Abstract
Notch signaling plays many important roles in homeostasis and remodeling in the vessel wall, and serves a critical role in the communication between endothelial cells and smooth muscle cells. Within blood vessels, Notch signaling integrates with multiple pathways by mechanisms including direct protein-protein interaction, cooperative or synergistic regulation of signal cascades, and co-regulation of transcriptional targets. After establishment of the mature blood vessel, the spectrum and intensity of Notch signaling changes during phases of active remodeling or disease progression. These changes can be mediated by regulation via microRNAs and protein stability or signaling, and corresponding changes in complementary signaling pathways. Notch also affects endothelial cells on a systems level by regulating key metabolic components. This review will outline the most recent findings of Notch activity in blood vessels, with a focus on how Notch signals integrate with other molecular signaling pathways controlling vascular phenotype.
Keywords: Notch signaling, endothelial cell, smooth muscle cell, microRNA, metabolism
1. Introduction
Notch signaling is a primary mediator of cell fate, differentiation, and intercellular communication in virtually all tissues. In this issue of Vascular Pharmacology, work from the Lilly lab expands upon the concept of Notch-mediated communication between vascular endothelial cells and smooth muscle cells, which is critical during vascular development and the pathogenesis of vascular disease. This work continues to expand our knowledge of endothelial and smooth muscle cell communication. This review will focus on recent concepts of Notch signal integration in the postnatal blood vasculature. We refer the reader to recent reviews on the related topics of Notch signaling in embryonic vascular development [1, 2], differentiation and function of vascular cells [3, 4], and an excellent comprehensive book on Notch signaling [5].
2. Vascular quiescence to activation - overview
Most blood vessels in adult organisms have a very low rate of cellular proliferation. Quiescent endothelium expresses Delta-like1 (DLL1), DLL4, Jagged1, Notch1 and Notch4. Vascular smooth muscle cells in the homeostatic condition express primarily Jagged1 and Notch3. Once the vasculature achieves its mature conformation and function, constant communication between vascular cells is required to maintain homeostasis, and to respond to external stimuli such as cytokines, hormones, changes in blood flow or shear stress, inflammation, and mechanical trauma. In particular, endothelial cell communication with vascular smooth muscle cells is mediated via Notch signaling on adjacent cells; however, unique mechanisms including paracrine signaling via exosomes are emerging, and will be discussed.
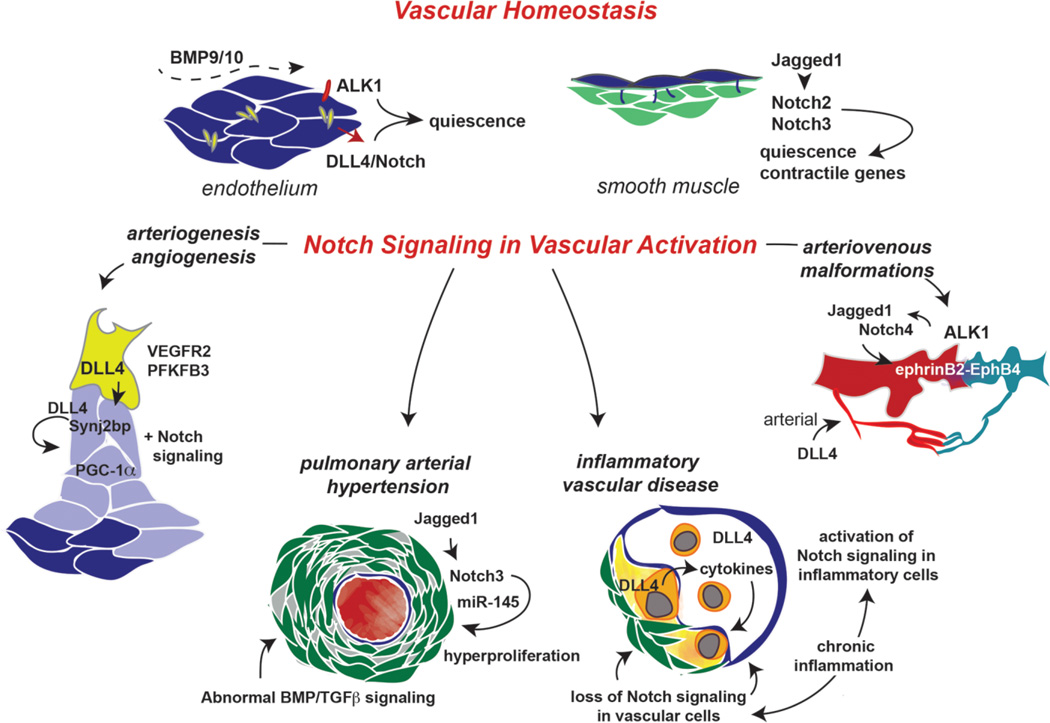
The endothelium is a primary mediator of homeostasis, forming a contact-inhibited monolayer with tight cellular junctions. DLL4 is highly expressed in arterial endothelial cells, and plays a critical role in maintaining endothelial cell quiescence. One tissue-specific exception to DLL4/Notch-mediated endothelial quiescence was recently reported in postnatal long bones, where Notch activity promoted endothelial proliferation in columnar and arched vessels in metaphyseal growth plates, and was responsible for osteoblast maturation and bone deposition in a paracrine manner [6]. Circulating bone morphogenetic proteins (BMP), BMP9 and BMP10 are additional quiescence signals that promote homeostasis in endothelial cells (Figure 1). Integration of Notch signaling with BMP signaling will be detailed below. With an intact endothelium, the smooth muscle cells also remain in a quiescent, contractile state, with high expression of smooth muscle cell markers. Highlights of the interaction between endothelium and smooth muscle cells via Notch signaling will be described.
Figure 1. Notch signaling in vascular homeostasis and remodeling.
In the mature vasculature, the endothelium is a quiescent monolayer with extensive cell adhesions that extend to underlying smooth muscle cells. Quiescence signals include BMP9/BMP10 activation of ALK1, and DLL4 activation of Notch. Normal expression of endothelial cell Jagged1 activates Notch3 in smooth muscle cells to maintain their differentiated, contractile phenotype. Vascular activation under conditions of stress, injury, or disease progression is accompanied by changes in Notch ligand and receptor expression in the vessel wall. These changes regulate cellular proliferation, cell identity, cell function, and overall phenotype.
The levels and expression patterns of Notch proteins and their ligands drastically change upon injury or pathological disease progression. These changes support the concept that Notch signaling is requisitioned during vascular remodeling, and also underscore the non-redundant roles of Notch ligands and receptors. Cardiovascular diseases are associated with dysregulation of Notch signaling, and specific genetic mutations have been mapped to JAG1 or NOTCH2 (Alagille syndrome, pulmonary artery stenoses, tetralogy of Fallot, cardiac septal defects, and coarctation of the aorta). In addition, mutations in NOTCH1 are associated with tetralogy of Fallot and aortic valve abnormalities. These mutations have been comprehensively reviewed [7]. Recently, mutations in NOTCH1 have also been linked to Adams-Oliver Syndrome, characterized by scalp aplasia cutis and terminal transverse limb defects, secondary to vasculopathy [8].
Several human vascular pathologies are associated with alterations in Notch signaling activity. Here we provide a few examples that include novel signaling interactions (Figure 1). It is well known that Notch signaling regulates the processes of angiogenesis and arteriogenesis following ischemic injury or during tumorigenesis. Recent insight has linked cellular metabolism with angiogenesis, and exciting developments in this area will be discussed. In addition, interacting proteins such as synaptojanin-2 binding protein have the potential to regulate Notch signaling during angiogenesis via direct proteinprotein interaction, and specific microRNAs are being discovered as regulatory factors affecting the Notch pathway.
Notch dysregulation also occurs during the pathogenesis of pulmonary arterial hypertension. Pulmonary arterial hypertension involves hyperproliferation of smooth muscle cells of the pulmonary arterioles that leads to decreased vessel lumen size and vessel elasticity, and increased pulmonary vascular resistance. Notch3 levels are increased under hypoxic conditions leading to pulmonary hypertension [9], suggesting a role in disease progression. Indeed, mice homozygous for Notch3 deletion are resistant to pulmonary hypertension, and inhibition of Notch by gamma secretase inhibitor can reverse the hypertensive phenotype in wild type mice [10]. Disease progression in pulmonary arterial hypertension is also associated with impaired BMP signaling and the activation of miR-145. It is interesting to consider the possibility that mutations leading to loss of BMP signals could lead to compensatory increases in Notch signaling. In addition, we previously showed that miR-145 is a transcriptional target of Notch in smooth muscle cells, and thus Notch activation would be predicted to further increase miR-145 levels, whose upregulation is also associated with BMPR2 mutation [11]. Associations of Notch signaling with microRNAs will be addressed in a later section.
Recent work has also highlighted the role of Notch signaling in inflammatory vascular diseases, such as atherosclerosis, where DLL4/Notch signaling has been implicated in macrophage and foam cell accumulation within the lesion [12]. In addition to chronic inflammation, Notch signaling also affected metabolic parameters such as insulin resistance and development of fatty liver [13]. Given the multiple cell types involved and unique activities of Notch on each, the role of Notch in inflammatory vascular diseases is complex. While Notch activity in endothelial and mural cells is necessary for maintenance of endothelial barrier function and smooth muscle contractile phenotype, multiple reports have demonstrated that suppression of Notch also inhibits inflammation, inflammatory cytokine release, atherosclerotic plaque formation, and activated macrophage infiltration [12, 14, 15]. It is important to distinguish between the function of Notch signaling in the different cell types involved during vascular remodeling and lesion formation, and also the timing of Notch activation in each lineage during disease progression. Activation of Notch on inflammatory cells exacerbates pathological lesion formation, but Notch activity in endothelial cells is necessary for prevention of remodeling. Further, inflammatory cytokines suppress endothelial Notch, leading to upregulation of adhesion molecules that recruit inflammatory cells [16, 17]. Notch signaling in bone marrow endothelial and stromal cells has also been linked to inhibition of a proinflammatory circuit involving suppression of miR-155 and subsequent NFκB-mediated myeloproliferative disease [18]. Another microRNA involved in inflammatory disease is miR-126, which regulates endothelial cell proliferation during atherogenesis and vascular repair. The mechanisms of activity of these microRNAs in the vasculature will be described below. Finally, complications of arteriovenous malformations are involved in multiple vascular diseases, and specific novel signal interactions with Notch in this context will be discussed further.
3. Endothelial cell and smooth muscle cell communication
Communication between endothelial cells and mural cells (smooth muscle cells or pericytes) occurs either by direct contact or by soluble or secreted factors, including ions that convey membrane potential information. Direct contact between endothelial cells and mural cells must overcome the distance imposed by the basal lamina and the extracellular matrix of the basement membrane, whose thickness varies based on vessel type and maturity. Myoendothelial gap junctions [19] and microprojections [20] may serve as conduits for cell contact mediated signaling, including juxtacrine Notch ligand-receptor interaction. There is strong evidence for the importance of heterotypic Notch signaling between endothelial and mural cells for proper vessel formation and integrity. Jagged1 signaling from endothelial cells to mural cells induces the contractile phenotype of differentiated smooth muscle cells [21], specifically via the induction and activation of Notch3 [22]. Reciprocal signaling from mural to endothelial cells is also important for proper postnatal angiogenesis [23]. Contact-mediated Notch signaling between endothelial and mural cells can also reinforce maturation signals by enhancing integrin-mediated binding to the basement membrane and activation of matrix-bound signaling peptides, such as endothelial-derived von Willebrand Factor [24]. The study by Lin et al. in this issue has explored these interactions using co-culture of human endothelial cells with vascular smooth muscle cells. Contact-mediated activation of Notch in smooth muscle cells was specific to co-culture with endothelial cells, which led to increased contractile protein expression, collagen secretion, and decreased proliferation. Both Notch2 and Notch3 in smooth muscle cells were mediators of the endothelial cell-induced differentiated phenotype, but Notch2 was specifically required for the suppression of cell proliferation. Thus, contact-mediated activation of Notch signaling plays an important role in cell and vessel maturation, survival, and homeostasis, and different Notch receptors may have specific roles in these processes.
Notch signaling between cells within the blood vessel microenvironment can also be transmitted via non-contact mediated signals, for example by membrane bound ligands on exosomes. Exosomal DLL4 has been shown to activate Notch signaling in endothelial cells to induce tip cell retraction [25], similar to the endogenous juxtacrine activity of DLL4 in angiogenic sprouting endothelial cells. By contrast, an earlier report showed cis-inhibition activity by exosomal DLL4, wherein exosome-incorporating endothelial cells decreased Notch and enhanced tip cell sprouting [26]. Exosome-mediated Notch signaling between endothelial and mural cells has not yet been reported.
4. Notch pathway crosstalk with other signaling networks in the vasculature
There are many reports of crosstalk between Notch signaling and other pathways within the vasculature. Due to the multi-step nature of Notch signaling, there are numerous levels of potential crosstalk. These include direct binding of nuclear transcription factors to one another or to shared promoter elements; cytoplasmic protein interactions; enzymatic modification such as ubiquitination; transmembrane and membrane-bound protein interactions, and binding of components in the extracellular environment such as extracellular matrix-bound and soluble proteins. We define crosstalk to include relationships wherein Notch signaling components bind directly to other proteins, or which share binding targets and cooperatively activate or diametrically antagonize one another. It is outside of our scope to discuss all instances of crosstalk between Notch and other proteins/pathways in vascular cells, and we refer the reader to recent excellent reviews that cover additional areas, including Hedgehog signaling as an upstream activator of Notch expression and vascular arterialization [27], VEGF and Notch interaction in endothelial cells [28], and Wnt/Sox 17 in arterial identity [29].
4a. Proteins directly enhancing or suppressing Notch activity in the vasculature
Post-translational regulation by protein interactions can enhance Notch signaling in both endothelial cells and mural cells. Synaptojanin-2 binding protein (Synj2bp) was identified as an enhancer of Notch signaling in endothelial cells [30]. Synj2bp, a mitochondrial outer membrane protein, interacts with the PDZ domains of DLL1 and DLL4. The activity of Synj2bp was anti-angiogenic, leading to decreased endothelial cell proliferation and migration. The mechanism of Synj2bp could partially be linked to activation of Notch signaling by stabilization of DLL4 ligand. In an angiogenic vessel, Synj2bp co-expression with DLL4 in a stalk cell could promote Notch signaling to suppress the development of tip cells. Indeed, suppression of Synj2bp in endothelial cells transplanted in vivo led to increased vascular density, similar to the suppression of DLL4 in an angiogenesis context. Non-canonical activation of Notch was also induced by estradiol in endothelial cells, especially in cooperation with DLL4 ligand stimulation, potentiating Notch expression of arterial genes and vessel stability [31]. In vascular smooth muscle cells, positive regulation of Notch was demonstrated in conjunction with angiotensin-2 (AngII) signaling. AngII rapidly promotes gamma secretase activity to cleave and activate Notch1, resulting in smooth muscle cell migration, proliferation, and neointima formation [32]. The effects of AngII were reversed by treatment with gamma secretase inhibitor.
Notch activity can also be negatively regulated via interacting proteins. EGFL7 is an endothelial cell-derived secreted protein that directly binds the extracellular domain of Notch and inhibits its activation. EGFL7 activity thus promotes vascular sprouting in an angiogenic context [33]. However, as vessels mature, EGFL7 expression is progressively restricted to veins and capillaries, where Notch maintenance of arterial fate is not needed [34]. Interestingly, nested within the EGFL7 gene is the intronic miR-126 gene, whose regulatory activity on Notch signaling will be described below. Inhibition of Notch1 can also occur in endothelial cells by direct binding to FKBP1a, a peptidylprolyl isomerase. Loss of FKBP1a led to hyperactive Notch signaling in the endocardium, causing hypertrabeculation and left ventricular heart disease [35]. Pathological inhibition of Notch signaling in cerebral microvascular mural cells can occur via von Willebrand Factor (vWF). vWF, when secreted by endothelial cells abluminally, can bind to and inhibit activation of Notch3, resulting in decreased expression of smooth muscle cell genes and vascular instability [36].
4b. Notch crosstalk with TGFβ
Notch crosstalk with the TGFβ pathway has been widely reported, comprehensive reviews are available [37, 38], and recent findings will be discussed here. In endothelial cells, crosstalk between Notch and TGFβ is often associated with endothelial-to-mesenchymal transition (EnMT) and pathological fibrosis. EnMT in corneal endothelial cells was inhibited by gamma secretase inhibitor, DAPT, suggesting that Notch signaling is a contributing factor [39]. Interestingly, EnMT was also inducible by treatment of the corneal endothelial cells with TGFβ, and it was also inhibited by DAPT, further implicating direct interaction and interdependence of the Notch and TGFβ pathways for this physiological process.
In a mouse model of chronic kidney disease, neointima formation and inflammatory cell invasion, secondary to arteriovenous fistulas, was attributed to crosstalk between Notch and TGFβ signaling. Increased TGFβ1 during uremia corresponds to activated Notch in endothelial cells and subsequent barrier dysfunction [40]. Not only were Notch ligands sufficient to elicit the same effect, but TGFβ-induced changes in phenotype were blocked with Notch inhibition or knockout of RPBJ. In cerebrovascular endothelial cells, direct binding of RBPJ to SMAD4 was shown to be responsible for cooperative, Notch-dependent expression of N-cadherin, which mediates endothelial cell-pericyte association. Loss of SMAD4 decreased the expression of N-cadherin, causing pericyte loss, vascular instability and intracranial hemorrhage [41].
4c. Notch crosstalk with BMP in the vasculature
Notch crosstalk with BMP signaling was recently reviewed in an excellent article that focused on regulation of oscillatory gene expression [42]. In endothelial cells, BMP signaling is generally thought to cooperate with Notch signaling to induce quiescence and promote vessel maturation and arterialization. Recent reports have highlighted this cooperative crosstalk between DLL4/Notch1 and the BMP9-ALK1-SMAD1/5 pathway. Endothelial-specific knockout and knockdown of SMAD1 and SMAD5 abrogate the ability of DLL4/Notch1 to properly coordinate tip and stalk angiogenic phenotypes, leading to hypersprouting vessels in vitro and embryonic lethality in vivo [43]. Inhibition of BMP9/ALK1 signaling resulted in similar consequences in a postnatal retinal angiogenesis model, where ALK1-Fc ligand trapping of circulating BMP9 induced hypersprouting, arteriovenous malformations, and decreased Hey1/2 expression. Additionally, inhibition of Notch activity with DAPT could be overcome with addition of excess BMP9, indicating that the cooperative signals between the pathways can be compensatory and fine-tuned [44]. The ability of Notch to compensate for loss of ALK1 signaling was also demonstrated in an in vitro model of hereditary hemorrhagic telangiectasia, where expression of the Notch target gene and arterial-specific marker EphrinB2 was analyzed. Decreased expression of EphrinB2 secondary to loss of ALK1 was reversed by co-culture with cells expressing DLL4 [45]. The converse was also demonstrated in an in vivo model of arteriovenous malformations in the matrix gla protein (MGP) knockout mouse. Since MGP is a BMP inhibitor, excess BMP and Notch target gene activation were associated with AVM development. The pathology was reversed after crossing the MGP null mice onto a Jag1/2 heterozygous background [46], thus demonstrating that cooperative signaling is required for AVM development in this model. In mural cells, BMP cooperation with Notch has been implicated in vascular calcification [47]. Stimulation with BMP2 and Notch cooperatively activates the expression of the osteogenic marker MSX2, and NotchICD complexed with SMAD1 on the MSX2 promoter. Finally, Notch1, BMP2, and MSX2 proteins were co-localized in atherosclerotic plaques from human vessels, suggesting cooperation in the pathogenesis of human disease progression.
5. Notch signal integration with cell metabolism
Vascular cell metabolism is of increasing interest, and several studies have focused on the role of metabolic changes in endothelial cells with respect to angiogenesis. The seminal study by De Bock et al. demonstrated that the primary method of endothelial metabolism and ATP generation is via anaerobic glycolysis, with glucose oxidation and mitochondrial respiration only activated during endothelial cell stress [48]. Minimizing oxygen consumption is consistent with the primary function of endothelial cells to deliver oxygen to tissues. This study showed that the most highly expressed glycolytic enzyme in endothelium is PFKFB3, a secondary fructose-6-phosphate kinase, whose product (fructose 2,6 bisphosphate) is an allosteric activator of the primary phosphofructokinase-1 glycolytic pathway. In this way, PFKFB3 levels could be regulated in endothelial cells without completely inhibiting glycolysis, which would cause cellular stress and apoptosis. While DLL4 suppressed the expression and activity of PFKFB3, the enzyme was upregulated in sprouting endothelial cells, induced by VEGF and FGF cytokines, and concentrated in the distal ends of filopodial tips where kinetic demand for ATP was high, and extracellular pO2 low. The increased glycolytic flux induced by PFKFB3 overexpression was sufficient to drive endothelial angiogenesis independent of VEGF signaling, and was sufficient to overcome Notch signaling, which would normally promote a stalk cell phenotype. Inhibition of PFKFB3 induced quiescence, even in the absence of Notch activation. Thus, glycolysis is a driver of angiogenesis, independent of activating and inhibitory signaling pathways. Schoors et al. demonstrated the therapeutic potential of regulating PFKFB3 in endothelial cells using the small molecule inhibitor of PFKFB3, 3PO [49]. Multiple in-vivo models of pathologic angiogenesis were successfully reversed using 3PO-mediated inhibition of glycolysis, including mouse models of choroidal neovascularization, psoriasis and colitis.
Sawada et al. showed that pathologic suppression of metabolism in endothelial cells was mediated by the PPARγ coactivator protein, PGC1α [50]. This protein induces expression of various metabolic genes, driving thermogenesis in brown fat [51] and mitochondrial biogenesis in cardiomyocytes and skeletal muscle [52, 53]. PGC1α induced the expression and activation of Notch1 and potently inhibited endothelial sprouting, migration and angiogenesis. Transgenic expression of PGC1α mimicked phenotypes of endothelial dysfunction secondary to diabetes, and vessel repair and wound healing were severely impaired. Consistent with these finding, knockout models of PGC1α rescued endothelial cell migration, angiogenesis, and diabetic endothelial dysfunction.
Although the role of metabolism and metabolic effector pathways has been thoroughly investigated in vascular smooth muscle cells, the interaction of Notch with the metabolic state of smooth muscle cells has not been investigated. However, some evidence suggests that Notch may have an impact on smooth muscle cell metabolism. In patients with the R133C CADASIL mutation, smooth muscle cells had reduced proliferation and an increased number of dysfunctional mitochondria [54]. The mitochondrial morphology was highly irregular, with decreased mitochondrial coupling or connectivity and decreased membrane potential. Thus, Notch3 signaling may contribute to normal smooth muscle cell metabolism by maintaining mitochondrial health.
Recent data have also implicated Notch signaling in various aspects of metabolism, although these pathways have not been addressed in vascular cells. In a human chronic myelogenous leukemia cell line, expression of the Notch1 intracellular domain was found to modify the mitochondrial proteome, leading to changes in genes involved in glutamine catabolism and the respiratory electron transport chain [55]. In addition, adipose tissue-specific inactivation of Notch1 led to the browning of white adipose tissue, leading to improved glucose metabolism and elevated energy expenditure via Ucp-1 [56]. These types of systemic metabolic changes mediated by Notch activity in endocrine organs such as adipose tissue are predicted to affect vascular homeostasis and remodeling.
6. Notch interaction with microRNAs
Many microRNA have been implicated in the regulation of angiogenesis and cardiovascular diseases [57, 58], but limited studies have identified specific microRNAs that regulate Notch ligand and receptor transcripts in endothelial cells or mural cells. One exception is DLL4 transcript, which is directly targeted by the miR-30 family [59, 60] as well as miR-27b [61] in endothelial cells. miR-30b and miR-30c directly bind to a conserved sequence in the DLL4 3’ UTR and decrease DLL4 protein in endothelial cells. Further, in zebrafish, expression of a miR-30 mimic suppressed DLL4 and led to hypersprouting and branching of vessels, similar to the DLL4 loss of function phenotype [59, 60]. DLL4 transcript is also targeted by miR-27b, and thus the levels of miR-27b can regulate vascular sprouting and arterial-venous identity [61]. Outside of the vasculature, particularly in cancer cells, multiple microRNAs have been validated to directly target and suppress the production of Notch ligands or receptors (Table 1). It will be of interest in future studies to determine if these interactions are also regulating Notch availability in the vessel wall, especially in conditions of vascular remodeling or tumor angiogenesis, when Notch components are dynamically regulated.
Table 1.
Validated miRNA-Notch target interactions compiled from PubMed, miRTarbase [120], and TarBase [121]. Included are direct target interactions confirmed by strong experimental evidence as defined in most cases by 3’UTR assay with wild type and mutant sequence, and qPCR and Western blot analysis after expression of the microRNA mimic or antagomir. By these criteria, there is no strong experimental evidence validating predicted miRNA target interactions for mammalian Jagged2.
| Notch1 | Cell Context | Jagged1 | Cell Context |
|---|---|---|---|
| miR-10b | glioma [75] | miR-21 | dendritic cells [76], breast cancer [77] |
| miR-30 | podocytes [78], dendritic cells (miR- 30b) [79], acute myeloid leukemia (miR-30c) [80] |
miR-34a | dendritic cells [76], osteoblasts [81], renal epithelial cells [82], cervical carcinoma and choriocarcinoma [83] |
| miR-34a | glioma, medulloblastoma [84], gliobastoma multiforme [85], renal epithelial cells [82], renal cell carcinoma [86], bladder carcinoma [87], colon cancer [88], breast and cervical carcinoma and choriocarcinoma [83, 89], mouse macrophage [90], keratinocytes and HeLa cells [91] |
miR-34b | breast cancer [92] |
| miR-124 | kidney epithelial and glioblastoma cells [93], neural progenitor cells (miR-124a) [94] |
||
| miR-193a | bone marrow stromal cells [95] | ||
| miR-34b | osteoblasts [96], neuroblastoma [97] | miR-199a | myogenesis [98] |
| miR-34c | osteoblasts [96], neuroblastoma [97] | miR-199b | HEK-293, ovarian cancer cells [99] |
| miR-139 | colorectal cancer [100] | miR-200 | multiple cancer cell lines [103] |
| miR-146a | glioma [101], dendritic cells [102] | miR-214 | kidney epithelial and glioblastoma cells [93] |
| miR-146b | myoblasts [104] | ||
| miR-200 | neuroblastoma [97], nasopharyngeal carcinoma (miR-200b) [105] |
Jagged2 | |
| miR-326 | glioma [106] | no strong experimental validation | |
| miR-449 | myeloid leukemia [107], airway mucociliary epithelial cells [108] |
||
| Notch2 | DLL1 | ||
| miR-34a | glioma, medulloblastoma [84] | miR-34a | choriocarcinoma [109], medulloblastoma [110] |
| miR-107 | glioma [111] | miR-449 | airway mucociliary epithelial cells [108] |
| miR-326 | glioma [106] | ||
| Notch3 | DLL3 | ||
| miR-1 | prostate cancer [112], colorectal cancer [113] |
miR-18a | glioma initiating cells [114] |
| miR-150 | T-cell development [115] | ||
| miR-206 | HeLa cells, mouse fibroblasts [116] | DLL4 | |
| Notch4 | miR-27b | endothelial cells [61] | |
| miR-34c | breast carcinoma [117] | miR-30a | endothelial cells [60] |
| miR-181c | gastric carcinoma [118] | miR-30b | endothelial cells [59] |
| miR-302a | melanoma [119] | miR-30c | endothelial cells [59] |
In addition to directly targeting transcripts of Notch pathway genes, microRNAs can also work upstream to alter ligand and receptor levels via indirect mechanisms. For example, delivery of miR-126 to injured mouse myocardium in transplanted mesenchymal stem cells increases secretion of VEGF and FGF2 and upregulates expression of DLL4, resulting in enhanced functional ischemic angiogenesis [62]. The hypoxia-induced miR-210 also appears to promote ischemic angiogenesis, possibly via upregulation of Notch1 as observed in human umbilical vein endothelial cells and rat models of ischemic stroke [63]. Specific targets of these microRNAs and mechanisms for their regulation of DLL4 and Notch1 in these contexts have yet to be defined.
MicroRNAs may also target Notch regulatory proteins, thereby affecting Notch signaling. One example is miR-126, which targets the non-canonical Notch ligand DLK1 [64]. In endothelial cells, expression of a miR-126 (miR-126-5p) mimic decreased DLK1 protein levels, and studies with a DLK1 3’UTR reporter confirmed miR-126 regulation via its predicted binding site. DLK1 functions as a Notch inhibitor, and Schober et al. have described the physiological consequences of miR-126 targeting of DLK1 in vascular repair and atherosclerosis [64]. Loss of function studies showed that mice lacking miR-126 on an ApoE null background had impaired endothelial cell recovery after injury, and increased atherosclerotic lesions. This effect was reversed when DLK1 was silenced, showing that the miR-126 null phenotype required increased DLK1. The proposed mechanism of mir-126 suppression of atherosclerosis is by promoting endothelial proliferation, creating a “reserve” of cells that can quickly repopulate areas of lumenal denudation, suppressing sclerotic lesion formation. Although miR-126 may target multiple transcripts in the vasculature, increased DLK1 in endothelial cells after vascular injury corresponded to decreased Notch1 activation in vivo. As described above, Notch signaling has complex, cell-specific roles in atherogenesis and other inflammatory vascular diseases.
Notch as a transcriptional activator has been confirmed for only a few microRNA gene targets in the vasculature. The miR-143/145 cluster, a known regulator of vascular smooth muscle cell phenotype [65], is a direct downstream transcriptional target activated by Jagged1/Notch signaling via RBPJ (CBF1) consensus binding sites [66]. The miR-143/145 cluster is involved in vascular remodeling following injury, and mediates phenotypic changes in vascular smooth muscle cells [67, 68]. Of note, miR-143/145 is also activated by serum response factor/myocardin transcriptionally via the CArG box [65], and this activity is modified by TGFβ and BMP [69]. TGFβ increases myocardin levels and BMP4 induces nuclear translocation of MRTF-A. Thus, miR-143 and miR-145 are examples of microRNAs that are regulated coordinately through multiple signal pathways. The miR-223 promoter was activated by Notch1ICD or Notch3ICD in Jurkat cells, and both receptors were immunoprecipitated in a complex with a promoter fragment containing an RBPJ consensus-binding site [70]. Although this has not been confirmed in vascular cells, miR-223 has been implicated in arterial remodeling [71].
Notch signaling can also suppress microRNA levels. In zebrafish, miR-221 was characterized as an essential angiogenic regulator that is negatively regulated by active Notch signaling [72]. In this case, loss of Notch led to hyper-angiogenesis and increased miR-221 levels, and knockdown of miR-221 rescued the loss of Notch phenotype. Although it is not clear whether Notch signaling regulates miR-221 gene transcription, miR-221 levels were decreased by Notch signaling. There is also some evidence that Notch signaling may lead to transcriptional repression of microRNAs. miR-155 is a flow-sensitive microRNA associated with inflammation and atherosclerosis, and has been described either as pro- or anti-inflammatory in the vasculature [73, 74]. Loss of function studies demonstrated that RBPJ downstream of Notch signaling repressed miR-155 and inflammation in bone marrow endothelial and stromal cells [18]. In this study, Notch/RBPJ appeared to act as a transcriptional repressor, which can be further validated with mutational analysis of the RPBJ consensus site in the miR-155 promoter. Alternatively, since the miR-155 promoter has multiple potential binding sites for the Hes/Hey family of transcriptional repressors, canonical Notch/RBPJ activation of Hes/Hey could be a second mechanism for repression of miR-155 transcription. The activity of miR-155 on inflammation and atherosclerosis appears to be temporally regulated, with basal expression of miR-155 inhibiting pathogenic inflammatory stimuli, which later becomes pro-inflammatory and detrimental after establishment of mature disease. Because Notch dysregulation is associated with atherogenesis, it will be of interest to determine whether Notch regulation of miR-155 plays a role in the inflammatory phenotype during atherosclerosis. Given the roles of these microRNAs in inflammation, angiogenesis, and vascular pathology, continued understanding of Notch interaction with microRNAs has implications for vascular therapeutics.
7. Conclusions
Balanced Notch signaling is required in endothelial and mural cells for proper blood vessel function. Too much or too little Notch activation causes cellular dysfunction and failure of vessel function or responsiveness. The potency of Notch signaling and the pathological consequences of imbalanced signaling requires careful regulation, which is aided by interacting proteins that fine-tune its activity. Identification of the regulators, binding partners, and targets of Notch in the vasculature will lead to better understanding of related vascular pathologies and strategies for restoration of vascular homeostasis.
Abbreviations
- AngII
angiotensin II
- AVM
arteriovenous malformation
- BMP
bone morphogenetic protein
- CADASIL
Cerebral Autosomal Dominant Arteriopathy with Sub-cortical Infarcts and Leukoencephalopathy
- DLL
delta-like
- EGFL7
EGF-like domain 7
- MGP
matrix Gla protein
- PGC1α
peroxisome proliferator activated receptor gamma coactivator 1 alpha
- PFKFB3
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase3
- RBPJ
recombination signal sequence-binding protein Jκ
- Synj2bp
synaptojanin-2 binding protein
- TGFβ
transforming growth factor beta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kume T. Ligand-dependent Notch signaling in vascular formation. Adv Exp Med Biol. 2012;727:210–222. doi: 10.1007/978-1-4614-0899-4_16. [DOI] [PubMed] [Google Scholar]
- 2.Domigan CK, Iruela-Arispe ML. Recent advances in vascular development. Curr Opin Hematol. 2012;19:176–183. doi: 10.1097/MOH.0b013e3283523e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher J, Gridley T, Liaw L. Molecular pathways of Notch signaling in vascular smooth muscle cells. Front Physiol. 2012;3:81. doi: 10.3389/fphys.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fouillade C, Monet-Lepretre M, Baron-Menguy C, Joutel A. Notch signalling in smooth muscle cells during development and disease. Cardiovasc Res. 2012;95:138–146. doi: 10.1093/cvr/cvs019. [DOI] [PubMed] [Google Scholar]
- 5.Bellen HJ, Yamamoto S. Notch Signaling, Methods and Protcols. In: Walker JM, editor. Methods in Molecular Biology. Vol. 1187. Humana Press; 2014. p. 351. [PubMed] [Google Scholar]
- 6.Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penton AL, Leonard LD, Spinner NB. Notch signaling in human development and disease. Semin Cell Dev Biol. 2012;23:450–457. doi: 10.1016/j.semcdb.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stittrich AB, Lehman A, Bodian DL, Ashworth J, Zong Z, Li H, Lam P, Khromykh A, Iyer RK, Vockley JG, Baveja R, Silva ES, Dixon J, Leon EL, Solomon BD, Glusman G, Niederhuber JE, Roach JC, Patel MS. Mutations in NOTCH1 Cause Adams-Oliver Syndrome. Am J Hum Genet. 2014 doi: 10.1016/j.ajhg.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu YR, Mao L, Piantadosi CA, Gunn MD. CCR2 deficiency, dysregulation of Notch signaling, and spontaneous pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2013;48:647–654. doi: 10.1165/rcmb.2012-0182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, Macias J, Yuan JX, Jamieson SW, Thistlethwaite PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med. 2009;15:1289–1297. doi: 10.1038/nm.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caruso P, Dempsie Y, Stevens HC, McDonald RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, Upton P, Xin M, van Rooij E, Olson EN, Morrell NW, MacLean MR, Baker AH. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res. 2012;111:290–300. doi: 10.1161/CIRCRESAHA.112.267591. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda D, Aikawa E, Swirski FK, Novobrantseva TI, Kotelianski V, Gorgun CZ, Chudnovskiy A, Yamazaki H, Croce K, Weissleder R, Aster JC, Hotamisligil GS, Yagita H, Aikawa M. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc Natl Acad Sci U S A. 2012;109:E1868–E1877. doi: 10.1073/pnas.1116889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda D, Aikawa M. Expanding role of delta-like 4 mediated notch signaling in cardiovascular and metabolic diseases. Circ J. 2013;77:2462–2468. doi: 10.1253/circj.cj-13-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoyama T, Takeshita K, Kikuchi R, Yamamoto K, Cheng XW, Liao JK, Murohara T. gamma-Secretase inhibitor reduces diet-induced atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2009;383:216–221. doi: 10.1016/j.bbrc.2009.03.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pabois A, Devalliere J, Quillard T, Coulon F, Gerard N, Laboisse C, Toquet C, Charreau B. The disintegrin and metalloproteinase ADAM10 mediates a canonical Notch-dependent regulation of IL-6 through Dll4 in human endothelial cells. Biochem Pharmacol. 2014;91:510–521. doi: 10.1016/j.bcp.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Quillard T, Coupel S, Coulon F, Fitau J, Chatelais M, Cuturi MC, Chiffoleau E, Charreau B. Impaired Notch4 activity elicits endothelial cell activation and apoptosis: implication for transplant arteriosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:2258–2265. doi: 10.1161/ATVBAHA.108.174995. [DOI] [PubMed] [Google Scholar]
- 17.Nus M, MacGrogan D, Martinez-Poveda B, Benito Y, Casanova JC, Fernandez-Aviles F, Bermejo J, de la Pompa JL. Diet-induced aortic valve disease in mice haploinsufficient for the Notch pathway effector RBPJK/CSL. Arterioscler Thromb Vasc Biol. 2011;31:1580–1588. doi: 10.1161/ATVBAHA.111.227561. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Zhang H, Rodriguez S, Cao L, Parish J, Mumaw C, Zollman A, Kamoka MM, Mu J, Chen DZ, Srour EF, Chitteti BR, HogenEsch H, Tu X, Bellido TM, Boswell HS, Manshouri T, Verstovsek S, Yoder MC, Kapur R, Cardoso AA, Carlesso N. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-kappaB-dependent manner. Cell Stem Cell. 2014;15:51–65. doi: 10.1016/j.stem.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. What's where and why at a vascular myoendothelial microdomain signalling complex. Clin Exp Pharmacol Physiol. 2009;36:67–76. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- 20.Nagaraja S, Kapela A, Tran CH, Welsh DG, Tsoukias NM. Role of microprojections in myoendothelial feedback--a theoretical study. J Physiol. 2013;591:2795–2812. doi: 10.1113/jphysiol.2012.248948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2008;105:1955–1959. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Kennard S, Lilly B. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ Res. 2009;104:466–475. doi: 10.1161/CIRCRESAHA.108.184846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang K, Proweller A. Vascular smooth muscle Notch signals regulate endothelial cell sensitivity to angiogenic stimulation. J Biol Chem. 2011;286:13741–13753. doi: 10.1074/jbc.M110.181842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheppke L, Murphy EA, Zarpellon A, Hofmann JJ, Merkulova A, Shields DJ, Weis SM, Byzova TV, Ruggeri ZM, Iruela-Arispe ML, Cheresh DA. Notch promotes vascular maturation by inducing integrin-mediated smooth muscle cell adhesion to the endothelial basement membrane. Blood. 2012;119:2149–2158. doi: 10.1182/blood-2011-04-348706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharghi-Namini S, Tan E, Ong LL, Ge R, Asada HH. Dll4-containing exosomes induce capillary sprout retraction in a 3D microenvironment. Sci Rep. 2014;4:4031. doi: 10.1038/srep04031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RC, Sargent I, Li JL, Harris AL. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 27.Katoh M. Therapeutics targeting angiogenesis: genetics and epigenetics, extracellular miRNAs and signaling networks (Review) Int J Mol Med. 2013;32:763–767. doi: 10.3892/ijmm.2013.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas JL, Baker K, Han J, Calvo C, Nurmi H, Eichmann AC, Alitalo K. Interactions between VEGFR and Notch signaling pathways in endothelial and neural cells. Cell Mol Life Sci. 2013;70:1779–1792. doi: 10.1007/s00018-013-1312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morini MF, Dejana E. Transcriptional regulation of arterial differentiation via Wnt, Sox and Notch. Curr Opin Hematol. 2014;21:229–234. doi: 10.1097/MOH.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 30.Adam MG, Berger C, Feldner A, Yang WJ, Wustehube-Lausch J, Herberich SE, Pinder M, Gesierich S, Hammes HP, Augustin HG, Fischer A. Synaptojanin-2 binding protein stabilizes the Notch ligands DLL1 and DLL4 and inhibits sprouting angiogenesis. Circ Res. 2013;113:1206–1218. doi: 10.1161/CIRCRESAHA.113.301686. [DOI] [PubMed] [Google Scholar]
- 31.Caliceti C, Aquila G, Pannella M, Morelli MB, Fortini C, Pinton P, Bonora M, Hrelia S, Pannuti A, Miele L, Rizzo P, Ferrari R. 17beta-estradiol enhances signalling mediated by VEGF-A-deltalike ligand 4-notch1 axis in human endothelial cells. PLoS ONE. 2013;8:e71440. doi: 10.1371/journal.pone.0071440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozasa Y, Akazawa H, Qin Y, Tateno K, Ito K, Kudo-Sakamoto Y, Yano M, Yabumoto C, Naito AT, Oka T, Lee JK, Minamino T, Nagai T, Kobayashi Y, Komuro I. Notch activation mediates angiotensin II-induced vascular remodeling by promoting the proliferation and migration of vascular smooth muscle cells. Hypertens Res. 2013;36:859–865. doi: 10.1038/hr.2013.52. [DOI] [PubMed] [Google Scholar]
- 33.Nichol D, Shawber C, Fitch MJ, Bambino K, Sharma A, Kitajewski J, Stuhlmann H. Impaired angiogenesis and altered Notch signaling in mice overexpressing endothelial Egfl7. Blood. 2010;116:6133–6143. doi: 10.1182/blood-2010-03-274860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poissonnier L, Villain G, Soncin F, Mattot V. Egfl7 is differentially expressed in arteries and veins during retinal vascular development. PLoS ONE. 2014;9:e90455. doi: 10.1371/journal.pone.0090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, Zhang W, Sun X, Yoshimoto M, Chen Z, Zhu W, Liu J, Shen Y, Yong W, Li D, Zhang J, Lin Y, Li B, VanDusen NJ, Snider P, Schwartz RJ, Conway SJ, Field LJ, Yoder MC, Firulli AB, Carlesso N, Towbin JA, Shou W. Fkbp1a controls ventricular myocardium trabeculation and compaction by regulating endocardial Notch1 activity. Development. 2013;140:1946–1957. doi: 10.1242/dev.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng H, Zhang X, Lee SJ, Wang MM. Von Willebrand factor inhibits mature smooth muscle gene expression through impairment of Notch signaling. PLoS ONE. 2013;8:e75808. doi: 10.1371/journal.pone.0075808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garside VC, Chang AC, Karsan A, Hoodless PA. Co-ordinating Notch, BMP, and TGF-beta signaling during heart valve development. Cell Mol Life Sci. 2013;70:2899–2917. doi: 10.1007/s00018-012-1197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kluppel M, Wrana JL. Turning it up a Notch: cross-talk between TGF beta and Notch signaling. Bioessays. 2005;27:115–118. doi: 10.1002/bies.20187. [DOI] [PubMed] [Google Scholar]
- 39.Li C, Dong F, Jia Y, Du H, Dong N, Xu Y, Wang S, Wu H, Liu Z, Li W. Notch signal regulates corneal endothelial-to-mesenchymal transition. Am J Pathol. 2013;183:786–795. doi: 10.1016/j.ajpath.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Liang A, Luo J, Liang M, Han G, Mitch WE, Cheng J. Blocking Notch in endothelial cells prevents arteriovenous fistula failure despite CKD. J Am Soc Nephrol. 2014;25:773–783. doi: 10.1681/ASN.2013050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Yang X. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell. 2011;20:291–302. doi: 10.1016/j.devcel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Beets K, Huylebroeck D, Moya IM, Umans L, Zwijsen A. Robustness in angiogenesis: notch and BMP shaping waves. Trends Genet. 2013;29:140–149. doi: 10.1016/j.tig.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Moya IM, Umans L, Maas E, Pereira PN, Beets K, Francis A, Sents W, Robertson EJ, Mummery CL, Huylebroeck D, Zwijsen A. Stalk cell phenotype depends on integration of Notch and Smad1/5 signaling cascades. Dev Cell. 2012;22:501–514. doi: 10.1016/j.devcel.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larrivee B, Prahst C, Gordon E, del Toro R, Mathivet T, Duarte A, Simons M, Eichmann A. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell. 2012;22:489–500. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JH, Peacock MR, George SC, Hughes CC. BMP9 induces EphrinB2 expression in endothelial cells through an Alk1-BMPRII/ActRII-ID1/ID3-dependent pathway: implications for hereditary hemorrhagic telangiectasia type II. Angiogenesis. 2012;15:497–509. doi: 10.1007/s10456-012-9277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao Y, Yao J, Radparvar M, Blazquez-Medela AM, Guihard PJ, Jumabay M, Bostrom KI. Reducing Jagged 1 and 2 levels prevents cerebral arteriovenous malformations in matrix Gla protein deficiency. Proc Natl Acad Sci U S A. 2013;110:19071–19076. doi: 10.1073/pnas.1310905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu T, Tanaka T, Iso T, Matsui H, Ooyama Y, Kawai-Kowase K, Arai M, Kurabayashi M. Notch signaling pathway enhances bone morphogenetic protein 2 (BMP2) responsiveness of Msx2 gene to induce osteogenic differentiation and mineralization of vascular smooth muscle cells. J Biol Chem. 2011;286:19138–19148. doi: 10.1074/jbc.M110.175786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquiere B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 49.Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquiere B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, Bifari F, Wang X, Blanco R, Tembuyser B, Cornelissen I, Bouche A, Vinckier S, Diaz-Moralli S, Gerhardt H, Telang S, Cascante M, Chesney J, Dewerchin M, Carmeliet P. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014;19:37–48. doi: 10.1016/j.cmet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Sawada N, Jiang A, Takizawa F, Safdar A, Manika A, Tesmenitsky Y, Kang KT, Bischoff J, Kalwa H, Sartoretto JL, Kamei Y, Benjamin LE, Watada H, Ogawa Y, Higashikuni Y, Kessinger CW, Jaffer FA, Michel T, Sata M, Croce K, Tanaka R, Arany Z. Endothelial PGC-1alpha mediates vascular dysfunction in diabetes. Cell Metab. 2014;19:246–258. doi: 10.1016/j.cmet.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 52.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 53.Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. 2004;94:525–533. doi: 10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- 54.Viitanen M, Sundstrom E, Baumann M, Poyhonen M, Tikka S, Behbahani H. Experimental studies of mitochondrial function in CADASIL vascular smooth muscle cells. Exp Cell Res. 2013;319:134–143. doi: 10.1016/j.yexcr.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 55.Basak NP, Roy A, Banerjee S. Alteration of mitochondrial proteome due to activation of Notch1 signaling pathway. J Biol Chem. 2014;289:7320–7334. doi: 10.1074/jbc.M113.519405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bi P, Shan T, Liu W, Yue F, Yang X, Liang XR, Wang J, Li J, Carlesso N, Liu X, Kuang S. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat Med. 2014;20:911–918. doi: 10.1038/nm.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang SH, Hla T. Post-transcriptional gene regulation by HuR and microRNAs in angiogenesis. Curr Opin Hematol. 2014;21:235–240. doi: 10.1097/MOH.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 58.Zampetaki A, Mayr M. MicroRNAs in vascular and metabolic disease. Circ Res. 2012;110:508–522. doi: 10.1161/CIRCRESAHA.111.247445. [DOI] [PubMed] [Google Scholar]
- 59.Bridge G, Monteiro R, Henderson S, Emuss V, Lagos D, Georgopoulou D, Patient R, Boshoff C. The microRNA-30 family targets DLL4 to modulate endothelial cell behavior during angiogenesis. Blood. 2012;120:5063–5072. doi: 10.1182/blood-2012-04-423004. [DOI] [PubMed] [Google Scholar]
- 60.Jiang Q, Lagos-Quintana M, Liu D, Shi Y, Helker C, Herzog W, le Noble F. miR-30a regulates endothelial tip cell formation and arteriolar branching. Hypertension. 2013;62:592–598. doi: 10.1161/HYPERTENSIONAHA.113.01767. [DOI] [PubMed] [Google Scholar]
- 61.Biyashev D, Veliceasa D, Topczewski J, Topczewska JM, Mizgirev I, Vinokour E, Reddi AL, Licht JD, Revskoy SY, Volpert OV. miR-27b controls venous specification and tip cell fate. Blood. 2012;119:2679–2687. doi: 10.1182/blood-2011-07-370635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang F, Zhu X, Hu XQ, Fang ZF, Tang L, Lu XL, Zhou SH. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int J Mol Med. 2013;31:484–492. doi: 10.3892/ijmm.2012.1200. [DOI] [PubMed] [Google Scholar]
- 63.Lou YL, Guo F, Liu F, Gao FL, Zhang PQ, Niu X, Guo SC, Yin JH, Wang Y, Deng ZF. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol Cell Biochem. 2012;370:45–51. doi: 10.1007/s11010-012-1396-6. [DOI] [PubMed] [Google Scholar]
- 64.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN, Weber C. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boucher JM, Peterson SM, Urs S, Zhang C, Liaw L. The miR-143/145 cluster is a novel transcriptional target of Jagged-1/Notch signaling in vascular smooth muscle cells. J Biol Chem. 2011;286:28312–28321. doi: 10.1074/jbc.M111.221945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, Hata A. downregulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J Biol Chem. 2011;286:28097–28110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar V, Palermo R, Talora C, Campese AF, Checquolo S, Bellavia D, Tottone L, Testa G, Miele E, Indraccolo S, Amadori A, Ferretti E, Gulino A, Vacca A, Screpanti I. Notch and NF-kB signaling pathways regulate miR-223/FBXW7 axis in T-cell acute lymphoblastic leukemia. Leukemia. 2014 doi: 10.1038/leu.2014.133. [DOI] [PubMed] [Google Scholar]
- 71.Wei Y, Schober A, Weber C. Pathogenic arterial remodeling: the good and bad of microRNAs. Am J Physiol Heart Circ Physiol. 2013;304:H1050–H1059. doi: 10.1152/ajpheart.00267.2012. [DOI] [PubMed] [Google Scholar]
- 72.Nicoli S, Knyphausen CP, Zhu LJ, Lakshmanan A, Lawson ND. miR-221 is required for endothelial tip cell behaviors during vascular development. Dev Cell. 2012;22:418–429. doi: 10.1016/j.devcel.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu J, Chen T, Yang L, Li Z, Wong MM, Zheng X, Pan X, Zhang L, Yan H. Regulation of microRNA-155 in atherosclerotic inflammatory responses by targeting MAP3K10. PLoS One. 2012;7:e46551. doi: 10.1371/journal.pone.0046551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, Weber C, Schober A. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122:4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin J, Teo S, Lam DH, Jeyaseelan K, Wang S. MicroRNA-10b pleiotropically regulates invasion, angiogenicity and apoptosis of tumor cells resembling mesenchymal subtype of glioblastoma multiforme. Cell Death Dis. 2012;3:e398. doi: 10.1038/cddis.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hashimi ST, Fulcher JA, Chang MH, Gov L, Wang S, Lee B. MicroRNA profiling identifies miR-34a and miR-21 and their target genes JAG1 and WNT1 in the coordinate regulation of dendritic cell differentiation. Blood. 2009;114:404–414. doi: 10.1182/blood-2008-09-179150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Selcuklu SD, Donoghue MT, Kerin MJ, Spillane C. Regulatory interplay between miR-21, JAG1 and 17beta-estradiol (E2) in breast cancer cells. Biochem Biophys Res Commun. 2012;423:234–239. doi: 10.1016/j.bbrc.2012.05.074. [DOI] [PubMed] [Google Scholar]
- 78.Wu J, Zheng C, Fan Y, Zeng C, Chen Z, Qin W, Zhang C, Zhang W, Wang X, Zhu X, Zhang M, Zen K, Liu Z. Downregulation of microRNA-30 facilitates podocyte injury and is prevented by glucocorticoids. J Am Soc Nephrol. 2014;25:92–104. doi: 10.1681/ASN.2012111101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su X, Qian C, Zhang Q, Hou J, Gu Y, Han Y, Chen Y, Jiang M, Cao X. miRNomes of haematopoietic stem cells and dendritic cells identify miR-30b as a regulator of Notch1. Nat Commun. 2013;4:2903. doi: 10.1038/ncomms3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katzerke C, Madan V, Gerloff D, Brauer-Hartmann D, Hartmann JU, Wurm AA, Muller-Tidow C, Schnittger S, Tenen DG, Niederwieser D, Behre G. Transcription factor C/EBPalpha-induced microRNA-30c inactivates Notch1 during granulopoiesis and is downregulated in acute myeloid leukemia. Blood. 2013;122:2433–2442. doi: 10.1182/blood-2012-12-472183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen L, Holmstrom K, Qiu W, Ditzel N, Shi K, Hokland L, Kassem M. MicroRNA-34a inhibits osteoblast differentiation and in vivo bone formation of human stromal stem cells. Stem Cells. 2014;32:902–912. doi: 10.1002/stem.1615. [DOI] [PubMed] [Google Scholar]
- 82.Du R, Sun W, Xia L, Zhao A, Yu Y, Zhao L, Wang H, Huang C, Sun S. Hypoxia-induced downregulation of microRNA-34a promotes EMT by targeting the Notch signaling pathway in tubular epithelial cells. PLoS One. 2012;7:e30771. doi: 10.1371/journal.pone.0030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pang RT, Leung CO, Ye TM, Liu W, Chiu PC, Lam KK, Lee KF, Yeung WS. MicroRNA-34a suppresses invasion through downregulation of Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells. Carcinogenesis. 2010;31:1037–1044. doi: 10.1093/carcin/bgq066. [DOI] [PubMed] [Google Scholar]
- 84.Li Y, Guessous F, Zhang Y, Dipierro C, Kefas B, Johnson E, Marcinkiewicz L, Jiang J, Yang Y, Schmittgen TD, Lopes B, Schiff D, Purow B, Abounader R. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69:7569–7576. doi: 10.1158/0008-5472.CAN-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li WB, Ma MW, Dong LJ, Wang F, Chen LX, Li XR. MicroRNA-34a targets notch1 and inhibits cell proliferation in glioblastoma multiforme. Cancer Biol Ther. 2011;12:477–483. doi: 10.4161/cbt.12.6.16300. [DOI] [PubMed] [Google Scholar]
- 86.Zhang C, Mo R, Yin B, Zhou L, Liu Y, Fan J. Tumor suppressor microRNA-34a inhibits cell proliferation by targeting Notch1 in renal cell carcinoma. Oncol Lett. 2014;7:1689–1694. doi: 10.3892/ol.2014.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang C, Yao Z, Zhu M, Ma X, Shi T, Li H, Wang B, Ouyang J, Zhang X. Inhibitory effects of microRNA-34a on cell migration and invasion of invasive urothelial bladder carcinoma by targeting Notch1. J Huazhong Univ Sci Technolog Med Sci. 2012;32:375–382. doi: 10.1007/s11596-012-0065-z. [DOI] [PubMed] [Google Scholar]
- 88.Bu P, Chen KY, Chen JH, Wang L, Walters J, Shin YJ, Goerger JP, Sun J, Witherspoon M, Rakhilin N, Li J, Yang H, Milsom J, Lee S, Zipfel W, Jin MM, Gumus ZH, Lipkin SM, Shen X. A microRNA miR-34a-regulated bimodal switch targets Notch in colon cancer stem cells. Cell Stem Cell. 2013;12:602–615. doi: 10.1016/j.stem.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li XJ, Ji MH, Zhong SL, Zha QB, Xu JJ, Zhao JH, Tang JH. MicroRNA-34a modulates chemosensitivity of breast cancer cells to adriamycin by targeting Notch1. Arch Med Res. 2012;43:514–521. doi: 10.1016/j.arcmed.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 90.Jiang P, Liu R, Zheng Y, Liu X, Chang L, Xiong S, Chu Y. MiR-34a inhibits lipopolysaccharideinduced inflammatory response through targeting Notch1 in murine macrophages. Exp Cell Res. 2012;318:1175–1184. doi: 10.1016/j.yexcr.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 91.Lefort K, Brooks Y, Ostano P, Cario-Andre M, Calpini V, Guinea-Viniegra J, Albinger-Hegyi A, Hoetzenecker W, Kolfschoten I, Wagner EF, Werner S, Dotto GP. A miR-34a-SIRT6 axis in the squamous cell differentiation network. EMBO J. 2013;32:2248–2263. doi: 10.1038/emboj.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee YM, Lee JY, Ho CC, Hong QS, Yu SL, Tzeng CR, Yang PC, Chen HW. miRNA-34b as a tumor suppressor in estrogen-dependent growth of breast cancer cells. Breast Cancer Res. 2011;13:R116. doi: 10.1186/bcr3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ahluwalia JK, Soni K, Sivasubbu S, Brahmachari V. Modeling SNP mediated differential targeting of homologous 3'UTR by microRNA. RNA Biol. 2012;9:351–360. doi: 10.4161/rna.19318. [DOI] [PubMed] [Google Scholar]
- 94.Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L, Chen C, Zhang ZG. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One. 2011;6:e23461. doi: 10.1371/journal.pone.0023461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balakrishnan I, Yang X, Brown J, Ramakrishnan A, Torok-Storb B, Kabos P, Hesselberth JR, Pillai MM. Genome-wide analysis of miRNA-mRNA interactions in marrow stromal cells. Stem Cells. 2014;32:662–673. doi: 10.1002/stem.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bae Y, Yang T, Zeng HC, Campeau PM, Chen Y, Bertin T, Dawson BC, Munivez E, Tao J, Lee BH. miRNA-34c regulates Notch signaling during bone development. Hum Mol Genet. 2012;21:2991–3000. doi: 10.1093/hmg/dds129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cama A, Verginelli F, Lotti LV, Napolitano F, Morgano A, D'Orazio A, Vacca M, Perconti S, Pepe F, Romani F, Vitullo F, di Lella F, Visone R, Mannelli M, Neumann HP, Raiconi G, Paties C, Moschetta A, Tagliaferri R, Veronese A, Sanna M, Mariani-Costantini R. Integrative genetic, epigenetic and pathological analysis of paraganglioma reveals complex dysregulation of NOTCH signaling. Acta Neuropathol. 2013;126:575–594. doi: 10.1007/s00401-013-1165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alexander MS, Kawahara G, Motohashi N, Casar JC, Eisenberg I, Myers JA, Gasperini MJ, Estrella EA, Kho AT, Mitsuhashi S, Shapiro F, Kang PB, Kunkel LM. MicroRNA-199a is induced in dystrophic muscle and affects WNT signaling, cell proliferation, and myogenic differentiation. Cell Death Differ. 2013;20:1194–1208. doi: 10.1038/cdd.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu MX, Siu MK, Liu SS, Yam JW, Ngan HY, Chan DW. Epigenetic silencing of microRNA-199b-5p is associated with acquired chemoresistance via activation of JAG1-Notch1 signaling in ovarian cancer. Oncotarget. 2014;5:944–958. doi: 10.18632/oncotarget.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang L, Dong Y, Zhu N, Tsoi H, Zhao Z, Wu CW, Wang K, Zheng S, Ng SS, Chan FK, Sung JJ, Yu J. microRNA-139-5p exerts tumor suppressor function by targeting NOTCH1 in colorectal cancer. Mol Cancer. 2014;13:124. doi: 10.1186/1476-4598-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mei J, Bachoo R, Zhang CL. MicroRNA-146a inhibits glioma development by targeting Notch1. Mol Cell Biol. 2011;31:3584–3592. doi: 10.1128/MCB.05821-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bai Y, Qian C, Qian L, Ma F, Hou J, Chen Y, Wang Q, Cao X. Integrin CD11b negatively regulates TLR9-triggered dendritic cell cross-priming by upregulating microRNA-146a. J Immunol. 2012;188:5293–5302. doi: 10.4049/jimmunol.1102371. [DOI] [PubMed] [Google Scholar]
- 103.Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J, Brabletz T. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30:770–782. doi: 10.1038/emboj.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khanna N, Ge Y, Chen J. MicroRNA-146b promotes myogenic differentiation and modulates multiple gene targets in muscle cells. PLoS One. 2014;9:e100657. doi: 10.1371/journal.pone.0100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang X, Ni W, Lei K. miR-200b suppresses cell growth, migration and invasion by targeting Notch1 in nasopharyngeal carcinoma. Cell Physiol Biochem. 2013;32:1288–1298. doi: 10.1159/000354527. [DOI] [PubMed] [Google Scholar]
- 106.Kefas B, Comeau L, Floyd DH, Seleverstov O, Godlewski J, Schmittgen T, Jiang J, diPierro CG, Li Y, Chiocca EA, Lee J, Fine H, Abounader R, Lawler S, Purow B. The neuronal microRNA miR-326 acts in a feedback loop with notch and has therapeutic potential against brain tumors. J Neurosci. 2009;29:15161–15168. doi: 10.1523/JNEUROSCI.4966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Weer A, Van der Meulen J, Rondou P, Taghon T, Konrad TA, De Preter K, Mestdagh P, Van Maerken T, Van Roy N, Jeison M, Yaniv I, Cauwelier B, Noens L, Poirel HA, Vandenberghe P, Lambert F, De Paepe A, Sanchez MG, Odero M, Verhasselt B, Philippe J, Vandesompele J, Wieser R, Dastugue N, Van Vlierberghe P, Poppe B, Speleman F. EVI1-mediated down regulation of MIR449A is essential for the survival of EVI1 positive leukaemic cells. Br J Haematol. 2011;154:337–348. doi: 10.1111/j.1365-2141.2011.08737.x. [DOI] [PubMed] [Google Scholar]
- 108.Marcet B, Chevalier B, Luxardi G, Coraux C, Zaragosi LE, Cibois M, Robbe-Sermesant K, Jolly T, Cardinaud B, Moreilhon C, Giovannini-Chami L, Nawrocki-Raby B, Birembaut P, Waldmann R, Kodjabachian L, Barbry P. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat Cell Biol. 2011;13:693–699. doi: 10.1038/ncb2241. [DOI] [PubMed] [Google Scholar]
- 109.Pang RT, Leung CO, Lee CL, Lam KK, Ye TM, Chiu PC, Yeung WS. MicroRNA-34a is a tumor suppressor in choriocarcinoma via regulation of Delta-like1. BMC Cancer. 2013;13:25. doi: 10.1186/1471-2407-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Antonellis P, Medaglia C, Cusanelli E, Andolfo I, Liguori L, De Vita G, Carotenuto M, Bello A, Formiggini F, Galeone A, De Rosa G, Virgilio A, Scognamiglio I, Sciro M, Basso G, Schulte JH, Cinalli G, Iolascon A, Zollo M. MiR-34a targeting of Notch ligand delta-like 1 impairs CD15+/CD133+ tumor-propagating cells and supports neural differentiation in medulloblastoma. PLoS One. 2011;6:e24584. doi: 10.1371/journal.pone.0024584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen L, Chen XR, Zhang R, Li P, Liu Y, Yan K, Jiang XD. MicroRNA-107 inhibits glioma cell migration and invasion by modulating Notch2 expression. J Neurooncol. 2013;112:59–66. doi: 10.1007/s11060-012-1037-7. [DOI] [PubMed] [Google Scholar]
- 112.Hudson RS, Yi M, Esposito D, Watkins SK, Hurwitz AA, Yfantis HG, Lee DH, Borin JF, Naslund MJ, Alexander RB, Dorsey TH, Stephens RM, Croce CM, Ambs S. MicroRNA-1 is a candidate tumor suppressor and prognostic marker in human prostate cancer. Nucleic Acids Res. 2012;40:3689–3703. doi: 10.1093/nar/gkr1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Furukawa S, Kawasaki Y, Miyamoto M, Hiyoshi M, Kitayama J, Akiyama T. The miR-1-NOTCH3-Asef pathway is important for colorectal tumor cell migration. PLoS One. 2013;8:e80609. doi: 10.1371/journal.pone.0080609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Turchi L, Debruyne DN, Almairac F, Virolle V, Fareh M, Neirijnck Y, Burel-Vandenbos F, Paquis P, Junier MP, Van Obberghen-Schilling E, Chneiweiss H, Virolle T. Tumorigenic potential of miR-18A* in glioma initiating cells requires NOTCH-1 signaling. Stem Cells. 2013;31:1252–1265. doi: 10.1002/stem.1373. [DOI] [PubMed] [Google Scholar]
- 115.Ghisi M, Corradin A, Basso K, Frasson C, Serafin V, Mukherjee S, Mussolin L, Ruggero K, Bonanno L, Guffanti A, De Bellis G, Gerosa G, Stellin G, D'Agostino DM, Basso G, Bronte V, Indraccolo S, Amadori A, Zanovello P. Modulation of microRNA expression in human T-cell development: targeting of NOTCH3 by miR-150. Blood. 2011;117:7053–7062. doi: 10.1182/blood-2010-12-326629. [DOI] [PubMed] [Google Scholar]
- 116.Song G, Zhang Y, Wang L. MicroRNA-206 targets notch3, activates apoptosis, and inhibits tumor cell migration and focus formation. J Biol Chem. 2009;284:31921–31927. doi: 10.1074/jbc.M109.046862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu F, Jiao Y, Zhu Y, Wang Y, Zhu J, Cui X, Liu Y, He Y, Park EY, Zhang H, Lv X, Ma K, Su F, Park JH, Song E. MicroRNA 34c gene down-regulation via DNA methylation promotes selfrenewal and epithelial-mesenchymal transition in breast tumor-initiating cells. J Biol Chem. 2012;287:465–473. doi: 10.1074/jbc.M111.280768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hashimoto Y, Akiyama Y, Otsubo T, Shimada S, Yuasa Y. Involvement of epigenetically silenced microRNA-181c in gastric carcinogenesis. Carcinogenesis. 2010;31:777–784. doi: 10.1093/carcin/bgq013. [DOI] [PubMed] [Google Scholar]
- 119.Costa FF, Seftor EA, Bischof JM, Kirschmann DA, Strizzi L, Arndt K, Bonaldo Mde F, Soares MB, Hendrix MJ. Epigenetically reprogramming metastatic tumor cells with an embryonic microenvironment. Epigenomics. 2009;1:387–398. doi: 10.2217/epi.09.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hsu SD, Tseng YT, Shrestha S, Lin YL, Khaleel A, Chou CH, Chu CF, Huang HY, Lin CM, Ho SY, Jian TY, Lin FM, Chang TH, Weng SL, Liao KW, Liao IE, Liu CC, Huang HD. miRTarBase update 2014: an information resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2014;42:D78–D85. doi: 10.1093/nar/gkt1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vergoulis T, Vlachos IS, Alexiou P, Georgakilas G, Maragkakis M, Reczko M, Gerangelos S, Koziris N, Dalamagas T, Hatzigeorgiou AG. TarBase 6.0: capturing the exponential growth of miRNA targets with experimental support. Nucleic Acids Res. 2012;40:D222–D229. doi: 10.1093/nar/gkr1161. [DOI] [PMC free article] [PubMed] [Google Scholar]