Abstract
Unlike antibody-mediated rejection (AMR) with clinical features, it remains unclear whether subclinical AMR should be treated, as its effect on allograft loss is unknown. It is also uncertain if AMR’s effect is homogeneous across donor (deceased/live) and (HLA/ABO) antibody types. We compared 219 patients with AMR (77 subclinical, 142 clinical) to controls matched on HLA/ABO-compatibility, donor type, prior transplant, PRA, age, and year. One and 5-year graft survival in subclinical AMR was 95.9% and 75.7%, compared to 96.8% and 88.4% in matched controls (P=0.0097). Subclinical AMR was independently associated with a 2.15-fold increased risk of graft loss (95%CI: 1.19–3.91; P=0.012) compared to matched controls, but not different from clinical AMR (P=0.13). 53.2% of subclinical AMR patients were treated with plasmapheresis within 3 days of their AMR-defining biopsy. Treated subclinical AMR patients had no difference in graft loss compared to matched controls (HR 1.73;95%CI: 0.73–4.05; P=0.21), but untreated subclinical AMR patients did (HR3.34;95%CI: 1.37–8.11; P=0.008). AMR’s effect on graft loss was heterogeneous when stratified by compatible deceased donor (HR=4.73;95%CI:1.57–14.26; P=0.006), HLA-incompatible deceased donor (HR=2.39;95%CI:1.10–5.19; P=0.028), compatible live donor (no AMR patients experienced graft loss), ABO-incompatible live donor (HR=6.13;95%CI:0.55–67.70; P=0.14), and HLA-incompatible live donor (HR=6.29;95%CI:3.81–10.39; P<0.001) transplant. Subclinical AMR substantially increases graft loss, and treatment seems warranted.
INTRODUCTION
Over 30% of the kidney transplant waitlist is comprised of sensitized patients (PRA>20%) [1]. In an effort to transplant these vulnerable patients, advances in desensitization protocols and kidney paired donation have promoted the widespread adoption of incompatible kidney transplantation at centers across the United States [2–5]. While antibody-mediated rejection (AMR) occurs in approximately 5% of compatible kidney transplants, the incidence seems to be significantly higher in incompatible kidney transplant recipients, as high as 40% in some reports [6–8]. As incompatible kidney transplantation and re-transplantation become more common [9], the incidence of antibody-mediated rejection (AMR) can only be expected to increase.
AMR has long been known to threaten graft survival. However, severity of presentation varies from subclinical rejection that is found only on protocol biopsies to severe, imminently graft-threatening rejection [10–12]. While the implications of developing AMR in the latter group on graft loss are clear, the effect of subclinical AMR on graft loss is less well defined. Studies to date have suggested that patients with a subclinical presentation of AMR have poorer graft function and worse pathological findings on subsequent allograft biopsies [10, 11], though an association between subclinical AMR and increased graft loss has not been directly established. Furthermore, while the magnitude of AMR’s effect on graft loss is estimated to range from 1.53 (95% CI: 1.21–1.91) to 4.58 (95% CI: 1.75–12.00) [13, 14], it remains unknown, both epidemiologically and mechanistically, if AMR differentially affects graft outcomes depending upon donor compatibility and donor type (deceased versus live donor). Indeed, the 2013 Banff Conference on Allograft Pathology reported that, for the first time, there will be a Banff Working Group formed to evaluate possible differences between non-sensitized patients who develop AMR and patients requiring desensitization who develop AMR [15].
The objective of this study was to understand the risk of allograft loss associated with AMR using a well-defined set of clinicopathologic criteria. Specifically, we sought to quantify the risk of graft loss associated with a clinical versus subclinical presentation of AMR and the risk of graft loss by the type of transplant (e.g., compatible deceased donor, HLA-incompatible deceased donor, compatible live donor, HLA-incompatible live donor, and ABO-incompatible live donor).
METHODS
Study Population
We studied all adult (≥18 years of age), kidney-only transplants performed at the Johns Hopkins Hospital from January 2000 through December 2012 (n=2,316) for the development of biopsy-proven AMR in the first-year post-transplant. Transplant type was categorized as: compatible deceased donor, HLA-incompatible deceased donor, ABO-incompatible live donor, compatible live donor, and HLA-incompatible live donor. HLA-incompatible live donor recipients were defined as those who had detectable anti-HLA DSA (or anti-angiotensin or anti-endothelial antibodies [n=6]) at any strength (single-bead, flow cytometric crossmatch, or cytotoxic crossmatch) that necessitated perioperative desensitization therapy [4]. Deceased donor HLA-incompatible recipients were those who had the presence of anti-HLA DSA identified prior to or at the time of transplant, either by single-bead assay or flow cytometric crossmatch, but with a negative cytotoxic crossmatch (anti-human globulin-enhanced). In other words, these patients had antibody strength at the level of a positive flow cytometric crossmatch or lower. Patients who were ABO-incompatible with their donor and also had anti-HLA DSA were studied as members of the HLA-incompatible live donor group. This study was approved by the Johns Hopkins Medical Institutions Institutional Review Board.
Peak PRA/CPRA
Because of Organ Procurement and Transplantation Network (OPTN) policy changes from PRA to CPRA in 2009, we combined all information available about PRA and/or CPRA, and those missing data (2.8%) were imputed to have the mean PRA/CPRA for patients of their same transplant type (e.g., compatible deceased donor, HLA-incompatible deceased donor, ABO-incompatible live donor, compatible live donor, HLA-incompatible live donor).
Performance of Biopsies
Ultrasound-guided needle allograft biopsies were performed as previously described [16]. Occasionally, biopsies were performed during an open re-operation under direct visualization. Protocol biopsies were performed at 1, 3, 6, and 12 months following ABO- and HLA-incompatible live donor transplants, but not routinely after deceased donor transplants or compatible live donor transplants. C4d staining was performed on frozen tissue using indirect immunofluorescence with anti-human C4d antibody (Quidel, San Diego, CA) at 1:40 dilution followed by secondary antibody (fluorescein isothiocyanate–conjugated goat anti-mouse IgG; Jackson Immunoresearch Laboratories, West Grove, PA) or on paraffin-embedded tissue sections using a rabbit polyclonal anti-human antibody (American, Pfungstadt, Germany) at a 1:50 dilution, in concert with a biotin-free polymer detection system (Leica, Wetzlar, Germany).
Diagnosis of AMR
The diagnosis of AMR occurring in the first year post-transplant was based on the 2013 international Banff Classification Criteria [15] and defined as the presence of circulating DSA and either: 1) peritubular capillary staining of C4d and at least one of the following: ptc score>0, g score>0, acute thrombotic microangiopathy in the absence of any other cause, or other features consistent with AMR (endothelial injury, fibrin thrombi, microinfarctions, interstitial hemorrhage), or 2) absence of capillary staining of C4d and the presence of ptc>0 and g>0 or ptc>0 or g>0 and acute thrombotic microangiopathy, in the absence of any other cause of thrombotic microangiopathy. Index episodes of AMR were also noted to be clinical or subclinical. Clinical episodes of AMR were defined as those that had evidence of graft dysfunction, manifested as oliguria/anuria, an increase in serum creatinine by >20% from baseline, treatment of cell-mediated rejection and/or thrombotic microangiopathy within the two prior weeks, the need for hemodialysis >7 days post-transplant, or new onset proteinuria at the time of the AMR-defining biopsy. Transplant glomerulopathy (cg) was measured using Banff histological parameters and defined as the presence (cg>1) or absence (cg=0) of transplant glomerulopathy [15] on biopsies performed 6 months (+3 months) and 12 months (+3 months) after the AMR-defining biopsy. To account for attrition, we created a composite of graft loss and presence of transplant glomerulopathy (versus neither graft loss nor presence of transplant glomerulopathy) in evaluating the relationship between AMR presentation, antibody class and subsequent transplant glomerulopathy.
Treatment of AMR
At our institution, we consider plasmapheresis to be the mainstay of treatment for AMR, although we do occasionally use adjunctive measures such as anti-CD20 therapy and complement inhibition. In the interest of homogeneity, patients were considered to be treated for AMR if they received plasmapheresis within 3 days of their AMR-defining biopsy.
Matched Controls Selection
Patients who developed AMR were matched to patients from our institution who did not develop AMR using iterative expanding radius matching as previously described [4, 17, 18]. AMR patients were matched in a 1:3 ratio on HLA-compatibility, ABO-compatibility, donor type (deceased versus live), history of previous transplant, peak PRA/CPRA, age at transplant, and year of transplant.
Outcome Ascertainment
Death-censored graft survival (DCGS) was defined as the time between date of transplant and either date of graft failure (marked by retransplantation, relisting, or a return to dialysis) or last date of follow-up with a functioning graft, censoring for death and administrative end of study. Death and graft failure ascertainment were augmented through linkage with the Social Security Death Master File and the Centers for Medicare and Medicaid Services by the SRTR. The SRTR includes information on all donors, wait-listed transplant candidates, and transplant recipients in the U.S. provided by members of the OPTN, and has been well described elsewhere [19]. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
Statistical Analysis
Between-group characteristics were compared using chi-square test for categorical variables, ANOVA for normally distributed continuous variables, and the Wilcoxon rank sum test for non-normally distributed continuous variables. Death-censored graft survival was estimated using the Kaplan-Meier method and compared between groups using log-rank testing and Cox models. Proportional hazards assumptions were confirmed by visual inspection of complimentary log-log plots. As a sensitivity analysis, AMR was treated as a time-varying covariate to account for immortal-time bias between the date of transplantation and the time until the AMR-defining biopsy. Analyses were performed using Stata 12.1/SE (Stata Corp, College Station, TX). A two-tailed p-value less than 0.05 was considered statistically significant.
Propensity Score Analysis
As a sensitivity analysis, AMR patients were compared with non-AMR controls matched on propensity for developing AMR. Propensity scores were generated using R (R Foundation for Statistical Computing, Vienna, Austria) with random forest methods, and 1:1 matches were assigned using nearest neighbor matching. Subsequent analyses were performed with Stata. Graft survival was estimated using the Kaplan-Meier method and compared between AMR patients and propensity score-matched non-AMR controls using log-rank testing and Cox models. There was no difference in the inferences of the sensitivity analysis and those of the main analyses described above (i.e., subclinical AMR versus AMR-free propensity score-matched patients, treated subclinical AMR versus AMR-free propensity score-matched patients, untreated subclinical AMR versus AMR-free propensity score-matched patients).
RESULTS
Study Population
Of 2,316 patients who underwent kidney-only transplantation during the study period, 219 developed biopsy-proven AMR (9.5%) at a median of 15 days post-transplant (IQR: 8–46). The overall mean follow-up time was 5.2 years (SD 3.5). The mean follow-up time was 4.7 years (SD 3.6) in compatible deceased donor transplant recipients, 3.9 years (SD 3.0) in HLA-incompatible deceased donor transplant recipients, 6.2 years (SD 3.5) in compatible live donor transplant recipients, 4.9 years (SD 3.0) in ABO-incompatible live donor transplant recipients, and 4.4 years (SD 3.0) in HLA-incompatible live donor transplant recipients. AMR patients were more frequently female (58.9% versus 42.9%; P<0.001), had a higher median peak PRA/CPRA (93 versus 2; P<0.001), spent more time on dialysis prior to the transplant (56.2% on dialysis ≥6 years versus 18.1%; P<0.001), and were more likely to have undergone a previous transplant (55.2% versus 16.7%; P<0.001) (Table 1). AMR patients were more likely to have been the recipients of HLA-incompatible live donor transplants (63.5% versus 7.3%), ABO-incompatible live donor transplants (5.0% versus 3.3%), and HLA-incompatible deceased donor transplants (21.0% versus 2.7%), and less likely to have been recipients of compatible live donor transplants (2.7% versus 38.6%) and compatible deceased donor transplants (7.8% versus 48.0%) (P<0.001).
Table 1.
Patient Characteristics of the Overall Cohort, the Antibody-Mediated Rejection Group (AMR) by Presentationa, and the AMR-Free Matched Controlsb
| Non-AMR Patients (n=2,097) | P- Valuec | AMR Patients | P-Valued | Matched Controls (n=231) c | P-Valuee | |||
|---|---|---|---|---|---|---|---|---|
| Overall (n=219) | Subclinical AMR (n=77) | Clinical AMR (n=142) | ||||||
| Transplant Type | <0.001 | 0.027 | 0.69 | |||||
| Deceased Donor | ||||||||
| Compatible | 48.0% | 7.8% | 1.3% | 11.3% | 90.9% | |||
| HLA-Incompatible | 2.7% | 21.0% | 22.1% | 20.4% | 74.2% | |||
| Live Donor | ||||||||
| Compatible | 38.6% | 2.7% | 5.2% | 1.4% | 63.6% | |||
| ABO-Incompatible | 3.3% | 5.0% | 2.6% | 6.3% | 75.0% | |||
| HLA-Incompatible | 7.3% | 63.5% | 68.8% | 60.6% | 75.0% | |||
| Age at Transplant (SD) | 50.8 (14.2) | <0.001 | 45.8 (13.4) | 44.8 (12.2) | 46.3 (14.1) | 0.43 | 45.5 (10.8) | 0.67 |
| Female | 42.9% | <0.001 | 58.9% | 61.0% | 57.7% | 0.64 | 64.1% | 0.63 |
| African-American | 34.8% | 0.003 | 24.7% | 23.4% | 25.3% | 0.75 | 27.7% | 0.46 |
| Public Insurance | 45.2% | <0.001 | 65.7% | 64.9% | 66.2% | 0.74 | 49.3% | 0.018 |
| BMI (SD) | 27.5 (5.9) | <0.001 | 25.9 (6.1) | 26.0 (6.7) | 25.9 (5.7) | 0.95 | 26.3 (6.3) | 0.72 |
| Cause of ESRD | <0.001 | 0.36 | 0.68 | |||||
| Glomerular Diseases | 23.9% | 38.4% | 35.1% | 40.1% | 34.2% | |||
| Diabetes Mellitus | 18.6% | 9.6% | 6.5% | 11.3% | 10.0% | |||
| Hypertensive Nephrosclerosis | 31.1% | 18.3% | 15.6% | 19.7% | 15.6% | |||
| Polycystic Kidney Disease | 9.8% | 9.6% | 10.4% | 9.1% | 12.5% | |||
| Renovascular & Other Vascular Diseases | 0.7% | 3.2% | 3.9% | 2.8% | 1.3% | |||
| Other or Missing (includes Tubular and Congenital) | 15.8% | 21.0% | 28.6% | 16.9% | 26.4% | |||
| Peak PRA/CPR (IQR) | 2 (0–33) | <0.001 | 93 (68–100) | 93 (85–100) | 92 (64–100) | 0.16 | 94 (83–100) | 0.89 |
| Previous Transplant | 16.7% | <0.001 | 55.2% | 61.0% | 52.1% | 0.20 | 57.6% | 0.91 |
| Zero HLA-mismatch | 7.9% | <0.001 | 0.9% | 0.0% | 1.4% | 0.29 | 1.7% | 0.24 |
| Time on Dialysis Prior to Recent Transplant | <0.001 | 0.10 | 0.20 | |||||
| Preemptive | 17.9% | 5.9% | 9.1% | 4.2% | 7.8% | |||
| ≤2years | 31.9% | 14.2% | 13.0% | 14.8% | 16.4% | |||
| 2–6 years | 32.1% | 23.7% | 15.6% | 28.2% | 25.5% | |||
| ≥6 years | 18.1% | 56.2% | 62.3% | 52.8% | 50.2% | |||
| Donor Age (SD) | 40.7 (14.8) | 0.16 | 42.2 (12.9) | 43.8 (12.2) | 41.3 (13.2) | 0.16 | 38.7 | 0.007 |
AMR=antibody-mediated rejection, SD=standard deviation, BMI=body mass index, ESRD=end stage renal disease, IQR=interquartile range, PRA/CPRA=panel reactive antibody/calculated panel reactive antibody
- Clinical AMR was distinguished from subclinical AMR by the presence of evidence of graft dysfunction, manifested as oliguria/anuria, an increase in serum creatinine by ≥20% from baseline, treatment of cell-mediated rejection and/or thrombotic microangiopathy within the two prior weeks, the need for hemodialysis>7 days post-transplant, or new onset proteinuria at the time of the AMR-defining biopsy.
– AMR patients were matched in a 1:3 ratio on HLA-compatibility, ABO-compatibility, donor type (deceased versus live), previous transplant, peak PRA/CPRA, age, and year of transplant to AMR-free patients.
- Refers to the statistical test that tested the difference between AMR patients and non-AMR patients.
- Refers to the statistical test that tested the difference between patients with a subclinical AMR presentation and patients with a clinical AMR presentation.
- Refers to the statistical test that tested the difference between subclinical AMR patients and matched controls.
Desensitization, Antibody Strength, and Antibody Class
All HLA-incompatible and ABO-incompatible live donor transplant recipients received pre-transplant desensitization with plasmapheresis and intravenous immunoglobulin (IVIg). Prior to desensitization, 30.7% of HLA-incompatible live donor kidney transplant recipients had a positive cytometric crossmatch (PCC), 49.8% had a positive flow but negative cytotoxic crossmatch (PFNC), and 19.5% had a positive Luminex but negative flow crossmatch (PLNF). In HLA-incompatible live donor kidney transplant recipients who developed AMR, 42.0%, 47.1%, and 10.9% were PCC, PFNC, and PLNF, compared to 20.1%, 52.3%, and 26.8% in those HLA-incompatible live donor kidney transplant recipients who did not develop AMR (P<0.001). In HLA-incompatible live donor kidney transplant recipients who developed subclinical AMR, 35.8%, 60.3%, and 3.8% were PCC, PFNC, and PLNF, compared to 45.9%, 38.8%, and 15.3% in those HLA-incompatible live donor kidney transplant recipients who developed clinical AMR (P=0.018).
Of the HLA-incompatible deceased donor kidney transplant recipients, 67.0% received post-transplant desensitization with plasmapheresis and IVIg, 1.9% received post-transplant IVIg alone, 6.8% received pre-transplant plasmapheresis and IVIg, and 24.3% received standard induction therapy alone. Of the HLA-incompatible deceased donor kidney transplant recipients, 55.3% were PFNC and 44.7% were PLNF. No HLA-incompatible deceased donor kidney transplant recipients were PCC with their donor. Amongst those that developed AMR, 78.3% were PFNC and 21.7% were PLNF, compared to 36.8% and 63.2% in those HLA-incompatible deceased donor kidney transplant recipients who did not develop AMR. In HLA-incompatible deceased donor kidney transplant recipients who developed subclinical AMR, 88.2% and 11.8% were PFNC and PLNF by Luminex testing, compared to 72.4% and 27.6% in those HLA-incompatible deceased donor kidney transplant recipients who developed clinical AMR (P=0.21).
Of those recipients with a subclinical presentation of AMR secondary to anti-HLA DSA, 29.2%, 26.4%, and 44.4% were from class I, class II, and both class I and II antibody. Of those recipients with a clinical presentation of AMR secondary to anti-HLA DSA, 30.7%, 29.1%, and 40.2% were from class I, class II, and both class I and II antibody. There was no statistically significant difference in the distribution of antibody class by AMR presentation (P=0.83).
Presentations of Antibody-Mediated Rejection
Of patients who developed AMR, 35.2% had a subclinical presentation of AMR at the time of their AMR-defining biopsy at a median of 29 days post-transplant (IQR: 15–54). Clinical AMR was diagnosed at a median of 11 days post-transplant (IQR: 7–29) (P<0.001). Mean follow-up time was 4.3 years (SD 2.6) for subclinical AMR patients, 4.2 years (SD 3.0) for clinical AMR patients, and 5.3 years (SD 3.6) for non-AMR patients. Clinical AMR patients were similar to subclinical AMR patients in terms of age at transplant (44.8 vs. 46.3; P=0.43), female sex (61.0% vs. 57.7%; P=0.64), black race (23.4% vs. 25.3%; P=0.75), median peak PRA (93 vs. 92; P=0.16), and history of prior transplant (61.0% vs. 52.1%; P=0.20) (Table 1).
Compared to patients with a clinical AMR presentation, those with a subclinical AMR presentation were less likely to be compatible deceased donor transplant recipients (1.3% versus 11.3%), more likely to be HLA-incompatible deceased donor transplant recipients (22.1% versus 20.4%), more likely to be compatible live donor recipients (5.2% versus 1.4%), less likely to be ABO-incompatible live donor transplant recipients (2.6% versus 6.3%), and more likely to be HLA-incompatible live donor transplant recipients (68.8% versus 60.6%) (P=0.027). They were otherwise similar to clinical AMR patients in terms of demographic characteristics (Table 1).
AMR Incidence
The incidence of AMR was 1.7% in compatible deceased donor transplant recipients, 44.7% in HLA-incompatible deceased donor transplant recipients, 0.7% in compatible live donor transplant recipients, 13.6% in ABO-incompatible live donor transplant recipients, and 47.4% in HLA-incompatible live donor transplant recipients (P<0.001) (Table 2A). By presentation, 5.9%, 37.0%, 66.7%, 18.2%, and 38.1% of AMR cases in compatible deceased donor, HLA-incompatible deceased donor, compatible live donor, ABO-incompatible live donor, and HLA-incompatible live donor transplants were subclinical.
Table 2.
| Table 2A. Antibody-Mediated Rejection Incidence and Presentationa, by Transplant Type | |||
|---|---|---|---|
| Transplant Type | Incidence of AMR | ||
| Overall (n=219) | Subclinical Presentationb (n=77) | Clinical Presentation (n=142) | |
| Deceased Donor: Compatible | 1.7% | 0.1% | 1.6% |
| Deceased Donor: HLA-Incompatible | 44.7% | 16.5% | 28.2% |
| Live Donor: Compatible | 0.7% | 0.5% | 0.2% |
| Live donor: ABO-Incompatiblec | 13.6% | 2.5% | 11.1% |
| Live Donor: HLA-Incompatible | 47.4% | 18.1% | 29.3% |
| Overall | 9.5% | 3.3% | 6.1% |
| Table 2B. Antibody-Mediated Rejection Risk of Graft Loss, by Transplant Type | ||
|---|---|---|
| Transplant Type | Hazard Ratiod | P-value |
| Deceased Donor: Compatible | 4.73 (1.57–14.26) | 0.006 |
| Deceased Donor: HLA-Incompatible | 2.39 (1.10–5.19) | 0.028 |
| Live Donor: Compatible | NAe | -- |
| Live donor: ABO-Incompatiblec | 6.13 (0.55–67.70) | 0.1 |
| Live Donor: HLA-Incompatible | 6.29 (3.81–10.39) | 0.001 |
| Overall | 4.61 (3.19–6.68) | <0.001 |
AMR=antibody-mediated rejection
- Clinical AMR was distinguished from subclinical AMR by the presence of evidence of graft dysfunction, manifested as oliguria/anuria, an increase in serum creatinine by ≥20% from baseline, treatment of cell-mediated rejection and/or thrombotic microangiopathy within the two prior weeks, the need for hemodialysis>7 days post-transplant, or new onset proteinuria at the time of the AMR-defining biopsy.
- The test for trend of differences in incidence of AMR presentation (subclinical vs. clinical) across transplant types was statistically significant (P=0.027).
- Patients who were ABO-incompatible with their donor and also had anti-HLA donor-specific antibody were studied as members of the HLA-incompatible live donor group (n=43; 14.7% of the HLA-incompatible live donor recipient population.
- Refers to hazard ratio comparing AMR patients of a transplant type with patients of the same transplant type who did not develop AMR.
- Hazard ratio could not be calculated as no compatible live donor recipients with AMR had a graft loss during the study period.
Transplant Glomerulopathy
Of the 165 (75.3%) AMR patients with allograft biopsies performed 6 months after their AMR-defining biopsy or with graft loss at that time, 40.6% had either transplant glomerulopathy or graft loss. Of the 130 (59.4%) AMR patients with allograft biopsies performed 12 months after their AMR defining biopsy or with graft loss at that time, 55.4% had either transplant glomerulopathy or graft loss. There was no statistically significant difference in the incidence of transplant glomerulopathy or graft loss at 6 months (P=0.40) or 12 months (P=0.13) by presentation of AMR (P=0.13) or by HLA antibody class (P=0.99).
Graft Outcomes by Presentation
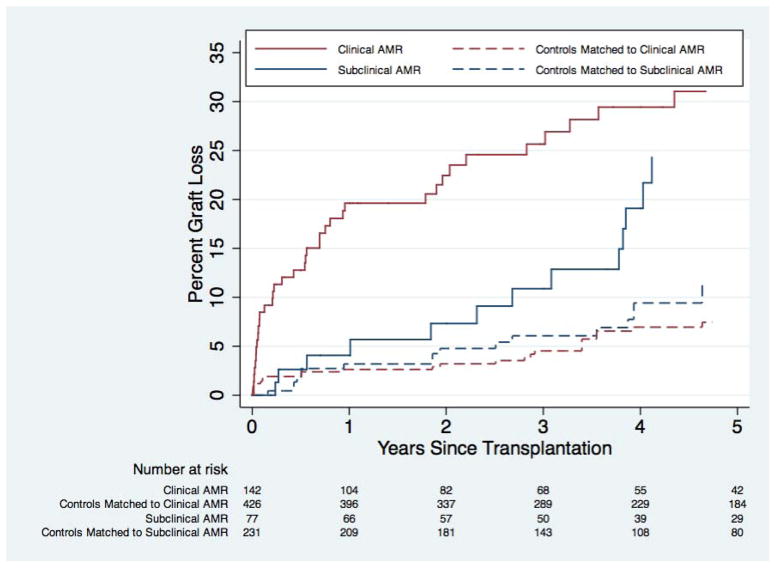
Death-censored graft survival in subclinical AMR patients was 95.9% at 1 year and 75.7% at 5 years, versus 96.8% and 88.4% for AMR-free matched controls (P=0.0097) (Table 3). Death-censored graft survival in clinical AMR patients was 80.4% at 1 year and 69.0% at 5 years, versus 97.4% and 92.5% for AMR-free matched controls (P<0.001) (Table 3, Figure 1). The risk of graft loss for patients developing subclinical AMR was 2.15-fold (95% CI: 1.19–3.91; P=0.012) higher than for matched controls without AMR. The risk of graft loss for patients developing clinical AMR was 5.79-fold (95% CI: 3.62–9.24; P<0.001) higher than for matched controls without AMR. The unadjusted risk of graft loss for patients with a clinical presentation of AMR was 1.52-fold (95% CI: 0.88–2.64; P=0.13) higher than for patients with a subclinical presentation of AMR.
Table 3.
| Death-Censored Graft Survival | P-Value | ||
|---|---|---|---|
| 1 Year (95% Confidence Interval) | 5 Year (95% Confidence Interval) | ||
| AMR--Clinical Presentation, Overall | 80.4% (72.7–86.1%) | 69.0% (59.2–76.8%) | <0.001 |
| AMR-Free Matched Controls c | 97.4% (95.3–98.5%) | 92.5% (88.9–95.0%) | |
| AMR--Clinical Presentation, Treated | 79.8% (70.8–86.4%) | 70.4% (59.2–79.1%) | <0.001 |
| AMR-Free Matched Controls c | 99.0% (93.3–99.9%) | 95.0% (87.1–98.1%) | |
| AMR--Clinical Presentation, Untreated | 82.0% (64.3–91.5%) | 65.5% (44.6–80.2%) | <0.001 |
| AMR-Free Matched Controls c | 97.1% (91.2–99.0%) | 91.2% (82.1–95.8%) | |
| AMR--Subclinical Presentation, Overall | 95.9% (87.9–98.7%) | 75.7% (61.2–85.4%) | 0.0097 |
| AMR-Free Matched Controls c | 96.8% (93.4–98.5%) | 88.4% (93.4–98.5%) | |
| AMR--Subclinical Presentation, Treated | 94.6% (80.1–98.6%) | 74.9% (53.4–87.5%) | 0.20 |
| AMR-Free Matched Controls c | 97.5% (92.4–99.2%) | 86.6% (75.6–92.8%) | |
| AMR--Subclinical Presentation, Untreated | 97.2% (81.9–99.6%) | 76.3% (53.7–88.9%) | 0.0048 |
| AMR-Free Matched Controls c | 97.1% (91.2–99.0%) | 90.6% (80.4–95.6%) | |
AMR=antibody-mediated rejection
- Clinical episodes of AMR were distinguished from subclinical episodes of AMR by the presence of evidence of graft dysfunction, manifested as oliguria/anuria, an increase in serum creatinine by >20% from baseline, treatment of cell-mediated rejection and/or thrombotic microangiopathy within the two prior weeks, the need for hemodialysis>7 days post-transplant, or new onset proteinuria at the time of the AMR-defining biopsy.
- Patients were considered treated if they underwent plasmapheresis within 3 days of their AMR-defining biopsy.
- Patients who developed AMR were matched in a 1:3 ratio on HLA-compatibility, ABO-compatibility, donor type (deceased versus live), history of previous transplant, peak panel-reactive antibody, age, and year of transplant to patients who did not develop AMR.
Figure 1. Death-Censored Graft Loss, by Presentationa of Antibody-Mediated Rejection Compared to AMR-Free Matched Controlsb.
AMR=antibody-mediated rejection
a- Clinical episodes of AMR were distinguished from subclinical episodes of AMR by the presence of evidence of graft dysfunction, manifested as oliguria/anuria, an increase in serum creatinine by >20% from baseline, treatment of cell-mediated rejection and/or thrombotic microangiopathy within the two prior weeks, the need for hemodialysis>7 days post-transplant, or new onset proteinuria at the time of the AMR-defining biopsy.
b- Patients who developed subclinical AMR were matched in a 1:3 ratio on HLA-compatibility, ABO-compatibility, donor type (deceased versus live), history of previous transplant, peak panel-reactive antibody, age, and year of transplant to patients who did not develop AMR.
There was no statistically significant difference in graft loss between clinical and subclinical AMR patients (P=0.13). Graft loss was significantly higher for subclinical AMR patients compared to AMR-free matched controls (P=0.0097) and for clinical AMR patients compared to AMR-free matched controls (P<0.001).
In an analysis of AMR as a time-varying covariate, the risk of graft loss for patients developing subclinical AMR was 2.22-fold (95% CI: 1.22–4.04; P=0.009) higher than for matched controls without AMR. The risk of graft loss for patients developing clinical AMR was 5.67-fold (95% CI: 3.54–9.07; P<0.001) higher than for matched controls without AMR. The unadjusted risk of graft loss with a clinical presentation of AMR was 1.48-fold higher (95% CI: 0.85–2.57; P=0.16) than for patients with a subclinical presentation of AMR.
Outcomes of AMR by Presentation and Treatment
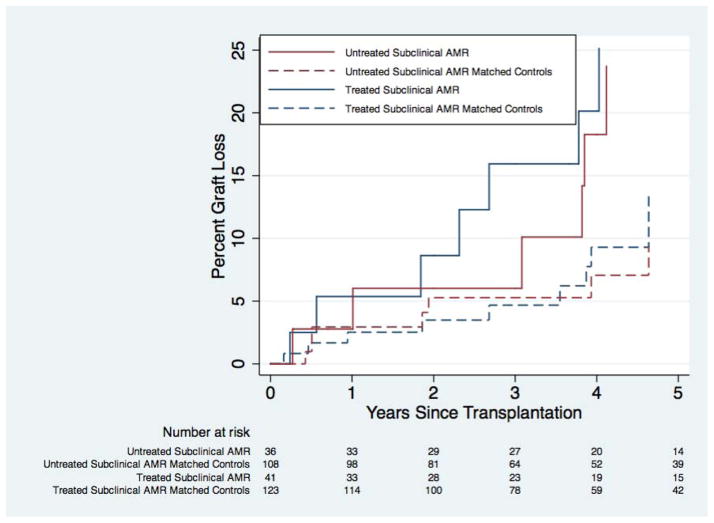
Overall, 53.2% of subclinical AMR patients were treated with plasmapheresis within 3 days of their AMR-defining biopsy. For treated subclinical AMR patients, graft survival at 1 and 5 years was 94.6% and 74.9%, compared to 97.5% and 86.6% in matched controls (P=0.20) (Figure 2). For untreated subclinical AMR patients, graft survival at 1 and 5 years was 97.2% and 76.3%, compared to 97.1% and 90.6% in matched controls (P=0.0048). Subclinical AMR patients who were treated had a 1.73-fold (95% CI: 0.73–4.05; P=0.21) increased risk of graft loss compared to matched controls. Subclinical AMR patients who did not receive treatment had a 3.34-fold (95% CI: 1.37–8.11; P=0.008) higher risk of graft loss compared to matched controls.
Figure 2. Death-Censored Graft Loss in Subclinicala Antibody-Mediated Rejection (AMR) Compared to AMR-Free Matched Controlsc, by Treatmentb.
a- Clinical episodes of AMR were distinguished from subclinical episodes of AMR by the presence of evidence of graft dysfunction, manifested as oliguria/anuria, an increase in serum creatinine by >20% from baseline, treatment of cell-mediated rejection and/or thrombotic microangiopathy within the two prior weeks, the need for hemodialysis>7 days post-transplant, or new onset proteinuria at the time of the AMR-defining biopsy.
b- Patients were considered treated if they underwent plasmapheresis within 3 days of their AMR-defining biopsy.
c- Patients who developed subclinical AMR that was untreated were matched in a 1:3 ratio on HLA-compatibility, ABO-compatibility, donor type (deceased versus live), history of previous transplant, peak panel-reactive antibody/calculated panel reactive antibody, age at transplant, and year of transplant to patients who did not develop AMR. The matching process was repeated for patients who developed subclinical AMR that was treated. An identical matching process was performed for patients with clinical AMR.
There was no statistically significant difference in graft loss for treated subclinical AMR patients compared to their matched controls (P=0.20); however graft loss was statistically significantly higher for untreated subclinical AMR patients compared to their matched controls (P=0.0048).
Overall, 75.3% of clinical AMR patients were treated within 3 days of their AMR-defining biopsy. For treated clinical AMR patients, graft survival at 1 and 5 years was 79.8% and 70.4%, compared to 99.0% and 95.0% in matched controls (P<0.001). For untreated clinical AMR patients, graft survival at 1 and 5 years was 82.0% and 65.5%, compared to 97.1% and 91.2% in matched controls (P<0.001). Despite treatment, clinical AMR patients had a 10.51-fold (95% CI: 3.72–29.71; P<0.001) higher risk of graft loss compared to matched controls. Clinical AMR patients who were untreated had a 5.68-fold (95% CI: 2.26–14.23; P<0.001) higher risk of graft loss compared to matched controls.
Graft Outcomes by Transplant Type
Recipients who developed AMR had a 4.61-fold higher risk of graft loss (95% CI: 3.19–6.68; P<0.001) (Table 2B) when compared to matched controls. This risk varied by donor type and compatibility, with 4.73-fold higher risk (95% CI: 1.57–14.26; P=0.006) in HLA-compatible deceased donor recipients, 2.39-fold higher risk (95% CI: 1.10–5.19; P=0.028) in HLA-incompatible deceased donor recipients, 6.13-fold higher risk (95% CI: 0.55–67.70; P=0.14) in ABO-incompatible live donor recipients, and 6.29-fold higher risk (95% CI: 3.81–10.39; P<0.001) in HLA-incompatible live donor recipients. No HLA-compatible live donor recipients with AMR experienced graft loss.
DISCUSSION
In this single-center study of 219-patients who developed biopsy-proven AMR consistent with 2013 Banff diagnostic criteria, we found that AMR was associated with an overall 4.15-fold (95% CI: 2.89–5.96; P<0.001) increase in the risk of graft loss and that even subclinical AMR was associated with an increased risk of graft loss. Specifically, the risk of graft loss for patients with a subclinical presentation of AMR was 2.15-fold (95% CI: 1.19–3.91; P=0.012) higher than for matched controls without AMR. The risk of graft loss for patients with a clinical presentation of AMR was 5.79-fold (95% CI: 3.62–9.24; P<0.001) higher than for matched controls without AMR. Timely treatment with plasmapheresis may attenuate that risk as subclinical AMR patients who were treated did not have a statistically significant increase in the risk of graft loss compared to AMR-free matched controls (HR 1.73; 95% CI: 0.73–4.05; P=0.21) and subclinical AMR patients who did not receive treatment had a statistically significant higher risk of graft loss compared to AMR-free matched controls (HR 3.34; 95% CI: 1.37–8.11; P=0.008). In the former group, the point estimate is high and the confidence interval is wide, making it difficult to determine the effect of treatment on the risk of graft loss compared to AMR-free patients. It is likely that early treatment attenuates the risk of graft loss, leading to an intermediate risk of graft loss between that seen for patients who do not develop AMR and those that develop subclinical AMR that is not treated early. We also quantified the risk of graft loss by transplant type. Specifically, the risk of graft loss for AMR patients was 4.73-fold (95% CI: 1.57–14.26; P=0.006) higher in HLA-compatible deceased donor recipients, 2.39-fold (95% CI: 1.10–5.19; P=0.028) higher in HLA-incompatible deceased donor recipients, 6.13-fold (95% CI: 0.55–67.70; P=0.14) higher in ABO-incompatible live donor recipients, and 6.29-fold (95% CI: 3.81–10.39; P<0.001) higher in HLA-incompatible live donor recipients. No HLA-compatible live donor recipients with AMR experienced graft loss.
Our finding of subclinical AMR associated with worse graft survival is novel, and consistent with previous findings of worse graft function and histologic findings in patients who previously had subclinical AMR. We and others have reported that, in general, antibody injury leads to transplant glomerulopathy, which is associated with reduced graft survival [1, 20]. Our group has previously reported that subclinical AMR specifically is associated with the development of chronic allograft nephropathy in HLA-incompatible live donor kidney transplant recipients [10]. Loupy and colleagues, in a study of HLA-incompatible deceased donor recipients, reported a number of adverse associations with subclinical AMR, including lower glomerular filtration rate, higher serum creatinine, and more peritubular capillaritis, C4d deposition, and transplant glomerulopathy on biopsy [11]. In a study of transplant glomerulopathy in compatible live and deceased donor transplant recipients, Gloor and colleagues reported that a history of AMR was associated with a three-fold increase in the risk of subsequent detection of transplant glomerulopathy on protocol biopsy [21]. Our finding of subclinical AMR associated with worse graft survival is novel, but consistent with these previous findings of worse graft function and histologic findings in patients who previously had subclinical AMR.
The rare occurrence of AMR in HLA-compatible recipients (0.7% live donor and 1.9% deceased donor) in our study is consistent with other single-center studies [22, 23]. For example, Everly et al. reported a 1.8% and 1.5% incidence of AMR in HLA-compatible live and deceased donors. The incidences of AMR among deceased donor recipients (44.3% HLA-incompatible and 1.9% HLA-compatible) were consistent with incidences of 43–54% and 5% reported in the literature [24, 25]. The high incidence of AMR in HLA-incompatible live donor recipients (46.5%) is also consistent with the 41–53% incidence reported by others [26–28]. AMR occurred in 14.8% of our ABO-incompatible live donor recipients, compared to 40% reported by Fidler and colleagues [29], although this is likely related to the fact that we defined the ABO-incompatible cohort to be purely ABO-incompatible, i.e. ABO-incompatible transplants with anti-HLA DSA were categorized as HLA-incompatible live donor transplants. When all ABO-incompatible transplants were considered, irrespective of anti-HLA DSA, our AMR incidence in ABO-incompatible recipients was a more similar 28.8%. Toki, et al., reported a 33% incidence of AMR in the first 3 months post-transplant in their ABO-incompatible live donor recipients [30].
Our findings of increased graft loss following AMR consolidate evidence of this causal pathway from several other studies. Loupy and colleagues reported that glomerular and peritubular inflammation, transplant glomerulopathy, and post-transplant C1q-binding DSA (pathologic and immunologic factors of AMR) on 1-year protocol biopsies were independently associated with graft loss [31]. Lefaucheur et al. reported a 4.1-fold (95% CI: 2.2–7.7) higher risk of graft loss in HLA-incompatible deceased donor recipients who developed AMR, consistent with but higher than our finding of a 2.39-fold (95% CI: 1.10–5.19) higher risk [32]. Couzi and colleagues reported a trend toward worse graft survival in this population as well [24]. Dunn and colleagues also demonstrated a 2.9-fold increase in the risk of graft loss for live and deceased donor recipients with pre-transplant DSA [26]. In a study of HLA-incompatible live donor recipients, Gloor and colleagues reported a nearly 3-fold increase in graft loss in AMR patients,, compared to our finding of a 6.3-fold increase in the risk of graft loss [27]. Among ABO-incompatible live donor recipients, however, Fidler, et al., did not find increased graft loss following AMR, nor did they find an increased incidence of chronic allograft nephropathy on 1-year protocol biopsies [29]. While Toki, et al., reported significantly worse graft survival in ABO-incompatible live donor AMR patients, all of the patients who experienced graft loss also had pre-transplant anti-HLA DSA [30], so these inferences are not generalizable to pure ABO-incompatibility. We did not detect a statistically significant difference in the risk of graft loss for ABO-incompatible patients who develop AMR (HR: 6.13; 95% CI: 0.55–67.70); however, the wide confidence interval for this estimate suggests that the risk associated with AMR in ABO-incompatible recipients remains incompletely characterized.
Strengths of this investigation include the timely, rigorous criteria for AMR consistent with the Banff 2013 criteria and the fact that this is the largest known cohort of patients with AMR [15]. Limitations of this study include the possibility of misclassification of AMR, particularly in the event of subclinical AMR, which is less likely to prompt a for-cause biopsy. This misclassification, if present, would likely bias towards the null, rendering our estimates of the risk of graft loss following subclinical AMR conservative. Furthermore, we performed protocol biopsies at 1, 3, 6, and 12 months following ABO- and HLA-incompatible live donor transplants, but not routinely after deceased donor transplants or compatible live donor transplants, introducing possible ascertainment bias. By stratifying graft outcomes in cases of clinical versus subclinical AMR by treatment status, we were unable to detect a statistically significant difference, though this may be a type II error secondary to a limited sample size.
Additionally, because not all subclinical AMR patients were treated, it did introduce significant heterogeneity into the study group; however, this did provide the opportunity to observe the natural history of untreated subclinical AMR and demonstrated that treatment is associated with improved graft survival. These findings confirm the importance of identifying early antibody-mediated injury via protocol biopsies. Finally, because this study includes patients from a single institution, there are limits to external validity (i.e. generalizability to the general transplant population); however, in quantifying the risk of graft loss by transplant type, our findings were consistent with those of a number of other high-volume centers, though it is unknown if these estimates can be extrapolated to lower-volume centers.
CONCLUSION
The development of AMR is associated with a significantly higher incidence of allograft loss, although the effects of AMR differ by transplant type. HLA-compatible live donor recipients who develop AMR do not have a higher risk of graft loss compared to their counterparts who do not develop AMR, and the risk for recipients of ABO-incompatible transplants remains inconclusive. However, deceased donor (both HLA-compatible and HLA-incompatible) and HLA-incompatible live donor recipients do have significantly higher risks of graft loss following AMR. The development of subclinical AMR is associated with a higher incidence of allograft loss compared to matched controls, though early treatment with plasmapheresis likely attenuates this risk. These findings highlight the need for strategies to prevent and aggressively treat subclinical AMR and strengthen the case for performing routine protocol biopsies in immunologically high-risk patients.
Acknowledgments
Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number F32DK093218 (BJO) and R01DK098431 (DLS).
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
The authors would like to thank Ms. Julie Houp for providing antibody data.
Abbreviations
- AMR
antibody-mediated rejection
- BMI
body mass index
- DSA
donor-specific antibody
- ESRD
end stage renal disease
- HRSA
Health Resources and Services Administration
- IQR
interquartile range
- OPTN
Organ Procurement and Transplantation Network
- PCC
positive cytotoxic crossmatch
- PFNC
positive flow; negative cytotoxic crossmatch
- PLNF
positive Luminex; negative flow crossmatch
- PRA/CPRA
panel reactive antibody/calculated panel reactive antibody
- SD
standard deviation
- SRTR
Scientific Registry for Transplant Recipients
Footnotes
Previous Presentations: A preliminary version of these findings was presented as an oral presentation at the 2013 American Society of Transplant Surgeons State-of-the-Art Winter Symposium in Miami, FL.
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
AUTHOR CONTRIBUTIONS:
Study concept and design: BJO, JRM, RAM, DLS
Acquisition of data: BJO, EHKC, AH, NG, JRM, BEL, NA, SMB, RAM
Analysis and interpretation of data: BJO, EHKC, KJVA, AH, NG, JRM, JMGW, BEL, NA, SMB, AMJ, RAM, DLS
Critical revision of the manuscript for important intellectual content: BJO, EHKC, KJVA, AH, NG, JRM, JMGW, BEL, NA, SMB, AMJ, RAM, DLS
Statistical analysis: BJO, EHKC, DLS
Obtained funding: NA
Administrative, technical, or material support: N/A
Study supervision: BJO, RAM, DLS
References
- 1.Bagnasco SM, et al. Time course of pathologic changes in kidney allografts of positive crossmatch HLA-incompatible transplant recipients. Transplantation. 2014;97(4):440–5. doi: 10.1097/01.TP.0000437177.40551.f4. [DOI] [PubMed] [Google Scholar]
- 2.Garonzik Wang JM, et al. Incompatible live-donor kidney transplantation in the United States: results of a national survey. Clin J Am Soc Nephrol. 2011;6(8):2041–6. doi: 10.2215/CJN.02940311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massie AB, et al. Center-level utilization of kidney paired donation. Am J Transplant. 2013;13(5):1317–22. doi: 10.1111/ajt.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery RA, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365(4):318–26. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 5.Segev DL, et al. Kidney paired donation and optimizing the use of live donor organs. JAMA. 2005;293(15):1883–90. doi: 10.1001/jama.293.15.1883. [DOI] [PubMed] [Google Scholar]
- 6.Racusen LC, et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3(6):708–14. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 7.Puttarajappa C, Shapiro R, Tan HP. Antibody-mediated rejection in kidney transplantation: a review. J Transplant. 2012:193724. doi: 10.1155/2012/193724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns JM, et al. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant. 2008;8(12):2684–94. doi: 10.1111/j.1600-6143.2008.02441.x. [DOI] [PubMed] [Google Scholar]
- 9.Matas AJS, JM, Skeans MA, Lamb KE, Gustafson SK, Samana CJ, Stewart DE, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2011 Annual Data Report: Kidney. American Journal of Transplantation. 2013;13(s1):11–46. doi: 10.1111/ajt.12019. [DOI] [PubMed] [Google Scholar]
- 10.Kraus ES, et al. Subclinical rejection in stable positive crossmatch kidney transplant patients: incidence and correlations. Am J Transplant. 2009;9(8):1826–34. doi: 10.1111/j.1600-6143.2009.02701.x. [DOI] [PubMed] [Google Scholar]
- 11.Loupy A, et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant. 2009;9(11):2561–70. doi: 10.1111/j.1600-6143.2009.02813.x. [DOI] [PubMed] [Google Scholar]
- 12.Orandi BJZA, Dagher NN, Bagnasco SM, Garonzik-Wang JM, Van Arendonk KJ, Gupta N, Lonze BE, Alachkar N, Kraus ES, Desai NM, Locke JE, Racusen LC, Segev DL, Montgomery RA. Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection following HLA-incompatible kidney transplantation. Transplantation. 2014 doi: 10.1097/TP.0000000000000298. (in press) [DOI] [PubMed] [Google Scholar]
- 13.Sellares J, et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant. 2013;13(4):971–83. doi: 10.1111/ajt.12150. [DOI] [PubMed] [Google Scholar]
- 14.Gaston RS, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90(1):68–74. doi: 10.1097/TP.0b013e3181e065de. [DOI] [PubMed] [Google Scholar]
- 15.Haas M, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272–83. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 16.Bagnasco SM, et al. CD20-positive infiltrates in renal allograft biopsies with acute cellular rejection are not associated with worse graft survival. Am J Transplant. 2007;7(8):1968–73. doi: 10.1111/j.1600-6143.2007.01885.x. [DOI] [PubMed] [Google Scholar]
- 17.Segev DL, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303(10):959–66. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 18.Muzaale AD, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579–86. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaubel DE, et al. Analytical approaches for transplant research, 2004. Am J Transplant. 2005;5(4 Pt 2):950–7. doi: 10.1111/j.1600-6135.2005.00837.x. [DOI] [PubMed] [Google Scholar]
- 20.Sharif A, et al. Histologic phenotype on 1-year posttransplantation biopsy and allograft survival in HLA-incompatible kidney transplants. Transplantation. 2014;97(5):541–7. doi: 10.1097/01.TP.0000442513.27641.7e. [DOI] [PubMed] [Google Scholar]
- 21.Gloor JM, et al. Transplant glomerulopathy: subclinical incidence and association with alloantibody. Am J Transplant. 2007;7(9):2124–32. doi: 10.1111/j.1600-6143.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo LG, et al. De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am J Transplant. 2009;9(11):2532–41. doi: 10.1111/j.1600-6143.2009.02800.x. [DOI] [PubMed] [Google Scholar]
- 23.Everly MJ, et al. Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant. 2009;9(5):1063–71. doi: 10.1111/j.1600-6143.2009.02577.x. [DOI] [PubMed] [Google Scholar]
- 24.Couzi L, et al. Interpretation of positive flow cytometric crossmatch in the era of the single-antigen bead assay. Transplantation. 2011;91(5):527–35. doi: 10.1097/TP.0b013e31820794bb. [DOI] [PubMed] [Google Scholar]
- 25.Lefaucheur C, et al. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet. 2013;381(9863):313–9. doi: 10.1016/S0140-6736(12)61265-3. [DOI] [PubMed] [Google Scholar]
- 26.Dunn TB, et al. Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant. 2011;11(10):2132–43. doi: 10.1111/j.1600-6143.2011.03640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gloor JM, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10(3):582–9. doi: 10.1111/j.1600-6143.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 28.Higgins R, et al. Human leukocyte antigen antibody-incompatible renal transplantation: excellent medium-term outcomes with negative cytotoxic crossmatch. Transplantation. 2011;92(8):900–6. doi: 10.1097/TP.0b013e31822dc38d. [DOI] [PubMed] [Google Scholar]
- 29.Fidler ME, et al. Histologic findings of antibody-mediated rejection in ABO blood-group-incompatible living-donor kidney transplantation. Am J Transplant. 2004;4(1):101–7. doi: 10.1046/j.1600-6135.2003.00278.x. [DOI] [PubMed] [Google Scholar]
- 30.Toki D, et al. Acute antibody-mediated rejection in living ABO-incompatible kidney transplantation: long-term impact and risk factors. Am J Transplant. 2009;9(3):567–77. doi: 10.1111/j.1600-6143.2008.02538.x. [DOI] [PubMed] [Google Scholar]
- 31.Loupy A, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369(13):1215–26. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 32.Lefaucheur C, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21(8):1398–406. doi: 10.1681/ASN.2009101065. [DOI] [PMC free article] [PubMed] [Google Scholar]