Summary
Oxidative stress and increased apoptosis are implicated in the pathogenesis of many disorders of pregnancy, including preeclampsia (PE) and fetal growth restriction (FGR). Since the transcription factor FOXO1 (forkhead box protein O1) is implicated in the regulation of a variety of cellular processes, including resistance to oxidative stress, apoptosis and morphogenesis of the placenta, we examined whether FOXO1 expression is abnormal in placentas from patients with PE or FGR. Paracentral sections from grossly unremarkable areas of 9 or 10 placentas each from early third trimester patients (31.7±5.0 weeks) with mild PE, severe PE, FGR and a gestational age-matched comparison group (GA controls) were double immunostained for FOXO1 and E-cadherin, the latter distinguishing villous cytotrophoblast cells (CTB) from syncytiotrophoblast (STB). The numbers of FOXO1-positive and FOXO1 negative STB and CTB nuclei were determined on ten 20× objective fields of each placenta section by three observers who were blinded to the clinical outcome. The results were evaluated by a generalized linear mixed model. In mild PE, FOXO1-positive STB nuclei were significantly decreased in number and FOXO1-negative STB nuclei were increased as compared to GA controls. However, the number of FOXO1-positive and FOXO1-negative CTB nuclei were not significantly changes as compared to GA controls. In severe PE and FGR, the numbers of FOXO-positive and FOXO1-negative STB and CTB were not statistically different from GA controls. Since FOXO1 is critical for placental cellular morphogenesis, abnormal FOXO1 expression may contribute in part to the abnormal trophoblast differentiation in mild PE. The differences in FOXO1 expression in mild and severe PE are consistent with other studies suggesting that the two forms of PE are different disease processes.
Keywords: Transcription factor, Immunohistochemistry, Villous trophoblast
Introduction
The transcription factors and other signaling molecules involved in the pathogenesis of placental disorders of human pregnancy are poorly understood. Since many studies implicate oxidative stress and increased apoptosis in the pathogenesis of several pathologic conditions of pregnancy, including preeclampsia (PE) (DiFederico et al., 1999; Ishihara et al., 2002; Yang et al., 2012) and fetal growth restriction (FGR) (Ishihara et al., 2002; Yung et al., 2008; Burton et al., 2009; Sharp et al., 2010,), we recently initiated studies to examine whether transcription factors that are important in the regulation of oxidative stress and apoptosis are abnormally expressed in PE and FGR placentas (Sheridan et al., 2012). In the current investigation, we focused on another transcription factor FOXO1. FOXO1 is expressed in the STB of chorionic villi and its acetylated (ac-FOXO1) and phosphorylated (p-FOXO1) forms are expressed in both STB and CTB (Lappas et al., 2009). In addition to being implicated in the regulation of oxidative stress and apoptosis, FOXO1 is implicated in the regulation of a variety of other cellular processes that are critical for the placenta, including cell cycle regulation, cellular differentiation and proliferation, DNA repair, and metabolism (Kitamura and Ido Kitamura, 2007; Kousteni, 2011; Monsalve and Olmos, 2011). FOXO1 is regulated in turn by oxidative stress, which promotes the transcriptional activity and protein stability of FOXO1 (Kousteni, 2011) and induces the phosphorylation, translocation to the nucleus, and acetylation-deacetylation of the transcription factor (Furukawa-Hibi et al., 2005). A possible role for FOXO1 in PE and other pathologic conditions of the placenta is further suggested by the recent observation that FOXO1 expression in the developing mouse embryo is essential for placental morphogenesis (Ferdous et al., 2011). PE is heterogeneous in its presentation and has been subclassified by clinical severity into mild and severe forms as determined by maternal and fetal characteristics (Cunningham et al., 2005). The link between the pathophysiology of abnormal placentation and the physiology of the maternal syndrome remains unclear, but oxidative stress is felt to be an important factor. The presence of chronic ischemia is essentially restricted to cases of severe preterm preeclampsia that are generally not present in the more common term or postpartum cases, suggesting that the pathogenesis of late onset preeclampsia and severe preterm preeclampsia are different.
FGR manifests as a variable syndrome of suboptimal growth and body disproportions rather than a specific well-defined entity (Scifres and Nelson, 2009). The causes are diverse and include aneuploidy and non-aneuploid syndromes, infections, metabolic factors and placental disorders (Scifres and Nelson, 2009). While the pathologic features of the FGR placenta have been studied extensively, very little is known about the cellular and molecular events involved in the pathogenesis of FGR.
We report here immunohistochemical analysis of villous trophoblasts cells from mainly early third trimester placentas that demonstrate that the total number of villous trophoblast nuclei that express FOXO1 is significantly less in mild PE placentas compared to GA control placentas, severe PE and FGR placentas. The findings suggest that mild and severe PE result from different pathologic processes (Roberts and Post, 2008).
Materials and methods
Inclusion criteria
The protocol for this research was approved by the institutional review boards of Cincinnati Children's Hospital Medical Center and the University of Cincinnati. Archival paraffin blocks of placentas from patients with mild PE, severe PE and FGR were selected from the tissue repository of the Department of Pathology of the University of Cincinnati Medical Center. The groups were matched for gestational age but were otherwise randomly selected from the database. A total of 37 placentas were chosen: 10 mild PE placentas and 9 each of severe PE, FGR and GA controls. 7 cases in control group, 7 cases in mild preeclampsia, 7 cases in severe preeclampsia, and 6 cases in fetal growth restriction were pre-term, the remaining cases being term. The placenta pathology reports and archived hematoxylin and eosin (H&E) slides were re-reviewed, and relevant gross, placental weight, and histologic findings were recorded. Clinical data were extracted from electronic medical records and diagnoses confirmed by chart review. The diagnosis of mild PE was based on the presence of blood pressure >140/90 mm Hg but less than 160/110 mm Hg, measured on at least 2 different occasions more than 12 hours apart, in a woman who was normotensive before 20 weeks' gestation, as well as the presence of proteinuria (>300 mg and <5 g/24h) (Lain and Roberts, 2002; Cunningham et al., 2005). Severe PE was defined as blood pressure >160/110 mm Hg and urinary protein excretion >5 g/24h. The mild and severe PE groups did not contain mothers who delivered infants with FGR. Placentas from infants whose weights were below the 10th percentile for gestational age and whose mothers did not have history of PE, chronic hypertension (HTN), or diabetes mellitus (DM) were included in the FGR group (Alexander et al., 1996). Thus the mild and severe PE groups did not contain mothers who delivered FGR infants; and the FGR group did not contain mothers with mild or severe PE. The comparison group consisted of gestational age-matched placentas (31.1±5.1 weeks gestation) from nonhypertensive, nonproteinuric, nondiabetic patients who delivered infants appropriate for gestational age following the spontaneous onset of labor (term delivery, 2 patients; spontaneous-onset preterm delivery, 7 patients).
Microscopy and immunohistochemistry
Microscopic and immunohistochemical studies were performed using paraffin blocks from grossly unremarkable paracentral areas of each placenta that had been previously fixed in 10% buffered formaldehyde solution. H&E-stained sections were used for routine microscopy. Evaluated histologic parameters included villous maturation, histologic evidence of acute ascending infection (acute chorioamnionitis, funisitis, and/or chorionic plate vasculitis), evidence of vascular lesions (decidual arteriolopathy, infarction, retroplacental hematoma, thrombosis in fetal vessels, and multifocal clusters of avascular villi), and chronic inflammation (decidual plasma cell infiltrates and chronic villitis of unknown etiology) (Kraus et al., 2004; Baergen, 2005; Redline, 2008; Stanek and Biesiada, 2014). Diffuse chronic hypoxic injuries were classified as preuterine, uterine or postuterine, based on previously reported criteria (Kingdom and Kaufmann, 1997; Stanek, 2012, 2013). Other placental lesions related to chronic placental hypoxia, such as excessive amount of extravillous trophoblast cells, microscopic chorionic pseudocysts, and clusters of multinucleated giant cells in decidua, were classified according to previously described criteria (Kraus et al., 2004; Stanek, 2013). Representative microphotographs of GA control, mild PE, severe PE and FGR placentas from early third trimester are shown in an earlier report from our laboratory (Sheridan et al., 2012).
Double immunostaining of the placenta slides with anti-FOXO1 and anti-E-cadherin antibodies was performed using a protocol identical to that previously used by us to study placental AP-2α expression (Sheridan et al., 2012) except that anti-FOXO1 serum was used in place of the anti-AP-2α serum. Briefly, 4-µm sections were mounted on Superfrost Plus slides and then stained using the automated Ventana immunostainer (Ventana Medical Systems, Tucson, AZ). The following protocol was used: deparaffinization, cell conditioning with EDTA for 60 minutes, incubation with primary rabbit polyclonal antibody anti–FOXO1 (Santa Cruz, Dallas TX, catalogue number sc-11350, working concentration 200 ug/ml) at 37°C with a dilution of 1:25 for 40 minutes, followed by basic Ultraview DAB detection system (Ventana Medical Systems) without any counterstaining or bluing agent. The anti- E-cadherin (Cell Marque, Rocklin, CA, catalogue number EP700Y, working concentration 200 ug/ml) primary monoclonal rabbit antibody is then applied at 37°C with a dilution of 1:50 for 92 minutes, followed by RedMap detection system (Ventana Medical Systems). The slides were then rinsed with reaction buffer and counterstained with hematoxylin for 4 minutes, followed by post counterstaining with bluing reagent for 4 minutes. The FOXO1 rabbit polyclonal antiserum was raised against amino acids 471–598 of human FOXO1 and is highly specific for FOXO1 (information provided by Santa Cruz). The optimal dilution for both primary antibodies was first worked up separately and then that information was applied to the dual protocol. Appropriate positive (term human placenta, human lung) and negative controls (non-immune serum, pre-diluted, Ventana Medical Systems were used for the anti-FOXO1 immunostaining. The negative control was run in the same fashion using the UltraView detection system, but instead of adding e-cadherin and FOXO1, the negative primary antibody (Ventana, catalog #760–2014) was applied.
Data analysis
Microscopic examinations were independently performed by three pathologists (R.S., C.B. and J.S.), who were blinded to the disease status. Ten random 40× objective fields (HPFs) from each transmural paracentral placental section without infarctions, intervillous thrombus, or massive perivillous fibrin deposition were examined for the numbers of chorionic villi and syncytial knots, total numbers of CTB and STB nuclei, and FOXO1-positive and FOXO1-negative CTB and STB nuclei per HPF. Because STB are multinucleated and the number of STB vary among HPF, FOXO1-positive and FOXO1-negative STB nuclei per HPR were expressed as a percentage of the total number of cells, total number of STB and total number of CTB per HPF. Syncytial knots (multinucleated protrusions from the villous surface with 10 or more nuclei (Kraus et al., 2004) were excluded from the total STB nuclei count. E-cadherin immunostaining (red membrane staining) highlights the entire CTB cell membrane (apical, lateral and basal surfaces) and the basal boundary of STB, thus distinguishing the 2 trophoblastic cell types (STB and CTB) from themselves and from all the other cells of the chorionic villous, such as fibroblasts, endothelial cells, and Hofbauer cells, which did not stain. It is conceivable that the basal boundary of STB may represent cytoplasmic extensions of CTB cells (Longtime et al., 2012), however for the purpose of this analysis this distinction was not attempted. Therefore, only mononuclear cells with apical, lateral and basal expression of e-cadherin in the cytoplasmic membranes were interpreted as being CTB. FOXO1-positive CTB (brown nuclear staining) featured FOXO1 nuclear and E-cadherin (red) membranous staining in the same cell (double positivity). FOXO1-positive STB featured FOXO1 (brown) nuclear and E-cadherin (red) positive staining present only in the basal boundary of STB. Nuclei were interpreted as negative or positive only, and intensity of positive staining was not scored. STB/CTB nuclei ratio and percentage of FOXO1-positive CTB per total number of CTB or FOXO1-positive STB nuclei per total number of STB nuclei were used as end points for analysis. Values were expressed as mean or geometric mean, as appropriate, and 95% confidence interval. Data were initially examined for deviation from normality. The average numbers of STB and CTB per HPF, and the percentage of FOXO1-negative STB nuclei, FOXO1-positive CTB, FOXO1-negative CTB, total CTB and ratio positive CTB/ total cells were transformed using the natural log for analysis. Untransformed raw data were used for other analyses. The analyses were performed using SAS, version 9.3 (SAS Institute, Cary, NC). Generalized linear models were used for analysis to account for the multiple readers (3) and multiple slides (10) per placenta. Further analysis was performed incorporating delivery method as a covariate to examine the potential effect on any differences that might be evident between the groups. All analyses were done assuming unequal variance, and using a Tukey-Kramer adjustment was used to account for the multiple post-hoc comparisons between groups. Statistical significance was assumed at p<0.05.
Statistical differences between selected clinical (Table 1) and pathologic (Table 2) features of the four placenta groups were examined using Fisher’s Exact test, due to the small numbers. A Bonferroni correction was used to correct for multiple testing. Analysis of variance was used to compare gestational week and placental weight between the groups, Dunnett’s test was used for poc-hoc analysis of individual group differences. Statistical significance was assumed at p<0.05.
Table 1.
Selected clinical features of study patients.
| CLINICAL FEATURES | GA control (N=9) | MPE (N=10) | SPE (N=9) | FGR (N=9) | p-value |
|---|---|---|---|---|---|
| Gestational age at delivery (average in weeks ± SD) | 31.1±5.1 | 32.1±4.8 | 30.9±4.7 | 31.3±5.3 | 0.95 |
| Premature rupture of membranes | 4 | 0 | 0 | 1 | 0.02 |
| Abnormal cardiotocographic findings | 1 | 5 | 5 | 2 | 0.14 |
| Perinatal (fetal) mortality | 0 | 1 | 0 | 1 | 1.00 |
| Cesarean section | 3 | 6 | 5 | 1 | 0.15 |
| Antepartum hemorrhage | 0 | 3 | 1 | 0 | 0.16 |
| Meconium-stained amniotic fluid | 1 | 2 | 2 | 0 | 0.72 |
| Induction of labor | 0 | 2 | 1 | 1 | 0.89 |
| Multiple fetuses | 0 | 2 | 1 | 0 | 0.59 |
Fisher’s exact test indicated that premature rupture of the membranes between the different placenta groups was statistically different (p=0.02). The other clinical features did not differ significantly among the groups.
Table 2.
Selected pathologic features of placentas.
| PLACENTAL FEATURES | GA control (N=9) | MPE (N=10) | SPE (N=9) | FGR (N=9) | P value |
|---|---|---|---|---|---|
| Placental weight (g, average + SD) | 305±135 | 347±109 | 281±113 | 257±78 | 0.37 |
| Choriodecidual hemosiderosis | 2 | 0 | 1 | 1 | 0.43 |
| Meconium macrophages | 2 | 0 | 1 | 1 | 0.43 |
| Acute chorioamnionitis | 4 | 3 | 2 | 0 | 0.18 |
| Laminar necrosis of membranes | 3 | 1 | 1 | 0 | 0.29 |
| X-cell microscopic cysts of membranes | 0 | 3 | 2 | 1 | 0.46 |
| Non-marginal infarction (> 5% of placental disc) | 1 | 6 | 3 | 4 | 0.18 |
| Perivillous fibrin deposition | 1 | 0 | 0 | 1 | 0.59 |
| Chronic villitis of unknown etiology | 2 | 1 | 2 | 0 | 0.52 |
| Multiple luminal vascular abnormalities of chorionic villi | 1 | 1 | 2 | 1 | 0.92 |
| Fibrosis | 0 | 1 | 1 | 0 | 1.00 |
| Intervillous thrombus | 0 | 1 | 1 | 2 | 0.72 |
| Hypertrophic decidual arteriolopathy | 1 | 8 | 8 | 1 | <0.001 |
| Acute atherosis | 1 | 1 | 3 | 0 | 0.29 |
| Multinucleated trophoblastic giant cells in decidua basalis | 0 | 3 | 4 | 0 | 0.03 |
| Intimal cushioning of stem or chorionic veins | 0 | 0 | 3 | 0 | 0.03 |
| Excessive amount of extravillous trophoblasts | 0 | 4 | 1 | 1 | 0.12 |
| Features of global hypoxia | 0 | 4 | 8 | 2 | <0.001 |
| Preuterine | 0 | 2 | 0 | 2 | 0.30 |
| Uterine | 0 | 2 | 8 | 0 | <0.001 |
| Postuterine | 0 | 0 | 0 | 0 | 1.0 |
Statistical differences between the groups for each pathologic feature was determined by Fisher’s exact test. Hypertrophic decidual arteriolopathy: MPE & SPE different from GA control & FGR, p<0.001. Multinucleated trophoblastic giant cells in decidua basalis: no individual differences, p>0.05. Intimal cushioning of stem or chorionic veins: no individual differences, p>0.05. Features of global hypoxia: SPE different from GA control & FGR, p<0.05. Uterine features of global hypoxia: SPE different from GA control, MPE & FGR, p<0.05
Results
Clinical and placental factors
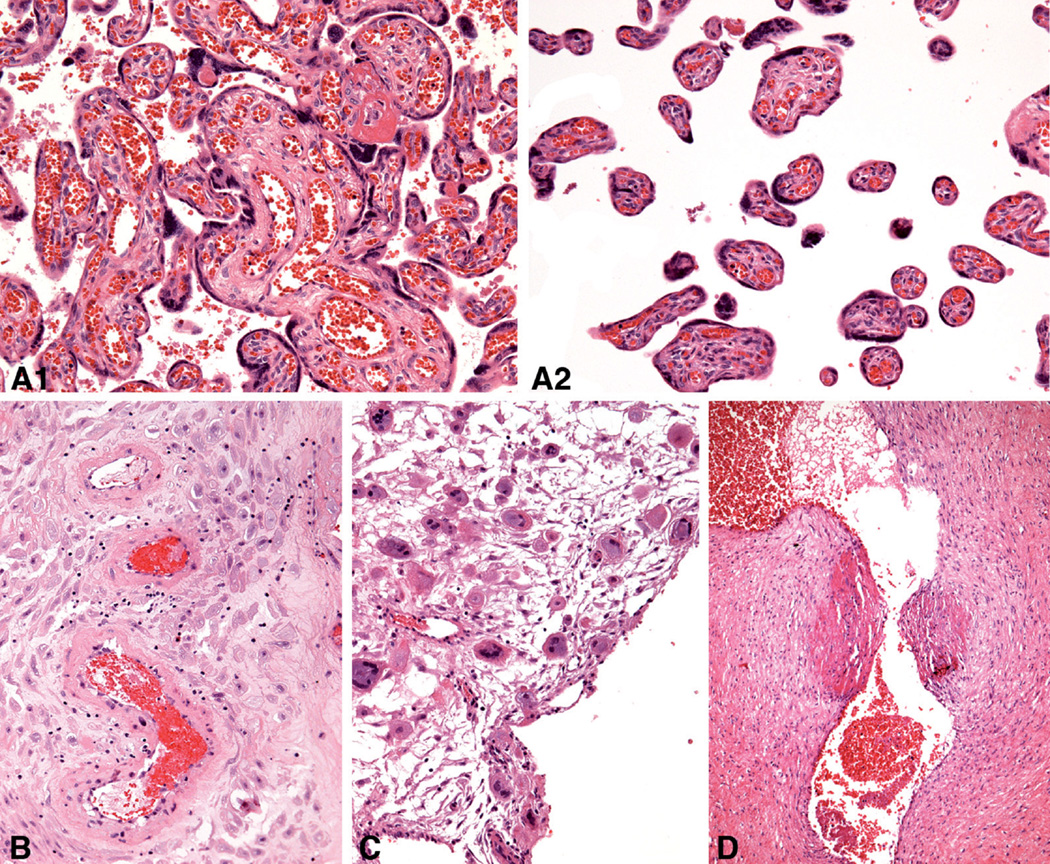
Some of the clinical and pathologic features of the GA control, mild PE, severe PE and FGR groups are shown in Tables 1 and 2. The mean gestational ages of the 4 groups of placentas (mean 31.4±4.8 weeks, range 25–39 weeks) were well matched and not significantly different between the groups. In addition, the mean placenta weights of the four groups were not statistically different, although the mean weight of the mild PE placentas was greater and the mean weight of the FGR placentas was less than the GA control placentas. As anticipated, abnormal cardiotocographic findings and cesarean delivery were more frequent in the mild and severe PE patients than in the GA control patients (Table 1). Histologic evidence of chronic hypoxic placental injury of the uterine type was observed in 8 of the 9 severe PE placentas and 2 of 10 mild PE placentas (Table 2). In contrast, this type of chronic hypoxic placental injury was not observed in any of the GA control or FGR placentas. Mild and severe PE placentas showed changes related to uteroplacental malperfusion, including hypertrophic decidual arteriolopathy, fetal thrombotic vasculopathy, increased placental site giant cells, and intimal cushioning of stem or chorionic veins. Histological placental lesions/patterns that were statistically significantly different among the groups are illustrated in Fig. 1.
Fig. 1.
Placental lesions for which statistically significant differences were found among 4 groups on hematoxylin-eosin stained sections: A. Uterine pattern of chronic hypoxic placental injury (1 and 2, representative different areas in same placenta), 27 weeks. B. Hypertrophic arteriolopathy in decidua parietalis, 37 weeks. C. Trophoblastic giant cells in decidua basalis, 26 weeks. D. Intimal cushions in a stem vein, 31 weeks. A–C,×20; D,×10
FOXO1 immunohistochemistry
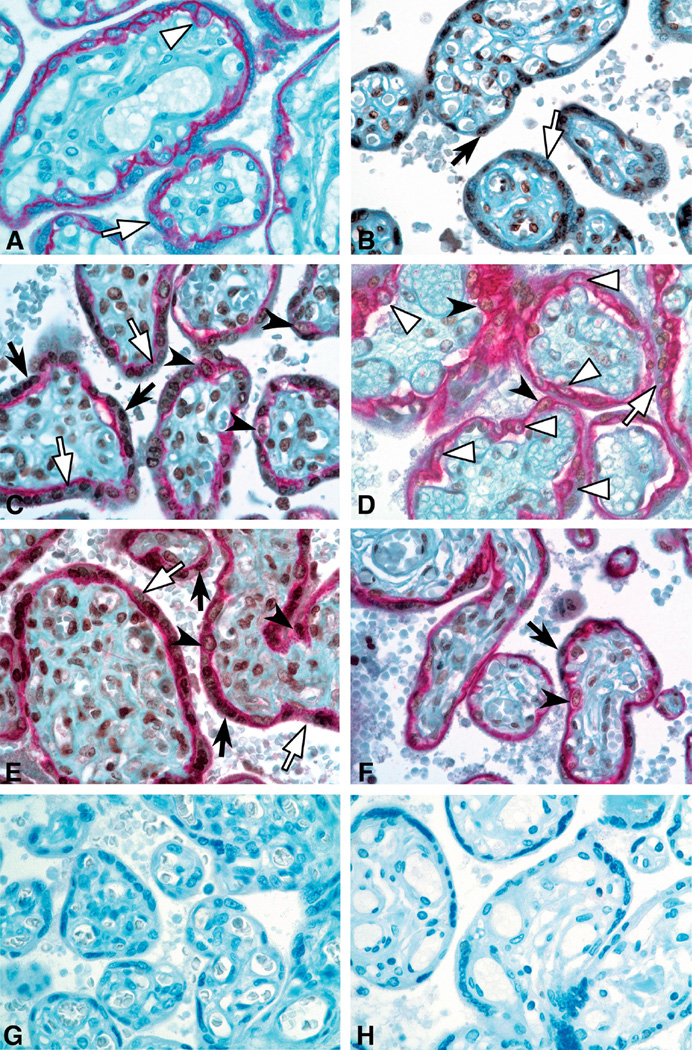
Representative sections of FOXO1 and E-cadherin localization in GA control, mild PE, severe PE and FGR placental villi are shown in Fig. 2. E-cadherin staining distinguished the mononuclear CTB from the multinucleated STB.
Fig. 2.
Immunohistochemical staining for FOXO1 (brown) and E-cadherin (red) in placental villi (40× magnification). A. E-cadherin antibody only: E-cadherin clearly distinguishes villous CTB, which are mononuclear cells encircled by red membranous staining from STB, which form a mononuclear syncytium with only the basal boundary of STB stained red. B. FOXO1 antibody only: positive cells display brown nuclear staining. Double immunostaining for FOXO1 and E-cadherin in gestational age matched control case (C), mild PE (D), severe PE (E) and FGR (F) placentas at 31 weeks gestation. Empty arrowheads point to FOXO1-negative CTB; solid arrowheads point to FOXO1-positive CTB nuclei; empty arrows point to FOXO1-negative STB nuclei; and solid arrows point to FOXO1-positive STB nuclei. (G, H) Negative control for ecadherin and FOXO1 antibodies, respectively.×40
As shown in Table 3, quantitative immunohistochemistry revealed no statistical differences in the total numbers of trophoblast nuclei (CTB and STB combined), STB nuclei and CTB nuclei among the GA control and pathologic placentas. However, FOXO1 immunostaining revealed that the total number of FOXO1-positive nuclei in the mild PE group was 35.4% less than in the GA control group (49.6 nuclei/HPF vs. 76.8 nuclei/HPF, p=0.0007); and the total number of FOXO1-negative nuclei in the mild PE group was 134.3% greater than in the GA control group (10.8 nuclei/HPF, p=0.0004). In contrast, the total numbers of FOXO1-positive and FOXO1-negative nuclei in the severe PE and FGR placenta groups were not different than those in the GA control group.
Table 3.
FOXO1 localization in trophoblast nuclei of GA control, mild PE, severe PE and FGR placentas.
| Mean and 95% CI | p-value | ||||||
|---|---|---|---|---|---|---|---|
| Variable | group | mean or geo mean | Lower 95% CI | Upper 95% CI | FGR | Mild PE | Severe PE |
| Total trophoblastic nuclei | GA control | 115.5 | 102.3 | 128.8 | 0.53 | 1.00 | 0.23 |
| FGR | 102.7 | 88.9 | 116.5 | 0.55 | 0.99 | ||
| Mild PE | 114.8 | 104.1 | 125.6 | 0.16 | |||
| Severe PE | 99.9 | 91.4 | 108.4 | ||||
| STB nuclei | GA control | 91.2 | 80.4 | 102.1 | 0.48 | 0.90 | 0.21 |
| FGR | 80.5 | 70.2 | 90.8 | 0.78 | 0.99 | ||
| Mild PE | 86.6 | 79.5 | 93.8 | 0.35 | |||
| Severe PE | 78.6 | 72.6 | 84.7 | ||||
| CTB nuclei | GA control | 16.9 | 12.0 | 23.7 | 0.99 | 0.94 | 0.99 |
| FGR | 17.9 | 14.0 | 22.9 | 0.98 | 0.91 | ||
| Mild PE | 19.0 | 15.3 | 23.6 | 0.72 | |||
| Severe PE | 15.7 | 11.8 | 20.9 | ||||
| Positive STB nuclei | Control | 76.8 | 68.3 | 85.3 | 0.23 | 0.0007 | 0.13 |
| IUGR | 63.7 | 52.9 | 74.6 | 0.24 | 1.00 | ||
| Mild PE | 49.6 | 40.3 | 58.9 | 0.05 | |||
| Severe PE | 64.6 | 58.3 | 70.9 | ||||
| Negative STB nuclei | Control | 10.8 | 8.1 | 14.4 | 0.99 | 0.0004 | 0.86 |
| IUGR | 11.6 | 8.6 | 15.5 | 0.002 | 0.83 | ||
| Mild PE | 25.3 | 19.7 | 32.5 | <0.0001 | |||
| Severe PE | 9.3 | 7.3 | 11.9 | ||||
| Positive CTB nuclei | Control | 12.0 | 8.6 | 16.7 | 0.99 | 0.34 | 0.65 |
| IUGR | 11.3 | 7.8 | 16.1 | 0.53 | 0.83 | ||
| Mild PE | 8.0 | 5.7 | 11.1 | 0.94 | |||
| Severe PE | 9.1 | 6.6 | 12.5 | ||||
| Negative CTB nuclei | Control | 4.1 | 2.7 | 5.9 | 1.00 | 0.06 | 0.95 |
| Mild PE | 7.8 | 5.7 | 10.7 | 0.20 | |||
| Severe PE | 4.7 | 3.1 | 6.9 | ||||
| Positive STB nuclei/total nuclei | GA control | 0.68 | 0.63 | 0.72 | 0.26 | <0.0001 | 0.84 |
| FGR | 0.60 | 0.54 | 0.67 | 0.01 | 0.62 | ||
| Mild PE | 0.44 | 0.36 | 0.51 | <0.0001 | |||
| Severe PE | 0.65 | 0.61 | 0.69 | ||||
| Positive STB nuclei/ total STB nuclei | GA control | 0.83 | 0.80 | 0.86 | 0.17 | <0.0001 | 0.95 |
| FGR | 0.74 | 0.67 | 0.82 | 0.04 | 0.3 | ||
| Mild PE | 0.57 | 0.47 | 0.67 | 0.0001 | |||
| Severe PE | 0.82 | 0.79 | 0.85 | ||||
| Positive CTB nuclei/ total nuclei | GA control | 0.11 | 0.08 | 0.14 | 1.00 | 0.28 | 0.90 |
| FGR | 0.11 | 0.08 | 0.15 | 0.25 | 0.83 | ||
| Mild PE | 0.07 | 0.05 | 0.10 | 0.61 | |||
| Severe PE | 0.09 | 0.07 | 0.12 | ||||
| Positive CTB nuclei/ total CTB nuclei | GA control | 0.74 | 0.68 | 0.81 | 0.81 | 0.002 | 0.14 |
| FGR | 0.70 | 0.60 | 0.79 | 0.06 | 0.76 | ||
| Mild PE | 0.52 | 0.43 | 0.61 | 0.26 | |||
| Severe PE | 0.63 | 0.56 | 0.71 | ||||
| Positive trophoblastic nuclei / total trophoblastic nuclei | GA control | 0.81 | 0.77 | 0.85 | 0.35 | <0.0001 | 0.5 |
| FGR | 0.74 | 0.66 | 0.82 | 0.02 | 0.91 | ||
| Mild PE | 0.55 | 0.46 | 0.64 | 0.0004 | |||
| Severe PE | 0.77 | 0.73 | 0.81 | ||||
Columns 3–5 depict the mean or geometric mean (CTB and SCB positive nuclei/ total nuclei) and the lower and higher 95% confidence levels for the cells and ratios in each placenta group. Columns 6–8 show the p values comparing the means or geometric means of the individual row values to the group indicated in the same row of column 2. P values <0.05 are bolded.
The percent of FOXO-1 positive STB nuclei in the mild PE group was also significantly less than in the GA control placentas. In the GA control placentas, 85% of the total trophoblast nuclei and 87% of the total STB nuclei were FOXO1-positive. In contrast, in the mild PE placentas, only 51% of the total trophoblast nuclei and 56% of the total STB were FOXO1-positive (p=0.001 in each instance). Thus the ratio of FOXO1-positive STB nuclei to total STB nuclei in mild PE placentas was 35.6% less than in the GA control group, indicating that the decrease in FOXO1-positive STB in mild PE is due to a relative reduction in the percent of STB that are FOXO1-positive and not to a reduction in the total amount of STB nuclei. In contrast, the ratio of FOXO1-positive STB to total STB nuclei in the severe PE and FGR placentas was not different than that in the GA control placentas. Analyses incorporating delivery method as a covariate in the model yielded similar results as the model without delivery method, allowing the same conclusions to be drawn regarding group differences.
Discussion
The results of this study indicate that FOXO1 expression in villous trophoblast nuclei of mild PE placentas from early in the third trimester (31.7±5 weeks) is significantly less than that in GA control placentas, regardless of the mode of delivery. However, villous trophoblast expression of FOXO1 in nuclei from early third trimester placentas from patients with severe PE or FGR was not different from the GA controls. The decreased FOXO1 expression in mild PE is not due to a reduced number of STB or CTB nuclei but is due rather to a decrease in the percentage of STB and CTB nuclei that express FOXO1. The amount of FOXO1 staining in the nuclei of mild PE and control placentas did not appear to be different, suggesting that the FOXO1 expression in the positive nuclei of the two groups of placentas was identical or nearly identical. However, the possibility that individual FOXO1-positive nuclei from the two groups of placentas express different amounts of the transcription factor cannot be excluded. Numerous studies have demonstrated that mode of delivery affects many aspects of the placenta, including metabolic parameters, the generation of oxidative and other stresses and the activation of signaling pathways and gene transcription (Burton et. al., 2014). However, statistical analyses taking mode of delivery into account as a covariate indicate that mode of delivery has no significant effect on the conclusions of this study.
In an earlier report, Lappas and coworkers observed that FOXO1 mRNA and protein levels in five PE placentas delivered vaginally at term were not different than those in GA control placentas (Lappas et al., 2009). The PE placentas were presumably obtained from women with mild PE although the magnitudes of the hypertension and proteinuria in these patients were not stated and the microscopic findings of the placentas were not presented. Taken together, our findings and those of Lappas suggest that the abnormally decreased FOXO1 expression in mild PE placentas is gestational age-dependent, normalizing at term. It is possible, however, that the difference in FOXO1 expression in the two studies is due to differences in methodology. Our study focused on FOXO1 expression localized in CTB and STB nuclei of the villous trophoblast layer by immunohistochemistry (IHC), while Lappas and coworkers examined FOXO1 protein and mRNA in whole placenta tissue that includes both nuclear and cytoplasmic FOXO1 as well as FOXO1 in non-villous trophoblast.
We demonstrated in an earlier study that the expression of the transcription factor AP-2α in villous trophoblast, as determined by immunohistochemistry (IHC), is also significantly decreased in mild PE but not in severe PE placentas. Decreased AP-2α expression by IHC was also noted in pregnancies complicated by fetal growth restriction, diabetes mellitus and chronic hypertension. Taken together, our studies indicate that mild PE placentas are characterized by decreased expression of both FOXO1 and AP-2a, while severe PE placentas are characterized by normal FOXO1 and AP-2a expression. FGR placentas differ from both the mild and severe PE placentas, with decreased AP-2a expression but normal FOXO1 expression.
The decreased expression of FOXO1 and AP-2α in mild PE placentas cannot be attributed to hypoxia and oxidative stress alone since placentas from severe PE and FGR pregnancies have characteristic morphological features of hypoxia. These striking differences in FOXO1 and AP-2α expression between mild and severe PE suggests that these two conditions not only are clinically diverse but also may have different pathophysiologic mechanisms. Previous studies have also noted significant differences in other parameters between the two forms of PE (Vatten and Skjaerven, 2004; Sebire et al., 2005; Roberts and Hubel, 2009; Nelson et al., 2014). Preterm PE is strongly associated with low birth weight; whereas term PE (>37 weeks) may be associated with unaffected infants, small- or even large-for-gestational-age infants, indicating variable degrees of placental dysfunction (Vatten and Skjaerven, 2004).
The reasons for the decreased FOXO1 and AP-2α expression are unknown. The question whether the differences in FOXO1 and AP-2α expression indicate an early intrinsic placental defect or an acquired abnormality secondary to systemic factors that suppress FOXO1 and AP-2α expression, and/or accelerated maternal hypertension remains to be answered. A recent study on global gene expression profile of 4 first-trimester placentas from patients who eventually developed PE approximately 6 months later in pregnancy revealed significant dysregulation of gene expression (Founds et al., 2008, 2009). Serum biomarkers of PE have been shown to be altered at as early as 7 weeks' gestation, indicating that an intrinsic abnormality of the placenta in PE is present much earlier than the onset of decreased flow of maternal blood, when failure of transformation of spiral arteries is thought to occur in PE (Roberts and Hubel, 2009).
PE was initially classified into severe and mild forms based primarily on maternal blood pressure measurements and proteinuria, followed by Doppler velocimetry and placental histology. However, genetic and molecular studies now indicate that PE is more complex and that severe and mild PE may represent different disease processes (Sheridan et al., 2012; Staff et al., 2013). Our current results demonstrating differences in FOXO1 and AP-2α expression in villous trophoblast between mild and severe PE placentas are also in keeping with this concept The various forms of PE may indeed result, at least in part, from differences in the villous trophoblast transcriptome.
Many studies have shown that both FOXO1 (McLoughlin et al., 2009; Alikhani et al., 2010) and AP-2α (Li et al., 2010; Zhang et al., 2012) stimulate apoptosis. Since the expressions of these transcription factors are decreased in mild PE vs severe PE, it is possible that the differences between the two forms of PE are due, at least in part, to the differences in FOXO1- and AP-2α-mediated apoptosis. However, additional studies are necessary to delineate the mechanistic roles for these transcription factors in PE and to determine whether decreased FOXO1 and AP-2α expression in villous trophoblast plays a role in the pathogenesis of mild PE.
Acknowledgements
We thank Betsy DiPasquale for technical assistance. This research was presented in part at the 25th European Congress of Pathology, Lisbon, Portugal, August 31-September 4, 2013.
References
- Alikhani M, Roy S, Graves DT. FOXO1 plays an essential role in aoptosis of retinal pericytes. Mol. Vis. 2010;16:408–415. [PMC free article] [PubMed] [Google Scholar]
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet. Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Baergen RN. Manual of Benirschke and Kaufmann’s pathology of the human placenta. New York, NY: Springer; 2005. [Google Scholar]
- Brosens JJ, Wilson MS, Lam EW. FOXO transcription factors: from cell fate decisions to regulation of human female reproduction. Adv. Exp. Med. Biol. 2009;665:227–241. doi: 10.1007/978-1-4419-1599-3_17. [DOI] [PubMed] [Google Scholar]
- Brown LM, Lacey HA, Baker PN, Crocker IP. E-cadherin in the assessment of aberrant placental cytotrophoblast turnover in pregnancies complicated by pre-eclampsia. Histochem. Cell Biol. 2005;124:499–506. doi: 10.1007/s00418-005-0051-7. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30(Suppl A):S43–S48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Sebire NJ, Myatt L, Tannetta D, Wang Y-L, Sadovsky Y, Staff AC, Redman CW. Optimising sample collection for placenta research. Placenta. 2014;35:9–22. doi: 10.1016/j.placenta.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Gilstrap LC, III, Wenstrom KD. In: Williams obstetrics. 22nd ed. Seils A, Edmonson KG, Davis K, editors. New York, NY: McGraw-Hill; 2005. [Google Scholar]
- Christian M, Lam EW, Wilson MS, Brosens JJ. FOXO transcription factors and their role in disorders of the female reproductive tract. Curr. Drug Targets. 2011;12:1291–1302. doi: 10.2174/138945011796150253. [DOI] [PubMed] [Google Scholar]
- DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am. J. Pathol. 1999;155:293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdous A, Morris J, Abedin MJ, Collins S, Richardson JA, Hill JA. Forkhead factor FoxO1 is essential for placental morphogenesis in the developing embryo. Proc. Natl. Acad. Sci. USA. 2011;108:16307–16312. doi: 10.1073/pnas.1107341108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Founds SA, Dorman JS, Conley YP. Microarray technology applied to the complex disorder of preeclampsia. J. Obstet. Gynecol. Neonatal. Nurs. 2008;37:146–157. doi: 10.1111/j.1552-6909.2008.00232.x. [DOI] [PubMed] [Google Scholar]
- Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30:15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid. Redox. Signal. 2005;7:752–760. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Nijland MJ, Knoblich P. Placental ischemia and cardiovascular dysfunction in preeclampsia and beyond: making the connections. Expert Rev. Cardiovasc. Ther. 2008;6:1367–1377. doi: 10.1586/14779072.6.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guller S, LaChapelle L. The role of placental Fas ligand in maintaining immune privilege at maternal-fetal interfaces. Semin. Reprod. Endocr. 1999;17:39–44. doi: 10.1055/s-2007-1016210. [DOI] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ. Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am. J. Obstet. Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- Kingdom JCP, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18:613–821. doi: 10.1016/s0143-4004(97)90000-x. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Ido Kitamura Y. Role of FoxO proteins in pancreatic beta cells. Endocr. J. 2007;54:507–515. doi: 10.1507/endocrj.kr-109. [DOI] [PubMed] [Google Scholar]
- Kousteni S. FoxOs: Unifying links between oxidative stress and skeletal homeostasis. Curr. Osteoporos. Rep. 2011;9:60–66. doi: 10.1007/s11914-011-0054-3. [DOI] [PubMed] [Google Scholar]
- Kraus FT, Redline RW, Gersell DJ, Nelson DM, Dicke JM. AFIP atlas of nontumor pathology: placental pathology. Washington, DC: American Registry of Pathology; 2004. Circulatory problems: thrombi and other vascular lesions; pp. 117–162. [Google Scholar]
- Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia. JAMA. 2002;287:3183–3186. doi: 10.1001/jama.287.24.3183. [DOI] [PubMed] [Google Scholar]
- Lappas M, Lim R, Riley C, Rice GE, Permezel M. Localisation and expression of FoxO1 proteins in human gestational tissues. Placenta. 2009;30:256–262. doi: 10.1016/j.placenta.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Li X, Glubrecht DD, Godbout R. AP2 transcription factor induces apoptosis in retinoblastoma cells. Genes Chromosom. Cancer. 2010;49:819–830. doi: 10.1002/gcc.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtime MS, Chen B, Odibo AO, Zhong Y, Nelson DM. Caspase-mediated apoptosis of trophoblasts in term human placental villi is restricted to cytotrophoblast and absent from the multinucleated syncytiotrophoblast. Reproduction. 2012;143:107–121. doi: 10.1530/REP-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin TJ, Smith SM, DeLong AD, Wang H, Unterman TG, Esser KA. FoxO1 induces apoptosis in skeletal myotubes in a DNA-binding-dependent manner. Am. J. Phiol. Cell Physiol. 2009;297:C548–C555. doi: 10.1152/ajpcell.00502.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsalve M, Olmos Y. The complex biology of FOXO. Curr. Drug Targets. 2011;12:1322–1350. doi: 10.2174/138945011796150307. [DOI] [PubMed] [Google Scholar]
- Nelson DB, Ziadie MS, McIntire DD, Rogers BB, Leveno KJ. Placental pathology suggesting that preeclampsia is more than one disease. Am. J. Obstet. Gynecol. 2014;210:66e1–66e7. doi: 10.1016/j.ajog.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Redline RW. Placental pathology: a systematic approach with clinical correlations. Placenta. 2008;29(Suppl A):S86–S91. doi: 10.1016/j.placenta.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J. Clin. Pathol. 2008;61:1254–1260. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32–S37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scifres CM, Nelson DM. Intrauterine growth restriction, human placental development and trophoblast cell death. J. Physiol. 2009;587:3453–3458. doi: 10.1113/jphysiol.2009.173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebire NJ, Goldin RD, Regan L. Term preeclampsia is associated with minimal histopathological placental features regardless of clinical severity. J. Obstet. Gynaecol. 2005;25:117–118. doi: 10.1080/014436105400041396. [DOI] [PubMed] [Google Scholar]
- Sharp AN, Heazell AE, Crocker IP, Mor G. Placental apoptosis in health and disease. Am J Reprod Immunol. 2010;64:159–169. doi: 10.1111/j.1600-0897.2010.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan RM, Stanek J, Khoury J, Handwerger S. Abnormal expression of transcription factor activator protein-2alpha in pathologic placentas. Hum. Pathol. 2012;43:1866–1874. doi: 10.1016/j.humpath.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff AC, Benton SJ, von Dadelszen P, Roberts JM, Taylor RN, Powers RW, Charnock-Jones DS, Redman CW. Redefining preeclampsia using placenta-derived biomarkers. Hypertension. 2013;61:932–942. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- Stanek J. Utility of diagnosing various histological patterns of diffuse chronic hypoxic placental injury. Pediatr. Dev. Pathol. 2012;15:13–23. doi: 10.2350/11-03-1000-OA.1. [DOI] [PubMed] [Google Scholar]
- Stanek J. Hypoxic patterns of placental injury: a review. Arch. Pathol. Lab. Med. 2013;137:706–720. doi: 10.5858/arpa.2011-0645-RA. [DOI] [PubMed] [Google Scholar]
- Stanek J, Biesiada J. Relation of placental diagnosis in stillbirth to fetal maceration and gestational age at delivery. J. Perinat. Med. 2014;42:457–471. doi: 10.1515/jpm-2013-0219. [DOI] [PubMed] [Google Scholar]
- Vatten LJ, Skjaerven R. Is pre-eclampsia more than one disease? BJOG. 2004;111:298–302. doi: 10.1111/j.1471-0528.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Yang X, Guo L, Li H, Chen X, Tong X. Analysis of the original causes of placental oxidative stress in normal pregnancy and pre-eclampsia: a hypothesis. J. Matern. Fetal Neonatal Med. 2012;25:884–888. doi: 10.3109/14767058.2011.601367. [DOI] [PubMed] [Google Scholar]
- Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones DS, Burton GJ. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am. J. Pathol. 2008;173:451–462. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jia L, Cui S, Shi Y, Chang A, Wang P, Zhang Z. AP-2α-dependent regulation of Bcl-2/Bax expression affects apoptosis in the trophoblast. J. Mol. Histol. 2012;43:681–689. doi: 10.1007/s10735-012-9439-6. [DOI] [PubMed] [Google Scholar]