Abstract
Objective
To describe associations between total and regional body fat mass loss and reduction of systemic levels of inflammation (C-reactive protein (CRP) and interleukin-6 (IL-6)) in obese, older adults with osteoarthritis, undergoing intentional weight loss.
Design
Data come from a single-blind, 18-month, randomized controlled trial in adults (age: 65.6±6.2; BMI: 33.6±3.7) with knee osteoarthritis. Participants were randomized to diet-induced weight loss plus exercise (D+E; n=150), diet-induced weight loss-only (D; n=149), or exercise-only (E; n=151). Total body and region-specific (abdomen and thigh) fat mass were measured at baseline and 18 months. High-sensitivity CRP and IL-6 were measured at baseline, six and 18 months. Intervention effects were assessed using mixed models and associations between inflammation and adiposity were compared using logistic and mixed linear regression models.
Results
Intentional total body fat mass reduction was associated with significant reductions in log-adjusted CRP (β=0.06 (95% CI=0.04,0.08) mg/L) and IL-6 (β=0.02 (95% CI=0.01,0.04) pg/mL). Loss of abdominal fat volume was also associated with reduced inflammation, independent of total body fat mass; although models containing measures of total adiposity yielded the best fit. The odds of achieving clinically desirable levels of CRP (<3.0 mg/L) and IL-6 (<2.5 pg/mL) were 3.8 (95% CI=1.6,8.9) and 2.2 (95% CI=1.1,4.6), respectively, with 5% total weight and fat mass loss.
Conclusions
Achievement of clinically desirable levels of CRP and IL-6 more than double with intentional 5% loss of total body weight and fat mass. Global, rather than regional, measures of adiposity are better predictors of change in inflammatory burden.
Clinical Trial Registration Number
Keywords: inflammation, weight loss, total body fat mass, regional fat mass, osteoarthritis
Introduction
Inflammation is a necessary response of the immune system to acute infection or trauma; however, a prolonged inflammatory state has detrimental health effects1. Old age is associated with higher circulating levels of inflammatory biomarkers2, and chronic, low-grade inflammation is involved in the pathophysiology of a number of aging-related conditions3, including osteoarthritis (OA)4. Indeed, levels of several inflammatory biomarkers are higher in older adults with OA compared to those without5. Therapies that control or reduce inflammation may be effective for prevention and/or improvement of OA and its debilitating symptoms.
Obesity is a strong modifiable risk factor for OA6, and is associated with significantly higher levels of C-reactive protein (CRP) and interleukin-6 (IL-6)7, two inflammatory biomarkers implicated in the onset and progression of OA4;8. Interventions that reduce excess body weight also reduce inflammatory burden9, especially for individuals with chronic conditions (like OA) characterized by a state of low-grade inflammation10. For overweight and obese individuals, achievement of at least 10% weight loss most consistently improves the inflammatory profile11–13; yet, the amount of weight and fat mass loss necessary to achieve a clinically desirable level of inflammation in this population is unknown. Moreover, whether there is a greater benefit of loss of adipose tissue from specific depots/regions on inflammation is not known. Fat stored in ectopic regions (such as the abdominal viscera and thigh muscle) is associated with higher circulating levels of CRP and IL-6 independent of total fat mass14–16, suggesting that greater reduction of fat in these regions may be associated with greater improvement in the inflammatory profile.
The primary purpose of this study was to present the effect of an intensive lifestyle-based weight loss intervention on CRP and IL-6 in obese, older adults with knee OA. Secondarily, we explored whether the relationship observed between fat loss and inflammatory burden was region specific. Lastly, we tested whether a 5% loss of total body mass and fat mass was associated with increased odds of achieving clinically desirable levels of CRP and IL-6. Clinical cut-points of <3.0 mg/L and <2.5 pg/mL of CRP and IL-6, respectively, were empirically selected based on cardiovascular disease (CVD)17 and disability18 risk prediction thresholds. We hypothesized that: (1) randomization to dietary-induced weight loss would significantly reduce inflammatory burden; (2) reduction in CRP and IL-6 would be most strongly associated with loss of ectopic (i.e., visceral, intermuscular) rather than global or subcutaneous fat stores; and (3) a 5% loss of total body mass and fat mass would be significantly associated with achievement of clinically desirable levels of systemic inflammation.
Method
Study Design
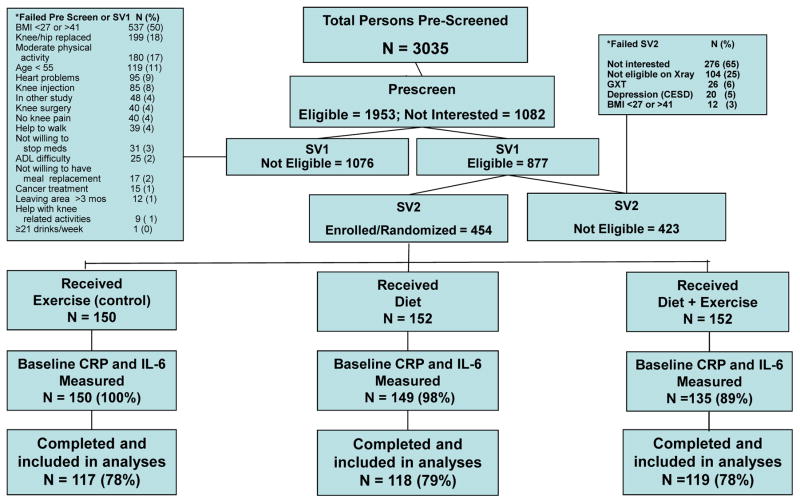
We performed an ancillary analysis of data collected as part of the Intensive Diet and Exercise for Arthritis (IDEA) trial (NCT00381290), a single-blinded, 18-month, randomized controlled trial comparing the effects of exercise-only (E), intensive dietary induced weight loss (D), or D+E on OA symptoms in 454 older adults with knee OA19. The Wake Forest University Health Sciences Institutional Review Board approved this study (IRB# 00000602), all study participants gave written informed consent to participate, and main study findings are published10. As previously reported, both diet groups lost significantly (p<0.01) more weight than the E group. Over 18-months, the D+E group lost an average of 11.4% (10.6 kg) and the D group lost 9.5% (8.9 kg); the E group lost 1.8 kg, or 2.0%, of baseline body weight. Further, compared with E participants, knee compressive forces were lower in D participants and IL-6 levels were lower in D and D+E participants10.
Study Participants
Detailed participant inclusion and exclusion criteria are published19. The original study sample consisted of 454 ambulatory, community-dwelling persons age ≥ 55 years with: 1) grade II-III (mild to moderate) radiographic tibiofemoral OA or tibiofemoral plus patellofemoral OA of one or both knees; 2) 27.0 ≤ BMI ≤ 40.5 kg/m2; and 3) a sedentary lifestyle, defined as not participating in a program that incorporates more than 30 minutes per week of formal exercise within the past six months. The sample for the present study include all IDEA participants with CRP and IL-6 measurements at baseline (n=450).
Interventions
Both the D and D+E groups received the same dietary intervention, consisting of group and individual nutrition education and behavioral sessions, as well as an individualized dietary prescription plan providing an energy-intake deficit of 800–1000 kcals/day to reach a study goal of 10% of baseline weight lost. The E-only group was not counseled to restrict caloric intake during the study intervention period. The D+E and E groups received the same exercise intervention, consisting of aerobic walking (15 minutes), strength training (20 minutes), a second aerobic phase (15 minutes), and cool-down (10 minutes), three days per week. During the first 6 months, participation was center-based; afterward, participants could remain in the facility program, opt for a home-based program, or combine the two.
Measurements
Body Composition
All body composition measures were collected at baseline and 18 months. Body mass index was measured in all participants and calculated as mass (measured in kilograms on a standard calibrated scale) divided by height squared (measured in meters). Baseline whole body fat mass (kg) was measured by dual-energy x-ray absorptiometry (DXA; Delphi A™, Hologic (Waltham, MA)) in 396 participants (n=58 participants declined baseline DXA due to concerns over radiation exposure and/or lack of time) with a coefficient of variation of 1.2% and following the manufacturer’s recommendations for patient position and scan protocols and analysis.
Computed tomography (CT) scans, using a GE 16-slice Light Speed Pro, quantified abdominal (intramuscular, subcutaneous, visceral) and thigh (intermuscular, subcutaneous) fat volume (cm3) in a random subset of IDEA participants pre and post-intervention (n=116). Participants were placed supine in the scanner with arms above the head and legs flat. Abdominal scanning technique was 120 KVp, 320 mA, 2.5-mm-thick slices, a helical pitch of 6.25 mm/rotation, and a gantry speed of 0.5 s. Scanning covered the lower abdomen, including the umbilicus and lower lumbar vertebra, using a 50-cm scan and display field that included the entire girth. Fat depots were defined by technicians segmenting volumes based on established anatomical boundaries. Subcutaneous fat was defined as outside the abdominal wall musculature; visceral fat as within the inner aspect of the abdominal wall; intermuscular fat as within the abdominal and paraspinal musculature. Thigh scans were conducted at 120 KVp, 350 mA, 10-mm helical with a pitch of 11.25 mm/rotation and a gantry speed of 0.8 s. The femur, from head to medial condyle, was measured and divided into 3 equal lengths. Measurements were performed on 50-mm-long slices centered at the boundary of the proximal and middle third of the femur.
Inflammatory Biomarkers
Blood samples were successfully collected at baseline in 450 participants in the early morning (between 7 and 9 AM) after a 12-hour fast (see Figure 1). Six-month (n=366 for CRP and n=368 for IL-6; n=84 and 82 loss to follow-up, respectively) and 18-month (n=350 for both CRP and IL-6; n=100 loss to follow-up) blood samples were collected at least 24 hours after the exercise session, and blood sampling was postponed (1–2 weeks after recovery of all symptoms) in the event of an acute respiratory, urinary tract, or other infection. All blood was collected, processed, divided into aliquots, stored locally at −80°C, and batch analyzed using previously published methods20. Briefly, high sensitivity IL-6 assays were run using Quantikine enzyme-linked immunosorbent kits from R&D systems (Minneapolis, MN) and high sensitivity CRP was assessed using an automated immunoanalyzer (IMMULITE; Diagnostics Products Corporation, Los Angeles, CA).
Figure 1.
IDEA Consort diagram
Statistical Analysis
Baseline characteristics are presented on all participants with baseline inflammatory biomarker data (n=450). Sample means and standard deviations are estimated for the continuous descriptive characteristics, and count and proportions were calculated for the discrete descriptive characteristics overall and by intervention group. For all inferential models, continuous outcomes were checked for approximate normality, and a log-transformation was applied for right-skewed inflammation variables. Regression model assumptions were checked using residual quantile-quantile plots for continuous outcomes to ensure appropriate fit. The intervention effect for variables collected at only baseline and 18 months (CT and DXA outcomes) was estimated using a one-way analysis of variance model for unadjusted estimates, and adjusted estimates were based on an analysis of covariance model that included baseline BMI, gender, and baseline values of the outcome to minimize model variance bias as specified by the IDEA analytic plan19. For outcomes collected at six and 18 months (body mass, BMI, CRP, and IL-6), a mixed linear model was utilized to account for within-subject variability assuming a first-order autoregressive covariance structure, and either no covariates (unadjusted) or baseline BMI, gender, and baseline values of the outcome (adjusted) were included in the model. Participants with missing follow-up visit data were excluded from visit-specific analyses, and prior publication indicate that attrition was likely unrelated to randomization assignment19. Overall group comparisons were performed at a 0.05 level, and when group comparisons were significant, pairwise comparisons between arms were deemed significant at a Bonferroni-adjusted 0.0167 level.
Associations between baseline body composition and inflammation data were performed using linear regression adjusting for randomization group, race, gender, and age. To determine the association between change in adiposity (resulting from intentional weight loss) and change in inflammatory biomarkers, analyses were limited to D and D+E arms, and all associations were adjusted for randomization arm, race, gender, age, and baseline CRP or IL-6 values. In both baseline and change analyses, model fit was compared using Akike’s Information Criteria (AIC) with a model limited to participants who had all adiposity measurements to ensure a comparable sample size across all models. Logistic regression was used to estimate the odds (95% Wald confidence intervals) of attaining clinically desirable biomarker values based on a 5% change in body mass, as weight loss of this magnitude is considered clinically meaningful.21 A similar threshold was selected for fat mass loss. Analyses were conducted for all groups and weight loss arms only, adjusted for randomization group, baseline BMI, gender, and baseline inflammation status. Tests of association were performed assuming a Type I error rate of 0.05. All statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Participant Baseline Characteristics
Baseline demographic characteristics of the study sample (n=450) are presented in Table 1. Briefly, participants were 65.6±6.2 year of age, 71% were women, and 83% were of Caucasian descent. On average, participants were classified as obese (BMI=33.6±3.7 kg/m2; percent body fat=40.0±6.7%), and median CRP and IL-6 levels were modestly elevated (5.25 (IQR 2.10, 10.70) mg/L and 2.31 (IQR 1.59, 3.94) pg/mL, respectively) at baseline, with 67% (n=303) of participants with CRP levels ≥3.0 mg/L and 46% (n=208) with IL-6 levels ≥2.5 pg/mL.
Table 1.
Baseline descriptive characteristics according to treatment group.
| Participant Characteristics | Exercise (n=150) | Diet (n=149) | Diet + Exercise (n=151) | Overall (n=450)* |
|---|---|---|---|---|
| Age (years) | 65.5±6.4 | 65.8±6.2 | 65.5±6.0 | 65.6±6.2 |
| Female, n (%) | 108 (72) | 105 (70) | 108 (72) | 321 (71) |
| White, n (%) | 123 (82) | 128 (86) | 123 (81) | 374 (83) |
| Weight (kg) | 92.3 (14.5) | 93.4 (15.2) | 92.9 (14.4) | 92.3 (14.7) |
| Body Mass Index (kg/m2) | 33.5±3.7 | 33.7±3.8 | 33.5±3.7 | 33.6±3.7 |
| Self-Reported Comorbidities, n (%) | ||||
| Hypertension | 89 (60) | 90 (62) | 91 (61) | 270 (61) |
| Cardiovascular disease | 135 (92) | 19 (13) | 11 (8) | 42 (10) |
| Diabetes | 18 (12) | 18 (12) | 23 (15) | 59 (13) |
| DXA-Acquired Adiposity Measures (n=396) | ||||
| Total body fat mass (kg) | 36.9±7.7 | 36.4±7.3 | 37.0±8.2 | 36.8±7.7 |
| Percent total body fat (%) | 40.0±7.0 | 39.9±6.6 | 40.1±6.7 | 40.0±6.7 |
| CT-Acquired Adiposity Measures (n=177) | ||||
| Total abdominal fat volume (cm3) | 8625.4±2031.5 | 8028.6±1731.9 | 7921.0±1991.8 | 8194.4±1940.5 |
| Abdominal visceral fat volume (cm3) | 3282.1±1390.8 | 3018.9±1348.9 | 2895.4±1222.8 | 3066.3±1324.9 |
| Abdominal subcutaneous fat volume (cm3) | 5064.7±1603.9 | 4779.9±1330.1 | 4772.0±1443.3 | 4873.8±1463.7 |
| Abdominal intermuscular fat volume (cm3) | 278.5±118.9 | 229.8±78.4 | 253.6±115.3 | 254.4±107.4 |
| Total thigh fat volume (cm3) | 988.1±310.4 | 910.3±286.0 | 937.9±308.5 | 946.0±302.1 |
| Thigh intermuscular fat volume (cm3) | 32.5±17.7 | 30.9±17.0 | 32.5±21.8 | 32.0±18.9 |
| Thigh subcutaneous fat volume (cm3) | 955.6±309.2 | 879.4±285.7 | 905.4±306.7 | 914.0±300.9 |
| CRP (mg/L) | 8.7±11.5 | 10.1±13.1 | 7.6±8.8 | 8.8±11.3 |
| IL-6 (pg/mL) | 3.0±2.1 | 3.2±2.3 | 3.2±2.22 | 3.1±2.2 |
Data are presented as means ± SD or n (%).
Inflammatory biomarkers assessed on 450 (out of 454) IDEA participants. DXA and CT-acquired adiposity measures are only available on a subset of IDEA participants (total n=396 and 177, respectively).
Intervention Effects on Adiposity and Inflammation
As previously reported10, both diet groups lost significantly more weight and fat mass than the E group (both p<0.001). Change in body mass for participants with baseline and follow-up data in the current study sample (n=339) was 9.3±0.6 kg, 8.4±0.6 kg, and 1.3±0.6 kg for the D+E, D and E groups, respectively, with no difference observed between D+E and D (p=0.32). Likewise, D+E and D groups had an 18% and 15% reduction, respectively, in total body fat mass over the 18-month intervention, while negligible change in total body fat mass (~1%) was observed in the E-only group.
All abdominal fat depots (e.g. total, visceral, subcutaneous, and intermuscular) were significantly reduced in the D+E and D groups, compared to E, at 18-months (all p<0.05; Table 2). Similarly, 18-month total, intermuscular, and subcutaneous thigh fat estimates were lower in the D+E and D groups, compared to E, although adjusted results did not reach statistical significance.
Table 2.
Unadjusted and adjusted 18-month treatment effects on regional fat mass.
| Regional Fat Volume Measures | Exercise | Diet | Diet + Exercise | Overall p-value* | |||
|---|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | ||
| Total abdominal fat (cm3) | |||||||
| Unadjusted | 8592.8 | 382.2 | 6813.4 | 358.7 | 6342.2 | 377.1 | <0.01* |
| Adjusted | 8151.4 | 244.5 | 6858.4 | 228.5 | 6802.3 | 234.7 | <0.01* |
|
| |||||||
| Abdominal visceral fat (cm3) | |||||||
| Unadjusted | 3082.6 | 211.8 | 2394.2 | 198.8 | 2276.2 | 209.0 | 0.02** |
| Adjusted | 2981.9 | 112.8 | 2406.3 | 106.7 | 2433.1 | 107.0 | <0.01* |
|
| |||||||
| Abdominal subcutaneous fat (cm3) | |||||||
| Unadjusted | 5233.5 | 265.6 | 4198.9 | 249.3 | 3852.4 | 262.1 | <0.01* |
| Adjusted | 4944.5 | 140.8 | 4239.1 | 137.6 | 4158.6 | 144.0 | <0.01* |
|
| |||||||
| Abdominal intermuscular fat (cm3) | |||||||
| Unadjusted | 276.8 | 17.6 | 220.3 | 16.5 | 213.6 | 17.3 | 0.02** |
| Adjusted | 252.4 | 9.9 | 229.4 | 9.3 | 218.7 | 9.5 | 0.04** |
|
| |||||||
| Total thigh fat (cm3) | |||||||
| Unadjusted | 1391.8 | 91.0 | 1132.3 | 84.3 | 1071.3 | 88.6 | 0.03** |
| Adjusted | 1322.4 | 66.8 | 1160.6 | 65.6 | 1151.5 | 67.0 | 0.11 |
|
| |||||||
| Thigh intermuscular fat (cm3) | |||||||
| Unadjusted | 46.8 | 4.8 | 40.2 | 4.4 | 40.4 | 4.6 | 0.54 |
| Adjusted | 48.8 | 3.7 | 41.3 | 3.5 | 41.7 | 3.6 | 0.25 |
|
| |||||||
| Thigh subcutaneous fat (cm3) | |||||||
| Unadjusted | 1345.0 | 89.3 | 1092.1 | 82.7 | 1030.8 | 86.9 | 0.03** |
| Adjusted | 1274.8 | 63.9 | 1122.4 | 63.0 | 1112.4 | 64.3 | 0.12 |
Unadjusted estimates are based on a one-way ANOVA at 18 months. Model-adjusted estimates control for gender, baseline BMI, and baseline outcome measure. In cases where statistical significance is noted,
indicates E differs significantly from D+E and D, and
indicates D+E differs significantly from E (all pairwise comparisons p<0.0167).
After adjustment for visit, gender, baseline BMI, and baseline biomarker value, a significant treatment effect on inflammatory burden was observed. As previously reported10, D+E and D participants presented with lower 18-month IL-6 than E participants [2.7 (0.2) and 2.7 (0.2) for D+E and D, versus 3.2 (0.2) pg/mL for E; p<0.01]. 18-month CRP was also reduced in the D+E and D groups [5.3 (SE=1.1) and 4.2 (1.1), respectively versus 6.9 (1.1) mg/L for E; p<0.01]; see Figure 2, and both diet groups had twice the odds of achieving clinically desirable levels of CRP at 18-months (i.e. <3.0 mg/L) compared with the E group (p=0.02). Although the odds of achieving IL-6<2.5 pg/mL was trending in a similar direction [1.5 (0.8–2.7) and 1.6 (0.9,2.8) for D+E and D versus E, respectively], a significant treatment effect was not seen (p=0.24).
Figure 2.
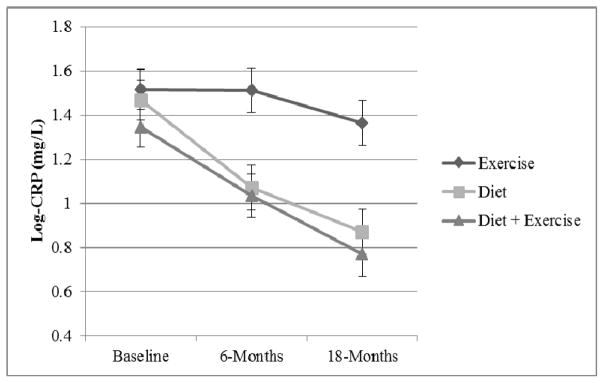
Mean log-CRP by group across the 18-month intervention
Associations between Total and Regional Fat Mass and Inflammation
Table 3 presents log-adjusted parameter estimates of the association between several measures of baseline adiposity and inflammation. Based on the magnitude of fat volume observed in Table 2, CT-derived measures of adiposity are presented per 1000 cm3. Lower total body mass, BMI, total body fat mass, and percent body fat were significantly associated with lower levels of CRP and IL-6 (all p<0.01); further, all regional fat depots (save associations between abdominal intermuscular and thigh intermuscular fat volume and CRP) were directly associated with log-adjusted CRP and IL-6 parameter estimates (all p≤0.04). After adjustment for total body fat mass, only associations between log-adjusted IL-6 and abdominal visceral fat volume (β=0.12 (95% CI: 0.03,0.21) log pg/mL per 1000 cm3; p=0.02) and thigh intermuscular fat volume (β=8.34 (95% CI: 3.12,13.56) log mg/L per 1000 cm3; p<0.01) remained. AIC analysis for individuals with baseline weight, BMI, DXA, and CT measures (n=162) suggest models containing total body mass and global measures of adiposity provide a better fit than models containing regional fat measures (lowest AIC found for BMI).
Table 3.
Log-adjusted parameter estimates and 95% confidence intervals of the association between baseline adiposity measures and baseline inflammation.
| Baseline body mass and adiposity measures | Log-Adjusted CRP (mg/L) | Log-Adjusted IL-6 (pg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | p-value | AIC* | Estimate | 95% CI | p-value | AIC* | |
| Total body mass (kg) | 0.02 | (0.01,0.03) | <0.01 | 496.0 | 0.01 | (0.01,0.02) | <0.01 | 303.5 |
| BMI (kg/m2) | 0.09 | (0.06,0.11) | <0.01 | 482.8 | 0.048 | (0.03,0.06) | <0.01 | 293.1 |
| Total body fat mass (kg) | 0.04 | (0.03,0.06) | <0.01 | 490.7 | 0.02 | (0.01,0.03) | <0.01 | 299.9 |
| Percent body fat (%) | 0.08 | (0.06,0.11) | <0.01 | 487.5 | 0.03 | (0.02,0.05) | <0.01 | 300.3 |
| Total abdominal fat volume (per 1000 cm3) | 0.12 | (0.04,0.20) | <0.01 | 504.6 | 0.08 | (0.04,0.13) | <0.01 | 311.5 |
| Abdominal visceral fat volume (per 1000 cm3) | 0.20 | (0.05,0.36) | 0.01 | 504.7 | 0.17 | (0.09,0.25) | <0.01 | 308.3 |
| Abdominal subcutaneous fat volume (per 1000 cm3) | 0.14 | (0.02,0.26) | 0.03 | 507.5 | 0.07 | (0.00,0.14) | 0.04 | 318.3 |
| Abdominal intermuscular fat volume (per 1000 cm3) | 0.85 | (−0.76,2.46) | 0.30 | 505.9 | 1.37 | (0.50,2.23) | <0.01 | 307.8 |
| Total thigh fat volume (per 1000 cm3) | 0.78 | (0.13,1.43) | 0.02 | 504.3 | 0.46 | (0.10,0.82) | 0.01 | 313.7 |
| Thigh intermuscular fat volume (per 1000 cm3) | 4.96 | (−3.85,13.76) | 0.27 | 502.0 | 9.60 | (4.95,14.25) | <0.01 | 295.5 |
| Thigh subcutaneous fat volume (per 1000 cm3) | 0.79 | (0.12,1.45) | 0.02 | 504.4 | 0.43 | (0.06,0.79) | 0.02 | 314.7 |
Models are adjusted for randomization group, race, gender and age. CT-derived measures of adiposity are expressed per 1000 cm3.
Akike’s Information Criteria (AIC) estimates are calculated among participants with baseline weight, BMI, DXA, and CT measures (n=162).
Associations between change in log-adjusted CRP and IL-6 per unit change in measures of body and fat mass for participants undergoing intentional weight loss (D and D+E arms, only) are presented in Table 4. Reductions in all measures of body fat mass and distribution, save change in thigh intermuscular fat volume, were associated with significant reductions in CRP (all p≤0.02); and, loss of total and abdominal fat, but not thigh fat, were associated with significant reductions in IL-6 (all p≤0.05). Only associations between change in total, visceral, and subcutaneous abdominal fat volume remained after further adjustment for change in total body fat mass (all p≤0.02, except the association between IL-6 and abdominal visceral fat volume; p=0.07). Similar to baseline findings, AIC analysis (n=98) revealed change in CRP and IL-6 models containing change in global measures of adiposity yielded the best fit.
Table 4.
18-month changes in log-adjusted inflammatory biomarker parameter estimates and 95% confidence intervals, per unit change in measures of body adiposity, for individual undergoing intentional weight loss, only.
| Change in body mass and adiposity measures | Δ Log-Adjusted CRP (mg/L) | Δ Log-Adjusted IL-6 (pg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | p-value | AIC* | Estimate | 95% CI | p-value | AIC* | |
| Δ Total body mass (kg) | 0.05 | (0.03,0.06) | <0.01 | 266.7 | 0.02 | (0.01,0.02) | <0.01 | 184.0 |
| Δ BMI (kg/m2) | 0.15 | (0.11,0.19) | <0.01 | 265.3 | 0.05 | (0.03,0.08) | <0.01 | 182.2 |
| Δ Total body fat mass (kg) | 0.06 | (0.04,0.08) | <0.01 | 271.2 | 0.02 | (0.01,0.04) | <0.01 | 181.3 |
| Δ Percent body fat (%) | 0.08 | (0.05,0.11) | <0.01 | 276.8 | 0.03 | (0.01,0.05) | <0.01 | 182.9 |
| Δ Total abdominal fat volume (per 1000 cm3) | 0.37 | (0.24,0.51) | <0.01 | 271.1 | 0.19 | (0.11,0.28) | <0.01 | 191.4 |
| Δ Abdominal visceral fat volume (per 1000 cm3) | 0.79 | (0.50,1.08) | <0.01 | 269.7 | 0.35 | (0.15.0.55) | <0.01 | 193.3 |
| Δ Abdominal subcutaneous fat volume (per 1000 cm3) | 0.57 | (0.33,0.80) | <0.01 | 276.5 | 0.33 | (0.18,0.48) | <0.01 | 189.2 |
| Δ Abdominal intermuscular fat volume (per 1000 cm3) | 5.00 | (0.66,9.34) | 0.02 | 284.8 | 2.75 | (0.02,5.49) | 0.05 | 191.0 |
| Δ Total thigh fat volume (per 1000 cm3) | 0.88 | (0.26,1.49) | <0.01 | 288.1 | 0.21 | (−0.20,0.61) | 0.31 | 195.4 |
| Δ Thigh intermuscular fat volume (per 1000 cm3) | 6.31 | (−4.14,16.76) | 0.23 | 286.9 | 3.61 | (−2.99,10.20) | 0.28 | 189.7 |
| Δ Thigh subcutaneous fat volume (per 1000 cm3) | 0.94 | (0.29,1.59) | <0.01 | 287.8 | 0.21 | (−0.21,0.64) | 0.32 | 195.3 |
Results presented for the D and D+E arms, only. Model estimates adjust for randomization group, race, gender, age and baseline outcome measure. CT-derived measures of adiposity are expressed per 1000 cm3.
Akike’s Information Criteria (AIC) estimates are calculated among participants with baseline and follow-up weight, BMI, DXA, and CT measures (n=98).
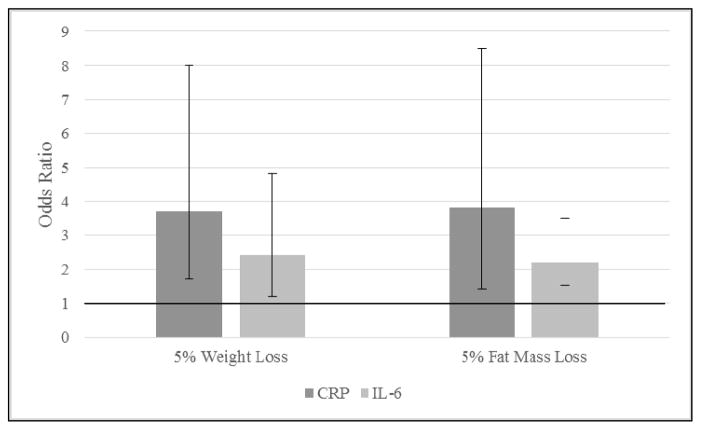
Lastly, a 5% reduction in baseline weight (selected a priori and considered clinically meaningful21) was utilized as a referent point to compare adjusted OR and 95% CI for achieving clinically desirable levels of CRP (<3.0 mg/L) and IL-6 (<2.5 pg/mL). Five percent weight loss for D+E and D participants was strongly associated with improvement in the odds of achieving CRP<3.0 mg/L [OR (95% CI): 3.7 (1.7,8.0)] and IL-6<2.5 pg/mL [2.4 (1.2,4.8)] (Figure 3), with similar results observed in analyses containing all groups: CRP<3.0 mg/L [3.3 (1.8,6.1)] and IL-6<2.5 pg/mL [2.1 (1.2,3.6)]. A nearly identical relationship was observed for fat mass, with the odds of achieving clinically desirable levels of CRP and IL-6 more than doubling with 5% loss of fat mass.
Figure 3.
Adjusted odds ratios (OR) and 95% confidence interval (CI) comparisons of achieving clinically desirable inflammation levels with 5% weight and fat mass loss, respectively.
Discussion
The purpose of this study was to determine the association between the magnitude of total body and regional fat loss and reduction of systemic levels of CRP and IL-6 in overweight and obese, older adults with knee OA partaking in an intensive lifestyle-based weight loss intervention. Reductions in all measures of body fat mass and distribution were associated with significant reductions in CRP, while only loss of total and abdominal (not thigh) fat was associated with reduced IL-6. Although associations between loss of abdominal fat and reduced inflammation were independent of total adiposity, goodness of fit analysis suggests loss of total body mass and fat mass may be better predictors. Importantly, results from this study show that the odds of achieving clinically desirable levels of CRP and IL-6 in this population more than doubles with achievement of 5% loss of total body or fat mass.
Although the exact role of inflammation in the OA disease pathway is debatable (i.e. a causal mediator22 or downstream consequence23), evidence suggesting overall health detriment associated with chronically elevated inflammatory biomarkers in old age is much clearer. Several prospective studies provide compelling data linking modest elevations in CRP to incident CVD24–26, especially for individuals with CRP concentration >3.0 mg/L (compared to those below 1.0 mg/L)17. Although IL-6 does not yet garner the same clinical importance as CRP, longitudinal data from the Health ABC cohort (community dwelling adults; 70–79 years at baseline) suggest IL-6 may be a better predictor of cardiovascular morbidity27 and mortality28 than CRP. Elevations in IL-6 are also associated with poor physical performance and muscle strength29, and plasma levels greater than 2.5 pg/mL are predictive of disability onset18. For these reasons, along with a direct role inflammation may play in OA progression, efforts to reduce chronically elevated CRP and IL-6 in this population below 3.0 mg/L and 2.5 pg/mL, respectively, appear prudent. Importantly, we demonstrate here that the magnitude of sustained weight and fat loss achieved in participants randomized to dietary-induced weight loss in the IDEA study is significantly associated with increased odds of lowering CRP and IL-6 levels to thresholds known to confer cardioprotective and functional benefit.
Data presented here confirm and extend prior work suggesting a direct relationship between weight and fat mass loss and decreased inflammation11–13. Previous data also suggest a significant role for ectopic fat depots in the production of inflammatory cytokines15, particularly in the abdominal cavity30. In accordance, our data show abdominal fat loss is associated with reduced inflammation, independent of total body fat mass. Loss of total body mass and fat mass, however, were found to explain more of the variability surrounding change in inflammation than loss of regional fat volume, likely driven by differential magnitude of change in these depots. This is not to say that downstream health effects of regional fat are inconsequential when compared to total body fat. On the contrary, several studies show increased regional, and in particular, ectopic fat stores are independently associated with increased risk of cardiometabolic31;32 and physical33;34 dysfunction. Indeed, recently published data from this study link thigh, but not total body, fat to the external knee abduction moment 35. Moreover, partitioning of general body obesity into abdominal and thigh compartments revealed that thigh fat had similar significant associations with knee joint forces as abdominal fat, despite its much smaller volume. To further this point, data from the Health ABC cohort show that both high and increasing thigh intermuscular fat area are important predictors of gait speed decline, independent of change in total body fat mass34. Thus, clinical effects of fat mass location are likely outcome specific, and true delineation of the effects of regional versus total body fat mass loss on inflammatory biomarkers necessitates results from a weight loss trial explicitly designed to test this question.
The current study capitalizes on previously collected total and regional adiposity data generated from a large, 18-month lifestyle based intervention that achieved significant weight and fat mass loss in a group of individuals presenting with subclinical inflammation. Additional strengths include serial measures of two robust biomarkers of inflammation in old age, as well use as sophisticated statistical techniques to clarify which adiposity measures are most associated with CRP and IL-6. This study is not without weaknesses, however. Although the reduction in sample size from baseline to 18-months was assumed to be random, the potential for differential drop out may have biased our results. The cut-points we used to define a clinically meaningful threshold for inflammatory biomarkers, while empirically driven, are not standardized and selection of different cut-points (or inflammatory biomarkers), may yield different results. Generalizability of study findings to diverse groups may be limited, as our study sample was represented primarily by overweight and obese, Caucasian women with OA. Our statistical approach attempted to include covariates parsimoniously; particularly we included variables that were relevant to the study design or known predictors of study outcomes. Thus, we cannot rule out the possibility that the addition of some covariates or exclusion of others may have led to bias in the observed associations36. Lastly, CT-derived images were only available on a subset of participants compared to the entire IDEA study sample.
In conclusion, diet-induced reductions in body and fat mass predict reduction of CRP and IL-6, with models of global adiposity found to provide the best fit. Additionally, the odds of achieving clinically desirable levels of inflammation more than double with 5% loss of body and fat mass. Future RCTs should be specifically designed to identify the exact amount and location of fat mass loss necessary to achieve clinically desirable levels of inflammation across varied chronic conditions in older adults, and discern how such reductions translate into risk of future disability, morbidity, and mortality.
Acknowledgments
This work was supported by Grant Number AR052528 (SPM) from the National Institute Of Arthritis And Musculoskeletal And Skin Diseases, the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332), the Arthritis and Musculoskeletal Disease Research Center at Wake Forest School of Medicine and the Wake Forest School of Medicine Translational Science Institute (RFL and DPB), and the Wake Forest University Science Research Fund and Department of Health and Exercise Science (KMB).
Footnotes
Author Contributions
The authors’ responsibilities were as follows—SPM, BJN and RFL designed the research; JJN, RFL, MFL, BJN, GDM, SLM, and SPM conducted the research; DPB and AMA analyzed the data; KMB, DPB, and BJN interpreted the data and drafted the manuscript; and KMB had primary responsibility for the final content. All authors read and approved the final manuscript.
Conflict of Interest
None of the authors had any conflicts of interest to report.
Role of the Funding Source
The funding sources had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kristen M. Beavers, Email: dbeavers@wakehealth.edu.
Daniel P. Beavers, Email: beaverkm@wfu.edu.
Andrea M. Anderson, Email: amanders@wakehealth.edu.
Richard F. Loeser, Jr., Email: richard_loeser@med.unc.edu.
Barbara J. Nicklas, Email: bnicklas@wakehealth.edu.
Mary F. Lyles, Email: mlyles@wakehealth.edu.
Gary D. Miller, Email: millergd@wfu.edu.
Shannon L. Mihalko, Email: mihalksl@wfu.edu.
Stephen P. Messier, Email: messier@wfu.edu.
References
- 1.Tracy RP. Emerging relationships of inflammation, cardiovascular disease and chronic diseases of aging. Int J Obes Relat Metab Disord. 2003;27 (Suppl 3):S29–S34. doi: 10.1038/sj.ijo.0802497. [DOI] [PubMed] [Google Scholar]
- 2.Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65:S173–S176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 3.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector TD, Hart DJ, Nandra D, Doyle DV, Mackillop N, Gallimore JR, et al. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997;40:723–727. doi: 10.1002/art.1780400419. [DOI] [PubMed] [Google Scholar]
- 5.Otterness IG, Swindell AC, Zimmerer RO, Poole AR, Ionescu M, Weiner E. An analysis of 14 molecular markers for monitoring osteoarthritis: segregation of the markers into clusters and distinguishing osteoarthritis at baseline. Osteoarthritis Cartilage. 2000;8:180–185. doi: 10.1053/joca.1999.0288. [DOI] [PubMed] [Google Scholar]
- 6.Rai MF, Sandell LJ. Inflammatory mediators: tracing links between obesity and osteoarthritis. Crit Rev Eukaryot Gene Expr. 2011;21:131–142. doi: 10.1615/critreveukargeneexpr.v21.i2.30. [DOI] [PubMed] [Google Scholar]
- 7.You T, Nicklas BJ. Chronic inflammation: role of adipose tissue and modulation by weight loss. Curr Diabetes Rev. 2006;2:29–37. doi: 10.2174/157339906775473626. [DOI] [PubMed] [Google Scholar]
- 8.Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, WIlliam FM, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheum. 2009;60:2037–2045. doi: 10.1002/art.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicklas BJ, Beavers KM. Exercise, weight loss, and effects on inflammation. Cardiovascular Risk Reports. 2012;4:284–292. [Google Scholar]
- 10.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsythe LK, Wallace JM, Livingstone MB. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21:117–133. doi: 10.1017/S0954422408138732. [DOI] [PubMed] [Google Scholar]
- 12.Esposito K, Giugliano G, Scuderi N, Giugliano D. Role of adipokines in the obesity-inflammation relationship: the effect of fat removal. Plast Reconstr Surg. 2006;118:1048–1057. doi: 10.1097/01.prs.0000232281.49432.ce. [DOI] [PubMed] [Google Scholar]
- 13.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167:31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 14.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 15.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 16.Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring) 2009;17:1062–1069. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 18.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 19.Messier SP, Legault C, Mihalko S, Miller GD, Loeser RF, DeVita P, et al. The Intensive Diet and Exercise for Arthritis (IDEA) trial: design and rationale. BMC Musculoskelet Disord. 2009;10:93. doi: 10.1186/1471-2474-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beavers KM, Hsu FC, Isom S, Kritchevsky SB, Church T, Goodpaster B, et al. Long-term physical activity and inflammatory biomarkers in older adults. Med Sci Sports Exerc. 2010;42:2189–2196. doi: 10.1249/MSS.0b013e3181e3ac80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3 (Suppl 2):211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 22.Brandt KD, Dieppe P, Radin EL. Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am. 2008;34:531–559. doi: 10.1016/j.rdc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Strandberg TE, Tilvis RS. C-reactive protein, cardiovascular risk factors, and mortality in a prospective study in the elderly. Arterioscler Thromb Vasc Biol. 2000;20:1057–1060. doi: 10.1161/01.atv.20.4.1057. [DOI] [PubMed] [Google Scholar]
- 25.Tice JA, Browner W, Tracy RP, Cummings SR. The relation of C-reactive protein levels to total and cardiovascular mortality in older U.S. women. Am J Med. 2003;114:199–205. doi: 10.1016/s0002-9343(02)01497-3. [DOI] [PubMed] [Google Scholar]
- 26.Cao JJ, Thach C, Manolio TA, Psaty BM, Kuller LH, Chaves PH, et al. C-reactive protein, carotid intima-media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation. 2003;108:166–170. doi: 10.1161/01.CIR.0000079160.07364.6A. [DOI] [PubMed] [Google Scholar]
- 27.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 28.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 29.Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 30.Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277:E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 31.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lara-Castro C, Garvey WT. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol Metab Clin North Am. 2008;37:841–856. doi: 10.1016/j.ecl.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 34.Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013;97:552–560. doi: 10.3945/ajcn.112.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messier SP, Beavers DP, Loeser RF, Carr JJ, Khajanchi S, Legault C, et al. Knee-joint loading in knee osteoarthritis: influence of abdominal and thigh fat. Med Sci Sports Exerc. 2014;46:1677–83. doi: 10.1249/MSS.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiological studies. Epidemiol. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]