Abstract
This study examined outcomes through 12 months from a randomized trial comparing computerized brief intervention (CBI) vs. in-person brief intervention (IBI) delivered by behavioral health counselors for adult community health center patients with moderate-level drug misuse (N= 360). Data were collected at baseline, 3-, 6-, and 12-month follow-up, and included the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) and laboratory analysis of hair samples. Repeated measures analyses examined differential change over time. There were no significant differences in drug-positive hair tests over time or by condition. Global ASSIST scores decreased in both conditions (p< .001), but there were no significant differences between conditions in overall change across 12 months of follow-up (p= .13). CBI produced greater overall reductions in alcohol (p= .04) and cocaine (p= .02) ASSIST scores than IBI, with initial differences dissipating over time. Computerized brief interventions present a viable alternative to traditional in-person brief interventions.
1. Introduction
Illicit substance use poses a serious public health problem in the United States and throughout the world. The vast majority of individuals who meet diagnostic thresholds for substance use disorders never receive treatment (Substance Abuse and Mental Health Administration (SAMHSA), 2012). Moreover, most of the aggregate health and social harms resulting from substance use are experienced by the large segment of the population whose substance use does not yet rise to such a level that it prompts treatment-seeking (Rossow & Romelsjo, 2006; Spurling & Vinson, 2005).
Primary care and other healthcare settings are promising venues in which to provide services along the full spectrum of substance use problems. Recent years have seen increased momentum for integrating screening, brief intervention, and referral to treatment (SBIRT) service models into medical settings. Brief interventions are designed to be short but potent encounters that can catalyze motivation and behavior change (Burke, Arkowitz, & Menchola, 2003; Madras et al., 2009; Moyer, Finney, Swearingen, & Vergun, 2002; Rubak, Sandbaek, Lauritzen, & Christensen, 2005).
There is a strong evidence base supporting the effectiveness of brief interventions (BIs) for alcohol misuse (Bertholet, Daeppen, Wietlisbach, Fleming, & Burnand, 2005; Cuijpers, Riper, & Lemmers, 2004; Moyer et al., 2002; Whitlock, Polen, Green, Orleans, & Klein, 2004; Wilk, Jensen, & Havighurst, 1997). Several randomized trials have found support for BIs in reducing drug use in non-treatment-seeking populations (Bernstein et al., 2009; Bernstein et al., 2005; D'Amico, Miles, Stern, & Meredity, 2008; Humeniuk et al., 2012; Ondersma, Svikis, & Schuster, 2007; Ondersma, Svikis, Thacker, Beatty, & Lockhart, 2014; Zahradnik, Otto, Crackau et al., 2009), although two recent large trials have not found such interventions to be effective (Roy-Byrne, Bumgardner, Krupski et al., 2014; Saitz, Palfai, Cheng et al., 2014).
Adoption and sustainability of BIs in clinical settings has been stymied by a number of factors. Screening and BI for alcohol misuse is among the highest ranked preventive services in terms of cost-effectiveness, yet it is highly underutilized compared to similarly ranked services (Solberg, Maciosek, & Edwards, 2008). Many health settings face substantial constraints with respect to time, personnel, and costs. For the typical primary care physician, simply delivering all of the preventive services alone that are currently recommended would take the entire working day (Yarnall, Pollak, Ostbye, Krause, & Michener, 2003).
One approach to providing screening and BI services in primary care is to have dedicated behavioral health staff that can deliver BIs. Yet not all clinics can afford to support such staff. Computerized, self-directed BIs represent another approach. A growing body of evidence shows that computerized interventions can be effective for health promotion and reducing risk behaviors (Portnoy, Scott-Sheldon, Johnson, & Carey, 2008), including alcohol misuse (Carey, Scott-Sheldon, Elliott, Bolles, & Carey, 2009), illicit drug use (Gilbert et al., 2008; Ondersma et al., 2007; Ondersma et al., 2014), and HIV sex risk behaviors (Gilbert et al., 2008; Grimley & Hook, 2009). Computerized BIs have the potential to avoid some of the common challenges that have stymied widespread adoption and sustainability of staff delivered BIs. Importantly, such interventions can be deployed by computer with minimal staff involvement. Eventually, integration of computerized self-administered screening and brief interventions could have major efficiency advantages. However, an important question is the comparative effectiveness of computerized and in-person brief interventions.
1.1. Focus of the present study
The current study examines outcomes through 12 months of follow-up from a randomized trial comparing a computerized brief intervention (CBI) with an in-person brief intervention (IBI) delivered by a behavioral health counselor for adult primary care patients with moderate-level illicit drug use. We originally hypothesized that both CBI and IBI conditions would show improvements from baseline, that the CBI condition would show greater improvements than the IBI condition in the first 3 months, and that CBI would maintain its advantage over IBI through 12 months. We made this hypothesis under the premise that the computerized, self-directed format may have a disarming quality for dealing with the potentially sensitive topic of drug use, thereby creating greater comfort in disclosing risky behaviors and higher receptivity to suggestions to modify behaviors. Moreover, the CBI would deliver the same “ideal form” intervention consistently, which may not be possible for IBI due to competing demands in a busy healthcare environment.
We previously reported outcomes from this study at a 3-month endpoint, which found no significant differences between CBI and IBI conditions in the primary outcomes of ASSIST global drug risk scores or drug-positive hair tests (Schwartz, Gryczynski, Mitchell et al., 2014). However, there were some encouraging secondary findings supporting the computerized intervention, which showed significantly lower marijuana and cocaine ASSIST scores at a 3 month endpoint compared to the in-person brief intervention.
The current study extends our earlier findings by considering a longer follow-up window and using an analytical strategy that examines change over time as opposed to status at a single endpoint.
2. Materials and Methods
2.1. Design
This study was a randomized controlled trial in which participants with moderate-risk drug use were randomly assigned to receive a single-session brief intervention delivered either by a computer or by a behavioral health counselor [see Schwartz et al. (2014), for a detailed description]. In summary, the IBI was conducted by experienced, Masters-level behavioral health counselors. The CBI was designed to have similar content as the IBI. Participants were randomly assigned to conditions using a block randomization procedure. The primary outcome was the reduction in global ASSIST score and results of hair testing for drug use. We also examined substance-specific ASSIST scores as secondary outcomes. The study was approved by the Institutional Review Boards of Friends Research Institute and Christus Health, and all participants provided written informed consent. The study was monitored by an independent Data and Safety Monitoring Board and registered on the national clinical trials registry (NCT01131520). Participants were paid $20 for completing each study assessment.
2.2. Setting
The study was conducted at two rural community health centers in New Mexico. Both of the clinics contracted with Sangre de Cristo Community Health Partnership (SDCCHP), the nonprofit organization that administered the State of New Mexico's SAMHSA SBIRT grant (Madras et al., 2009).
2.3. Participants
Participants were adult clinic patients, of whom 46% were female, 90% were white, and 47% were of Hispanic ethnicity. The mean age was 36.2 years (SD=14.6). The majority were unemployed (59%), 78% had completed high school or equivalent education, 22% were married, and 66% owned a computer at home. There were no significant differences between conditions in demographics or computer ownership (Schwartz et al., 2014).
2.4. Eligibility and recruitment
Patients were approached in the clinic waiting area by a research assistant and invited to be screened for a “health study.” The research assistant then administered the ASSIST in a private office. The eligibility criteria were designed to reflect the criteria of the World Health Organization ASSIST brief intervention trial (Humeniuk et al., 2012). Adult patients (ages 18 and older) were eligible if they scored in the moderate risk range (ASSIST scores between 4-26) for non-medical use of any of the following: marijuana, cocaine, amphetamines or methamphetamine, inhalants, sedatives, hallucinogens, or opioids. Patients were excluded and referred to the behavioral health counselor if they scored in the high risk range for any of the drugs listed above, or alcohol (ASSIST score >26). Other exclusion criteria included past 3-month drug abstinence, receipt of drug abuse treatment within the past year, receipt of a brief intervention within the past month, or plans to move out of New Mexico in the next year (to allow for appropriate follow-up).
2.5. Random assignment
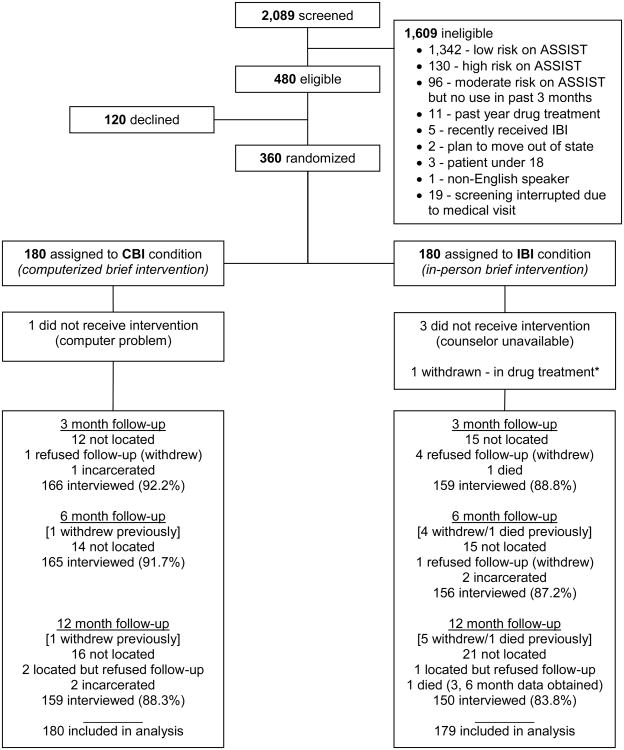
Following the informed consent and baseline assessment, participants were randomized within each site to either CBI or IBI using a block randomization approach (Figure 1). Three hundred sixty participants were enrolled in the study and randomized, but one was withdrawn post-randomization because of the participant's subsequent disclosure of being enrolled in buprenorphine treatment for opiate dependence. Research assistants and participants were blinded to the assignment at the time of the baseline assessment, after which the research assistant would open the next opaque envelope to reveal the participant's condition. For those assigned to the in-person brief intervention, the research assistant accompanied the participant to the clinic behavioral health counselor, who would deliver the IBI. For those assigned to the CBI, the research assistant set up the tablet computer with headphones, gave the participant a brief tutorial on navigating the intervention, and allowed the participant to complete the computerized intervention privately.
Figure 1.
CONSORT diagram.
Note: *Case excluded from analysis.
2.6. Study conditions
2.6.1. In-Person Brief Intervention (IBI)
The IBI was based on motivational interviewing, and was the standard BI that the behavioral health counselors had been delivering at the clinics for several years as part of the SAMHSA-supported SBIRT initiative. These licensed, Masters-level behavioral health counselors worked on-site in the primary care clinics, were trained in motivational interviewing, and received ongoing weekly clinical supervision by senior clinical supervisors at Sangre de Cristo Community Health Partnership. The study sought to test CBI against IBI as it is normally delivered under “real-world” conditions. Therefore, we did not record IBI sessions or otherwise monitor fidelity, because such practices would not be typical in normal clinical delivery of IBI services. The IBI interventions followed the principles of motivational interviewing and lasted approximately 14 minutes on average as estimated by the behavioral health counselors in their post-intervention record reports (SD= 6.6; min= 5; max= 45).
2.6.2. Computerized Brief Intervention (CBI)
The CBI was designed to mirror the content of the “ideal” in-person BI, and was developed in close collaboration with the SDCCHP clinical supervisors and reviewed by the behavioral health counselors prior to study implementation. The CBI was developed on the Computerized Intervention Authoring Software (CIAS) platform developed by SO and marketed by Interva, Inc. (Ondersma, Chase, Svikis, & Schuster, 2005; Ondersma et al., 2012; Ondersma et al., 2007; Ondersma et al., 2014). The CBI used an animated avatar to ask questions and guide the participant through the intervention. Content was tailored based on the participant's reported motivation level for reducing or stopping substance use, as well as their perceived confidence in their ability to stop or cut down such use. The intervention also included a gender-specific normative feedback component using data on substance use quit rates from the National Survey on Drug Use and Health (normative data was also provided to the counselors for use in the IBI if they wished). Participants were permitted to complete two substance-specific modules, and they could choose to focus on their alcohol use for the second module after completing one module on an illicit drug of their choice. As timed by the software, the average duration of the CBI was just under 7 minutes (SD= 5.0; min= 1.5; max= 35.1).
2.7. Follow-up
Participant follow-ups were conducted at 3, 6, and 12 months post-study enrollment. While approximately 90% of participants were located at each follow-up point, several refused to continue participation, were incarcerated, or had died for reasons not related to study participation (see Figure 1). Thus, 325 completed the 3-month assessment (91%), 321 completed the 6-month assessment (89%), and 309 completed the 12-month assessment (86%).
2.8. Measures
A brief assessment battery was administered at baseline, and again at each follow-up. The assessment consisted of an individual interview and collection of a hair sample.
Alcohol, Smoking and Substance Involvement Screening Test (ASSIST)
The ASSIST was used to determine study eligibility as well as an outcome measure. Substance-specific risk scores were computed for each substance, as well as the “Global Continuum of Illicit Drug Risk (GCIDR)” score which served as the primary outcome (Newcombe, Humeniuk, & Ali, 2005). Consistent with the international World Health Organization trial, substance-specific risk scores were examined as outcomes in the subsample of participants who met the moderate-risk classification for each substance at baseline.
Hair Testing
A 3.8 cm hair sample (corresponding to approximately a 3-month time period) was collected from participants from the scalp (if scalp hair was not available, an attempt was made to collect hair from the leg or underarm) at baseline, and at each follow-up. Hair samples were sent to an external commercial laboratory and analyzed for marijuana, cocaine, amphetamine or methamphetamine, and opioids (morphine, heroin metabolite, codeine) via assay screening with gas chromatography/mass spectrometry (GC/MS) confirmation. At 6 months, hair samples were obtained for 83% of participants interviewed, but 12% (33/267) of samples could not be processed by the laboratory due to insufficient hair quantity. At 12 months, hair samples were obtained for 89% of participants interviewed, but 11% (29/263) of samples could not be processed by the laboratory due to insufficient quantity. Although we initially intended to examine quantitative levels of drugs in hair, the prevalence of the substances for which such an analysis was appropriate (e.g., opioids, cocaine) was too low to pursue this strategy. (The laboratory advised that the level of marijuana in hair does not correlate reliably with level of usage). Thus, we analyzed the hair testing data as binary (positive vs. negative).
2.9. Power
Power for this study was estimated using Stroup's four-step power estimation procedure (Stroup, 1999; Littell et al., 2006) for general linear mixed models under a variety of assumptions regarding the covariance structure of the repeated data and in the presence of unbalancing effects due to missing data. This approach involves calculating power for the non-centrality parameter associated with a given effect in the statistical model of interest based on a simulation in which the parameters of interest, the sample size and attrition of observations are specified. At a target N of 360, under the assumption of 10% attrition, power to detect “small” mean differences over time (Cohen, 1988) exceeded .80 in all simulations.
2.10. Statistical analysis
Global and substance-specific ASSIST scores were examined using a generalized linear mixed modeling framework to account for repeated measurement and nesting of participants in clinic site. Fixed predictors in the models included assigned study condition (a categorical variable representing IBI vs. CBI), assessment time point (a categorical variable with baseline as the reference category, 3 months, 6 months, and 12 months), and the Condition × Time interaction (the effect of interest, assessing the additional change for the CBI condition, over and above the IBI condition). Hair testing data was analyzed using Generalized Estimating Equations (GEE). Predictors in the GEE models included Condition, Time, their interaction, and clinic site. The GEE analyses were fit as logistic regression-style models for binary data, with an unstructured correlation structure and robust standard errors.
We report the overall contrast for the Time effect to test whether there were significant changes over time for the entire sample. We report the overall condition × time interaction as the omnibus test of CBI vs. IBI (i.e., the joint test of the interaction parameter estimates). We also report the single degree-of-freedom contrasts for each level of the interaction, comparing the conditions on change from baseline to each follow-up point in order to characterize differences between IBI and CBI over time.
3. Results
3.1. Substance use characteristics of the study sample
Marijuana was by far the most commonly reported drug used by participants. The majority of the sample (88%) scored as moderate risk for marijuana on the ASSIST at baseline. Non-medical use of opioids was the second most common moderate-risk drug category (20% moderate risk), followed by cocaine (18% moderate risk), sedatives (12% moderate risk), and amphetamines (11% moderate risk). Moderate risk alcohol use was reported by 28%. At baseline, only 62% of hair samples were positive for any drug (47% positive for marijuana, 20% were positive for cocaine, 8% were positive for amphetamines, and 3% were positive for opiates). Thus, the hair testing did not track well with self-reported use or risk level. This lack of close concordance suggests that hair testing at the standard laboratory cut-offs may have missed a substantial number of cases in this moderate-risk primary care population. Opioid rates of self-report and hair testing may have been non-concordant in part because, at the time the study was planned, the laboratory did not offer testing for many pharmaceutical opioids (e.g., oxycodone, hydrocodone, methadone). A detailed analysis of concordance between hair testing and self-report in this trial has been reported separately (Gryczynski, Schwartz, Mitchell, O'Grady, & Ondersma, in press).
3.2. Hair Test Results
There were no significant reductions in drug-positive hair tests (positive for any drug) for the sample as a whole (χ2 (3)=3.91; p=.27), and no significant between-condition differences in drug-positive hair tests (overall contrast of the condition × time interaction: χ2(3)=1.73; p=.63; Table 1). Similar findings were evident when examining specific drugs in hair as secondary outcomes. For marijuana, the most common drug used in the sample, there was no significant overall reduction over time in marijuana-positive hair tests (χ2 (3)=2.74; p=.43), and no differences between IBI and CBI conditions (χ2 (3)= 1.67; p=.64). This pattern of null findings, both in terms of no significant overall change and no significant between-condition differences, was consistent for hair tests for amphetamines and cocaine. The GEE model failed to converge for opiate hair tests, due to the extremely low number of positive tests (<4% at each time point).
Table 1.
Generalized Estimating Equation (GEE) model of drug-positive hair tests.
| Any Drug OR (95% CI) |
Marijuana OR (95% CI) |
Cocaine OR (95% CI) |
Amphetamines OR (95% CI) |
|
|---|---|---|---|---|
| Time (ref= baseline) | ||||
| 3 months | 0.92 (0.67 - 1.27) | 0.91 (0.67 - 1.24) | 1.02 (0.79 - 1.32) | 1.08 (0.61 - 1.91) |
| 6 months | 1.01 (0.70 - 1.45) | 1.01 (0.73 - 1.41) | 0.92 (0.67 - 1.28) | 1.43 (0.80 - 2.55) |
| 12 months | 0.72 (0.49 - 1.04) | 0.82 (0.58 - 1.15) | 0.83 (0.57 - 1.20) | 0.71 (0.33 - 1.55) |
| Condition (ref= IBI) | ||||
| CBI | 0.74 (0.47 - 1.18) | 0.83 (0.53 - 1.29) | 0.88 (0.49 - 1.60) | 1.18 (0.52 - 2.66) |
| Condition × Time | ||||
| 3 months × CBI | 1.12 (.74 – 1.67) | 1.13 (0.76 - 1.67) | 0.99 (0.69 - 1.43) | 1.14 (0.55 - 2.37) |
| 6 months × CBI | 0.95 (0.61 - 1.49) | 0.87 (0.57 - 1.33) | 0.96 (0.63 - 1.45) | 0.78 (0.36 - 1.70) |
| 12 months × CBI | 1.24 (0.75 - 2.07) | 1.02 (0.63 - 1.66) | 1.01 (0.61 - 1.70) | 0.97 (0.34 - 2.75) |
| Overall Contrasts | ||||
| Time Effect (overall change) | χ2(3)= 3.91; p= .27 | χ2(3)= 2.74; p= .43 | χ2(3)= 2.75; p= .43 | χ2(3)= 6.25; p= .10 |
| Condition × Time (differential change by condition) | χ2(3)= 1.73; p= .63 | χ2(3)= 1.67; p= .65 | χ2(3)= 0.07; p= .99 | χ2(3)= 1.33; p= .72 |
Notes: OR= Odds Ratio; CI= Confidence Interval. Due to missing data, for any positive test: n= 334 (1,024 person-time observations); for marijuana n= 332 (1,001 person-time observations); for cocaine n= 334 (1,023 person-time observations); for amphetamines n= 334 (1,022 person-time observations). GEE models failed for opiates due to a very low number of positive tests (<4% at each time point).
3.3. ASSIST Scores
3.3.1. Global ASSIST Drug Risk Scores
Table 2 shows the results from the analysis of the global ASSIST drug risk scores, alongside the substance-specific ASSIST scores for alcohol, cocaine, and marijuana. The time main effect showed that the sample as a whole had significant reductions in global ASSIST scores over the 12-month study period (χ2 (3)= 45.25; p< .001); however, the overall contrast of the condition × time interaction was non-significant (χ2(3)=5.64; p=.13), indicating that the pattern of change over time did not differ between CBI and IBI conditions. Although the model showed a significant difference in change from baseline to 3 months between CBI and IBI conditions (p=.030), this trend was accentuated by a chance difference between conditions at baseline, and global scores for IBI and CBI tracked closely at 3, 6, and 12 month follow-up points (Figure 2, upper left). Single-degree-of-freedom contrasts showed no significant differences for subsequent follow-up points (Table 2).
Table 2.
Changes in Global Continuum of Illicit Drug Risk (GCIDR) and substance-specific ASSIST risk scores by condition.
| Model-Predicted Means | Statistical Tests | |||
|---|---|---|---|---|
|
| ||||
| IBI | CBI | Condition × Time Interaction | Time Main Effect | |
| Mean (SE) | Mean (SE) | (differential change for IBI vs. CBI) | (overall change) | |
| GCIDR (n= 359; 1,314 PTO) | Overall Test: χ2(3)= 5.64; p=.131 | χ2(3)=45.25; p<.001 | ||
| Baseline | 30.00 (1.27) | 33.11 (1.27) | ||
| 3 months | 28.75 (1.32) | 28.25 (1.30) | Baseline to 3m: χ2(1)=4.72 p= .030 | |
| 6 months | 27.36 (1.33) | 27.34 (1.30) | Baseline to 6m: χ2(1)= 3.51; p= .061 | |
| 12 months | 25.89 (1.34) | 26.51 (1.32) | Baseline to 12m: χ2(1)= 2.16; p= .141 | |
|
| ||||
| Marijuana (n= 314; 1,152 PTO) | Overall Test: χ2(3)= 7.79; p=.050 | χ2(3)=56.08; p<.001 | ||
| Baseline | 11.09 (0.49) | 11.03 (0.49) | ||
| 3 months | 11.08 (0.51) | 9.08 (0.50) | Baseline to 3m: χ2(1)=7.64 p= .006 | |
| 6 months | 9.52 (0.51) | 8.76 (0.50) | Baseline to 6m: χ2(1)= 0.99; p= .321 | |
| 12 months | 9.05 (0.52) | 8.10 (0.51) | Baseline to 12m: χ2(1)= 1.57; p= .210 | |
|
| ||||
| Cocaine (n= 66; 250 PTO) | Overall Test: χ2(3)= 10.35; p=.016 | χ2(3)=25.70; p<.001 | ||
| Baseline | 11.73 (2.01) | 10.35 (1.91) | ||
| 3 months | 10.85 (2.05) | 5.44 (1.93) | Baseline to 3m: χ2(1)= 4.54; p= .033 | |
| 6 months | 9.52 (2.05) | 4.22 (1.92) | Baseline to 6m: χ2(1)= 4.33; p= .037 | |
| 12 months | 7.30 (2.06) | 6.59 (1.92) | Baseline to 12m: χ2(1)= 0.12; p= .727 | |
|
| ||||
| Alcohol (n= 101; 367 PTO) | Overall Test: χ2(3)= 8.37; p=.039 | χ2(3)=61.68; p<.001 | ||
| Baseline | 16.13 (0.91) | 17.95 (1.10) | ||
| 3 months | 13.19 (0.94) | 11.08 (1.16) | Baseline to 3m: χ2(1)= 5.84; p= .016 | |
| 6 months | 12.63 (0.96) | 12.02 (1.15) | Baseline to 6m: χ2(1)= 2.23; p= .135 | |
| 12 months | 10.38 (0.98) | 12.26 (1.17) | Baseline to 12m: χ2(1)= 0.00; p= .967 | |
Notes: PTO= person-time observations. Substance-specific analyses are restricted to participants who were at moderate risk for the substance at baseline. Data are derived from generalized linear mixed models of ASSIST scores.
Figure 2.
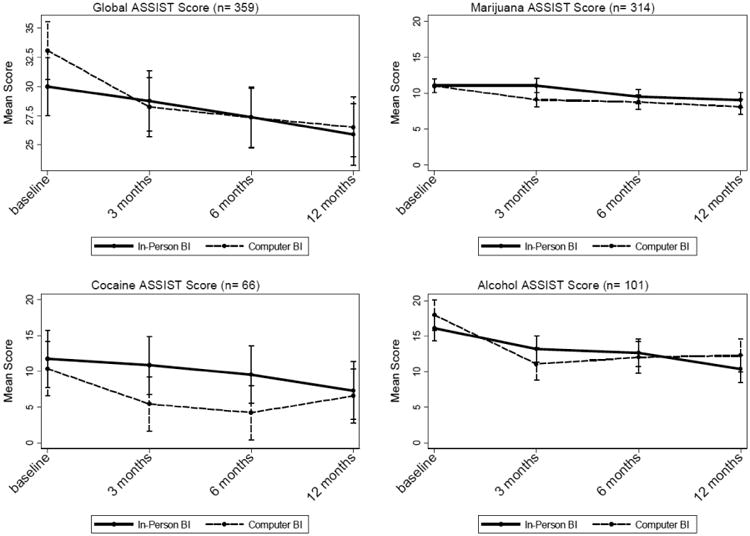
Change in ASSIST scores by study condition.
3.3.2. Marijuana
There was a significant overall reduction in marijuana risk scores over time for the combined sample as indicated by the omnibus test of the time main effect (χ2(3)=56.08; p<.001). The overall contrast of the condition × time interaction just exceeded the .05 boundary (χ2(3)=7.79; p=.0505). Participants in the CBI condition had significantly sharper reductions in marijuana risk scores from baseline to 3 months compared to the IBI condition (χ2(1)=7.64; p=.006), with the CBI condition showing an additional 1.95-point decrease in marijuana scores relative to the IBI condition. However, this advantage of the CBI over IBI did not hold subsequent to 3 months. Figure 2 (upper right) plots changes in marijuana risk scores over time by condition.
3.3.3. Cocaine
Participants in both conditions had significant reductions in cocaine risk scores on the ASSIST (χ2(3)= 25.70; p< .001). The overall joint contrast of the condition × time interaction was statistically significant, favoring the CBI condition (χ2(3)= 10.35; p= .016). The CBI condition had significantly greater reductions in cocaine risk scores than the IBI condition from baseline to 3 months (χ2(1)=4.54; p=.033), an advantage that persisted through 6 months(χ2(1)=4.33; p=.038). As seen in Figure 2 (lower left), the CBI condition had approximately a 4-point greater reduction in cocaine risk scores from baseline to 3 month follow-up compared to the IBI condition. By 12 months, cocaine risk scores in the IBI and CBI conditions had converged.
3.3.4. Amphetamines, Sedatives, & Opiates
For each of these substance-specific ASSIST scores, participants in both groups reported significant reductions from baseline (ps< .001 for amphetamine-type stimulants, sedatives, and opioids). There were no significant between-condition differences for amphetamine/methamphetamine risk scores (p= .416), sedative risk scores (p= .752), or opiate risk scores (p= .408).
3.3.5. Alcohol
There was a significant overall reduction in alcohol risk scores over time for both conditions (χ2(3)= 61.68; p<.001). The overall contrast of the condition × time interaction was statistically significant, indicating differential change between the study conditions, favoring the computer intervention (χ2(3)=8.37; p=.039). Participants in the CBI condition had significantly sharper reductions in alcohol risk scores from baseline to 3 months than their counterparts in the IBI condition, with the CBI condition showing a 3.9-point decrease above and beyond that of the IBI condition (χ2(1)=5.84; p=.016). However, the advantage of the CBI was short-lived (Figure 2, bottom right) as alcohol risks scores for the two conditions began to converge by 6 month follow-up.
4. Discussion
In this randomized trial comparing a single-session computerized brief intervention (CBI) to an in-person brief intervention (IBI) among community health center patients with moderate-risk drug use, there were no significant overall differences between CBI and IBI in reducing ASSIST global drug risk scores or drug-positive hair tests through 12 months of follow-up. On the secondary outcomes of substance-specific ASSIST scores, CBI was superior to IBI in facilitating overall reductions in alcohol and cocaine scores, but differences dissipated by 6 month follow-up for alcohol and by 12 month follow-up for cocaine. The finding for cocaine should be interpreted cautiously given the relatively small number of moderate-risk cocaine users in the sample. The majority of participants in this sample used marijuana, and there was some evidence of a possible advantage of CBI over IBI in decreasing marijuana ASSIST scores over the 3 month short-term. However, caution is warranted because the overall between-group comparison (jointly for all time points) exceeded the,05 significance level.
Thus, for most outcomes examined, there were no significant differences between IBI and CBI. However, it is important to note that participants in the CBI condition did not appear to fare worse than their counterparts in the IBI condition on any of the outcomes examined. Given the overall pattern of findings, it seems reasonable to conclude that the drug use risk outcomes obtained by the computerized brief intervention were no worse than those obtained by in-person brief intervention, and the computerized intervention may even show some modest advantages for certain substances. Notably, there were significant reductions in all self-reported drug risks scores for the sample as a whole (although no significant reductions in drug-positive hair tests).
While the overall contrast between CBI and IBI (jointly for all time points) was not significant for the global ASSIST score, the CBI condition had a sharper reduction on this score from baseline to 3 month follow-up than the IBI condition. However, upon closer inspection, the CBI condition had somewhat higher scores at baseline. Although the baseline difference itself was not statistically significant at the .05 level, it makes it difficult to interpret whether the observed differences were due to a genuine but short-lived treatment effect, or regression to the mean. As previously reported, differences on this outcome were non-significant in a 3-month endpoint analysis (Schwartz et al., 2014). The present report employs a different analysis strategy (and thus answers subtly different questions) over a longer time horizon. The focus of the current analysis was on examining change over the course of the entire 12 months of the trial, for the sample as a whole and differentially by study condition.
An important question, but one that is difficult to answer with precision, is the extent to which observed changes in ASSIST scores would be clinically meaningful, whether for the overall reductions from baseline in the full sample, or in the magnitude of CBI's advantage for those few outcomes in which CBI outperformed IBI. Based on the ASSIST questions and scoring weights, it is plausible that even small decreases in scores could have potentially important clinical implications. For example, a three-point decrease in a substance-specific ASSIST score can be driven by a reduction in frequency of drug use from a daily to a monthly level, or a reduction in craving from a daily occurrence to a single craving episode in the span of three months, or complete elimination of other people expressing concerns about the individual's substance use. Importantly, even relatively small effects could yield substantial public health impact under wide scale implementation. Although the operational challenges and costs of implementing and sustaining brief intervention services are not trivial, computerized brief interventions offer some options that could enhance their reach and utility compared with traditional in-person interventions (for example, web-based deployment; integration with electronic medical records systems).
It is important to note that this study examined a single-session BI. It is not known what effect a booster session would have had in this study. In the alcohol literature, studies in which BI is delivered over multiple contacts have been more consistent in finding intervention effects than studies employing single-encounter BIs (Whitlock et al., 2004). However, there are significant challenges to providing follow-up interventions or boosters to primary care patients whose drug use does not rise to the level of dependence and who are not seeking assistance for their substance use. With such patients, computer-delivered sessions completed in a healthcare setting could be followed with tailored messaging via print, email, or text messages; this may allow for boosters without having to rely on patient motivation to return for subsequent visits.
There were no significant differences in drug-positive hair specimens for any drug category. This finding is in contrast to a recent study comparing a computerized brief intervention to an attention control condition for postpartum women, which found significant effects at 6 months using hair testing (Ondersma et al., 2014). Likewise, in an earlier study that used hair testing, Bernstein and colleagues (2005) found that participants who received BI were more likely than a control condition to be abstinent from cocaine and heroin at 6 months.
As in our study, Bernstein and colleagues (2005) found that self-reported drug use at study entry was not always confirmed by hair testing, and in their analysis they discarded the data for participants with baseline-negative hair tests because they viewed this discrepancy as a potential indication that participants either did not adequately recall the time frame of their drug use, or were untruthful about their drug use in order to qualify for the study (and receive the incentive). We also examined outcomes while restricting the analysis to participants with baseline-positive hair samples, a process that yielded findings consistent with those presented here. Although the $20 incentive in the current study was a nominal amount, the study was conducted in a rural community during the economic recession. However, eligibility criteria was defined by a substance-specific ASSIST score within the moderate-risk range (and no high-risk use), which would add some degree of difficulty for falsifying study eligibility on the part of the participants. Moreover, during eligibility screening participants were told only that this was a health study; no details were given about the focus of the study or how to satisfy eligibility criteria. Nevertheless, it is possible that information about the study spread within the communities. Given the relatively high rates of discrepancy between self-reported use and hair test results, another possibility is that hair testing at the standard laboratory cut-offs used in this study may have limited ability to capture intermittent levels of use in populations with moderate-level drug use that does not rise to the level of dependence (Gryczynski et al., 2014).
One in four patients who were screened as eligible for the study declined to enroll, a participation rate that is well in line with research of this type but that may not reflect the participation rate for IBI or CBI in a purely clinical context. Although we did not track reasons for refusal quantitatively, anecdotally most of those who were eligible but declined did so because of lack of time. Although the interventions were very short, participation in the trial involved additional time commitment to complete the written informed consent process and to complete the baseline assessment. Recruitment was conducted opportunistically during an unrelated medical visit, and even the brief time commitment for the study could have been prohibitive for some patients (particularly if the medical visit itself was already extended beyond patients' expectations). In regular clinical practice, delivery of either intervention would be unencumbered by research-related activities, and may produce lower refusal rates.
In this study, nearly 1 in 4 clinic patients screened reported substance use at a moderate-risk level that would meet clinical standards for brief intervention (and another 6% that were high-risk and could benefit from more intensive services). However, most study participants used marijuana. If screening and brief intervention for drug use in general is to be implemented widely in primary care, it is important to recognize that the majority of interventions are bound to focus on marijuana use, with other drug use being less frequent. Part of the challenge of studying and disseminating screening and brief intervention has been the heterogeneity of substance use and a corresponding lack of clarity regarding the assumed public health impact of brief interventions. Different substances have different profiles with respect to adverse health consequences, different prevalence rates that vary geographically and across subpopulations, different abuse potential following initial exposure, and quite possibly different degrees of responsiveness to motivational interventions. Ultimately, the public health benefits of brief interventions for drug use, and whether such interventions constitute a cost-effective use of resources, are empirical questions. Future studies that aim to estimate the public health impact of brief interventions for drug use should attempt to take these nuances into account.
4.1. Limitations
There are several limitations to this study. Self-reported drug use and associated problems may be subject to underreporting. In the present study, self-report data were gathered for research purposes only. To increase the accuracy of self-reports, the research assistants emphasized confidentiality safeguards during the informed consent process, including that data would not be shared with clinic staff and that participant records were protected by a Certificate of Confidentiality. Another potential limitation is that the research assistants who conducted the follow-up interviews were not blind to study condition. For a minority of participants, there were some difficulties obtaining an adequate quantity of hair for their specimen sample, leading to either no sample being collected or the collection of a hair sample of insufficient quantity for lab analysis. Additionally, at the time of the study, the laboratory was unable to test for opioids other than morphine, heroin, or codeine, which likely resulted in under-detection of opioid use. The lack of corroboration between the self-reported ASSIST scores and hair test results may call into question the validity of self-report, although it is important to note that the ASSIST risk scores are heavily weighted towards capturing problems resulting from substance use. Substance use frequency per se plays a role in the ASSIST's scoring, but it is just one of several factors considered. Thus, our finding of significant overall reductions in ASSIST risk scores is not necessarily inconsistent with the finding of no concurrent reductions in drug-positive hair tests.
It is also important to note that we did not apply a statistical correction for testing multiple secondary outcomes. Rather, we viewed each substance as a distinct phenomenon under the rationale that BIs may work differently for different drugs, and that participants may be more responsive to CBI for certain drug problems but not others. Although some other brief intervention trials have not applied such corrections (e.g., Humeniuk et al., 2012), not doing so may lead to rejection of the null hypothesis more frequently than would be warranted. Finally, a potentially important limitation of this study is that the experimental design did not include a no-intervention control condition. As such, definitive conclusions about the effectiveness of either intervention cannot be drawn, and all conclusions are limited to how the interventions compare to one another. More research is needed comparing computerized to in-person BIs that include no-intervention control groups, in order to rule out the possibility that observed changes in substance use behaviors would have occurred naturally due to regression to the mean (Barnett, van der Pols, & Dobson, 2005), or as an artifact of the research through inadvertent reactivity to assessment (McCambridge & Kypri, 2011; Walters, Vader, Harris, & Jouriles, 2009).
4.2. Conclusion
This randomized controlled trial comparing computerized vs. in-person brief intervention for illicit drug use through 12 months of follow-up found that participants in both conditions had significant improvements in self-reported drug risks (but not in drug-positive hair test results). The computerized intervention was generally not superior to an in-person brief intervention in reducing drug use or general drug risks. Although the computerized brief intervention was not superior to the in-person brief intervention on the primary outcomes, neither did it perform worse than the in-person intervention delivered by experienced Master's-level behavioral health counselors. Moreover, CBI outperformed IBI on some secondary outcomes examining substance-specific risks. These findings suggest that computerized brief interventions may be useful in primary care settings, particularly those with limited availability of behavioral health staff. In addition to their potential for similar outcomes, computerized brief interventions are likely to be relatively cost-effective and easier to implement than in-person brief interventions, although this will need to be established empirically in future research. Cost effectiveness, ease of implementation, and scalability are important considerations that, along with effectiveness, determine the extent to which a given intervention can have a meaningful public health impact.
Highlights.
This study compared in-person v. computerized brief intervention (BI) for drug use.
The conditions had similar outcomes on hair drug tests and global drug risk scores.
Both conditions had reductions in self-reported drug risks, but not in drug-positive hair tests.
The computerized condition was superior for decreasing alcohol and cocaine risks.
The findings support the potential utility of computerized BIs in primary care.
Acknowledgments
Declarations of interest and source of funding: The study was supported through National Institute on Drug Abuse (NIDA) Grant R01 DA026003 (PI Schwartz). NIDA had no role in the design and conduct of the study; data acquisition, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Disclosures: No financial disclosures were reported by Drs. Gryczynski, Mitchell, O'Grady, Gonzales, Moseley, Peterson, or Schwartz. Dr. Ondersma is part owner of Interva, Inc., which markets the intervention authoring tool that was used to develop the intervention for this study.
Clinical Trials Registration: Clinicaltrials.gov NCT01131520
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. International Journal of Epidemiology. 2005;34(1):215–220. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Edwards E, Dorfman D, Heeren T, Bliss C, Bernstein J. Screening and brief intervention to reduce marijuana use among youth and young adults in a pediatric emergency department. Academic Emergency Medicine. 2009;16(11):1174–1185. doi: 10.1111/j.1553-2712.2009.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J, Bernstein E, Tassiopoulos K, Heeren T, Levenson S, Hingson R. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug and Alcohol Dependence. 2005;77(1):49–59. doi: 10.1016/j.drugalcdep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Bertholet N, Daeppen JB, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Archives of Internal Medicine. 2005;165(9):986–995. doi: 10.1001/archinte.165.9.986. [DOI] [PubMed] [Google Scholar]
- Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. Journal of Cconsulting and Clinical Psychology. 2003;71(5):843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- Carey KB, Scott-Sheldon LA, Elliott JC, Bolles JR, Carey MP. Computer-delivered interventions to reduce college student drinking: a meta-analysis. Addiction. 2009;104(11):1807–1819. doi: 10.1111/j.1360-0443.2009.02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Second. Hillsdale, NJ: LEA; 1988. [Google Scholar]
- Cuijpers P, Riper H, Lemmers L. The effects on mortality of brief interventions for problem drinking: a meta-analysis. Addiction. 2004;99(7):839–845. doi: 10.1111/j.1360-0443.2004.00778.x. [DOI] [PubMed] [Google Scholar]
- D'Amico E, Miles JN, Stern S, Meredity LS. Brief motivational interviewing for teens at risk of substance use consequences: a randomized pilot study in a primary care clinic. Journal of Substance Abuse Treatment. 2008;35(1):53–61. doi: 10.1016/j.jsat.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Gilbert P, Ciccarone D, Gansky SA, Bangsberg DR, Clanon K, McPhee SJ, et al. Interactive “Video Doctor” counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. PLoS One. 2008;3(4):e1988. doi: 10.1371/journal.pone.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales A, Westerberg VS, Peterson TR, Moseley A, Gryczynski J, Mitchell SG, Buff G, Schwartz RP. Implementing a state-wide SBIRT service in rural health settings: New Mexico SBIRT. Substance Abuse. 2012;33:114–123. doi: 10.1080/08897077.2011.640215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley DM, Hook EW., 3rd A 15-minute interactive, computerized condom use intervention with biological endpoints. Sexually Transmitted Diseases. 2009;36(2):73–78. doi: 10.1097/OLQ.0b013e31818eea81. [DOI] [PubMed] [Google Scholar]
- Gryczynski J, Schwartz RP, Mitchell SG, O'Grady KE, Ondersma S. Hair Drug Testing Results and Self-reported Drug Use among Primary Care Patients with Moderate-Risk Illicit Drug Use. Drug and Alcohol Dependence. 2014;141:44–50. doi: 10.1016/j.drugalcdep.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor T, Souza-Formigoni ML, de Lacerda RB, Ling W, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107(5):957–966. doi: 10.1111/j.1360-0443.2011.03740.x. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. Second. Cary, NC: SAS Institute, Inc; 2006. [Google Scholar]
- Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug and Alcohol Dependence. 2009;99(1-3):280–295. doi: 10.1016/j.drugalcdep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge J, Kypri K. Can simply answering research questions change behaviour? Systematic review and meta analyses of brief alcohol intervention trials. PLoS One. 2011;6(10):e23748. doi: 10.1371/journal.pone.0023748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97(3):279–292. doi: 10.1046/j.1360-0443.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug and Alcohol Review. 2005;24(3):217–226. doi: 10.1080/09595230500170266. [DOI] [PubMed] [Google Scholar]
- Ondersma SJ, Chase SK, Svikis DS, Schuster CR. Computer-based brief motivational intervention for perinatal drug use. Journal of Substance Abuse Treatment. 2005;28(4):305–312. doi: 10.1016/j.jsat.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Lam PK, Connors-Burge VS, Ledgerwood DM, Hopper JA. A randomized trial of computer-delivered brief intervention and low-intensity contingency management for smoking during pregnancy. Nicotine & Tobacco Research. 2012;14(3):351–360. doi: 10.1093/ntr/ntr221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Schuster CR. Computer-based brief intervention a randomized trial with postpartum women. American Journal of Preventive Medicine. 2007;32(3):231–238. doi: 10.1016/j.amepre.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Thacker LR, Beatty JR, Lockhart N. Computer-delivered screening and brief intervention (e-SBI) for postpartum drug use: A randomized trial. Journal of Substance Abuse Treatment. 2014;46(1):52–59. doi: 10.1016/j.jsat.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy DB, Scott-Sheldon LA, Johnson BT, Carey MP. Computer-delivered interventions for health promotion and behavioral risk reduction: a meta-analysis of 75 randomized controlled trials, 1988-2007. Preventive Medicine. 2008;47(1):3–16. doi: 10.1016/j.ypmed.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow I, Romelsjo A. The extent of the ‘prevention paradox’ in alcohol problems as a function of population drinking patterns. Addiction. 2006;101(1):84–90. doi: 10.1111/j.1360-0443.2005.01294.x. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P, Bumgardner K, Krupski A, et al. Brief intervention for problem drug use in safety-net primary care settings: A randomized clinical trial. Journal of the American Medical Association. 2014;312(5):492–501. doi: 10.1001/jama.2014.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. British Journal of General Practice. 2005;55(513):305–312. [PMC free article] [PubMed] [Google Scholar]
- Saitz R, Palfai TPA, Cheng DM, et al. Screening and brief intervention for drug use in primary care. The ASPIRE randomized clinical trial. Journal of the American Medical Association. 2014;312(5):502–513. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Gryczynski J, Mitchell SG, Gonzales A, Moseley A, Peterson TR, et al. Computerized v. in-person brief intervention for drug misuse: a randomized clinical trial. Addiction. 2014;109(7):1091–1098. doi: 10.1111/add.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg LI, Maciosek MV, Edwards NM. Primary care intervention to reduce alcohol misuse ranking its health impact and cost effectiveness. American Journal of Preventive Medicine. 2008;34(2):143–152. doi: 10.1016/j.amepre.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Spurling MC, Vinson DC. Alcohol-related injuries: evidence for the prevention paradox. Annals of Family Medicine. 2005;3(1):47–52. doi: 10.1370/afm.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup WW. Mixed model procedures to assess power, precision, and sample size in the design of experiments. Lincoln, NE: University of Nebraska, American Statistical Association; 1999. pp. 19–24. [Google Scholar]
- Substance Abuse and Mental Health Administration (SAMHSA) Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Administration; 2012. (NSDUH Series H-44). HHS Publication No. (SMA) 12-4713. [Google Scholar]
- Walters ST, Vader AM, Harris TR, Jouriles EN. Reactivity to alcohol assessment measures: an experimental test. Addiction. 2009;104(8):1305–1310. doi: 10.1111/j.1360-0443.2009.02632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock EP, Polen MR, Green CA, Orleans T, Klein J. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2004;140(7):557–568. doi: 10.7326/0003-4819-140-7-200404060-00017. [DOI] [PubMed] [Google Scholar]
- Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. Journal of general internal medicine. 1997;12(5):274–283. doi: 10.1046/j.1525-1497.1997.012005274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? American journal of public health. 2003;93(4):635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradnik A, Otto C, Crackau B, Lohrmann I, Bischof G, John U, Rumpf HJ. Randomized controlled trial of a brief intervention for problematic prescription drug use in non-treatment-seeking patients. Addiction. 2009;104:109–117. doi: 10.1111/j.1360-0443.2008.02421.x. [DOI] [PubMed] [Google Scholar]