Abstract
Approximately 10% of infants born in the United States are of low birth weight. Growth failure during the neonatal period is a common occurrence in low birth weight infants due to their inability to tolerate full feeds, concerns about advancing amino acid supply, and high nutrient requirements for growth. An improved understanding of the nutritional regulation of growth during this critical period of development is vital for the development of strategies to improve lean growth. Past studies with animal models have demonstrated that muscle protein synthesis is increased substantially following a meal and that this increase is due to the postprandial rise in amino acids as well as insulin, which independently stimulate protein synthesis in a mammalian target of rapamycin (mTOR)-dependent manner. Further studies have elucidated that leucine, in particular, as well as its metabolites, α-ketoisocaproic acid and β-hydroxy-β-methylbutyrate, have unique anabolic properties. Supplementation with leucine, provided either parenterally or enterally, has been shown to enhance muscle protein synthesis in neonatal pigs, making it an ideal candidate for stimulating growth of low birth weight infants.
Keywords: amino acids, leucine, low birth weight, mTOR, neonatal, protein synthesis
Introduction
The rate of growth during the neonatal period is higher than at any other stage of postnatal development (Davis et al. 1996). The majority of this grow this comprised of skeletal muscle (Davis and Fiorotto 2009) and, therefore, muscle isa significant determinant of amino acid and energy requirements at this stage of development. Approximately 10% of newborn infants in the United States are of low birth weight (Ehrenkranz 2007). Many remain small at hospital discharge and are of short stature as adults (Ehrenkranz 2007). Poor growth in the immediate neonatal period is due to a number of factors including reduced nutrient intake as a result of overall feeding intolerance or inability to handle full protein feeding. An understanding of the mechanisms underlying growth in the neonatal period, especially with respect to muscle growth, is crucial for the development of strategies to improve survival and long-term health of low birth weight infants. Previously, we demonstrated that the increase in muscle protein synthesis following a meal is due to the postprandial rise in amino acids as well as insulin (Wray-Cahen et al. 1998; Davis et al. 2002; O'Connor et al. 2003). In particular, the branched-chain amino acid, leucine, and its metabolites, α-ketoisocaproic acid and β-hydroxy-β-methylbutyrate, haveunique anabolic properties and the potential to enhance protein synthesis and growth during the neonatal period. In this review we will discuss the regulation of skeletal muscle growth during the neonatal period, through mTOR-related pathways, and how nutrient ingestion, particularly of leucine and its metabolites, can be used to modulate the protein synthetic machinery in muscle.
Muscle growth and development in the neonatal period
The neonatal period is characterized by a rapid rate of growth and is, therefore, a critical stage of development (Senterre and Rigo 2013). Growth faltering during this time often leads to both short- and long-term consequences including short stature, neuronal deficits (Ford et al. 2000; Hay 2008), and increased risk of cardiovascular disease and metabolic syndrome, including obesity, in later life (Barker 2004; Yajnik 2004; Senterre and Rigo 2013; Brown 2014). For the low birth weight infant (≤ 2500 g; Ehrenkranz 2007) the risk of poor growth and adverse developmental outcomes is especially high (Brown 2014).Regardless of technological advances and improvements in the nutritional management of these infants, many low birth weight infants are discharged weighing less than the 10th percentile of intrauterine growth standards (Ehrenkranz et al. 1999). Due to concerns regarding the ability of infants to tolerate full feeds or to metabolize nutrients and the potential for hyperammonemia, metabolic acidosis, and necrotizing enterocolitis (Johnson et al. 1972; Thureen and Hay 2001; Hay 2008; Abdelhamid et al. 2011), the move to feed with higher protein content is often delayed resulting in reduced nutrient consumption and a failure to meet the infant’s requirements (Senterre and Rigo 2013). This is despite evidence of improved growth with greater parenteral infusion of amino acids (Thureen et al. 2003; Ibrahim et al. 2004) ordietary protein intake (Kashyap et al. 1986, 1988; Cooke et al. 2006; Premji et al. 2006). A more complete understanding of nutrient metabolism in infants, as well as the physiology of muscle growth, will aid in the development of nutritional interventions aimed at optimization of lean growth during the neonatal period.
In the neonate, skeletal muscle represents 30% of body mass (Davis and Fiorotto 2009) and is the most rapidly growing body compartment(Davis et al. 1989; Davis et al. 1996). The muscle protein pool, therefore, is an important determinant of overall body protein metabolism and amino acid requirements in the young, growing animal (Liu and Barrett 2002; Lobley 2003). It has been demonstrated previously that feeding stimulates protein synthesis in all tissues in the young rat (Davis et al. 1989), pig (Burrin et al. 1992; Davis et al. 1993), and human infant (Denne et al. 1991) and that this rise is most pronounced for skeletal muscle (Davis and Fiorotto 2009). The response in protein synthesis to a meal is rapid, reaching peak activation within 30 min and remaining elevated for a minimum of 2 h post-meal (Wilson et al. 2009).
Role of amino acids and insulin in the post-prandial stimulation of muscle protein synthesis
A number of potential positive regulators of muscle protein synthesis have been discussed previously (Lobley 1998; Liu and Barrett 2002) including IGF-1, insulin, glucose, growth hormone, and amino acids. Thus, the rise in plasma levels of insulin, glucose, and amino acids in response to a mealare of potential importance for the regulation of protein synthesis. Indeed, the circulating levels of these plasma metabolites were shown by Wilson et al. (2009) to parallel the changes in protein synthesis in muscle tissue. The importance of the rise in plasma metabolites in response to a meal with respect to initiation of protein synthesis was also demonstrated by El-Kadi et al. (2012) who examined the effect of bolus versus continuous feeding in young pigs. It was shown that bolus feeding, which elicits a rapid rise in plasma insulin, glucose, and amino acids, resulted in greater rates of muscle protein synthesis than in those pigs fed an equivalent amount of the same diet continuously, which produced steady and low plasma levels of the same metabolites (El-Kadi et al. 2012). The importance of the postprandial rise in plasma metabolites was also demonstrated in studies which showed that a twofold increase in plasma aminoacidemia is required in order to increase protein synthesis (Tessari et al. 1987; Giordano et al. 1996; Dangin et al. 2001).
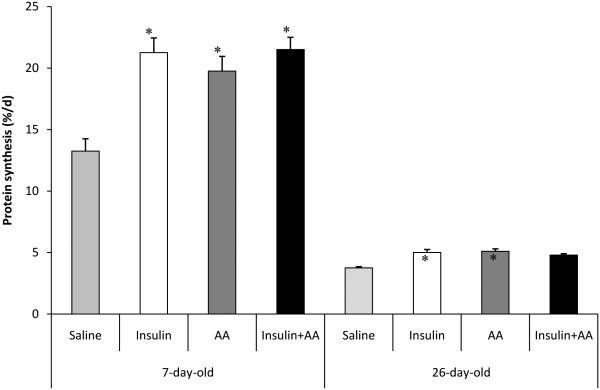
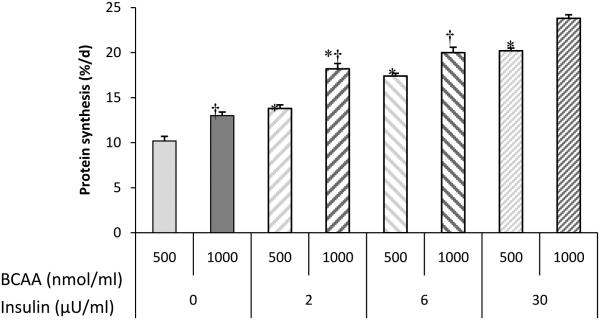
Elucidating which dietary component or hormonal response to dietary intake is responsible for the postprandial rise in protein synthesis is confounded by the concurrent changes in a number of plasma metabolites and hormones. This is especially difficult to determine when several potential anabolic signals are interrelated, as is the case with plasma insulin, glucose, and amino acids. The pancreatic-substrate clamp technique allows for independent assessment of the effects of insulin, glucose, and amino acids by blocking pancreatic insulin secretion, maintaining constant glucose, and manipulating plasma levels of insulin and other hormones and nutrients (Wray-Cahen et al. 1998). Using this technique, we demonstrated that the feeding-induced stimulation of muscle protein synthesis is independently regulated by the postprandial rise in amino acids and insulin (Fig. 1; Wray-Cahen et al. 1998; Davis et al. 2002; O'Connor et al. 2003)and the ability of both amino acids and insulinto stimulate protein synthesis is unique to skeletal muscle (Davis et al. 2002).Raising plasma insulin to levels similar to those observed in the fed state, while maintaining amino acids at fasting levels, resulted in maximum stimulation of protein synthesis, with no further stimulation of protein synthesis at insulin levels above fed values (Wray-Cahen et al. 1998). Similarly, raising plasma amino acid levels to the fed state resulted in an increase in protein synthesis (Fig. 2; O’Connor et al. 2003), although it is not known whether higher levels of amino acids would have continued to increase muscle protein synthesis.
Fig. 1.
Fractional protein synthesis rates in longissimusdorsi muscle of 7- and 26-d-old neonatal pigs in which saline, insulin, amino acids (AA), or both amino acids and insulin were infused during a pancreatic clamp. Euglycemia was maintained at the fasting level. *Significantly different than control within an age group (P< 0.05). Adapted from Davis et al. (2002) with permission.
Fig. 2.
Fractional protein synthesis rates in longissimusdorsi muscle of neonatal pigs at different levels of insulin and amino acids. *Significantly different than previous insulin level within same amino acid level (P < 0.05). †Significantly different than 500 nmol/ml branched-chain amino acids (BCAA) within same insulin level (P< 0.05). Adapted from O’Connor et al (2003) with permission.
Although these results are in agreement with previous studies that suggest amino acids alone can stimulate protein synthesis (Bennet et al. 1989; Bennet et al. 1990; Watt et al. 1992), this has not been a consistent finding. For example, Preedy and Garlick (1986) and Garlick and Grant (1988) suggested that amino acids only act to enhance insulin-stimulated protein synthesis in skeletal muscle and do not act independently of insulin. One possible explanation for this discrepancy is the plasma insulin level at which the effect of amino acids was examined in the different studies. O’Connor et al. (2003) demonstrated a dose-response of protein synthesis to both insulin and amino acids (Fig. 2), with the effect of amino acids and insulin being additive up to plasma insulin concentrations equivalent to those observed in the fed state. Davis et al. (2002) also demonstrated that there was no difference in protein synthesis when insulin and amino acids were independently or concurrently increased to fed levels (Fig. 1).
In addition, when comparing results from different studies, the age at which the anabolic effects of insulin and amino acids must be considered. The response of protein synthesis to a meal has been shown to rapidly decline with age in the rat (Baille and Garlick 1991), human (Davis et al. 1989; McNurlan et al. 1993), and pig (Fig. 1; Davis et al. 1996) and this decline is more evident for skeletal muscle than forvisceral tissue protein synthesis (Davis et al. 1996). Moreover, the developmental decline in the response of protein synthesis to insulin and amino acids parallels the decline in protein synthesis observed with feeding (Suryawan et al. 2007). Those studies in which amino acids were shown to have little or no effect on protein synthesis may have been at an age in which the anabolic response is reduced, making it more difficult to detect differences across treatments, or in tissues that respond differently to insulin and amino acid signaling.
Regulation of protein synthesis through the mTOR pathway
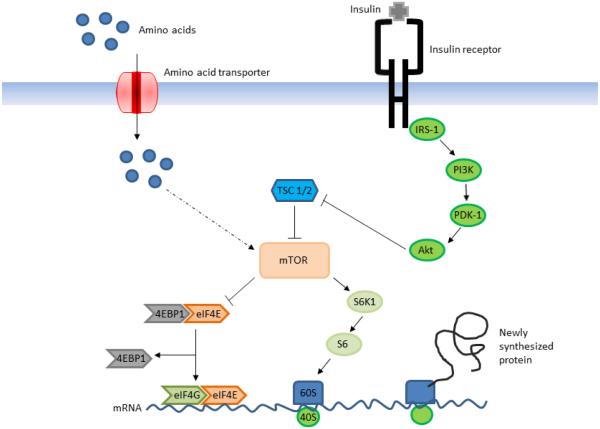
The postprandial increase in protein synthesis is regulated through the effects of amino acids, growth factors, and hormones, such as insulin, on the mammalian target of rapamycin (mTOR) signaling pathway (Fig. 3; Burrin and Davis 2013; Kimball 2013). There are two distinct mTOR complexes, mTOR complex 1 (mTORC1) and complex 2 (mTORC2), with mTORC1 clearly being associated with regulation of protein turnover (Kimball 2013). This review will focus on the regulatory importance of mTORC1 and the use of mTOR will refer solely to this complex. The pathway through which insulin activates mTOR is well characterized and has been presented in a number of reviews (Wullschleger et al. 2006; Hietakangas and Cohen 2009). Briefly, insulin binds to and activates its receptor which in turn initiates a signaling cascade, activating phosphoinositide 3-kinase and phosphoinositide-dependent kinase 1. This in turn leads to activation of Akt (also known as protein kinase B) which phosphorylates and inactivates tuberous sclerosis complex 1 and 2, a repressor of mTOR activity (Kimball 2013). The mechanism by which amino acids act to stimulate protein synthesis through mTOR is less well understood. However, it is widely accepted that amino acids stimulate protein synthesis through a pathway independent of the insulin signaling cascade (Efeyan et al. 2012). Evidence for this is provided by observations that amino acid infusion alone fails to result in the activation of Akt (Liu et al. 2002; Suryawan et al. 2012) indicating mTOR regulation by amino acids occurs through a distinct pathway. Moreover, the in vivo infusion of rapamycin, an inhibitor of mTOR activity, is capable of eliminating the stimulatory effect of amino acids on protein synthesis (Suryawan et al. 2008; Dickinson et al. 2011) indicating that amino acids do act to regulate mTOR activity. Regardless of upstream events, stimulation by both insulin and amino acids result in activation of mTOR. Activation of mTOR results in phosphorylation and activation of the 70-kDa ribosomal protein S6 kinase 1 (S6K1) which, in turn, phosphorylates ribosomal protein S6 (S6).mTOR also phosphorylates the eukaryotic initiation factor (eIF) repressor 4E-binding protein 1 (4EBP1), releasing eukaryotic initiation factor-4E (eIF4E) which can then bind to eIF4G (Kimball 2013). Phosphorylation of ribosomal protein S6 and the formation of the active eIF4E·eIF4G complex stimulate translation initiation and therefore protein synthesis.
Fig. 3.
Regulation of protein synthesis through the mTOR signaling pathway by amino acids and insulin. Insulin binds to its receptor and activates insulin receptor substrate-1 (IRS-1) which in turn activates phosphoinositide-3 kinase (PI3K), phosphoinositide-dependent kinase (PDK), and Akt. Activation of Akt removes the inhibition of mTOR by the tuberous sclerosis complex 1 and 2 (TSC 1/2). Both amino acids and insulin activatemTOR, which results in inactivation of the eukaryotic initiation factor repressor 4E-binding protein 1 (4EBP1), release of eukaryotic initiation factor (eIF) 4E, and formation of the active eIF4E·eIF4G complex. mTOR also activates ribosomal protein S6 kinase 1 (S6K1) which in turn activates ribosomal protein S6 (S6) allowing recruitment of the 60S and 40S ribosomal subunits. Phosphorylation of S6 and formation of the active eIF4E·eIF4G complex stimulate translation initiation and protein synthesis.
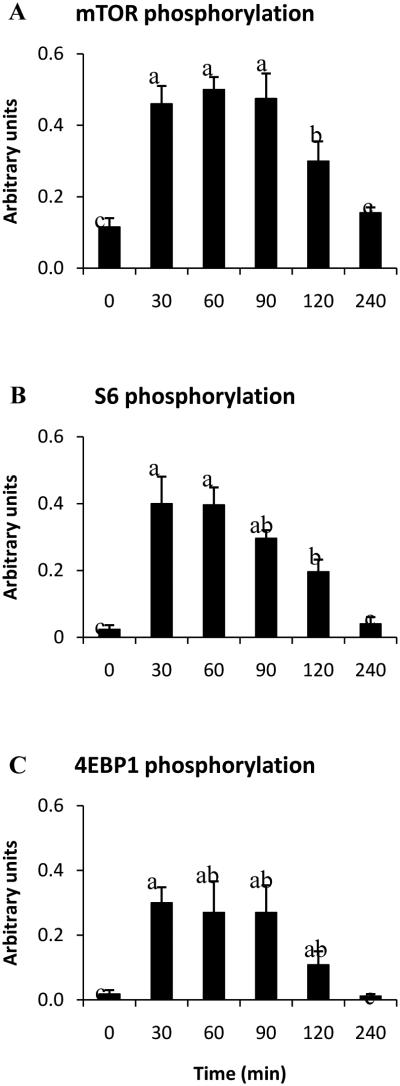
The rapid increase in protein synthesis following a meal was shown by Wilson et al. (2009) to be highly correlated with phosphorylation of key signals in the mTOR pathway such as Akt, mTOR, S6, and 4EBP1 (Fig. 4). Given the central role of mTOR signaling for translation initiation, mTOR activation has been utilized to indicate an increase in protein synthesis rate, however, it has been demonstrated that the phosphorylation status of targets of mTOR (i.e., 4EBP1 and S6K1) does not always correlate with measured rates of fractional protein synthesis (Escobar et al. 2005; Greenhaff et al. 2008; Atherton et al. 2010a). There are several possible reasons for this including the timing of muscle tissue sampling for determination of translation initiation signaling versus the period over which protein synthesis is determined as well as the stimulation of the protein synthetic machinery versus the availability of protein precursors. It is, therefore, important to validate observations of mTOR activation with actual rates of protein synthesis and caution should be used when interpreting results of studies in which the latter are not reported.
Fig. 4.
Changes in phosphorylation of mTORSer2448 (A), ribosomal protein S6Ser235/236 (B), and 4EBP1Thr70 (C) over 240 min in longissimusdorsi muscle of neonatal pigs following a meal providing one-sixth of their daily requirements. Means without a common letter differ (P< 0.05).Adapted from Wilson et al. (2009) with permission.
Anabolic effect of dietary amino acids on muscle protein synthesis
The dose response of protein synthesis to increasing intake of dietary protein has been clearly demonstrated in human adults (Moore et al. 2009; Churchward-Venne et al. 2014; Witard et al. 2014), infants (Kashyap et al. 1986; Kashyap 1988; Thureen et al. 2003) and young animals (O’Connor et al. 2003; Frank et al. 2006). Furthermore, it has been demonstrated that muscle protein synthesis will continue to increase with additional dietary protein but eventually reaches a plateau beyond which no further increase is achieved (Frank et al. 2006). The lack of a growth response to further increases in dietary protein indicates that the protein requirement for maximal muscle growth has been reached or another nutrient, such as energy, has become limiting (NRC 2012). In addition, Frank et al. (2006) demonstrated that there is no further increase in activation of mTOR signaling components in piglets at levels of dietary protein above the requirement (NRC 1998), indicating a molecular basis for this plateau as well.
Due to the importance of protein and amino acids in stimulating muscle protein synthesis, the amino acids responsible for this effect have been the subject of a number of investigations. The amino acid composition of dietary protein sources can differ drastically (FAO 1970; NRC 2012) resulting in very different rates of protein synthesis when equivalent amounts of different protein sources are fed (Norton et al. 2012). It is generally assumed that indispensable amino acids are primarily responsible for the anabolic response of muscle protein synthesis to dietary protein (Borsheim et al. 2002; Liu et al. 2002; Volpi et al. 2003) with little impact of dispensable amino acids. However, regardless of their impact on intracellular signaling of protein synthesis, dispensable amino acids may play an important role in maintaining the increased rates of synthesis (Davis et al. 2002) most likely due to their presence in muscle protein and role as precursors for a number of other protein metabolites (Wu et al. 2013). In adult male humans, approximately 10 g of total indispensable amino acids appear to be required to maximize the synthetic response to ingestion of a protein meal(Cuthbertson et al. 2005; Moore et al. 2009) which is equivalent to about 20-25 g of high quality protein such as whey, casein, egg white, or soy. The variability in the maximum protein synthetic rate observed with differing protein sources (Norton et al. 2012) is likely due to the total indispensable amino acid content but may also be due to the ability of individual amino acids, such as leucine, to stimulate protein synthesis.
Regulation of muscle protein synthesis by leucine
The stimulatory ability of individual amino acids is still a topic of debate, with studies demonstrating that indispensable amino acids are capable of enhancing protein synthesis through mTOR dependent pathways in muscle (Atherton et al. 2010b). Of all the indispensable amino acids, it is clear that leucine, a branched-chain amino acid, has anabolic properties distinct from its function as a component of proteins. When comparing the anabolic response of indispensable amino acids, Atherton et al. (2010b) demonstrated in C2C12 skeletal muscle cells that while phosphorylation of S6K1 was increased with all indispensable amino acids, this response was greatest with leucine and leucine was the only amino acid also capable of activation of mTOR and deactivation of 4EBP1. Nonetheless, some studies have demonstrated that the combined anabolic response to a mixture of indispensableamino acids may be sufficient to stimulate protein synthesis. Churchward-Venne et al. (2012), for example, demonstrated that supplementing a low protein meal with a mix of indispensable amino acids without leucine resulted in the same degree of increase in protein synthesis as supplementing with leucine alone. However, it is possible that the leucine content of the low protein meal was sufficient to activate protein synthesis and the amino acid mix simply provided more substrate to enable synthesis to occur.
Evidence that leucine is an important anabolic factor was first provided by Buse and Reid (1975) who observed that in vitro incubation of rat hemidiaphragms with leucine increased muscle protein synthesis. The importance of leucine was further demonstrated in vivo by Anthony et al. (2000) and Lynch et al. (2002) who observed that an oral gavage of leucine stimulated protein synthesis in rats in an mTOR-dependent manner. However, these studies used a pharmacological dose of leucine that would never be achieved with normal feeding (Lynch et al. 2002).To determine the efficacy of physiological increases in leucine for stimulating muscle protein synthesis, Escobar et al. (2005) infused leucine intravenously in overnight fasted neonatal pigs at rates that achieved plasma leucine levels similar to those observed following a meal,(i.e., approximately two- to three-fold increase above postabsorptive levels). Leucine infusion increased protein synthesis in skeletal muscle and activated key targets of translation initiation including S6K1 and 4EBP1, indicating that the effect of leucine was mTOR-dependent (Escobar et al. 2005). Escobar et al. (2005) further demonstrated that a minimum two-fold increase in plasma leucine levels was required to maximize protein synthesis, a finding supported by other studies (Escobar et al. 2006, Escobar et al. 2007, Boutry et al. 2013).In a follow-up study, Escobar et al. (2006) confirmed that, among the branched-chain amino acids, the response of muscle protein synthesis is unique to leucine and that valine and isoleucine failed to stimulate activation of them TOR pathway or protein synthesis (Fig. 5).
Fig. 5.
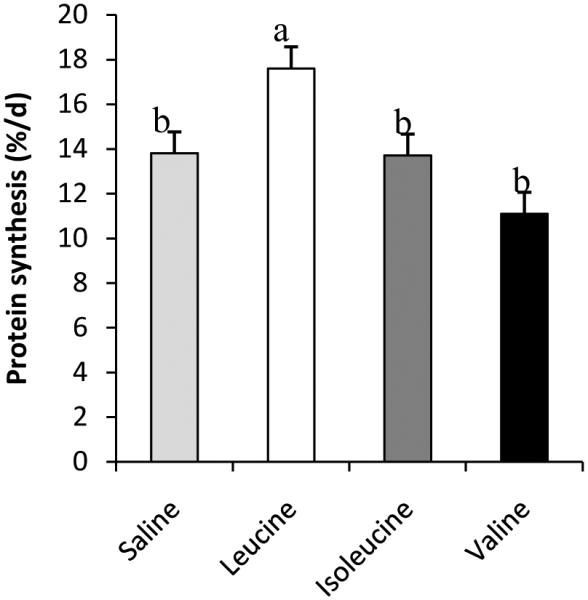
Fractional protein synthesis rates in longissimusdorsi muscle of fasted neonatal pigs intravenously infused with saline, leucine, isoleucine, or valine. Means without a common letter differ (P < 0.05).Adapted from Escobar et al. (2006) with permission.
Another key finding of these leucine infusion studies was that, although leucine stimulated muscle protein synthesis, this stimulation was maintained only for a short period of time (1 h) and protein synthesis rates returned to baseline values within 2 h of infusion despite continued activation of the mTOR pathway (Escobar et al. 2005). This response is most likely due to the utilization of amino acids for protein synthesis to the point that there is insufficient substrate for synthesis to continue, regardless of the continued activation of translation initiation signals. Thus, although leucine alone can enhance muscle protein synthesis, this increase cannot be maintained indefinitely without provision of a source of amino acids to maintain plasma euaminoacidemia. This conclusion was substantiated by the observation that after 2 h of leucine infusion, the plasma levels of other amino acids had decreased from basal levels (Escobar et al. 2005). To test whether the reduction in protein synthesis after long-term leucine infusion was dependent on substrate availability, Escobar et al. (2007) infused leucine to achieve fed levels in overnight fasted pigs with or without a replacement amino acid infusion to keep all other amino acids at the fasted level. Indeed, when plasma amino acid concentrations were maintained, protein synthesis continued to be enhanced beyond 2 h. This enhancement of protein synthesis by leucine could be extended up to 24 h so long as the decline in plasma amino acid concentrations was prevented (Wilson et al. 2010). Therefore, the provision of sufficient substrate is critical for the leucine-induced increase in muscle protein synthesis. Among the essential amino acids, isoleucine and valinehas shown a more dramatic decline in plasma levels with leucine supplementation in some studies (Yin et al. 2010; Suryawan et al. 2012). Although it has been suggested that all branched chain amino acids may need to be supplied in diets with supplemental leucine, few studies have been performed to date to address this hypothesis. Churchward-Venne et al. (2014) reported that while supplementation of a low protein meal resulted in a reduction in plasma valine and isoleucine, intracellular levels of the branched chain amino acids were not affected, suggested that the drop in plasma branched chain amino acids may be due to uptake to maintain intracellular levels. Moreover, in a study by Yin et al. (2010) the fall in isoleucine and valine did not appear to limit protein synthesis as supplementation with leucine still resulted in an increase in weight gain.
Leucine metabolites and muscle protein synthesis
Although the anabolic effects of leucine have been well-documented, the exact mechanism by which leucine exerts its anabolic effects is not clear. Moreover, it is not known whether leucine specifically or a molecular characteristic or metabolite unique to leucine is responsible for the increase in protein synthesis. The use of leucine metabolites, such as α-ketoisocaproic acid (KIC) and β-hydroxy-β-methylbutyrate (HMB),may also be useful in therapeutic programs in which muscle growth is desirable but nitrogen intake must be restricted, as in patients with kidney disease. The first step in leucine breakdown is the deamination by branched-chain aminotransferase to produce glutamate andKIC (Suryawan et al. 1998). When Escobar et al. (2010) infused either leucine or KIC into neonatal pigs it was found that leucine and KIC increased muscle protein synthesis to the same extent. The infusion of both KIC and leucine also increased the phosphorylation of 4EBP1 and formation of the active eIF4G·eIF4E complex. It should be noted, however, that the deamination of leucine by branched-chain aminotransferase is a reversible step in the catabolism of leucine. Indeed, infusion of KIC resulted in an increase in plasma leucine concentration (Escobar et al. 2010), indicating that a significant portion of the KIC infused was transaminated to leucine and, therefore, may not have been directly responsible for the increase in protein synthesis. In an early study, Tischler et al. (1982) demonstrated that incubation of muscle tissue with leucine in the presence or absence of an inhibitor of leucine transaminase resulted in the same increase in protein synthesis, indicating that breakdown of leucine to KIC is not required for the stimulatory ability of leucine. The use of KIC to enhance protein synthesis is still a viable alternative in those situations in which reduced dietary protein intake is desirable as no additional nitrogen is provided with KIC.
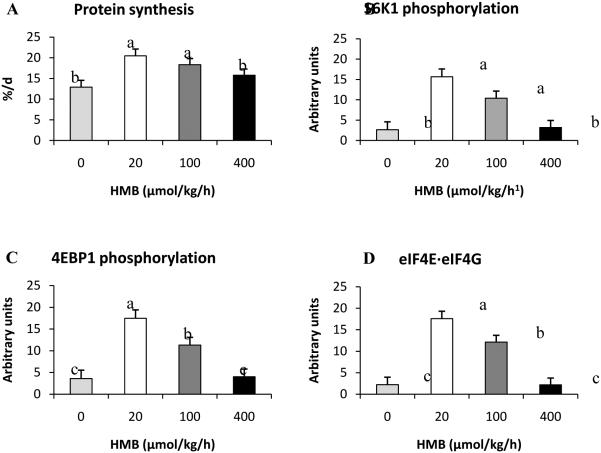
Another leucine metabolite that has been shown to have potential anabolic properties in adults is HMB (Nissen et al. 1996; Flakoll et al. 2004). HMB is a breakdown product of leucine metabolism produced by the action of α-ketoisocaproatedioxygenase on KIC (Van Koevering and Nissen 1992), with approximately 5% of daily leucine metabolism being channeled to this fate (Nissen et al. 2000). The ability of HMB to stimulate lean gain in the young or neonatal animal has been largely ignored but, as with KIC, has the potential to be used a therapeutic alternative to promote protein anabolism when reduced dietary protein is required. Unlike KIC, however, the breakdown of leucine to HMB is irreversible and, therefore, any observed responses to HMB cannot be attributed to production of leucine. One study has examined the ability of HMB to stimulate protein synthesis in the neonatal animal. In this study, intravenous infusion of increasing doses of HMB into fasted, neonatal piglets for 1hresulted in the activation of the mTOR pathway and anincrease in muscle protein synthesis (Wheatley et al. 2014; Fig. 6). Interestingly, it was reported that the greatest response was observed with the dose that resulted in plasma HMB levels of 90 μU/ml, whereas higher doses showed either a reduced anabolic effector failed to stimulate protein synthesis above the fasting baseline levels. The most effective doses of HMB produced plasma levels of HMB similar to those seen in HMB supplemented adults (Zanchi et al. 2010).
Fig. 6.
Fractional protein synthesis rates(A),phosphorylation of S6K1Thr389 (B) and 4EBP1Thr46 (C), and formation of the active eIF4G·eIF4E complex (D) in longissimusdorsi muscle of fasted neonatal pigs intravenously infused with 0, 20, 100, or 400 μmol/kg/hHMB for 1 h. Means without a common letter differ (P< 0.05). Adapted from Wheatley et al. (2014) with permission.
Application of leucine to increase lean gain in neonates
Many low birth weight infants experience extrauterine growth failure due to our inability to provide an ideal diet which is sufficiently high in protein and other nutrients to meet their needs (Hay 2008; Berseth 2001). The limitations can be attributed to feeding intolerances and other complications associated with the escalation of oral feeds. Additionally, there are concerns of amino acid toxicity, uremia, and acidosis (Hay 2008) when advancing the amount of protein fed to premature infants. Although it is not known whether the ability of low birth weight infants to efficiently increase protein synthesis in response to feeding is impaired, growth is increased with feeding a high protein diet (Kashyap et al. 1986, 1987). Thus, the reduction in growth during this period is likely due, at least in part, to inadequate nutrition to meet requirements for growth (Hay 2008). Thus, nutritional therapies that can optimize growth without substantial increases in dietary protein or feeding would be beneficial. Given its anabolic effects, leucine has the potential to improve lean gain in neonates where growth has been restricted. The supplementation of protein-restricted diets with leucine may enhance the efficiency of utilization of amino acids for growth through activation of mTOR signaling. To determine if leucine can improve muscle protein synthesis in protein restricted neonates, Torrazza et al. (2010) fed neonatal pigs a low protein meal, a high protein meal, or a low protein meal supplemented with leucine. Supplementation of the low protein meal with leucine resulted in an increase in protein synthesis in both muscle and visceral tissues, (Torrazza et al. 2010). This same effect was maintained when the leucine supplemented diet was fed for 24 h, althoughthe extent of this increase was not to the same level as the increase seen with a high protein meal (Suryawan et al. 2012). It is likely that amino acid supply may have begun to limit protein synthesis in the low protein fed pigs after 24 h or that the higher level of insulin produced in the high protein fed pigs independently enhanced protein synthesis. Leucine supplementation of a low protein diet has also been shown to increase muscle protein synthesis in weaned pigs (Yin et al. 2010) and adults (Churchward-Venne et al. 2012, 2014), indicating that the effect of leucine to improve anabolism under dietary restriction is not lost with age.
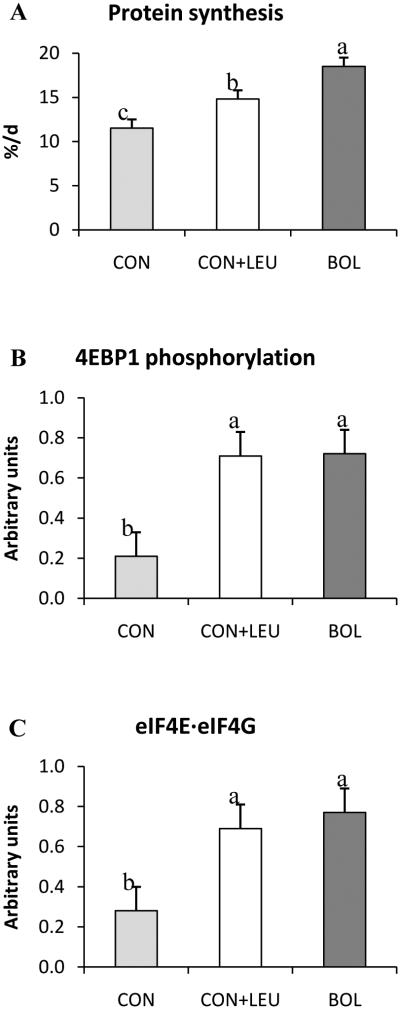
Many neonates are incapable of feeding properly, with orogastric tube feeding commonly used in these situations to deliver feed. Under normal conditions intermittent bolus delivery of food is the ideal feeding modality for optimizing growth (Gazzaneo et al. 2011; El-Kadi et al. 2012), however, many infants are incapable of tolerating full feeds which necessitates the use of continuous feeding (Dollberg et al. 2000). It was hypothesized by Boutry et al. (2013) that a pulse of leucine could mimic the effect of bolus feeding in neonatal pigs fed continuously by orogastric tube. Over a 24 h period, pigs were fed either by intermittent bolus every 4 h or were fed continuously, with or without a leucine pulse administered every 4 h. Rates of protein synthesis in muscle were greater in continuously fed pigs administered leucine pulses than in those fed continuously with no leucine pulse, but were lower than in those fed by intermittent bolus fed (Fig. 7). This was despite leucine stimulating 4EBP1 phosphorylationand formation of the active eIF4E·eIF4G complex to the same extent as bolus feeding (Fig. 7; Boutry et al. 2013) suggesting that other factors may be involved.
Fig. 7.
Fractional protein synthesis rates (A), phosphorylation of S6K1Thr389 (B) and 4EBP1Thr46 (C), and formation of the active eIF4G·eIF4E complex (D) in longissimusdorsi muscle of neonatal pigscontinuously fed (CON), continuously fed and pulsed with leucine (CON+LEU), or bolus fed (BOL). Means without a common letter differ (P< 0.05). Adapted from Boutry et al. (2013) with permission.
Clearly, leucine has the potential to improve lean tissue growth in low birth weight infants where proteinintake is restricted or normal meal feeding is not possible. However, there have been no studies to date on the efficacy of the long-term use of supplemental leucine. Therefore, it is unknown if the increase in muscle protein synthesis observed in these studies will translate into an increase in overall body weight and lean body composition. Future studies should attempt to determine the long-term efficacy of leucine supplementation to improve growth in low birth weight infants in the immediate neonatal period.
Conclusions
The increase in protein synthesis following a meal is due to the postprandial rise in insulin and amino acids, which can act independently to increase muscle protein synthesis in an mTOR-dependent manner. Leucine specifically has been shown to have unique anabolic properties and the supplementation of leucine or its metabolites, α-ketoisocaproic acid and β-hydroxy-β-methylbutyrate, have been shown to enhance muscle protein synthesis in neonates. Leucine in particular has been evaluated extensively for its ability to enhance muscle protein synthesis with low protein dietsand its potential to improve growth in low birth weight infants whose growth has been compromised due to insufficient feed or protein intake merits further investigation.
Acknowledgements
The work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR-044474 (Davis) and AR-46308 (Fiorotto), National Institute of Child Health and Human Development HD-072891 (Davis), United States Department of Agriculture National Institute of Agriculture grant 2013-67015-20438 (Davis), and by the USDA/ARS under Cooperative Agreement no. 6250-510000-055 (Davis). This work is a publication of the USDA, Agricultural Research Service (USDA/ARS) Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX. The contents of this publication do not necessarily reflect the views or politics of the USDA, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- 4EBP1
4E binding protein 1
- AA
amino acids
- Akt/PKB
protein kinase B
- BCAA
branched-chain amino acids
- BOL
bolus fed
- CON
continuously fed
- CON+LEU
continuously fed plus leucine
- eIF4E
eukaryotic initiation factor 4E
- eIF4G
eukaryotic initiation factor 4G
- HMB
β-hydroxy-β-methylbutyrate
- KIC
α-ketoisocaproic acid
- mTOR
mammalian target of rapamycin
- S6
ribosomal protein S6
- S6K1
ribosomal protein S6 kinase 1
Footnotes
The authors have no conflicts of interests.
References
- Abdelhamid AE, Chuang SL, Hayes P, Fell JM. In vitro cow’s milk protein-specific inflammatory and regulatory cytokine responses in preterm infants with necrotizing enterocolitis and sepsis. Pediatr Res. 2011;69:165–169. doi: 10.1203/PDR.0b013e31820263e7. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010a;92:1080–1088. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signaling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010b;38:1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- Baille AGS, Garlick PJ. Attenuated responses of muscle protein synthesis to fasting and insulin in adult female rats. Am J Physiol Endocrinol Metab. 1991;25:E1–E5. doi: 10.1152/ajpendo.1992.262.1.E1. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Scrimgeour RT, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1-13C] leucine. Clin Sci (Lond) 1989;76:447–454. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Scrimgeour CM, Jung RT, Rennie MJ. Euglycemic hyperinsulinemia augments amino acid uptake by human leg tissues during hyperaminoacidemia. Am J Physiol Endocrinol Metab. 1990;259:E185–E194. doi: 10.1152/ajpendo.1990.259.2.E185. [DOI] [PubMed] [Google Scholar]
- Berseth CL. Feeding methods for the preterm infant. Semin Neonatol. 2002;6:417–424. doi: 10.1053/siny.2001.0062. [DOI] [PubMed] [Google Scholar]
- Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- Boutry C, El-Kadi SW, Suryawan A, Wheatley SM, Orellana RA, Kimball SR, Nguyen HV, Davis TA. Leucine pulses enhance skeletal muscle protein synthesis during continuous feeding in neonatal pigs. Am J Physiol Endocrinol Metab. 2013;305:E620–E631. doi: 10.1152/ajpendo.00135.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LD. Endocrine regulation of fetal skeletal muscle growth: impact on future metabolic health. J Endocrinol. 2014;221:R13–R29. doi: 10.1530/JOE-13-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrin DG, Shulman RJ, Reeds PJ, Davis TA, Gravitt KR. Porcine colostrum and milk stimulate visceral organ and skeletal muscle protein synthesis in neonatal piglets. J Nutr. 1992;122:1205–1213. doi: 10.1093/jn/122.6.1205. [DOI] [PubMed] [Google Scholar]
- Burrin DG, Davis TA. Mechanisms of nutrient sensing. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, editors. Modern Nutrition in Health and Disease. 11th. Williams and Wilkins Publishers; PA: 2013. pp. 626–632. Part II. Nutritional roles in integrated biologic systems, Section B. Digestive, endocrine, immune, and neural mechanisms. [Google Scholar]
- Buse MG, Reid SS. Leucine: A possible regulator of protein turnover in muscle. J Clin Invest. 1975;56:1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward-Venne TA, Burd NA, Mitchell CJ, West DWD, Philip A, Marcotte GR, Baker SK, Baar K, Phillips M. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Pysiol. 2012;590:2751–2765. doi: 10.1113/jphysiol.2012.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, Phillips SM. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr. 2014;99:276–286. doi: 10.3945/ajcn.113.068775. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Cooke R, Embleton N, Rigo J, Carrie A, Haschke F, Ziegler E. High protein pre-term infant formula: effect on nutrient balance, metabolic status and growth. Pediatr Res. 2006;59:265–270. doi: 10.1203/01.pdr.0000196376.99101.34. [DOI] [PubMed] [Google Scholar]
- Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballévre O, Beaufrére B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280:E340–E348. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of suckling rats. Am J Physiol Reg. 1989;26:R1141–R1146. doi: 10.1152/ajpregu.1989.257.5.R1141. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol Reg. 1993;265:R334–R340. doi: 10.1152/ajpregu.1993.265.2.R334. [DOI] [PubMed] [Google Scholar]
- Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol Endocrinol Metab. 1996;33:E802–E809. doi: 10.1152/ajpendo.1996.270.5.E802. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O’Connor PM. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab. 2002;282:E880–E890. doi: 10.1152/ajpendo.00517.2001. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care. 2009;2:78–85. doi: 10.1097/MCO.0b013e32831cef9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denne SC, Rossi EM, Kalhan SC. Leucine kinetics during feeding in normal newborns. Pediatr Res. 1991;30:23–27. doi: 10.1203/00006450-199107000-00005. [DOI] [PubMed] [Google Scholar]
- Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–862. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollberg S, Kuint J, Mazkereth R, Mimouni FB. Feeding tolerance in preterm infants: randomized trial of bolus and continuous feeding. J Am CollNutr. 2000;19:797–800. doi: 10.1080/07315724.2000.10718080. [DOI] [PubMed] [Google Scholar]
- Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med. 2012;18:524–533. doi: 10.1016/j.molmed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenkranz RA, Younes N, Lemons JA, Fanaroff AA, Donovan EF, Wright LL, Katsikiotis V, Tyson JE, Oh W, Shankaran S, Bauer CR, Korones SB, Stoll BJ, Stevenson DK, Papile LA. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104:280–289. doi: 10.1542/peds.104.2.280. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz RA. Early, aggressive nutritional management for very low birth weight infants: what is the evidence? Semin Perinatol. 2007;31:48–55. doi: 10.1053/j.semperi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- El-Kadi SW, Suryawan A, Gazzaneo MC, Srivastava N, Orellana RA, Nguyen HV, Lobley GE, Davis TA. Anabolic signaling and protein deposition are enhanced by intermittent compared with continuous feeding in skeletal muscle of neonates. Am J Physiol Endocrinol Metab. 2012;302:E674–E686. doi: 10.1152/ajpendo.00516.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab. 2005;288:E914–E921. doi: 10.1152/ajpendo.00510.2004. [DOI] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E612–E621. doi: 10.1152/ajpendo.00402.2005. [DOI] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Davis TA. Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am J Physiol Endocrinol Metab. 2007;293:E1615–E1621. doi: 10.1152/ajpendo.00302.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Van Horn CG, Hutson SM, Davis TA. Leucine and α-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J Nutr. 2010;140:1418–1424. doi: 10.3945/jn.110.123042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) Amino-acid content of foods and biological data on proteins. FAO; Rome, Italy: 1970. Amino-acid content of foods. [Google Scholar]
- Flakoll P, Sharp R, Baier S, Levenhagen D, Carr C, Nissen S. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition. 2004;20:445–451. doi: 10.1016/j.nut.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Ford GW, Doyle LW, Davis NM, Callanan C. Very low birth weight and growth into adolescence. Arch Pediatr Adolesc Med. 2000;154:778–784. doi: 10.1001/archpedi.154.8.778. [DOI] [PubMed] [Google Scholar]
- Frank JW, Escobar J, Suryawan A, Kimball SR, Nguyen HV, Jefferson LS, Davis TA. Protein synthesis and translation initiation factor activation in neonatal pigs fed increasing levels of dietary protein. J Nutr. 2005;135:1374–1381. doi: 10.1093/jn/135.6.1374. [DOI] [PubMed] [Google Scholar]
- Frank JW, Escobar J, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Dietary protein and lactose increase translation initiation factor activation and tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:3225–E233. doi: 10.1152/ajpendo.00351.2005. [DOI] [PubMed] [Google Scholar]
- Garlick PJ, Grant I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin: effect of branched-chain amino acids. Biochem J. 1988;254:579–584. doi: 10.1042/bj2540579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaneo MC, Suryawan A, Orellana RA, Torrazza RM, El-Kadi SW, Wilson FA, Kimball SR, Srivastava N, Nguyen HV, Fiorotto ML, Davis TA. Intermittent bolus feeding has a greater stimulatory effect on protein synthesis in skeletal muscle than continuous feeding in neonatal pigs. J Nutr. 2011;141:2152–2158. doi: 10.3945/jn.111.147520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M, Castellino P, DeFronzo RA. Differential responsiveness of protein synthesis and degradation to amino acid availability in humans. Diabetes. 1996;45:393–399. doi: 10.2337/diab.45.4.393. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay WW. Strategies for feeding the preterm infant. Neonatology. 2008;94:245–254. doi: 10.1159/000151643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietakangas V, Stephen Cohen SM. Regulation of tissue growth through nutrient sensing. Annu Rev Genet. 2009;43:389–410. doi: 10.1146/annurev-genet-102108-134815. [DOI] [PubMed] [Google Scholar]
- Ibrahim HM, Jeroudi MA, Baier RJ, Dhanireddy R, Krouskop RW. Aggressive early total parenteral nutrition in low-birth-weight infants. J Perinatol. 2004;24:482–486. doi: 10.1038/sj.jp.7211114. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Albritton WL, Sunshine P. Hyperammonemia accompanying parenteral nutrition in newborn infants. J Pediatr. 1972;81:154–161. doi: 10.1016/s0022-3476(72)80395-0. [DOI] [PubMed] [Google Scholar]
- Kashyap S, Forsyth M, Zucker C, Ramakrishnan R, Dell RB, Heird WC. Effects of varying protein and energy intakes on growth and metabolic responses in low birth weight infants. J Pediatr. 1986;108:955–963. doi: 10.1016/s0022-3476(86)80940-4. [DOI] [PubMed] [Google Scholar]
- Kashyap S, Okamoto E, Kanaya S, Zucker C, Abildskov K, Dell RB, Heird WC. Growth, nutrient retention, and metabolic response in low birth weight infants fed varying intakes of protein and energy. J Pediatr. 1988;133:713–721. doi: 10.1016/s0022-3476(88)80388-3. [DOI] [PubMed] [Google Scholar]
- Kimball SR. Integration of signals generated by nutrients, hormones, and exercise in skeletal muscle. Am J Clin Nutr. 2013;99:237S–242S. doi: 10.3945/ajcn.113.068387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Barrett EJ. Human protein metabolism: its measurement and regulation. Am J Physiol Endocrinol Metab. 2002;283:E1105–E1112. doi: 10.1152/ajpendo.00337.2002. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jahn LA, Wei L, Long W, Barrett EJ. Amino acids stimulate translation initiation and protein synthesis through an Akt-independent pathway in human skeletal muscle. J Clin Endocrinol Metab. 2002;87:5553–5558. doi: 10.1210/jc.2002-020424. [DOI] [PubMed] [Google Scholar]
- Lobley GE. Nutritional and hormonal control of muscle and peripheral tissue metabolism in farm species. Livest Prod Sci. 1998;56:91–114. [Google Scholar]
- Lobley GE. Protein turnover – What does it mean for animal production? Can J AnimSci. 2003;83:327–340. [Google Scholar]
- Lynch CJ, Patson BJ, Anthony J, Vaval A, Jefferson LS, Vary TC. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am J Physiol Endocrinol Metab. 2002;283:E503–513. doi: 10.1152/ajpendo.00084.2002. [DOI] [PubMed] [Google Scholar]
- McNurlan MA, Essen P, Milne E, Vinnars E, Garlick PJ, Wernerman J. Temporal response of protein synthesis in human skeletal muscle to feeding. Br J Nutr. 1993;69:117–126. doi: 10.1079/bjn19930014. [DOI] [PubMed] [Google Scholar]
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89:161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- Nissen S, Sharp R, Ray M, Rathmacher JA, Rice D, Fuller JC, Connelly AS, Abumrad N. Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol. 1996;81:2095–2104. doi: 10.1152/jappl.1996.81.5.2095. [DOI] [PubMed] [Google Scholar]
- Nissen S, Sharp RL, Panton L, Vukovich M, Trappe S, Fuller JC. β-hydroxy-β-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr. 2000;130:1937–1945. doi: 10.1093/jn/130.8.1937. [DOI] [PubMed] [Google Scholar]
- Norton LE, Wilson GJ, Layman DK, Moulton CJ, Garlick PJ. Leucine content of dietary proteins is a determinant of postprandial skeletal muscle protein synthesis in adult rats. NutrMetab. 2012;9:67–75. doi: 10.1186/1743-7075-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . Nutrient Requirements of Swine. 10th National Academies Press; Washington, D.C.: 1998. [Google Scholar]
- NRC . Nutrient Requirements of Swine. 11th National Academies Press; Washington, D.C.: 2012. [Google Scholar]
- O’Connor PMJ, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E110–E119. doi: 10.1152/ajpendo.00326.2002. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Garlick PJ. The response of muscle protein synthesis to nutrient intake in postabsorptive rats: The role of insulin and amino acids. Biosci Rep. 1986;6:177–183. doi: 10.1007/BF01115004. [DOI] [PubMed] [Google Scholar]
- Premji SS, Fenton TR, Sauve RS. Higher versus lower protein intake in formula-fed low birth weight inants. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003959.pub2. CD003959. [DOI] [PubMed] [Google Scholar]
- Senterre T, Rigo J. Update on nutritional management of the premature infants. P Belg Roy Acad Med. 2013;2:164–178. [Google Scholar]
- Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr. 1998;68:72–81. doi: 10.1093/ajcn/68.1.72. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Fleming JR, Davis TA. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol Endocrinol Metab. 2007;293:E1597–E1605. doi: 10.1152/ajpendo.00307.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of the neonatal pig by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab. 2008;295:E868–E875. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawan A, Torrazza RM, Gazzaneo MC, Orellana RA, Fiorotto ML, El-Kadi SW, Srivastava N, Nguyen HV, Davis TA. Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr Res. 2012;71:324–331. doi: 10.1038/pr.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari P, Inchiostro S, Biolo G, Trevisan R, Fantin G, Marescotti MC, Iori E, Tiengo A, Crepaldi G. Differential effects of hyperinsulinemia and hyperaminoacidemia on leucine-carbon metabolism in vivo. Evidence for distinct mechanisms in regulation of net amino acid deposition. J Clin Invest. 1987;79:1062–1069. doi: 10.1172/JCI112919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thureen PJ, Hay WW. Early aggressive nutrition in preterm infants. Semin Neonatol. 2001;6:403–415. doi: 10.1053/siny.2001.0061. [DOI] [PubMed] [Google Scholar]
- Thureen PJ, Melara D, Fennessey PV, Hay WW. Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res. 2003;53:24–32. doi: 10.1203/00006450-200301000-00008. [DOI] [PubMed] [Google Scholar]
- Tischler ME, Desautels M, Goldberg AL. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem. 1982;257:1613–1621. [PubMed] [Google Scholar]
- Torraza RM, Suryawan A, Gazzaneo MC, Orellana RA, Frank JW, Nguyen HV, Fiorotto ML, El-Kadi S, Davis TA. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J Nutr. 2010;140:2145–2152. doi: 10.3945/jn.110.128421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Koevering M, Nissen S. Oxidation of leucine and α-ketoisocaproate to β-hydroxy-β-methylbutyrate in vivo. Am J Physiol Endocrinol Metab. 1992;262:E27–E31. doi: 10.1152/ajpendo.1992.262.1.E27. [DOI] [PubMed] [Google Scholar]
- Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt PW, Corbett ME, Rennie MJ. Stimulation of protein synthesis in pig skeletal muscle by infusion of amino acids during constant insulin availability. Am J Physiol Endocrinol Metab. 1992;263:E453–E460. doi: 10.1152/ajpendo.1992.263.3.E453. [DOI] [PubMed] [Google Scholar]
- Wheatley SM, El-Kadi SW, Suryawan A, Boutry C, Orellana RA, Nguyen HV, Davis SR, Davis TA. Protein synthesis in skeletal muscle of neonatal pigs is enhanced by administration of β-hydroxy-β-methylbutyrate. Am J Physiol Endocrinol Metab. 2014;306:E91–E99. doi: 10.1152/ajpendo.00500.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FA, Suryawan A, Orellana RA, Kimball SR, Gazzaneo MC, Nguyen HV, Fiorotto ML, Davis TA. Feeding rapidly stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing translation initiation. J Nutr. 2009;139:1873–1880. doi: 10.3945/jn.109.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr. 2010;140:264–270. doi: 10.3945/jn.109.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr. 2014;99:86–95. doi: 10.3945/ajcn.112.055517. [DOI] [PubMed] [Google Scholar]
- Wray-Cahen D, Nguyen HV, Burrin DG, Beckett PR, Fiorotto ML, Reeds PJ, Wester TJ, Davis TA. Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. Am J Physiol Endocrinol Metab. 1998;275:E602–E609. doi: 10.1152/ajpendo.1998.275.4.E602. [DOI] [PubMed] [Google Scholar]
- Wu G, Wu Z, Dai Z, Yang Y, Wang W, Liu C, Wang B, Wang J, Yin Y. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids. 2013;44:1107–1113. doi: 10.1007/s00726-012-1444-2. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2007;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yajnik CS. Obesity epidemic in India: intrauterine origins? Proc Nutr Soc. 2004;63:387–396. doi: 10.1079/pns2004365. [DOI] [PubMed] [Google Scholar]
- Yin Y, Yao K, Liu Z, Gong M, Ruan Z, Den D, Tan B, Liu Z, Wu G. Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids. 2010;39:1477–1486. doi: 10.1007/s00726-010-0612-5. [DOI] [PubMed] [Google Scholar]
- Zanchi NE, Gerlinger-Romero F, Guimaraes-Ferreira L, de Siqueira Filho MA, Felitti V, Lira FS, Seelaender M, Lancha AH. HMB supplementation: clinical and athletic performance-related effects and mechanisms of action. Amino Acids. 2010;40:1015–1025. doi: 10.1007/s00726-010-0678-0. [DOI] [PubMed] [Google Scholar]