Abstract
Background
Our objective was to evaluate the effect of obesity on pre-treatment quality of life (QoL) in gynecologic oncology patients.
Methods
We analyzed collected data from an institution-wide cohort study of women with gynecologic cancers enrolled from 8/2012 to 6/2013. The Functional Assessment of Cancer Therapy-General (FACT-GP), site-specific symptom scales, and the National Institutes of Health Patient Reported Outcomes Measurement Information System (PROMIS©) global mental (GMH) and physical health (GPH) tools were administered. Survey results were linked to clinical data abstracted from medical records (demographics and comorbid conditions). Bivariate tests and multivariate linear regression models were used to evaluate factors associated with QoL scores.
Results
182 women with ovarian, uterine, cervical, and vulvar/vaginal cancers were identified; 152 (82%) were assessed prior to surgery. Mean body mass index (BMI) was 33.5 kg/m2 and race included white (120 [79%]), black (22 [15%]), and other (10 [6.5%]). 98 (64.5%) patients were obese (BMI ≥ 30). On multivariate analysis, subscales for functional (17 vs 19, P=0.04), emotional (16 vs 19, P=0.008), and social (22 vs 24, P=0.02) well-being as well as overall FACT-GP scores (77 vs 86, P=0.002) and PROMIS GPH (45 vs 49, P=0.003) were significantly lower in obese versus non-obese patients.
Conclusions
Prior to cancer treatment, obese gynecologic oncology patients have worse baseline QoL than their normal-weight counterparts. Emerging models of QoL-based cancer outcome measures may disproportionately affect populations with high obesity burden. The potential disparate impact of cancer therapy on longitudinal QoL in the obese vs. non-obese needs to be evaluated.
Keywords: Quality of life, Female Genital Neoplasms, Preoperative period, Obesity, Patient-Centered Outcomes Research
Introduction
Obesity is a growing epidemic with 35% of the general population defined as obese (body mass index (BMI) ≥ 30 by World Health Organization (WHO) criteria) (1). This trend is evident in gynecologic cancer patients, with estimated rates of obesity of 19 - 44%(2). Obesity can complicate the incidence, diagnosis, and treatment of many malignancies affecting women. Additionally, in breast, colorectal, and uterine cancer studies, obese patients have been shown to have worse outcomes (3-5), however the mechanisms driving these disparities are not well understood (6).
Health-related quality of life (QoL) measures are patient-reported, and offer a novel way to assess patient experiences without provider interpretation or modification. For patients with cancer, QoL scores can be used to assess effectiveness of an intervention, to facilitate patient-provider treatment discussions, and to predict healthcare outcomes (7). The limited studies that have evaluated the relationship between obesity and QoL in gynecologic cancers have focused largely on early stage, post-operative uterine cancer populations (8-10), and we found one study(11) to report a negative correlation between BMI and QoL that included 33 ovarian cancer patients. To our knowledge, there are no studies focused on the relationship between QoL and obesity in women with vulvar or cervical cancer and few that have examined QoL preoperatively(11-13).
In patients with breast cancers, increasing BMI has been associated with lower QoL scores assessed at initiation of chemotherapy (post-operatively), with lower scores persisting after treatment (14-16). In addition, during the survivorship phase - after completion of all cancer treatment - obesity has been consistently associated with lower QoL (8, 17). The contribution of obesity to baseline QoL of gynecologic oncology patients presenting for treatment is unknown.
The aim of this study was to evaluate the effect of obesity on pre-operative QoL in a cohort of women with gynecologic cancers.
Methods
Study Design, Enrollment, and Data Collection
We conducted an analysis of data collected for a large hospital-based observational cancer cohort. The Health Registry/Cancer Survivorship Cohort (HR/CSC) is an institutional review board approved (IRB 09-0605) University of North Carolina at Chapel Hill (UNC) Health Care registry of cancer patients that integrates a comprehensive database of clinical, epidemiological, and interview data, with repositories of biologic specimens and tumor tissue. Patients are identified and recruited through UNC Health Care oncology outpatient clinics with the following eligibility criteria: age 18 years or older; North Carolina mailing address; and English or Spanish language proficiency. Patients who are unable to provide informed consent or participate in interview questionnaires are excluded. For this analysis, eligibility was further restricted to HR/CSC patients recruited through the gynecologic oncology clinics and who completed the baseline interview prior to any cancer treatments.
Interviews were conducted within 2 weeks of enrollment by trained staff using a computer-assisted telephone interview software tool specifically developed for the HR/CSC. Interview questionnaire topics include medical and social histories, and general and cancer-specific health assessments. The following structured and validated questionnaires were included in the analysis: Functional Assessment of Cancer Therapy - General Population (FACT-GP), NIH Patient Reported Outcomes Measurement Information System (PROMIS©) global mental (GMH) and physical health (GPH), and cancer-specific FACTs (Endometrial – En, Ovarian –O, Vaginal/Vulvar – V, Cervical – Cx). The FACT-GP version 4 is a 21-item scale that measures health related QoL using four subscales: physical (PWB), functional (FWB), emotional (EWB), and social (SWB) wellbeing. The cancer-specific FACT scales include the FACT-GP in addition to multi-item subscales that measure cancer site-specific symptoms(18-21). The PROMIS© v1.0 global is a 10-item scale that measures the domains of fatigue, physical function, pain, emotional distress, and social health(22). Minimally important score differences, that translate to meaningful clinical differences, have been defined for the FACT-GP (5 points)(23) and PROMIS global (4-6 points)(24) scales.
Patient age, self-reported race/ethnicity, and employment status were also available from the HR/CSC baseline interview. The electronic medical record was reviewed (physician, nursing, and case management staff documentation) to abstract clinical data at the time of new patient visit (BMI, co-morbid conditions, mental health history, and cancer site). Insurance status, at the time of new patient visit, was also abstracted from the medical record. All medical record data was limited to encounters at the UNC Health Care. Data from all sources was merged using an honest broker model. The HR/CSC subsequently provided a de-identified analytic file containing the medical record, demographic, and QoL data for analysis.
Statistical Analysis
Summary statistics were generated using simple frequencies for categorical variables and mean/medians for continuous variables. Composite variables of major medical comorbidity, and mental health diagnoses were created. The major comorbidity variable included notation in the record for at least one of these conditions: diabetes, pulmonary disease (chronic obstructive pulmonary disease (COPD), restrictive lung disease, home oxygen requirement), cardiac disease (congestive heart failure, history of myocardial infarction, coronary artery disease), immunocompromised states (Human immunodeficiency virus seropositive, chronic steroid use), and chronic kidney disease. For the composite mental health variable, we combined any notated diagnosis of anxiety, depression, and chronic pain. QoL scores were treated as continuous variable and were analyzed in relation to obesity by WHO classification and with BMI as a continuous variable. Baseline characteristics between obese vs. non-obese groups were compared using two-sided Fisher's exact tests for categorical variables, and Student's t-tests for continuous variables. Simple linear regression was used to evaluate the relationship between potential confounders and QoL scores. The relationship between obesity and the various QoL domains was then evaluated using multivariate linear regression models with obesity defined in a binary fashion (BMI ≥ 30).
In multivariate modeling, both clinical factors (major comorbidity, anxiety/depression/pain, cancer site, cancer stage) and demographic factors (age, insurance status, employment) were explored as possible confounders. Insurance status and employment were included in this list as we considered them to be broad surrogate markers of socioeconomic status. Bivariate relationships between each factor and obesity (our primary exposure) and QoL domain scores (our primary outcome) were analyzed and noted. Factors that were both 1) significantly associated with QoL scores and 2) differed substantially between obese compared to non-obese groups, were considered to be likely confounders, although all were included in the initial model. Using multiple linear regression, we constructed a full model with all identified possible confounders (anxiety/depression/pain, major comorbidity, insurance status, employment, cancer site, age, and cancer stage) and systematically removed non-significant (p>0.05) variables that did not meaningfully influence (change in odds ratio > 10%) the primary relationship between obesity and the specific QoL domain scores. The AIC statistic was calculated to confirm the best model fit. Using this best fit model, multivariate adjusted means were calculated using linear regression and setting covariate values to the sample mean. Additionally, oneway analysis of variance (ANOVA) was used to analyze QoL scores across different cancer sites. A graph demonstrating the relationship between BMI and overall FACT score was constructed. All testing was based on a significance level of p< 0.05 and we did not include correction for multiple testing.
Results
Demographic and clinical characteristics
Of 182 gynecologic oncology patients enrolled in the HR/CSC, there were 152 who met inclusion criteria for the study cohort. Of the 30 excluded, 27 did not complete baseline interviews (refusal (n=20), contacted after surgery (n=6), incomplete interview (n=1)) and 3 received treatment at outside facilities. Median age was 58.9 years (range 28 – 90, SD 13.0). There was substantial racial, economic, and social diversity, with 32 patients of racial/ethnic minorities (21%), 44 patients having either Medicare/Medicaid-only or who were uninsured (29%), as well as a variety of employment statuses, including 77 patients who were unemployed or retired (50.1%).There were 44 patients (29.0%) with no documented medical comorbidity, 65 (42.7%) with some medical comorbidity, and 43 (28.3%) with major medical comorbidity as defined by the composite variable. With regard to mental health, 39 patients (25.7%) had diagnoses of depression, anxiety, or chronic pain. All gynecologic cancer sites and stages were represented (Table 1).
Table 1.
Overall Study Cohort Characteristics
| Variable | N | % | |
|---|---|---|---|
| Age | 18 - 30 years | 3 | 2.0 |
| 31 – 50 years | 30 | 19.7 | |
| 51 – 70 years | 92 | 60.5 | |
| Over 70 years | 27 | 17.8 | |
| BMI | |||
| Under 25.0 | 27 | 17.8 | |
| 25.0 – 29.9 | 27 | 17.8 | |
| 30.0 – 39.9 | 68 | 44.7 | |
| Over 40.0 | 30 | 19.7 | |
| Race1 | |||
| White | 120 | 79.0 | |
| Black | 22 | 14.5 | |
| Other | 10 | 6.5 | |
| Insurance | |||
| Private | 108 | 71.0 | |
| Uninsured | 32 | 21.0 | |
| Medicare only | 7 | 4.6 | |
| Medicaid | 5 | 3.2 | |
| Employment | |||
| Unemployed/Retired | 77 | 50.1 | |
| Full Time | 51 | 33.6 | |
| Part Time | 24 | 15.8 | |
| Marital Status | |||
| Unknown | 68 | 44.5 | |
| Married/Partnered | 67 | 44.0 | |
| Single/Divorced/Widowed | 17 | 11.2 | |
| Past Medical History | |||
| HTN | 75 | 49.3 | |
| Prior Laparotomy | 35 | 23.0 | |
| Diabetes2 | 30 | 19.7 | |
| History of Cancer | 28 | 18.4 | |
| Substance Abuse | 18 | 11.8 | |
| Cardiac Disease (CHF/CAD/MI)2 | 15 | 9.9 | |
| Arthritis | 13 | 8.6 | |
| Pulmonary Disease2 | 4 | 2.6 | |
| Chronic Kidney Disease2 | 2 | 1.3 | |
| Immunocompromise2 | 2 | 1.3 | |
| Major Comorbidity3 | 43 | 28.3 | |
| Mental Health | |||
| Depression | 24 | 15.8 | |
| Anxiety | 18 | 11.8 | |
| Chronic Pain | 5 | 3.3 | |
| Cancer Site | |||
| Uterine | 94 | 61.8 | |
| Ovarian | 26 | 17.1 | |
| Cervical | 17 | 11.1 | |
| Vulva/Vaginal | 9 | 5.9 | |
| Gyn NOS4 | 3 | 2.0 | |
| GI5 | 3 | 2.0 | |
| Cancer Stage | |||
| Stage I - II | 98 | 64.5 | |
| Stage III - IV | 46 | 30.3 | |
| Unstaged | 8 | 5.3 | |
White (non-Hispanic), Black (Non-Hispanic), and Other: Hispanic, Asian, Native American, and Asian participants. Self-reported
Indicates inclusion in Major Comorbidity composite variable. All conditions abstracted from the medical record. CHF – congestive heart failure, CAD – coronary artery disease, MI – myocardia infarction.
Indicates inclusion in Major Comorbidity composite variable. All conditions abstracted from the medical record. CHF – congestive heart failure, CAD – coronary artery disease, MI – myocardia infarction.
Gyn Not Otherwise Specified – Either gynecologic cancer of unknown origin (2) or dual gynecologic primaries (1).
Gastrointestinal – 3 patients with suspected ovarian cancer that had primary GI malignancy on final pathology.
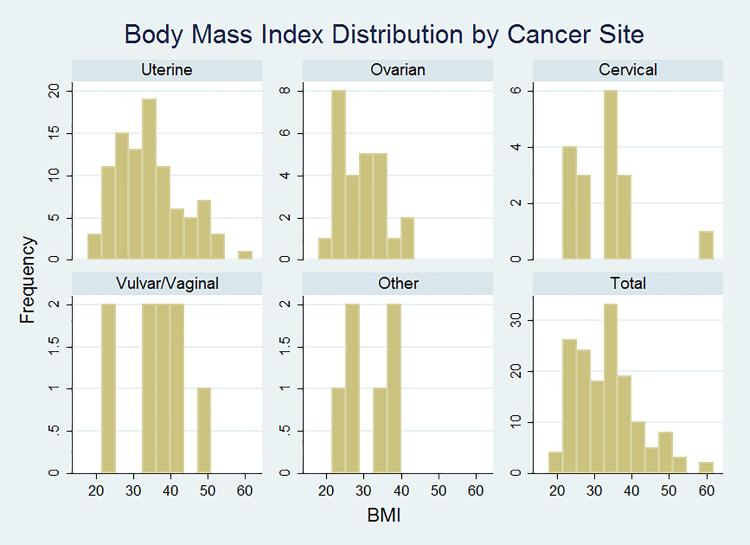
The majority of patients, (98/152, 64.5%), were obese (BMI ≥ 30 kg/m2) (Figure 1). BMI values ranged from 18 to 62, with a median BMI of 33.5 (IQR 26.5 – 38.5). Obesity was prevalent in all cancer sites, including cervical and vulvar cancers (Figure 1). Of the possible confounders (covariates) we identified, mean age, insurance status, and the presence of major comorbidity were noted to differ among the obese vs. non-obese (Table 2), although only age was statistically different. On bivariate analysis of possible confounders, cancer site, in particular, was strongly associated with several QoL domains (Table 3). In addition, age, anxiety/depression/pain, and insurance status were also related to QoL domains (not shown) and considered in multivariate modeling.
Figure 1.
The distribution of BMI of patients in within each cancer site. Other includes patients with gynecologic not-otherwise-specified (GYN-NOS) and gastrointestinal tumors. Obesity was prevalent in all cancer sites.
Table 2.
Distribution of covariates by Obese vs. Non-Obese (BMI ≥ 30)
| Non-obese (%) | Obese (%) | p-value | |
|---|---|---|---|
| Anxiety/Depression/Chronic Pain | 24 | 27 | 0.7 |
| Major Comorbidity | 19 | 34 | 0.05 |
| Uninsured | 13 | 25 | 0.07 |
| Employment | |||
| Unemployed/Retired | 50 | 50 | |
| Part-time Employed | 19 | 14 | |
| Full-time Employed | 31 | 35 | 0.8 |
| Cancer site | |||
| Uterine | 54 | 66 | |
| Ovarian | 24 | 13 | |
| Cervical | 13 | 10 | |
| Vulvar/Vaginal | 4 | 7 | |
| Other | 6 | 3 | 0.3 |
| Age (mean yrs) | 62 | 57 | 0.02 |
| Stage | |||
| Stage I – II | 56 | 69 | |
| Stage III – IV | 39 | 25 | |
| Unstaged | 6 | 5 | 0.2 |
Table 3.
Health Related Quality of Life Domain Scores by Gynecologic Cancer Site
| QoL Instrument | Cancer Site Mean (SD) | |||||
|---|---|---|---|---|---|---|
| Uterine N=94 | Ovarian N=26 | Cervical N=17 | Vulvar/Vaginal N=9 | p-value | ||
| FACT – GP1 | 85 (17) | 75 (18) | 66 (18) | 65 (23) | 0.0001 | |
| Functional | 20 (7) | 15 (6) | 14 (7) | 13 (8) | 0.0001 | |
| Physical | 23 (6) | 20 (7) | 18 (8) | 18 (7) | 0.002 | |
| Emotional | 18 (5) | 17 (7) | 13 (8) | 14 (7) | 0.010 | |
| Social | 23 (5) | 24 (5) | 21 (4) | 20 (3) | 0.14 | |
| PROMIS2 Global | ||||||
| Physical Health | 48 (8) | 44 (10) | 43 (7) | 41 (9) | 0.02 | |
| Mental Health | 50 (8) | 50 (8) | 46 (7) | 41 (6) | 0.002 | |
Functional Assessment of Cancer Therapy – General
Patient Reported Outcomes Measurement Information System
QoL and Obesity
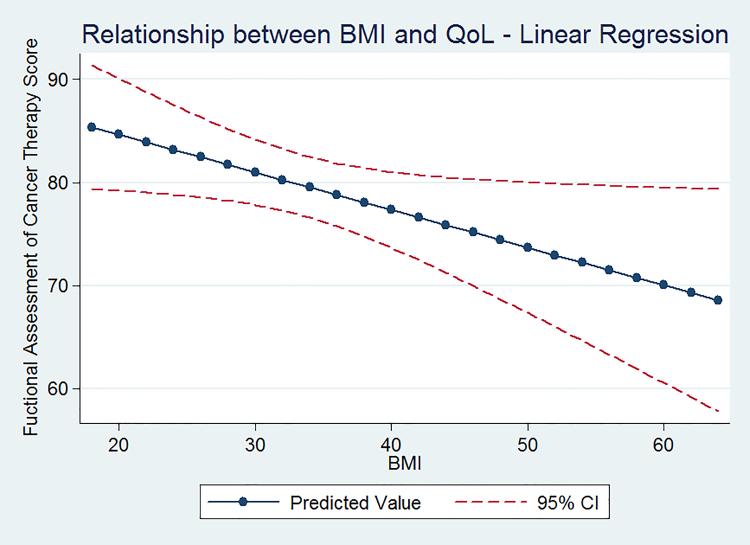
Mean QoL scores for each WHO-defined BMI category are listed in Table 4. On univariate analysis, mean QoL domain scores decreased with increasing BMI category, with the PROMISGPH scale reaching statistical significance. The linear relationship between FACT-GP score and BMI (unadjusted) is shown in Figure 2. A binary (obese vs. non-obese) categorization was used in multivariate modeling. In our final model, adjusted for age, insurance status, and cancer site, obese patients had lower mean overall FACT-GP scores, as well as lower mean functional, emotional, and social wellbeing scores, compared to non-obese patients (Table 5). Mean PROMIS GPH were also lower in obese patients, compared to non-obese (Table 5). For each 5 point increase in BMI, there was a concomitant 1.7 point decrease in FACT score.
Table 4.
Mean Health Related Quality of Life Score for Each BMI Category – Unadjusted Means (SD)
| Normal Weight N=27 | Overweight N=27 | Class I Obese N=36 | Class II Obese N=62 | p-value | ||
|---|---|---|---|---|---|---|
| FACT-GP1 | 85 (13) | 86 (14) | 78 (18) | 76 (22) | 0.06 | |
| Functional | 18 (8) | 20 (60) | 16 (7) | 18 (8) | 0.2 | |
| Physical | 22 (5) | 24 (6) | 22 (6) | 20 (8) | 0.07 | |
| Emotional | 19 (5) | 19 (4) | 17 (6) | 16 (7) | 0.09 | |
| Social | 25 (3) | 23 (4) | 22 (5) | 22 (5) | 0.05 | |
| PROMIS2 Global | ||||||
| Physical Health | 49 (8) | 50 (9) | 46 (9) | 44 (8) | 0.01 | |
| Mental Health | 51 (8) | 51 (7) | 50 (9) | 47 (7) | 0.06 |
Functional Assessment of Cancer Therapy – General
Patient Reported Outcomes Measurement Information System
Figure 2.
The relationship between BMI and the Functional Assessment of Cancer Therapy – General (FACT-GP) scores. As BMI increased, overall health related quality of life as assessed by the FACT-GP, decreased.
Table 5.
Mean Health Related Quality of Life Score for Obese vs. Non-Obese – Adjusted analysis1
| HRQoL | Non-Obese | Obese | p-value | |
|---|---|---|---|---|
| FACT-GP2 | 86 | 77 | .002 | |
| Functional subscale | 19 | 17 | .04 | |
| Physical subscale | 23 | 21 | .09 | |
| Emotional subscale | 19 | 16 | .008 | |
| Social subscale | 24 | 22 | .02 | |
| Site-specific symptom scales3 | ||||
| Ovarian | 30 | 29 | .67 | |
| Endometrial | 54 | 53 | .55 | |
| Vulvar/Vaginal/Cervical | 38 | 41 | .40 | |
| PROMIS4 Global | ||||
| Physical Health | 49 | 45 | .003 | |
| Mental Health | 51 | 48 | .08 | |
Adjusted for age, insurance status, and cancer site except where noted
Functional Assessment of Cancer Therapy – General
Adjusted for age only due to subgroup sizes
Patient Reported Outcomes Measurement Information System
Discussion
In this prospective cohort study of cancer patients, we found that obese uterine, ovarian, cervical, and vulvar/vaginal cancer patients have worse baseline QoL scores on both traditional FACT-GP and the PROMIS© Global scales, compared to their normal weight counterparts. This relationship is present at the time of diagnosis, prior to any gynecologic oncology treatment initiation.
These findings corroborate and add to previous work published on the relationship of QoL and obesity. In a survey of women undergoing surgery for pelvic masses and/or suspected gynecologic malignancy, higher BMI was correlated with lower physical and social wellbeing (11). Endometrial cancer is known to have close correlation with obesity status, with increasing BMI associated with decreased physical and functional well-being and fatigue in early stage patients (8). Other studies have echoed these findings in endometrial cancer survivors (9, 25). These studies are limited in that they were conducted with cohorts of primarily Caucasian women, studied only one cancer site, and did not use multiple surveys to assess QoL. In this study, we use two different systems of measurement – the FACT and the PROMIS scales – and report on both global and cancer site-specific domain scores. The FACT scales have been in use for decades and are increasingly incorporated as critical endpoints in major clinical trials. A popular QoL endpoint in clinical trials is the trial outcome index (TOI), which is a summation of the physical and functional wellbeing subscales of the FACT, along with the cancer-site specific symptom index. This report indicates that obesity significantly impacts each component of the TOI. Clinical trial populations that underrepresent obesity may not adequately reflect expected QoL outcomes in the general population. In addition, as an observational study of a clinic population, we noted significant racial and socioeconomic diversity, allowing our results to be more reflective of the general population than those from clinical trials, including those within gynecologic oncology(26, 27). The PROMIS scales are a more recent health-related QoL instrument and data has been reported on only a small population of gynecologic oncology patients. To our knowledge our cohort represents the largest study of gynecologic cancer patients to use this newly developed instrument (24, 28, 29).
Defining the change in score on a QoL scale that signifies a clinically important change for patient and practitioner can be challenging. The concept of minimal clinically important difference (MCIDs) is a method used to determine this. In this study, obese patients had a FACT-GP score of 8.5 points lower than their non-obese counterparts. This is well beyond the minimally clinically important difference of 5 points in this scale (23) which represents a one-unit change in Gynecologic Oncology Group (GOG) performance status (30). In contrast, the statistical difference in PROMIS Global physical health score of 4.3 between obese and normal weight patients may not hold clinical significance, as the MCID for this scale is estimated to be between 4-6 points (24). As a measure of global health developed for general populations, this scale may not be sensitive to the impact of obesity in patients with a new cancer diagnosis.
By including all gynecologic cancer sites in our cohort, we were able to explore differences in baseline QoL by cancer site. In fact, exploratory analyses suggested that patients with cervical and vulvar cancers had much lower QoL scores prior to treatment initiation than patients with uterine and ovarian cancer. This study was not designed to evaluate this difference, but these are intriguing findings to follow up in a larger study. The etiology of these QoL differences may be site-specific. For example, disparate QoL in cervical cancer may be due to a high incidence of poverty in this population(31), whereas vulvar cancer is frequently characterized by delayed diagnosis long after symptom onset (32, 33). Regardless of etiology, pre-treatment QoL scores have been associated with surgical and medical (chemotherapy) cancer treatment outcomes (34-38), this may indicate that certain gynecologic cancer patients are at differential risk of poor treatment outcomes.
Strengths of this study include the racial and socioeconomic diversity that is the reality of the majority of gynecologic oncology practices and the inclusion of a broad representation of the various gynecologic malignancies. This study is limited by enrollment at a single institution. The sample size was adequate for evaluating obesity in a binary fashion, but we were unable to construct multivariate models within each WHO-defined obesity sub-classification. In addition, QoL scores were collected at a single point in time, prior to treatment, and so we cannot assess changes in scores over time or the impact that surgery, chemotherapy and radiation, alone or in combination, may play. Finally not all statistical differences seen may be clinical meaningful ones as the MCIDs for each scale were not initially evaluated in the pre-operative period and this time frame may have unique pressures on patients that are not captured in the scales.
It is important to understand the impact of QoL on treatment outcomes and cancer survivorship. There have been calls for more studies evaluating the effect of obesity on QoL before and after cancer treatment (6, 39). This study is an important step in delineating this relationship. If QoL scores are to be an outcome measure of care delivery in our gynecologic cancer patients, and in light of our findings, we are obligated to investigate the contribution of obesity to these outcomes. Further research should address the QoL trends of obese patients during and after gynecologic cancer treatment. As we transition many of our patients from active therapy to follow-up, it will be important to assess the efficacy and sustainability of lifestyle modifications on quality of life and ultimately its effects on patient survival.
Acknowledgements
The authors thank the HR/CSC participants for their important contributions. The UNC Health Registry/Cancer Survivorship Cohort is funded in part by the UNC Lineberger Comprehensive Cancer Center's University Cancer Research Fund.
This project was reviewed and approved by the Human Research Protections Program (IRB Number: 09-0605) at the University of North Carolina at Chapel Hill.
Funding:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA116339. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no financial disclosures.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA : the journal of the American Medical Association. 2006;295(13):1549–55. doi: 10.1001/jama.295.13.1549. Epub 2006/04/06. [DOI] [PubMed] [Google Scholar]
- 2.Beesley VL, Eakin EG, Janda M, Battistutta D. Gynecological cancer survivors' health behaviors and their associations with quality of life. Cancer causes & control : CCC. 2008;19(7):775–82. doi: 10.1007/s10552-008-9140-y. Epub 2008/03/07. [DOI] [PubMed] [Google Scholar]
- 3.Arem H, Park Y, Pelser C, Ballard-Barbash R, Irwin ML, Hollenbeck A, et al. Prediagnosis body mass index, physical activity, and mortality in endometrial cancer patients. Journal of the National Cancer Institute. 2013;105(5):342–9. doi: 10.1093/jnci/djs530. Epub 2013/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62(6):933–47. doi: 10.1136/gutjnl-2013-304701. [DOI] [PubMed] [Google Scholar]
- 5.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123(3):627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz KH, Neuhouser ML, Agurs-Collins T, Zanetti KA, Cadmus-Bertram L, Dean LT, et al. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst. 2013;105(18):1344–54. doi: 10.1093/jnci/djt223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia SF, Cella D, Clauser SB, Flynn KE, Lad T, Lai JS, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(32):5106–12. doi: 10.1200/JCO.2007.12.2341. Epub 2007/11/10. [DOI] [PubMed] [Google Scholar]
- 8.Fader AN, Frasure HE, Gil KM, Berger NA, von Gruenigen VE. Quality of life in endometrial cancer survivors: what does obesity have to do with it? Obstet Gynecol Int. 2011;2011:308609. doi: 10.1155/2011/308609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oldenburg CS, Boll D, Nicolaije KA, Vos MC, Pijnenborg JM, Coebergh JW, et al. The relationship of body mass index with quality of life among endometrial cancer survivors: a study from the population-based PROFILES registry. Gynecologic oncology. 2013;129(1):216–21. doi: 10.1016/j.ygyno.2012.12.041. Epub 2013/01/09. [DOI] [PubMed] [Google Scholar]
- 10.von Gruenigen VE, Huang HQ, Gil KM, Gibbons HE, Monk BJ, Rose PG, et al. Assessment of factors that contribute to decreased quality of life in Gynecologic Oncology Group ovarian cancer trials. Cancer. 2009;115(20):4857–64. doi: 10.1002/cncr.24520. Epub 2009/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gil KM, Gibbons HE, Jenison EL, Hopkins MP, von Gruenigen VE. Baseline characteristics influencing quality of life in women undergoing gynecologic oncology surgery. Health and quality of life outcomes. 2007;5:25. doi: 10.1186/1477-7525-5-25. Epub 2007/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Gruenigen VE, Frasure HE, Jenison EL, Hopkins MP, Gil KM. Longitudinal assessment of quality of life and lifestyle in newly diagnosed ovarian cancer patients: the roles of surgery and chemotherapy. Gynecologic oncology. 2006;103(1):120–6. doi: 10.1016/j.ygyno.2006.01.059. Epub 2006/03/25. [DOI] [PubMed] [Google Scholar]
- 13.von Gruenigen VE, Gil KM, Frasure HE, Jenison EL, Hopkins MP. The impact of obesity and age on quality of life in gynecologic surgery. American journal of obstetrics and gynecology. 2005;193(4):1369–75. doi: 10.1016/j.ajog.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Elme A, Utriainen M, Kellokumpu-Lehtinen P, Palva T, Luoto R, Nikander R, et al. Obesity and physical inactivity are related to impaired physical health of breast cancer survivors. Anticancer research. 2013;33(4):1595–602. Epub 2013/04/09. [PubMed] [Google Scholar]
- 15.Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118(8 Suppl):2277–87. doi: 10.1002/cncr.27466. Epub 2012/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang P, Tan KS, Troxel AB, Rengan R, Freedman G, Lin LL. High body mass index is associated with worse quality of life in breast cancer patients receiving radiotherapy. Breast cancer research and treatment. 2013;141(1):125–33. doi: 10.1007/s10549-013-2663-2. Epub 2013/08/15. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard CM, Stein K, Courneya KS. Body mass index, physical activity, and health-related quality of life in cancer survivors. Med Sci Sports Exerc. 2010;42(4):665–71. doi: 10.1249/MSS.0b013e3181bdc685. [DOI] [PubMed] [Google Scholar]
- 18.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9. doi: 10.1200/JCO.1993.11.3.570. Epub 1993/03/01. [DOI] [PubMed] [Google Scholar]
- 19.Cella D. FACT-En (Version 4) 1997.
- 20.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, Webster K, Cella D, Hu S, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol. 2001;19(6):1809–17. doi: 10.1200/JCO.2001.19.6.1809. Epub 2001/03/17. [DOI] [PubMed] [Google Scholar]
- 21.Janda M, Obermair A, Cella D, Crandon AJ, Trimmel M. Vulvar cancer patients' quality of life: a qualitative assessment. Int J Gynecol Cancer. 2004;14(5):875–81. doi: 10.1111/j.1048-891X.2004.14524.x. Epub 2004/09/14. [DOI] [PubMed] [Google Scholar]
- 22.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–80. doi: 10.1007/s11136-009-9496-9. Epub 2009/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringash J, O'Sullivan B, Bezjak A, Redelmeier DA. Interpreting clinically significant changes in patient-reported outcomes. Cancer. 2007;110(1):196–202. doi: 10.1002/cncr.22799. Epub 2007/06/05. [DOI] [PubMed] [Google Scholar]
- 24.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64(5):507–16. doi: 10.1016/j.jclinepi.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smits A, Lopes A, Das N, Bekkers R, Galaal K. The impact of BMI on quality of life in obese endometrial cancer survivors: Does size matter? Gynecologic oncology. 2014;132(1):137–41. doi: 10.1016/j.ygyno.2013.11.018. Epub 2013/11/23. [DOI] [PubMed] [Google Scholar]
- 26.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–45. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kornblith AB, Huang HQ, Walker JL, Spirtos NM, Rotmensch J, Cella D. Quality of life of patients with endometrial cancer undergoing laparoscopic international federation of gynecology and obstetrics staging compared with laparotomy: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27(32):5337–42. doi: 10.1200/JCO.2009.22.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenton BW, Palmieri P, Diantonio G, Vongruenigen V. Application of Patient-Reported Outcomes Measurement Information System to chronic pelvic pain. Journal of minimally invasive gynecology. 2011;18(2):189–93. doi: 10.1016/j.jmig.2010.12.001. Epub 2011/02/08. [DOI] [PubMed] [Google Scholar]
- 29.Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS). J Clin Epidemiol. 2010;63(11):1195–204. doi: 10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof. 2005;28(2):172–91. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 31.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975-2000. Cancer. 2004;101(5):1051–7. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 32.Senn B, Gafner D, Happ MB, Eicher M, Mueller MD, Engberg S, et al. The unspoken disease: symptom experience in women with vulval neoplasia and surgical treatment: a qualitative study. Eur J Cancer Care (Engl) 2011;20(6):747–58. doi: 10.1111/j.1365-2354.2011.01267.x. [DOI] [PubMed] [Google Scholar]
- 33.Vandborg MP, Christensen RD, Kragstrup J, Edwards K, Vedsted P, Hansen DG, et al. Reasons for diagnostic delay in gynecological malignancies. Int J Gynecol Cancer. 2011;21(6):967–74. doi: 10.1097/IGC.0b013e31821d2770. [DOI] [PubMed] [Google Scholar]
- 34.von Gruenigen VE, Huang HQ, Gil KM, Frasure HE, Armstrong DK, Wenzel LB. The association between quality of life domains and overall survival in ovarian cancer patients during adjuvant chemotherapy: a Gynecologic Oncology Group Study. Gynecol Oncol. 2012;124(3):379–82. doi: 10.1016/j.ygyno.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wenzel L, Huang HQ, Monk BJ, Rose PG, Cella D. Quality-of-life comparisons in a randomized trial of interval secondary cytoreduction in advanced ovarian carcinoma: a Gynecologic Oncology Group study. Journal of Clinical Oncology. 2005;23(24):5605–12. doi: 10.1200/JCO.2005.08.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wenzel LB, Huang HQ, Armstrong DK, Walker JL, Cella D, Gynecologic Oncology G. Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: a Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2007;25(4):437–43. doi: 10.1200/JCO.2006.07.3494. [DOI] [PubMed] [Google Scholar]
- 37.Ihemelandu CU, McQuellon R, Shen P, Stewart JH, Votanopoulos K, Levine EA. Predicting postoperative morbidity following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CS+HIPEC) with preoperative FACT-C (Functional Assessment of Cancer Therapy) and patient-rated performance status. Ann Surg Oncol. 2013;20(11):3519–26. doi: 10.1245/s10434-013-3049-8. Epub 2013/06/12. [DOI] [PubMed] [Google Scholar]
- 38.Doll KM, Snavely AC, Kalinowski A, Irwin DE, Bensen JT, Bae-Jump V, et al. Preoperative quality of life and surgical outcomes in gynecologic oncology patients: A new predictor of operative risk? Gynecologic oncology. 2014 doi: 10.1016/j.ygyno.2014.04.002. Epub 2014/04/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tao W, Lagergren J. Clinical management of obese patients with cancer. Nature reviews Clinical oncology. 2013;10(9):519–33. doi: 10.1038/nrclinonc.2013.120. Epub 2013/07/17. [DOI] [PubMed] [Google Scholar]