, Nath A, Ming GL, Song H. Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci. 2007;64(16):2120–2132. doi: 10.1007/s00018-007-7063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A, Uzasci L, Chen Z, Rajbhandari L, Anderson C, Lee MH, et al. Impairment of adult hippocampal neural progenitor proliferation by methamphetamine: role for nitrotyrosination. Mol Brain. 2011;4:28. doi: 10.1186/1756-6606-4-28. 1756-6606-4-28 [pii] Retrieved from PM:21708025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C. Extracellular membrane microvesicles and nanotubes in the brain: understanding their nature, their function in cell-to-cell communication, their role in transcellular spreading of pathological agents and their therapeutic potential. Front Physiol. 2013;4:163. doi: 10.3389/fphys.2013.00163. Retrieved from PM:23847543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Muzio L, Turola E, Bergami A, Novellino L, Ruffini F, et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Annals of Neurology. 2012;72(4):610–624. doi: 10.1002/ana.23627. Retrieved from PM:23109155. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10(3):241–252. doi: 10.1016/S1474-4422(10)70325-2. S1474-4422(10)70325-2 [pii] Retrieved from PM:21349439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Parpura V. Astroglia in neurological diseases. Future Neurol. 2013;8(2):149–158. doi: 10.2217/fnl.12.90. Retrieved from PM:23658503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21(23):9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. 21/23/9414 [pii]. Retrieved from PM:11717374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annual Review of Pharmacology and Toxicology. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. Retrieved from PM:21961707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6(8):626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Research. 1980;181(1):151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu F, Liu YY, Zhao CH, You Y, Wang L, et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011;21(11):1534–1550. doi: 10.1038/cr.2011.83. cr201183 [pii] Retrieved from PM:21577236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127(Pt 11):2452–2458. doi: 10.1093/brain/awh269. Retrieved from PM:15319273. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Plane JM, Jiang P, Zhou CJ, Deng W. Concise review: Quiescent and active states of endogenous adult neural stem cells: identification and characterization. Stem Cells. 2011;29(6):907–912. doi: 10.1002/stem.644. Retrieved from PM:21557389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, et al. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312(1):60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108(6):1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. JNC5886 [pii] Retrieved from PM:19154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, et al. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193(8):905–915. doi: 10.1084/jem.193.8.905. Retrieved from PM:11304551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2(6):699–703. doi: 10.1038/nm0696-699. Retrieved from PM:8640565. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kish SJ. The vesicular monoamine transporter, in contrast to the dopamine transporter, is not altered by chronic cocaine self-administration in the rat. Journal of Neuroscience. 1996;16(10):3507–3510. doi: 10.1523/JNEUROSCI.16-10-03507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler C, Modi W, Smith MW, Nelson GW, Wu X, Carrington M, et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. ALIVE Study, Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC) Science. 1998;279(5349):389–393. doi: 10.1126/science.279.5349.389. Retrieved from PM:9430590. [DOI] [PubMed] [Google Scholar]
- Wong AW, McCallum GP, Jeng W, Wells PG. Oxoguanine glycosylase 1 protects against methamphetamine-enhanced fetal brain oxidative DNA damage and neurodevelopmental deficits. J Neurosci. 2008;28(36):9047–9054. doi: 10.1523/JNEUROSCI.2557-08.2008. 28/36/9047 [pii] Retrieved from PM:18768699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Hung CJ, Shen CH, Chen WY, Chang CY, Pan HC, et al. Prenatal buprenorphine exposure decreases neurogenesis in rats. Toxicol Lett. 2014;225(1):92–101. doi: 10.1016/j.toxlet.2013.12.001. S0378-4274(13)01457-4 [pii] Retrieved from PM:24321744. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM. A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain. J Pharmacol Exp Ther. 2009;330(1):316–325. doi: 10.1124/jpet.109.153775. jpet.109.153775 [pii] Retrieved from PM:19364908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu M, Bolan E, Gilbert AK, Pasternak GW, Pan YX. Isolating and characterizing three alternatively spliced mu opioid receptor variants: mMOR-1A, mMOR-1O, and mMOR-1P. Synapse. 2014;68(4):144–152. doi: 10.1002/syn.21727. Retrieved from PM:24375714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovleva T, Bazov I, Watanabe H, Hauser KF, Bakalkin G. Transcriptional control of maladaptive and protective responses in alcoholics: a role of the NF-kappaB system. Brain Behav Immun. 2011;25(Suppl 1):S29–S38. doi: 10.1016/j.bbi.2010.12.019. Epub@2010 Dec 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Duan M, Yang L, Buch S. Platelet-derived growth factor-BB restores human immunodeficiency virus Tat-cocaine-mediated impairment of neurogenesis: role of TRPC1 channels. J Neurosci. 2012;32(29):9835–9847. doi: 10.1523/JNEUROSCI.0638-12.2012. 32/29/9835 [pii] Retrieved from PM:22815499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Kim K, Duan M, Hayashi T, Guo M, Morgello S, et al. Cocaine hijacks sigma1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J Neurosci. 2011;31(16):5942–5955. doi: 10.1523/JNEUROSCI.5618-10.2011. 31/16/5942 [pii] Retrieved from PM:21508219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Yang Y, Kim KJ, Bethel-Brown C, Gong N, Funa K, et al. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115(23):4951–4962. doi: 10.1182/blood-2010-01-266221. blood-2010-01-266221 [pii] Retrieved from PM:20354174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y, Lee C, Liu QH, Freedman BD, Collman RG. Chemokine receptor utilization and macrophage signaling by human immunodeficiency virus type 1 gp120: Implications for neuropathogenesis. J Neurovirol. 2004;10(Suppl 1):91–96. doi: 10.1080/753312758. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kawada K, Gotoh Y, Shiba T, Ogita K. Endogenous reactive oxygen species are essential for proliferation of neural stem/progenitor cells. Neurochem Int. 2010;56(6–7):740–746. doi: 10.1016/j.neuint.2009.11.018. S0197-0186(09)00323-4 [pii] Retrieved from PM:19958807. [DOI] [PubMed] [Google Scholar]
- You ZB, Herrera-Marschitz M, Nylander I, Goiny M, Kehr J, Ungerstedt U, et al. Effect of morphine on dynorphin B and GABA release in the basal ganglia of rats. Brain Research. 1996;710(1–2):241–248. doi: 10.1016/0006-8993(95)01402-0. [DOI] [PubMed] [Google Scholar]
- Yuan CJ, Quiocho JM, Kim A, Wee S, Mandyam CD. Extended access methamphetamine decreases immature neurons in the hippocampus which results from loss and altered development of neural progenitors without altered dynamics of the S-phase of the cell cycle. Pharmacology, Biochemistry and Behavior. 2011;100(1):98–108. doi: 10.1016/j.pbb.2011.08.004. S0091–3057(11)00270-X [pii] Retrieved from PM:21855565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Arnatt CK, El-Hage N, Dever SM, Jacob JC, Selley DE, et al. A bivalent ligand Targeting the putative mu opioid receptor and chemokine receptor CCR5 heterodimers: binding affinity versus functional activities. Medchemcomm. 2013;4(5):847–851. doi: 10.1039/C3MD00080J. Retrieved from PM:23682308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Arnatt CK, Li G, Haney KM, Ding D, Jacob JC, et al. Design and synthesis of a bivalent ligand to explore the putative heterodimerization of the mu opioid receptor and the chemokine receptor CCR5. Org Biomol Chem. 2012;10(13):2633–2646. doi: 10.1039/c2ob06801j. Retrieved from PM:22354464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Butelman ER, Ho A, Morgello S, Kreek MJ. Neurocognitive and neuroinflammatory correlates of PDYN and OPRK1 mRNA expression in the anterior cingulate in postmortem brain of HIV-infected subjects. J Neuroinflammation. 2014;11(1):5. doi: 10.1186/1742-2094-11-5. 1742-2094-11-5 [pii] Retrieved from PM:24405578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Levran O, Proudnikov D, Nielsen DA, Kreek MJ. Search for genetic markers and functional variants involved in the development of opiate and cocaine addiction and treatment. Ann NY Acad Sci. 2010;1187:184–207. doi: 10.1111/j.1749-6632.2009.05275.x. Retrieved from PM:20201854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zamecnik J, Homola A, Cicanic M, Kuncova K, Marusic P, Krsek P, et al. The extracellular matrix and diffusion barriers in focal cortical dysplasias. Eur J Neurosci. 2012;36(1):2017–2024. doi: 10.1111/j.1460-9568.2012.08107.x. Retrieved from PM:22536791. [DOI] [PubMed] [Google Scholar]
- Zhang L, Looney D, Taub D, Chang SL, Way D, Witte MH, et al. Cocaine opens the blood-brain barrier to HIV-1 invasion. J Neurovirol. 1998;4(6):619–626. doi: 10.3109/13550289809114228. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20(5):588–594. doi: 10.1016/j.conb.2010.06.005. Retrieved from PM:20655735. [DOI] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Mactutus CF, Booze RM. Recombinant human immunodeficiency virus-1 transactivator of transcription1-86 allosterically modulates dopamine transporter activity. Synapse. 2011;65(11):1251–1254. doi: 10.1002/syn.20949. Retrieved from PM:21538554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009;329(3):1071–1083. doi: 10.1124/jpet.108.150144. jpet.108.150144 [pii] Retrieved from PM:19325033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF, et al. Nov 18, 2011 [Epub ahead of print]). Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at μ-opioid receptor-expressing glia. Brain. 2011;134(12):3613–3628. doi: 10.1093/brain/awr281. Retrieved from http://brain.oxfordjournals.org.proxy.library.vcu.edu/content/early/2011/11/16/brain.awr281.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development [see comments] Nature. 1998;393(6685):595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22(12):5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. 22/12/5100 [pii]. Retrieved from PM:12077205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman MD, Boyer EW. HIV and club drugs in emerging adulthood. Curr Opin Pediatr. 2012;24(2):219–224. doi: 10.1097/MOP.0b013e32834faa9b. Retrieved from PM:22227785. [DOI] [PubMed] [Google Scholar]