Abstract
Objective
To estimate the association between maternal hydroxyvitamin D (25-hydroxyvitamin D) concentrations and risk of preterm birth subtypes.
Methods
We performed a case-cohort study using data and banked samples from patients at a teaching hospital in Pittsburgh, Pennsylvania. Eligible participants were women with a prenatal aneuploidy screening serum sample at or before 20 weeks of gestation who subsequently delivered a singleton, live-born infant. Of the n=12,861 eligible women, we selected n=2327 at random as well as all remaining preterm birth cases for a total of n=1126 cases. Serum 25-hydroxyvitamin D was measured using liquid chromatography–tandem mass spectrometry. Multivariable log-binomial regression models were used to estimate associations between maternal vitamin D status and preterm birth <37 weeks (separately by spontaneous or indicated) and preterm birth <34 weeks.
Results
The incidence of preterm birth <37 weeks was 8.6% overall and 11.3%, 8.6%, and 7.3% among mothers with serum 25-hydroxyvitamin D<50, 50–74.9, and ≥75nmol/L, respectively (p<0.01). After adjustment for maternal race and ethnicity, prepregnancy BMI, season, smoking, and other confounders, the risk of preterm birth <37 weeks significantly decreased as 25-hydroxyvitamin D increased to approximately 90 nmol/L and then plateaued (test of nonlinearity p<0.01). Results were similar when limiting to cases that were medically indicated or occurred spontaneously and cases occurring at <34 weeks of gestation.
Conclusions
Our data support a protective association maternal vitamin D sufficiency and preterm birth that combined with extant epidemiologic data may provide justification for a randomized clinical trial of maternal vitamin D replacement or supplementation to prevent preterm birth.
Introduction
Every year, 15 million infants worldwide are born preterm (1). Of these, 1.1 million die due to complications of being born too soon and even more suffer from serious prematurity- related complications including learning disabilities (1). In the United States, 1 in 9 infants is born preterm (2), but rates are higher among socially disadvantaged groups, older mothers, and racial and ethnic minorities (3). First-year medical costs required to care for a preterm infant in the United States are nearly $30,000 more than for a term infant (1). Prevention of preterm birth is a global priority (1). There is increasing conviction that preterm birth is not a singular disease, but a syndrome that has a multitude of potentially independent causes (4, 5). While much about the pathophysiology of preterm birth remains obscure, there is strong evidence that intrauterine infection is a frequent and important mechanism causing early delivery (6).
Vitamin D may be relevant for preterm birth prevention. 1,25-dihydroxyvitamin D is known to reduce bacterial infections by inducing cathelicidin in many tissues, including maternal and fetal cells of the placenta (7, 8). While laboratory studies have elegantly demonstrated links between maternal vitamin D status, as measured by 25-hydroxyvitamin D, and placental antibacterial responses (9–11), findings from the one randomized trial (12) and epidemiologic studies of vitamin D and preterm birth are equivocal (13–18). Our objective was to study the association between maternal 25-hydroxyvitamin D concentrations and risk of clinical subtypes of preterm birth in a large contemporary cohort of U.S. women.
Materials and Methods
The Epidemiology of Vitamin D Study (EVITA) is a case-cohort study designed to evaluate associations between maternal vitamin D status and adverse pregnancy outcomes. EVITA uses existing data and banked prenatal aneuploidy screening samples from deliveries at Magee-Womens Hospital of UPMC in Pittsburgh, Pennsylvania. The hospital houses the Center for Medical Genetics and Genomics, which provides integrated clinical and laboratory service for reproductive screening. Data for EVITA came from a detailed and validated electronic perinatal database at the hospital, described in detail previously (19, 20), which was merged with a database of all clinical genetics encounters and laboratory results performed by the Center for Medical Genetics and Genomics. Data are populated from various electronic sources (e.g., procedure coding) and medical chart abstractors. A data administrator reviews and cleans these data regularly.
We used existing maternal serum samples that the Center banked as part of the second-trimester multiple marker (quad) screening test in 1999, 2000, 2001, 2003, 2009, and 2010 (lack of adequate freezer space prevented storing samples in 2002 and 2004 – 2008). We also used samples stored from 2007 to 2010 for first-trimester aneuploidy screening. The Center began performing first-trimester aneuploidy screening in 2007. EVITA used de-identified data and was approved by the University of Pittsburgh Institutional Review Board.
We used a case-cohort study because (1) this design provides nearly equal statistical efficiency as a cohort study while reducing laboratory costs; (2) controls can be selected from the sub-cohort for multiple endpoints such as preterm birth and preeclampsia; and (3) information from the randomly selected sub-cohort can be used to estimate the prevalence vitamin D deficiency and other exposures in the original cohort (21).
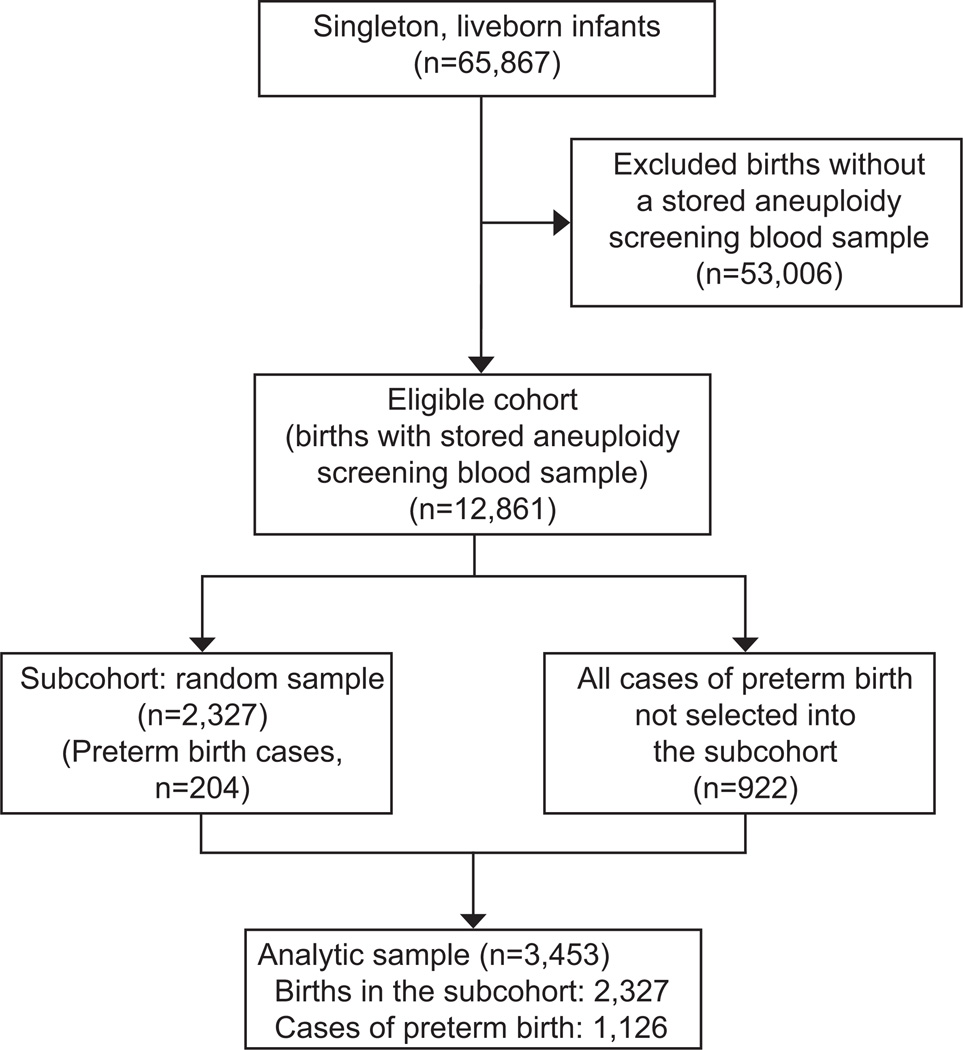
There were 65,867 singleton live-born infants that delivered at Magee-Womens Hospital in the 8 years when genetics samples were stored (1999–2001, 2003, 2007–2010). Of these, 12,861 received aneuploidy screening at the Center for Medical Genetics and Genomics at or before 20 weeks and were therefore eligible for EVITA (Figure 1). Based on a priori power calculations, we randomly sampled 2,327 of these deliveries to form a representative subcohort (n=204 were preterm birth cases). We then augmented the subcohort with all remaining cases of preterm birth in the eligible cohort (n=922) for a total of 1,126 cases. In the analysis, the 204 cases from the subcohort serve in both the subcohort and in the case group, resulting in 3453 total observations from 3249 unique records. Sampling weights were 1 for cases and 5.52 (1/sampling probability) for non-cases.
Figure 1.
Study flow diagram.
Gestational age at delivery was determined using customary derivation of best obstetric estimate based on a comparison of menstrual dating and ultrasound dating (22) derived from the perinatal database. We defined preterm birth as the delivery of a live-born infant at <37 weeks of gestation (3). We also examined cases at <34 weeks. Spontaneous preterm births were preterm births occurring after preterm labor with intact membranes or preterm prelabor rupture of the fetal membranes, and remaining preterm deliveries were classified as indicated preterm births. Throughout the study period, policies at the hospital prevent inductions or cesarean deliveries before 37 weeks without a medical indication.
An unpublished validation study demonstrated excellent agreement between gestational age at delivery determined by the perinatal database compared with physician chart abstraction (r = 0.96, n=184). The database has 100% sensitivity for identifying preterm birth cases and 96% specificity. Additionally, clinical presentation of preterm birth in the perinatal database was appropriately classified as following spontaneous preterm labor, preterm premature rupture of membranes or medical induction in 95% of cases (174/184).
Maternal serum samples were stored at ™20°C for 3 months and transferred to long-term storage at ™80°C. There were no recorded interim thaws. We sent samples to the laboratory of Dr. Michael Holick at Boston University (Boston, Massachusetts), which is Vitamin D External Quality Assessment Scheme–proficient (DEQAS, London, United Kingdom) and Clinical Laboratory Improvement Amendments–certified (Centers for Disease Control and Prevention, Atlanta, Georgia). Samples were assayed for total 25-hydroxyvitamin D (calculated as 25(OH)D2 + 25(OH)D3) by using liquid chromatography–tandem mass spectrometry according to the requirements of the National Institute of Standards and Technology (Gaithersburg, Maryland) (23). The intra- and inter-assay variation was 9.6% and 10.9%, respectively. There is no universally accepted definition of vitamin D deficiency, so we used multiple cut-points (<50, 50–74.9, and ≥75 nmol/L (24, 25)). We elected to group all women with 25-hydroxyvitamin D<50 nmol/L because only 6% of pregnancies had serum 25-hydroxyvitamin D<30 nmol/L.
Maternal race and ethnicity, marital status, education, smoking status, and parity were ascertained from the electronic perinatal database based on self-report. Prepregnancy body mass index (weight (kg)/height (m)2) was defined using prepregnancy weight and height recalled at the first prenatal visit. The season of blood sampling classified as winter (December–February), spring (March–May), summer (June–August), or fall (September–November) to avoid misclassification bias due to having only a single 25-hydroxyvitamin D measurement (26).
Of the 3453 observations in the analytic sample, 1241 were missing maternal height because height was not collected in the perinatal database before 2003. A total of 15 were missing prepregnancy weight, 396 were missing maternal education, 6 were missing smoking, and 1 was missing parity (n=1633 with any missing data). We addressed the missing data using multiple imputation. Five imputed datasets that assumed a multivariable normal distribution with a Markov chain Monte Carlo approach were created (27, 28). Prepregnancy weight, height, parity, smoking, education, and weight at delivery were jointly imputed by including preterm birth, 25-hydroxyvitamin D, race and ethnicity, marital status, age, season, gestational age at blood sampling, year, and the sampling weight in the imputation model. We also performed sensitivity analyses using 25 imputations as well as only observations with complete data (n=1820).
Pearson’s chi-squared tests adjusted for the case-cohort design were used to test for independence in 25-hydroxyvitamin D categories across maternal characteristics. We tested for a trend in the weighted incidence of preterm birth across categories of 25-hydroxyvitamin D. Multivariable log-binomial regression models were used to estimate risk ratios and 95% confidence intervals for the association between maternal 25-hydroxyvitamin D and risk of preterm birth after adjusting for potential confounders defined a priori using theory-based causal diagrams (29) (maternal race and ethnicity, prepregnancy BMI, education, marital status, parity, smoking, delivery year, clinic or private prenatal care, season and gestational age of blood sampling, assay batch, and type of aneuploidy screening (first-trimester or multiple marker)). We did not adjust for medical comorbidities such as preeclampsia or fetal growth restriction because these variables may be on the causal pathway from vitamin D to preterm birth. To account for the case-cohort design (204 cases also in the subcohort), we used robust standard errors and sampling weights (21). Serum 25-hydroxyvitamin D was modeled using categorical variables for ease of interpretation, but we also used restricted cubic spline terms with 4 knots in default locations (30) to capture non-linear relations. We also tested for effect modification by race and ethnicity and prepregnancy BMI (31, 32).
Results
The 12,861 women with singleton live-born deliveries in the hospital who received prenatal aneuploidy screenings (the eligible cohort) were similar to the source population of 65,867 singleton live-born deliveries with regard to maternal age, marital status, prepregnancy BMI, and smoking status (Appendix 1, available online at http://links.lww.com/xxx), but were slightly more likely to be college graduates (49.1% vs. 45.4%), non-Hispanic black (21.2% vs. 18.8%), receiving care at the hospital outpatient resident clinic (23.1% vs. 16.0%), and to be nulliparous (51.8% vs. 55.2%).
The 2327 women randomly selected from the eligible cohort into the EVITA subcohort were predominantly non-Hispanic white, married, and non-smokers, and received care at a hospital-affiliated private practice (Table 1). Approximately half of the subcohort was multiparous and normal weight before pregnancy and had graduated from college. The subcohort was almost evenly divided among those who had received the multiple marker screening and those who received first-trimester screening. Compared with the subcohort, preterm birth cases were more likely to be younger, unmarried, non-Hispanic black, obese, and smokers. They also had fewer years of education and were more likely to have received prenatal care at the hospital outpatient resident clinic.
Table 1.
Characteristics of women in the random sample of the eligible cohort and cases of preterm birth <37 weeks, Magee-Womens Hospital, Pittsburgh, Pennsylvania.
| Random subsample of cohort n(%) |
Preterm birth cases n(%) |
|
|---|---|---|
| Maternal age, years | ||
| <20 | 149(6.4) | 91(8.1) |
| 20–29 | 966(41.5) | 476(42.3) |
| ≥30 | 1212(52.1) | 558(49.6) |
| Marital status | ||
| Unmarried | 845(36.3) | 491(43.6) |
| Married | 1482(63.7) | 635(56.4) |
| Maternal education | ||
| Less than high school | 142(6.1) | 93(8.3) |
| High school or equivalent | 477(20.5) | 298(26.5) |
| Some college | 535(23.0) | 262(23.3) |
| College graduate | 1173(50.4) | 472(41.9) |
| Maternal race and ethnicity | ||
| Non-Hispanic White | 1727(74.2) | 744(66.1) |
| Non-Hispanic Black | 444(19.1) | 318(28.2) |
| Other | 156(6.7) | 64(5.7) |
| Parity | ||
| 0 | 1152(49.5) | 571(50.7) |
| 1 or more | 1175(50.5) | 555(49.3) |
| Prepregnancy body mass index, kg/m2 | ||
| <18.5 | 100(4.3) | 48(4.3) |
| 18.5–24.9 | 1191(51.2) | 538(47.8) |
| 25.0–29.9 | 586(25.2) | 279(24.8) |
| ≥30.0 | 449(19.3) | 260(23.1) |
| Smoking status | ||
| Nonsmoker | 2076(89.2) | 960(85.3) |
| Smoker | 251(10.8) | 166(14.7) |
| Type of provider | ||
| Hospital-affiliated private practice | 1831(78.7) | 808(71.8) |
| Hospital outpatient resident clinic | 496(21.3) | 318(28.2) |
| Season of blood collection | ||
| Winter | 486(20.9) | 247(21.9) |
| Spring | 661(28.4) | 304(27.0) |
| Summer | 572(24.6) | 309(27.4) |
| Fall | 607(26.1) | 267(23.7) |
| Aneuploidy screening test | ||
| First-trimester | 1019(43.8) | 458(40.7) |
| Multiple marker (quad) test | 1308(56.2) | 668(59.3) |
The prevalence of maternal serum 25-hydroxyvitamin D<50, 50–74.9, ≥75 nmol/L in the subcohort was 21.4%, 36.7%, and 41.9%, respectively. 25-hydroxyvitamin D was assayed in samples drawn at a median (IQR) of 15.9 (12.6–17.3) weeks of gestation. Women in the subcohort who were older, married, college-educated, non-Hispanic white, parous, non-smokers, or lean were significantly more likely to have serum 25-hydroxyvitamin D≥75 nmol/L compared with their counterparts (Table 2). Patients receiving care at a private practice or whose blood was collected in the summer or fall were also more likely to have higher serum 25-hydroxyvitamin D.
Table 2.
Association between maternal characteristics and serum 25-hydroxyvitamin D concentration at or before 20 weeks of gestation in the random subcohort, Magee-Womens Hospital, Pittsburgh, Pennsylvania.
| Serum 25(OH)D, nmol/L |
|||
|---|---|---|---|
| <50 n(%) |
50–74.9 n(%) |
≥75 n(%) |
|
| Maternal age, years (%)* | |||
| <20 | 71(48.0) | 48(32.4) | 29(19.6) |
| 20–29 | 267(27.6) | 343(35.5) | 356(36.9) |
| ≥30 | 159(13.1) | 463(38.2) | 591(48.7) |
| Marital status (%)* | |||
| Unmarried | 312(37.0) | 281(33.3) | 251(29.7) |
| Married | 185(12.5) | 572(38.6) | 725(48.9) |
| Maternal education (%)* | |||
| Less than high school | 67(47.4) | 54(38.3) | 20(14.3) |
| High school or equivalent | 150(31.4) | 155(32.5) | 173(36.1) |
| Some college | 124(23.0) | 192(35.7) | 222(41.3) |
| College graduate | 156(13.3) | 453(38.7) | 562(48.0) |
| Maternal race and ethnicity (%)* | |||
| Non-Hispanic White | 169(9.8) | 655(38.5) | 893(51.7) |
| Non-Hispanic Black | 267(60.1) | 128(28.8) | 49(11.0) |
| Other | 61(39.1) | 61(39.1) | 34(21.8) |
| Parity (%)* | |||
| 0 | 255(22.1) | 451(39.1) | 447(38.8) |
| 1 or more | 242(20.6) | 404(34.4) | 528(45.0) |
| Prepregnancy body mass index, kg/m2 (%)* | |||
| <18.5 | 20(21.5) | 35(37.1) | 39(41.4) |
| 18.5–24.9 | 201(17.0) | 433(36.5) | 549(46.4) |
| 25.0–29.9 | 118(19.8) | 230(38.5) | 249(41.7) |
| ≥30.0 | 157(34.8) | 157(34.7) | 138(30.5) |
| Smoking status (%)* | |||
| Nonsmoker | 417(20.1) | 768(37.0) | 890(42.9) |
| Smoker | 79(31.3) | 87(34.5) | 86(34.1) |
| Type of provider (%)* | |||
| Hospital-affiliated private practice | 288(15.7) | 696(38.0) | 848(46.3) |
| Hospital outpatient resident clinic | 209(42.2) | 158(31.9) | 86(28.9) |
| Season of blood collection (%)* | |||
| Winter | 140(28.8) | 180(37.0) | 166(34.2) |
| Spring | 160(24.2) | 282(42.7) | 218(33.0) |
| Summer | 92(16.0) | 197(34.3) | 285(49.7) |
| Fall | 105(17.3) | 195(32.1) | 307(50.6) |
| Aneuploidy screening test (%) | |||
| First-trimester | 221(21.7) | 399(39.1) | 401(39.3) |
| Multiple marker (quad) test | 276(21.1) | 456(34.9) | 575(44.0) |
p<0.05 based on a Pearson’s chi-squared test of independence adjusted for the case-cohort design.
25OH(D), 25-hydroxyvitamin D.
There was an 8.6% incidence of preterm birth <37 weeks and a 2.1% incidence of preterm birth <34 weeks (weighted sample). Approximately 55% of preterm births <37 weeks were spontaneous (621/1126). The incidence of preterm birth <37 weeks was 11.3%, 8.6%, and 7.3% among mothers with serum 25-hydroxyvitamin D<50, 50–74.9, and ≥75 nmol/L, respectively (p<0.01; Table 3). The incidence of spontaneous and medically indicated preterm birth <37 weeks and preterm birth <34 weeks also declined significantly as 25-hydroxyvitamin D improved.
Table 3.
Association between maternal 25-hydroxyvitamin D concentrations at or before 20 weeks of gestation and preterm birth.
| Maternal serum 25(OH)D, nmol/L |
Cases (n) | Incidence per 100 birthsa |
Unadjusted relative risk (95% CI) |
Adjusted b relative risk (95% CI) |
|---|---|---|---|---|
| Preterm birth <37 weeks | ||||
| <50 | 317 | 11.3 | 1.6 (1.3, 1.9) | 1.8 (1.3, 2.6) |
| 50–74.9 | 414 | 8.6 | 1.2 (1.1, 1.4) | 1.4 (1.1, 1.8) |
| ≥75 | 395 | 7.3* | referent | referent |
| Spontaneous preterm birth <37 weeks | ||||
| <50 | 177 | 6.6 | 1.6 (1.3, 2.0) | 1.8 (1.2, 2.7) |
| 50–74.9 | 222 | 4.8 | 1.1 (0.9, 1.4) | 1.3 (0.9, 1.8) |
| ≥75 | 222 | 4.2* | referent | referent |
| Medically indicated preterm birth <37 weeks | ||||
| <50 | 140 | 5.3 | 1.6 (1.3, 2.1) | 1.9 (1.3, 3.0) |
| 50–74.9 | 192 | 4.2 | 1.3 (1.1, 2.6) | 1.6 (1.1, 2.3) |
| ≥75 | 173 | 3.3* | referent | referent |
| Preterm birth <34 weeks | ||||
| <50 | 83 | 3.2 | 2.5 (1.6, 3.8) | 2.1 (1.3, 3.6) |
| 50–74.9 | 109 | 2.4 | 2.2 (1.5, 3.2) | 2.2 (1.4, 3.4) |
| ≥75 | 71 | 1.4* | referent | referent |
Risk ratios and 95% CI that reach statistical significance at p<0.05 are presented in boldface.
25(OH)D, 25-hydroxyvitamin D; CI, confidence interval
Incidence is based on the weighted sample.
Adjusted for maternal race and ethnicity, prepregnancy body mass index, parity, maternal education, marital status, smoking status, season and gestational age of blood sampling, assay batch, and year of delivery.
P <0.05 based on a test for trend adjusted for the case-cohort design.
After adjustment for maternal race and ethnicity, prepregnancy BMI, parity, education, marital status, age, smoking, season and gestational age of blood sampling, assay batch, and year of delivery, the risk of preterm birth <37 weeks was 1.8-fold (95% CI 1.3, 2.6) and 1.4-fold (1.1, 1.8) higher for among mothers with serum 25-hydroxyvitamin D<50, and 50–74.9 nmol/L compared with ≥75 nmol/L (Table 3). Additionally, the adjusted risk of spontaneous preterm birth <37 weeks, indicated preterm birth <37 weeks, or preterm birth <34 weeks among mothers with serum 25-hydroxyvitamin D<50 nmol/L were 1.8-fold to 2.1-fold greater than mothers with serum 25-hydroxyvitamin D≥75 nmol/L.
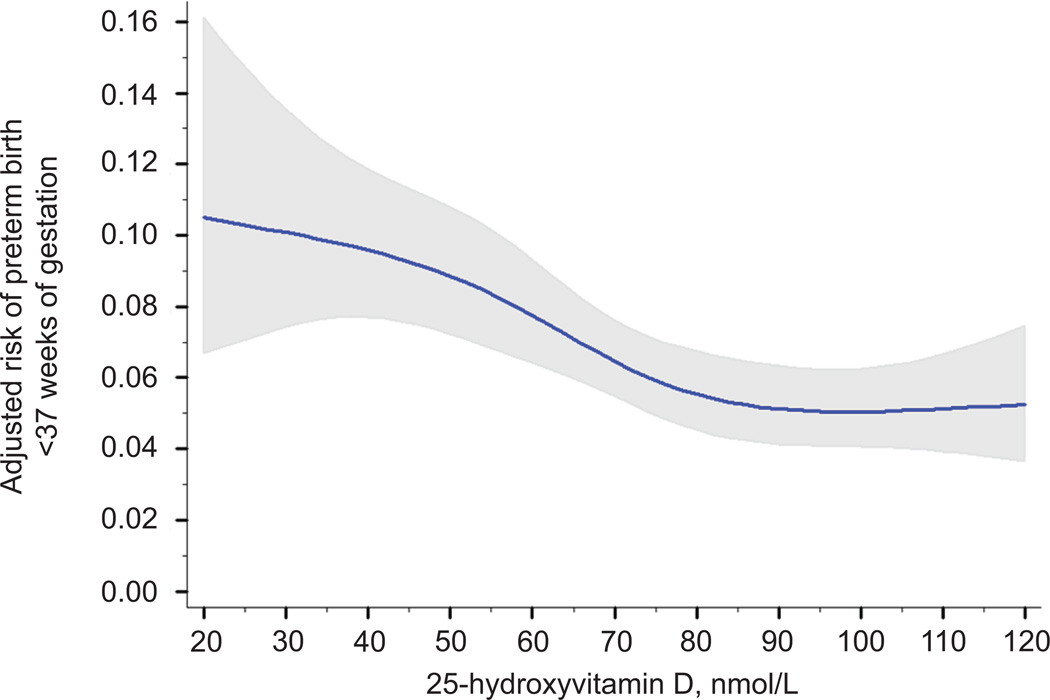
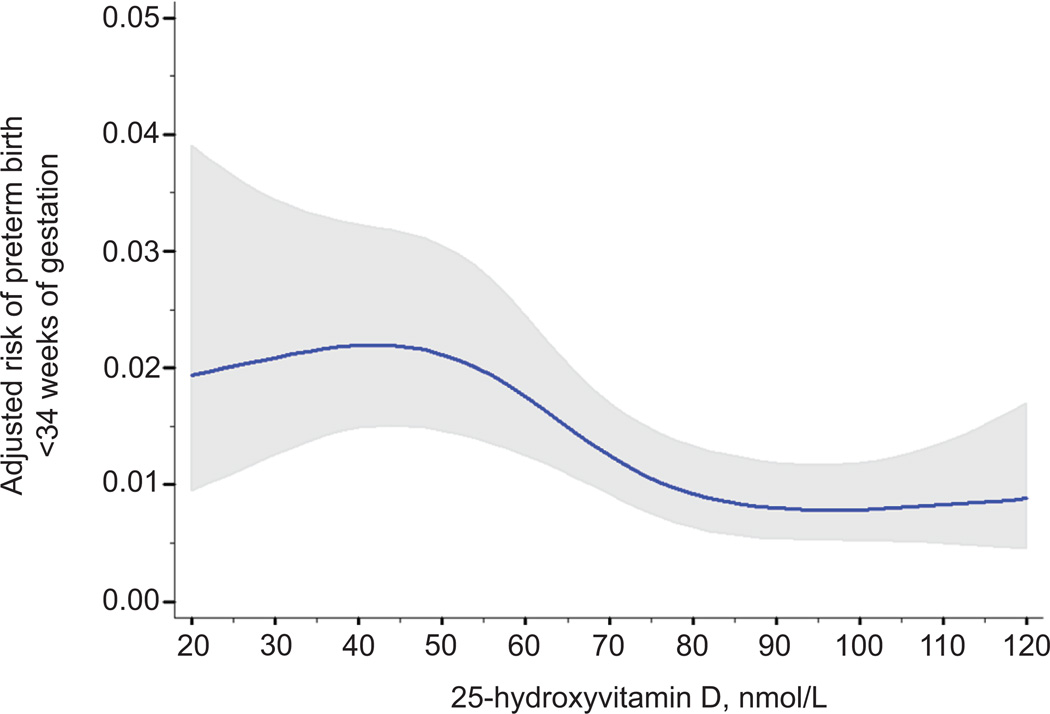
Spline regression revealed curvilinear associations between 25-hydroxyvitamin D and risk of preterm birth. After confounder adjustment, the risk of preterm birth <37 weeks significantly decreased as 25-hydroxyvitamin D rose to approximately 90 nmol/L and then plateaued (test of nonlinearity p<0.01; Figure 2). Similar non-linear risk curves were observed for spontaneous and medically indicated preterm birth (tests of nonlinearity p<0.01; data not shown) and for preterm birth <34 weeks (test of nonlinearity p<0.01; Figure 3).
Figure 2.
Association between maternal 25-hydroxyvitamin D and the adjusted risk of preterm birth at less than 37 weeks of gestation. The solid line represents the point estimate and the gray area represents its 95% confidence interval. 25-hydroxyvitamin D was modeled as a restricted cubic spline with 4 knots (test of nonlinearity, P<.01). The estimates were adjusted for maternal race and ethnicity, prepregnancy body mass index, parity, maternal education, marital status, smoking status, season and gestational age of blood sampling, assay batch, and year of delivery. All covariates were set to the mean value for graphing.
Figure 3.
Association between maternal 25-hydroxyvitamin D and the adjusted risk of preterm birth at less than 34 weeks of gestation. The solid line represents the point estimate and the gray area represents its 95% confidence interval. 25-hydroxyvitamin D was modeled as a restricted cubic spline with 4 knots (test of nonlinearity, P<.01). The estimates were adjusted for maternal race and ethnicity, prepregnancy body mass index, parity, maternal education, marital status, smoking status, season and gestational age of blood sampling, assay batch, and year of delivery. All covariates were set to the mean value for graphing.
None of these results varied by race and ethnicity. Findings were similar when we imputed 25 datasets rather than 5 (data not shown) and when we limited the analysis to those with complete data (Appendixes 2–4, available online at http://links.lww.com/xxx).
Discussion
We found that the confounder-adjusted risk of preterm birth was highest when serum 25- hydroxyvitamin D was less than 50 nmol/L, declined as 25-hydroxyvitamin D increased to approximately 90 nmol/L, and then plateaued. Findings were spontaneous or medically indicated preterm birth and preterm birth <34 weeks.
Our findings generally agree with our previous work in two multi-center U.S. samples of women in the general obstetric population. In a case-cohort study that included 767 cases of spontaneous preterm birth <35 weeks (15), maternal 25-hydroxyvitamin D<30 nmol/L at 20 weeks were associated with 50% increase in confounder-adjusted risk compared with 25-hydroxyvitamin D≥75 nmol/L among non-white mothers (n=556 cases), but vitamin D was not associated among white mothers (n=211 cases). In a study of twin pregnancies, the risks of preterm birth <35 and <32 significantly declined as serum 25-hydroxyvitamin D at 24–28 weeks increased (33), and like the results in our present analysis, did not vary by race and ethnicity.
Our results disagree with two recent studies of mothers receiving prenatal aneuploidy screening that reported no association between vitamin D at 10–14 weeks (18) or 15–21 weeks (17) and preterm birth. Similar null results were reported in women with prior preterm birth (14) and in a sample of HIV-infected Tanzanian mothers (13). A randomized trial of vitamin D supplementation recently showed no effect of 2000 or 4000 IU vitamin D3 per day starting at 16 weeks versus placebo in reducing preterm birth risk (12). This trial was designed to assess safety and did not have the statistical power to test whether supplementation had a causal role in adverse birth outcomes.
A limitation of our work and the aforementioned studies is a lack of additional markers of vitamin D metabolism. Vitamin D binding protein concentrations affect the amount of bioavailable 25-hydroxyvitamin D (34, 35). Because the vast majority of 25-hydroxyvitamin D is bound to vitamin D binding protein (36), serum 25-hydroxyvitamin D alone may be an inadequate indicator vitamin D function, particularly in ethnically-diverse populations like ours, where the vitamin D binding protein affinity is highly variable (37).
Deepening our understanding of the causal role (if any) of vitamin D in preterm birth will require a recognition that preterm birth has a heterogeneous pathophysiology (38). Our previous epidemiologic study combining gestational age information with placental histology data (15) as well as laboratory data support a role for vitamin D in placental infection and inflammation (7, 9, 11). In the present paper, we attempted to disaggregate cases by distinguishing spontaneous from indicated preterm births as well as those occurring at <34 weeks, but found a similar association with vitamin D deficiency with all subtypes. Our perinatal database lacked detailed information on key phenotypic components of the preterm birth syndrome, such as placental pathologic conditions and some signs of parturition initiation (38). We therefore could not to provide further insight into the causal pathways of preterm birth influenced by vitamin D. Future work is needed to fill this important knowledge gap.
Women who elect prenatal aneuploidy screening are different than women who chose not to be screened. We reduced the likelihood of selection bias by adjusting for measured variables that influence self-selection and by choosing the term births from the same population as the cases. Bias would result if vitamin D status affected self-selection (i.e., if vitamin D caused diabetes, and this led to the choice to be screened with the multiple marker test) but this seems like an unlikely. Moreover, we found only modest differences in our eligible subcohort with a screening sample compared with the full cohort (Appendix 1, http://links.lww.com/xxx), which suggests these results may generalize well to eligible deliveries at our hospital and other tertiary care centers. We adjusted for many measured confounders in this analysis, but cannot rule out the potential for unmeasured confounding. However, we have shown previously that unmeasured confounding by social status, fish intake, and physical activity has little impact on vitamin D- preterm effect estimates (15).
Data from our large observational pregnancy cohort support a dose-response association between vitamin D and preterm birth. Our data, as well as several large extant epidemiological studies, provide a key element of justification for the conduct of a randomized clinical trial. We believe that before well-powered randomized trials are undertaken, more research is needed into whether the intervention should be given universally, in a selected population, or based on screening; the most effective mode of intervention; patient preferences regarding screening and route of vitamin D administration, and fundamental pharmacokinetic and pharmacodynamic data regarding vitamin D replacement/supplementation in pregnancy.
Supplementary Material
Acknowledgements
Supported by CDC cooperative agreement U01 DP003177.
Footnotes
Presented at the Annual meeting of the Society for Epidemiologic Research, Seattle, Washington, June 23–25, 2014.
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization; 2012. March of Dimes PMNCH, Save the Children, WHO. [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: Final data for 2012. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 3.Institute of Medicine. Preterm birth: causes, consequences, and prevention. Washington, D.C.: National Academy of Sciences; 2007. [Google Scholar]
- 4.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008 Jan 5;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Annals of the New York Academy of Sciences. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. British Journal of Obstetrics & Gynaecology. 2006 Dec;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;7(6):337–345. doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 8.Liu NQ, Hewison M. Vitamin D, the placenta and pregnancy. Arch Biochem Biophys. 2012 Jul 1;523(1):37–47. doi: 10.1016/j.abb.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, et al. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biology of reproduction. 2009 Mar;80(3):398–406. doi: 10.1095/biolreprod.108.073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, et al. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod. 2006 Dec;75(6):816–22. doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- 11.Liu NQ, Kaplan AT, Lagishetty V, Ouyang YB, Ouyang Y, Simmons CF, et al. Vitamin D and the regulation of placental inflammation. J Immunol. 2011 May 15;186(10):5968–5974. doi: 10.4049/jimmunol.1003332. [DOI] [PubMed] [Google Scholar]
- 12.Wagner CL, McNeil RB, Johnson DD, Hulsey TC, Ebeling M, Robinson C, et al. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: A combined analysis. J Steroid Biochem Mol Biol. 2013 Jan 10; doi: 10.1016/j.jsbmb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. Journal of Infectious Diseases. 2009 Oct 1;200(7):1022–1030. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorp JM, Camargo CA, McGee PL, Harper M, Klebanoff MA, Sorokin Y, et al. Vitamin D status and recurrent preterm birth: a nested case-control study in high-risk women. BJOG. 2012 Dec;119(13):1617–1623. doi: 10.1111/j.1471-0528.2012.03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodnar LM, Klebanoff MA, Gernand AD, Platt RW, Parks WT, Catov JM, et al. Maternal vitamin D status and spontaneous preterm birth by placental histology in the US Collaborative Perinatal Project. American Journal of Epidemiology. 2014 Jan 15;179(2):168–176. doi: 10.1093/aje/kwt237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodnar LM, Rouse DJ, Momirova V, Peaceman AM, Sciscione A, Spong CY, et al. Maternal 25-hydroxyvitamin D and preterm birth in twin gestations. Obstet Gynecol. 2013 Jul;122(1):91–98. doi: 10.1097/AOG.0b013e3182941d9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetta LA, Biggio JR, Cliver S, Abramovici A, Barnes S, Tita AT. Is midtrimester vitamin D status associated with spontaneous preterm birth and preeclampsia? American Journal of Perinatology. 2014 Jun;31(6):541–546. doi: 10.1055/s-0033-1356483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneuer FJ, Roberts CL, Guilbert C, Simpson JM, Algert CS, Khambalia AZ, et al. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. American Journal of Clinical Nutrition. 2014 Feb;99(2):287–295. doi: 10.3945/ajcn.113.065672. [DOI] [PubMed] [Google Scholar]
- 19.Bodnar LM, Siega-Riz AM, Simhan HN, Himes KP, Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. American Journal of Clinical Nutrition. 2010 Jun;91(6):1642–1648. doi: 10.3945/ajcn.2009.29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodnar LM, Hutcheon JA, Platt RW, Himes KP, Simhan HN, Abrams B. Should gestational weight gain recommendations be tailored by maternal characteristics? American Journal of Epidemiology. 2011 Jul 15;174(2):136–146. doi: 10.1093/aje/kwr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cologne J, Preston DL, Imai K, Misumi M, Yoshida K, Hayashi T, et al. Conventional case-cohort design and analysis for studies of interaction. Int J Epidemiol. 2012;41(4):1174–1186. doi: 10.1093/ije/dys102. [DOI] [PubMed] [Google Scholar]
- 22.Abuhamad AZ. ACOG Practice Bulletin, clinical management guidelines for obstetrician-gynecologists number 98, October 2008 (replaces Practice Bulletin number 58, December 2004). Ultrasonography in pregnancy. Obstetrics & Gynecology. 2008 Oct;112(4):951–961. doi: 10.1097/AOG.0b013e31818b1fba. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005 Jun;90(6):3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, D.C.: National Academy Press; 2010. [Google Scholar]
- 25.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Jacobs EJ, McCullough ML, Rodriguez C, Thun MJ, Calle EE, et al. Comparing methods for accounting for seasonal variability in a biomarker when only a single sample is available: insights from simulations based on serum 25-hydroxyvitamin d. American Journal of Epidemiology. 2009 Jul 1;170(1):88–94. doi: 10.1093/aje/kwp086. [DOI] [PubMed] [Google Scholar]
- 27.Royston P. Multiple imputation of missing values: Further update of ice, with an emphasis on categorical variables. Stata J. 2009;9(3):466–477. [Google Scholar]
- 28.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glymour MM, Greenland S. Causal diagrams. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 30.Harrell FE. Regression modeling strategies with applications to linear models, logistic regression and survival analysis. New York, USA: Springer-Verlag; 2001. [Google Scholar]
- 31.Rothman KJ. The estimation of synergy or antagonism. Am J Epidemiol. 1976 May;103(5):506–511. doi: 10.1093/oxfordjournals.aje.a112252. [DOI] [PubMed] [Google Scholar]
- 32.Skrondal A. Interaction as departure from additivity in case-control studies: a cautionary note. Am J Epidemiol. 2003 Aug 1;158(3):251–258. doi: 10.1093/aje/kwg113. [DOI] [PubMed] [Google Scholar]
- 33.Bodnar LM, Pugh SJ, Abrams B, Himes KP, Hutcheon JA. Gestational weight gain in twin pregnancies and maternal and child health: a systematic review. Journal of Perinatology. 2014 Apr;34(4):252–263. doi: 10.1038/jp.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. New England Journal of Medicine. 2013 Nov 21;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: The free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2013 Oct 4; doi: 10.1016/j.jsbmb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986 Oct;63(4):954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 37.Kamboh MI, Ferrell RE. Ethnic variation in vitamin D-binding protein (GC): a review of isoelectric focusing studies in human populations. Hum Genet. 1986 Apr;72(4):281–293. doi: 10.1007/BF00290950. [DOI] [PubMed] [Google Scholar]
- 38.Villar J, Papageorghiou AT, Knight HE, Gravett MG, Iams J, Waller SA, et al. The preterm birth syndrome: a prototype phenotypic classification. American Journal of Obstetrics and Gynecology. 2012;206(2):119–123. doi: 10.1016/j.ajog.2011.10.866. [DOI] [PubMed] [Google Scholar]
- 39.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol. 2001 Jul;15(Suppl 2):78–89. doi: 10.1046/j.1365-3016.2001.00010.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.