Abstract
Background
Leptomeningeal metastases (LM) are an increasingly frequent and devastating complication of anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer (NSCLC). Currently, the optimal management of LM in ALK-positive patients remains poorly understood as these patients have been routinely excluded from clinical trials.
Methods
We describe four ALK-positive patients with LM who were treated with the next-generation ALK inhibitor alectinib through single-patient, compassionate use protocols at two institutions. All patients had previously been treated with both FDA-approved ALK inhibitors—crizotinib and ceritinib. Patients received alectinib at a starting dose of 600 mg twice daily.
Results
Four ALK-positive NSCLC patients with symptomatic leptomeningeal disease were identified. Three of four patients experienced significant clinical and radiographic improvements in LM upon treatment with alectinib. A fourth patient had stable intracranial disease for four months before eventual systemic disease progression. Overall, alectinib was well tolerated. One patient required dose reduction due to grade 2 hyperbilirubinemia.
Conclusions
Alectinib is active in ALK-rearranged NSCLC patients with LM, including in patients previously treated with crizotinib and ceritinib. Additional prospective studies of alectinib in ALK-positive patients with LM are warranted.
Keywords: ALK, anaplastic lymphoma kinase, leptomeningeal metastases, alectinib
Introduction
Anaplastic lymphoma kinase (ALK) rearrangements are important therapeutic targets in non-small cell lung cancer (NSCLC) that confer sensitivity to ALK tyrosine kinase inhibitors (TKIs).1, 2 Crizotinib was the first ALK TKI approved for ALK-positive NSCLC based upon randomized studies demonstrating significant improvements in objective response rates (ORRs) and progression-free survival (PFS) compared to cytotoxic chemotherapy.3 Despite this activity, however, patients ultimately progress on therapy, at which time management approaches commonly include treatment with second-generation ALK inhibitors (e.g., ceritinib, AP26113 and alectinib) or chemotherapy.
Recently, the central nervous system (CNS) has emerged as an important sanctuary site in ALK-positive NSCLC. Approximately 26% of ALK-positive patients with newly diagnosed, metastatic disease have CNS metastases.4 The incidence of CNS metastases also appears to increase with disease course. For example, among crizotinib-treated patients enrolled on trials of second-generation ALK inhibitors, rates of CNS metastases have been as high as 60%.5 ALK-positive patients may also experience less common forms of CNS involvement, such as spinal cord intramedullary and leptomeningeal metastases (LM).6, 7
Herein, we present a series of four ALK-positive NSCLC patients who developed LM on or after treatment with crizotinib and ceritinib, and were treated with the second-generation ALK inhibitor alectinib (CH5424802/RO5424802). Alectinib is a novel, highly potent and selective ALK inhibitor that has demonstrated promising anti-tumor activity in early phase trials.8
Methods
Patients were treated with alectinib through single-patient, compassionate use protocols at two institutions: Massachusetts General Hospital and the Medical University of South Carolina. All patients received alectinib at a starting dose of 600 mg twice daily, which is the established recommended phase II dose.8 Consistent with prior reports, patients were considered to have LM if they had cytologically confirmed malignant cells in cerebrospinal fluid (CSF) or gadolinium-enhanced magnetic resonance imaging (MRI) consistent with LM (i.e., presence of abnormal leptomeningeal enhancement or enhancing subarachnoid nodules).9
Results
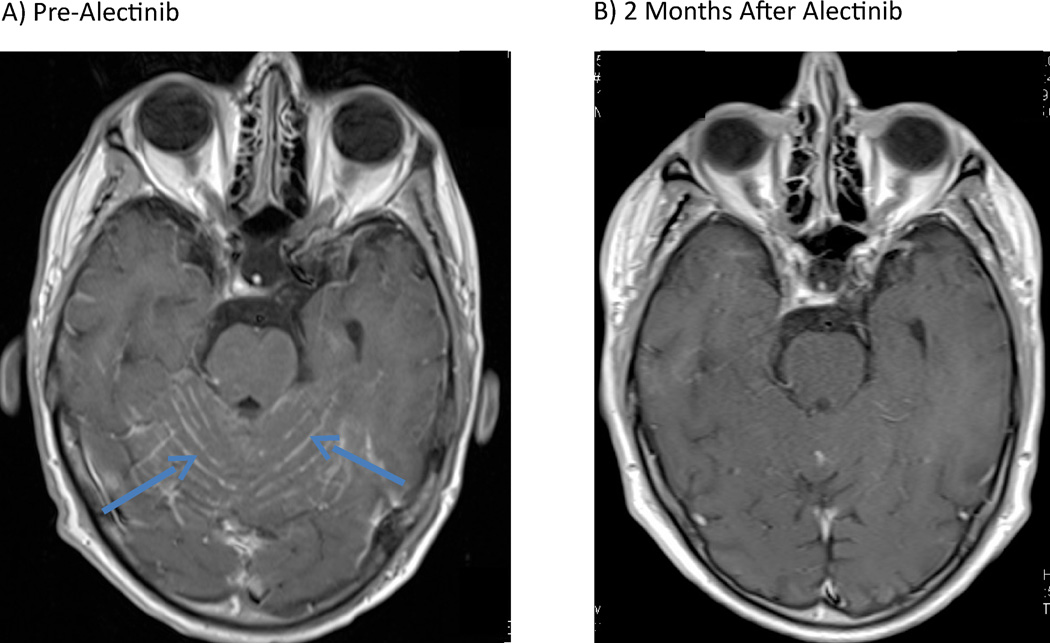
Case 1 involves a 56 year-old man with metastatic, ALK-rearranged NSCLC. A baseline MRI was negative for intracranial metastases. He was initially treated with crizotinib, achieving a significant radiographic response. Sixteen months later, however, he developed osseous metastases and multiple new, asymptomatic brain parenchymal metastases. Radiation therapy (RT) was deferred in favor of treatment with the second-generation ALK inhibitor ceritinib. Despite significant improvement of his systemic disease on ceritinib, repeat neuroimaging demonstrated progressive CNS disease, including new leptomeningeal enhancement. Soon thereafter, the patient experienced a generalized seizure, prompting treatment with corticosteroids and whole brain radiotherapy (WBRT). His post-RT course was complicated by persistent fatigue, word-finding difficulties and intermittent confusion. Ceritinib, which had been held throughout radiation, was resumed. Repeat neuroimaging two months later demonstrated interval improvement in numerous brain metastases, but unchanged leptomeningeal enhancement. The patient’s mental status and mobility continued to decline, ultimately prompting the transition to alectinib (600 mg twice daily). Within several weeks, he experienced significant improvements in cognition, energy, and mobility. Alectinib was well tolerated without significant adverse events (AEs). Repeat imaging two months later demonstrated near complete resolution of leptomeningeal enhancement (Figure 1) and stable systemic disease. The patient has remained on alectinib for five months with continued intracranial response.
Figure 1.
T1, post-gadolinium magnetic resonance images (MRI) depicting near complete resolution of leptomeningeal enhancement in a patient treated with alectinib. A) Before treatment with alectinib (blue arrows = abnormal leptomeningeal enhancement). B) Two months after initiation of treatment with alectinib.
Case 2 involves a 50 year-old man with metastatic ALK-positive NSCLC treated with first-line crizotinib. He initially responded to crizotinib, but developed grade 4 transaminase elevation, ultimately prompting discontinuation of therapy. He subsequently received carboplatin/pemetrexed, maintenance pemetrexed, and ceritinib. Notably, a pre-ceritinib brain MRI revealed asymptomatic CNS parenchymal metastases, which initially improved on therapy. Repeat neuroimaging seven months later, however, demonstrated innumerable new brain parenchymal metastases as well as leptomeningeal enhancement. He underwent radiosurgery (Gamma Knife®) to multiple target lesions followed by eventual WBRT. Four months later, the patient developed left-sided ptosis, diplopia, slurred speech and headaches. Repeat imaging showed worsening LM, including new diffuse enhancement throughout the leptomeninges of the spine. Ceritinib was discontinued. He began alectinib in March 2014. After three weeks of therapy, he had dramatic improvements in headaches, speech, diplopia and performance status. Repeat imaging two months later demonstrated significant interval improvement in leptomeningeal enhancement throughout the neuraxis. No significant AEs were observed while on alectinib. After six months of therapy, however, the patient developed recurrent neurologic symptoms. Imaging confirmed progressive LM, as well as interval progression in the liver. He was ultimately transitioned to hospice and died in October 2014.
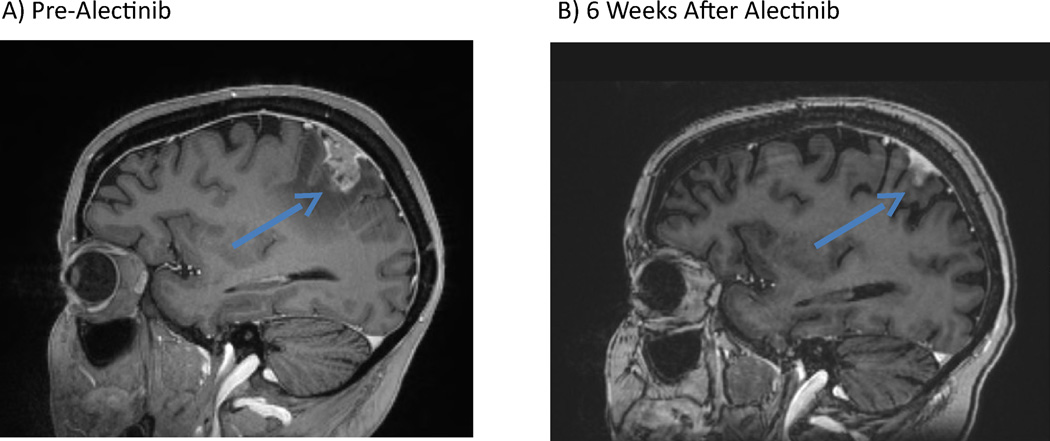
Case 3 involves a 39 year-old woman with metastatic, ALK-positive NSCLC initially treated with cisplatin/pemetrexed (one cycle) followed by crizotinib. She remained on therapy with good systemic disease control until February 2013, when a brain MRI revealed new left parietal dural enhancement with extension into the leptomeninges. She was treated with WBRT, resuming crizotinib upon completion. Two months later, she was found to have systemic disease progression with stable neuroimaging. She subsequently received ceritinib followed by carboplatin/pemetrexed/crizotinib. In August 2014, she developed headaches, right-sided weakness, visual hallucinations and focal seizure activity. A brain MRI showed an enlarging left parietal leptomeningeal-based lesion with extension of LM enhancement (Figure 2). She was placed on corticosteroids and levetiracetam. Intrathecal chemotherapy was deferred due to its unclear efficacy in large nodular dural-based disease. She began alectinib 600 mg twice daily. She tolerated alectinib well with no significant treatment-related AEs. Her right-sided weakness gradually improved and her seizures resolved, and her corticosteroids were tapered off. After six weeks of alectinib, repeat neuroimaging demonstrated significant interval reduction in nodular dural-based and LM enhancement. The patient remains on alectinib at this time with no evidence of progression in her CNS disease.
Figure 2.
Regression of a nodular leptomeningeal metastasis in an ALK-positive patient treated with alectinib. Sagittal, T1 post-gadolinium magnetic resonance images A) prior to treatment with alectinib (blue arrows = abnormal, nodular leptomeningeal enhancement) and B) six weeks after starting alectinib.
Case 4 involves a 49 year-old woman diagnosed with stage IIA (T2bN0M0) NSCLC in February 2013. She underwent surgical resection and four cycles of adjuvant cisplatin/pemetrexed. In October 2013, she developed pulmonary nodules and a pleural effusion, consistent with recurrent disease. ALK FISH performed on her resection specimen revealed an ALK rearrangement. She began treatment with crizotinib. Of note, a brain MRI performed shortly after starting crizotinib was negative for intracranial metastases. She remained on crizotinib for seven months, transitioning to ceritinib upon disease progression. After one month of ceritinib, she required hospitalization for worsening fatigue, dypsnea, and dysgeusia. Brain MRI revealed innumerable brain parenchymal metastases with leptomeningeal involvement. Ceritinib was discontinued. She was started on corticosteroids and experienced an improvement in her fatigue and performance status. Upon discharge, she began alectinib 600 mg twice daily. Treatment was briefly interrupted due to grade 2 hyperbilirubinemia, which required dose reduction to 450 mg twice daily. Her CNS disease remained stable over the next four months; however, she ultimately developed disease progression in the chest.
Discussion
LM in NSCLC have been historically associated with a dismal prognosis (median survival 3.0–4.3 months).10, 11 Importantly, patients with LM have been routinely excluded from clinical trials; thus, data on management largely comes from retrospective analyses involving heterogeneous patient populations. Common treatment strategies have included WBRT, intrathecal or systemic chemotherapy, and palliative ventriculoperitoneal shunting. Among NSCLC patients with EGFR mutations, “pulsatile” EGFR inhibition has also been explored, and data suggests that this achieves higher cerebrospinal fluid (CSF) drug concentrations, controlling LM in a subset of patients.12
In ALK-positive NSCLC, LM are present in ~4% of patients,6 but the optimal management of this complication is poorly defined. Early studies focused on the use of crizotinib, which has been associated with a 12-week intracranial disease control rate of 56% in patients with untreated brain parenchymal metastases; however, only 7% of patients have objective intracranial responses.13 It has also been recognized that the CNS is a frequent site of relapse on crizotinib, commonly in the setting of continued systemic disease control—likely reflecting a pharmacokinetic failure of therapy. Indeed, in one case report, the CSF-to-plasma ratio of crizotinib was very low (0.0026) in a patient following crizotinib administration, suggesting poor blood-brain barrier (BBB) penetration.14 Investigators have since explored using high-dose crizotinib (1000 mg once daily) or high-dose crizotinib (600 mg once daily) plus pemetrexed (900 mg/m2) in patients with brain metastases, but such reports were small and didn’t include patients with LM.15, 16 Crizotinib has also been combined with intrathecal methotrexate in one report, producing cytological responses in two patients with LM; however, the relative contributions of crizotinib versus methotrexate on treatment effects in this series were unclear.7
Given crizotinib’s limited CNS activity, emphasis has been placed on identifying new ALK inhibitors with improved CNS penetration. Here, we report a series of four ALK-positive patients with LM who experienced clinical benefit on alectinib. All four patients were diagnosed with LM based on characteristic MRI features and symptoms. Although CSF cytology has been considered the historical gold standard for LM, it has low sensitivity.17 Contrast-enhanced MRI, with its improved visualization of the subarachnoid space, is now considered sufficient to establish the diagnosis in a patient with typical clinical features and has become the initial, and frequently sole, diagnostic tool.9, 18 Impressively, three of four patients with LM experienced radiographic and clinical improvement, while a fourth had stable imaging findings. In animal models, alectinib shows high brain-to-plasma ratios (0.63–0.94) and activity in intracranial tumor implantation models.19 In contradistinction to crizotinib and ceritinib,20 preclinical studies also suggest that alectinib is not a substrate of P-glycoprotein (P-gp), a key drug efflux pump typically expressed in the BBB.19 Clinically, objective responses have been seen in 55% of crizotinib-resistant/intolerant patients treated with alectinib in an ongoing phase I/II trial.8 Importantly, among 21 patients with baseline CNS lesions in this study, 52% had objective responses in the CNS. Moreover, measurable concentrations of alectinib were found in 5/5 patients who underwent CSF sampling, confirming the CNS penetration of alectinib.
In addition to alectinib, data on the CNS activity of other next-generation ALK inhibitors has recently emerged. For example, in an ongoing phase I/II trial of the ALK inhibitor AP26113, 10/14 (71%) patients with untreated/progressive brain metastases experienced intracranial tumor regressions on therapy.21 CNS antitumor activity has also been described with ceritinib. Specifically, intracranial responses were seen in 10/29 (34.5%) patients who entered the phase I study of ceritinib with measurable CNS metastases.22 More recently, radiographic improvements were also reported in an ALK-positive patient with LM who was treated with ceritinib.23 However, as illustrated by this report, patients may relapse in the CNS despite treatment with ceritinib. Indeed, CNS concentrations of ceritinib are predicted to be ~15% of plasma levels based upon animal models.24 It is therefore possible that the CNS activity of alectinib in this series may have been due to enhanced CNS bioavailability, thereby overcoming incomplete ALK inhibition.
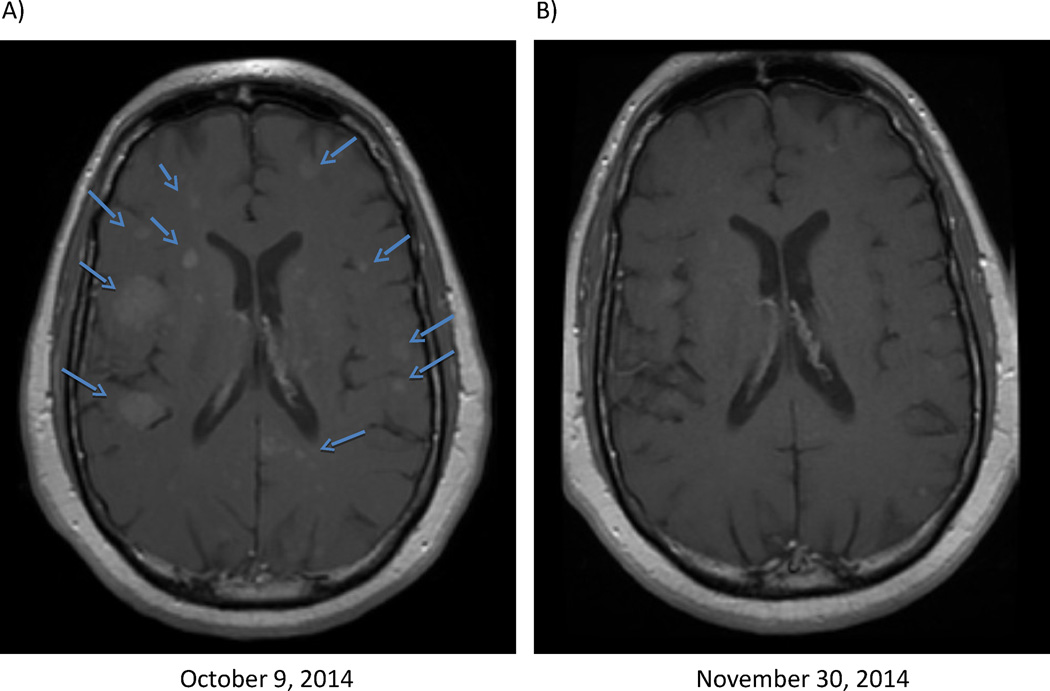
In summary, we demonstrate that alectinib has significant antitumor activity in ALK-positive patients with LM. Moreover, alectinib was active in this cohort despite prior exposure to both crizotinib and ceritinib (the two ALK inhibitors currently approved by the FDA), suggesting that alectinib may have greater CNS activity. Of note, in addition to responses among these patients with LM, we have also observed antitumor activity in brain parenchymal metastases in a patient treated with compassionate-use alectinib after CNS relapses on crizotinib and ceritinib (Figure 3). Additional prospective studies of alectinib in ALK-positive patients with CNS metastases, including LM, are therefore warranted. Indeed, one such study, the ALEX study, is already ongoing. This phase III randomized trial is evaluating first-line crizotinib versus alectinib in treatment-naive, ALK-positive lung cancer patients (NCT02075840). Of note, the ALEX trial permits patients with asymptomatic brain or leptomeningeal metastases to enroll. Moreover, time to CNS progression is a key secondary endpoint of the study, which may in turn provide important prospective data on the CNS antitumor activity of both agents.
Figure 3.
Marked regression in brain parenchymal metastases in a 46 year-old, ALK-positive lung cancer patient treated with alectinib. This patient had previously received both crizotinib and ceritinib, relapsing in the central nervous system after each agent. Axial, T1 post-gadolinium magnetic resonance images A) prior to alectinib and B) on alectinib.
As is common with targeted therapies in NSCLC, acquired resistance developed in two patients in this series, highlighting the need for additional therapies or therapeutic combinations with activity in the CNS. One approach has been to use still more potent and/or structurally distinct ALK inhibitors. For example, PF-06463922 is a novel ALK inhibitor that was recently developed and rationally designed to minimize P-gp-mediated drug efflux and optimize CNS penetration.25 In preclinical models, this agent has shown anti-tumor activity in the CNS and has demonstrated strong activity against all known ALK resistance mutations identified in patients with crizotinib-resistant disease. Moving forward, clinical trials of novel, brain penetrable targeted therapies may help inform the optimal management of CNS metastases in advanced ALK-positive NSCLC.
Table 1.
Baseline Characteristics of ALK-Positive Patients with Leptomeningeal Metastases
| Pt. | Age | Sex | Prior ALK Inhibitors |
Previous Radiation Therapy |
Interval From Diagnosis to Development of LM |
Concomitant Brain Metastases |
Neurologic Symptoms |
|---|---|---|---|---|---|---|---|
| 1 | 56 | M | Crizotinib, Ceritinib | WBRT | 23 Months | Yes | Seizure, confusion, word-finding difficulties |
| 2 | 50 | M | Crizotinib, Ceritinib | Radiosurgery×2, WBRT | 18 Months | Yes | Headaches, diplopia, slurred speech, nausea, ptosis |
| 3 | 39 | F | Crizotinib, Ceritinib | WBRT | 9 Months | No | Focal seizures, right-sided weakness, visual hallucinations |
| 4 | 49 | F | Crizotinib, Ceritinib | None | 16 Months | Yes | Fatigue |
Abbreviations: ALK, anaplastic lymphoma kinase; F, female; LM, leptomeningeal metastases; M, male; WBRT, whole brain radiation therapy
Acknowledgements
We would like to thank the members of the Massachusetts General Hospital Cancer Center Protocol Office for their assistance.
Funding: This work was supported by grants from the U.S. National Institutes of Health 5R01CA164273 and C06CA059267. F Hoffmann La-Roche supplied alectinib on a compassionate use basis.
Footnotes
Disclosures: JFG has served as a paid consultant to Boehringer Ingelheim, Jounce Therapeutics and Kyowa Hakko Kirin. ATS has served as a paid consultant for Pfizer, Novartis, Genentech, Roche, Ariad, Chugai, Ignyta, and Daiichi-Sankyo.
References
- 1.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 3.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 4.Mok T, Kim D, Wu Y, et al. First-line crizotinib versus pemetrexed-cisplatin or pemetrexed-carboplatin in patients (pts) with advanced ALK-positive non-squamous non-small cell lung cancer (NSCLC): results of a phase III study (PROFILE 1014) J Clin Oncol. 2014;32:5s. (suppl; abstr 8002) [Google Scholar]
- 5.Kim D-W, Mehra R, Tan DS, et al. Ceritinib in advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCLC): Results of the ASCEND-1 trial. J Clin Oncol. 2014;32:5S. (suppl; abstr 8003) [Google Scholar]
- 6.Gainor JF, Ou SH, Logan J, Borges LF, Shaw AT. The Central Nervous System as a Sanctuary Site in ALK-Positive Non-Small-Cell Lung Cancer. J Thorac Oncol. 2013;8(12):1570–1573. doi: 10.1097/JTO.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 7.Ahn HK, Han B, Lee SJ, et al. ALK inhibitor crizotinib combined with intrathecal methotrexate treatment for non-small cell lung cancer with leptomeningeal carcinomatosis. Lung Cancer. 2012;76(2):253–254. doi: 10.1016/j.lungcan.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15(10):1119–1128. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 9.Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010;74(18):1449–1454. doi: 10.1212/WNL.0b013e3181dc1a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JH, Kim YJ, Lee JO, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer. 2012;76(3):387–392. doi: 10.1016/j.lungcan.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7(2):382–385. doi: 10.1097/JTO.0b013e3182398e4f. [DOI] [PubMed] [Google Scholar]
- 12.Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010;99(2):283–286. doi: 10.1007/s11060-010-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa D, Shaw A, Ou S, et al. Clinical experience with crizotinib in patients with advanced, ALK-rearranged non-small cell lung cancer and brain metastases in PROFILE 1005 and PROFILE 1007. J Thorac Oncol. 2013;8 doi: 10.1200/JCO.2014.59.0539. (suppl; abstr MO07.02) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–e445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi L, Drappatz J, Ramaiya NH, Otterson GA. High-dose pemetrexed in combination with high-dose crizotinib for the treatment of refractory CNS metastases in ALK-rearranged non-small-cell lung cancer. J Thorac Oncol. 2013;8(1):e3–e5. doi: 10.1097/JTO.0b013e3182762d20. [DOI] [PubMed] [Google Scholar]
- 16.Kim YH, Ozasa H, Nagai H, et al. High-dose crizotinib for brain metastases refractory to standard-dose crizotinib. J Thorac Oncol. 2013;8(9):e85–e86. doi: 10.1097/JTO.0b013e31829cebbb. [DOI] [PubMed] [Google Scholar]
- 17.Glass JP, Melamed M, Chernik NL, Posner JB. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology. 1979;29(10):1369–1375. doi: 10.1212/wnl.29.10.1369. [DOI] [PubMed] [Google Scholar]
- 18.Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995;38(1):51–57. doi: 10.1002/ana.410380111. [DOI] [PubMed] [Google Scholar]
- 19.Kodama T, Hasegawa M, Takanashi K, Sakurai Y, Kondoh O, Sakamoto H. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74(5):1023–1028. doi: 10.1007/s00280-014-2578-6. [DOI] [PubMed] [Google Scholar]
- 20.Chuan Tang S, Nguyen LN, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer. 2014;134(6):1484–1494. doi: 10.1002/ijc.28475. [DOI] [PubMed] [Google Scholar]
- 21.Gettinger S, Bazhenova L, Salgia R, et al. ALK Inhibitor AP26113 in Patients with Advanced Malignancies, including ALK+ Non-Small Cell Lung Cancer (NSCLC): Updated Efficacy and Safety Data. Ann Oncol. 2014;25(suppl_4):iv426–iv470. 10.1093/annonc/mdu349. [Google Scholar]
- 22.Shaw A, Mehra R, Tan D, et al. Evaluation of ceritinib-treated patients (pts) with anaplastic lymphoma kinase rearranged (ALK+) non-small cell lung cancer (NSCLC) and brain metastases in the ASCEND-1 study. Ann Oncol. 2014;25(suppl_4):iv426–iv470. 10.1093/annonc/mdu349. [Google Scholar]
- 23.Arrondeau J, Ammari S, Besse B, Soria JC. LDK378 compassionate use for treating carcinomatous meningitis in an ALK translocated non-small-cell lung cancer. J Thorac Oncol. 2014;9(8):e62–e63. doi: 10.1097/JTO.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 24.United States Food and Drug Administration. [Accessed 12/1/2014]; Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205755lbl.pdf.
- 25.Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57(11):4720–4744. doi: 10.1021/jm500261q. [DOI] [PubMed] [Google Scholar]