Abstract
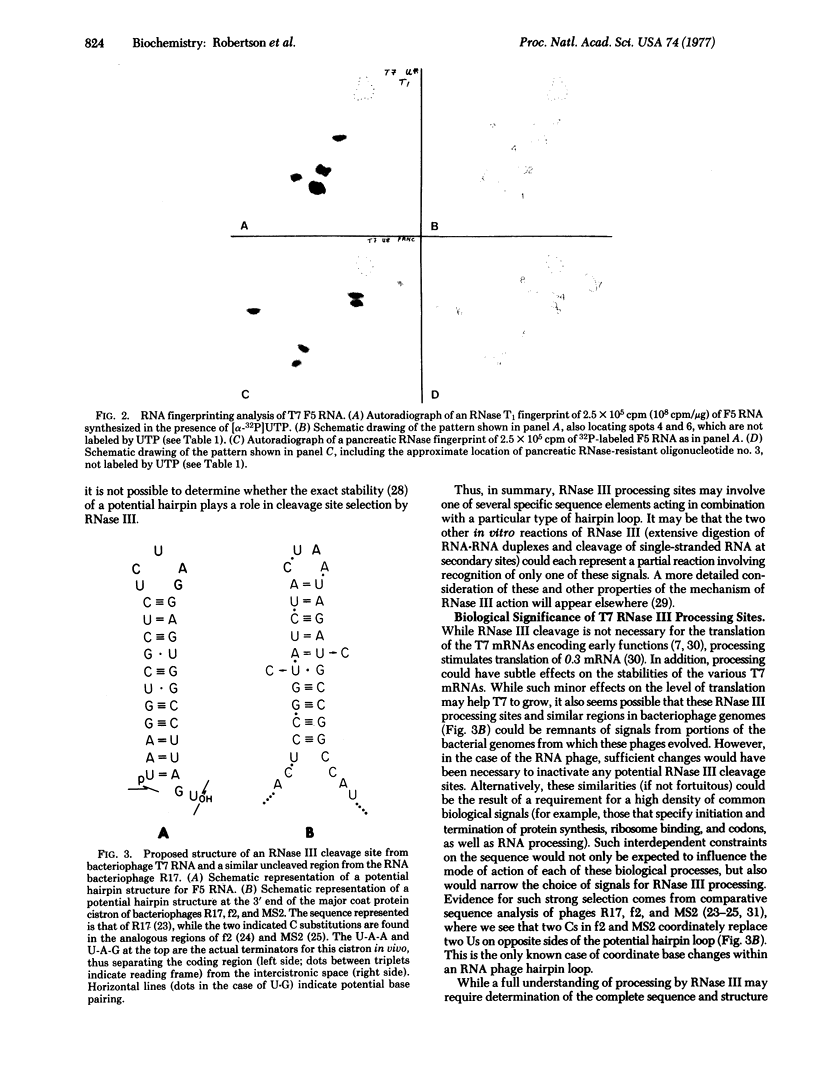
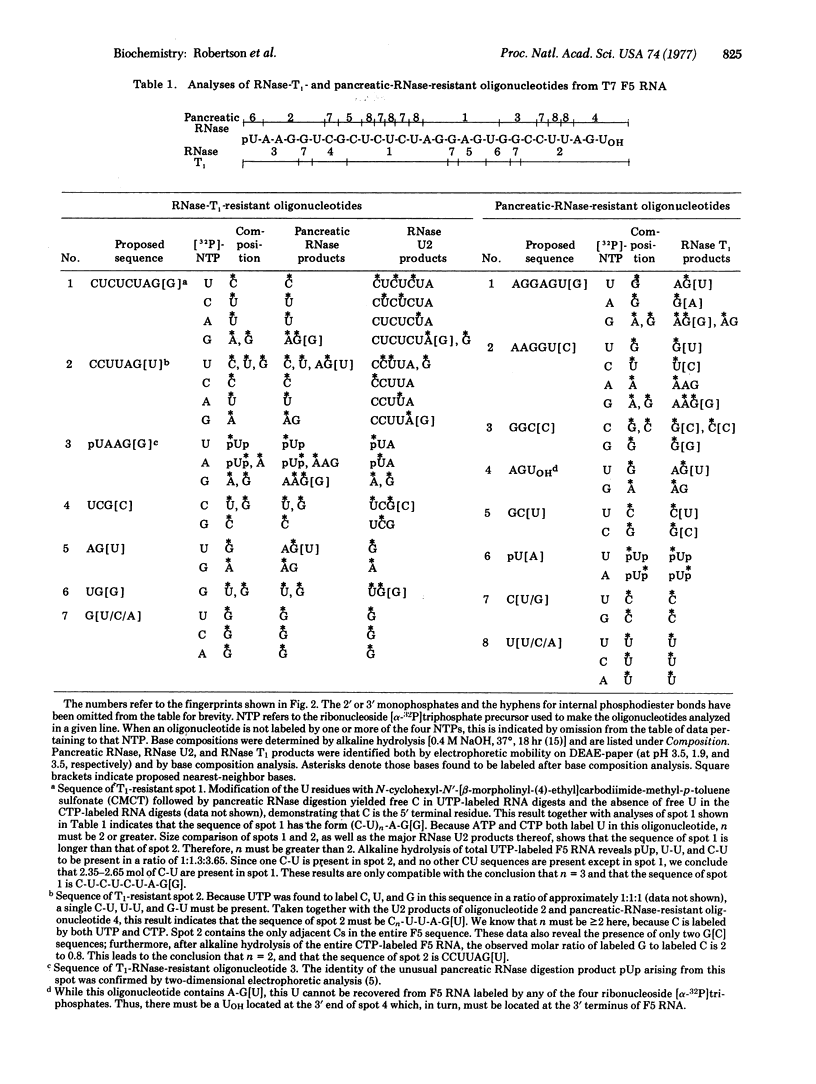
Transcription of that portion of the bacteriophage T7 genome encoding early functions yields RNA molecules about 7500 nucleotides long representing this entire early region. These long transcripts can be cleaved in vitro by highly purified Escherichia coli ribonuclease III (endoribonuclease III; EC 3.1.4.24), yielding five messenger RNAs identical to those produced in vivo. During this reaction, a small RNA fragment called F5 RNA is released, which is specified by the region of the T7 genome between genes 1.1 and 1.3. The following sequence of 32P-labeled F5 RNA has been determined using standard RNA sequencing techniques: pU-A-A-G-G-U-C-G-C-U-C-U-C-U-A-G-G-A-G-U-G-G-C-C-U-U-A-G-Uoh. The relative contributions of sequence and structure to ribonuclease III processing signals are considered in light of these findings.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branlant C., Widada J. S., Krol A., Ebel J. P. Extensions of the known sequences at the 3' and 5' ends of 23S ribosomal RNA from Escherichia coli, possible base pairing between these 23S RNA regions and 16S ribosomal RNA. Nucleic Acids Res. 1976 Jul;3(7):1671–1687. doi: 10.1093/nar/3.7.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Crouch R. J. Ribonuclease 3 does not degrade deoxyribonucleic acid-ribonucleic acid hybrids. J Biol Chem. 1974 Feb 25;249(4):1314–1316. [PubMed] [Google Scholar]
- Dunn J. J. RNase III cleavage of single-stranded RNA. Effect of ionic strength on the fideltiy of cleavage. J Biol Chem. 1976 Jun 25;251(12):3807–3814. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Effect of RNAase III, cleavage on translation of bacteriophage T7 messenger RNAs. J Mol Biol. 1975 Dec 15;99(3):487–499. doi: 10.1016/s0022-2836(75)80140-9. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Processing transcription, and translation of bacteriophage T7 messenger RNAs. Brookhaven Symp Biol. 1975 Jul;(26):267–276. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg D., Steitz J. A. The 30 S ribosomal precursor RNA from Escherichia coli. A primary transcript containing 23 S, 16 S, and 5 S sequences. J Biol Chem. 1975 Jul 25;250(14):5647–5654. [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Nichols J. L. Nucleotide sequence from the polypeptide chain termination region of the coat protein cistron in bacteriophage R17 RNA. Nature. 1970 Jan 10;225(5228):147–151. doi: 10.1038/225147a0. [DOI] [PubMed] [Google Scholar]
- Nichols J. L., Robertson H. D. Sequences of RNA fragments from the bacteriophage f2 coat protein cistron which differ from their R17 counterparts. Biochim Biophys Acta. 1971 Feb 11;228(3):676–681. doi: 10.1016/0005-2787(71)90731-3. [DOI] [PubMed] [Google Scholar]
- Paddock G. V., Fukada K., Abelson J., Robertson H. D. Cleavage of T4 species I ribonucleic acid by Escherichia coli ribonuclease III. Nucleic Acids Res. 1976 May;3(5):1351–1371. doi: 10.1093/nar/3.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieczenik G., Model P., Robertson H. D. Sequence and symmetry in ribosome binding sites of bacteriophage f1 RNA. J Mol Biol. 1974 Dec 5;90(2):191–124. doi: 10.1016/0022-2836(74)90368-4. [DOI] [PubMed] [Google Scholar]
- Planterose D. N., Birch P. J., Pilch D. J., Sharpe T. J. Antiviral activity of double stranded RNA and virus-like particles from Penicillium stoloniferum. Nature. 1970 Aug 1;227(5257):504–505. doi: 10.1038/227504a0. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dunn J. J. Ribonucleic acid processing activity of Escherichia coli ribonuclease III. J Biol Chem. 1975 Apr 25;250(8):3050–3056. [PubMed] [Google Scholar]
- Robertson H. D., Hunter T. Sensitive methods for the detection and characterization of double helical ribonucleic acid. J Biol Chem. 1975 Jan 25;250(2):418–425. [PubMed] [Google Scholar]
- Robertson H. D., Jeppesen P. G. Extent of variation in three related bacteriophage RNA molecules. J Mol Biol. 1972 Jul 28;68(3):417–428. doi: 10.1016/0022-2836(72)90096-4. [DOI] [PubMed] [Google Scholar]
- Robertson H., Webster R. E., Zinder N. D. Bacteriophage coat protein as repressor. Nature. 1968 May 11;218(5141):533–536. doi: 10.1038/218533a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A. Nucleotide sequence surrounding a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977 Mar;74(3):984–988. doi: 10.1073/pnas.74.3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A., Steitz J. A. The specificity of RNase III cleavage of bacteriophage T7 early messenger RNAs. Brookhaven Symp Biol. 1975 Jul;(26):277–285. [PubMed] [Google Scholar]
- Rubin G. M. The nucleotide sequence of Saccharomyces cerevisiae 5.8 S ribosomal ribonucleic acid. J Biol Chem. 1973 Jun 10;248(11):3860–3875. [PubMed] [Google Scholar]
- Schweitz H., Ebel J. P. A study of the mechanism of action of E. coli ribonuclease 3. Biochimie. 1971;53(5):585–593. doi: 10.1016/s0300-9084(71)80014-7. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Westphal H., Crouch R. J. Cleavage of adenovirus messenger RNA and of 28S and 18S ribosomal RNA by RNase III. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3077–3081. doi: 10.1073/pnas.72.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]