Abstract
Background/Aims
Stress exacerbates neuron loss in many CNS injuries via the actions of adrenal glucocorticoid (GC) hormones. For some injuries, this GC-endangerment of neurons is accompanied by greater immune cell activation in the CNS, a surprising outcome given the potent immunosuppressive properties of GCs.
Methods
To determine whether the effects of GCs on inflammation contribute to neuron death or result from it, we tested whether non-steroidal anti-inflammatory drugs could protect neurons from GCs during kainic acid excitotoxicity in adrenalectomized male rats. We next measured GC effects on (i) chemokine production (CCL2, CINC-1), (ii) signals that suppress immune activation (CX3CL1, CD22, CD200, and TGF-b), and (iii) NF-kB activity.
Results
Concurrent treatment with minocycline but not indomethacin prevented GC-endangerment. GCs did not substantially affect CCL2, CINC-1, or baseline NF-kB activity, but they did suppress CX3CL1, CX3CR1, and CD22 expression in the hippocampus, factors that normally restrain inflammatory responses.
Conclusions
These findings demonstrate that cellular inflammation is not necessarily suppressed by GCs in the injured hippocampus; instead, GCs may worsen hippocampal neuron death, at least in part, by increasing the neurotoxicity of CNS inflammation.
Keywords: Inflammation, excitotoxicity, stress, minocycline, NF-kappaB
Introduction
Glucocorticoid (GC) stress hormones have long been appreciated for their potent anti-inflammatory properties, but closer investigation has revealed that depending on the context and duration of exposure, GCs can increase some of the same inflammatory responses they normally inhibit [1–5]. In particular, acute GC exposure (from a single stressor) prior to an immune challenge increases leukocyte recruitment to sites of peripheral inflammation in rodents [6–8] and in humans [9, 10]. This priming effect [11] of GCs switches to the classical immunosuppressive effect if GCs are given after injury [6, 12] or chronically (for weeks) [13]. Timing, concentration, and duration of GC exposure are therefore all critical in determining whether these hormones will be immune-activating or immunosuppressive [3, 4, 14], but the specific situations leading to each outcome are not well-documented.
In the CNS, these immune-augmenting GC effects may be detrimental to neuron survival. Subacute or chronic GC exposure that would normally suppress inflammatory responses in the periphery instead lead to increased CNS inflammation in response to bacterial lipopolysaccharide (LPS) [15, 16] and excitotoxin [2, 17], particularly in GR-rich regions like the frontal cortex and hippocampus. Hallmarks of this increased immune response include increased activity of the pro-inflammatory transcription factor NF-kB and a failure of GCs to induce some of their normal anti-inflammatory targets like MKP-1, IL-1ra, and IkBa in the forebrain [1]. In sub-cortical regions like the hypothalamus or in the periphery, these same GC treatments still lead to a classical decrease in NF-kB activation [16]. Prolonged GC exposure can lead to compensatory GR down-regulation, but immune cell-specific GR knockouts and a GR antagonist or GC-synthesis inhibitor can block GC-enhanced inflammation, arguing against this explanation [2, 16, 17].
Exposure to GCs also impairs neuron survival during many types of acute CNS injury including hypoxia-ischemia, excitotoxicity, and hypoglycemia [18]. GCs impair several processes that are necessary for neurons to survive an injury: the re-uptake of excitotoxic glutamate from the synapse [19], sequestration and extrusion of free cytosolic calcium [20], and oxygen radical quenching [21]. Classical anti-inflammatory GC effects would also include suppressing NF-kB activity [22], and reducing the abundance of pro-inflammatory mediators like IL-1beta [23] and prostaglandins [24]. This might be expected to be neuroprotective because inhibiting IL-1beta [25], PGE2 [26, 27], or NF-kB activation [28] protects against excitotoxic neuron death. As described, however, in some situations GCs can increase the production of these inflammatory mediators, so it could also be the case that GC effects on inflammation further contribute to neuron death.
We therefore sought to investigate whether the effects of GCs on the inflammatory response also contribute to neuron death during excitotoxic injury. To see whether we could manipulate GC-augmented inflammation post-injury, we attempted to inhibit it using two anti-inflammatory drugs with different mechanisms of action, indomethacin and minocycline. Indomethacin is a non-selective inhibitor of the COX enzymes that are the rate-limiting step in the production of prostaglandins. Two months of treatment with indomethacin only slightly reduces microglial activation post-irradiation [29] so we did not expect this drug to necessarily have a large effect unless COX enzymes were specifically required. In contrast, minocycline is reported to be a potent inhibitor of microglial activation [30].
We present evidence that blocking certain aspects of the inflammatory response can protect neurons from GC-endangerment. We also investigate the possibility that GCs condition the CNS in unique ways, priming it to have a greater and potentially more damaging immune response to subsequent injury. The dogma that GCs are only immunosuppressive is being refined as the actions of these hormones are able to be explored with greater contextual resolution.
Materials and Methods
Experimental Design
Male Sprague-Dawley rats between 250–300 g (Charles River, Gilroy, CA) were housed in groups of three in a 12 h light–dark cycle and fed ad libitum. Animals were given a minimum of one week to acclimate to our vivarium after purchase.
To measure the effects of different GC exposures, rats were anesthetized with 2% halothane and bilaterally adrenalectomized (ADX) or sutured without ADX (sham). ADX rats were given 100 mg s.c. pellets with either 15, 30, 60, or 100% corticosterone/cholesterol mixture. These treatments yield blood GC levels within physiological limits [17, 31]. Rats given 15% pellets (low GCs) have blood GC levels around 2–4 μg/dL, corresponding with basal hormone levels. Rats given 100% pellets were also given daily 10 mg/kg s.c. injections of corticosterone in peanut oil to maintain high blood GC levels between 30–40 μg/dL. Intermediate 30% and 60% pellets produce blood hormone levels that correspond with moderate stress, or 15–20 μg/dL and 20–30 μg/dL [1]. These animals were compared with each other to isolate the effects of GC concentration, but they were also compared with sham animals that received a sham surgery to account for the effects of ADX. For comparison, the injury-induced increase above baseline in peripheral corticosterone levels in sham animals post-KA is: 30 min post-injury: 32 ± 2 μg/dL, 1 hour post-injury: 27 ± 4 μg/dL, 3 hours post-injury: 14 ± 8 μg/dL, 6 hours post-injury: 12 ± 4 μg/dL, 12 hours post-injury: 3 ± 2 μg/dL [19, 31].
To study the effects of GCs on neuron death, we used the kainic acid (KA) model of excitotoxicity, where rats are given stereotactic injections of KA into the hilus of the dentate gyrus in the hippocampus. This model leads to reproducible amounts of damage and we previously found that sub-acute exposure to high GCs (~30μg/dL) can increase the inflammatory response to this injury in vivo, with similar effects in primary culture [17, 32]. After three days of exposure to GC treatments, rats were anesthetized with ketamine/xylazine/acepromazine cocktail (77/7.7/1.54 mg/kg, i.p.) and were counter-balanced to first receive either 1μl of phosphate-buffered saline (PBS) in the left hemisphere or 1μl of 0.06 μg of kainic acid (KA) dissolved in PBS in the right hemisphere at an infusion rate of 0.2 μl/min. Coordinates: ±3.2 mm lateral, 4.5 mm posterior to bregma, 3.3 mm ventral to dura. Buprenorphine (0.03 mg/kg, s.c.) was given as postoperative analgesic. In animals receiving additional drug treatments, indomethacin was injected i.p. at 10 mg/kg beginning 24 h prior to KA and every 12 h until rats were killed. Minocycline was given s.c. at 22.5 mg/kg 30 min prior to KA, and daily for the next 72 h. All drugs were purchased from Sigma (St. Louis, MO). Unless otherwise indicated, rats were sacrificed between 2–4 hours after lights on. All rats were treated in accordance with the Stanford University Administrative Panel on Laboratory Care regulations and the NIH guide for the care and use of laboratory animals.
Lesion quantification
Rats were transcardially perfused 72 h post-KA with 0.9% saline followed by 4% PFA. Brains were post-fixed in 4% PFA and 30% sucrose for 24 h prior to cutting 20 μm frozen cryostat sections. Sections were stained with cresyl violet, and 40x images of the CA3 region were acquired using an Olympus IX70 microscope and Hamamatsu digital camera. CA3 damage was calculated by dividing the remaining CA3 cell area in the KA-injected hemisphere by that in the contralateral PBS-injected hemisphere as previously described [17].
Immunohistochemistry
Sections were air-dried for 20 min, blocked in 5% normal goat serum in PBS + 0.3% Triton-X 100 (PBST) for 1 h at room temperature, and incubated overnight at 4°C with mouse anti-rat CD11b/c at 1:100 dilution in PBST (OX-42 clone, Serotec). Sections were washed in PBST 3x for 10 min and incubated for 1 h at room temperature with goat anti-mouse Alexa 488 (1:200 in PBST, Invitrogen). Sections were washed again in PBST 3x for 10 min and stored in polyvinyl alcohol with DABCO (Sigma). Metamorph (Universal Imaging) was used to quantify the amount of CD11b/c signal according to guidelines for quantifying fluorescent signal [30]. Briefly, 5 evenly-spaced sections from the hippocampus of each rat were stained and uniformly background corrected. Signal was only included if it was 10 pixel intensity units above background and between 3 to 17 μm in diameter. These parameters were empirically determined based on the average size of microglia cell bodies [33] to eliminate autofluorescent debris and artifacts. Total signal estimates are therefore not an approximation of microglia number, but are an approximation of the area they encompass [34].
Quantitative PCR
Total RNA was extracted using the RNEasy Plus Mini Kit (Qiagen), and was reverse-transcribed using the iScript cDNA synthesis kit (BioRad). Intron-spanning primers used were (sense, antisense): CX3CL1 (NM_134455): 5′-GCATGACGAAATGCAACATC-3′, 5′-TTGGACCCATTTCTCCTTTG-3′; CD22 (NM_001107503): 5′-CTGTGGCCGTGGAGATAGAT-3′, 5′-AAGAGGGTTTGGGGATGTTC-3′; CD200 (NM_031518): 5′-TGTTTGGATCTGGGAAGGTC-3′, 5′-GGAGGATGCTGGTGACAGAT-3′; TGF-b1 (NM_021578): 5′-GCGGACTACTACGCCAAAGA-3′, 5′-CGTGTTGCTCCACAGTTGAC-3′; IL-1ra (NM_001039701): 5′-GAAAAGACCCTGCAAGATGC-3′, 5′-GATGCCCAAGAACACATTCC-3′. Quantitative PCR was performed on a BioRad PCR machine using SyBRGreen and PCR Miner software [35].
Electrophoretic mobility shift assay
Following GC treatment, the CA3 was microdissected and nuclear extracts were prepared [16]. EMSA to NF-kB was performed using the gel shift assay kit from Promega and the consensus oligonucleotide probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′) [36]. Autoradiographs of non-denaturing acrylamide electrophoresis were quantified with the ChemImager detection system (Alpha-Innotech, San Leandro, CA), and several exposure times to ensure linearity of band intensities.
ELISAs
Protein was prepared from dissected CA3 tissue in NP-40 lysis buffer (150mM sodium chloride, 1.0% Nonidet-P40, 50mM Tris, pH 8.0) and quantified by the BCA method (Pierce). ELISAs for MCP-1, CINC-1, and IL-1b (R&D) were run as per manufacturer instructions and quantified on a Molecular Devices plate-reader. No values fell outside of the manufacturer-reported limits for these ELISAs: MCP-1: 10 pg/mL, CINC-1: 1.3 pg/mL, IL-1b: 5 pg/mL.
Statistics
Data are presented as mean ± SEM. Statistical tests and sample sizes used to establish significance are indicated in figure legends. All statistical tests were performed using GraphPad Prism.
Results
GC-augmented Immune Cell Activation in the Injured Hippocampus is Blocked by Minocycline but not Indomethacin
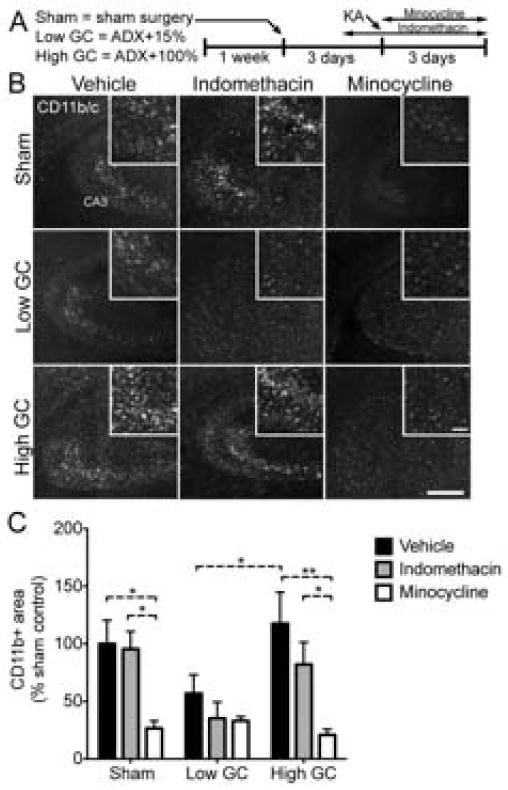
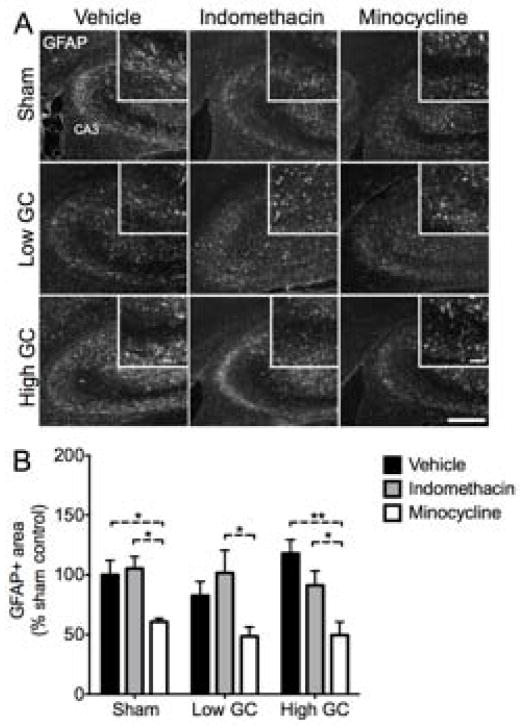
We selected two different anti-inflammatory drugs that block different components of the inflammatory response to injury. Rats were given injections of either indomethacin or minocycline concurrent with kainic acid-induced excitotoxic injury (Fig. 1A). Three days post-injury, animals were killed and brain sections were stained for activated infiltrating and resident immune cells (CD11b/c) in the injury site (Fig. 1B,C). The same sections were also co-stained with GFAP to indicate the area of gliosis resulting from the injury (Fig. 2).
Fig. 1.
CD11b/c+ area is increased by GCs and reversed by minocycline following KA injury. (A) Experimental design: Male rats were given 1 week to acclimate after arriving at our animal facility. GC manipulations were performed three days prior to KA, and brains were analyzed three days later. Indomethacin was injected every 12 h starting 24 h before KA. Minocycline was injected every 24 h starting 30 min before KA. (B) Representative images of CD11b/c staining in the hippocampal CA3 region 72 h post-injury. Scale bars = 500 μm, 100 μm inset. (C) The total amount of CD11b/c+ area was significantly lower in low GC treated rats compared with high GC rats. Minocycline significantly reduced CD11b/c signal in sham rats and high GC rats, but not low GC rats. Significance levels * p < 0.05 and ** p < 0.01 established by 2-way ANOVA followed by Holm-Sidak post hoc test. Significant 2-way ANOVA main effect of GC treatment (p < 0.05) and drug treatment (p < 0.0001). Sham, vehicle n = 7; low GC, vehicle n = 12; high GC, vehicle n = 6; Sham, indomethacin n = 6; low GC, indomethacin n = 5; high GC, indomethacin n = 9; Sham, minocycline n = 7; low GC, minocycline n = 7; high GC, minocycline n = 6.
Fig. 2.
GFAP+ area is reduced by minocycline following KA injury. (A) Representative images of GFAP staining in the hippocampal CA3 region 72 h post-injury. Scale bars = 500 μm, 100 μm inset. (B) The total amount of GFAP+ area was reduced by minocycline treatment but not by indomethacin treatment or GC manipulations. Significance levels * p < 0.05 and ** p < 0.01 established by 2-way ANOVA followed by Holm-Sidak post hoc test. Significant 2-way ANOVA main effect of drug treatment (p < 0.0001). Sham, vehicle n = 7; low GC, vehicle n = 12; high GC, vehicle n = 6; Sham, indomethacin n = 6; low GC, indomethacin n = 5; high GC, indomethacin n = 9; Sham, minocycline n = 7; low GC, minocycline n = 7; high GC, minocycline n = 6.
Low GC animals had approximately 50% of the CD11b/c+ area in sham animals. This decrease, although not significant, was significantly increased in animals given high GCs to an amount of CD11b/c+ area similar to that observed in sham animals. Concurrent treatment with the non-selective COX-inhibitor indomethacin had no significant effects on CD11b/c signal in any of the groups. In contrast, minocycline, an inhibitor of NF-kB and microglia activation [30,37,38], significantly reduced CD11b/c signal in both sham and high GC animals. The amount of GFAP signal was similar between all groups, but was reduced by approximately 50% in animals receiving minocycline (Fig. 2B). These results demonstrate that elevated GC concentrations are sufficient to increase inflammatory cell activation and show that these increases are sensitive to minocycline but not indomethacin treatment.
GC-augmented Cellular Inflammation Impairs Neuron Survival
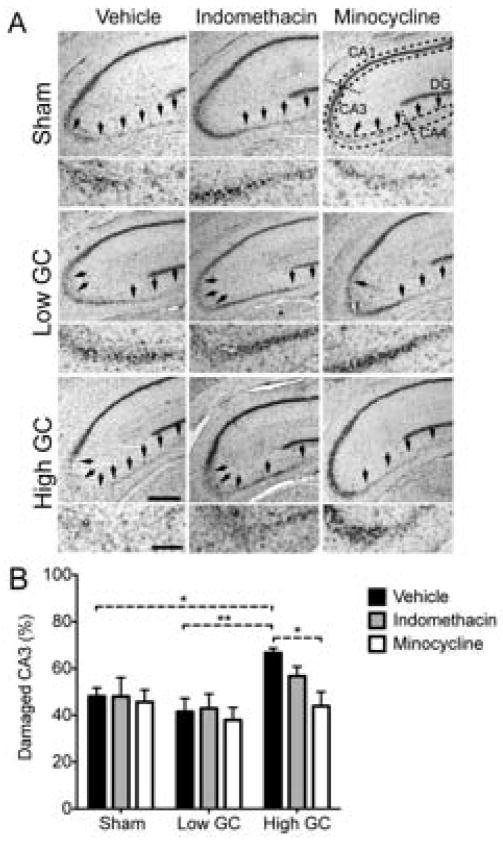
We next measured whether these anti-inflammatory drug treatments could protect neurons from GC endangerment. To quantify neuron death, the damaged area in the CA3 hippocampal field was measured in subsequent tissue sections (Fig. 3A, arrows). The dose of KA delivered to the hilus of the dentate gyrus resulted in approximately 50% CA3 cell loss. In agreement with previous examples of GC endangerment [17, 31, 39], high GCs significantly worsened CA3 neuron death (Fig. 3B). Indomethacin was unable to protect CA3 neurons from this GC endangerment, but minocycline restored CA3 damage to levels observed in sham animals. This result is consistent with the differing ability of these drugs to block the cellular response to injury and supports the hypothesis that augmented inflammatory responses contribute to GC endangerment of neurons. Importantly, we did not observe a neuroprotective effect of reducing inflammation in all groups (e.g. low GC animals). We next sought to improve our understanding of how these GC manipulations alter post-injury inflammation.
Fig. 3.
High GCs increase excitotoxic injury and this is reversed by minocycline. (A) Representative images of Nissl-stained sections following each treatment combination at 72 h post-KA. Damage was quantified in the CA3 region (magnified below each image). Hippocampal sub-regions are indicated with dashed lines. Arrows point to areas of neuron loss. Scale bars = 500 μm, (200 μm, magnification). (B) Quantification of CA3 lesioned area 72 h post-injury. High GCs significantly increased CA3 damage relative to low GCs and sham rats. This was reversed by minocycline, but not indomethacin treatment. Significance levels * p < 0.05 and ** p < 0.01 established by 2-way ANOVA followed by Holm-Sidak post hoc test. Significant 2-way ANOVA main effect of GC treatment (p < 0.01). Sham, vehicle n = 7; low GC, vehicle n = 12; high GC, vehicle n = 6; Sham, indomethacin n = 6; low GC, indomethacin n = 5; high GC, indomethacin n = 9; Sham, minocycline n = 7; low GC, minocycline n = 7; high GC, minocycline n = 6.
GCs Dose-Dependently Influence Chemokine Release Post-Injury
One possible explanation for GC-enhanced immune activation is that GCs increase the signals that activate or recruit immune cells. The injured CA3 region was micro-dissected at several time points post-injury to measure the levels of two principal chemokines involved in recruiting monocytes (CCL2) and granulocytes (CINC-1) (Fig. 4). A careful time-course revealed that both low and high GC animals had earlier CCL2 and higher CINC-1 levels in the injury site (Fig. 4A,C). For CINC-1, the chemokine release was also sharper, increasing from and decreasing to levels well below those measured in sham animals. Both chemokine levels had nearly returned to baseline by 48 hours post-injury. Because both low and high GC groups responded similarly, we wondered if these were effects of ADX, so we picked the 8 hour time point to compare intermediate GC concentrations of 30 and 60% (Fig. 4B,D). Chemokine levels at this time post-injury revealed a U-shaped dose-response to GC hormone levels. A non-linear fit of a second-order polynomial curve produced R-squared values of 0.89 for CCL2 and 0.82 for CINC-1.
Fig. 4.
GCs alter chemokine release post-injury. CA3 tissue was collected at 2, 4, 8, 12, 18, 24, and 48 h post-KA injury. n = 3–6 per group. (A) CCL2 production peaked at 18 h post-KA and was significantly elevated over sham animals in both low GC and high GC groups at 8 h and continued to be elevated in low GC animals from 18 to 24 h. Significance levels * p < 0.05 and ** p < 0.01 established by 2-way ANOVA followed by Holm-Sidak post hoc test. Significant 2-way ANOVA main effect of time post-injury (p < 0.0001). (B) CCL2 production at 8 hours post-injury was significantly higher in low GC animals compared to sham. Significance established by 1-way ANOVA followed by Holm-Sidak post hoc test. Significant 1-way ANOVA main effect (p < 0.05). (C) CINC-1 production peaked at 8 h post-KA and was increased in high GC animals relative to sham. Significance established by 2-way ANOVA followed by Holm-Sidak post hoc test. Significant 2-way ANOVA main effect of time post-injury (p < 0.0001) and significant interaction (p < 0.01). (D) CINC-1 production at 8 hours post-injury was significantly higher in animals given 100% GC pellets compared to those given 30%. Significance established by 1-way ANOVA followed by Holm-Sidak post hoc test. Significant 1-way ANOVA main effect (p < 0.01).
These results suggested that GCs can alter chemokine levels with a classical U-shaped dose-response, but also that the large increases in inflammatory cell activation post-injury are not due to a corresponding large increase in chemokine signaling.
GCs Suppress Anti-Inflammatory Signals in the Hippocampus Prior to Injury
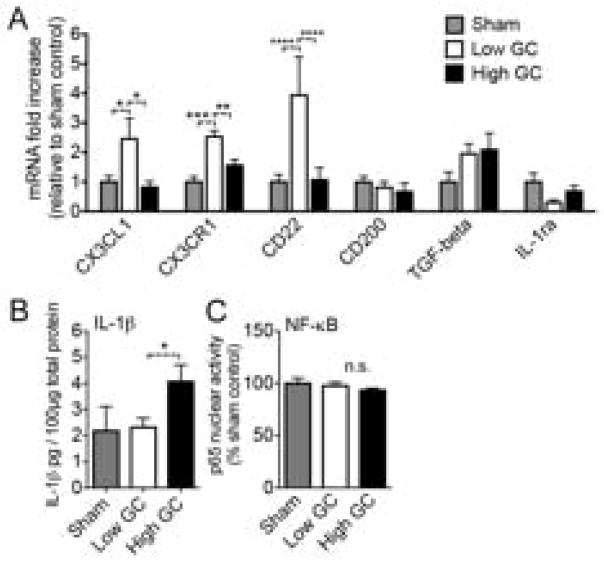
Based on the hypothesis that GCs might prime the CNS to be hyper-responsive to subsequent injury [6], we measured the effects of exposure to these GC treatments on the expression of several genes that keep the immune response in check within the CNS. Neurons produce multiple signals that suppress microglia activation, including CX3CL1, CD200, and CD22 [40–43]. We also examined TGF-b1 because GCs normally induce this gene as a component of their anti-inflammatory actions [22, 44]. To determine whether GCs alter the expression of these genes, their levels were measured by qPCR at the time when KA injury was previously given (Fig. 5A).
Fig. 5.
GCs suppress anti-inflammatory gene expression. Rats were treated with GCs and the hippocampus was dissected at the time when the injury would normally be given. n = 5–6 per group. (A) Anti-inflammatory gene expression in the uninjured hippocampus following each GC treatment, expressed as fold-increase relative to sham. Low GC animals had elevated CX3CL1, CX3CR1, and CD22 expression relative to sham or high GC treated animals (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001). Significance established by 2-way ANOVA followed by Holm-Sidak post hoc test. (B) IL-1beta protein levels in the uninjured hippocampus following each GC treatment. High GC animals had higher IL-1beta levels than low GC animals (* p < 0.05). Significance established by two-way unpaired t test. (C) Quantification of EMSA autoradiographs for the p65 subunit of NF-kB in the uninjured hippocampus. Basal NF-kB activation was not affected by GC-treatments. (* p < 0.05, ** p < 0.01, **** p < 0.0001). Significance established by 2-way ANOVA followed by Holm-Sidak post hoc test.
GCs significantly altered the expression of several of these anti-inflammatory signaling molecules in the hippocampus. Low GC rats had 2 to 4-fold increases in the expression of CX3CL1, its receptor CX3CR1, and CD22 relative to either sham or high GC rats (Fig. 5A). This suggested that removing GCs by ADX leads to a compensatory increase in these anti-inflammatory signaling molecules. Furthermore, this increase could be reversed in ADX animals by treatment with high GCs, suggesting that GCs might be the necessary factor removed by ADX. Because GCs suppressed these anti-inflammatory signals, we next examined whether this resulted in an increase in pro-inflammatory mediators prior to injury.
GCs Increase Pro-Inflammatory Signals in the Hippocampus Prior to Injury
Previous reports of GC-augmented inflammation have detected increased production of the pro-inflammatory cytokine IL-1beta in response to an inflammatory challenge [1, 15–17, 32]. If GCs also increase the production of this cytokine prior to the injury, it might lead to augmented and potentially more neurotoxic immune responses, particularly in the context of reduced anti-inflammatory signaling by molecules like CX3CL1 [45].
Although IL-1 beta levels were quite low in the uninjured hippocampus, high GC animals had significantly more IL-1 beta than low GC rats (Fig. 5B). Because IL-1 beta production is stimulated by NF-kB transcriptional activity, and NF-kB is normally a target of the anti-inflammatory actions of GCs, we next investigated whether the increased IL-1 beta might result from increased NF-kB activity. We measured the DNA-binding activity of the p65 subunit of NF-kB via electrophoretic mobility shift assay (EMSA) following each GC treatment but there were no differences in DNA binding activity (Fig. 5C), suggesting that the IL-1beta increase was NF-kB independent.
Discussion
Our findings suggest that GCs can increase cellular inflammatory responses to excitotoxic injury in the hippocampus and we identify several anti-inflammatory signaling molecules that are suppressed by GCs and could contribute to an augmented cellular response. This increased inflammation was detrimental to the ability of neurons to survive excitotoxic injury and represents a new mechanism whereby GCs can endanger neurons.
GCs Increase Immune Cell Activation in the Injured Hippocampus
Comparison of low and high GC rats revealed that high GC concentrations increased CD11b/c+ area. This agrees with previous reports that GCs can increase immune cell activation both basally [46] and in this same excitotoxicity model [17]. Previous work using this same excitotoxicity model found that ADX decreased inflammatory cell activation and sham rats given additional GCs had higher inflammatory cell activation [17]. These effects could potentially be due to compensatory decreases in endogenous GC synthesis (comparing sham with sham given additional GCs) or they could be an artifact of ADX (comparing sham with ADX). The present finding that GC-augmented inflammation still occurs when GC dose is an isolated variable in ADX rats argues against these alternative possibilities.
The different abilities of the two drugs to inhibit GC-augmented cellular activation also narrows the possible mechanisms responsible. The ineffectiveness of indomethacin argues that prostaglandin synthesis is not necessary for GCs to increase inflammation. While it was expected that minocycline would generally reduce inflammatory cell activation, it does this at least in part by inhibiting NF-kB activation [30], and this is the same target of normal GC anti-inflammatory effects. If GCs were already inhibiting NF-kB, minocycline would be expected to be less effective. Instead, we found the opposite: minocycline suppressed immune cell activation in sham and high-GC rats but could not further reduce cellular activation in low-GC rats. Thus we conclude that GCs increase immune cell activation in the injured hippocampus and minocycline can reverse these effects whereas indomethacin cannot.
GCs Do Not Augment Inflammation via Increased Chemokine Release
Given that GCs doubled immune cell activation, a likely possibility is that they do so by accelerating and/or increasing the magnitude of chemokine release in the injury site. The predominant chemokine source is inflammatory cells themselves, so increased cellular activation in the presence of GCs might correspond with higher chemokine levels. Instead, we found that CCL2 and CINC-1 levels post-injury were only slightly changed by GC-treatments (Fig. 4) suggesting either that GCs are suppressing chemokine production, or that greater cellular activation does not equate with more chemokine production. From this we concluded that GCs must affect other mechanisms to increase immune activation.
GCs Suppress Anti-inflammatory Signals in the Hippocampus Prior to Injury
We asked whether GCs suppress the expression of anti-inflammatory genes and found that they did affect several genes in this way (Fig. 5A). The anti-inflammatory genes that GCs suppress are at least partially redundant in their ability to restrain the immune response to injury. CX3CR1 is a receptor present on immune cells and its ligand CX3CL1 is a soluble factor expressed in neurons throughout the CNS [47]. CX3CR1 knockout mice have a more neurotoxic microglial response to a number of different CNS injuries [45]. CD22 is less-extensively studied, but is a soluble ligand for the pan-leukocyte marker CD45 and it inhibits microglial release of cytokines in response to LPS [42]. That GCs affect these molecules in the CNS is not unprecedented as they have been seen to reduce CX3CL1 expression in respiratory epithelial cells [48]. In peripheral injury, GCs suppress CX3CL1 expression by displacing NF-kB from the CX3CL1 promoter [48], but in the absence of a stimulus NF-kB is not present on this promoter, suggesting that GCs must be able to suppress CX3CL1 via an alternative method and that GC suppression of CX3CL1 expression might be even greater post-injury. Thus, exposure to GCs reduces the expression of several critical inhibitory inflammatory signals in the hippocampus, in agreement with our findings in immune-cell GR knockout mice [2].
GC-mediated suppression of these anti-inflammatory genes corresponds with the amount of cellular activation post-injury (Fig. 1B). Low-GC rats have one-half the immune activation of high-GC rats and have correspondingly elevated anti-inflammatory gene expression. Because high-GCs did not suppress anti-inflammatory genes beyond the level of sham rats, it could be that GC signaling is required in a permissive fashion to facilitate an appropriately robust inflammatory response. It is possible that if sham rats were given high-GC treatment that these genes might be even further suppressed since sham rats given additional GCs have greater hippocampal immune cell responses to excitotoxicity than sham rats alone [17].
We next measured GC effects on the pro-inflammatory cytokine IL-1beta. This cytokine is increased by GCs in the uninjured hippocampus [17] and after generalized inflammation from peripheral or centrally injected LPS [1, 15, 16], or excitotoxicity [17, 34]. In the present work, high GC animals had two-times the IL-1beta protein levels of low GC animals in the uninjured hippocampus (Fig. 5B). High GCs should suppress IL-1beta relative to low if GCs were acting in their normal anti-inflammatory manner. This suggested that in addition to suppressing anti-inflammatory signals, GCs might also have a deficit in their normal ability to inhibit pro-inflammatory signals. Supporting this, GCs failed to induce IL-1ra, the anti-inflammatory antagonist of IL-1beta (Fig. 5A). To further test this hypothesis we measured the ability of GCs to inhibit NF-kB, a normal target of their anti-inflammatory actions. GC treatment did not affect basal NF-kB activation (Fig. 5C), indicating that the cytokine changes prior to injury were not associated with changes in NF-kB DNA-binding activity.
These results narrow down the possible causes of the GC-augmented cellular response. It is not due to GCs increasing the production of chemokines in the injury site. Instead, GCs suppress the expression of several important anti-inflammatory genes in a manner that could lead to increased immune activation during a subsequent injury. Furthermore, GCs have an impaired ability to inhibit NF-kB in the hippocampus and fail to induce some of their normal anti-inflammatory targets. Although we did not determine whether GR or mineralocorticoid receptors (MR) are important for these effects, these changes were observed in the high GC group relative to the low GC group, suggesting that the increased occupancy of the GR could be responsible. Additionally, previous work has demonstrated reversal of GC-augmented inflammation by mifepristone administration (blocking GR) [16]. There is some precedent for GCs having concentration-dependent effects via mechanisms other than the often-opposing effects of GR and MR activation. While GCs post-injury can potently suppress pro-inflammatory cytokines, they also simultaneously increase the expression of the corresponding cytokine receptor [49]. This results in minimal cytokine signaling at extreme GC concentrations and peak signaling at an intermediate GC concentration. This could serve to optimize the course of inflammation post-injury, however it remains an open question whether prior exposure to high GCs affects the receptors for these inflammatory mediators in the same manner as GC release post-injury.
GC effects on inflammation contribute to GC endangerment of neurons
These results suggested that the effects of GCs on inflammation might be detrimental to neurons. The reduced ability of GCs to inhibit pro-inflammatory signals in the hippocampus like IL-1beta could imperil neurons because increased neurotoxicity of microglia lacking CX3CR1 is mediated via IL-1 beta [45]. Furthermore, NF-kB activation in immune cells during excitotoxicity increases neuron death [28] and GCs had an impaired ability to inhibit NF-kB.
Treatment with minocycline blocked GC endangerment, arguing that GC-augmented inflammation is necessary for GCs to endanger neurons. Indomethacin did not reduce immune activation and was also unable to protect neurons. GC-augmented inflammation alone is not sufficient to endanger neurons, however, because minocycline also suppressed cellular responses in sham rats but was not neuroprotective, and low GC animals had reduced cellular activation but no neuroprotection. The fact that decreasing the cellular inflammation in the context of high-GCs was protective, argues that GCs either make neurons more vulnerable to the potential toxicity of an augmented inflammatory response or they increase the toxicity of that response itself. Minocycline can also have anti-apoptotic effects, but GCs do not endanger neurons by increasing apoptosis [50], making this an unlikely explanation for the effectiveness of minocycline. Thus, GC-effects on inflammation are likely to contribute, at least in part, to GC endangerment.
During the energy crisis caused by excessive excitatory neurotransmitter release, GCs impair multiple processes including glucose transport, glutamate re-uptake, calcium sequestration, and the scavenging of reactive oxygen species [18]. None of these effects can individually account for GC endangerment, supporting the notion that GC endangerment emerges from the varied effects of these hormones. The findings we report here add an additional source of endangerment as the anti-inflammatory properties of GCs might have normally been expected to be protective.
Conclusion
GC-augmented inflammation in the hippocampus is therefore likely to worsen neuron death and is not simply a consequence of GCs making more neurons die. This fits with the finding that the amount of GC-augmented immune cell activation prior to (but not after) the emergence of damage predicts the extent of subsequent GC-endangerment [17]. Furthermore, GCs can also increase inflammation in the hippocampus in response to a peripheral inflammatory challenge that causes no neuron death (i.e. LPS) [1, 16]. GCs can also augment inflammation in humans in a pattern consistent with rodent studies. GC treatments prior to LPS challenge in humans potentiate plasma IL-6 and TNF-a levels [9, 51]. Moreover, even pharmacological GCs like dexame-thasone have been reported to induce the expression of factors associated with innate immune responses (amid decreasing components of adaptive immunity) in human blood mononuclear cells [10].
These findings demonstrate a functional role for GC-augmented inflammation in CNS injury. Future studies are required to verify the physiological relevance of the injury model in this study and to further identify the molecular changes associated with exposure to high GCs. One critical caveat is the artificial nature of our glucocorticoid manipulations. Despite this, we conclude that exposure to high levels of GCs for as little as a few days prior to hippocampal injury is sufficient to increase cellular and molecular inflammatory responses with detrimental consequences for neuron survival. These results support the idea that prior GC exposure modifies the immune environment in the hippocampus making it more toxic to injured neurons, and could reflect a unique response of the hippocampus to GCs [1, 16].
Acknowledgments
The authors thank Angela Lee, Javier Caso, Marta Perez, Trevor Sorrells, Norman Ruby, Jessie Ansari, and Kevin Kinney for help with experiments and discussions about the manuscript. This work was supported by NIH F31 NS063491 (S.F.S.), and NIH R01 NS059918 (R.M.S.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Munhoz CD, Sorrells SF, Caso JR, Scavone C, Sapolsky RM. Glucocorticoids exacerbate lipopolysaccharide-induced signaling in the frontal cortex and hippocampus in a dose-dependent manner. J Neurosci. 2010;30:13690–13698. doi: 10.1523/JNEUROSCI.0303-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorrells SF, Caso JR, Munhoz CD, Hu CK, Tran KV, Miguel ZD, Chien BY, Sapolsky RM. Glucocorticoid signaling in myeloid cells worsens acute cns injury and inflammation. J Neurosci. 2013;33:7877–7889. doi: 10.1523/JNEUROSCI.4705-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed cns: When glucocorticoids aggravate inflammation. Neuron. 2009;64:33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeager MP, Guyre PM, Munck AU. Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol Scand. 2004;48:799–813. doi: 10.1111/j.1399-6576.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- 6.Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to e. Coli lipopolysaccharide. Brain Behav Immun. 2009;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Smyth GP, Stapleton PP, Freeman TA, Concannon EM, Mestre JR, Duff M, Maddali S, Daly JM. Glucocorticoid pretreatment induces cytokine overexpression and nuclear factor-kappab activation in macrophages. J Surg Res. 2004;116:253–261. doi: 10.1016/S0022-4804(03)00300-7. [DOI] [PubMed] [Google Scholar]
- 8.Viswanathan K, Dhabhar FS. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc Natl Acad Sci U S A. 2005;102:5808–5813. doi: 10.1073/pnas.0501650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber AE, Coyle SM, Marano MA, Fischer E, Calvano SE, Fong Y, Moldawer LL, Lowry SF. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol. 1993;150:1999–2006. [PubMed] [Google Scholar]
- 10.Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O’Shea JJ, Chrousos GP, Bornstein SR. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. Faseb J. 2002;16:61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- 11.Perry VH. Stress primes microglia to the presence of systemic inflammation: Implications for environmental influences on the brain. Brain Behav Immun. 2007;21:45–46. doi: 10.1016/j.bbi.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Goujon E, Parnet P, Laye S, Combe C, Kelley KW, Dantzer R. Stress downregulates lipopolysaccharide-induced expression of proinflammatory cytokines in the spleen, pituitary, and brain of mice. Brain Behav Immun. 1995;9:292–303. doi: 10.1006/brbi.1995.1028. [DOI] [PubMed] [Google Scholar]
- 13.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 14.Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 2008;22:105–113. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, de Lima LS, Avellar MC, Sapolsky RM, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappab in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinkel K, MacPherson A, Sapolsky RM. Novel glucocorticoid effects on acute inflammation in the cns. J Neurochem. 2003;84:705–716. doi: 10.1046/j.1471-4159.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- 18.Sapolsky RM. Stress, glucocorticoids, and damage to the nervous system: The current state of confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- 19.Stein-Behrens BA, Elliott EM, Miller CA, Schilling JW, Newcombe R, Sapolsky RM. Glucocorticoids exacerbate kainic acid-induced extracellular accumulation of excitatory amino acids in the rat hippocampus. J Neurochem. 1992;58:1730–1735. doi: 10.1111/j.1471-4159.1992.tb10047.x. [DOI] [PubMed] [Google Scholar]
- 20.Elliott EM, Sapolsky RM. Corticosterone impairs hippocampal neuronal calcium regulation--possible mediating mechanisms. Brain Res. 1993;602:84–90. doi: 10.1016/0006-8993(93)90245-i. [DOI] [PubMed] [Google Scholar]
- 21.McIntosh LJ, Sapolsky RM. Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin-induced toxicity in neuronal culture. Exp Neurol. 1996;141:201–206. doi: 10.1006/exnr.1996.0154. [DOI] [PubMed] [Google Scholar]
- 22.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappab or activator protein-1: Molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 23.Goujon E, Parnet P, Cremona S, Dantzer R. Endogenous glucocorticoids down regulate central effects of interleukin-1 beta on body temperature and behaviour in mice. Brain Res. 1995;702:173–180. doi: 10.1016/0006-8993(95)01041-9. [DOI] [PubMed] [Google Scholar]
- 24.Uz T, Dwivedi Y, Savani PD, Impagnatiello F, Pandey G, Manev H. Glucocorticoids stimulate inflammatory 5-lipoxygenase gene expression and protein translocation in the brain. J Neurochem. 1999;73:693–699. doi: 10.1046/j.1471-4159.1999.0730693.x. [DOI] [PubMed] [Google Scholar]
- 25.Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z, Shimizu-Sasamata M, Yuan J, Moskowitz MA. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci U S A. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi K, Hickey RW, Rose ME, Zhu L, Chen J, Graham SH. Cyclooxygenase-2 expression is induced in rat brain after kainate-induced seizures and promotes neuronal death in ca3 hippocampus. Brain Res. 2005;1050:130–137. doi: 10.1016/j.brainres.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 27.Takemiya T, Maehara M, Matsumura K, Yasuda S, Sugiura H, Yamagata K. Prostaglandin e2 produced by late induced cox-2 stimulates hippocampal neuron loss after seizure in the ca3 region. Neurosci Res. 2006;56:103–110. doi: 10.1016/j.neures.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Cho IH, Hong J, Suh EC, Kim JH, Lee H, Lee JE, Lee S, Kim CH, Kim DW, Jo EK, Lee KE, Karin M, Lee SJ. Role of microglial ikkbeta in kainic acid-induced hippocampal neuronal cell death. Brain. 2008;131:3019–3033. doi: 10.1093/brain/awn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 30.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sapolsky R, Brooke S, Stein-Behrens B. Methodologic issues in studying glucocorticoid-induced damage to neurons. J Neurosci Methods. 1995;58:1–15. doi: 10.1016/0165-0270(94)00155-a. [DOI] [PubMed] [Google Scholar]
- 32.MacPherson A, Dinkel K, Sapolsky R. Glucocorticoids worsen excitotoxin-induced expression of pro-inflammatory cytokines in hippocampal cultures. Exp Neurol. 2005;194:376–383. doi: 10.1016/j.expneurol.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 34.Waters JC. Accuracy and precision in quantitative fluorescence microscopy. J Cell Biol. 2009;185:1135–1148. doi: 10.1083/jcb.200903097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12:1047–1064. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rong Y, Baudry M. Seizure activity results in a rapid induction of nuclear factor-kappa b in adult but not juvenile rat limbic structures. J Neurochem. 1996;67:662–668. doi: 10.1046/j.1471-4159.1996.67020662.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang DD, Englot DJ, Garcia PA, Lawton MT, Young WL. Minocycline- and tetracycline-class antibiotics are protective against partial seizures in vivo. Epilepsy Behav. 2012;24:314–318. doi: 10.1016/j.yebeh.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196:168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 39.Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: Therapeutic implications. Science. 1985;229:1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- 40.Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘on’ and ‘off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with ox2 (cd200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 42.Mott RT, Ait-Ghezala G, Town T, Mori T, Vendrame M, Zeng J, Ehrhart J, Mullan M, Tan J. Neuronal expression of cd22: Novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46:369–379. doi: 10.1002/glia.20009. [DOI] [PubMed] [Google Scholar]
- 43.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 44.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 45.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 46.Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through nmda receptor activation. J Neuroimmunol. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and cx3cr1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhavsar PK, Sukkar MB, Khorasani N, Lee KY, Chung KF. Glucocorticoid suppression of cx3cl1 (fractalkine) by reduced gene promoter recruitment of nf-kappab. Faseb J. 2008;22:1807–1816. doi: 10.1096/fj.07-094235. [DOI] [PubMed] [Google Scholar]
- 49.Wiegers GJ, Reul JM. Induction of cytokine receptors by glucocorticoids: functional and pathological significance. Trends Pharmacol Sci. 1998 Aug;19(8):317–21. doi: 10.1016/s0165-6147(98)01229-2. [DOI] [PubMed] [Google Scholar]
- 50.Roy M, Sapolsky RM. The exacerbation of hippocampal excitotoxicity by glucocorticoids is not mediated by apoptosis. Neuroendocrinology. 2003;77:24–31. doi: 10.1159/000068337. [DOI] [PubMed] [Google Scholar]
- 51.Yeager MP, Rassias AJ, Pioli PA, Beach ML, Wardwell K, Collins JE, Lee HK, Guyre PM. Pretreatment with stress cortisol enhances the human systemic inflammatory response to bacterial endotoxin. Crit Care Med. 2009;37:2727–2732. doi: 10.1097/ccm.0b013e3181a592b3. [DOI] [PMC free article] [PubMed] [Google Scholar]