Abstract
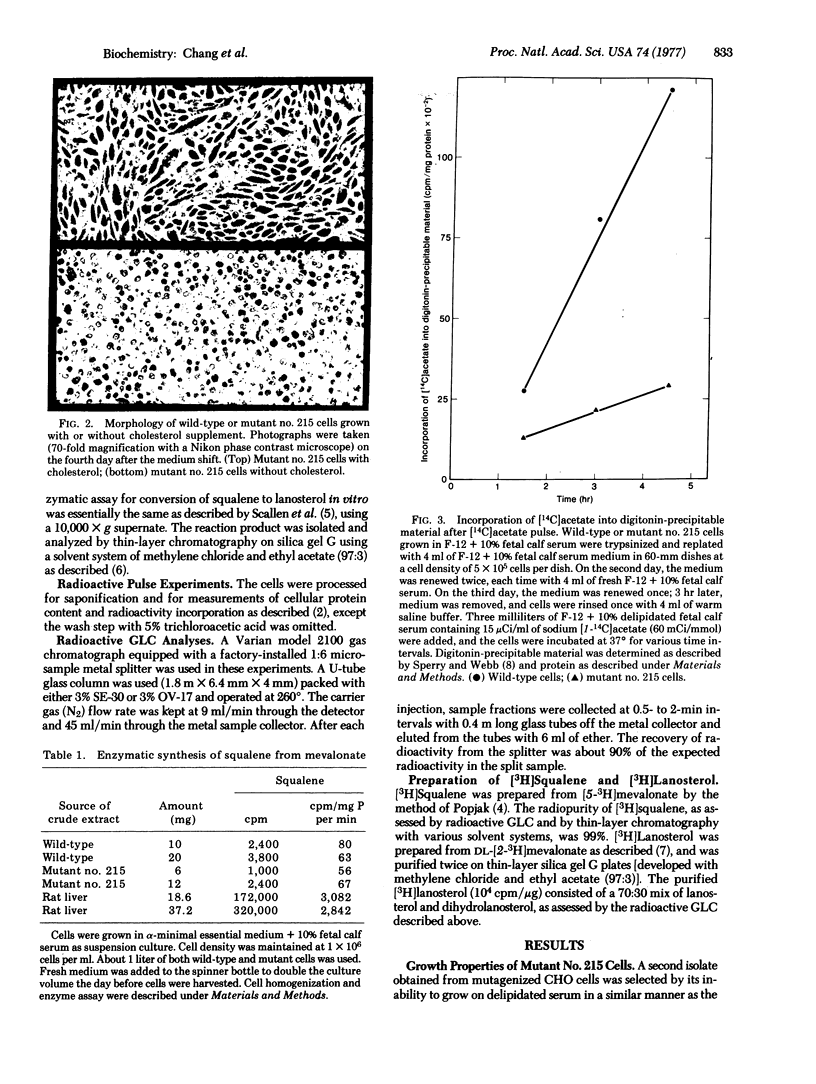
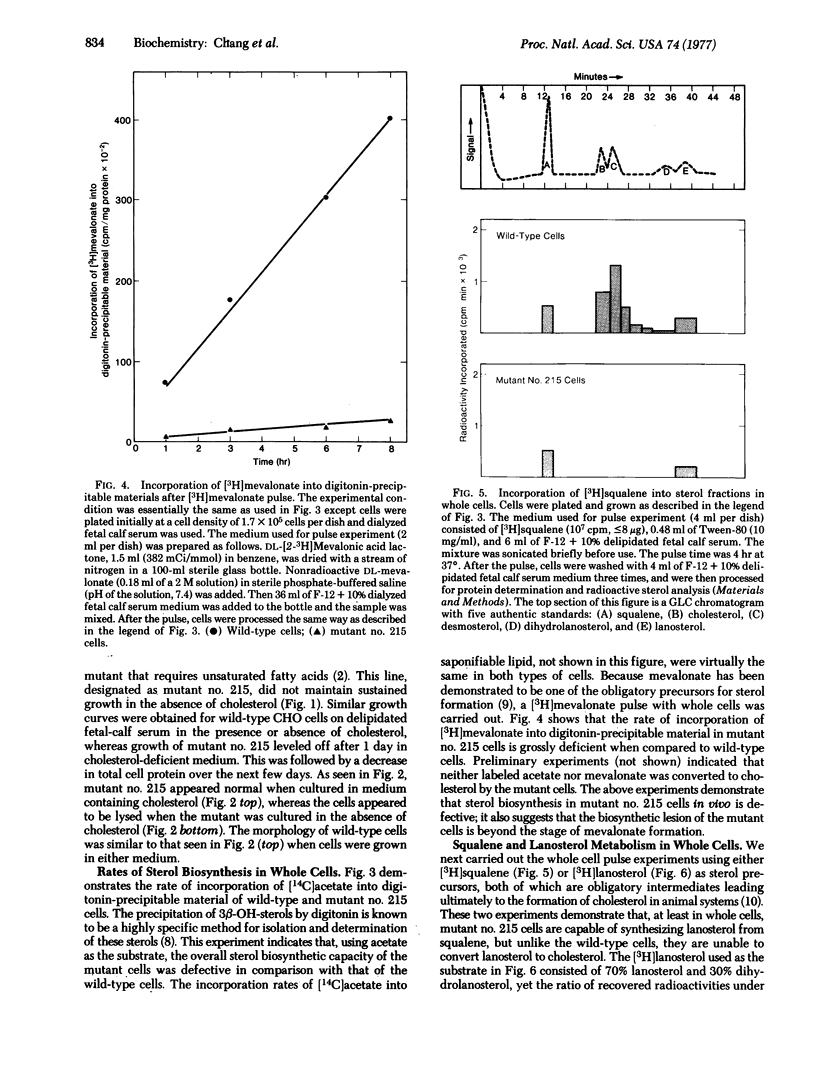
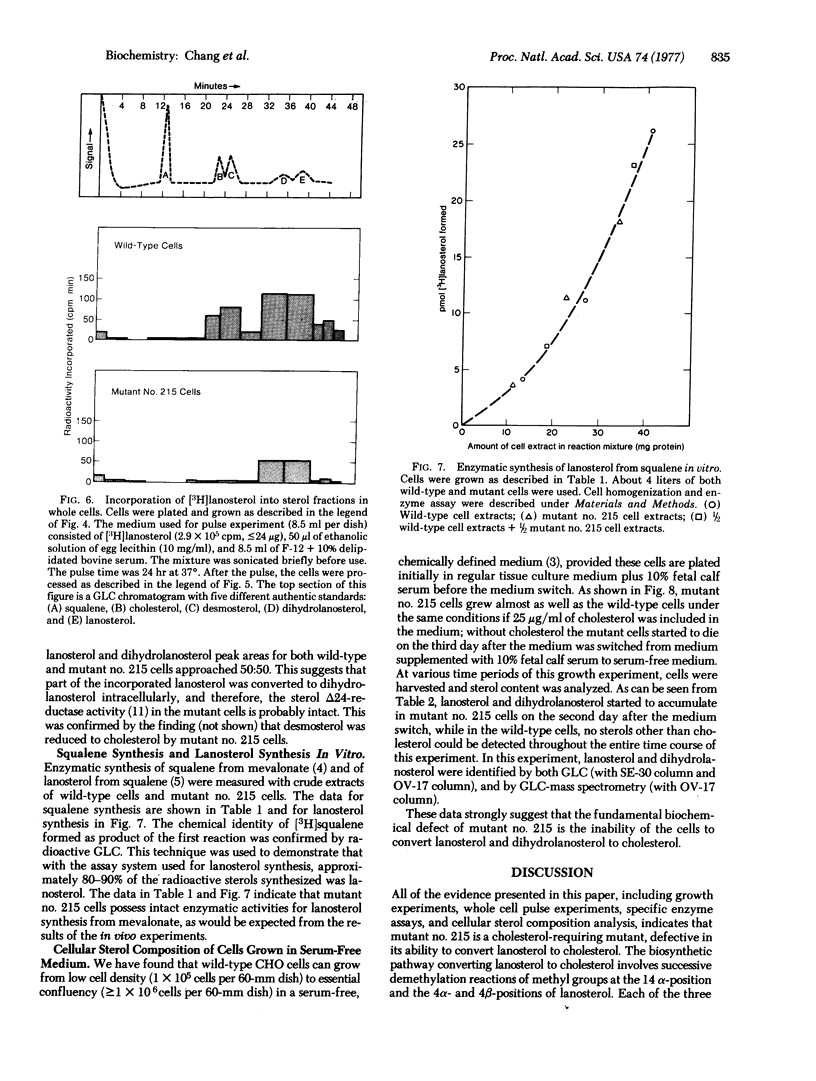
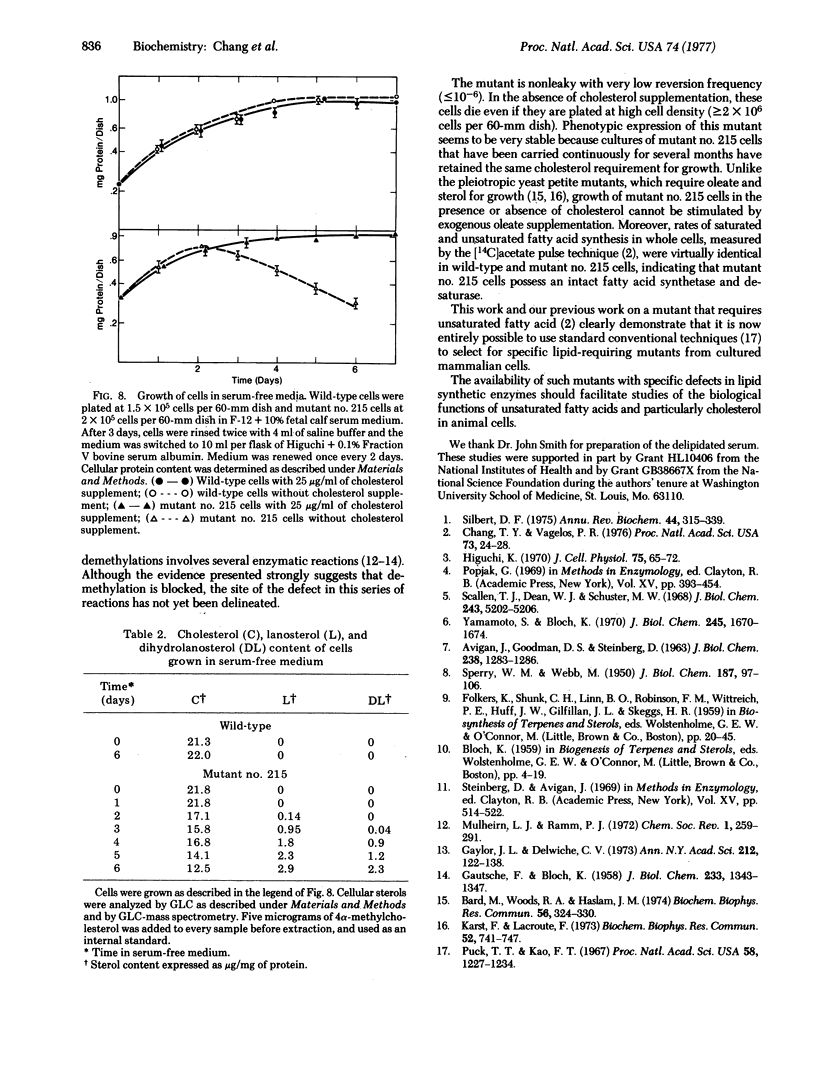
A sterol-requiring mutant has been isolated from mutagenized Chinese hamster ovary cells. This mutant grows normally only when cholesterol is present in the medium. Cell lysis occurs within 3 days in the absence of cholesterol. The frequency of reversion of this mutant to prototrophic growth is low (less than or equal to 10(-6). Whole cell pulse experiments with [14C]acetate or [3H]mevalonate indicate that the rate of synthesis of digitonin-precipitable material is greatly diminished in the mutant cells as compared to that in normal Chinese hamster ovary cells. Enzyme assays in vitro with crude cell extracts show that the biosynthetic conversion of mevalonate to squalene and the conversion of squalene to lanosterol are not impaired in the mutant cells. Gas-liquid chromatographic analyses of radioactive sterol composition after whole cell pulse experiments with [3H]squalene and with [3H]anosterol suggest that the fundamental enzymatic defect of the mutant is at the stage of lanosterol demethylation. When cells were grown in serum-free medium, lanosterol and dihydrolanosterol accumulated intracellularly in the mutant cells before cell lysis occurred; neither of these two intermediary sterols was detected in the wild-type cells grown under the same condition.
Full text
PDF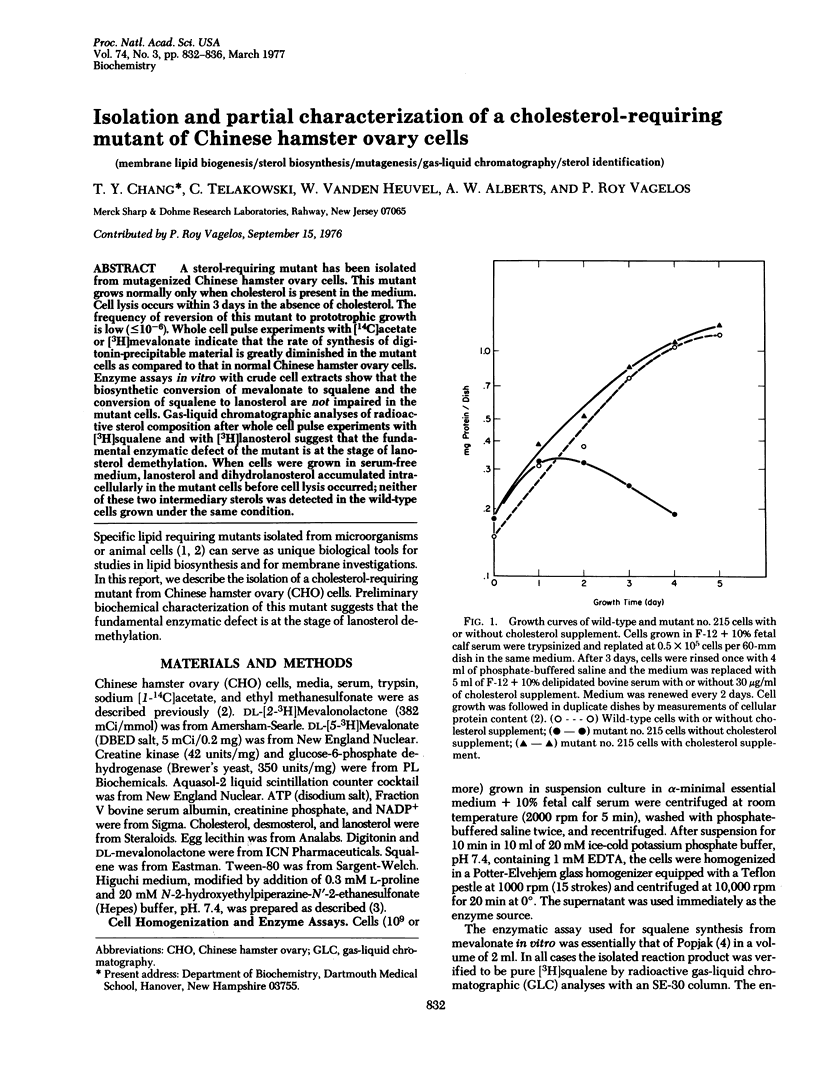
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAN J., GOODMAN D. S., STEINBERG D. Studies of cholesterol biosynthesis. IV. Reduction of lanosterol to 24,25-dihydrolanosterol by rat liver homogenates. J Biol Chem. 1963 Apr;238:1283–1286. [PubMed] [Google Scholar]
- Bard M., Woods R. A., Haslam J. M. Porphyrine mutants of Saccharomyces cerevisiae: correlated lesions in sterol and fatty acid biosynthesis. Biochem Biophys Res Commun. 1974 Jan 23;56(2):324–330. doi: 10.1016/0006-291x(74)90845-6. [DOI] [PubMed] [Google Scholar]
- Chang T. Y., Vagelos P. R. Isolation and characterization of an unsaturated fatty acid-requiring mutant of cultured mammalian cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):24–28. doi: 10.1073/pnas.73.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUTSCHI F., BLOCH K. Synthesis of isomeric 4,4-dimethylcholestenols and identification of a lanosterol metabolite. J Biol Chem. 1958 Dec;233(6):1343–1347. [PubMed] [Google Scholar]
- Gaylor J. L., Delwiche C. V. Investigation of the multienzymic system of microsomal cholesterol biosynthesis. Ann N Y Acad Sci. 1973;212:122–138. doi: 10.1111/j.1749-6632.1973.tb47591.x. [DOI] [PubMed] [Google Scholar]
- Higuchi K. An improved chemically defined culture medium for strain L mouse cells based on growth responses to graded levels of nutrients including iron and zinc ions. J Cell Physiol. 1970 Feb;75(1):65–72. doi: 10.1002/jcp.1040750108. [DOI] [PubMed] [Google Scholar]
- Karst F., Lacroute F. Isolation of pleiotropic yeast mutants requiring ergosterol for growth. Biochem Biophys Res Commun. 1973 Jun 8;52(3):741–747. doi: 10.1016/0006-291x(73)90999-6. [DOI] [PubMed] [Google Scholar]
- Puck T. T., Kao F. T. Genetics of somatic mammalian cells. V. Treatment with 5-bromodeoxyuridine and visible light for isolation of nutritionally deficient mutants. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1227–1234. doi: 10.1073/pnas.58.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERRY W. M., WEBB M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950 Nov;187(1):97–106. [PubMed] [Google Scholar]
- Scallen T. J., Dean W. J., Schuster M. W. Enzymatic conversion of squalene to cholesterol by an acetone powder of rat liver microsomes. J Biol Chem. 1968 Oct 10;243(19):5202–5206. [PubMed] [Google Scholar]
- Silbert D. F. Genetic modification of membrane lipid. Annu Rev Biochem. 1975;44:315–339. doi: 10.1146/annurev.bi.44.070175.001531. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Bloch K. Studies on squalene epoxidase of rat liver. J Biol Chem. 1970 Apr 10;245(7):1670–1674. [PubMed] [Google Scholar]