Summary
The metabolome of the nematode Caenorhabditis elegans, like that of other model organisms, remained largely uncharacterized until recent studies demonstrated the importance of small molecule-based signaling cascades for many aspects of nematode biology. These studies revealed that nematodes are amazingly skilled chemists: using simple building blocks from primary metabolism and a strategy of modular assembly, nematodes create complex molecular architectures that serve as signaling molecules. These nematode-derived modular metabolites (NDMMs) are based on the dideoxysugars ascarylose or paratose, which serve as scaffolds for attachment of moieties from lipid, amino acid, neurotransmitter, and nucleoside metabolism. Although preliminary biosynthetic studies have confirmed the primary metabolism origin of some of the building blocks incorporated into NDMMs, the mechanisms that underlie their highly specific assembly are not understood. Overall, I argue that identification of new variants of primary metabolism-derived structures that serve important signaling functions in C. elegans and other nematodes provides a strong incentive for a comprehensive re-analysis of metabolism in higher animals, including humans.
Introduction
In contrast to bacteria, fungi, and plants, whose genomes have revealed a great variety of “secondary” small-molecule biosynthetic pathways, animal genomes aren’t generally known to encode biosynthetic machinery for complex secondary metabolites. Many times structurally intriguing metabolites have been identified from animal sources, the compounds ultimately turned out to be derived from microorganisms or plants. Examples include the identification of tetrodotoxin from pufferfish and the great number of polyketides identified from marine sponges (Fisch et al., 2009; Piel, 2009). The great diversity of insect natural products forms a notable exception; however, at least some groups of insect-derived natural products also have been shown to be of microbial origin (Gronquist et al., 2010; Piel, 2009).
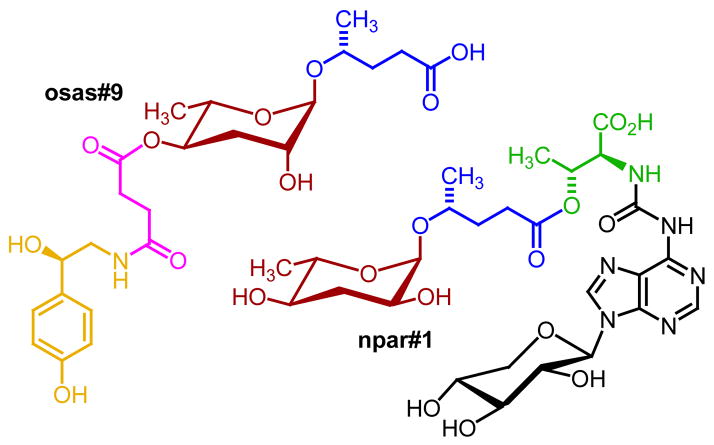
Recent analyses of nematodes, the most abundant animals on earth, have revealed a family of natural products that embodies a new assembly strategy for secondary metabolites. These nematode-derived modular metabolites (NDMMs) are based on modular assembly that uses glycosides of the dideoxysugars ascarylose (“ascarosides”) and paratose (“paratosides”) as a central scaffold and attaches different building blocks derived from primary metabolic pathways, for example carbohydrate, amino acid, lipid, nucleoside, or neurotransmitter metabolism, via ester, amide, or glycosidic linkages (Figure 1).
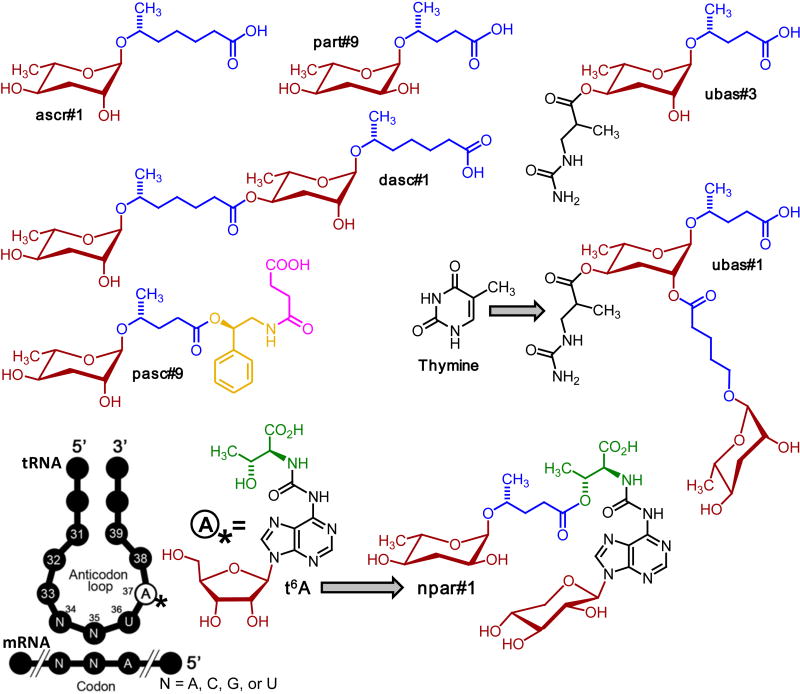
Figure 1. Examples for nematode-derived modular metabolites (NDMMs).
The ascaroside osas#9 from C. elegans L1 larvae serves as a dispersal signal (Artyukhin et al., 2013), whereas the paratoside npar#1 identified from P. pacificus promotes entry into a long-lived and stress resistant larval diapause (dauer larva formation) (Bose et al., 2012). Structures of the two NDMMs incorporate building blocks from lipid (blue), amino acid (green), neurotransmitter (yellow), nucleoside (black), and short-chain fatty acid (purple) metabolism. Ascarosides are named using Small Molecule IDentifiers (“SMIDs”), representing searchable, gene-style identifiers that consist of four lower case non-italicized letters followed by a pound sign and a number, e.g., ascr#3, osas#9, or npar#1. See http://www.smid-db.org/ and the WormBook chapter on ascarosides for additional information on compound naming (Ludewig and Schroeder, 2013).
NDMMs serve important signaling functions in nematodes, for example as regulators of organismal development, lifespan, and social communication. The study of these biological activities in the model nematode C. elegans has been contributing greatly to our understanding of how conserved signaling pathways (e.g. insulin signaling) regulate aging, metabolism, and behavior (Ludewig and Schroeder, 2013). Notably, available evidence indicates that NDMMs are produced by the nematodes, not associated microbiota, and that conserved primary metabolic pathways contribute to their biosynthesis. In this review, I will begin with a brief description of the biological phenomena that triggered the discovery of the first NDMMs, followed by a summary of NDMM structures and their biological activities, highlighting the role of comparative metabolomics for the discovery of new compounds and activities. Finally, I will summarize current knowledge of NDMM biosynthesis and possible connections to conserved primary metabolism.
Nematodes as model organisms
Nematodes are arguably the most numerous animals (Blaxter, 1998). They are of great relevance to human health, on one hand, because they infect 25% of the world’s population and significantly impact agricultural crops and animals and, on the other, because the non-parasitic soil nematode Caenorhabditis elegans (literally “elegant empty stick”) is an important model organism for biomedical research (Kaletta and Hengartner, 2006). C. elegans was selected as the first fully differentiated animal for complete genome sequencing (The C. elegans Sequencing Consortium, 1998) and has become a very productive model system, for several reasons. This nematode has a short life cycle of only three days, it is small enough for high-throughput whole-organism screens, and much of its biology is controlled by evolutionarily conserved molecular pathways. This high level of genetic conservation allows ancient features of endocrine pathways to be explored in C. elegans. Significantly, C. elegans has been developed as a unique platform for the study of conserved mechanisms regulating metabolism, development, reproductive maturation, and longevity, which revealed a deeply intertwined regulatory network that remains, at best, partly understood (Fielenbach and Antebi, 2008). As part of this network, small-molecule signals, including steroids and the NDMMs play major roles in connecting metabolism with behavior, development, and aging.
Similar to C. elegans, Pristionchus pacificus is a free-living nematode that has been established as a model organism for the study of developmental and evolutionary biology (Dieterich et al., 2008). In contrast to C. elegans, P. pacificus forms a necromenic association with beetles, which may represent a pre-adaptation to the evolution of true parasitism (Rae et al., 2008). Importantly, P. pacificus is used as a “satellite” model organism alongside C. elegans. Satellite models are species that are sufficiently closely related to well-known model organisms so that the genetic regulation of homologous biosynthetic, cellular, and developmental processes can be studied, enabling the identification of the molecular changes that underlie phenotypic and biochemical differences or variation.
The dauer pheromone: the first ascaroside-based signaling molecules
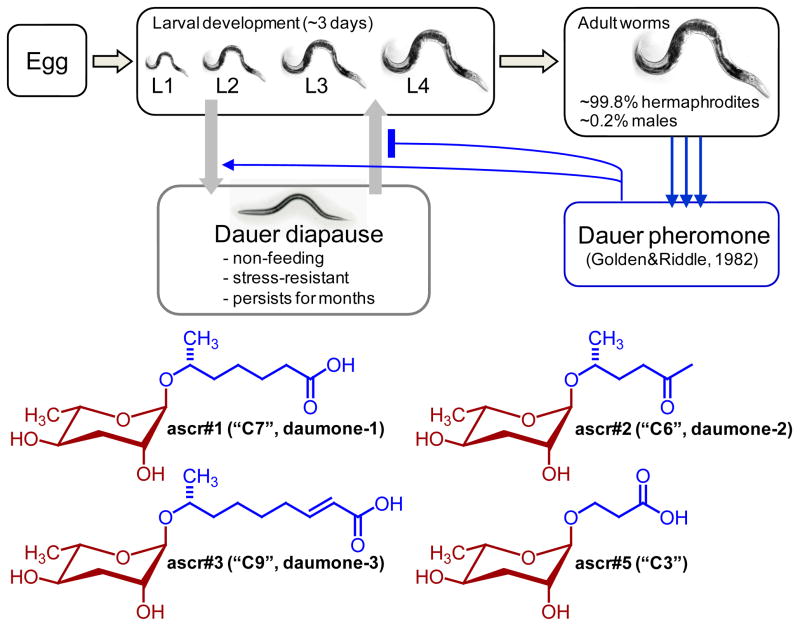
Studies of longevity and development in C. elegans initially focused on one unique feature of its development: an alternative larval stage called “dauer” (German, “enduring”). When exposed to environmental stress, e.g. starvation, high temperature, or excessive crowding, C. elegans larvae abort normal reproductive development and enter the highly stress-resistant dauer diapause (Figure 2). The non-feeding dauer larvae are seemingly “non-aging” as they can persist for several months under adverse conditions, compared to a normal adult lifespan of only about 2–3 weeks (Hu, 2007; Riddle and Albert, 1997). When environmental conditions improve, dauer larvae resume reproductive development, resulting in adults with normal physiology and lifespan. Genetic screens for mutants that either cannot attain the dauer stage or form dauer larvae constitutively identified more than three dozen DAF (DAuer Formation) genes (Hu, 2007). Subsequent studies revealed important roles for DAF genes in C. elegans lifespan regulation, and orthologs of DAF genes were found to serve similar roles in the regulation of aging in higher organisms (Kenyon, 2010).
Figure 2. C. elegans life cycle and the dauer pheromone.
Under favorable conditions, C. elegans larvae develop rapidly into reproductive adults, whereas environmental stress, in combination with the dauer pheromone, leads to formation of stress-resistant and long-lived dauer larvae. Recent work showed that the dauer pheromone, originally described in 1982 by Golden and Riddle, consists of a mixture of ascarosides, including ascr#1, ascr#2, ascr#3, and ascr#5 (Ludewig and Schroeder, 2013).
More than 30 years ago, Golden and Riddle showed that then unknown small molecule signals (the “dauer pheromone”) that are constitutively excreted by the worms control dauer entry and exit (Golden and Riddle, 1982, 1984). The identification of a mutation, named daf-22, that did not produce the dauer pheromone, confirmed that this signal must be worm-derived (Golden and Riddle, 1985). Based on solubility and other physical properties of the dauer-inducing signal, Golden and Riddle suggested that the dauer pheromone may consist of a mixture of short-chain hydroxylated lipid derivatives (Golden and Riddle, 1982, 1984); however, the precise chemical identity of this small-molecule signal remained undetermined for almost 25 years. Finally, in 2005 Jeong et al. described the isolation and identification of “daumone” (also “ascr#1”, or “C7”, see www.smid-db.org for ascaroside nomenclature), a glycoside of the dideoxysugar ascarylose featuring a seven-carbon carboxylic acid side chain, as one component of the dauer pheromone (Figure 2) (Jeong et al., 2005). The dideoxysugar ascarylose and derived glycosides (“ascarosides”) had originally been identified several decades earlier from parasitic nematodes of the genus Ascaris. The ascarosides obtained from Ascaris feature very long aliphatic side chains (predominantly 29 and more carbons in length) and were shown to form a protective coating of Ascaris eggs, providing remarkable resilience to harsh environmental conditions (Jezyk and Fairbairn, 1967). More recently, additional variants of such lipophilic ascarosides have been described from Ascaris suum (Bartley et al., 1996); however, none of these lipid-like ascarosides are believed to have signaling functions.
More detailed investigations of the biological properties of the ascaroside ascr#1 revealed that it accounted only for a small portion of the dauer-promoting activity of C. elegans metabolite extracts (Butcher et al., 2007; Gallo and Riddle, 2009), suggesting that the dauer pheromone must include additional components. Subsequent analyses of the C. elegans exo-metabolome (the entirety of all excreted or secreted metabolites) then demonstrated that the dauer pheromone in fact consists of a mixture of several different ascarosides, including ascr#2, ascr#3, and ascr#5, which are much more potent dauer inducers than the originally identified ascr#1 (Figure 2) (Butcher et al., 2007; Butcher et al., 2008). These dauer-inducing ascarosides are produced constitutively by developing and adult worms and thus function as a population density signal, similar to N-acyl homoserine lactones in bacterial quorum sensing (Dickschat, 2010; Miller and Bassler, 2001).
Comparative metabolomics reveals modular ascaroside derivatives
Following the identification of ascarosides as components of the C. elegans dauer pheromone, it soon became apparent that ascarosides control many other aspects of C. elegans’ life history, including a variety of social behaviors as well as adult lifespan (Ludewig and Schroeder, 2013). It was shown that ascr#2 and ascr#3 also function as a male-attracting sex pheromone, and that ascr#3 and ascr#5 promote dispersal of hermaphrodites (C. elegans forms primarily self-fertile hermaphrodites, as well as small numbers of males). In addition, ascr#2 and ascr#3 were shown to increase adult lifespan in C. elegans (Ludewig et al., 2013). However, synthetic samples of the initially identified ascarosides ascr#1-ascr#4 consistently failed to reproduce the full potency of complete, unfractionated C. elegans exo-metabolome extracts, suggesting that the chemical characterization of C. elegans pheromone signals, which had been based on activity-guided fractionation, remained incomplete. The observation that different components of the sex pheromone synergize with each other (Srinivasan et al., 2008) suggested a possible explanation for this loss of activity: chromatographic separation of a synergistic mixture must lead to partial loss of activity, increasing chances that some components of a signal are missed.
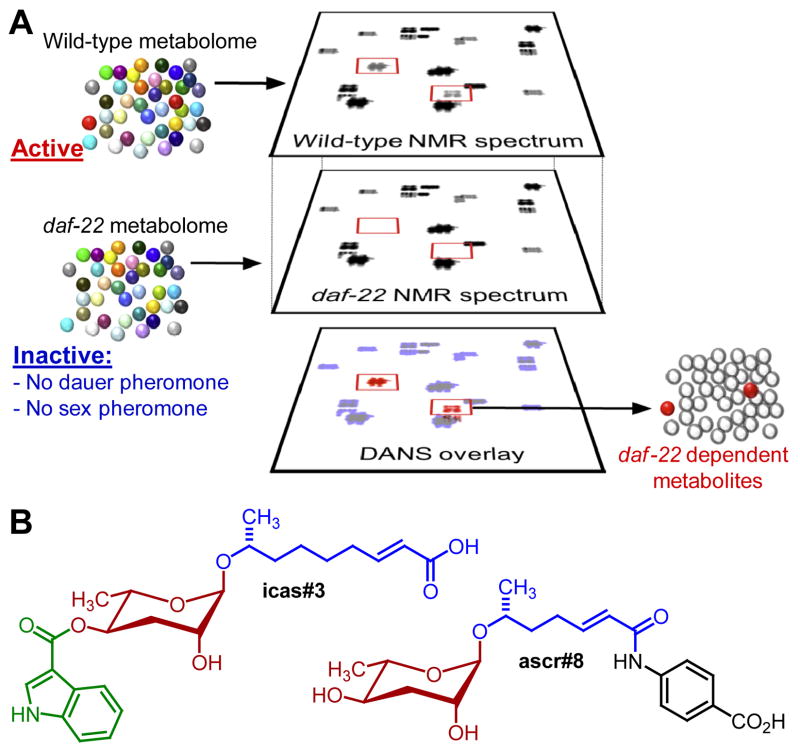
The limitations of activity-guided fractionation-based approaches prompted the introduction of comparative metabolomics as a tool for the identification of small-molecule signals, which ultimately revealed a much greater diversity of nematode-produced small molecules. In a first example, Pungaliya et al. used 2D NMR-based comparative metabolomics (Differential Analysis by 2D NMR Spectroscopy, DANS) to identify missing components of the dauer and sex pheromone blends. Earlier work had shown that daf-22 mutant worms do not produce these pheromone signals (Golden and Riddle, 1985; Simon and Sternberg, 2002), and therefore comparison of 2D NMR spectra of the exo-metabolomes of wildtype worms with that of daf-22 mutant worms should reveal NMR signatures (groups of crosspeaks) that represent the sought-after signaling molecules. 2D NMR-based comparison of the daf-22 mutant- and wildtype-metabolomes revealed a large number of additional ascaroside derivatives in the wildtype-metabolome that were absent in the metabolome of the daf-22 mutant, suggesting that they may represent additional pheromone signals (Figure 3). Notably, the identified compounds included the first examples for modular ascaroside derivatives, NDMMs, in which the ascarylose sugar or side chain is linked to an additional moiety. The first NDMM, ascr#8, includes a p-aminobenzoic acid moiety, which is uncommon among known primary and secondary metabolites as a building block, except for its role in folic acid (vitamin B9) metabolism. ascr#8 synergizes strongly with the previously identified components of the dauer and sex pheromones, validating the use of comparative metabolomic approaches to complement activity-guided fractionation.
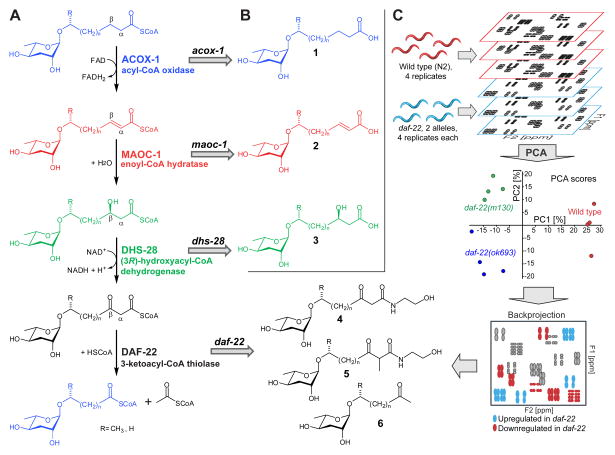
Figure 3. Identification of multi-modular ascaroside derivatives (NDMMS) via 2D NMR-based comparative metabolomics (DANS).
(A) Schematic representation of differential 2D NMR spectroscopy (DANS) applied to comparing C. elegans wildtype exo-metabolome with that of daf-22 mutant worms (Pungaliya et al., 2009). (B) NDMMs detected via DANS, including lipid- (blue), amino acid- (green), and folate- (black) derived building blocks.
In addition, DANS-based comparison of the daf-22 and wildtype exo-metabolomes revealed another group of NDMMs, bearing an indole carboxy moiety in position 4 of the ascarylose ring. Although some indole ascarosides were found to contribute to dauer formation (Butcher et al., 2009a), it was initially unclear whether the addition of an indole carboxy moiety to the ascaroside scaffold was associated with any specific signaling function. Screening indole ascarosides in a wide array of behavioral assays, it was eventually shown that indole ascarosides function as potent aggregation pheromones. The two most abundant indole ascarosides, icas#3 and icas#9, were shown to attract C. elegans hermaphrodites at concentrations as low as 100 femtomolar (Srinivasan et al., 2012). Notably, the existence of an aggregation pheromone in C. elegans had not previously been suspected, and thus this discovery was a direct consequence of chemical analysis of the C. elegans metabolome.
Targeted mass spectrometric analyses reveal additional chemical diversity
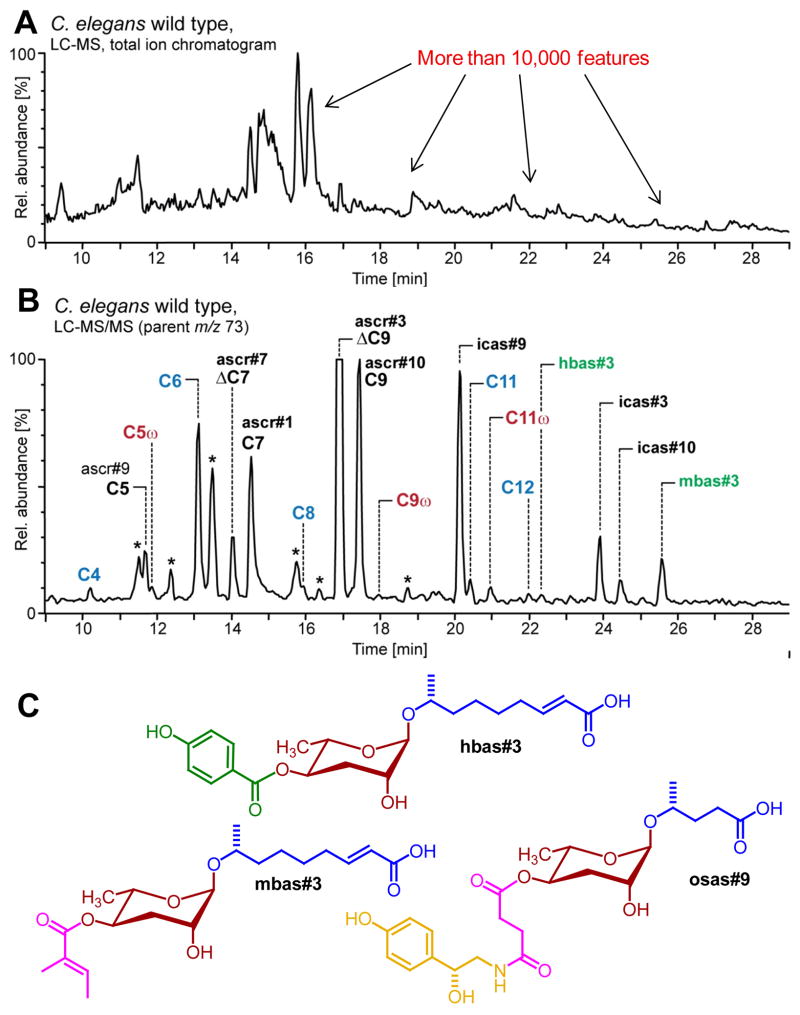
Although ascarosides represent only a relatively small fraction of the C. elegans metabolome, their important biological roles motivated efforts to identify additional ascarosides, including compounds that are produced in a sex-specific or life stage-specific manner. Serendipitously, it was found that mass-spectrometric fragmentation of ascarosides characteristically produced a fragment m/z 73, corresponding to C3H5O2 (Figure 4) (von Reuss et al., 2012). Screening the entire C. elegans exo-metabolome for compounds that fragment in this manner, two additional 4-subsituted ascarosides were identified, including tiglyl- (mbas#3, Figure 4) and p-hydroxybenzoyl (hbas#3, Figure 4) substituted derivatives, which appear to be part of the C. elegans aggregation pheromone. The most potent of all so-far identified NDMMs, hbas#3 is active at low femtomolar concentrations. To put this into perspective, at this concentration a given worm likely senses no more than one hbas#3 molecule every few minutes (von Reuss et al., 2012). Using the MS/MS screen for the analysis of the exo-metabolomes of different larval stages led to the identification of osas#9 (Figure 4), an ascaroside connected to a succinyl octopamine moiety, which is specifically produced during the first larval stage and acts as a dispersal signal under starvation conditions (Artyukhin et al., 2013). Similar to dopamine in higher animals, octopamine in nematodes serves as a neurotransmitter mediating starvation responses, and thus the chemical structure of osas#9 appears to integrate ascaroside and neurotransmitter signaling.
Figure 4. Ascaroside-targeted HPLC-MS/MS.
(A) Total ion current chromatogram of C. elegans exo-metabolome sample, representing more than 10,000 features, most of which belong to yet undescribed compounds (von Reuss et al., 2012). (B) Chromatogram of ascaroside targeted HPLC-MS/MS analysis (precursors of m/z 73) of the same C. elegans exo-metabolome sample reveals known ascarosides (black), new homologs (blue), new (ω)-oxygenated isomers (red) and new 4′-acylated derivatives (green) (* non-ascarosides). (C) Structures of hbas#3, mbas#3, and osas#9, including building blocks from lipid (blue), amino acid (green), neurotransmitter (yellow), and short-chain fatty acid (purple) metabolism. For compound naming, see http://www.smid-db.org/.
Multi-modular ascarosides from Pristionchus pacificus
The discovery of ascaroside-based signaling molecules as central mediators of C. elegans development and behavior suggested that other nematode species may produce similar types of signaling molecules. Given that the C. elegans ascarosides are derived from modular assembly of building blocks of conserved primary metabolism, it seemed possible that animal species from other phyla also may employ similar strategies for the biosynthesis of small molecule signals. Therefore, metabolomic analysis of the satellite model P. pacificus, a nematode species that diverged from C. elegans more than 200 million years ago, has been of particular significance.
As in C. elegans, harsh environmental conditions, for example food shortage, trigger developmental arrest as highly stress-resistant dauer larvae (Weller et al., 2010). P. pacificus further exhibits a unique dimorphism in mouth development, representing an example for phenotypic plasticity in an adult metazoan (Ogawa et al., 2011). Previous studies had suggested that both dauer formation and mouth dimorphism are regulated by specific small-molecule signals that target evolutionarily conserved downstream pathways, including the nuclear hormone receptor DAF-12, a homolog of mammalian vitamin D and liver-X receptors (Bento et al., 2010; Ogawa et al., 2011; Sommer and Ogawa, 2011).
Detailed 2D NMR-spectroscopic analyses of the P. pacificus exo-metabolome revealed that dauer induction and mouth-form dimorphism in P. pacificus are controlled by a series of structurally unusual small molecules (Figure 5) (Bose et al., 2014; Bose et al., 2012). These NDMMs are based on glycosides of two different deoxy sugars, the ascarosides and paratosides, which are combined with building blocks derived from fatty acid, carbohydrate, amino acid, and nucleoside metabolism. Modular assembly of building blocks from these pathways generates diverse molecular architectures, which are further distinguished from any previously known animal metabolites by several unprecedented structural features, including the sugar L-paratose (e.g. in part#9), dimeric ascarosides such as dasc#1, the 3′-ureido isobutyrate moieties in ubas#1 and ubas#2, and, notably, a xylopyranose-based adenosine as part of npar#1. This nucleoside is likely derived from canonical (ribo)-threonylcarbamoyl adenosine (t6A), a highly conserved nucleoside found directly adjacent to the anticodon triplet of a subset of tRNAs (Deutsch et al., 2012). t6A plays an important role for maintaining translational fidelity; however, it usually accounts for only a very small fraction of tRNA, and the production of large quantities of a xylopyranose derivative in P. pacificus is surprising. Similarly, the 3′-ureido isobutyrate sidechains in ubas#1 and ubas#2 likely originate from thymine metabolism (Bose et al., 2012). Different combinations of these NDMM’s regulate dauer formation and mouth-form dimorphism in different P. pacificus wildtype strains. For example, in some wildtype strains, the nucleoside derivative npar#1 regulates dauer formation, whereas in other strains the N-succinyl 1-phenylethanolamide derivative pasc#9 serves this function (Bose et al., 2014). Similarly, the quantitative profiles of NDMMs vary significantly between different strains, suggesting that both NDMM biosynthesis and receptors evolve rapidly in nematodes (Bose et al., 2014).
Figure 5.
Structures of NDMMs from P. pacificus and putative origin of building blocks from nucleoside metabolism (Bose et al., 2012). The shown NDMMs incorporate building blocks from lipid (blue), amino acid (green), neurotransmitter (yellow), nucleoside (black), and short-chain fatty acid (purple) metabolism. For compound naming, see http://www.smid-db.org/.
NDMM functions and biosyntheses are highly selective
Extensive bioassays with C. elegans and P. pacificus using synthetic samples of the identified NDMMs have shown that compounds that differ in the presence of a single structural feature may have strikingly different biological effects. For example, ascr#3 strongly induces developmental arrest and attracts male C. elegans, whereas a two-carbon shorter variant, ascr#7, has neither activity (Hollister et al., 2013; Ludewig and Schroeder, 2013). Similarly, the indole ascaroside icas#3 functions as a potent aggregation signal, whereas ascr#3, lacking the indole carboxy moiety, acts as a dispersal signal (Srinivasan et al., 2012). In P. pacificus, dasc#1, a dimeric NDMM derived from the simple ascaroside ascr#1, regulates adult body shape, whereas the monomer, ascr#1, is completely inactive in this assay (Bose et al., 2012). Even the addition of a single double bond can greatly affect biological activity. For example, whereas ascr#3, the most abundant ascaroside produced by hermaphrodites, attracts male worms and repulses other hermaphrodites, its saturated derivative, the predominantly male-produced ascr#10, is strongly attractive to hermaphrodites (Izrayelit et al., 2012).
Correspondingly, biosynthesis of the NDMMs identified from C. elegans and P. pacificus proceeds with high selectivity. For example, even though ascarosides with a 7-carbon sidechain (e.g. ascr#1) are much more abundant than 5-carbon sidechain ascarosides (e.g. ascr#9) in P. pacificus, only the 5-carbon variant is further decorated with a 3′-ureido isobutyrate substituent: there are no 7-carbon sidechain homologs of ubas#1 or ubas#2. Even more strikingly, an ω-oxygenated 5-carbon sidechain ascaroside is selectively attached to the 2-position in ubas#1, whereas all other identified compounds feature (ω-1)-oxygenated sidechains (Bose et al., 2012).
A similarly high level of selectivity is observed for some of the modular C. elegans compounds. For example, the likely folate-derived p-aminobenzoic acid moiety in ascr#8 is selectively attached to an unsaturated 7 carbon sidechain, although ascarosides based on saturated 7-carbon and unsaturated 9-carbon sidechain are much more abundant (Pungaliya et al., 2009). Similarly, the indole ascarosides icas#9 (5-carbon sidechain) and icas#3 (9-carbon sidechain) are about equally abundant, although the unmodified 5-carbon ascaroside ascr#9 is orders of magnitude less abundant than the 9-carbon variant ascr#3 (Srinivasan et al., 2012).
Lastly, comparison of NDMM profiles in the exo-metabolomes of C. elegans and P. pacificus with those found in the endo-metabolome (the entirety of extractable metabolites in the worm bodies) revealed that NDMM excretion is highly selective. For example, P. pacificus worm bodies retain large amounts of the simple, unmodified ascarosides ascr#1 and ascr#9, whereas the paratosides part#9 and npar#1 are primarily excreted (Bose et al., 2014; Bose et al., 2012).
NDMM biosynthesis
The selective combination of building blocks and chain lengths in the NDMMs from P. pacificus and C. elegans, along with their structure-specific biological functions, suggests that NDMMs are the products of carefully controlled biosynthetic pathways. Considering the structures of their diverse building blocks, it seems likely that these are derived more of less directly from conserved primary metabolism. For example, the incorporation of hydroxybenzoyl and indole carboxyl moieties in hbas#3 and icas#3 is suggestive of the involvement of amino acid metabolism, which was confirmed for the case of icas#3 by feeding C. elegans with tryptophan-d5 (Srinivasan et al., 2012). However, the involvement of conserved primary metabolic enzymes has been confirmed only for the biosynthesis of the lipid-derived sidechains, which were shown to be derived from conserved peroxisomal β-oxidation in C. elegans (Figure 6) (Butcher et al., 2009b; Joo et al., 2010; von Reuss et al., 2012). Ascaroside-targeted mass spectrometric analysis of null-mutants of the acyl-CoA oxidase ACOX-1, the enoyl-CoA hydratase MAOC-1, and the 3-hydroxyacyl-CoA dehydrogenase DHS-28 revealed almost complete abolishment of the biosynthesis of ascaroside-based signaling molecules as well as accumulation of long-chain ascarosides as shunt metabolites, consistent with enzymatic functions as predicted by homology (Butcher et al., 2009b; von Reuss et al., 2012). Targeted MS analysis of null-mutants of the predicted 3-ketoacyl-CoA thiolase DAF-22, catalyzing the last step in the 4-step peroxisomal β-oxidation cycle, also indicated abolishment of the biosynthesis of short-chain ascarosides; however, the MS-based analysis did not uncover any shunt metabolites that would directly support the proposed enzymatic function. Ultimately, daf-22-dependent shunt metabolites were identified using untargeted NMR-based metabolomics via multivariate DANS (mvaDANS), a program that partially automates comparative 2D NMR spectroscopic analysis (Izrayelit et al., 2013). Using mvaDANS, it was shown that daf-22 mutant worms accumulate large quantities of long-chain β-keto ethanolamides (4 and 5) and methylketones (6, Figure 6C) The accumulation of these long-chain ascarosides in daf-22(0) worms confirmed that daf-22 serves as a thiolase in ascaroside biosynthesis, as had previously been proposed based on homology (Butcher et al., 2009b; Pungaliya et al., 2009). Furthermore, this study demonstrated that accumulation of ascaroside ethanolamides in response to inhibited peroxisomal β-oxidation dramatically reduces endocannabinoid levels, revealing an unexpected connection between fatty acid peroxisomal β-oxidation and endocannabinoid signaling (Izrayelit et al., 2013).
Figure 6. Role of conserved peroxisomal β-oxidation in ascaroside side-chain biosynthesis.
(A) Homology-based model of side-chain biosynthesis. (B) Structures of shunt metabolites 1–3 identified in acox-1, maoc-1, and dhs-28 mutants via targeted MS-based metabolomics (Butcher et al., 2009b; von Reuss et al., 2012). (C) Use of untargeted NMR-based metabolomics (mvaDANS) for the identification of shunt metabolites 4–6 from daf-22 mutant worms (Izrayelit et al., 2013).
Although these results clearly demonstrate that NDMM biosynthesis and primary metabolism are deeply intertwined, the biosynthetic origin of most building blocks of the C. elegans and P. pacificus NDMMs has not been determined. Similarly, the enzymes that create complex architectures by combining different modules via ester and amide bonds in a highly selective manner remain unknown. Growing C. elegans and P. pacificus for several generations axenically, i.e. under sterile conditions using a bacteria-free nutrient solution, demonstrated that bacteria are not required for the biosynthesis any of the identified NDMMs (Bose et al., 2012; Srinivasan et al., 2012). Characterization of this biosynthetic machinery may reveal how input from conserved primary metabolism is transduced to create small-molecule signals that regulate aging, development, and behavior in nematode model organisms. If NDMM biosynthesis turns out to rely mostly on conserved enzymes, their identification may have additional implications for the metabolism of other animal groups.
Downstream pathways and receptors
The NDMM-mediated lifespan and developmental phenotypes have been shown to depend on evolutionarily conserved downstream signaling pathways, including sirtuin and insulin signaling (Ludewig et al., 2013; Ludewig and Schroeder, 2013; Sommer and Ogawa, 2011). As endogenous small molecules, the NDMMs thus provide exciting new opportunities to study the role of conserved signaling cascades in aging and development. To take full advantage of these possibilities will require detailed knowledge of the chemosensory mechanisms that underlie NDMM perception, which given the NDMMs’ diverse biological activities, may involve a large diversity of receptor proteins. Notably, the C. elegans genome encodes more than 1000 G-protein coupled receptors (GPCRs), of which many are expressed in chemosensory neurons that have been shown to be required for NDMM-mediated phenotypes (Bargmann, 2006; Ludewig and Schroeder, 2013). Several GPCRs have recently been described as receptors of the main components of the C. elegans sex and dauer pheromones (Kim et al., 2009; McGrath et al., 2011); however, direct binding to a GPCR has been demonstrated for only one compound, ascr#2 (Park et al., 2012). Moreover, little is known about the mechanisms that underlie the observed structure-activity relationships or the synergistic activity of many compounds.
Ascaroside production is widely conserved among nematodes
Recent analyses of a larger selection of parasitic and free-living nematodes revealed the presence of ascaroside derivatives in the metabolomes of most of the analyzed species (Choe et al., 2012a; Choe et al., 2012b; Noguez et al., 2012). For example, the ethanolamide easc#18 was shown to regulate development of the insect parasitic Heterorhabditis bacteriophora and the side chain-dihydroxylated dhas#18 serves as a mating signal in the sour paste nematode Panagrellus redivivus (Figure 7). The use of ascarosides and the related paratosides, neither of which have been found in other animals, as scaffolds for signaling molecules in nematodes may be the result of a peculiarity of nematode lipid metabolism: long-chain ascarosides (chain length 29 carbons and more) have been shown to account for a considerable portion of total lipid content in C. elegans and other nematode species, e.g. Ascaris spp. (Bartley et al., 1996; Jezyk and Fairbairn, 1967). Therefore, short-chain ascarosides appear as endproducts of lipid metabolism, which then are combined with building blocks from amino acid catabolism and other metabolic pathways. However, the fact that all so-far characterized members of this “modular language” of nematode signaling molecules are based on glycosides of the two deoxysugars, ascarylose and paratose, may also reflect bias toward identifying additional ascaroside derivatives rather than characterizing other groups of nematode metabolites, whose potential relevance for nematode biology may seem less clear. Nonetheless, mass spectrometric and 2D NMR spectroscopic analyses of the C. elegans and P. pacificus metabolomes show that ascarosides make up for only a small fraction of nematode metabolomes and have provided evidence for the presence of a large diversity of yet uncharacterized, non ascarylose-derived compounds (Bose et al., 2012; von Reuss et al., 2012).
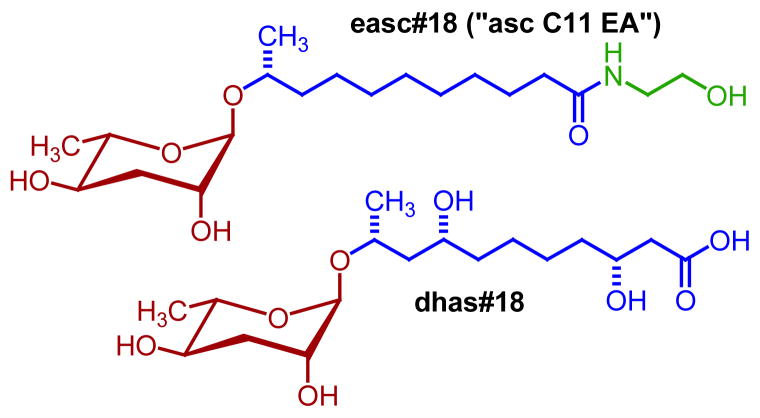
Figure 7.
Examples for ascaroside-based signaling molecules identified from the insect parasite Heterorhabditis bacteriophora (easc#18, (Noguez et al., 2012)) and the sour paste nematode Panagrellus redivivus (dhas#18, (Choe et al., 2012a)).
Outlook and future directions
NDMMs represent a new class of natural products that transcends the dichotomy between “primary” and “secondary” metabolism. Much of the NDMMs’ chemical originality and appeal derives from the fact that they combine well-known primary metabolites into new structures, sometimes with unusual chemical modifications, demonstrating unexpected biosynthetic capabilities in animals (Figure 8).
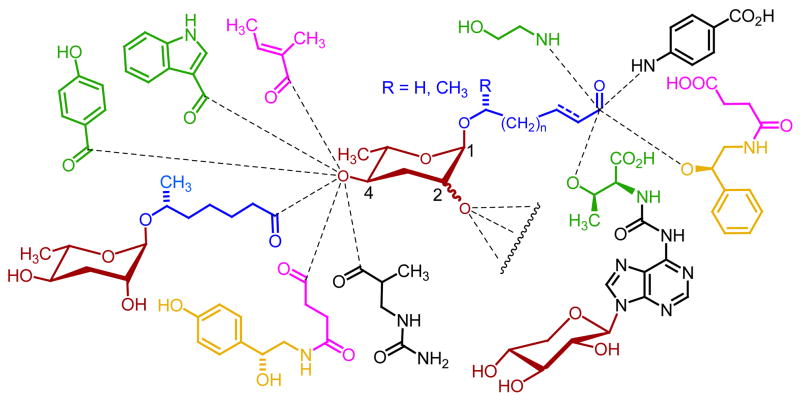
Figure 8.
NDMMs form a modular library of signaling molecules, derived from combination of building blocks from diverse primary metabolic pathways, e.g. neurotransmitter (yellow), amino acid (green), short-chain fatty acid (pink), folate and nucleoside (black), long-chain fatty acid (blue), and carbohydrate (crimson) metabolism. Acylation or glycosylation at position 2 of the ascarylose is also observed, although much less frequently than at position 4.
More than one hundred ascaroside-based NDMMs have been identified over the past few years, and, not surprisingly, the characterization of their biological properties has not kept pace with this high rate of chemical discovery. Given the large number of yet entirely uncharacterized metabolites known to exist in C. elegans and other model organisms, one may ask whether this unexplored chemical diversity is likely to be of any significance to the biology of these organisms. The discovery of unexpected phenotypes as a result of the identification of new NDMMs, e.g. the discovery of an aggregation pheromone as a result of the identification of the indole ascarosides, or the finding that ascarosides regulate C. elegans lifespan via sirtuin-dependent pathways (see above), suggests that many yet to be identified metabolites serve important signaling functions. More generally, it should be noted that, in fact, any molecule produced by a living organism – or a community of organisms – carries specific information about the state of the producer(s), simply by virtue of the molecule’s biosynthetic history (Meinwald, 2011). Therefore, it is not surprising that many different types of biological small molecules have acquired signaling roles; within a single cell, as hormones or second messengers between different cells or tissues of one organisms, or as pheromones and quorum sensing signals between individuals of the same or several different species. For these small molecule signals, biosynthetic pathways have evolved to transduce specific messages most effectively, along with dedicated perception mechanisms, for example in the form of nuclear or membrane-bound receptor proteins that are finely tuned to respond to specific small molecules, often at very low concentrations.
It seems certain that many more new small molecule structures and functions will be discovered in nematodes, contributing to the value of C. elegans and P. pacificus as model systems for basic biology, but also providing a clear rationale for a re-analysis of the metabolomes of other groups of animals, including mammals.
Acknowledgments
The author thanks Robert J. Micikas and Dr. Joshua C. Judkins for a critical reading of the manuscript and many helpful suggestions. Support from the National Institutes of Health (GM088290) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artyukhin AB, Yim JJ, Srinivasan J, Izrayelit Y, Bose N, von Reuss SH, Jo Y, Jordan JM, Baugh LR, Cheong M, et al. Succinylated octopamine ascarosides and a new pathway of biogenic amine metabolism in Caenorhabditis elegans. J Biol Chem. 2013;288:18778–18783. doi: 10.1074/jbc.C113.477000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI. Chemosensation in C. elegans. WormBook, editor. The C. elegans Research Community. 2006 doi: 10.1895/wormbook.1.123.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Bartley JP, Bennett EA, Darben PA. Structure of the ascarosides from Ascaris suum. J Nat Prod. 1996;59:921–926. doi: 10.1021/np960236+. [DOI] [PubMed] [Google Scholar]
- Bento G, Ogawa A, Sommer RJ. Co-option of the hormone-signalling module dafachronic acid-DAF-12 in nematode evolution. Nature. 2010;466:494–497. doi: 10.1038/nature09164. Epub 2010 Jun 2030. [DOI] [PubMed] [Google Scholar]
- Blaxter M. Caenorhabditis elegans is a Nematode. Science. 1998;282:2041–2046. doi: 10.1126/science.282.5396.2041. [DOI] [PubMed] [Google Scholar]
- Bose N, Meyer JM, Yim JJ, Mayer MG, Markov GV, Ogawa A, Schroeder FC, Sommer RJ. Natural variation in dauer pheromone production and sensing supports intraspecific competition in nematodes. Curr Biol. 2014;24:1536–1541. doi: 10.1016/j.cub.2014.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose N, Ogawa A, von Reuss SH, Yim JJ, Ragsdale EJ, Sommer RJ, Schroeder FC. Complex small-molecule architectures regulate phenotypic plasticity in a nematode. Angew Chem Int Ed Engl. 2012;51:12438–12443. doi: 10.1002/anie.201206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- Butcher RA, Ragains JR, Clardy J. An indole-containing dauer pheromone component with unusual dauer inhibitory activity at higher concentrations. Org Lett. 2009a;11:3100–3103. doi: 10.1021/ol901011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RA, Ragains JR, Kim E, Clardy J. A potent dauer pheromone component in Caenorhabditis elegans that acts synergistically with other components. Proc Natl Acad Sci U S A. 2008;105:14288–14292. doi: 10.1073/pnas.0806676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RA, Ragains JR, Li W, Ruvkun G, Clardy J, Mak HY. Biosynthesis of the Caenorhabditis elegans dauer pheromone. Proc Natl Acad Sci U S A. 2009b;106:1875–1879. doi: 10.1073/pnas.0810338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe A, Chuman T, von Reuss SH, Dossey AT, Yim JJ, Ajredini R, Kolawa AA, Kaplan F, Alborn HT, Teal PE, et al. Sex-specific mating pheromones in the nematode Panagrellus redivivus. Proc Natl Acad Sci U S A. 2012a;109:20949–20954. doi: 10.1073/pnas.1218302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe A, von Reuss SH, Kogan D, Gasser RB, Platzer EG, Schroeder FC, Sternberg PW. Ascaroside signaling is widely conserved among nematodes. Curr Biol. 2012b;22:772–780. doi: 10.1016/j.cub.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C, El Yacoubi B, de Crecy-Lagard V, Iwata-Reuyl D. Biosynthesis of threonylcarbamoyl adenosine (t6A), a universal tRNA nucleoside. J Biol Chem. 2012;287:13666–13673. doi: 10.1074/jbc.M112.344028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickschat JS. Quorum sensing and bacterial biofilms. Nat Prod Rep. 2010;27:343–369. doi: 10.1039/b804469b. [DOI] [PubMed] [Google Scholar]
- Dieterich C, Clifton SW, Schuster LN, Chinwalla A, Delehaunty K, Dinkelacker I, Fulton L, Fulton R, Godfrey J, Minx P, et al. The Pristionchus pacificus genome provides a unique perspective on nematode lifestyle and parasitism. Nat Genet. 2008;40:1193–1198. doi: 10.1038/ng.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22:2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch KM, Gurgui C, Heycke N, van der Sar SA, Anderson SA, Webb VL, Taudien S, Platzer M, Rubio BK, Robinson SJ, et al. Polyketide assembly lines of uncultivated sponge symbionts from structure-based gene targeting. Nat Chem Biol. 2009;5:494–450. doi: 10.1038/nchembio.176. [DOI] [PubMed] [Google Scholar]
- Gallo M, Riddle DL. Effects of a Caenorhabditis elegans dauer pheromone ascaroside on physiology and signal transduction pathways. J Chem Ecol. 2009;35:272–279. doi: 10.1007/s10886-009-9599-3. [DOI] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol. 1984;102:368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. A gene affecting production of the Caenorhabditis elegans dauer-inducing pheromone. Mol Gen Genet. 1985;198:534–536. doi: 10.1007/BF00332953. [DOI] [PubMed] [Google Scholar]
- Gronquist M, Schroeder FC, Lew M, Hung-Wen L. Comprehensive Natural Products II. Oxford: Elsevier; 2010. Insect Natural Products; pp. 67–108. [Google Scholar]
- Hollister KA, Conner ES, Zhang X, Spell M, Bernard GM, Patel P, de Carvalho AC, Butcher RA, Ragains JR. Ascaroside activity in Caenorhabditis elegans is highly dependent on chemical structure. Bioorg Med Chem. 2013;21:5754–5769. doi: 10.1016/j.bmc.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu PJ. Dauer. WormBook, editor. The C. elegans Research Community. 2007 http://www.wormbook.org.
- Izrayelit Y, Robinette SL, Bose N, von Reuss SH, Schroeder FC. 2D NMR-based metabolomics uncovers interactions between conserved biochemical pathways in the model organism Caenorhabditis elegans. ACS Chem Biol. 2013;8:314–319. doi: 10.1021/cb3004644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrayelit Y, Srinivasan J, Campbell SL, Jo Y, von Reuss SH, Genoff MC, Sternberg PW, Schroeder FC. Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS Chem Biol. 2012;7:1321–1325. doi: 10.1021/cb300169c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim YH, Kim K, Paik YK. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- Jezyk PF, Fairbairn D. Ascarosides and ascaroside esters in Ascaris lumbricoides (Nematoda) Comp Biochem Physiol. 1967;23:691–705. doi: 10.1016/0010-406x(67)90334-9. [DOI] [PubMed] [Google Scholar]
- Joo HJ, Kim KY, Yim YH, Jin YX, Kim H, Kim MY, Paik YK. Contribution of the peroxisomal acox gene to the dynamic balance of daumone production in Caenorhabditis elegans. J Biol Chem. 2010;285:29319–29325. doi: 10.1074/jbc.M110.122663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;5:387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kim K, Sato K, Shibuya M, Zeiger D, Butcher R, Ragains J, Clardy J, Touhara K, Sengupta P. Two Chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science. 2009;326:994–998. doi: 10.1126/science.1176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig AH, Izrayelit Y, Park D, Malik RU, Zimmermann A, Mahanti P, Fox BW, Bethke A, Doering F, Riddle DL, et al. Pheromone sensing regulates Caenorhabditis elegans lifespan and stress resistance via the deacetylase SIR-2.1. Proc Natl Acad Sci U S A. 2013;110:5522–5527. doi: 10.1073/pnas.1214467110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig AH, Schroeder FC. Ascaroside signaling in C. elegans. WormBook, editor. The C. elegans Research Community. 2013 doi: 10.1895/wormbook.1.155.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- McGrath PT, Xu Y, Ailion M, Garrison JL, Butcher RA, Bargmann CI. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature. 2011;477:321–325. doi: 10.1038/nature10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinwald J. Natural products as molecular messengers. J Nat Prod. 2011;74:305–309. doi: 10.1021/np100754j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- Noguez JH, Conner ES, Zhou Y, Ciche TA, Ragains JR, Butcher RA. A novel ascaroside controls the parasitic life cycle of the entomopathogenic nematode Heterorhabditis bacteriophora. ACS Chem Biol. 2012;7:961–966. doi: 10.1021/cb300056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Bento G, Bartelmes G, Dieterich C, Sommer RJ. Pristionchus pacificus daf-16 is essential for dauer formation but dispensable for mouth form dimorphism. Development. 2011;138:1281–1284. doi: 10.1242/dev.058909. [DOI] [PubMed] [Google Scholar]
- Park D, O’Doherty I, Somvanshi RK, Bethke A, Schroeder FC, Kumar U, Riddle DL. Interaction of structure-specific and promiscuous G-protein-coupled receptors mediates small-molecule signaling in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2012;109:9917–9922. doi: 10.1073/pnas.1202216109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J. Metabolites from symbiotic bacteria. Nat Prod Rep. 2009;26:338–362. doi: 10.1039/b703499g. [DOI] [PubMed] [Google Scholar]
- Pungaliya C, Srinivasan J, Fox BW, Malik RU, Ludewig AH, Sternberg PW, Schroeder FC. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2009;106:7708–7713. doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae R, Riebesell M, Dinkelacker I, Wang Q, Herrmann M, Weller AM, Dieterich C, Sommer RJ. Isolation of naturally associated bacteria of necromenic Pristionchus nematodes and fitness consequences. J Exp Biol. 2008;211:1927–1936. doi: 10.1242/jeb.014944. [DOI] [PubMed] [Google Scholar]
- Riddle DL, Albert PS. Genetic and environmental regulation of dauer larva development. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Cold Spring Harbor (NY): 1997. [PubMed] [Google Scholar]
- Simon JM, Sternberg PW. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:1598–1603. doi: 10.1073/pnas.032225799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer Ralf J, Ogawa A. Hormone signaling and phenotypic plasticity in nematode development and evolution. Curr Biol. 2011;21:R758–R766. doi: 10.1016/j.cub.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PEA, Malik RU, Edison AS, Sternberg PW, Schroeder FC. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J, von Reuss SH, Bose N, Zaslaver A, Mahanti P, Ho MC, O’Doherty OG, Edison AS, Sternberg PW, Schroeder FC. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012;10:e1001237. doi: 10.1371/journal.pbio.1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- von Reuss SH, Bose N, Srinivasan J, Yim JJ, Judkins JC, Sternberg PW, Schroeder FC. Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small molecule signals in C. elegans. J Am Chem Soc. 2012;134:1817–1824. doi: 10.1021/ja210202y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller AM, Mayer WE, Rae R, Sommer RJ. Quantitative assessment of the nematode fauna present on Geotrupes dung beetles reveals species-rich communities with a heterogeneous distribution. J Parasitol. 2010;96:525–531. doi: 10.1645/GE-2319.1. [DOI] [PubMed] [Google Scholar]