Abstract
Introduction
Erectile dysfunction (ED) is associated with cardiovascular disease (CVD), however the association between change in ED status over time and future underlying CVD risk is unclear.
Aim
To investigate the association between change in ED status and Framingham CVD risk, as well change in Framingham risk.
Main outcome measures
Framingham CVD risk and change in Framingham CVD risk.
Methods
We studied 965 men free of CVD in the Boston Area Community Health Survey (BACH), a longitudinal cohort study with three assessments. ED was assessed with the 5-item International Index of Erectile Function (IIEF-5) at BACH I (2002-2005) and BACH II (2007-2010) and classified as no ED/transient ED/persistent ED. CVD risk was assessed with 10-year Framingham CVD risk algorithm at BACH I and BACH III (2010-2012). Linear regression models controlled for baseline age, socio-demographic and lifestyle factors, as well as baseline Framingham risk. Models were also stratified by age (≥/< 50 years).
Results
Transient and persistent ED were significantly associated with increased Framingham risk and change in risk over time in univariate and age-adjusted models. In younger men, persistent ED was associated with a Framingham risk that was 1.58 percentage points higher (95% CI: 0.11-3.06) and in older men, a Framingham risk that was 2.54 percentage points higher (95% CI: -1.5, 6.59), compared to those without ED. Change in Framingham risk over time was also associated with transient and persistent ED in men <50 years, but not in older men.
Conclusions
Data suggest that even after taking into account other CVD risk factors, transient and persistent ED are associated with Framingham CVD risk and a greater increase in Framingham risk over time, particularly in younger men. Findings further support clinical assessment of CVD risk in men presenting with ED, especially those under 50 years.
Keywords: male, sexual dysfunction, prospective cohort, changes in erectile function
Introduction
Erectile dysfunction (ED) affects 16 to 20 million men in the US (1) and commonly co-exists with cardiovascular disease (CVD). Both share similar risk factors (e.g. age, diabetes, hypertension, obesity, smoking) (2, 3) and an underlying pathophysiology involving endothelial dysfunction (4-6). Previous studies have investigated ED as a pre-cursor of CVD (4, 7-9), showing that CVD generally develops 2-5 years after ED development (10). In a recent meta-analysis, risk of cardiovascular events was greatest among those with ED with younger age and intermediate Framingham Risk Score (8). Studies also show that Framingham coronary risk is associated with increased ED risk (11) and that men with ED are at increased risk of death from all causes and CVD (12, 13).
ED symptoms are not necessarily progressive however and have been shown to abate spontaneously in up to one-third of men (14). ED may also occur as a consequence of CVD and associated risk factors, and clinical and experimental data show changes in lifestyle and diet can improve ED (15, 16). Yet, how changes in ED over time may impact CVD risk is unclear, and whether persistence of ED predicts CVD independent of traditional risk factors is unknown. Although studies have assessed associations between ED and future CV events, as well as cross-sectional associations between Framingham Risk Score and ED, no studies have assessed changes in ED status and Framingham CVD risk. Such information can provide a clearer understanding of risk of CVD among those with persistent and non-persistent ED.
Using data from the Boston Area Community Health Survey (BACH), a population-based longitudinal cohort study with detailed interview and questionnaire data, the objectives of this paper were to investigate the association between changes in ED status, as measured by a validated questionnaire, and future CVD risk as measured by the general Framingham 10-year CVD risk (17). We also investigated the relationship between changes in ED status and changes in Framingham risk over time. As previous studies have indicated that coronary artery disease (CAD) and cardiovascular events associated with ED are stronger in younger men (18-20), we also sought to investigate associations between change in ED and Framingham risk by age.
Methods
Study population
The study population consisted of men who participated in all three waves of the BACH Survey and were free of CVD during the first two surveys (n=965). BACH, a population-based survey of urologic symptoms, used a two-stage cluster sample design to recruit a random sample of 2,301 men and 3,201 women from Boston, MA ages 30-79 years. Participants completed an in-person interview at baseline (2002-2005) and approximately every 5 years (BACH II: 2006-2010; BACH III: 2010-2012). Completed follow-up interviews were obtained for 4,144 and 3,155 individuals at BACH II and III, resulting in 80.5% and 81.4% conditional retention rates. At each survey, a home visit was conducted for anthropometric measurements, blood pressure, and interview regarding symptoms, comorbidities, and lifestyle. Additionally, blood samples were collected at BACH I and III. Further details on methods have been previously published (21, 22). Two-hundred-twelve men reported CVD (defined as having reported myocardial infarction, angina, stroke, surgery or angioplasty for arterial disease of the leg, peripheral vascular disease, congestive heart failure, or carotid artery surgery) at either BACH I or II and were excluded from analyses. ED status was determined at BACH I and II for men, but not at BACH III. A schematic of the study design and primary measures are provided in Figure 1 and described in detail below. All participants gave written informed consent at each survey. The BACH Survey protocol was approved by the Institutional Review Board of New England Research Institutes.
Figure 1.
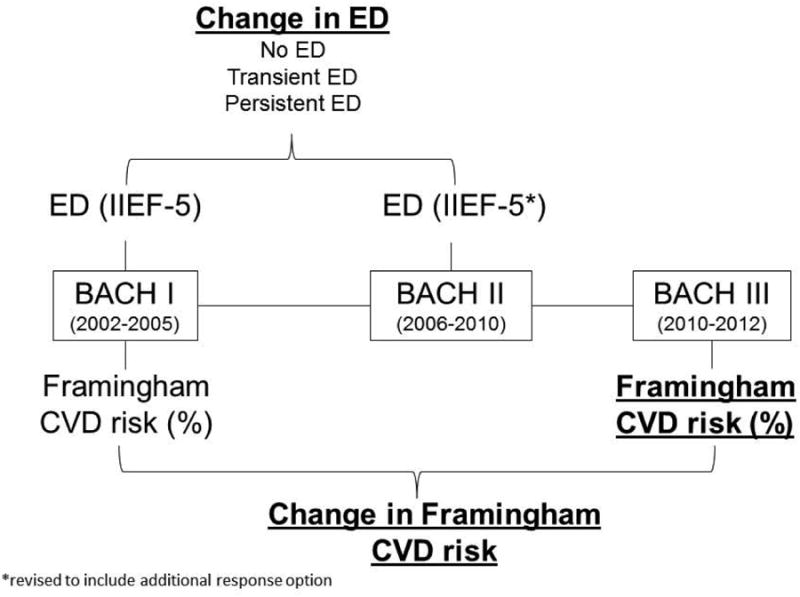
Study design and data collection scheme. Variables used in models are in bold and underlined.
Assessment of Erectile Dysfunction
ED was assessed using the International Index of Erectile Function (IIEF-5), a validated, self-administered 5-item questionnaire (23). At baseline, an early IIEF-5 version was administered in which each question was graded on a scale of 1-5 and the total score ranged from 5-25. ED was classified as: severe (5-7); moderate (8-11); mild-moderate (12-16); mild (17-21); and no ED (22-25). At BACH II, a slightly modified version of the IIEF-5 was administered, in which an additional response option reflecting no sexual activity or no attempt at sexual activity was provided for the 2nd through 5th questions, resulting in a score range of 1-25, with severe ED as 1-7 (24). Because of this modification at BACH II, we focused on categorical changes in ED status, rather than continuous IIEF-5 change scores. We defined ED as a dichotomous variable (IIEF-5 <22). Change in ED status from baseline to BACH II was classified as no ED (no ED at either time points), transient ED (ED at one time point), and persistent ED (ED at both time points).
Framingham Cardiovascular Disease Risk
The general 10-year CVD Framingham risk (17), expressed as a percent, assesses risk of atherosclerotic CVD events (i.e. coronary heart disease, cerebrovascular disease, peripheral vascular disease, and heart failure) and was assessed at BACH I and III using age, high-density lipoprotein, total cholesterol level, systolic blood pressure, antihypertensive medication use, diabetes, and current smoking status. Current smoker was defined as having smoked ≥100 cigarettes in their lifetime and reported currently smoking. Diabetes was defined by self-report or fasting blood glucose level ≥126mg/dl. Change in Framingham risk was calculated as the risk at BACH III minus risk at BACH I. Because blood was not collected at BACH II, Framingham risk was not computed at BACH II.
Statistical analysis
We assessed the association between 1) change in ED status and Framingham risk (%) at BACH III, and 2) change in ED and change in Framingham risk (%) from BACH I to III. Unadjusted and multivariable-adjusted associations between change in ED status, Framingham risk at BACH III, and change in Framingham risk were estimated with linear regression models, as well as corresponding 95% confidence intervals (CI). For models assessing the association between change in ED status and Framingham risk at BACH III, we considered baseline age, demographic and socioeconomic factors [race/ethnicity (white/black/Hispanic), married/living with a partner (y/n), education level (≤high school/some college/≥college), income (<20k/20-<50k/≥50k)], BMI, physical activity (low/medium/high), alcohol use (<1/1-3/>3 drinks/day), depression (y/n), and baseline Framingham risk as potential confounders. We also considered use of antihypertensive medications and statins as potential confounders but model results were largely unchanged and thus we did not include these variables to maintain parsimony of the models. Baseline age was not considered as a covariate in fully-adjusted models with baseline Framingham risk, as age is included in the Framingham algorithm. Analyses were also stratified by 50 years of age, close to the median age.
Similar models were constructed for the secondary analysis assessing change in ED status and change in Framingham risk score. However as both age and baseline Framingham risk were accounted for in the outcome, we did not control for these in the models. In sensitivity analyses, we excluded 38 men who reported taking ED medications at either BACH I or II. In separate analyses, we also excluded 200 men classified as having ED at BACH II and answered “no sexual activity at all” or “did not attempt intercourse” to any of the IIEF-5 questions. In this subset we also assessed associations with continuous change scores in the IIEF-5.
In the Framing CVD risk models, betas reflect the difference in percentage Framingham risk among those with persistent or transient ED compared to those with no ED. In the change in Framingham CVD risk models, betas reflect the difference in the change in percentage Framingham risk over time among those with persistent or transient ED compared to the change in those without ED.
To preserve the maximum available sample size, multiple imputation was used to impute missing values by gender and race/ethnicity. Fifteen imputations were performed in SAS 9.1.3 (SAS Institute, Cary, NC). Because participants were selected using a stratified sampling scheme, observations were weighted inversely to their probability of selection, and weights were post-stratified to the Boston population in 2000. All percentages presented, unless otherwise noted, are weighted. Statistical significance for all testing was considered at the α=0.05 level. To accommodate use of multiple imputation datasets and survey weights, analyses were performed with SUDAAN 11.0.0 (RTI, Research Triangle Park, NC).
Results
Among the 965 men, mean age at baseline was 44.3 years (range 29.4-79.7) and 39.6% were white (Table 1). At baseline, the majority was overweight or obese (74.8%) and more than one-quarter were smokers (27.6%). Very few (< 3%) used ED medications at BACH I or II. Mean follow-up time was 4.8 years between BACH I and II and 7.1 years between BACH I and III. At BACH III, a total of 49 men (3.0%) reported CVD and the mean Framingham risk was 14.7% (SE 0.57) regardless of CVD status, with a mean change of 5.61% (SE 0.30) from baseline.
Table 1. Characteristics of male study participants free of CVD at BACH I and BACH II (n=965).
| No. | Weighted percent | |
|---|---|---|
| Age, mean (SE) (years)* | 44.3 | 0.57 |
| Race/ethnicity | ||
| White | 375 | 38.9† |
| Black | 297 | 30.8 |
| Hispanic | 293 | 30.4 |
| Married/living with partner (Y/N)* | 477 | 48.2 |
| Education* | ||
| HS or less | 432 | 55.6 |
| Some College | 325 | 29.7 |
| Advanced degree | 146 | 9.4 |
| Income ($) | ||
| <20,000 | 316 | 21.1 |
| 20,000-50,000 | 315 | 27.8 |
| >50,000 | 334 | 51.1 |
| BMI (kg/m2)* | ||
| Normal (<25) | 241 | 25.2 |
| Overweight (25-30) | 391 | 44.7 |
| Obese (>30) | 334 | 30.1 |
| Blood pressure (mmHg)* | ||
| Systolic | 123.3 | 0.65 |
| Diastolic | 77.5 | 0.52 |
| Smoking status* | ||
| Current | 404 | 45.5 |
| Former | 270 | 26.9 |
| Never | 291 | 27.6 |
| Physical activity* | ||
| Low | 235 | 21.1 |
| Medium | 482 | 49.5 |
| High - | 246 | 29.4 |
| Alcohol use (drinks/day)* | ||
| none | 285 | 22 |
| <1 | 358 | 41.2 |
| 1-3 | 219 | 27.3 |
| >3 | 103 | 9.5 |
| Depression (Y/N)* | 206 | 17.0 |
| Use of antihypertensives | ||
| BACH I | 205 | 13.2 |
| BACH II | 308 | 21.3 |
| Use of statins | ||
| BACH I | 112 | 8.8 |
| BACH II | 181 | 16.2 |
| Use of ED medication* | ||
| BACH I | 18 | 1.94 |
| BACH II | 29 | 2.92 |
| Change in ED status from BACH I to BACH II | ||
| No ED | 263 | 37.7 |
| Transient ED | 288 | 30.1 |
| Persistent ED | 414 | 32.6 |
| IIEF-5 | ||
| BACH I, mean (SE) | 21.0 | 0.21 |
| BACH II, mean (SE) | 18.34 | 0.47 |
determined at baseline;
Percents are unweighted
Forty-four percent and 50.9% of men had ED at BACH I and II, respectively, resulting in 30.1% with transient ED and 32.6% with persistent ED (Table 1). Distributions of ED severity at BACH I and II, as well changes from BACH I to II are shown in Table 2. Men with persistent ED had highest Framingham risk at BACH I and III, and also the largest increase in Framingham risk over time, followed by men with transient ED, and those with no ED (Table 3). A similar pattern was observed by age, with those ≥50 years having greater Framingham risk and change in risk over time than younger men.
Table 2. Distribution of ED status at BACH I and BACH II, no. (%).
| BACH II | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| BACH I | None | Mild | Mild-Moderate | Moderate | Severe | |
|
|
|
|||||
| None | 432 (55.6%) | 263 | 82 | 42 | 20 | 26 |
| Mild | 325 (29.7%) | 95 | 93 | 70 | 25 | 41 |
| Mild-Moderate | 146 (9.4%) | 19 | 38 | 43 | 18 | 28 |
| Moderate | 43 (3.7%) | 3 | 4 | 16 | 6 | 14 |
| Severe | 19 (1.6%) | 2 | 1 | 2 | 7 | 8 |
| 382 (49.1%) | 218 (18.5%) | 173 (12.9%) | 76 (6.8%) | 117 (12.7%) | ||
Table 3. Mean (SE) Framingham CVD risk (%) and change in Framingham CVD risk by change in ED status from BACH I to BACH II, overall and by age.
| Overall | <50 | ≥50 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| mean | SE | mean | SE | mean | SE | |
| Framingham CVDrisk, BACH I | ||||||
| No ED | 6.2 | 0.46 | 4.5 | 0.35 | 15.3 | 1.23 |
| Transient ED | 9.2 | 0.69 | 5.9 | 0.46 | 19.2 | 1.48 |
| Persistent ED | 13.0 | 1.07 | 6.0 | 0.56 | 24.1 | 1.67 |
| FraminghamCVD risk, BACH III | ||||||
| No ED | 10.4 | 0.65 | 8.4 | 0.56 | 22.0 | 1.68 |
| Transient ED | 15.5 | 0.93 | 11.4 | 0.85 | 27.8 | 1.73 |
| Persistent ED | 19.7 | 1.27 | 12.1 | 0.88 | 31.9 | 1.71 |
| Change in Framinghamrisk | ||||||
| No ED | 4.3 | 0.35 | 3.9 | 0.33 | 6.7 | 1.26 |
| Transient ED | 6.3 | 0.59 | 5.5 | 0.61 | 8.7 | 1.48 |
| Persistent ED | 6.7 | 0.64 | 6.0 | 0.70 | 7.7 | 1.24 |
In univariate analyses, transient (β=5.05, 95% CI: 2.8, 7.3) and persistent ED (β=9.22; 95% CI: 6.45, 12.0) were strongly associated with increased Framingham risk at BACH III as compared to those with no ED at either time point (Figure 2). Associations were largely attributable to age, as control for age substantially attenuated parameter estimates. In models fully adjusted for demographics, lifestyle factors, and baseline Framingham risk, transient and persistent ED were both associated with Framingham risk [transient ED β=1.89 (95% CI: 0.49, 3.30); persistent ED β=2.23 (95% CI: 0.79, 3.68)].
Figure 2.
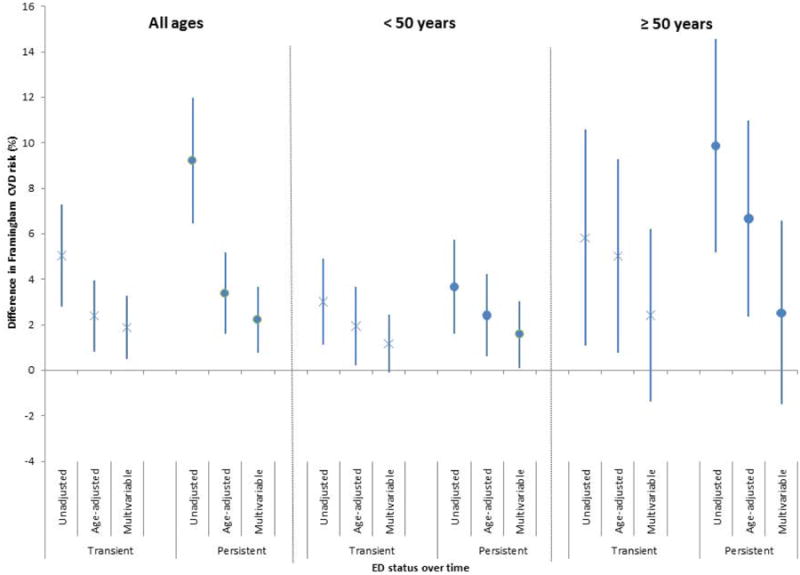
Difference in Framingham CVD risk (%) in men with transient and persistent ED compared to men without ED (all ages and stratified by age). Bars represent 95% CIs. Results are from unadjusted, age-adjusted, and multivariable-adjusted (demographics, lifestyle factors and baseline Framingham CVD risk) linear regression models. Men with transient and persistent ED over time had greater Framingham CVD risk compared to men without ED. Associations remained significant in men under 50 years after adjusting for baseline Framingham risk, but not in men over 50 years.
When stratified by age, transient ED was marginally associated with Framingham risk (β=1.17; 95% CI: -0.09, 2.44) while persistent ED was significantly associated with Framingham risk (β=1.58; 95% CI: 0.11, 3.06) in men <50 years in a fully-adjusted model. In men ≥ 50 years larger parameter estimates were observed between both transient and persistent ED and Framingham risk as compared to younger men in both univariate and adjusted models, however associations were not statistically significant in the full-adjusted models (Figure 2).
When we assessed associations between change in ED status and change in Framingham risk over time, transient and persistent ED were both associated with a greater change in risk as compared to those without ED at either time points in univariate models [β=2.01 (95% CI: 0.69, 3.33) for transient ED and β=2.38 (0.99, 3.76) for persistent ED] (Figure 3). With adjustment for demographic and lifestyle variables, associations were attenuated but remained statistically significant [β=1.77 (0.39, 3.15) for transient ED and β=1.96 (0.57, 3.35) for persistent ED].
Figure 3.
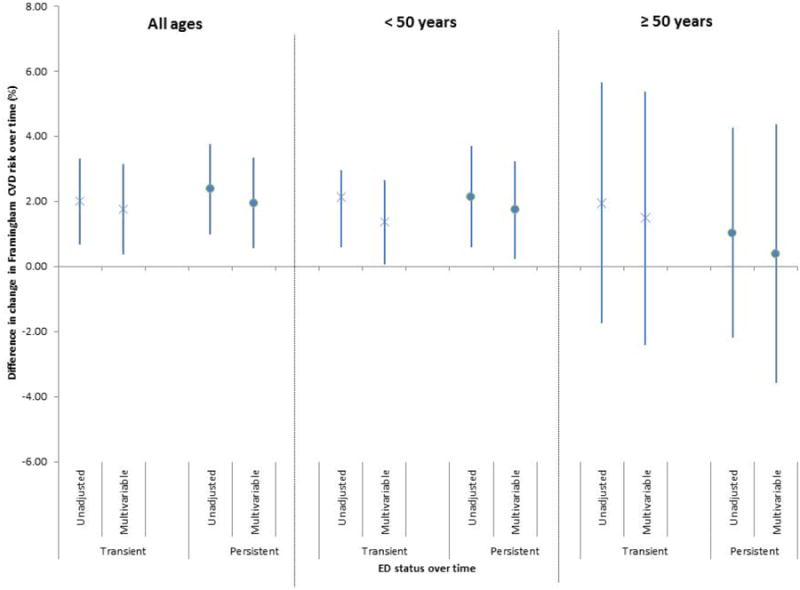
Difference in change in Framingham CVD risk over time (%) in men with transient and persistent ED compared to men without ED (all ages and stratified by age). Bars represent 95% CIs. Results are from unadjusted and multivariable-adjusted (demographics and lifestyle factors) linear regression models. Both transient and persistent ED were associated with a greater change in Framingham risk over time in men under 50 years, but not over 50 years.
When stratified by age, both transient and persistent ED in younger men were associated with change in Framingham risk over time in univariate and multivariable models, with a greater magnitude of change in those with persistent ED (Figure 3). In older men, neither transient nor persistent ED was associated with change in Framingham risk over time in univariate or multivariable models.
Sensitivity analyses
In analyses excluding 38 men taking ED medication at BACH I or BACH II, associations between both transient and persistent ED with Framingham risk were more pronounced in fully adjusted models, with significant associations in those <50 years, but not those ≥50 years as in previous models. Similarly for the analysis of change in Framingham risk, parameter estimates became larger, however findings remained the same.
In analyses excluding 200 men classified as having ED at BACH II because they endorsed no attempt at sexual intercourse on the IIEF-5, parameter estimates were generally reduced however overall findings remained the same with persistent ED associated with Framingham risk in younger men even after accounting for baseline Framingham risk, and neither transient nor persistent ED associated with Framingham risk in older men. Similarly, persistent ED in men <50 years remained significantly associated with change in Framingham risk while no associations were observed for transient or persistent ED in older men, as in the main analysis. When outcomes were regressed on continuous change scores in this subset of individuals, associations were null in univariate and multivariable models.
Discussion
In an observational population-based study of 965 men, Framingham CVD risk, and change in Framingham CVD risk over time were highest in men with persistent ED, measured by a validated questionnaire, regardless of severity. Both transient and persistent ED were associated with elevated Framingham risk, approximately seven years from baseline, however a large portion of these associations were attributable to age. In men <50 years, persistent ED was associated with increased Framingham risk, even after adjustment for baseline Framingham risk and other factors. Associations between Framingham risk and transient ED trended towards significance in the expected direction. In men ≥50 years, associations between Framingham risk and both transient and persistent ED were larger than that of men < 50 years, but multivariable associations were not significant. When we examined associations with change in Framingham risk over time from baseline to BACH III, transient and persistent ED were both associated with an increased Framingham risk over time in younger men while no associations were observed in older men, which is likely attributable to higher baseline CVD risk in older men generally. Findings were robust to sensitivity analyses excluding individuals taking ED medications at BACH I or BACH II, as well as individuals classified as having ED at BACH II based on endorsements of no sexual activity on the later version of the IIEF-5. We also stratified by an age cutoff of 55, and our findings remained the same. These findings suggest that in younger men, having ED, especially persistent ED, is a significant marker of subclinical CVD risk.
While previous studies have not assessed changes in ED status and Framingham risk, our findings are consistent with results from cross-sectional and longitudinal studies that suggest ED is a marker of increased CVD risk in younger men (age <50 or <60 years), but less obviously so in older men. In a case control study of 114 men with CAD and 128 controls, risk of CAD among men with ED as assessed with the IIEF, was higher in those <60 years (20). In a population-based cross-sectional study of 2,869 men ages 20-60 years, increased CHD (assessed using the FRS) and stroke risk in men was associated with moderate to severe ED but not mild ED, with especially pronounced risk of CHD in men 40-49 years (25). Longitudinal data from the Olmsted County Study on 1,402 men ≥40 years with no known CAD at baseline, show that baseline ED was associated with a marked increase in the risk of future cardiac events in men <50 years of age over a 10-year follow-up period, whereas in older men the prognostic importance of ED was diminished (19). Similarly, a retrospective cohort study design of Australian men also observed that ED was predictive of atherosclerotic CV events, with stronger associations in men presenting with ED at a younger age, and decreasing predictive value of ED among older men (18).
Endothelial dysfunction, characterized by impaired nitric oxide bioavailability, is a precursor of atherosclerotic lesions and has been established as an important underlying mechanism linking ED and CVD (26, 27). Given the smaller diameter of the penile arteries relative to the heart and brain, it is thought that the penile corpora may be more susceptible to the effects of reduced vasodilation resulting from impaired NO uptake into the arterial wall, leading to earlier clinical manifestation in the penis (28).
We were able to take advantage of longitudinal data on ED and CVD risk factors in a large population-based study including a wide age range of adult men, to assess for the first time associations between changes in ED status, Framingham CVD risk and changes in Framingham CVD risk, however this study is not without limitations. Most notably, we were not able to assess associations between change in ED status and risk of clinical CVD. Our findings however remain informative in showing that changes in ED status may be associated with pre-clinical CVD. Further, with only two assessments of ED status, we could not assess with confidence incident ED and therefore assessed transient ED. It is possible that CVD risk among men with incident ED versus non-incident ED differed. Nonetheless our study found that the presence of ED at either time point, whether incident or not, was marginally associated with increased Framingham risk particularly among those <50 years, and associated with an increase in Framingham risk over time. Of note, the American College of Cardiology and the American Heart Association released a revised 10-year CVD risk calculator in November 2013, shortly after our study was conducted. The revised calculator has been shown to overestimate risk in several large external validation cohorts (29), as discussed in an on-going debate (e.g. (30, 31)), and as such additional data are needed before implementation of this new risk calculator. Another potential limitation is assessment of ED with a modified IIEF-5 at BACH II, which may have misclassified men as having severe ED if they responded “no sexual activity” or “did not attempt sexual intercourse” for reasons other than ED (i.e. no sexual partner). Despite this potential misclassification, our findings largely persisted with exclusion of these individuals.
Conclusions
Using longitudinal data from a population-based sample of men without CVD, our findings suggest that younger men with persistent ED have an increased Framingham risk that is independent of traditional CVD risk factors. Findings further support clinical assessment of CVD risk in men <50 years presenting with ED. Future longitudinal studies with additional assessments of ED and CVD risk in younger men will to help elucidate the relationship of ED status over time and risk and development of CVD.
Acknowledgments
This study was funded by grant no. 5R01DK080662 from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK).
Footnotes
Conflict of Interest: None
References
- 1.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120(2):151–7. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Feldman HA, Johannes CB, Derby CA, Kleinman KP, Mohr BA, Araujo AB, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med. 2000;30(4):328–38. doi: 10.1006/pmed.2000.0643. [DOI] [PubMed] [Google Scholar]
- 3.Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. 2006;166(2):207–12. doi: 10.1001/archinte.166.2.207. [DOI] [PubMed] [Google Scholar]
- 4.Schouten BW, Bohnen AM, Bosch JL, Bernsen RM, Deckers JW, Dohle GR, et al. Erectile dysfunction prospectively associated with cardiovascular disease in the Dutch general population: results from the Krimpen Study. Int J Impot Res. 2008;20(1):92–9. doi: 10.1038/sj.ijir.3901604. [DOI] [PubMed] [Google Scholar]
- 5.Solomon H, Man JW, Jackson G. Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart. 2003;89(3):251–3. doi: 10.1136/heart.89.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billups KL, Bank AJ, Padma-Nathan H, Katz S, Williams R. Erectile dysfunction is a marker for cardiovascular disease: results of the minority health institute expert advisory panel. J Sex Med. 2005;2(1):40–50. doi: 10.1111/j.1743-6109.2005.20104_1.x. discussion -2. [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294(23):2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 8.Vlachopoulos CV, Terentes-Printzios DG, Ioakeimidis NK, Aznaouridis KA, Stefanadis CI. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: a systematic review and meta-analysis of cohort studies. Circulation Cardiovascular quality and outcomes. 2013;6(1):99–109. doi: 10.1161/CIRCOUTCOMES.112.966903. [DOI] [PubMed] [Google Scholar]
- 9.Araujo AB, Hall SA, Ganz P, Chiu GR, Rosen RC, Kupelian V, et al. Does erectile dysfunction contribute to cardiovascular disease risk prediction beyond the Framingham risk score? J Am Coll Cardiol. 2010;55(4):350–6. doi: 10.1016/j.jacc.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vlachopoulos C, Ioakeimidis N, Terentes-Printzios D, Stefanadis C. The triad: erectile dysfunction--endothelial dysfunction--cardiovascular disease. Current pharmaceutical design. 2008;14(35):3700–14. doi: 10.2174/138161208786898716. [DOI] [PubMed] [Google Scholar]
- 11.Grover SA, Lowensteyn I, Kaouache M, Marchand S, Coupal L, DeCarolis E, et al. The prevalence of erectile dysfunction in the primary care setting: importance of risk factors for diabetes and vascular disease. Arch Intern Med. 2006;166(2):213–9. doi: 10.1001/archinte.166.2.213. [DOI] [PubMed] [Google Scholar]
- 12.Bohm M, Baumhakel M, Teo K, Sleight P, Probstfield J, Gao P, et al. Erectile dysfunction predicts cardiovascular events in high-risk patients receiving telmisartan, ramipril, or both: The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (ONTARGET/TRANSCEND) Trials. Circulation. 2010;121(12):1439–46. doi: 10.1161/CIRCULATIONAHA.109.864199. [DOI] [PubMed] [Google Scholar]
- 13.Araujo AB, Travison TG, Ganz P, Chiu GR, Kupelian V, Rosen RC, et al. Erectile dysfunction and mortality. J Sex Med. 2009;6(9):2445–54. doi: 10.1111/j.1743-6109.2009.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Travison TG, Shabsigh R, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB. The natural progression and remission of erectile dysfunction: results from the Massachusetts Male Aging Study. J Urol. 2007;177(1):241–6. doi: 10.1016/j.juro.2006.08.108. discussion 6. [DOI] [PubMed] [Google Scholar]
- 15.Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D'Andrea F, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291(24):2978–84. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 16.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. Jama. 2004;292(12):1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 17.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 18.Chew KK, Finn J, Stuckey B, Gibson N, Sanfilippo F, Bremner A, et al. Erectile dysfunction as a predictor for subsequent atherosclerotic cardiovascular events: findings from a linked-data study. J Sex Med. 2010;7(1 Pt 1):192–202. doi: 10.1111/j.1743-6109.2009.01576.x. [DOI] [PubMed] [Google Scholar]
- 19.Inman BA, Sauver JL, Jacobson DJ, McGree ME, Nehra A, Lieber MM, et al. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc. 2009;84(2):108–13. doi: 10.4065/84.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riedner CE, Rhoden EL, Fuchs SC, Wainstein MV, Goncalves SC, Wainstein RV, et al. Erectile dysfunction and coronary artery disease: an association of higher risk in younger men. J Sex Med. 2011;8(5):1445–53. doi: 10.1111/j.1743-6109.2011.02224.x. [DOI] [PubMed] [Google Scholar]
- 21.McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) Survey. Eur Urol. 2007;52(2):389–96. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccolo RS, Araujo AB, Pearce N, McKinlay JB. Cohort Profile: The Boston Area Community Health (BACH) survey. International journal of epidemiology. 2012 doi: 10.1093/ije/dys198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11(6):319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 24.Cappelleri JC, Siegel RL, Glasser DB, Osterloh IH, Rosen RC. Relationship between patient self-assessment of erectile dysfunction and the sexual health inventory for men. Clin Ther. 2001;23(10):1707–19. doi: 10.1016/s0149-2918(01)80138-7. [DOI] [PubMed] [Google Scholar]
- 25.Ponholzer A, Temml C, Obermayr R, Wehrberger C, Madersbacher S. Is erectile dysfunction an indicator for increased risk of coronary heart disease and stroke? Eur Urol. 2005;48(3):512–8. doi: 10.1016/j.eururo.2005.05.014. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 26.Ganz P. Erectile dysfunction: pathophysiologic mechanisms pointing to underlying cardiovascular disease. Am J Cardiol. 2005;96(12B):8M–12M. doi: 10.1016/j.amjcard.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Billups KL. Sexual dysfunction and cardiovascular disease: integrative concepts and strategies. Am J Cardiol. 2005;96(12B):57M–61M. doi: 10.1016/j.amjcard.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Montorsi P, Ravagnani PM, Galli S, Rotatori F, Briganti A, Salonia A, et al. The artery size hypothesis: a macrovascular link between erectile dysfunction and coronary artery disease. Am J Cardiol. 2005;96(12B):19M–23M. doi: 10.1016/j.amjcard.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382(9907):1762–5. doi: 10.1016/S0140-6736(13)62388-0. [DOI] [PubMed] [Google Scholar]
- 30.Muntner P, Safford MM, Cushman M, Howard G. Comment on the reports of over-estimation of ASCVD risk using the 2013 AHA/ACC risk equation. Circulation. 2014;129(2):266–7. doi: 10.1161/CIRCULATIONAHA.113.007648. [DOI] [PubMed] [Google Scholar]
- 31.Cook NR, Ridker PM. Response to Comment on the reports of over-estimation of ASCVD risk using the 2013 AHA/ACC risk equation. Circulation. 2014;129(2):268–9. doi: 10.1161/CIRCULATIONAHA.113.007680. [DOI] [PubMed] [Google Scholar]


