Abstract
Concurrent damage to the lateral frontal and parietal cortex is common following middle cerebral artery infarction leading to upper extremity paresis, paresthesia and sensory loss. Motor recovery is often poor and the mechanisms that support, or impede this process are unclear. Since the medial wall of the cerebral hemisphere is commonly spared following stroke, we investigated the long-term (6 and 12 month) effects of lateral frontoparietal injury (F2P2 lesion) on the terminal distribution of the corticospinal projection (CSP) from intact, ipsilesional supplementary motor cortex (M2) at spinal levels C5 to T1. Isolated injury to the frontoparietal arm/hand region resulted in a significant loss of contralateral corticospinal boutons from M2 compared to controls. Specifically, reductions occurred in the medial and lateral parts of lamina VII and the dorsal quadrants of lamina IX. There were no statistical differences in the ipsilateral corticospinal projection. Contrary to isolated lateral frontal motor injury (F2 lesion) which results in substantial increases in contralateral M2 labeling in laminae VII and IX (McNeal et al., Journal of Comparative Neurology 518:586-621, 2010), the added effect of adjacent parietal cortex injury to the frontal motor lesion (F2P2 lesion) not only impedes a favorable compensatory neuroplastic response, but results in a substantial loss of M2 CSP terminals. This dramatic reversal of the CSP response suggests a critical trophic role for cortical somatosensory influence on spared ipsilesional frontal corticospinal projections, and that restoration of a favorable compensatory response will require therapeutic intervention.
Keywords: Pyramidal Tract, Frontal Lobe, Parietal Lobe, Corticofugal, Neurosurgical Resection, Plasticity, Spinal Cord, Motor Recovery, Hand Movements
INTRODUCTION
The corticospinal projection (CSP) is the longest fiber pathway in the central nervous system. Its circuitry specializations, especially pronounced in some higher-order primates, establish the underlying capacity to produce highly coordinated, fractionated movements of the digits. Although an extensive region of the cerebral cortex is known to harbor corticospinal projection neurons (Biber et al., 1978; Murray and Coulter, 1981; Nudo and Masterton, 1988, 1990; Dum and Strick 1991; Galea and Darian-Smith, 1994; Luppino et al., 1994; Rozzi et al., 2006) the projection from the primary motor cortex (M1) is widely known for its special structural and functional contributions to generating contralateral skilled voluntary hand movements. These coordinated hand movements include opposition of the tips of the fingers to the thumb, grip formation, and object manipulation (e.g., Cheney et al., 1991; Porter and Lemon, 1993; Lemon et al., 2004; Park et al., 2004; Martin, 2005; Lemon and Griffiths, 2005; Schieber, 2007; Lemon, 2008; Griffin et al., 2009; Boudrias et al. 2010a).
Numerous experimental approaches have been instrumental in defining the unique structural and functional characteristics of the M1 corticospinal projection in non-human primates and one of the oldest and enduring methods includes precentral cortical resection (e.g., Ferrier, 1886, Graham Brown and Sherrington, 1913; Leyton and Sherrington, 1917; for review see Vilensky and Gilman, 2002; Darling et al., 2011; Wiesendanger, 2011). After isolated resection of M1, a short period of flaccid paralysis ensues that is followed by remarkable recovery of upper extremity movements. However, a hallmark clinical feature of extensive precentral resection which includes the anterior bank of the central sulcus, is more lasting deficits in fine control of contralesional digit movements for manipulating small objects in macaques (Ogden and Franz 1917, Fulton and Kennard 1934) and humans (Bucy, 1949). In non-human primates, adaptive mechanisms that accompany precentral injury and upper limb recovery include physiological reorganization of adjacent lateral premotor cortex (e.g., Glees and Cole, 1950; Black et al., 1974; Nudo et al., 1996; Rouiller et al., 1998; Liu and Rouiller, 1999; Frost et al., 2003; Dancause et al., 2006) and rewiring of corticocortical connections from the ventrolateral premotor cortex (Dancause et al., 2005). In the supplementary motor cortex (M2, SMC or SMA-proper), increased unit activity (Aizawa et al., 1991), expansion of the distal forelimb region (Esiner-Janowicz et al., 2008) and enhanced terminal labeling of corticospinal projections (McNeal et al., 2010) have been shown to occur after lateral frontal motor injury.
In contrast to the abundant studies examining the effects of isolated lateral frontal motor injury on recovery of upper extremity movements in non-human primates, there have been few experimental studies examining the effects of isolated postcentral injury on upper extremity movement. The available evidence from some highly regarded authorities show that localized parietal lobe lesions may produce serious motor deficits. Notably, in a rarely cited study, Kennard and Kessler (1940) reported that isolated postcentral (primary somatosensory cortex or S1) resection primarily affects fine motor acts of the digits. These deficits were characterized by a lack of precision in both direction and extent of movements, especially during grooming activities. Permanent loss of tactile placing was noted and accuracy of manipulation by the digits improved only when visual attention was used. Remarkably, motor recovery deficits were found to be more enduring following postcentral (somatosensory) injury than after precentral (somatomotor) lesions, with progressive improvements in recovery occurring over longer periods of time following precentral injury. Denny-Brown (1950) described postcentral lesions as having a devastating effect on motor function that is equivalent to area 4 (M1) ablation with additional persistent depression of posture and postural reflexes. Yet adaptive frontomotor mechanisms that accompany motor recovery following isolated postcentral injury have not been studied in the non-human primate model. However, higher than normal current levels are required to elicit movement from M1 following dorsal column lesions that disrupt ascending proprioceptive and tactile input to S1 (Kambi et al., 2011), indicating that frontomotor mechanisms are likely to be altered following somatosensory deprivation. This change in stimulation threshold is also accompanied by reorganization of the M1 thumb area map such that intracortical microstimulation primarily causes abduction-adduction movements instead of primarily flexion-extension movements as in control monkeys.
It is clear that studies applying localized lesions confined to either the precentral (M1) or postcentral (primary somatosensory cortex or S1) region were designed, in part, with the intent to determine each representative contribution to altered patterns of motor performance after injury, as well as the negative or positive effects that each lesion type may have on the motor recovery process. However, it would be of substantial interest to study the effects of combined lateral frontal (M1) and lateral parietal (S1) lobe injury on the recovery process of upper extremity movements in the non-human primate model because this cortical territory is often compromised as a collective unit in a large number of stroke patients as indicated by the high incidence of motor weakness and sensory abnormalities resulting from middle cerebral artery (MCA) embolic disease (Yoo et al., 1998). Indeed, the MCA supplies the lateral part of the frontal and parietal lobes (e.g., Tatu et al., 1998; Duvernoy, 1999) and is the most frequently occluded artery in cerebrovascular pathology (e.g., Bogousslavsky et al., 1990; Carrera et al., 2007). The poor motor recovery often noted in these patients suggests different neuronal response mechanisms, that are perhaps more unfavorable and limiting, may accompany this type of injury versus isolated lesions limited to either the precentral or postcentral regions alone.
One cortical area that would be of significant interest to study compensatory recovery mechanisms following lateral frontoparietal injury is the medial frontal motor region. Indeed, the anterior cerebral artery supplies the medial wall of the cerebral hemisphere and is spared in the majority (>97%) of stroke patients (Bogousslavsky and Regli, 1990; Kazui et al, 1993; Kumral et al., 2002; Carrera et al., 2007). As we have previously noted (McNeal et al., 2010), it is of particular significance that the supplementary motor cortex (M2), resides in the vascular territory of the anterior cerebral artery. Moreover, M2 gives rise to the second strongest (only to M1) corticospinal projection (Dum and Strick 1991; Galea and Darian-Smith, 1994) and is involved in higher-order motor functions including motor planning and movement sequencing (Tanji, 1994; Nachev et al., 2008; Passingham et al., 2010). As such, M2 may represent an important resource in the recovery process of upper extremity movement following common, lateral frontoparietal cortical injury. Furthermore this cortex may contribute to the recovery of fine motor control of the digits since M2 has a relatively prominent projection to alpha motoneuron rich lamina IX (Dum and Strick, 1996; Maier et al., 2002; McNeal et al., 2010) which has recently been characterized as a monosynaptic projection (Maier et al., 2002; Boudrias et al., 2006, 2010b).
Hence, the objective of this study was to examine the effects of lateral frontoparietal injury on the spared corticospinal projection from the supplementary motor cortex (M2) following spontaneous long-term (6 and 12 months) recovery. In this report, we find that in contrast to isolated lateral frontal motor injury (F2 lesion), which gives rise to a substantial increase in the contralateral corticospinal projection from M2 in the form of greater numbers of terminal boutons and enhanced terminal fiber length versus controls (McNeal et al., 2010), the added effect of anterior parietal lobe injury (F2P2 lesion) has devastating consequences on the contralateral corticospinal projection from M2. This has important implications for humans who probably rely extensively, if not exclusively, on the corticospinal projection for performing precisely coordinated hand and finger movements.
METHODS AND MATERIALS
All monkeys (Macaca mulatta) were housed and cared for in a United States Department of Agriculture (USDA) and Association for Assessment and Accreditation of Laboratory Animal Care (AALAC) approved and inspected facility. All behavioral, surgical and experimental protocols were approved by the University of South Dakota Institutional Animal Care and Use Committee, and conducted in accordance with USDA, National Institutes of Health, and Society for Neuroscience guidelines for the ethical treatment of animals. Prior to initiating the study, each monkey was evaluated by a veterinarian with primate experience and judged to be healthy and free of any neurological deficit.
Study Design
To accomplish the aims of this investigation, the organization of the CSP arising from M2 was studied at spinal levels C5 to T1 in rhesus monkeys (Macaca mulatta). Four control monkeys from our previous work (McNeal et al., 2010 – see Table 1) and one additional monkey (SDM84) served as the control group (Table 1). Four other monkeys received an isolated neurosurgical lesion (F2P2 lesion) using coagulation/subpial aspiration that involved removal of the arm representation of M1 (area 4), lateral premotor cortex (LPMC or area 6), and arm region of the adjacent somatosensory cortex (S1 or areas 3, 1 and 2) including the rostral part of area PE (or area 5) of the superior parietal lobule. Detailed descriptions of the neurosurgical exposure, intracortical microstimulation method, and aspiration method have been reported previously (Morecraft et al., 2001; 2002, 2007a; McNeal et al., 2010). In the control group (Table 1), each monkey was injected with the tract tracer fluorescein dextran (FD) into the central region of the arm representation of M2 and survived for 33 days prior to sacrifice. The 4 F2P2 lesion monkeys were also injected with the same tracer (FD) into the central region of the arm representation of M2 in the ipsilesional hemisphere 33-34 days prior to sacrifice but after 6 or 12 months of recovery from the induced lateral frontoparietal lesion. Following immunohistochemical tissue processing for microscopic visualization of the FD tract tracer in all control and lesion cases, terminal boutons were estimated in Rexed’s lamina at spinal levels C5 to T1 using stereological counting methods, which is widely acknowledged as the most accurate method to quantify these neuronal structures (Glaser et al., 2007; West, 2012). Terminal fiber lengths were also estimated in lamina VII and IX and within the lateral corticospinal tract (LCST) at C5 and C8 using stereology. Definitions of general anatomical terminology adopted for this study have been described previously (McNeal et al., 2010, see Fig. 7). For the present report M1 was further subdivided into gyral, or rostral part (M1r) and a sulcal, or caudal part (M1c) (Rathelot and Strick, 2009) (Table 2). Similarly, the somatosensory cortex was subdivided into a rostral (S1r) component that lined the fundus and posterior bank of the central sulcus (cytoarchitectonic areas 3, and 1) and caudal part (S1c) that resides on the gyral surface of parietal cortex (cytoarchitectonic areas 1 and 2) (Table 2).
TABLE 1.
Description of the Experimental Parameters for Each Case
| Case | Sex | Age (yrs.) |
Weight (kg) |
Area Injected |
Tracer/ Injections |
Total Vol. (μL) |
Injection Core vol. (mm3) |
Injection Halo vol. (mm3) |
Post- Injection survival (days) |
|---|---|---|---|---|---|---|---|---|---|
| Control | |||||||||
| SDM41 | male | 4.6 | 4.8 | M2 arm | FD/3 | 1.2 | 38.7 | 84.6 | 33 |
| SDM54 | male | 9.0 | 9.2 | M2 arm | FD/3 | 1.2 | 33.0 | 67.7 | 33 |
| SDM62 | female | 3.2 | 3.2 | M2 arm | FD/3 | 1.2 | 31.5 | 60.4 | 33 |
| SDM77 | male | 8.3 | 9.6 | M2 arm | FD/3 | 1.2 | 14.7 | 51.6 | 33 |
| SDM84 | male | 15.3 | 11.7 | M2 arm | FD/3 | 1.2 | 20.9 | 85.9 | 33 |
| 6 Mo. Survival | |||||||||
| SDM87 | female | 17.5 | 10.9 | M2 arm | FD/3 | 0.9 | 15.5 | 47.8 | 33 |
| SDM91 | female | 8.3 | 5.6 | M2 arm | FD/3 | 1.2 | 36.6 | 119.7 | 34 |
| 12 Mo. Survival | |||||||||
| SDM81 | female | 16.0 | 6.0 | M2 arm | FD/3 | 0.9 | 19.0 | 55.5 | 33 |
| SDM83 | female | 4.7 | 7.0 | M2 arm | FD/3 | 0.9 | 19.5 | 89.5 | 33 |
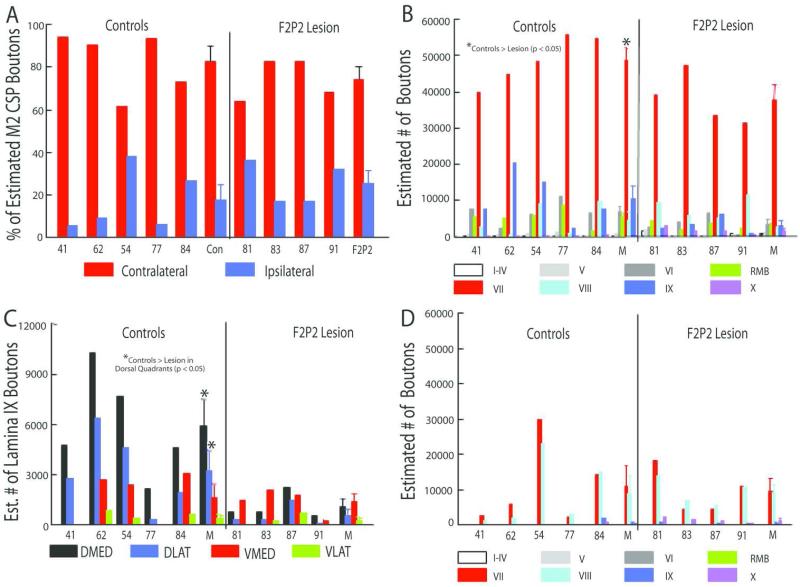
Figure 7.
A: Percentages of all boutons in the contralateral and ipsilateral projections from M2 to C5-T1 for each control and F2P2 lesioned monkey. B: Estimated numbers of labelled boutons in the contralateral CSP from M2 to C5-T1 laminae for each control and F2P2 lesioned monkey. C: Estimated numbers of lamina IX boutons in each quadrant in each control and F2P2 lesioned monkey. D: Estimated numbers of labeled boutons in the ipsilateral CSP from M2 to C5-T1 laminae in each control and F2P2 lesioned monkey. Mean (M) values of controls and lesioned monkeys are also provided in each graph. Error bars on the bars of mean values are 1 S.E.M.
TABLE 2.
Lesion/Spared Volume Data for F2P2 Cases (mm3)
| Case | Total lesion (white matter) |
Total lesion (grey matter) |
LPMCd lesion |
LPMCv lesion |
M1 ros lesion |
M1 ros spared |
M1 caudal lesion |
M1 caudal spared |
S1 ros lesion |
S1 ros spared |
S1 caudal lesion |
PE/ area5 lesion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDM 81 | 13.55 | 179.54 | 26.26 | 8.01 | 51.65 | 9.12 | 22.88 | 52.61 | 15.29 | 63.05 | 38.35 | 14.50 |
| SDM 83 | 16.11 | 261.18 | 83.59 | -.- | 68.12 | -.- | 29.38 | 29.13 | 24.11 | 46.08 | 41.21 | 11.43 |
| SDM 87 | 56.21 | 329.87 | 41.61 | 42.07 | 107.65 | -.- | 32.70 | 38.24 | 7.30 | 22.64 | 47.31 | 47.68 |
| SDM 91 | 52.94 | 274.30 | 39.44 | -.- | 80.45 | -.- | 72.72 | -.- | 42.80 | 18.40 | 25.61 | 7.80 |
Before the frontoparietal lesion was induced, the preferred hand in all 4 F2P2 lesion group monkeys was determined by deriving a handedness index for each animal (Nudo et al., 1992; Pizzimenti et al., 2007) which served to identify the hemisphere that would be lesioned (contralateral to the preferred hand). In addition, all monkeys in the lesion group were trained on two fine motor behavioral tasks that involved reaching for small food targets using a modified movement assessment panel (mMAP) (Darling et al. , 2006) and a modified dexterity board (mDB) (Pizzimenti et al. , 2007). Specifically, after reaching stable levels of motor performance on each task (approximately 18-28 testing sessions after learning the task), each monkey was lesioned and then tested once every week (on both tasks) for the first 2 months post-injury and once every other week (on both tasks) thereafter. Motor performances on individual trials were quantified from 3-dimensional video recordings of movements to acquire small food pellets in the mDB task (Pizzimenti et al. , 2007) and from recordings of 3-dimensional forces applied to small carrot chips for the mMAP task (Darling et al. , 2006). The animals in the control group were provided with daily distal upper extremity motor enrichment activities (such as a foraging board) to compensate for potential learning/training induced effects of the F2P2 lesioned animals during the brief manual testing sessions (maximum of 40 trials with each hand to acquire the food targets).
Neurosurgical and Neuroanatomical Procedures
Neurosurgical and Tract Tracing Procedures
Frontal lobe exposure was performed following neurosurgical procedures previously described (Morecraft et al., 2001, 2002, 2007a; McNeal et al., 2010). Briefly, with each animal under isofluorane anesthesia, a skin flap was made over the cranium followed by a bone flap over the midline region. In control cases and lesion cases, the medial surface of the frontal lobe was exposed by incising the dura matter and ligating bridging vessels emptying into the superior sagittal sinus with the aid of a surgical microscope. Small pieces of cottonoid pads were then gently packed into the interhemispheric sulcus to slowly increase the interhemispheric space allowing for the cortex lining the medial frontal surface to be clearly visible.
In both the control and lesion cases the location of the arm representation of M2 was determined by intracortical microstimulation (McNeal et al., 2010). Then the anterograde neural tract tracer FD (10% solution in saline comprised of an equal quantity of both 3,000 MW and 10,000 MW volumes) (Molecular Probes, Eugene, OR) was injected into the central region of the physiologically localized arm representation of M2. Specifically, graded pressure injections with a Hamilton microsyringe were made into three separate penetration sites spaced 1-1.5mm apart in a triangular pattern, 2.5 - 3.5 mm below the medial cortical surface at approximately a 10-20° angle from the vertical axis. In the control cases, a total of 1.2 μl of neural tracer was injected (i.e., 0.4 μl per penetration site) (Table 1). The tract tracer injections in the F2P2 lesion cases were performed in identical fashion with the exception that slightly less tracer volume (total of 0.9 μl; 0.3 μl per penetration site) was injected in 3 of the lesion cases (Table 1) and a volume that was equal to the controls (1.2 μl) was injected in one lesion case (SDM91) (Table 1). The smaller volume of FD injected into first the 3 F2P2 lesion animals were originally made to match the experimental design for our F2 lesion animals (removal of M1 arm/hand region and adjacent LPMC; n=4; see McNeal et al., 2010). In the F2 lesion experiments, we were able to clearly demonstrate that 0.9 μl of FD injected into the spared M2 arm area provided more than adequate amount of tracer volume to label 2-3 times the number of labeled CSP terminal boutons (and terminal axon fiber length) versus all the control cases that received 1.2 μl of FD (McNeal et al., 2010). To verify that the experimental results of the F2P2 lesion cases receiving 0.9 μl of FD reflected a true loss of corticospinal terminal labeling, we injected 1.2 μl of FD in our final experimental F2P2 lesion case (SDM91). The craniotomy was closed as described previously (Morecraft et al., 2001, 2002, 2007a).
Tissue Processing and Immunohistochemical Procedures
Following the survival period after tract tracer injection, each monkey was deeply anesthetized with an overdose of pentobarbital (50 mg/kg or more) and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde and sucrose as previously described (Morecraft et al., 2013). Following an appropriate period for cryoprotection, the cerebral cortex was frozen and cut in the coronal plane at a thickness of 50 μm in cycles of 10 and the spinal cord was cut horizontally at a thickness of 50 μm in cycles of 6. One series of cortical and spinal cord tissue sections was stained for Nissl substance for cytoarchitectural analysis using thionin (Morecraft et al., 1992, 2004, 2012, 2013). Subsequent series of tissue sections through the cortex and spinal cord were then processed using single and double label immunohistochemical procedures for visualization of the injected neural tracers as previously described (Morecraft et al., 2007a, 2013). To verify that the FD antibody and subsequent tissue labeling process resulted in staining only the injected and transported tract tracer, sections from the prefrontal cortex and occipital lobe were immunohistochemically processed and parts of these cortices that are not connected to M2 (Luppino et al., 1993; Morecraft etal., 2012) were examined for false FD labeling. Neuronal immunohistochemical labeling was not found in these control tissue sections demonstrating that false labeling was not present in the final tissue specimens used for analysis.
Stereological Analysis
The methods used to calculate unbiased estimated bouton numbers at specific spinal levels and within specific spinal cord laminae have been provided in detail in our previous papers (McNeal et al., 2010; Morecraft et al., 2007b, 2013). Immunoreactive terminal-like varicosities (i.e., putative boutons or terminal-like profiles) were defined as small swellings along the terminal fibers that were 0.5 – 3.5 um in diameter (Lawrence et al., 1985; Wouterlood and Groenewegen, 1985: Freese and Amaral, 2006; Morecraft et al., 2007b) (Fig. 2). Unbiased estimates of the total number of terminal boutons within Rexed’s laminae (and subdivisions) were determined using the Optical Fractionator (Microbrightfield, Colchester, VT, USA). Briefly, we calculated the average section thickness, overall fraction of tissue thickness that would be analyzed, the overall fraction of sectional area, and the overall number of tissue sections under analysis that contain the region of interest (ROI) (i.e., spinal cord laminae and respective subdivisions). Using the sectional area and the average tissue thickness we then constructed counting bricks and counted axon terminal boutons using unbiased counting rules (Larsen et al., 1998, West et al., 1991; West, 2012). The stereological parameters included the counting brick dimensions, tissue thickness, counting brick placement, guard zone and disector height. The same X/Y counting frame (109.2/71.4 μm) and X/Y grid placement (125.3/241.9 μm) was applied to all case material when performing stereology. To obtain an accurate estimate of the total number of labeled particles of interest (i.e., terminal boutons) in each individual monkey spinal cord, every other tissue section in a complete series of processed sections was used for microscopic stereological analysis and the total number of boutons within each ROI were expressed as total number counted per ROI per animal as previously described (Courtine et al., 2008; McNeal et al., 2010).
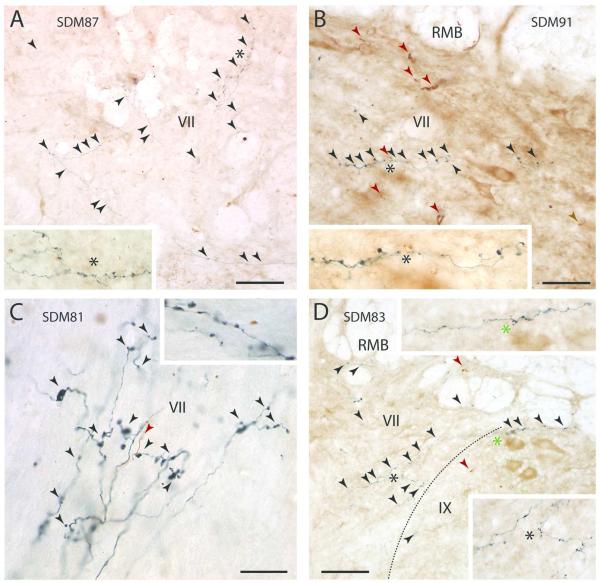
Figure 2.
Plate of high-power digital photomicrographs of immunohistochemically processed tissue sections through the spinal cord illustrating fluorescein dextran (FD) labeled terminal axon fibers and boutons (black arrowheads) in the gray matter contralateral to the FD injection site of F2P2 lesion cases SDM87 (A, spinal level C8), SDM91 (B, spinal level T1), SDM81 (C, spinal level T1) and SDM83 (D, spinal level C8). In panels A, B and D each inset is a higher power view of the field marked by the color matched asterisk. In panel C, the inset is an enlarged image of labeled terminals at superior levels of T1 whereas the main panel shows a field of labeled terminals at inferior levels of T1. In panels B, C and D, the red arrowheads indicate the locations of brown labeled fibers resulting from an injection of the tract tracer biotinylated dextran amine (BDA) that was placed into another spared cortical region of interest. The dotted line on paned D indicates the boundary between laminae VII and IX. Roman numerals represent Rexed’s laminae. Abbreviations: RMB, reticulated marginal border. Scale bar = 100 μm in A; 50 μm in B (also applies to D); 20 μm in C. Scale bars apply only to main panels in A-D.
To estimate terminal fiber length within these ROIs, we employed the Spaceballs probe. This probe was uniquely designed to satisfy isotropic requirements for tissue processing that may not be feasible. Specifically, because the probe is a virtual sphere, by definition all orientations on the surface of the sphere have an equal probability of being used (Mouton et al., 2002; West, 2012). To implement the probe, a computer software package (StereoInvestigator, Microbrightfield, Colchester VT, USA), allowed us to set the radius of the sphere and to maintain the same stereological parameters (i.e., area section fraction, series section fraction, section thickness) as the ones employed for the Optical Fractionator. The sphere was also designed to be less than the thickness of the tissue. The designed virtual sphere was then superimposed on the microscope image. At successive focal planes with a 100x oil immersion objective, labeled fibers that intersected the edge of the virtual sphere were counted for every sight within a given ROI. Estimates of terminal fiber length and associated coefficient of errors were then calculated according to previously derived formulas (Mouton et al, 2002; West, 2012)
The cortical ROIs to which stereology was applied included the FD tract tracer injection site and the extirpated lesion site (Tables 1, 2). Unbiased estimates of the total injection site volume (which included the core volume) and core injection site volume were determined using the Cavalieri probe in the same StereoInvestigator software. To accomplish this, every cortical tissue section spaced 500 μm apart through the injection site in the FD immunostained series of tissue sections was used for analysis. The same probe and method was used to determine the lesion site volume (gray and white matter) as reported in our previous papers (Pizzimenti et al., 2007, Darling et al., 2009). Briefly, after carefully evaluating the gray matter and white matter extent of the lesion site from Nissl stained sections through the lesioned hemisphere, the lesion site boundaries were superimposed onto gray and white matter regions on matching Nissl stained sections through the non-lesioned hemisphere (see Fig. 4 of Pizzimenti et al. , 2007, right column). The sections were spaced 500 μm apart then the Cavalieri probe was used to determine the respective volumes (Table 2).
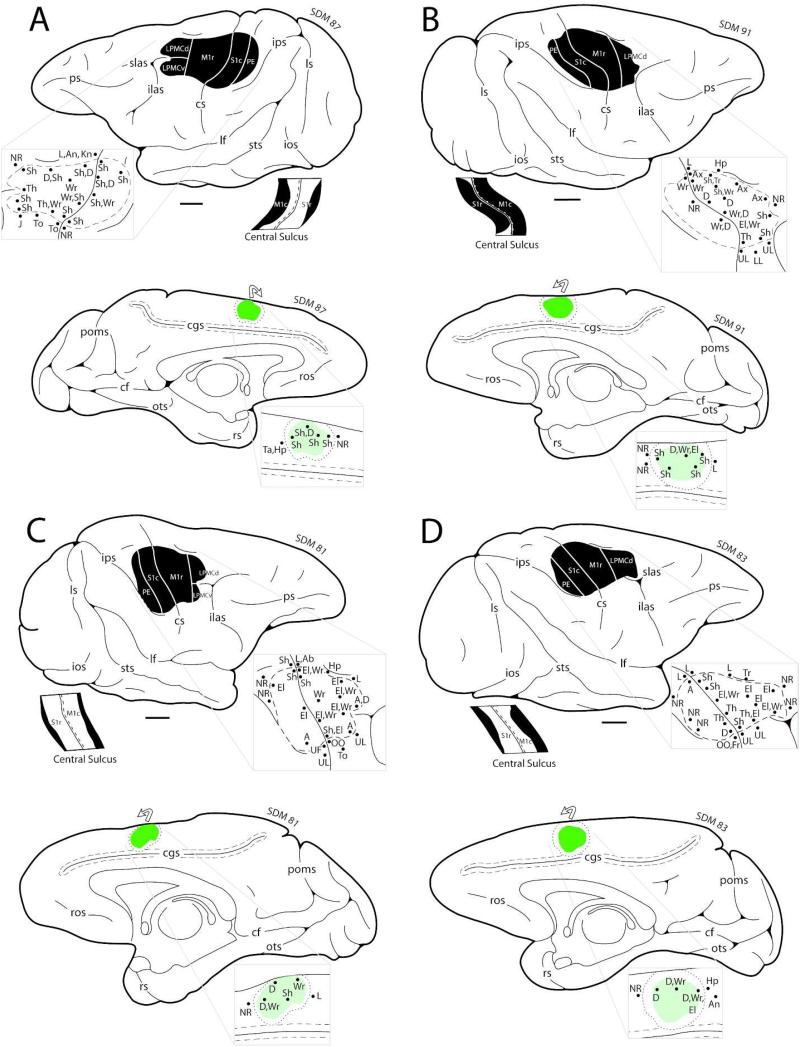
Figure 4.
Line drawings of the lateral (top) and medial (bottom) surfaces of the cerebral cortex in F2P2 lesion cases SDM87 (A), SDM91 (B), SDM81 (C) and SDM83 (D) depicting the locations of the lesion site (blackened area on lateral surface) and core of the fluorescein dextran (FD) injection site (green irregular shape on the medial surface). On the medial wall the dotted line around the injection site core represents the external boundary of the injection site halo. The curved arrow above the injection site indicates that some of the injection site halo was present on the dorsal convexity. On the lateral surface, the pullout depicts the physiological map of movement representation obtained with intracortical microstimulation used to localize the arm representations of the primary motor cortex (M1), lateral premotor cortex (LPMC), and primary somatosensory cortex (S1) prior to neurosurgical resection of the gray matter forming these cortical regions. Below the occipital lobe on the lateral surface drawing, is a flattened map showing cortex lining the rostral and caudal banks of the central sulcus. The black region represents the lesion site that extended into the sulcal cortex. The pullout on the medial surface is the physiological map of movement representation obtained with intracortical microstimulation to localize the arm representation of M2 prior to injection of the tract tracer FD. Scale bar = 5 mm and applies to the lateral surface, medial surface and flattened map of the cortex lining the central sulcus. For abbreviations see list.
Statistical Analysis of Neuroanatomical Data
Statistical analyses were performed to determine if significant differences occurred in bouton numbers and terminal fiber lengths between the control and lesion animals. Specifically, separate mixed 2-way repeated measures ANOVAs were used to compare these dependent variables in control versus lesioned animals across the spinal laminae (I-IV, V, VI, VII, VIII, IX, X and RMB for spinal segments C5-T1). We also used a mixed 3-way repeated measures ANOVA to compare bouton numbers within quadrants of lamina IX for segments C5-T1. In this case the repeated measures factors were: dorsal/ventral regions each subdivided into medial and lateral quadrants. Mauchley’s test was used to determine whether the assumption of sphericity was met when there were three or more levels in a repeated-measures factor (i.e., laminae). Adjustments in degrees of freedom for F-tests were made on the basis of the Huynh-Feldt epsilon values resulting in adjusted P-values, which are reported in the Results section. Statistical tests were accomplished using the GraphPad InStat 3 statistical software package (GraphPad Software Inc., San Diego, CA) or Statistica software (Tulsa, OK).
Neuroanatomical Data Reconstruction and Presentation
Publication quality images of injection sites and labeled fibers were captured using a Spotflex 64 mega pixel camera, (Diagnostic Instruments Inc., Sterling Heights, MI, USA, version 4.6), mounted on an Olympus BX51 microscope. Photographic montages of the injection sites and labeled fibers were created using Adobe PhotoShop 7.0 (Adobe Systems Inc., San Jose, CA, USA). Only brightness and contrast were adjusted to maximize discrimination and normalize images for comparative purposes. Cortical reconstructions and reconstruction of the physiological stimulation maps were developed as previously described using metrically calibrated digital images of the cortical surface (Morecraft and Van Hoesen 1992, Morecraft et al., 2002, 2013). Publication quality line illustrations were created using Adobe Illustrator 10.0 (Adobe Systems Inc., San Jose, CA, USA).
Movement Analysis Procedures
Two apparati were used to test fine hand/digit motor function: the modified movement assessment panel (mMAP) (Darling et al., 2006) and the modified dexterity board (mDB) (Pizzimenti et al., 2007) as previously described (McNeal et al., 2010). Forces applied during manipulation of the carrot chip in the mMAP task were recorded at 200 samples/s using Datapac 2k2 (Run Technologies). Movements of the hand during the mMAP task were recorded using a single digital video camera (Sony, model DCR-DVD301) to provide qualitative assessments of movement strategy and success/failure on each trial. Quantitative video recordings of hand movements during the mDB task from 4 cameras were used to assess spatial and temporal variables (e.g., accuracy and duration of the initial reach, grip aperture at touchdown).
Behavioral Procedures
Prior to an experimental session the monkey was food restricted for 12-24 hours. The initial training sessions used a “standard” rectangular dexterity board to assess the preferred hand of each monkey as described previously (Nudo et al., 1992). Hand preference was measured over 150 trials over a 3 day period (with 50 trials conducted each day). We recorded: 1) the hand used on the initial reach for that trial; 2) the hand used on subsequent reaches and; 3) the hand used to retrieve the pellet. A reach was defined when the animals hand passed through the plane of the square portal window located directly above the Kluver board. A subsequent reach was defined if the hand was withdrawn into the cage then extended back through the portal plane and over the Kluver board. The handedness index (HI) was computed as: (P-50)*2 where P is the average percentage of initial reaches and retrievals with the preferred hand (hand with the higher percentage of initial reaches and retrievals). HI ranges from 0 (50% of initial reaches and retrievals with each hand) to 100 (all initial reaches and retrievals with the preferred hand).
Training with the mMAP (Darling et al., 2006) and mDB (Pizzimenti et al., 2007) devices commenced after hand preference was determined. Full testing sessions with the mDB included 5 retrieval attempts for each of the wells (A-E) for both limbs proceeding from the easiest well (E) to the most difficult (A), for a total of 25 trials with each hand. During post-lesion tests, the more impaired hand (contralateral to the surgically induced lesion) was always tested first to ensure high motivation. Full testing sessions with the mMAP included blocks of 5 trials at each difficulty level with each hand, thereby giving the monkey 30 opportunities to retrieve carrot chips, 15 with each hand. During the first few post-lesion tests, the more impaired hand was tested first on the flat surface task (easiest task) to ensure high motivation. Thus, when considering both tests, a single testing session consisted of only 40 reaches for each hand.
Pre-lesion data were collected every 1-3 weeks for a total of 18-28 sessions according to each monkey’s ability to learn the task and perform consistently. The final five pre-lesion experiments demonstrated relatively stable levels of performance before lesions were made to cortical motor areas. Post-lesion data were collected from both limbs during weekly experimental sessions for the first two months after the surgery (i.e., one testing session per week). Thereafter, tests were conducted biweekly (i.e., one testing session every 2 weeks). After initial training, it was only during the pre- and post-lesion experimental sessions that the monkeys had exposure to the mMAP and mDB devices.
mMAP Data Analysis
Force data from the mMAP task were analyzed by visually identifying the first touch of the carrot chip or plate/rod supporting the carrot chip to the end of force application (i.e., when the carrot chip was removed from the plate supported by the load cell or the rod) on each trial using force recordings displayed in Datapac 2k2. The accompanying video data was also analyzed to verify these times and to identify trial outcome. The duration and total applied 3-dimensional absolute impulse were computed for each trial and, along with trial outcome used to compute a performance score. After pre-lesion data collection was completed, performance scores were computed and normalized to individual monkey’s abilities (i.e., maximum and minimum applied impulse and duration) for each trial at each difficulty level (McNeal et al. 2010).
mDB Data Analysis
Temporal characteristics of reaching, manipulation, and 3D locations of the tips of the index finger and thumb were determined from the digital video files as described previously and used to compute performance scores for reaching, manipulation and an overall score on each pre- and post-lesion trial (Pizzimenti et al., 2007). Measurements taken from video were used to compute reach duration, accuracy, grip aperture, manipulation duration and manipulation attempts (# of times contact between the pellet and a digit was lost and then re-established) on each trial. These measurements were each normalized to the performance ranges for each monkey prior to the lesion (i.e., to maximum/minimum reach and manipulation duration, least/most accurate reach, largest/smallest grip aperture, maximum/minimum number of manipulation attempts) and used to compute the performance scores (McNeal et al., 2010).
Analysis of Hand Motor Skill
We quantitatively assessed overall motor skill by computing mean divided by the standard deviation of manipulation performance scores over 5 consecutive testing sessions (i.e., 25 trials over an approximately 5 week period) (Pizzimenti et al. 2007; McNeal et al. 2010). These were computed for the performance scores on the mMAP curved rod task (which are computed from manipulation duration and forces exerted during manipulation) and for the manipulation scores on the mDB task (well with highest pre-lesion skill for each monkey). Note that higher mean performance scores (lower duration, impulse on mMAP; lower duration and fewer lost contacts with the food pellet in the mDB tasks) and lower variability of performances will result in higher skill values. Skill was computed for the best 5 consecutive and last 5 pre-lesion test sessions and for the 5 consecutive test sessions with the highest skill during post-lesion recovery. We also qualitatively analyzed each lesioned monkey’s contralesional hand/digit motions in the mDB and mMAP tasks to determine whether the monkey’s strategy changed post-lesion to use additional digits or different digits to perform the task.
Statistical Analysis of Motor Performance
We evaluated lesion effects on hand motor function from the duration (in weeks) from the time of the lesion until the first testing session with an attempt, successful acquisition and 5 successful acquisitions on the mMAP (flat surface task) and mDB (any well) tasks in each monkey. Recovery of fine motor skill was defined as the ratio of post-lesion skill (highest skill for 5 consecutive test sessions) to pre-lesion skill measured over the last 5 testing sessions before the lesion on the best well (well with highest pre-lesion skill) and 2nd smaller well (with pre-lesion skill of about 50% of that on the best well) of the mDB task and on the curved rod task of the mMAP. These measures of recovery were entered into single linear regression analyses to test whether recovery was correlated with the ratio of bouton numbers in individual lesioned monkeys to average number of boutons in control monkeys. Specifically, we considered the number of boutons in laminae VII and IX in lesioned monkeys, which we previously showed were highly correlated with recovery of skill in monkeys with lesions of M1 and lateral premotor cortex (LPMC) but without damage to parietal lobe (McNeal et al. 2010).
RESULTS
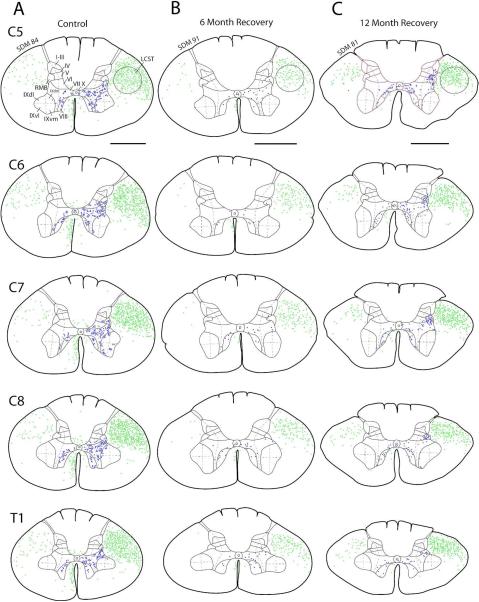
Microscopic analysis of the new control case (SDM84 – Fig. 3) and all F2P2 lesion cases (Figs. 1, 4) revealed that all FD injections were confined to the physiologically defined arm representation of M2 (cytoarchitectonic area 6m) and did not extend into the fundus and lower bank of the cingulate sulcus to involve the cingulate motor field. The core region of all injection sites was largely confined to the medial wall of the frontal cortex (Fig. 1) with the addition to the lip of the dorsal convexity which collectively is considered to be part of the supplementary motor cortex (Woolsey et al., 1952; Tanji and Kurata 1982, 1985). Importantly, all injection sites were found to involve cortical layer V which harbors the cells of origin of the CSP (Kuypers, 1981). The M2 corticospinal projection of the new control case (SDM84) was added to our previous control group of 4 control animals (Tables 1, 3-5). In SDM84, the general distribution of the contralateral terminal projection was similar to all other control cases with the highest number of labeled boutons being located in lamina VII and IX (Fig. 5; Table 3). The strongest projection to lamina IX in case SDM84 was localized to the dorsal lateral quadrant followed by the ventromedial quadrant (Table 5). The total number of estimated contralateral labeled boutons in case SDM84 was strikingly similar to case SDM54 (Table 3). Like the 4 previous control cases, the ipsilateral M2 corticospinal projection in case SDM84 terminated primarily in lamina VIII and the medial part of lamina VII (Table 4).
Figure 3.
Composite illustration showing the FD injection site (panels A and B) and terminal labeling in the spinal cord (panel C) in control case SDM84. A: Line drawing of the medial wall of the cerebral hemisphere depicting the location of the FD injection site core (green irregular shape) in the arm/hand representation of M2. The dotted line around the injection site represents the external boundary of the injection site halo. The curved arrow above the injection site indicates that some of the injection site halo was present on the dorsal convexity. The pullout depicts the physiological map of movement representation obtained with intracortical microstimulation used to localize the arm representation of M2 prior to injection of the tract tracer FD. B: A low-power digital photomicrograph of an immunohistochemically processed coronal tissue section through the fluorescein dextran (FD) injection site in the arm/hand region of M2. The dashed line on the injection site represents the external boundary of the injection site core and the dotted line the external boundary of the injection site halo. The blue arrow identifies a coalesced descending labeled fiber bundle emerging from the FD injection site. C: High-power digital photomicrographs of an immunohistochemically processed tissue section through spinal C7 illustrating FD labeled terminal axon fibers and boutons (black arrowheads) in contralateral laminae VII and IX. The blue arrows show fibers in passage. The inset is from the same tissue section but a different microscopic focal plane showing labeled boutons (black arrowheads) in lamina IX. Abbreviations: as, arcuate spur; cgs, cingulate sulcus. For other abbreviations see list. Scale bar = 2 mm in B, 50 μm in C.
Figure 1.
Plate of low-power digital photomicrographs of immunohistochemically processed coronal tissue sections illustrating the fluorescein dextran (FD) injection site in the arm/hand region of M2 in F2P2 lesion cases SDM87 (A), SDM91 (B), SDM81 (C) and SDM83 (D). The dashed line represents the external boundary of the injection site core and the dotted line the external boundary of the injection site halo. The white arrows identify a coalesced descending labeled fiber bundle emerging from the FD injection site. The curved black arrow indicates the location of gray matter lining the depths of the arcuate spur. Abbreviations: as, spur of arcuate sulcus; cb, cingulum bundle; cgs, cingulate sulcus. The scale bar in A = 2mm and applies to all panels.
TABLE 3.
Contralateral Bouton Counts in Each Control Case by Spinal Lamina1
| Bouton | I-III | IV | V | VI | VII | VIII | IX | X | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | # | Med | Lat | Med | Lat | Med | Lat | Med | Lat | RMB | Med | Lat | |||
| SDM41 | 64,075 | 0 | 21 (0.03) |
0 | 0 | 0 | 627 (1) |
2,006 (3) |
5,766 (9) |
5,515 (9) |
18,050 (28) |
21,811 (34) |
2,507 (4) |
7,521 (12) |
251 (0.4) |
| SDM62 | 73,706 | 0 | 0 | 0 | 0 | 0 | 612 (1) |
122 (0.2) |
2,326 (3) |
5,265 (7) |
12,856 (17) |
31,833 (43) |
490 (0.7) |
20,202 (27) |
0 0 |
| SDM54 | 85,130 | 0 | 12 (0.01) |
0 | 294 (0.3) |
0 | 784 (1) |
588 (1) |
5,485 (6) |
5,779 (7) |
21,255 (25) |
26,936 (32) |
9,011 (11) |
14,986 (18) |
0 0 |
| SDM77 | 80,504 | 0 | 0 | 0 | 0 | 375 (0.005) |
900 (1) |
7,428 (9) |
3,826 (5) |
8,703 (11) |
32,862 (41) |
22,884 (28) |
975 (1) |
2,401 (3) |
150 (0) |
| SDM84 | 86,829 | 0 | 0 | 0 | 0 | 0 | 88 (0.1) |
352 (0.4) |
1,676 (2) |
1,852 (2) |
32,566 (38) |
25,503 (29) |
13,415 (15) |
10,231 (12) |
1,146 (1) |
| MEAN | 78,048.8 | 0.0 | 6.6 | 0.0 | 58.8 | 75.0 | 602.2 | 2,099.2 | 3,815.8 | 5,422.8 | 23,517.8 | 25,793.4 | 5,279.6 | 11,068.2 | 309.4 |
Percentages of total label in parentheses. Values are rounded to the nearest whole number, except when the value is 0.7 or less. Lat, lateral; Med, medial; RMB, reticulated marginal border.
TABLE 5.
Contralateral Bouton Counts in Each Control Case Within Each Quadrant of Lamina IX 1
| Bouton | IX | ||||
|---|---|---|---|---|---|
| Case | # | DMED | DLAT | VMED | VLAT |
| SDM41 | 7,521 | 4,763 (63) |
2,758 (37) |
0 | 0 |
| SDM62 | 20,202 | 10,285 (51) |
6,367 (32) |
2,693 (13) |
857 (4) |
| SDM54 | 14,986 | 7,640 (51) |
4,604 (31) |
2,351 (16) |
391 (3) |
| SDM77 | 2,401 | 2,101 (88) |
300 (12) |
0 | 0 |
| SDM84 | 10,231 | 4,588 (45) |
1,940 (19) |
3,086 (30) |
617 (6) |
| MEAN | 11,068.2 | 5,875.4 | 3,193.8 | 2,032.5 | 466.3 |
Percentages of total label in parentheses. Values are rounded to the nearest whole number, except when the value is 0.7 or less. Lat, lateral; Med, medial; RMB, reticulated marginal border.
Figure 5.
Line drawings depicting a representative transverse section through spinal levels C5 to T1 in case SDM84 (control) (A), SDM91 (6 month recovery) (B) and SDM81 (12 month recovery) (C) showing regions in the lateral corticospinal tract, posterior funiculus and anterior funiculus containing labeled axons (green dots) and regions of Rexed’s laminae containing labeled boutons and bouton clusters (blue dots). Roman numerals in section C5 designate Rexed’s laminae and apply to all spinal sections. Laminae I-VII were subdivided into medial and lateral halves (see dashed line) and lamina IX into quadrants (see dashed lines) for stereological analysis. Note the sparse terminal labeling in cases SDM91 and SDM81 denoted by the presence of significantly fewer blue dots located in the spinal gray matter compared to the robust terminal labeling in control case SDM84. For orientation dorsal is located on the top of each section and ventral at the bottom. Abbreviations: dm, dorsomedial; dl, dorsal lateral; LCST, lateral corticospinal tract; RMB, reticulated marginal border; vm, ventromedial; vl, ventrolateral). Scale bar = 2 mm.
TABLE 4.
Ipsilateral Bouton Counts in Each Control Case by Spinal Lamina1
| Bouton | I-III | IV | V | VI | VII | VIII | IX | X | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | # | Med | Lat | Med | Lat | Med | Lat | Med | Lat | RMB | Med | Lat | |||
| SDM41 | 4,012 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2,257 (56) |
501 (12) |
1,254 (31) |
0 | 0 |
| SDM62 | 7,836 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5,265 (67) |
734 (9) |
1,837 (23) |
0 | 0 |
| SDM54 | 53,187 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 27,524 (52) |
2,547 (5) |
23,116 (43) |
0 | 0 |
| SDM77 | 5,552 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2,101 (38) |
225 (4) |
3,076 (55) |
0 | 150 (3) |
| SDM84 | 31,942 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12,794 (15) |
1,411 (2) |
15,180 (17) |
1,764 (2) |
793 (1) |
| MEAN | 20,505.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9,988.2 | 1,083.6 | 8,892.6 | 352.8 | 188.6 |
Percentages of total label in parentheses. Values are rounded to the nearest whole number, except when the value is 0.7 or less. Lat, lateral; Med, medial; RMB, reticulated marginal border.
General Experimental Observations of the F2P2 Lesion Cases
Immediately after the F2P2 lesion, all animals showed clear impairment with flaccid paresis of the contralesional hand. Opposite the lesioned hemisphere, a pendulous hand hung limply at the side and was not used for postural support or grasping of objects. This paresis lasted for 7-10 days after the lesion when hand function began to be reinstated to assist the unimpaired hand during feeding and typical cage behaviors such as grasping of the cage bars and climbing. There was a clear tactile sensory impairment in all cases immediately after the lesion as the animals apparently did not notice when the affected hand and finger tips came into contact with the cage floor or cage walls. The sensory deficit was also evident when the animals attempted to pick up objects in the cage with the affected hand as they often groped at an object and did not successfully grasp the object until the animal visually attended to it. This behavior was also observed during the mDB and mMAP tasks when the animals had to pick up very small food items. The lesion volumes for each extirpated area are provided in Table 2. A detailed description of the lesion site in each experimental case is provided below.
Lesion Site Analysis
Lesion Site Analysis of SDM 87 (6 month recovery)
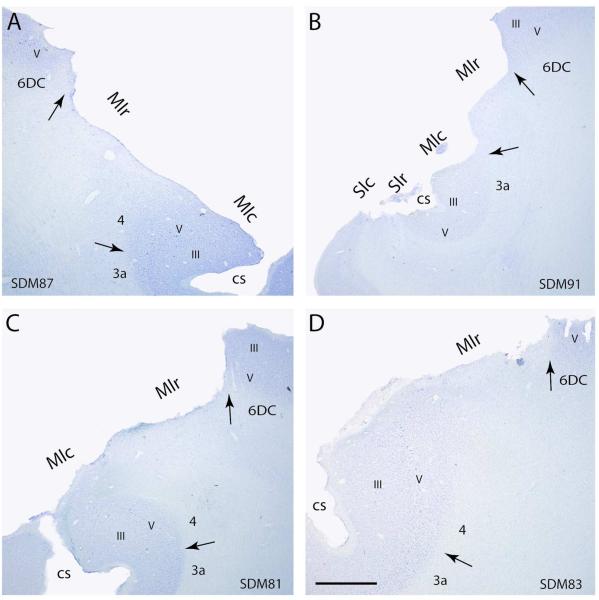
In the frontal lobe, the LPMC lesion involved the inferior part of LPMCd (area 6DC) and dorsal part of LPMCv (area 6Vd) of which all gray matter was removed with the exception of the rostral-most portion of the lesion site (1-2mm) which involved removal of only layers I-III. In this vicinity, the gray matter lining the depths of the arcuate spur was spared. The lesion spread caudally to involve all gray matter corresponding to the arm/hand region of M1r (or the gryal, or “old” part of area 4 – Rathelot and Strick, 2009) (Figs. 4, 6A; Table 2). In the anterior bank of the central sulcus extensive removal of M1c was noted, involving nearly one-half of the anterior bank (or “new” part of area 4-Rathelot and Strick, 2009) (Figs. 4, 6A; Table 2). In the posterior bank of the central sulcus the upper one-quarter of S1r was removed (Fig. 4). On the lateral parietal surface, all of the arm/hand region of S1c was ablated which extended posteriorly to involve the rostral part of area PE of the superior parietal lobule (Fig. 4).
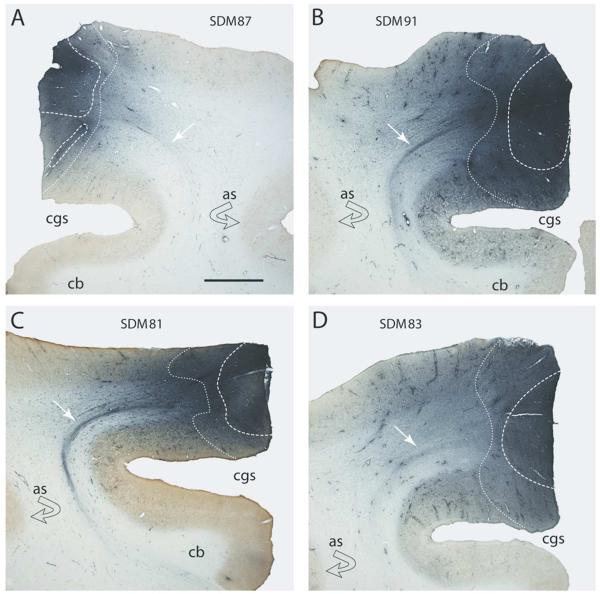
Figure 6.
Panel of photomicrographs of representative Nissl stained sections through the lesioned hemisphere of F2P2 lesion cases SDM87 (A), SDM91 (B), SDM81 (C) and SDM83 (D). In all sections the black arrow marks the location of the cytoarchitectonic border between either area 4 (M1) and area 3a (S1) or area 4 and lateral premotor area 6DC. Cortical layers are identified using Roman numerals. The regions of extirpated cortex are identified by M1c, M1r, S1r and S1c. Note the extensive removal of M1c, M1r, S1c and Sr in case SDM91. Abbreviation: cs, central sulcus; M1c, caudal primary motor cortex; M1r, rostral primary motor cortex; S1c, caudal primary somatosensory cortex; S1r, rostral primary somatosensory cortex. Scale bar in D = 2 mm and applies to all other panels.
Overall, the subcortical white matter lesion was limited to the region immediately below the gray matter extirpation (Figs. 6A; Table 2). However, on the lateral surface in the central region of the frontal lobe resection, a small part of the lesion spread inferiorly forming a vacated v-shape that appeared to part the uppermost portions of the frontal occipital fasciculus (FOF) and superior longitudinal fasciculus II (SLFII) (nomenclature according to Schmahmann and Pandya 2006). Much of this subcortical lesion may have been a consequence of lost subcortical fiber pathway that originally emerged with the resected gray matter cortex. At its deepest level, the vacated cortex corresponded to the horizontal level containing the fundus of the cingulate sulcus.
Lesion site analysis of SDM 91 (6 month recovery)
On the lateral frontal surface the lesion involved the very caudal and inferior part of LPMCd (area 6DC). Posteriorly all cortex forming M1r was completely removed (Figs. 4, 6B; Table 2). In the anterior bank of the central sulcus all of M1c was extirpated (Figs. 4, 6B; Table 2). On the posterior bank of the central sulcus most of the gray matter forming S1r was removed except a very small part of S1r in the very depth of the fundus (Figs. 4, 6B). In the Nissl stained preparations this cortex was found to be disorganized, possibly by aspiration tip trauma during the extirpation process. The lesion extended posteriorly onto the gyral surface of the parietal lobe fully removing S1c and extending into the rostral part of area PE of the superior parietal lobule (Fig. 4).
Like case SDM87, white matter damage was found in the central region of the frontal lobe lesion on the lateral surface that spread inferiorly. This formed a vacated V-shape that appeared between the uppermost portions of the frontal occipital fasciculus (FOF) and superior longitudinal fasciculus II (SLFII) (Table 2). At its deepest level, the vacated cortex corresponded to the horizontal level containing the fundus of the cingulate sulcus.
Lesion site analysis of SDM 81 (12 month recovery)
On the lateral surface of the frontal lobe the lesion effectively removed nearly all of the rostral part of M1 (Figs. 4, 6C). For example, throughout the lesion site, there was no evidence of cellular layers I-VI with the exception of a very small region near the shoulder/leg border that only had layers I-III resected. The lateral frontal lesion extended rostrally to involve a small portion of the inferior-posterior part of dorsolateral premotor cortex (LPMCd) removing layers I-III. This part of the lesion corresponded to architectonic area 6DC. A small part of the posterior region of architectonic area 6V of the ventrolateral premotor cortex (LPMCv) was fully resected. The M1 lesion extended into the anterior bank of the central sulcus, involving only the dorsal most part of M1c (Table 2). Thus, a large portion of M1c was spared (Figs. 4, 6C). Likewise, in the caudal bank of the central sulcus, most of the cortex forming the rostral part of the arm/hand part of the primary somatosensory cortex (S1r, or areas 3 and 1) was spared with the exclusion of layers I-VI forming the dorsal part of area 1 which was fully resected (Fig. 4). From this location, the lesion extended posteriorly, effectively removing all layers (I-VI) of cortex forming the arm/hand part of caudal somatosensory cortex (S1c, or the gyral part of S1 corresponding to areas 1 and 2). The parietal lobe lesion extended into the superior parietal lobule removing layers I-VI of the rostral part of area PE (or area 5) (Fig. 4) and only layers I-III in the posterior tip (1.5mm) of the excised cortical region.
In both the frontal and parietal regions there was minimal white matter lesion damage that was confined to the location immediately below the extirpated gray matter (Fig. 6C; Table 2). There was no vacated region of white matter located below the gray matter resection as found in monkeys SDM87 and SDM91.
Lesion site analysis of SDM 83 (12 month recovery)
The rostral part of M1 (M1r), on the gyral surface, was completely removed (Figs. 4, 6D) and anterior to this cortex a small portion of LPMCd was ablated (i.e., layers I-VI were not found) (Fig. 4) with the exception of the anterior-most region (1 mm) of the extirpation where only layers I-III were removed. The M1r lesion extended into the anterior bank of the central sulcus, involving slightly more cortex on the anterior bank of the central sulcus (i.e., M1c) than case SDM81 (Fig. 4; Table 2). Like SDM81, much of the cortex lining the fundus of the sulcus was spared which extended onto the caudal bank of the central sulcus (Fig. 6D). However, slightly more cortex in the dorsal half of S1r was removed including all gray matter layers. From this location, the lesion extended posteriorly, effectively removing all layers (I-VI) of cortex forming the arm/hand part of S1c on the gyral convexity. The parietal lobe lesion extended very slightly into the superior parietal lobule removing layers I-VI of the rostral-most part of area PE (Fig. 4).
Like SDM 81, there was minimal white matter lesion damage that was confined to the location immediately below the excised gray matter (Fig. 6D; Table 2). There was also no evidence of a vacated region of white matter located below the gray matter resection as found in monkeys SDM87 and SDM91.
Summary of Lesion Site Analyses
In all monkeys our histological analysis showed that the cortical lesion was confined to the intended lateral frontal motor cortex, adjacent primary somatosensory cortex and rostral-most part of the superior parietal lobule. A significant component of gray matter on the cortical surface was removed in the ablation cases with differing amounts of cortex being extirpated along the banks lining the depths of the central sulcus. The amount of extirpated cortex lining the anterior part of the central sulcus gradually increased with SDM81 having the smallest percentage of extirpated M1c cortex (30%), cases SDM83 and SDM87 having approximately 50% of M1c removed, and SDM91 having all of M1c ablated (100%) (Table 2). Likewise, the smallest percentage of extirpated S1r cortex was in case SDM81 (20%) and the greatest amount in case SDM91 (80%) with intermediate levels of S1r removal occurring in cases SDM83 (34%) and SDM87 (24%) (Table 2). In all cases M1r and S1c were effectively removed with the exception of a small part of M1r near the shoulder/leg transition region dorsally in case SDM81. In all cases, there was minimal subcortical white matter involvement limited to the region immediately below the cortical extirpation and without subcortical gray matter involvement (i.e., basal ganglia and thalamus).
Neuroanatomical Observations of the F2P2 Lesion Cases versus the Controls
The corticospinal projection from the arm representation of M2 was examined in 4 F2P2 animals and the estimated numbers of labeled boutons for each case within each lamina are provided in Table 6 (contralateral projection) and Table 7 (ipsilateral projection). In the F2P2 lesion cases, the terminal bouton estimate for the M2 contralateral corticospinal projection to the 4 quadrants of lamina IX is provided in Table 8.
TABLE 6.
Contralateral Bouton Counts in Each F2P2 Lesion Case by Spinal Lamina1
| Bouton | I-III | IV | V | VI | VII | VIII | IX | X | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | # | Med | Lat | Med | Lat | Med | Lat | Med | Lat | RMB | Med | Lat | |||
| SDM 81 | 62,240 | 0 | 1,097 (2) |
0 | 411 (1) |
0 | 1,851 (3) |
137 (0.2) |
2,606 (4) |
4,254 (7) |
21,758 (35) |
15,102 (24) |
9,404 (15) |
2,465 (4) |
3,155 (5) |
| SDM 83 | 64,157 | 0 | 0 | 0 | 0 | 0 | 0 | 394 (1) |
3,545 (6) |
1,902 (3) |
25,681 (40) |
21,739 (34) |
5,976 (9) |
3,279 (5) |
1,641 (3) |
| SDM 87 | 56,630 | 0 | 0 | 0 | 0 | 0 | 125 (0.2) |
2,641 (5) |
3,838 (7) |
3,901 (7) |
18,822 (33) |
14,602 (26) |
5,098 (9) |
6,096 (11) |
1,507 (3) |
| SDM 91 | 47,951 | 0 | 176 (0.4) |
0 | 620 (1) |
0 | 354 (1) |
88 (0.002) |
619 (1) |
2,126 (4) |
22,875 (48) |
8,330 (17) |
11,349 (24) |
795 (2) |
619 (1) |
| MEAN | 57,744.5 | 0 | 318.3 | 0.0 | 257.8 | 0 | 582.5 | 815.0 | 2,652.0 | 3,045.8 | 22,284.0 | 14,943.3 | 7,956.8 | 3,158.8 | 1,730.5 |
Percentages of total label in parentheses. Values are rounded to the nearest whole number, except when the value is 0.7 or less. Lat, lateral; Med, medial; RMB, reticulated marginal border.
TABLE 7.
Ipsilateral Bouton Counts in Each F2P2 Lesion Case by Spinal Lamina1
| Bouton | I-III | IV | V | VI | VII | VIII | IX | X | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | # | Med | Lat | Med | Lat | Med | Lat | Med | Lat | RMB | Med | Lat | |||
| SDM81 | 35,066 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16,196 (46) |
1,920 (5) |
13,796 (39) |
890 (3) |
2,264 (6) |
| SDM83 | 13,456 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4,199 (31) |
393 (3) |
7,159 (53) |
197 (1) |
1,508 (11) |
| SDM87 | 11,693 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3,834 (33) |
689 (6) |
5,539 (47) |
439 (4) |
1,192 (10) |
| SDM91 | 22,517 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9,662 (43) |
1,506 (7) |
10,552 (47) |
443 (2) |
354 (2) |
| MEAN | 20,683.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 8,472.8 | 1,127.0 | 9,261.5 | 492.3 | 1,329.5 |
Percentages of total label in parentheses. Values are rounded to the nearest whole number, except when the value is 0.7 or less. Lat, lateral; Med, medial; RMB, reticulated marginal border.
TABLE 8.
Contralateral Bouton Counts in Each F2P2 Lesion Case within Each Quadrant of Lamina IX1
| Bouton | IX | ||||
|---|---|---|---|---|---|
| Case | # | DMED | DLAT | VMED | VLAT |
| SDM81 | 2,465 | 753 (31) |
273 (11) |
1,439 (58) |
0 |
| SDM83 | 3,279 | 721 (22) |
327 (10) |
2,034 (62) |
197 (6) |
| SDM87 | 6,096 | 2,200 (36) |
1,446 (24) |
1,760 (29) |
690 (11) |
| SDM91 | 795 | 531 (67) |
88 (11) |
176 (22) |
0 |
| MEAN | 3,158.8 | 1,051.3 | 533.5 | 1,352.3 | 221.8 |
Percentages of total label in parentheses. Values are rounded to the nearest whole number, except when the value is 0.7 or less. Lat, lateral; Med, medial; RMB, reticulated marginal border.
The F2P2 lesion cases averaged about 75% of the mean estimated number of boutons of control cases for the M2 CSP projection in the contralateral spinal gray matter of C5-T1. Two-way [group – lesion/control; region – dorsal horn (laminae I-IV), intermediate zone (laminae V-VII and RMB), ventral horn (laminae VIII-IX), lamina X] repeated measures ANOVA showed that the number of boutons was lower in F2P2 lesion subjects than in controls (Fig. 7, group main effect: F1,7 = 5.45, p = 0.052) and that the distribution of boutons among spinal regions was altered by the lesion (Fig. 7, group x region interaction effect: F3,21 = 5.03, p = 0.009). Specifically, there were fewer boutons in the intermediate zone in subjects with F2P2 lesions than in controls (Fig. 7B, p = 0.009 on post-hoc tests), but not in other regions (Fig. 7B, p > 0.05). Further statistical analysis of boutons within the intermediate zone laminae and RMB showed that only lamina VII had fewer boutons in F2P2 lesion monkeys than controls (Fig. 7 – F3,21 = 3.75, p = 0.063; p = 0.009 on post-hoc test for lamina VII, p > 0.98 for lamina V, VI and RMB). Analysis of lamina IX boutons by quadrants showed that the F2P2 lesion subjects had significantly fewer boutons only in the dorsal quadrants (Fig. 7C, group x quadrant interaction: F3,21 = 8.74, p = 0.005, p < 0.05 on post-hoc tests comparing specific quadrants of controls and lesion subjects). The neuroplastic response was limited to the contralateral pathway as the estimated numbers of labelled boutons in the ipsilateral M2 CSP was similar for lesion and control cases (Fig. 7D, F1,7 = 0.007, p = 0.937).
On average M2 fiber lengths in the contralateral lateral corticospinal tract at C5 and C8 were longer in controls than subjects with F2P2 lesions but there were no significant differences between the two groups (F1,7 = 1.25, p = 0.3) and no group by segmental level (C5, C8) interaction effect (F1,7 = 0.16, p = 0.7). In contrast, the lesion cases had shorter M2 terminal fiber lengths than controls in contralateral spinal gray matter. Specifically, within the spinal gray matter at segmental levels C5 and C8, the distribution of M2 terminal fiber lengths within the medial and lateral regions of lamina VII and the dorsal regions of lamina IX differed between F2P2 lesioned monkeys and controls (group x segment x lamina region effects: F3,21 = 7.61, p = 0.001). Post-hoc tests showed that these differences were confined to the lateral and medial regions of lamina VII at C5 in which F2P2 lesioned monkeys had shorter fiber lengths than controls (p < 0.05). Fiber lengths were similar for lamina VII medial and lateral regions at C8 and for lamina IX dorsolateral and dorsomedial regions at C5 and C8 (p > 0.93).
Correlation of Neuroanatomical and Behavioral Observations
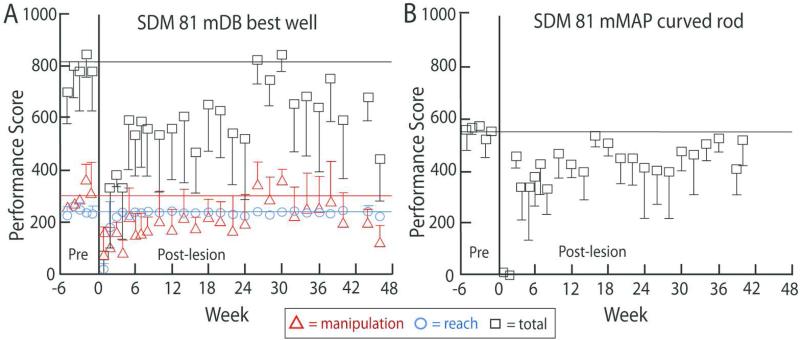
There were varying levels of recovery of reaching and grasp/manipulation of the food targets on the mDB and mMAP tasks among the four lesion cases. Three cases (SDM81, 83, 87) showed good recovery of performance scores (Fig. 8A,B) and post-lesion/pre-lesion manipulation skill ratios (Fig. 9A,B – manipulation skill ratios between about 0.4 and 1.2) in the mDB and mMAP tasks (e.g., Figs. 8,9), comparable to recovery of manipulation skill we have reported previously for most monkeys with M1 + LPMC lesions (Darling et al. 2009). However, one case (SDM91) showed very poor recovery. Specifically, SDM91 had 5 successful acquisitions in a single post-lesion test for only well E of the mDB task (actually a shallow dimple in the Plexiglas plate to hold the pellet) and never on the easiest mMAP task (grasping a carrot chip from a flat surface). There were also some successful post-lesion acquisitions of the small food pellet used in the mDB task wells from wells B, C, D and of the carrot chip in the mMAP flat surface task, but not in the mMAP straight rod or curved rod tasks. Thus, some recovery clearly occurred, but learned nonuse developed in SDM91 beginning after the 8th post-lesion week as there were no further attempts to retrieve the food targets with the contralesional hand on the mDB or mMAP tasks (e.g., Fig. 8B).
Figure 8.
A shows mean pre- and post-lesion total (black), reach (blue) and manipulation (red) performance scores (+/− 1 SD) for the mDB best well task by Case SDM81. Note that reach performance scores recovered rapidly to mean pre-lesion levels (indicated by the blue horizontal line) whereas total (black) and manipulation (red) performance scores remained lower than mean pre-lesion levels on most tests and were also more variable. B shows mean pre- and post-lesion performance scores in the mMAP curved rod task by SDM81.
Figure 9.
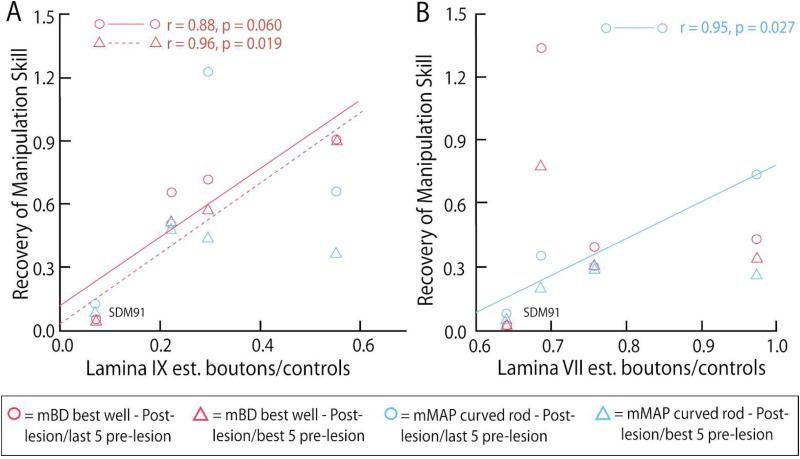
A and B show relationships between recovery of manipulation skill (ratio of best post-lesion skill ratio to skill of last 5 pre-lesion tests and to best 5 consecutive pre-lesion tests) in the mDB best well and mMAP (curved rod) tasks and the ratio of lamina IX and lamina VII boutons of C5-T1 for each lesion monkey to the mean of controls. Note that recovery of skill data for SDM91 are indicated as the plotted points on the bottom left (i.e., poorest recovery) in A and B. Linear correlation coefficients for statistically significant relationships between recovery of skill and numbers of laminae VII and IX boutons are also shown.
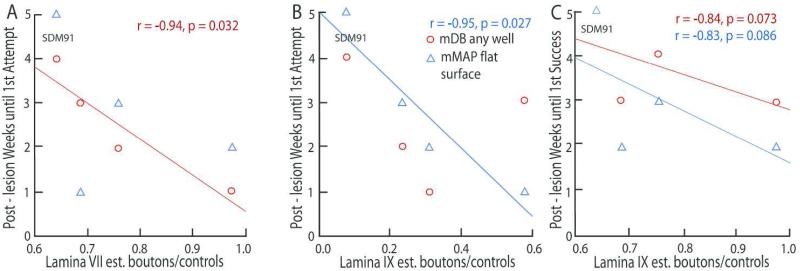
There was evidence that the post-lesion duration until behavioral attempts and successes were made, correlated with the remaining numbers of boutons from M2 in laminae containing interneurons and motor neurons. Specifically there were significant negative correlations of the ratios of lamina VII and IX boutons in lesion cases to average numbers of boutons in controls with post-lesion duration to first attempt and first success in the mDB and/or the easiest mMAP (flat surface) tasks. In the mDB task the post-lesion duration until the 1st attempt on any well was strongly negatively correlated with the lamina VII bouton ratio (Fig. 10A, r = −0.94, p = 0.032). In the easiest (flat surface) mMAP task there was a significant negative correlation of the lamina IX bouton ratio with post-lesion duration until first attempt (Fig. 10B, r = −0.95, p = 0.027) and also evidence of a correlation with post-lesion duration until the 1st success on this task (Fig. 10C, r = −0.83, p = 0.086).
Figure 10.
Scattergraphs showing post-lesion duration in weeks until 1st attempt (A,B) and 1 success (C) in the mDB (any well) and mMAP (flat surface) tasks versus estimated number of labeled boutons in individual cases with F2P2 lesions relative to mean number of labeled boutons in control cases in lamina VII (A) or lamina IX (B,C) of C5-T1. Each plotted point is data from a single case with a F2P2 lesion. Note the data for SDM91 (longest duration until first attempt and success) are indicated in the top left of each graph. Linear correlation coefficients for statistically significant relationships between post-lesion duration until first attempt and success in the mDB and mMAP tasks and numbers of laminae VII and IX boutons are also shown.
Recovery of fine motor function varied substantially among the 4 lesion cases and was also closely related to the number of M2 CSP boutons remaining in laminae VII and IX in individual lesion cases. The ratio of the number of labeled lamina IX boutons in lesion cases to the average number of labeled lamina IX boutons in controls was highly positively correlated with post-lesion recovery of manipulation skill (measured as the ratio of post-lesion skill to skill of the last 5 pre-lesion tests and skill of the best 5 consecutive pre-lesion tests (Fig. 9A, r = 0.88, 0.99 respectively, p < 0.003). Similarly, recovery of manipulation skill in the mMAP curved rod task was positively correlated with the ratio of labeled boutons in lamina VII of lesion cases to the average number of labeled lamina VII boutons in controls (Fig. 9B, r = 0.95, p = 0.027).
DISCUSSION
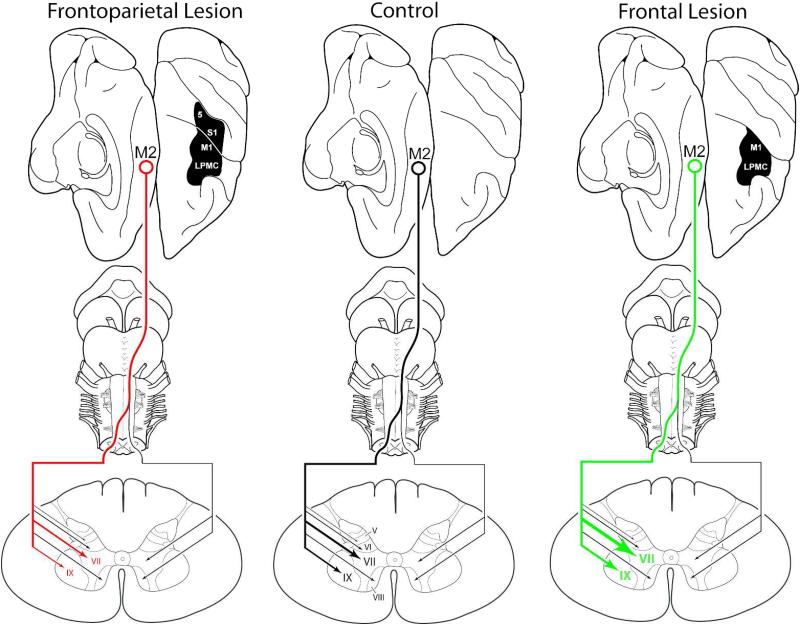
Lateral frontoparietal damage is common following stroke due to the fact that the middle cerebral artery (MCA) supplies this part of the cortical mantle (Tatu et al., 1998) and is the most commonly affected vessel in stroke (e.g., Ameriso and Sahai, 1997; Castillo and Bogousslavsky, 1997; Pessin, 1997; Carrera et al., 2007; González Delgado and Bogousslavsky, 2012). Our findings reveal that the collective loss of the lateral frontal somatomotor cortex and lateral parietal somatosensory cortex (F2P2 lesion) not only impedes a favorable compensatory neuroplastic response from the spared M2 corticospinal projection, but results in a substantial loss of M2 corticospinal terminal boutons and terminal fibers when compared to controls (Fig. 11). This response is in striking contrast to our previous findings following isolated lateral frontal somatomotor cortex injury (F2 lesion) showing that the corticospinal projection from M2 substantially increases in terms of the number of terminal boutons and terminal fiber lengths in laminae VII and IX at spinal levels C5 to T1 compared to controls (Fig. 11) (McNeal et al., 2010). We did not find any statistical differences between corticospinal fiber lengths in the lateral corticospinal tract (LCST) in the F2P2 lesion cases versus the controls. This suggests that the breakdown of the intact M2 corticospinal pathway occurred at the putative synaptic level within the confines of the spinal gray matter, at least with respect to the parameters investigated in our study, and this condition persists 6 to 12 months after lateral frontoparietal injury. What our data does not reveal is whether this long-term loss of corticospinal input represents a gradual decline, or conversly, a gradual recovery of input following a potentially massive loss of M2 corticospinal input in the acute and subacute phases following lateral frontoparietal injury. Indeed, application of the current experimental design on cases terminated at earlier post-injury time intervals (1 and 3 months after injury) will be required to address these important issues.
Figure 11.
Model of the effects of lateral cortical lesions on M2 CSP to spinal cord laminae of C5-T1 in controls and monkeys with F2P2 lesions and F2 lesions. In the present study we found substantial reductions in the total number of terminal labeled boutons of the supplementary motor cortex (M2) corticospinal projection (CSP) in lamina VII and IX at spinal levels C5 to T1 following removal of the arm/hand region of the lateral frontal somatomotor cortex (M1 + LPMC) and removal of the arm/hand region of the lateral parietal somatosensory cortex (S1 + rostral part of area 5/PE) (far left – F2P2 lesion) compared to controls (middle of figure). In our previous study, we found that isolated removal of the arm/hand region of the lateral frontal somatomotor cortex (M1 + LPMC) alone (far right - F2 lesion) results in substantial increases in contralateral terminal labeling of the M2 CSP in laminae VII and IX compared to controls (McNeal et al., Journal of Comparative Neurology 518:586-621, 2010). Thus, we demonstrate in the present study that the added effect of adjacent parietal cortex injury to the frontal motor lesion not only impedes a favorable compensatory neuroplastic response, but results in a substantial loss of M2 CSP terminal boutons. Clinically, our findings suggest that localized lateral peri-Rolandic cortical injury in stroke patients, which is very common, may undermine the structural and functional integrity of intact corticospinal projections located at remote sites from the lesion site. (Note: The relative intensity of the projection to spinal cord laminae is indicated by line thickness and arrow size. Denser terminal projections are represented by increased line thickness and arrow head size. Progressively lighter terminal projections are indicated by progressively thinner lines and arrowheads).
Effects of Sensory Denervation/Deprivation on Motor System Output
It is well beyond the scope of this research article to summarize the literature concerning the effects of sensory denervation on movement. However, some discussion is appropriate in the context of our experimental findings following partial destruction of S1. It has long been noted in non-human primates that section of the dorsal roots which subserve upper limb sensory impulses has devastating effects on motor function of the upper limb. Mott and Sherrington (1895) found that sectioning the dorsal roots in monkeys produced more severe and lasting paralysis than motor cortex resection. Similarly, more recent studies in monkeys have shown cervical dorsal rhizotomy that completely abolishes afferents to a subset of the digits is associated with poor recovery of grasping such that only digits that were incompletely deafferented were used to grasp objects (Darian-Smith and Ciferri, 2005; Darian-Smith, 2009). Interestingly, after sectioning of dorsal roots with receptive fields on the first three digits, recovery on a grasp retrieval task was accompanied by M1 corticospinal sprouting in the dorsal horn indicating an adaptive and possibly supportive role for spared M1 corticospinal projections in the recovery process (Darian-Smith, et al., 2013). However, the S1 corticospinal projection retracted which is similar to the reduction of spared M2 corticospinal projections observed in the present study following lateral frontoparietal injury. Taken together, the findings of Darian-Smith and colleagues indicate the loss of peripheral sensory afferent input to S1 as a result of dorsal rhizotomy results in a retraction of the S1 CSP in the affected dorsal horn and increase in the M1 CSP in the affected dorsal horn. However, it is unclear whether this sensory deprivation results in a decrease in the M1 CSP in the intermediate and anterior spinal gray matter regions as would be predicted by our findings.
Dorsal column injury, which disrupts the delivery of tactile and proprioceptive sensation information to S1 has devastating effects on fractionated finger movements (i.e., Beck, 1976; Eidelberg et al., 1976; Vierck, 1978; Cooper et al., 1993). Most of the investigative work examining the CNS adaptations that accompany dorsal column lesions have focused on adaptive reorganization at the level of the cuneate nucleus, sensory thalamus and primary somatosensory cortex (for review see Qi et al., 2014). Much like dorsal root lesions, reactivation and reorganization of S1 that correlates with behavioral motor recovery appears to depend on preserved subcomponents of the ascending sensory pathways. Some recovery may occur following complete dorsal column lesions through preserved ascending second order neuron axons arising from the dorsal horn neurons, and spared ascending sensory fibers that travel in the anterior and lateral funiculi (see Qi et al., 2014 for review). Notably, recovery of manual dexterity has been correlated with reactivation of lost components of the ascending somatosensory relay nuclei through local sprouting mechanisms from intact sensory fiber systems, including at the level of S1. It seems logical to assume that the immediate deficits in manual dexterity may be primarily due to the loss of somatosensory input to guide appropriate motor output, but it remains unclear if this condition has an acute or long-term detrimental effect on what appears to be an otherwise intact frontal motor neural network. Indeed, since the literature indicates that fractionated movements appear to be highly susceptible to impairments after dorsal column lesions, it is possible that this sensory loss may have a negative impact on intact corticospinal projections from the frontal cortex to lamina IX which contains motoneurons innervating the distal upper extremity musculature. Supporting this possibility is recent work showing that increased current levels are required to evoke movements from M1 following long-term dorsal column lesions (Kambi et al., 2011).
It is clear that our parietal lobe lesions did not fully disrupt the arm/hand area of the primary somatosensory cortex with the possible exception of SDM91 (extensive damage to S1r and S1c - Fig. 4; Table 2), and that alternate, indirect routes of ascending somatosensory input (e.g., from the cuneate nucleus as well as from cerebellum which receives cutaneous/proprioceptive inputs from spinocerebellar tracts) were likely to reach and influence M2. However, our results show that partial S1r and substantial S1c damage of the lateral parietal cortex, along with lateral frontal motor cortex injury completely changes the recovery dynamics/mechanisms of spared medial motor cortex CSP output to one that is maladaptive, blocking a favorable recovery mechanism that we have previously shown for the M2 CSP response following lateral frontal motor cortex injury alone (McNeal et al., 2010). Collectively, this work strongly suggests a critical trophic influence for intact S1 on a remote component of the frontal motor cortical network (i.e., M2) that does not receive direct S1 cortical afferents with the exception of a small projection from the posterior part of area 2 (Luppino et al., 1993; Morecraft et al., 2012). Indeed, it will be of great interest to study the effects of isolated parietal cortex injury on spared frontomotor corticospinal projections to determine the nature of trophic input from different parietal lobe regions on the maintenance and integrity of lateral and medial frontomotor corticospinal pathways.
It is possible that a trophic influence of parietal cortex neurons on motor cortex is mediated through release of nerve growth factors from parietal cortex affecting distant cortical and subcortical sites or by parietal neural activity influencing long term potentiation in cortical and subcortical areas receiving parietal inputs. There is evidence that cortical activation with repetitive transcranial magnetic stimulation (rTMS) of the brain enhances production of mRNA for brain-derived neurotrophic factor (BDNF) specifically in parietal cortex with some increase also in hippocampus and piriform cortex of rats (Hausmann et al. 2000; Muller et al. 2000). Kindling seizures induced by electrical stimulation of the ventral hippocampus in rats also increases production of mRNA for BDNF in parietal cortex (Ernfors et al. 1991; Kokaia et al. 1996). Normal activation of parietal cortex by stimulation of peripheral sensory receptors may also increase production and release of BDNF from parietal lobe which may enter the cerebrospinal fluid and help maintain neural tissue in various areas of the CNS including motor areas. There is also evidence that BDNF is an anterograde neurotrophic factor transported and released by axons originating from subcortical areas and parietal lobe to cause neurotrophic responses in specific targets (Fawcett et al. 1998; Kokaia et al. 1998). Damage to parietal lobe would presumably reduce this production and release of BDNF which may decrease its availability in motor areas for maintenance of healthy neural tissue, including connections onto spinal motor neurons and interneurons from intact motor areas. Action potentials in parietal cortex neurons that normally occur during voluntary limb motions may help to maintain synaptic connections among parietal and motor areas as suggested from studies of paired associative stimulation and functional connectivity between PPC and motor cortex (Veniero et al. 2013). Removal of parietal cortex and the subsequent loss of connections to motor areas may decrease health of neural tissues in those areas, perhaps leading to loss of output connections as we have observed in the case of the M2 CSP.
Motor Recovery Considerations
Given the previously described effects of dorsal root and dorsal column lesions on upper limb movements we were surprised that recovery of dexterous hand/digit movements was good in 3 of the 4 F2P2 lesion cases (e.g., Fig. 8A, B). Indeed, SDM81, SDM83 and SDM87 showed recovery of fine movement control that was similar to monkeys with lesions limited to frontal motor areas (Darling et al. 2009). Only SDM91 showed very poor long-term recovery (Figs. 8, 9, 10), which was probably due to the more extensive damage to both caudal M1 and rostral S1 than in the other F2P2 cases reported here (Fig. 4; Table 2). However, SDM91 did show recovery of precision grasping of small food pellets at 6 and 7 weeks post-lesion on the mDB task (unpublished observations) before learned nonuse set in and never used the contralesional hand again in either task. Degeneration of the M2 CSP may have occurred over several weeks after the lesion and may have contributed to the apparent arrest of motor recovery and onset of learned nonuse. Identification of therapies that can prevent deterioration of the CSP from spared frontal motor areas after large parietal lobe lesions may be important in generating good motor recovery and preventing learned nonuse of the contralesional limb, especially in humans who rely extensively on the corticospinal projection to perform individuated fractionated digit movements (Porter and Lemon, 1993; Schieber, 2007; Lemon, 2008).
The positive fine motor recovery of 3 of the 4 cases (SDM81, SDM83 and SDM87) suggests that spared subcortical and/or cortical motor areas had access to upper limb somatosensory inputs despite the lesion to S1. Sparing of a large portion of S1r in the posterior bank of the central sulcus in these 3 cases would have allowed some somatosensory input from the upper limb to project to spared frontal motor areas (including the spared part of M1c located in the depths of the central sulcus) and is also consistent with the notable recovery reported when some cervical dorsal roots are spared in studies of effects of dorsal rhizotomy (Mott and Sherrington 1895; Darian-Smith and Ciferri 2005). However, the 3 cases with good recovery usually confirmed grasp of the food with vision during post-lesion testing whereas during pre-lesion testing the tasks were often performed without vision as the monkey would watch the experimenter rather than view their hand during these tasks. Such pre and post-lesion behaviors during fine motor tasks have been reported for monkeys experiencing dorsal column lesions (e.g., Qi et al. 2014) and postcentral cortical ablation (Kennard and Kessler, 1940; Peele 1944).
There was evidence that recovery rate and quality depended on numbers of remaining M2 CSP boutons in laminae VII and IX of C5-T1 (Figs. 9A, B, 10). That is, there were inverse relationships between post-lesion duration until first attempt and first success with the contralesional hand in the mDB and mMAP tasks with estimated numbers of boutons in laminae VII and IX relative to numbers of boutons in controls (Fig. 10). Furthermore, there were positive relationships of numbers of laminae VII and IX boutons relative to controls with recovery of manipulation skill in the mDB and mMAP tasks (Fig. 9A, B). These relationships are consistent with our previous work showing correlations of motor function recovery following an F2 lesion with increased numbers of M2 CSP boutons in these laminae (McNeal et al., 2010). However, a large increase in M2 CSP boutons in laminae VII and IX following a subtotal F2P2 lesion in the non-human primate model is apparently not required for good recovery since we observed significant loss of M2 CSP boutons in the present work. It is important to note that we were able to demonstrate a contributing role in motor recovery for the increased CSP from M2 following isolated lateral frontal motor injury (McNeal et al., 2010). Specifically, after motor recovery from the lateral frontomotor (F2) lesion, subsequent destruction of M2 with ibotenic acid reinstated the initial deficits in fine movements of the hand (see SDM58, Fig. 11 in McNeal et al., 2010). It is possible in the three F2P2 lesion cases that showed good recovery in the current study, other cortical and subcortical motor areas were able to compensate for the apparent degeneration of the M2 CSP and re-establish innervation of spinal interneurons, and possibly motor neurons controlling arm/hand/digit muscles. Likely candidates include spared parts of the M1c arm/hand area located in the depths of the central sulcus as previously mentioned, the cingulate motor areas and subcortical motor structures that exert influence directly on spinal neurons (i.e., red nucleus, reticular formation). Indeed, recent work in non-lesioned monkeys has shown that the reticulospinal projection has excitatory disynaptic and monosynaptic connections with motor neurons innervating upper limb muscles, including the intrinsic hand muscles (Riddle et al., 2009). The correlation analyses discussed above suggest that the remaining small numbers of M2 CSP boutons in laminae VII and IX also contribute to recovery of dexterity in SDM81, SDM83 and SDM87, perhaps due to maintenance of some direct corticomotoneuronal projections (Table 8).
Clinical Considerations
Of the many postulated brain areas contributing to motor recovery following brain injury, the role of spared corticospinal projection areas are among the most promising as they represent highly accessible targets for non-invasive therapeutic intervention. Furthermore, spared corticospinal projections represent the only direct linkage between the cerebral cortex and spinal cord. Thus, therapeutic interventions directed on this system may have the most immediate and significant impact on the motor recovery process, particularly for restored hand movements. However, findings from the current study suggest that what may appear to be spared corticospinal projection fields following frontoparietal cortical injury, may in fact be highly vulnerable to long-term deterioration, and over time become part of an “extended” cortical lesion. Indeed, our findings suggest that the loss of critical parietal somatosensory input on spared components of the frontal motor system may contribute significantly to the breakdown of intact corticospinal projections, and may subsequently contribute to the failure to recover fine sensorimotor control of dexterous hand/digit movements. Specifically, the findings from the current study suggest that the medial frontal motor system, which at the cortical level is likely to be spared in at least 97% of stroke victims since it is supplied by the anterior cerebral artery (ACA) (Bogousslavsky and Regil, 1990; Kazui et al., 1993; Kumral et al., 2002; Carrera et al., 2007), may be particularly vulnerable to the eventual loss of descending influence on spinal interneurons and motoneurons following common MCA infarctions that compromise the peri-Rolandic region. The importance of maintaining the integrity of the CSP from M2 is underscored by the fact that it represents the second largest corticospinal projection only to M1 (Dum and Strick, 1991; Galea and Darian-Smith, 1994) and is the only cortical area other than M1 known to give rise to direct corticomotoneuronal projections supplying muscles acting on the wrist and hand (Maier et al., 2002; Boudrias et al., 2006, 2010a,b). It is important to note that it is unclear if M2 CSP vulnerability identified in the present report is due to the combined loss of lateral M1 and S1, or if parietal lobe injury alone is enough to render it susceptible to long-term degeneration in the absence of therapeutic intervention. It is also possible that diaschisis, or adverse neurophysiological changes that occur at remote sites from the brain lesion, may have played a role in the deterioration of the M2 CSP (for review see Carrera and Tononi, 2014). However, if this were the case it is likely that we would have not found the favorable neuroplastic response for the M2 CSP following our focal lateral frontal lobe injury experiments (McNeal et al., 2010). Indeed, understanding the status of spared corticospinal projections following subtotal cortical injury will be critical to designing targeted interventions that can not only maintain their integrity following injury, but also include them in the recovery process during rehabilitative intervention.
Technical Considerations
In the current study design, a lower volume (0.9 μl) of FD was injected into the central region of the arm representation of M2 in 3 of the 4 F2P2 lesion monkey cases compared to a greater volume (1.2 μl) in the controls (Table 1). Thus, it is possible that this volume difference may account for the lower number of labeled boutons being estimated in the 3 F2P2 lesion cases versus the control experiments. Indeed, our initial study design hypothesized that the much larger F2P2 lesion, compared to the smaller sized F2 lesion would potentially result in much greater CSP neuroplastic response (e.g., more labeled terminal boutons) than found in our previous F2 lesion experiments. We therefore opted to initiate the F2P2 experiments by injecting the same tracer volume as we injected in the F2 cases (0.9 μl) (McNeal et al., 2010). Several observations point to the conclusion that the lower number of labeled boutons found in all the F2P2 experimental cases versus the controls is not only truly reflective of inhibited neuroplastic response, but also of a true reduction, or deterioration of the spared M2 CSP following parietal lobe inclusion in our lateral frontomotor lesion model. First, our F2P2 lesion case SDM91 received 1.2 μl of FD and resulted in the lowest number of labeled CSP boutons in all cases presented in this paper. Second, we have previously demonstrated in 4 monkey experiments with a F2 lesion, that a total FD injection volume of 0.9 μl is more than enough tracer volume to not only adequately label terminal boutons throughout spinal levels C5 to T1, but label a far greater number of terminal boutons (i.e., 2-3 times) versus all 5 of our M2 control cases (see Tables 5 and 7 of McNeal et al., 2010). For instance, our F2 lesion cases SDM45 and SDM70 received 0.9 μl of FD tracer and each gave rise to greater than 200,000 labeled terminal boutons compared to an average control value of 75,854 labeled boutons (McNeal et al., 2010). The lack of significant changes in the ipsilateral corticospinal projection would also seem to rule out differences attributable to different injection volumes associated with these large injection sites. Other considerations would also support a compatible tracer transport environment when assessing the estimated volumes of the injection site core and halo (Table 1). The range of halos (which included the core volume) for the controls was 51.6 mm3 to 85.9 mm3 whereas the range for the F2P2 cases was 47.8 mm3 to 119.7 mm3. Similarly, the range of the injection site cores for the controls was 14.7 to 38.7 mm3 and for the F2P2 lesion cases 15.5 to 36.6 mm3. Thus, in each set of experimental animals there were representative cases with similar injection site characteristics in terms of tracer core density and peripheral spread (i.e., halo). Indeed, F2P2 case SDM 91 had the largest halo (119.7 mm3) and second largest core value (36.6 mm3) and the fewest labeled boutons suggesting that the total number of labeled boutons was not critically dependent on the size of the injection site halo/core or the total volume (0.9 - 1.2 μl) injected. It is notable that in all 4 F2P2 cases we were able to find solidly filled terminal axons and boutons throughout C5 to T1 (Fig. 2) and at levels T2 and T3 which were not part of this investigation. Indeed, these lower levels (T2 and T3) were processed to assure we had a full set of tissue sections through T1 and to evaluate the quality of tracer transport to spinal levels below the cervical enlargement. Finally, our histological analysis demonstrated that a dense, solid core region was present in the FD injection site in all 4 F2P2 lesion cases (Fig. 1) showing that an extensive, and concentrated amount of tracer was still available for active transport 33-34 days after the administration of FD into the arm/hand region of M2.
It is important to note that 3 of the 4 study subjects were male in the previous report of increased M2 CSP boutons after M1 + LPMC lesions (McNeal et al. 2010) but in the present report of decreased M2 CSP boutons all subjects were female. Moreover, the lone female (SDM48) of the previous study had fewer M2 CSP boutons after the M1 + LPMC lesion than 2 of the 3 male monkeys (see Table 5 in McNeal et al. 2010). Thus, gender differences in capacity for intraspinal sprouting of axons and boutons after cortical lesions may partially account for the different results. Unfortunately we could not locate any studies of gender differences in axonal sprouting within the spinal cord after lesions to cerebral cortex. However, there are investigations of sex differences in axonal sprouting within hippocampus following lesions to the medial septal nucleus or fimbria/fornix which show that females exhibit much greater axonal sprouting than males (e.g., Loy and Milner 1980). Moreover, other research shows that estrogen stimulates axonal sprouting of the nucleus retroambiguus-spinal pathway in female mice, cats and rhesus monkeys (Van der Horst and Holstege 1998; Vanderhorst et al. 2002; Vanderhorst 2005) and that axonal sprouting after cutting of peripheral nerves is greater in females than in males (Kovacic et al. 2003). Given these findings, it seems unlikely that the reduced numbers of M2 CSP boutons after F2P2 lesions observed in the current study can be attributed to less capacity for axonal sprouting in females than in males. Future study of gender differences in compensatory axonal sprouting responses following cortical brain lesions is warranted in the monkey model.
Summary and Conclusions
We have found that concomitant removal of the peri-Rolandic frontal and parietal arm/hand region blocks a favorable M2 corticospinal neuroplastic response but also results in a devastating loss of corticospinal projections from spared ipsilesional M2 at the chronic stage of spontaneous recovery (Fig. 11). These findings suggest that spared frontal corticospinal projections that are secluded from the grossly identifiable lateral frontoparietal lesion site, are in fact vulnerable and, over time eventually become part of the lesion. It is important to note that our experimental animals were allowed to move freely in their cage over a 6 and 12 month time period and use either hand during their activities of daily living (including feeding, climbing and playing with large toys for environmental enrichment). Despite such extensive motor activity over an extended time frame, the CSP from M2 still deteriorated indicating that some form of therapeutic intervention will be required to reinstate a positive compensatory response for the spared M2 projection, as we have found following spontaneous recovery following isolated resection of M1 and LPMC alone (McNeal et al., 2010). Clinically, our findings suggest it is possible that the common occurrence of lateral frontoparietal cortical injury in stroke patients may also undermine the structural integrity and functional operations of intact corticospinal projections located at remote sites from the lesion, and much like our experimental rhesus monkey model, may lead to the gradual deterioration of intact CSP neurons in the absence of effective therapeutic intervention. It is also possible that the involvement of parietal lobe injury, which is the most frequently damaged part of the cortical mantle in stroke patients (Yoo et al., 1998), may undermine the motor recovery process in humans, and be an overlooked factor that contributes to the poor motor recovery outcomes that often accompany superficial middle cerebral artery stroke.
Acknowledgments
GRANT SUPPORT: Grant sponsor: National Institutes of Health; Grant numbers: NS 046367.
LIST OF ABBREVIATIONS
- A
arm
- Ab
abdomen
- An
ankle
- As
arcuate spur of the arcuate sulcus
- Ax
axial
- cb
cingulum bundle
- cf
calcarine sulcus
- cgs
cingulate sulcus
- cs
central sulcus
- CSP
corticospinal projection
- D
digit
- El
elbow
- FD
Fluorescein dextran
- Fr
frontalis
- Hp
hip
- IC
internal capsule
- ilas
inferior limb of the arcuate sulcus
- ios
inferior occipital sulcus
- ips
intraparietal sulcus
- J
jaw (mandible)
- Kn
knee
- L
leg
- LCST
lateral corticospinal tract
- lf
lateral fissure
- LL
lower lip
- LPMC
lateral premotor cortex
- LPMCd
dorsal lateral premotor cortex
- LPMCv
ventral lateral premotor cortex
- ls
lunate sulcus
- M1
primary motor cortex
- M1c
primary motor cortex, caudal part
- M1r
primary motor cortex, rostral part
- M2
supplementary motor cortex
- mDB
modified dexterity board
- mMAP
modified movement assessment panel
- N
neck
- NR
no response
- OO
orbicularis oculi
- ots
occipito-temporal sulcus
- poms
medial parieto-occipital sulcus
- ps
principle sulcus
- RMB
reticulated marginal border
- ros
rostral sulcus
- rs
rhinal sulcus
- S1
primary somatosensory cortex
- S1c
primary somatosensory cortex, caudal part
- S1r
primary somatosensory cortex, rostral part
- Sh
shoulder
- slas
superior limb of the arcuate sulcus
- spcd
superior precentral dimple
- sts
superior temporal sulcus
- Ta
tail
- Th
thumb
- To
tongue
- Tr
trunk
- UL
upper lip
- v
ventricle
- Wr
wrist
- 3a,4,6
Cortical architectonic divisions according to Brodmann (1905)
- I-X
Laminae of the spinal cord according to Rexed (1954)
Footnotes
Conflict of interest statement.
All authors do not have any conflict of interest that could inappropriately influence, or be perceived to influence, this work.
LITERATURE CITED
- Aizawa H, Inase M, Mushiake H, Shima K, Tanji J. Reorganization of activity in the supplementary motor area associated with motor learning and functional recovery. Exp Brain Res. 1991;84:668–671. doi: 10.1007/BF00230980. [DOI] [PubMed] [Google Scholar]
- Ameriso SF, Sahi S. Mechanisms of ischemia in in situ vascular disease. In: Welch KMA, Caplan LR, Reis DJ, Siesjö BK, Weir B, editors. Primer on cerebrovascular diseases. Academic Press; New York: 1997. pp. 279–285. 1997. [Google Scholar]
- Beck C. Forelimb performance by squirrel monkeys (Saimiri sciureus) before and after dorsal column lesions. J Comp Physiol Psychol. 1976;90:353–362. doi: 10.1037/h0077206. [DOI] [PubMed] [Google Scholar]
- Biber MP, Kneisley LW, LaVail JH. Cortical neurons projecting to the cervical and lumbar enlargements of the spinal cord in young and adult rhesus monkeys. Exp Neurol. 1978;59:492–508. doi: 10.1016/0014-4886(78)90240-6. [DOI] [PubMed] [Google Scholar]
- Black P, Markowitz RS, Cianci S. In search of the motor engram: a behavioral study of somatotopic localization in motor cortex of monkey. Trans Am Neurol Assoc. 1974;99:188–190. [PubMed] [Google Scholar]
- Bogousslavsky J, Regli F. Anterior cerebral artery territory infarction in the Lausanne stroke registry. Arch Neurol. 1990;47:144–150. doi: 10.1001/archneur.1990.00530020040012. [DOI] [PubMed] [Google Scholar]
- Boudrias MH, Belhaj-Saïf A, Park MC, Cheney PD. Contrasting properties of motor output from the supplementary motor area and primary motor cortex in rhesus macaques. Cereb Cortex. 2006;16:632–638. doi: 10.1093/cercor/bhj009. [DOI] [PubMed] [Google Scholar]
- Boudrias MH, Lee SP, Svojanovsky S, Cheney PD. Forelimb muscle representations and output properties of motor areas in the mesial wall of rhesus macaques. Cereb Cortex. 2010b;20:704–719. doi: 10.1093/cercor/bhp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudrias MH, McPherson RL, Frost SB, Cheney PD. Output properties and organization of the forelimb representation of motor areas on the lateral aspect of the hemisphere in rhesus macaques. Cereb Cortex. 2010a;20:169–186. doi: 10.1093/cercor/bhp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Beiträge zur histologischen lokalisation der grosshirnrinde: dritte mitteilung: die rindenfelder der niederen affen. J Psychol Neurol. 1905;4:177–226. [Google Scholar]
- Bucy PC. Effects of extirpation in man. In: Bucy PC, editor. The precentral motor cortex. 2nd The University of Illinois Press; Urbana, Illinois: 1949. pp. 353–394. [Google Scholar]
- Carrera E, Maeder-Ingvar M, Rossetti AO, Devuyst, Bogousslavsky G. Trends in risk factors, patterns and causes in hospitalized strokes over 25 years: the Lausanne stroke registry. Cerebrovas Dis. 2007;24:97–103. doi: 10.1159/000103123. [DOI] [PubMed] [Google Scholar]
- Carrera E, Tononi G. Diaschisis: past, present, future. Brain. 2014;137:2408–2422. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- Castillo V, Bogousslavsky J. Brain embolism. In: Welch KMA, Caplan LR, Reis DJ, Siesjö BK, Weir B, editors. Primer on cerebrovascular diseases. Academic Press; New York: 1997. pp. 286–289. [Google Scholar]
- Cheney PD, Fetz EE, Mewes K. Neural mechanisms underlying corticospinal and rubrospinal control of limb movements. Prog Brain Res. 1991;87:213–252. doi: 10.1016/s0079-6123(08)63054-x. [DOI] [PubMed] [Google Scholar]
- Cooper BY, Glendinning DS, Vierck CJ. Finger movement deficits in the stumptail macaque following lesions of the fasciculus cuneatus. Somatosens Mot Res. 1993;10:17–29. doi: 10.3109/08990229309028820. [DOI] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C, Ciferri M. Loss and recovery of voluntary hand movements in the macaque following a cervical dorsal rhizotomy. J Comp Neurol. 2005;491:27–45. doi: 10.1002/cne.20686. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Lilak A, Alarcón C. Corticospinal sprouting occurs selectively following dorsal rhizotomy in the macaque monkey. J Comp Neurol. 2013;521:2359–2372. doi: 10.1002/cne.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling WG, Peterson CR, Herrick JL, McNeal D, Stilwell-Morecraft KS, Morecraft RJ. Measurement of coordination of object manipulation in non-human primates. J Neurosci Methods. 2006;154:38–44. doi: 10.1016/j.jneumeth.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Darling WG, Pizzimenti MA, Morecraft RJ. Recovery of Function following Motor Cortex Injury in Non-human Primates: Experimental Implications for Human Stroke Patients. J Integr Neurosci, 2011;10:353–384. doi: 10.1142/S0219635211002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling WG, Pizzimenti MA, Rotella DL, Peterson CR, Ge J, Cline K, McNeal DW, Stilwell-Morecraft KS, Morecraft RJ. Volumetric Effects of Frontal Motor Cortex Injury on Recovery of Dexterous Movements. Exp Neurol. 2009;220:90–108. doi: 10.1016/j.expneurol.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Zoubina EV, Plautz EJ, Mahnken JD, Nudo RJ. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J Neurophysiol. 2006;96:3506–3511. doi: 10.1152/jn.00792.2006. [DOI] [PubMed] [Google Scholar]
- Denny-Brown D. Disintegration of motor function resulting from cerebral lesions. J Nerv Mental Dis. 1950;112:1–45. [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. 2nd SpringerWein; New York: 1999. The human brain: Surface, blood supply and three-dimensional sectional anatomy. [Google Scholar]
- Eidelberg E, Woolf B, Kreinick CJ, Davis F. Role of dorsal funiculi in movement control. Brain Res. 1976;114:427–438. doi: 10.1016/0006-8993(76)90964-1. [DOI] [PubMed] [Google Scholar]
- Eisner-Janowicz I, Barbay S, Hoover E, Stowe AM, Frost SB, Plautz EJ, Nudo RJ. Early and late changes in the distal forelimb representation of the supplementary motor area after injury to frontal motor areas in the squirrel monkey. J Neurophysiol. 2008;100:1498–1512. doi: 10.1152/jn.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Fawcett JP, Bamji SX, Causing CG, Aloyz R, Ase AR, Reader TA, McLean JH, Miller FD. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci. 1998;18:2808–2821. doi: 10.1523/JNEUROSCI.18-08-02808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier D. The functions of the brain. GP Putnam’s Sons; New York: 1886. [Google Scholar]
- Freese JL, Amaral DG. 2006 Synaptic organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. J Comp Neurol. 2006;496:655–667. doi: 10.1002/cne.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2008;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- Fulton JF, Kennard MA. A study of flaccid and spastic paralyses produced by lesions of the cerebral cortex in primates. Chapter VII. Res Publ Assoc Res Nerv Ment Dis. 1934;13:158–210. [Google Scholar]
- Galea MP, Darian-Smith I. Corticospinal projection patterns following unilateral section of the cervical spinal cord in the newborn and juvenile macaque monkey. J Comp Neurol. 1997;381:282–306. [PubMed] [Google Scholar]
- Glaser J, Greene G, Hendricks S. Stereology for biological research: with a focus on neuroscience. MBF Press; Williston VT: 2007. [Google Scholar]
- Glees P, Cole J. Recovery of skilled motor functions after small repeated lesions in motor cortex in macaque. J Neurophysiol. 1950;13:137–148. [Google Scholar]
- González Delgado M, Bogousslavsky J. Superficial middle cerebral artery territory infarction. Front Neurol Neurosci. 2012;30:111–114. doi: 10.1159/000333604. [DOI] [PubMed] [Google Scholar]
- Graham Brown T, Sherrington CS. Note on the functions of the cortex cerebri. J Physiol (London) 1913;46(suppl):xxii. [Google Scholar]
- Griffin DM, Hudson HM, Belhaj-Saïf A, Cheney PD. Stability of output effects from motor cortex to forelimb muscles in primates. J Neurosci. 2009;29:1915–1927. doi: 10.1523/JNEUROSCI.4831-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann A, Weis C, Marksteiner J, Hinterhuber H, Humpel C. Chronic repetitive transcranial magnetic stimulation enhances c-fos in the parietal cortex and hippocampus. Brain Res Mol Brain Res. 2000;76:355–362. doi: 10.1016/s0169-328x(00)00024-3. [DOI] [PubMed] [Google Scholar]
- Kambi N, Tandon S, Mohammed H, Lazar L, Jain N. Reorganization of the primary motor cortex of adult macaque monkeys after sensory loss resulting from partial spinal cord injuries. J Neurosci. 2011;31:3696–3707. doi: 10.1523/JNEUROSCI.5187-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazui S, Sawada T, Naritomi H, Kuriyama Y, Yamaguchi T. Angiographic evaluation of brain infarction limited to the anterior cerebral artery territory. Stroke. 1993;24:549–553. doi: 10.1161/01.str.24.4.549. [DOI] [PubMed] [Google Scholar]
- Kennard MA, Kessler MM. Studies of motor performance after parietal ablations in monkeys. J Neurophysiol. 1940;3:248–257. [Google Scholar]
- Kokaia Z, Andsberg G, Yan Q, Lindvall O. Rapid alterations of BDNF protein levels in the rat brain after focal ischemia: evidence for increased synthesis and anterograde axonal transport. Exp Neurol. 1998;154:289–301. doi: 10.1006/exnr.1998.6888. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Kelly ME, Elmer E, Kokaia M, McIntyre DC, Lindvall O. Seizure-induced differential expression of messenger RNAs for neurotrophins and their receptors in genetically fast and slow kindling rats. Neuroscience. 1996;75:197–207. doi: 10.1016/0306-4522(96)00257-6. [DOI] [PubMed] [Google Scholar]
- Kovacic U, Sketelj J, Bajrovic FF. Sex-related difference in collateral sprouting of nociceptive axons after peripheral nerve injury in the rat. Exp Neurol. 2003;184:479–488. doi: 10.1016/s0014-4886(03)00269-3. [DOI] [PubMed] [Google Scholar]
- Kumral E, Bayulkem G, Evyapan D, Yunten N. Spectrum of anterior cerebral artery territory infarction: clinical and MRI findings. Eur J Neurol. 2002;9:615–624. doi: 10.1046/j.1468-1331.2002.00452.x. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Anatomy of the descending motor pathways. In: Brooks VB, editor. Handbook of physiology, section I, The nervous system, Vol II, Motor control, Pt I. American Physiological Society; Bethesda: 1981. pp. 567–666. [Google Scholar]
- Larsen JO, Gundersen HJ, Nielsen J. Global spatial sampling with isotropic virtual planes: estimators of length density and total length in thick, arbitrarily orientated sections. J Microsc. 1998;191:238–248. doi: 10.1046/j.1365-2818.1998.00365.x. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Porter R, Redman SJ. Corticomotoneuronal synapses in the monkey: light microscopic localization upon motoneurons of intrinsic muscles of the hand. J Comp Neurol. 1985;232:499–510. doi: 10.1002/cne.902320407. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Kirkwood PA, Maier MA, Nakajima K, Nathan P. Direct and indirect pathways for corticospinal control of upper limb motoneurons in the primate. Prog Brain Res. 2004;143:263–279. doi: 10.1016/S0079-6123(03)43026-4. [DOI] [PubMed] [Google Scholar]
- Leyton ASF, Sherrington CS. Observations on the excitable cortex of the chimpanzee, orangutan and gorilla. Exp Physiol. 1917;11:135–222. [Google Scholar]
- Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp Brain Res. 1999;128:149–159. doi: 10.1007/s002210050830. [DOI] [PubMed] [Google Scholar]
- Loy R, Milner TA. Sexual dimorphism in extent of axonal sprouting in rat hippocampus. Science. 1980;208:1282–1284. doi: 10.1126/science.7375941. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticospinal projections from mesial frontal and cingulate areas in the monkey. Neuroreport. 1994;5:2545–2548. doi: 10.1097/00001756-199412000-00035. [DOI] [PubMed] [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex. 2002;12:281–296. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- Martin JH. The corticospinal system: from development to motor control. The Neuroscientist. 2005;11:161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon KM, Hynes SM, Pizzimenti MA, Rotella DL, Vanadurongvan T, Morecraft RJ. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010;518:586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Cioplloni PB, Stilwell-Morecraft KS, Gedney MT, Pandya DN. Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. J Comp Neurol. 2004;469:37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Pizzimenti MA, Darling WG. Terminal distribution of the corticospinal projection from the hand/arm area of the primary motor cortex to the cervical enlargement in rhesus monkey. J Comp Neurol. 2013;521:4205–4235. doi: 10.1002/cne.23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Herrick JL, Stilwell-Morecraft KS, Louie JL, Schroeder CM, Ottenbacher JG, Schoolfield MW. Localization of arm representation in the corona radiata and internal capsule in the non-human primate. Brain. 2002;125:176–198. doi: 10.1093/brain/awf011. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124:176–208. doi: 10.1093/brain/124.1.176. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Dvanajscak Z, Ge J, Schneider P. Localization of arm representation in the cerebral peduncle of the non-human primate. J Comp Neurol. 2007a;504:149–167. doi: 10.1002/cne.21438. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Gedney M, Ge J, Schroeder CM, Van Hoesen GW. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J Comp Neurol. 2007b;500:134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal D, Pandya DN. Cytoarchitecture and Cortical Connections of the Anterior Cingulate and adjacent Somatomotor Fields in the Rhesus Monkey. Brain Res Bull. 2012;87:457–497. doi: 10.1016/j.brainresbull.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Stillwell-Morecraft KS, Solon-Cline KM, Ge J, Darling WG. Cortical innervation of the hypoglossal nucleus in the non-human primate (Macaca mulatta) J Comp Neurol, 2014b;522:3456–3484. doi: 10.1002/cne.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Ugollini G, Lanciego JL, Wouterlood FG, Pandya DN. Classic and Contemporary Neural Tract Tracing Techniques. Diffusion MRI: From Quantitative Measurement to In-Vivo Neuroanatomy. In: Johansen-Berg H, Behrens T, editors. 2nd Oxford University Press; Oxford: 2014a. pp. 359–399. [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J Comp Neurol. 1992;322:471–489. doi: 10.1002/cne.903220403. [DOI] [PubMed] [Google Scholar]
- Mott FW, Sherrington CS. Experiments upon the influence of sensory nerves upon movement and nutrition of the limbs. Proc R Soc London B. 1895;57:481–488. [Google Scholar]
- Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956:30–35. doi: 10.1016/s0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]
- Muller MB, Toschi N, Kresse AE, Post A, Keck ME. Long-term repetitive transcranial magnetic stimulation increases the expression of brain-derived neurotrophic factor and cholecystokinin mRNA, but not neuropeptide tyrosine mRNA in specific areas of rat brain. Neuropsychopharm. 2000;23:205–215. doi: 10.1016/S0893-133X(00)00099-3. [DOI] [PubMed] [Google Scholar]
- Murray EA, Coulter JD. Organization of corticospinal neurons in the monkey. J Comp Neurol. 1981;195:339–365. doi: 10.1002/cne.901950212. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12:2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Descending pathways to the spinal cord: a comparative study of 22 mammals. J Comp Neurol. 1988;277:53–79. doi: 10.1002/cne.902770105. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Descending pathways to the spinal cord, IV: some factors related to the amount of cortex devoted to the corticospinal tract. J Comp Neurol. 1990;296:584–597. doi: 10.1002/cne.902960406. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Ogden R, Franz SI. On cerebral motor control: the recovery from experimentally produced hemiplegia. Psychobiology. 1917;1:33–47. [Google Scholar]
- Park MC, Belhaj-Saïf A, Gordon M, Cheney PD. Properties of primary motor cortex output to forelimb muscles in rhesus macaques. J Neurophysiol. 2004;92:2968–2984. doi: 10.1152/jn.00649.2003. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Bengtsson SL, Lau HC. Medial frontal cortex: from self-generated action to reflection on one's own performance. Trends Cogn Sci. 2010;14:16–21. doi: 10.1016/j.tics.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peele TL. Acute and chronic parietal lobe ablations in monkeys. J Neurophysiol. 1944;7:269–286. [Google Scholar]
- Pessin MS. Anterior circulation – large artery occlusive disease and embolism. In: Welch KMA, Caplan LR, Reis DJ, Siesjö BK, Weir B, editors. Primer on cerebrovascular diseases. Academic Press; New York: 1997. pp. 293–298. [Google Scholar]
- Pizzimenti MA, Darling WG, Rotella DL, McNeal DW, Herrick JL, Ge J, Stilwell-Morecraft KS, Morecraft RJ. Measurement of reaching kinematics and prehensile dexterity in nonhuman primates. J Neurophysiol. 2007;98:1015–1029. doi: 10.1152/jn.00354.2007. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal functions and voluntary movement. Clarendon Press; Oxford: 1993. [Google Scholar]
- Qi HX, Kass JH, Reed JL. The reactivation of somatosensory cortex and behavioral recovery after sensory loss in mature primates. Front Syst Neurosci. 2014;8:1–14. doi: 10.3389/fnsys.2014.00084. article 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathelot J-A, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexed B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954;100:297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29:4993–4999. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, Yu XH, Moret V, Tempini A, Wiesendanger M, Liang F. Dexterity in adult monkeys following early lesion of the motor cortical hand area: the role of cortex adjacent to the lesion. Eur J Neurosci. 1998;10:729–740. doi: 10.1046/j.1460-9568.1998.00075.x. [DOI] [PubMed] [Google Scholar]
- Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, Luppino G. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb Cortex. 2006;16:1389–1417. doi: 10.1093/cercor/bhj076. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Comparative anatomy and physiology of the corticospinal system. Handbook Clin Neurol. 2007;82:15–37. doi: 10.1016/S0072-9752(07)80005-4. [DOI] [PubMed] [Google Scholar]
- Tanji J. The supplementary motor area in the cerebral cortex. Neurosci Res. 1994;19:251–268. doi: 10.1016/0168-0102(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Tanji J, Kurata K. Comparison of movement related activity in two cortical motor areas of primates. J Neurophysiol. 1982;48:633–653. doi: 10.1152/jn.1982.48.3.633. [DOI] [PubMed] [Google Scholar]
- Tanji J, Kurata K. Contrasting neuronal activity in supplementary and precentral motor cortex of monkeys. I. Responses to instructions determining motor responses to forthcoming signals of different modalities. J Neurophysiol. 1985;53:129–141. doi: 10.1152/jn.1985.53.1.129. [DOI] [PubMed] [Google Scholar]
- Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: cerebral hemispheres. Neurology. 1998;50:1699–1708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG. Nucleus retroambiguus-spinal pathway in the mouse: localization, gender differences, and effects of estrogen treatment. J Comp Neurol. 2005;488:180–200. doi: 10.1002/cne.20574. [DOI] [PubMed] [Google Scholar]
- Van der Horst VG, Holstege G. Sensory and motor components of reproductive behavior: pathways and plasticity. Behav Brain Res. 1998;92:157–167. doi: 10.1016/s0166-4328(97)00188-5. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Terasawa E, Ralston HJ. Axonal sprouting of a brainstem-spinal pathway after estrogen administration in the adult female rhesus monkey. J Comp Neurol. 2002;454:82–103. doi: 10.1002/cne.10446. [DOI] [PubMed] [Google Scholar]
- Veniero D, Ponzo V, Koch G. Paired associative stimulation enforces the communication between interconnected areas. J Neurosci. 2013;33:13773–13783. doi: 10.1523/JNEUROSCI.1777-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ. Comparison of forelimb and hindlimb motor deficits following dorsal column section in monkeys. Brain Res. 1978;146:279–294. doi: 10.1016/0006-8993(78)90974-5. [DOI] [PubMed] [Google Scholar]
- Vilensky JA, Gilman S. Lesions of the precentral gyrus in nonhuman primates: a pre-medline bibliography. International J Primatology. 2002;23:1319–1333. [Google Scholar]
- West MJ. Basic stereology for biologists and neuroscientists. Cold Spring Harbor Laboratory Press; New York: 2012. [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M. Postlesion recovery of motor and sensory cortex in the early twentieth century. J Hist Neurosci. 2011;20:42–57. doi: 10.1080/09647041003775446. [DOI] [PubMed] [Google Scholar]
- Woolsey CN, Settlage PH, Meyer DR, Sencer W, Pinto Hamuy T, Travis AM. Patterns of localization in precentral and "supplementary" motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis. 1952;30:238–264. [PubMed] [Google Scholar]
- Wouterlood FG, Groenewegen HJ. Neuroanatomical tracing by use of Phaseolus vulgaris-leucoagglutinin (PHA-L): electron microscopy of PHA-L-filled neuronal somata, dendrites, axons and axon terminals. Brain Res. 1985;326:188–191. doi: 10.1016/0006-8993(85)91402-7. [DOI] [PubMed] [Google Scholar]
- Yoo K-M, Shin H-K, Chang H-M, Caplan LR. Middle cerebral artery occlusive disease: the New England Medical Center stroke registry. J Stroke Cerebrovas Dis. 1998;7:344–351. doi: 10.1016/s1052-3057(98)80053-0. [DOI] [PubMed] [Google Scholar]