Abstract
Acute and chronic rejection impact distinct compartments of cardiac allografts. Intramyocardial mononuclear cell infiltrates define acute rejection, whereas chronic rejection affects large arteries. Hearts transplanted from male to female C57BL/6 mice undergo acute rejection with interstitial infiltrates at 2 weeks that resolve by 6 weeks when large arteries develop arteriopathy. These processes are dependent on T cells because no infiltrates developed in T cell deficient mice and transfer of CD4 T cells restored T cell as well as macrophage infiltrates and ultimately neointima formation. Markers of inflammatory macrophages were upregulated in the interstitium acutely and decreased as markers of wound healing macrophages increased chronically. Programmed cell death protein, a negative costimulator, and its ligand PDL1 were upregulated in the interstitium during resolution of acute rejection. Blocking PDL1:PD1 interactions in the acute phase increased interstitial T cell infiltrates. Toll Like Receptor 4 and its endogenous ligand hyaluronan were increased in arteries with neointimal expansion. Injection of hyaluronan fragments increased intragraft production of chemokines. Our data indicate that negative co-stimulatory pathways are critical for the resolution of acute interstitial infiltrates. In the arterial compartment recognition of endogenous ligands including hyaluronan by the innate toll like recepetors may support the progression of arteriopathy.
Immune responses to cardiac allografts can result in acute and chronic rejection. In addition to differences in kinetics, acute and chronic rejection attack two different compartments of the heart. Acute rejection is defined by interstitial mononuclear cell infiltrates with associated myocyte damage in endomyocardial biopsies (1). In contrast, chronic rejection primarily involves large coronary arteries. Chronic rejection is characterized by diffuse intimal hyperplasia containing mononuclear infiltrates. Additional mononuclear infiltrates are frequently present in the media and adventitia (2-4). This pathological process is diagnosed by angiography or intravascular ultrasound and is labeled cardiac allograft vasculopathy (CAV). The incidence of interstitial pathology decreases with time after transplantation in most patients, and one multicenter study concluded that routine endomyocardial biopsies were not of diagnostic value after 5 years except in patients with high risk for acute rejection (5). In contrast, the incidence of CAV increases progressively after transplantation. As a result, advanced CAV is often reported with little or no infiltrates in endomyocardial biopsies (2).
Mechanisms underlying compartmentalization of acute and chronic rejection are not known. However, the infiltrates in acute and chronic rejection are predominantly comprised of T cells and macrophages, which are regulated by many positive and negative co-stimulatory signals. The most extensively studied co-stimulatory receptors on T cells belong to the CD28 family and include the activating receptor CD28 and inhibitory receptor CTLA-4, both of which bind B7-1/CD80 and B7-2/CD86 ligands. Another member of the CD28 family is PD1 (CD279), an inhibitory receptor that binds to PDL1 and PDL2 (CD274 and CD273) that are expressed on antigen presenting cells (6-8). In addition, PDL1 is constitutively expressed by various parenchymal cells including cardiomyocytes and can be induced on endothelial cells (6, 9). Therefore, compartmentalized pathology could result from differentially expressed ligands for positive and negative co-stimulatory receptors on T cells during the process of rejection.
T cells in turn produce cytokines that direct macrophages to differentiate into performing acute inflammatory functions or chronic wound healing functions (10). Clinically, macrophages are routinely identified in biopsies by a universal macrophage marker, such as CD68 (1, 11, 12). Inflammatory macrophages (M1 macrophages) release cytokines including IL-1β and TNFα, chemokines such as MIG/CXCL9 and MCP-1/CCL2, as well as reactive oxygen species that promote acute interstitial infiltrates (10). Increased expression of MIG/CXCL9 in endomyocardial biopsies has been associated with acute rejection in clinical studies (13), and infiltrating macrophages have been identified as a source of this chemokine (12). In contrast, wound healing macrophages (M2 macrophages) produce growth factors such as TGFβ and VEGF that are elevated in fibrotic processes.
Another set of genes associated with acute rejection was identified in the multicentered Cardiac Allograft Rejection Gene Expression Observational (CARGO) study. This study was designed to establish gene profiles in peripheral blood mononuclear cells that distinguish patients with stable transplants from patients who developed acute rejection (14). In the CARGO study, the gene encoding the decoy receptor for IL-1 (IL-1R2) correlated most strongly with resolution of acute rejection and stable graft function (15, 16). Although IL-1R2 is the predominant receptor for IL-1 on monocytes (17), the expression of this decoy receptor has not been examined on macrophages in the interstitial or arterial compartments of cardiac transplants.
Based on these clinical observations, we hypothesized that differential expression of critical molecules in the interstitial and arterial compartments accounts for the distinct localization of acute and chronic rejection in cardiac transplants.
Materials and Methods
Mice
Male and female B6 (H2b), and female B6.129S7-Rag1tm1Mom/J mice were purchased from Jackson Laboratories (Bar Harbor, ME) for use at 8-12 weeks of age. Female C57BL/10NA;-(Tg)TCR Marilyn-(KO)Rag2 mice (H-2b, Marilyn), were a generous gift from Polly Matzinger (National Institutes of Health, Bethesda, MD) and Olivier Lantz (INSERM, Paris, France). All animal studies were approved by the Cleveland Clinic institutional animal care and use committee.
Donor hearts were transplanted heterotopically into the abdomen of recipients as previously described (18).
CD4 T cell transfer
Splenocytes from female Marilyn transgenic mice were incubated with the following mixture of FITC-labeled antibodies (BD Pharmingen, San Jose, CA): CD8a, CD19, CD11c, CD11b, CD117, NK1.1. Labeled cells were removed by flow sorting and unlabeled CD4 cells were transferred to SCID mice 7 days following transplantation. These cells were >95% CD4+ by flow cytometry.
Treatment with blocking antibody or hyaluronan fragments
Recipients were injected intraperitoneally with 3 doses of 200ug of purified IgG2a rat monoclonal antibody to PDL1 (clone 10F.9G2) or isotype control (clone LTF-2) antibody (BioXCell, West Lebanon, NH) on alternate days in the second or sixth week after transplantation.
Highly purified hyaluronan fragments (Lifecore Biomedical, LLC) were electrophoretically separated as previously described (19). A 100ug dose of low molecular weight (4.7 or 35kD) hyaluronan was administered intraperitoneally daily from the day of transplantation for 2 weeks. Controls were administered equal volumes of the PBS diluent.
Immunohistochemistry
Full cross-sections through cardiac grafts were fixed in methanol acetic acid and immunohistology was performed as previously described (18, 20). The following primary reagents were used: polyclonal rabbit antibody to CD3 (Abcam, Cambridge, MA), rat monoclonal antibody to Galectin-3 (Mac-2; Cedarlane, Burlington, NC), rabbit polyclonal antibody to Ym-1(Stem Cell Technologies, Vancouver, Canada), polyclonal goat antibody to PD-1 (R&D systems Inc, Minneapolis, MN), rat monoclonal antibody to mouse Foxp3 (eBiosciences, San Diego, CA), and biotinylated hyaluronic acid binding protein (Millipore, Billerica, MA).
Laser capture microdissection and real time RT PCR
Frozen sections on PET-Membrane slides (Leica, Buffalo Grove, IL) stained with Arcturus Histogen solution (Life Technologies, Grand Island, NY) were microdissected on a Leica AS-LMD microscope. Samples were collected in RNAlater. Total RNA was isolated using the RNeasy micro kit and reverse transcribed with the RT2 PreAMP cDNA synthesis kit (QIAGEN, Gaithersburg, MD). Expressions of 86 genes (supplemental Table 1) was screened with a customized PCR array kit (QIAGEN). Eighteen genes were analyzed in additional samples by qPCR using the ddCT method (21), calibrated to (control sample) and normalized to B-actin. TaqMan assay numbers for all genes measured are listed in supplemental table 2.
Isolation of graft infiltrating cells for flow cytometry
Cells infiltrating the graft were isolated as previously described (18). Briefly, grafts were removed after perfusing the recipient with RPMI media to flush cells from the circulation. The apical half of the graft was weighed and incubated 1 h at 37C in RPMI with Type II collagenase (Sigma-Aldrich) before pressing through a 40 μm filter. The collected cells were washed twice in RPMI, counted and stained for phenotypic surface markers (CD45, CD4, CD11b, F4/80, PDL1, PD1 and IL1-R2 from BD Bioscience, San Jose, CA; eBioscience, San Diego, CA, USA). Flow cytometry was performed using a FACSCalibur (BD Biosciences) cytometer and FlowJo analysis software (Tree Star Inc., Ashland, OR, USA). The forward scatter and FL1 (CD45+) channels were used to gate on leukocytes followed by analysis of the specific leukocyte populations.
Quantitation of chemokines, hyaluronan and IL-1 receptors in allografts
Grafts were removed and homogenized in 500ul of proteinase inhibitor. Then 1 ml of 1.5% Triton X-100 in PBS was added before shaking 30 minutes at 4C. After pelleting cell debris, the supernatants were collected and total protein concentration quantified by Coomassie Plus Protein Assay Reagent kit (Pierce, Thermo Fisher Scientific, Rockford, IL). Protein concentrations were determined using ELISA kits for CXCL9, CCL5, CCL2 and hyaluronan (R&D Systems, Minneapolis MN), and IL-1R1 and IL-1R2 (US Biological, Marblehead MA).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software Inc. San Diego, CA). Differences between groups were evaluated using an unpaired Student’s t-test.
Results
Hearts transplanted from male to female B6 undergo acute rejection that transitions into chronic rejection
Female recipients of male B6 hearts were sacrificed 2 or 6 weeks after transplantation to assess acute and chronic manifestations of rejection. At 2 weeks, allografts contained diffuse interstitial infiltrates of T cells and macrophages with limited periadventitial involvement of larger arteries (Figure 1A,B). By 6 weeks interstitial infiltrates diminished, and about half of the large arteries developed adventitial and intimal mononuclear infiltrates predominantly composed of macrophages (Figure 1C,D). Isograft controls did not exhibit pathological changes (Supplemental Figure 1A,B).
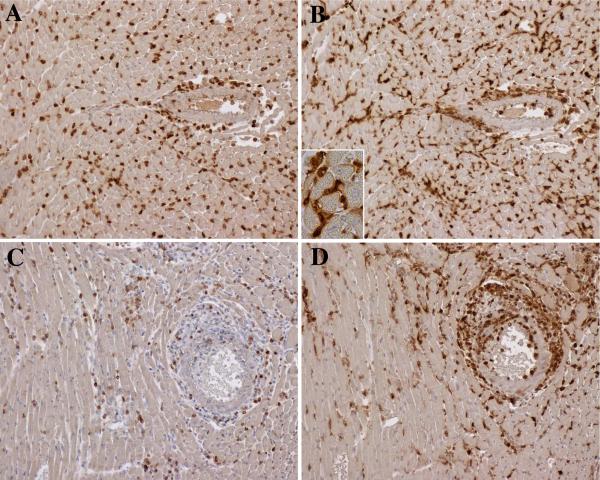
Figure 1.
Immunoperoxidase stains of acute and chronic infiltrates in cardiac allografts. At 2 weeks (top row), allografts contained a diffuse interstitial infiltration of CD3 T cells (A) and Galectin-3+ macrophages (B) with limited periadventitial involvement of larger arteries (right side of figures). The macrophages displayed cytoplasmic projections on high power (inset). At 6 weeks, the interstitial infiltrates diminished, and large arteries developed adventitial and intimal infiltrates composed of CD3 T cells (C) and larger numbers of macrophages (D). Original magnifications 200x (inset 600x).
Most macrophages in the acute and chronic lesions were intensely positive for Galectin-3, a marker of activated macrophages that contribute to cardiovascular disease (22, 23). These macrophages were elongated with cytoplasmic projections and frequently formed clusters (Figure 1B inset). Few macrophages in either the acute or chronic infiltrates were positive for Ym-1, a marker for M2 macrophages (10). Moreover, these M2 macrophages were scattered as individual large ovoid cells (Supplemental Figure 2A,B). We also found limited numbers of Foxp3 positive regulatory T cells in interstitial and arterial compartments (Supplemental Figure 2C,D).
CD4 T-cells orchestrate acute and chronic rejection
Because H-Y peptides are presented in the context of MHC class II molecules, we tested the requirement for CD4 T cells in generating acute and chronic pathology. Hearts were transplanted from wild type male B6 mice into female B6 Rag1 deficient recipients (B6.RAG−/−), which lack mature T and B cells. One week later we reconstituted the recipients with 2-3×104 CD4 T cells from transgenic female B6 mice that express T cell receptors for H-Y peptides in the context of H2-IAb (Marilyn mice). B6.RAG−/− recipients were sacrificed at 2 and 6 weeks after cell transfer. In the absence of T cell reconstitution, no infiltrates developed in the male B6 cardiac allografts to female B6.RAG−/− recipients (Supplemental Figure 1C,D). CD4 T cells from Marilyn mice did not cause infiltrates in control hearts that were isografted from female B6 mice into female B6.RAG−/− recipients. However, CD4 T cells from Marilyn mice reconstituted acute interstitial infiltrates that progressed to CAV in male B6 cardiac allografts to female B6.RAG−/− recipients. As in wild type recipients, both acute and chronic infiltrates were composed of T cells and macrophages, but activated macrophages predominated (Figure 2A-D). An additional group of mice sacrificed at 10 weeks had more extensive arterial lesions in their allografts, but the T cells had diminished (Figure 2E) and macrophages increased (Figure 2F). At this time, the neointima had progressed to contain alpha-smooth muscle expressing cells (Supplemental Figure 3).
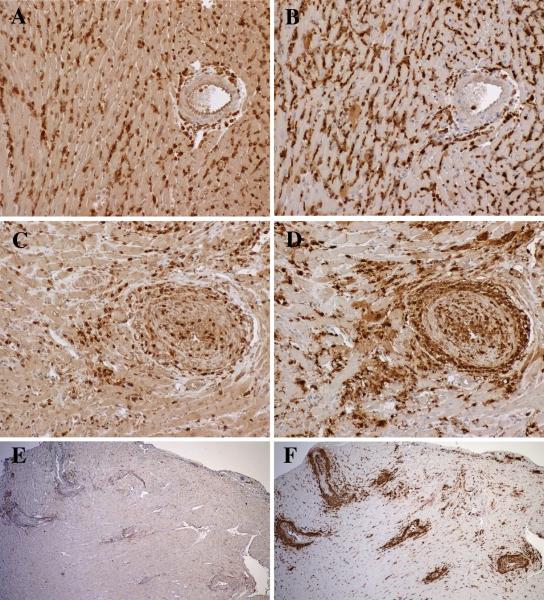
Figure 2.
Male hearts transplanted to female RAG−/− recipients reconstituted with Marilyn CD4 T cells. At 2 weeks, allografts contained diffuse interstitial infiltrates of CD3 T cells (A) and Galectin-3+ macrophages (B) with limited periadventitial involvement of larger arteries. At 6 weeks, the interstitial infiltrates diminished, and large arteries developed adventitial and intimal infiltrates of CD3 T cells (C) and large numbers of macrophages (D). At 10 weeks, the arterial lesions contained decreased numbers of CD3 T cells (E), and increased macrophages (F). Original magnifications 200x (A-D) and 40x (E, F).
Mediators expressed more highly in the interstitial than in the arterial compartments
Laser capture microdissection was used to isolate tissue from the interstitial and arterial compartments of allografts and isografts. The expression of 86 different genes was evaluated by real time PCR in a single plate. Table 1 lists the most highly upregulated genes in the interstitium of allografts compared to isografts at 2 and 6 weeks. These included the chemokines MIG/CXCL9, RANTES/CCL5 and MCP-1/CCL2; the co-stimulatory molecules B7-1/CD80, B7-2/CD86, and PDL1 (CD274); IL-1 receptors (IL1R1 and IL1R2); and the Toll like Receptors TLR2 and TLR4. The chemokine genes were more highly upregulated at 2 weeks than 6 weeks in the interstitium of allografts compared to isografts. In contrast, TLR2 and 4 increased with time after transplantation.
Table 1.
Genes upregulated in microdissected allografts expressed as fold change compared to isograft.
| GENE | 2 week Interstitium |
6 week Interstitium |
2 week Artery |
6 week Artery |
|---|---|---|---|---|
| MIG (CXCL9) | 139 | 21 | 16 | 51 |
| RANTES (CCL5) | 46 | 12 | 7.5 | 34 |
| MCP-1 (CCL2) | 15 | 7 | 4.5 | 8 |
| CD80/B7-1 | 50 | 6 | 2.5 | 2 |
| CD86/B7-2 | 225 | 225 | 41 | 8 |
| CTLA4 | 7 | 4 | 2.5 | 2 |
| CD274 (PDL1) | 70 | 40 | 47 | 8.5 |
| IL1R1 | 10 | 20 | 10 | 20 |
| IL1R2 | 205 | 4 | 540 | 2 |
| TLR2 | 10 | 175 | 30 | 11 |
| TLR4 | 75 | 225 | 76 | 700 |
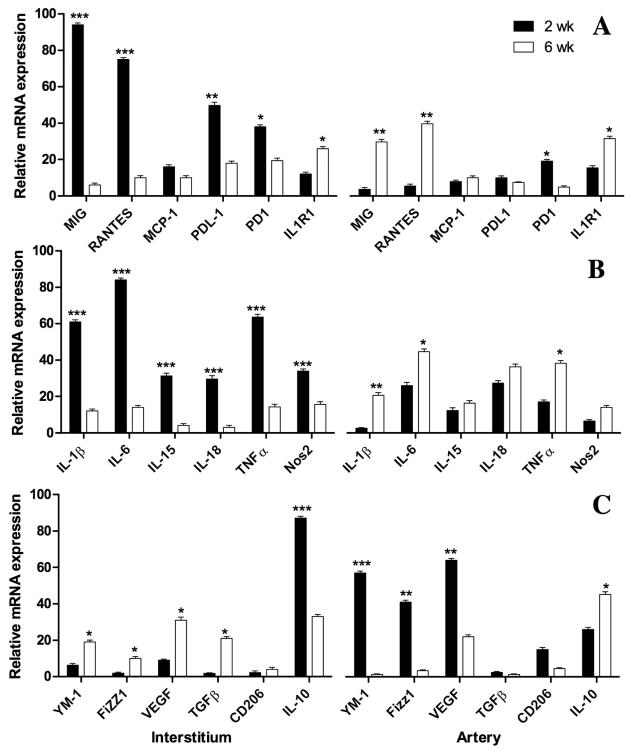
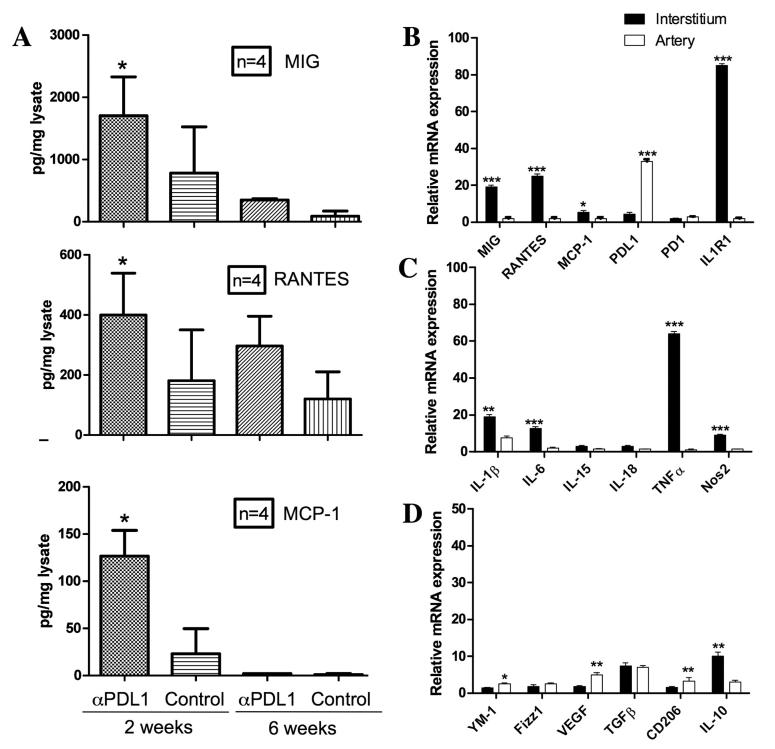
Because MIG and MCP-1 are produced by M1 inflammatory macrophages, additional microdissections were performed and the captured tissues were probed for 6 markers for M1 macrophages (IL-1β, IL-6, IL-15, IL-18, TNFα and Nos2), all of which were increased more in the interstitium than in the arterial compartment at 2 weeks (Figure 3B). By 6 weeks, all of the M1 markers had decreased in the interstitium and increased in the arterial compartment. Changes in MIG and MCP-1 were congruent with the M1 markers in these samples (Figure 3A). The converse was found for 5 markers for M2 macrophages (Ym1, Fizz1, VEGF, TGFβ, and CD206), which were more elevated in the arterial compartment than in the interstitum at 2 weeks (Figure 3C). By 6 weeks, these M2 markers had decreased in the arterial compartment and increased in the interstitium. The exception to this pattern was IL-10, which was most elevated acutely in the interstitium, but was elevated in at 2 and 6 weeks in both compartments (Figure 3C).
Figure 3.
Quatitative PCR on 3 microdissected allografts at 2 and 6 weeks expressed as fold change relative to isografts from the same time points. Confirmation of key cytokines, receptors and ligands from the initial PCR array (A). Markers for M1 inflammatory macrophages are consistently higher in the interstium (left panel) at 2 weeks (filled bars) than at 6 weeks (open bars), whereas in the arterial compartment (right panel) the these markers are low at 2 weeks and increase by 6 weeks (B). In contrast, the converse occurs for markers of M2 wond healing macrophages with the exception of IL-10 (C). Error bars represent standard errors of the mean of 3 allografts. Differences between 2 and 6 week values were significant at the P<0.05*; <0.01**; or <0.001*** level as indicated.
Chemokine expression was confirmed on the protein level by ELISA on tissue homogenates of allografts. These homogenates were prepared from the apex of the heart that contains myocardium but no large arteries, and therefore, sampled the interstitial compartment. MIG, RANTES and MCP-1 were all elevated at 2 weeks and diminished by 6 weeks in parallel with interstitial infiltrates (Figure 4A). IL-1R2 protein was also elevated in the graft homogenates at 2 weeks by ELISA (Figure 4B).
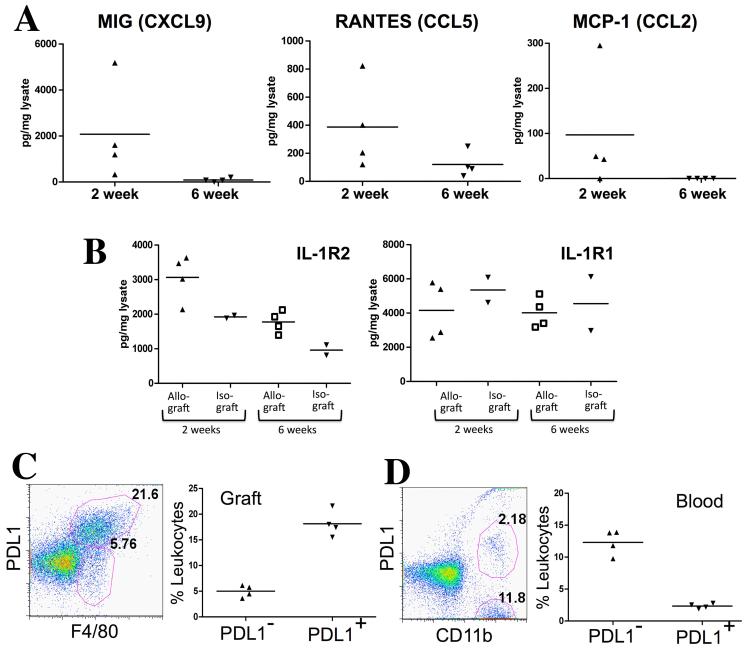
Figure 4.
Confirmation of expression of chemokines, IL-1R2 and PDL1 by ELISA and flow cytometry. MIG, RANTES and MCP-1 were elevated at 2 weeks and diminished by 6 weeks in cardiac allografts (A). IL-1R2 was elevated in allografts compared to isografts by ELISA (B). PDL1 was expressed by most F4/80+ macrophages infiltrating allografts at 2 weeks (P<0.05) (C). This represented a major enrichment compared to PDL1 expression on circulating monocytes (D). Each symbol in the scattergrams represents an individual animal.
PDL1 expression was assessed by flow cytometry on cells isolated from the allografts at 2 weeks. About 25-30% of CD45 labeled cells were F4/80+ macrophages, and PDL1 was expressed by almost 80% of these macrophages (Figure 4C). This represented a major enrichment compared to the low percentage of circulating monocytes that expressed PDL1 (16±0.6%; Figure 4D).
Blocking PDL1 early prevents resolution of acute interstitial infiltrates
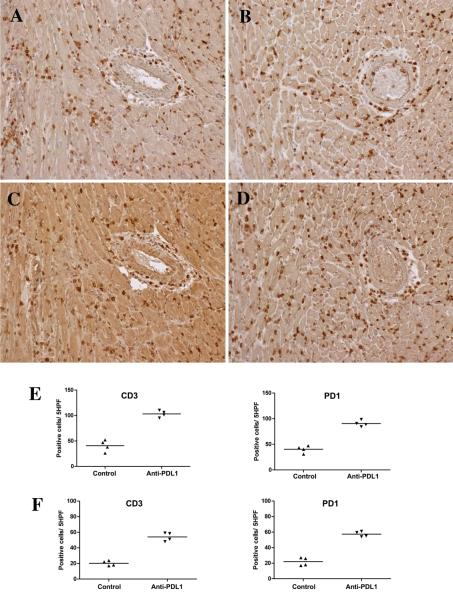
The effects of blocking the negative signals delivered by PDL1 on acute interstitial infiltrates was tested by treating female B6 recipients of male cardiac allografts with monoclonal antibody to PDL1 or isotype control antibody on days 8, 10 and 12. By 15 days, blockade of PDL1 caused a greater than two-fold increase in interstitial infiltrates of CD3+ T cells and PD1 expressing cells compared to the control group (Figure 5A-E), but did not increase arterial pathology. Double staining demonstrated that many cells co-expressed CD3 and PD1; single positive CD3 and PD1 cells were also detected (Supplemental Figure 4).
Figure 5.
Immunohistology and cell counts from mice treated with blocking antibodies to PDL1. Administering blocking antibody to PDL1 on days 8, 10 and 12 increased interstitial infiltrates of CD3+ T cells (B) and PD1+ (D) cells compared to controls (A and C, respectively) at 2 weeks. Blocking PDL1 did not increase arterial pathology at 2 weeks (right side of panels A-D). Cell counts per 5 high power fields verified about a 2-fold increase in CD3 and PD1 expressing cells at 2 weeks (E) and 6 weeks (F), but there was an overall decrease in cells from 2 to 6 weeks. Each symbol in the scattergrams represents an individual animal. All differences between control and anti-PDL-1 treated mice were significant <0.05.
To determine whether blockade of PDL1 would modulate chronic arterial pathology, we treated with antibody to PDL1 on days 34, 36 and 38, and sacrificed mice 6 weeks after transplantation. Blocking PDL1 caused about a 2 fold increase in CD3 and PD1 cells in the interstitium compared to controls, but the absolute numbers of cells was almost 50% lower than at 2 weeks after transplantation (Figure 5E,F). Treatment with PDL1 antibody at this later period after transplantation did not increase vascular pathology.
Effect of blocking PDL1 early on mediators in the interstitial and arterial compartments
Allografts to recipients treated with blocking antibody to PDL1 and control recipients were microdissected for PCR analysis to determine changes associated with accelerated rejection. Blocking PDL1:PD1 interactions caused an additional increase in MIG, RANTES, MCP-1 and IL-1R1 as well as IL-1β, IL-6, TNFα and Nos2 in the interstitium, but not in the arterial compartment at 2 weeks (Figure 6B,C). M2 macrophage markers were increased to a lesser extent (Figure 6D).
Figure 6.
Treatment with blocking antibodies to PDL1 resulted in increased expression of MIG, RANTES and MCP-1 in allografts by ELISA that was greater at 2 weeks than 6 weeks (A). These samples were taken from the apex of the hearts which contains few large arteries. Microdissection of allografts at 2 weeks demonstrated levels of MIG, RANTES, MCP-1, IL-1R1, IL-1β, IL-6 TNFa and Nos2 were greater in the interstitium than the arterial compartment (B, C). M2 macrophage markers were changed to a lesser extent (D). Bars represent average of 3-4 samples in each group. PCR results represent fold changes compared to allografts treated with control antibody. Differences between interstitial and arterial values were significant at the P<0.05*; <0.01**; or <0.001*** level as indicated.
The chemokine expression was confirmed on the protein level by ELISA on homogenates of control and experimental allografts. All three chemokines were upregulated by treatment with antibodies to PDL1 (Figure 6A). However, these chemokines were more highly expressed at 2 weeks than at 6 weeks.
TLR4 and hyaluronan are upregulated in the arterial compartment during chronic rejection
PCR array analysis showed IL1R2 and TLR4 were the most upregulated genes in the microdissected arterial compartment in allografts compared to isografts at 2 weeks. By 6 weeks, IL1R2 had decreased and TLR4 had dramatically increased (Table 1). This was confirmed by qPCR analysis (Supplemental Figure 5).
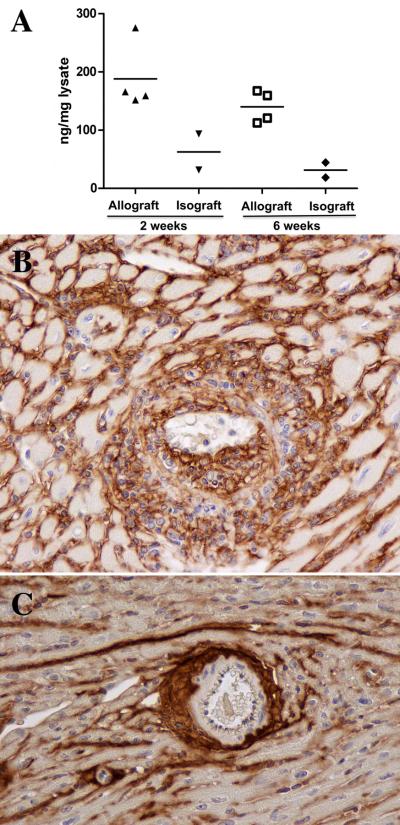
Among the endogenous ligands reported for TLR4 is fragmented hyaluronan, an extracellular matrix component that is known to be upregulated in various forms of arterial injury. Increased amounts of hyaluronan were detected by ELISA in homogenates of allografts at 2 and 6 weeks compared to isografts (Figure 7A). Immunohistology demonstrated increased hyaluronan in the interstitium at 2 weeks and in the neointimal lesions and surrounding adventitial infiltrates of arteries as well as the interstitium of cardiac allografts at 6 weeks (Figure 7B). In isografts, hyaluronan was present as a compact band in the adventitia (Figure 7C).
Figure 7.
Upregulation of hyaluronan in the arterial compartment at 6 weeks. ELISA measurements of hyaluronan in allografts and isografts (A). Differences at 6 weeks were significant P<0.01. Hyaluronan surrounded infiltrating mononuclear cells in the neointimal lesions and adventitial of arteries as well as the interstitium of (B). In isografts, hyaluronan formed a compact band in the adventitia of large and smaller arteries with limited amounts in the interstitum (C). Original magnifications 200x.
Low molecular weight hyaluronan increases MIG and MCP-1 production in cardiac allografts
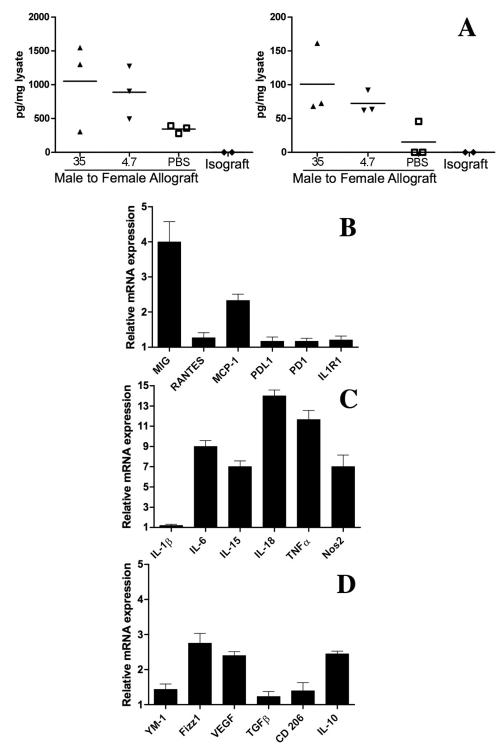
Inflammation can cause fragmentation of hyaluronan and circulating hyaluronan fragments have been found to stimulate chemokine production by macrophages through a TLR4- and TLR2-dependent mechanism (24). To test whether hyaluronan fragments increased MIG and MCP-1 in cardiac allografts, we administered 100ug of hyaluronan fragments daily to female recipients of male allografts. Controls were administered an equal volume of the PBS diluent. Both 4.7 or 35kD fragments of hyaluronan stimulated MIG and MCP-1 production in the cardiac allografts by 2 weeks (Figure 8A). Infiltrating cells were isolated from cardiac transplants to mice treated with PBS or 35kD fragments of hyaluronan, and after cell sorting for F4/80 expressing macrophages, mRNA was isolated and markers of M1 and M2 macrophages were probed by PCR. In addition to MIG and MCP-1, the M1 markers IL-6, IL-15, IL-18, TNFa and Nos2 were increased (Figure 8B-D).
Figure 8.
Both 4.7 or 35kD fragments of hyaluronan stimulated MIG and MCP-1 measure by ELISA on homogenates from the apex of cardiac allografts at 2 weeks (A). PCR on macrophages isolated from 3 heart allografts at 2 weeks demonstrated increased expression of MIG and MCP-1 as well as even greater increases in IL-6, IL-15, IL-18 TNFα and Nos2 (B,C). Markers for M2 macrophages were changed to a lesser extent (C). Each symbol in the ELISA scattergram represents results from an individual graft. PCR results are expressed as fold increase in macrophages from mice treated with hyaluronan fragments compared to PBS (n=3)
Discussion
This study was designed to discover mediators that differentially modulate acute mononuclear infiltrates in the myocardium and chronic infiltrates in the large arteries. As in human cardiac transplants, the infiltrates in acute and chronic rejection were predominantly composed of mononuclear cells with macrophages outnumbering T cells (1, 11). However, the acute and chronic pathology were dependent on T cells because no infiltrates developed in the absence of T cells, and passive transfer of CD4 T cells restored not only the characteristic T cell infiltrates, but also the extensive macrophage infiltrates and ultimately neointima formation with smooth muscle cells.
Macrophages are identified routinely in clinical endomyocardial biopsy by universal macrophage markers, such as CD68 (1, 11, 12). Subpopulations of macrophages are now recognized to have critical functional differences (10). The most clearly defined types of macrophages are classically activated or inflammatory M1 macrophages and alternatively activated or wound healing M2 macrophages. Sustained production of IFNγ by T helper 1 (Th1) cells induces inflammatory macrophages during adaptive immune responses; whereas wound healing macrophages develop in response to production of IL-4 by T helper 2 (Th2) cells. No clinical or experimental studies have assessed macrophage subpopulations in cardiac allografts, but Famulski et al (25) have reported that M2 macrophages increase with time during T cell-mediated rejection in mouse renal allografts. We found relatively few Ym-1 expressing M2 macrophages in the acute interstitial infiltrates that increased modestly with time. Moreover, the M2 macrophages were largely ovoid without cytoplamic extensions. These histological findings were supported by 5 molecular markers of M2 macrophages (Ym1, Fizz1, VEGF, TGFβ, and CD206), which were expressed at low levels in the interstitium and increased at 6 weeks.
In contrast, 6 markers of M1 macrophages (IL-1β, IL-6, IL-15, IL-18, TNFα and Nos2) were markedly elevated in the interstitium at 2 weeks and decreased by 6 weeks.
These changes in M1 markers paralleled the numbers and morphology of galectin-3 expressing macrophages, which had many cytoplasmic extensions that made contact with endothelial cells and myocytes. In hearts, galectin-3 has been associated with fibrosis (22). Furthermore, MIG, a signature cytokine produced by inflammatory macrophages, was elevated in interstitial samples by microarray and ELISA. MIG has been reported to co-localize with infiltrating CD68+ macrophages in clinical endomyocardial biopsies during acute cellular rejection (12).
In the transition to chronic rejection, galectin-3-expressing macrophages and MIG decreased in the interstitium, and increased in the arterial compartment. In vitro, galectin-3 stimulates human macrophages to upregulate inflammatory genes including RANTES and MCP-1 (23). These 2 chemokines were upregulated acutely in the macrophage-rich interstitium and chronically in the arterial compartment.
In addition to increased expression of chemoattractants for macrophages and T cells, ligands for both positive and negative co-stimulatory receptors on T cells were upregulated in the intersititum. While CD80 and CD86 can stimulate T cells through CD28, PDL1 delivers a negative signal to T cells that express PD1. The majority of T cells in the interstitium expressed PD1 on immunohistology. The functional relevance of the interaction between PDL1 and PD1 was demonstrated by treating mice with a blocking antibody to PDL1. Blocking PDL1:PD1 interaction has been reported to increase acute and chronic rejection of cardiac allografts in other murine models depending upon the histoincompatibility and treatment schedule (9, 26-28). In our model, treatment in the acute phase caused a doubling of the number of PD1 positive cells in the interstitium and resulted in increased tissue injury. Blocking PDL1 in the chronic phase also increased the number of PD1 positive cells in the interstitium, but to a lesser extent than in the acute phase. Increased infiltrates of macrophages and T cells were accompanied by increased MIG and RANTES production in the interstitium. Yang et al (29) reported that blocking PDL1 decreased FoxP3 expressing T regulatory cells in the spleen, but not in cardiac allografts. We also found only limited numbers of FoxP3 cells in the interstitium or arterial compartment of cardiac allografts. Of note, blocking PDL1 changed the balance of IL-1 receptors. At 2 weeks IL-1R2 predominated in untreated mice, but blocking PDL1 resulted in the upregulation of IL-1R1. IL-1R2 is structurally similar to IL-1R1, but has a truncated cytoplasmic domain that prevents transmembrane signaling (17). IL-1R2 competes with IL-1R1 for ligands and for the IL-1 receptor accessory protein. By acting as a decoy receptor for IL-1 on macrophages, IL-1R2 modulates the inflammatory effects of IL-1. The counterbalance between IL-1R2 and PD1 in our model parallels the clinical findings in the multicenter CARGO study of gene profiles in peripheral blood mononuclear cells, which reported that IL-1R2 was the gene most highly correlated with stable graft function and PD1 with acute rejection of cardiac transplants (15, 16). Our examination of graft infiltrates by flow cytometry indicated that macrophages were equipped to modulate acute rejection because the majority expressed PDL1.
By microdissecting tissue sections from cardiac allografts rather than homogenizing entire samples, we examined the compartmentalization of mediators and ligands. On microarray screen, only IL-1R2, TLR2 and 4 were more highly expressed in the arterial than interstitial compartment in the acute phase. By 6 weeks, TLR4 increased in both compartments, but more in arteries. TLR4 has several endogenous ligands that are upregulated in injured arteries including galectin-3 and hyaluronan (30, 31). The extracellular matrix macromolecule hyaluronan has been found to increase in the hyperplastic intima of restenotic arteries (32). Macrophages engage in extracellular matrix remodeling both degrading and synthesizing hyaluronan (33). Changes in distribution of hyaluronan are not studied routinely in clinical cardiac transplants, but have been implicated in rejection of human lung transplants (34, 35). Hyaluronan levels are also increased in male skin 2 weeks after allotransplantation to female recipients (35). Similarly, in our model, hyaluronan was increased in homogenates of cardiac transplants at 2 and 6 weeks. In addition, we demonstrated that increased amounts of hyaluronan were localized in the neointima of arteries in cardiac allografts, which contained activated macrophages that could contribute to fragmentation of the hyaluronan. Circulating hyaluronan fragments stimulate chemokine production by macrophages through a TLR4- and TLR2-dependent mechanism (24). In our model the administration of low molecular weight hyaluronan fragments stimulated MIG and MCP-1 expression as well as M1 markers in macrophages isolated from allografted hearts.
In summary, we found a differential expression of inflammatory signals in the interstitial and arterial compartments of cardiac transplants that changed from the acute to chronic phases of rejection. Our data indicate that upregulation of PDL1 in the interstitium contributes to the resolution of acute interstitial infiltrates. In the arterial compartment recognition of endogenous ligands including hyaluronan by TLR4 may promote progression of arteriopathy.
Supplementary Material
Figure S1. Control cardiac grafts stained for CD3 and Mac2. Female B6 to female B6 isograft control containes only scattered CD3 T cells (A) and Mac2 macrophages (B). In the absence of T cell reconstitution, no CD3 infiltrates developed in the male B6 cardiac allografts to female B6.RAG−/− recipients (C). Mac2 stained scattered interstitial resident macrophages (D). Original magnifications 100x (A,B) and 200x (C,D).
Figure S2. Few macrophages expressed Ym-1 (Chitinase 3-like 3) at 2 (A) or 6 weeks (B). These M2 macrophages were scattered large ovoid cells (inset). Few Foxp3 T cells were present in interstitial and arterial compartments at 2 (C) and 6 weeks (D). Treatment with blocking antibody to PDL1 did not appreciably alter the FoxP3 T cell infiltrates at 2 (E) or 6 weeks (F). Original magnifications 200x (inset 600x).
Figure S3. Male hearts transplanted to female RAG−/− recipients reconstituted with Marilyn CD4 T cells and sacrificed at 10 weeks. Large artery with limited numbers of T cells (A), large numbers of macrophages in the neointima as well as adventitia (B), and alpha-smooth muscle expressing cells in the neointima (C). Original magnifications 200x.
Figure S4. Immunohistology of acute interstitial infiltrates in allograft following treatment with blocking antibodies to PDL1. Double stain for CD3 (brown) and PD1 (blue) demonstrates the majority of cells express both CD3 and PD1 (original magnification 600x).
Figure S5. TLR4 quantification by qPCR analysis on interstital and arterial tissue from allografts relative to isografts at 2 and 6 weeks.
Acknowledgments
We thank Katayoun Ayasoufi for providing us with Marilyn mice; Denise A. Hatala for sectioning tissues to microdissect; and Drs. Hong Yu, Xi Wang and Ran Fan for perfoming cardiac grafts. This work was supported by NIH grant 1P01 AI087586.
Abbreviations
- CARGO
Cardiac Allograft Rejection Gene Expression Observational Study
- CAV
Cardiac allograft vasculopathy
- IL-1R2
Decoy receptor for IL-1
- PD1; CD279
Programmed cell death protein 1
- PDL1; CD274
Programmed cell death protein ligand 1
- PDL2; CD273
Programmed cell death protein ligand 2
- SDF-1; CXCL12
Stromal cell derived factor 1
- TLR
Toll Like Receptor
Footnotes
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Lu WH, Palatnik K, Fishbein GA, Lai C, Levi DS, Perens G, et al. Diverse morphologic manifestations of cardiac allograft vasculopathy: a pathologic study of 64 allograft hearts. J Heart Lung Transplant. 2011;30(9):1044–1050. doi: 10.1016/j.healun.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 3.van Loosdregt J, van Oosterhout MF, Bruggink AH, van Wichen DF, van Kuik J, de Koning E, et al. The chemokine and chemokine receptor profile of infiltrating cells in the wall of arteries with cardiac allograft vasculopathy is indicative of a memory T-helper 1 response. Circulation. 2006;114(15):1599–1607. doi: 10.1161/CIRCULATIONAHA.105.597526. [DOI] [PubMed] [Google Scholar]
- 4.Wehner JR, Fox-Talbot K, Halushka MK, Ellis C, Zachary AA, Baldwin WM., 3rd B cells and plasma cells in coronaries of chronically rejected cardiac transplants. Transplantation. 2010;89(9):1141–1148. doi: 10.1097/TP.0b013e3181d3f271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stehlik J, Starling RC, Movsesian MA, Fang JC, Brown RN, Hess ML, et al. Utility of long-term surveillance endomyocardial biopsy: a multi-institutional analysis. J Heart Lung Transplant. 2006;25(12):1402–1409. doi: 10.1016/j.healun.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. The Journal of experimental medicine. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116(18):2062–2071. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- 8.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozkaynak E, Wang L, Goodearl A, McDonald K, Qin S, O'Keefe T, et al. Programmed death-1 targeting can promote allograft survival. J Immunol. 2002;169(11):6546–6553. doi: 10.4049/jimmunol.169.11.6546. [DOI] [PubMed] [Google Scholar]
- 10.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michaels PJ, Kobashigawa J, Laks H, Azarbal A, Espejo ML, Chen L, et al. Differential expression of RANTES chemokine, TGF-beta, and leukocyte phenotype in acute cellular rejection and quilty B lesions. J Heart Lung Transplant. 2001;20(4):407–416. doi: 10.1016/s1053-2498(00)00318-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhao DX, Hu Y, Miller GG, Luster AD, Mitchell RN, Libby P. Differential expression of the IFN-gamma-inducible CXCR3-binding chemokines, IFN-inducible protein 10, monokine induced by IFN, and IFN-inducible T cell alpha chemoattractant in human cardiac allografts: association with cardiac allograft vasculopathy and acute rejection. J Immunol. 2002;169(3):1556–1560. doi: 10.4049/jimmunol.169.3.1556. [DOI] [PubMed] [Google Scholar]
- 13.Fahmy NM, Yamani MH, Starling RC, Ratliff NB, Young JB, McCarthy PM, et al. Chemokine and chemokine receptor gene expression indicates acute rejection of human cardiac transplants. Transplantation. 2003;75(1):72–78. doi: 10.1097/00007890-200301150-00013. [DOI] [PubMed] [Google Scholar]
- 14.Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. The New England journal of medicine. 2010;362(20):1890–1900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 15.Mehra MR, Kobashigawa JA, Deng MC, Fang KC, Klingler TM, Lal PG, et al. Transcriptional signals of T-cell and corticosteroid-sensitive genes are associated with future acute cellular rejection in cardiac allografts. J Heart Lung Transplant. 2007;26(12):1255–1263. doi: 10.1016/j.healun.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Mehra MR, Kobashigawa JA, Deng MC, Fang KC, Klingler TM, Lal PG, et al. Clinical implications and longitudinal alteration of peripheral blood transcriptional signals indicative of future cardiac allograft rejection. J Heart Lung Transplant. 2008;27(3):297–301. doi: 10.1016/j.healun.2007.11.578. [DOI] [PubMed] [Google Scholar]
- 17.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nature reviews. 2010;10(2):89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 18.Setoguchi K, Hattori Y, Iida S, Baldwin WM, 3rd, Fairchild RL. Endogenous memory CD8 T cells are activated within cardiac allografts without mediating rejection. Am J Transplant. 2013;13(9):2293–2307. doi: 10.1111/ajt.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill DR, Kessler SP, Rho HK, Cowman MK, de la Motte CA. Specific-sized hyaluronan fragments promote expression of human beta-defensin 2 in intestinal epithelium. The Journal of biological chemistry. 2012;287(36):30610–30624. doi: 10.1074/jbc.M112.356238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erdinc Sunay MM, Fox-Talbot K, Velidedeoglu E, Baldwin WM, 3rd, Wasowska BA. Absence of FcgammaRIII Results in Increased Proinflammatory Response in FcgammaRIII-KO Cardiac Recipients. Transplantation. 2013 doi: 10.1097/TP.0b013e31829c2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110(19):3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 23.Papaspyridonos M, McNeill E, de Bono JP, Smith A, Burnand KG, Channon KM, et al. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(3):433–440. doi: 10.1161/ATVBAHA.107.159160. [DOI] [PubMed] [Google Scholar]
- 24.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature medicine. 2005;11(11):1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 25.Famulski KS, Kayser D, Einecke G, Allanach K, Badr D, Venner J, et al. Alternative macrophage activation-associated transcripts in T-cell-mediated rejection of mouse kidney allografts. Am J Transplant. 2010;10(3):490–497. doi: 10.1111/j.1600-6143.2009.02983.x. [DOI] [PubMed] [Google Scholar]
- 26.Koga N, Suzuki J, Kosuge H, Haraguchi G, Onai Y, Futamatsu H, et al. Blockade of the interaction between PD-1 and PD-L1 accelerates graft arterial disease in cardiac allografts. Arteriosclerosis, thrombosis, and vascular biology. 2004;24(11):2057–2062. doi: 10.1161/01.ATV.0000145015.23656.e4. [DOI] [PubMed] [Google Scholar]
- 27.Tao R, Wang L, Han R, Wang T, Ye Q, Honjo T, et al. Differential effects of B and T lymphocyte attenuator and programmed death-1 on acceptance of partially versus fully MHC-mismatched cardiac allografts. J Immunol. 2005;175(9):5774–5782. doi: 10.4049/jimmunol.175.9.5774. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Popoola J, Khandwala S, Vadivel N, Vanguri V, Yuan X, et al. Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation. 2008;117(5):660–669. doi: 10.1161/CIRCULATIONAHA.107.741025. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Riella LV, Chock S, Liu T, Zhao X, Yuan X, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol. 2011;187(3):1113–1119. doi: 10.4049/jimmunol.1100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuff CA, Kothapalli D, Azonobi I, Chun S, Zhang Y, Belkin R, et al. The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. The Journal of clinical investigation. 2001;108(7):1031–1040. doi: 10.1172/JCI12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circulation research. 2011;108(9):1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashima Y, Takahashi M, Shiba Y, Itano N, Izawa A, Koyama J, et al. Crucial role of hyaluronan in neointimal formation after vascular injury. PloS one. 2013;(3):8, e58760. doi: 10.1371/journal.pone.0058760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang MY, Chan CK, Braun KR, Green PS, O'Brien KD, Chait A, et al. Monocyte-to-macrophage differentiation: synthesis and secretion of a complex extracellular matrix. The Journal of biological chemistry. 2012;287(17):14122–14135. doi: 10.1074/jbc.M111.324988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirali AC, Goldstein DR. Activation of the innate immune system by the endogenous ligand hyaluronan. Current opinion in organ transplantation. 2008;13(1):20–25. doi: 10.1097/MOT.0b013e3282f3df04. [DOI] [PubMed] [Google Scholar]
- 35.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant. 2006;6(11):2622–2635. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Control cardiac grafts stained for CD3 and Mac2. Female B6 to female B6 isograft control containes only scattered CD3 T cells (A) and Mac2 macrophages (B). In the absence of T cell reconstitution, no CD3 infiltrates developed in the male B6 cardiac allografts to female B6.RAG−/− recipients (C). Mac2 stained scattered interstitial resident macrophages (D). Original magnifications 100x (A,B) and 200x (C,D).
Figure S2. Few macrophages expressed Ym-1 (Chitinase 3-like 3) at 2 (A) or 6 weeks (B). These M2 macrophages were scattered large ovoid cells (inset). Few Foxp3 T cells were present in interstitial and arterial compartments at 2 (C) and 6 weeks (D). Treatment with blocking antibody to PDL1 did not appreciably alter the FoxP3 T cell infiltrates at 2 (E) or 6 weeks (F). Original magnifications 200x (inset 600x).
Figure S3. Male hearts transplanted to female RAG−/− recipients reconstituted with Marilyn CD4 T cells and sacrificed at 10 weeks. Large artery with limited numbers of T cells (A), large numbers of macrophages in the neointima as well as adventitia (B), and alpha-smooth muscle expressing cells in the neointima (C). Original magnifications 200x.
Figure S4. Immunohistology of acute interstitial infiltrates in allograft following treatment with blocking antibodies to PDL1. Double stain for CD3 (brown) and PD1 (blue) demonstrates the majority of cells express both CD3 and PD1 (original magnification 600x).
Figure S5. TLR4 quantification by qPCR analysis on interstital and arterial tissue from allografts relative to isografts at 2 and 6 weeks.