Abstract
MicroRNAs (miRNAs) regulate gene expression and biological processes including embryonic development, innate immunity and infection in many species. Emerging evidence indicates that miRNAs are involved in drug resistance. However, little is known about the relationship between the miRNAs and insecticide resistance in mosquitos. Here, we reported that conserved miR-278-3p and its target gene are critical for pyrethroid resistance in Culex pipiens pallens. We found that CYP6AG11 is the target of miR-278-3p, through bioinformatic analysis and experimental verification. The expression level of miR-278-3p was lower, whereas the level of CYP6AG11 was higher in deltamethrin-resistant strain, which were detected using qRT-PCR. We also found that CYP6AG11 was regulated by miR-278-3p via a specific target site with the 3′UTR by luciferase reporter assay. In addition, overexpression of CYP6AG11 in the mosquito C6/36 cells showed better prolification than the cells with empty vector when treated by deltamethrin at different concentrations. Moreover, the overexpression of miR-278-3p through microinjection led to a significant reduction in the survival rate, and the level of CYP6AG11 was simultaneously reduced. These results indicated that miR-278-3p could regulate the pyrethroid resistance through CYP6AG11.
Keywords: Culex pipiens pallens, microRNA, CYP6AG11, pyrethroid resistance
Introduction
Mosquitos are one of the most medically important vectors, which transmit reemerging diseases such as malaria, dengue fever, yellow fever, and West Nile fever (Govindarajan and Sivakumar 2014; Huber et al. 2014). In recent years, mosquito borne diseases have emerged as a serious public health concern in the world (Azizullah et al. 2014; Bouzid et al. 2014). Among the diseases, only dengue fever, affects the life of 3.6 billion people (Zellweger et al. 2013). Chemical control is a major method to manage the mosquito borne diseases (Marimuthu et al. 2012). Pyrethroids are usually used for the impregnation of bed nets and indoor residual spray (White et al. 2014). Unfortunately, excessive and continuous application of pyrethroids has caused the development of pyrethroid resistance, which has become the major obstacle to control the mosquito borne diseases. The mechanisms responsible for pyrethroid resistance are mainly associated with target site modification and metabolic resistance (Bouvier et al. 2002; Somwang et al. 2011). Overexpression and/or elevated activity of P450s may enhance biodegradation of the pyrethroids (Lertkiatmongkol et al. 2011). High levels of P450 activity are frequently observed in pyrethroid resistance mosquitoes, and some of P450 have been shown to metabolize pyrethroids (Chandor-Proust et al. 2013).
MicroRNAs (miRNAs) are endogenous small non-coding RNAs of 20~25 nucleotides in length that have been shown to be responsible for the post-transcriptional regulation of mRNAs (Huang et al. 2012). MiRNAs can bind to the 3′ untranslated region (3′UTR) of their targets and silence gene expression in a sequence specific manner (Qiu et al. 2008). Since the discovery of miRNAs in Caenorhabditis elegans (Lee et al. 1993), numerous studies have demonstrated their essential role in regulating development, cell differentiation, apoptosis, and other critical biological events in both animals and plants (Hewezi et al. 2012; Pio et al. 2014; Samaraweera et al. 2014).
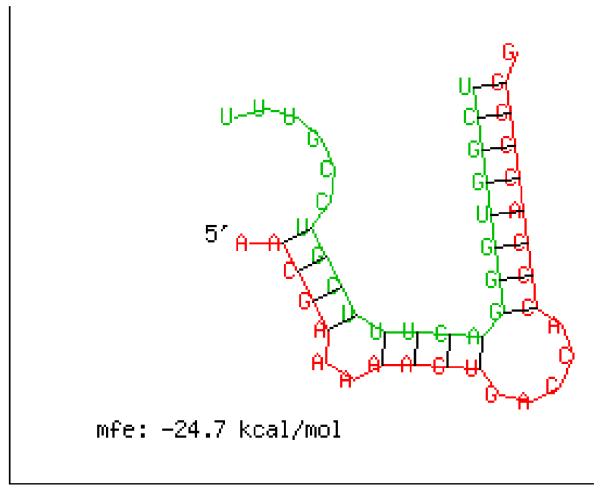
In previous study, we obtained many miRNAs in Culex pipiens pallens, which have different expression levels between pyrethroid-resistant strain and the susceptible strain of Lab population, using a high throughput Solexa sequencing (Shanchao Hong, manuscript submitted for publication). One of them is miR-278-3p, which is upregulated in the susceptible strain. To elucidate if miR-278-3p is involved in pyrethroid resistance through P450s, the 3′UTR sequences of all P450s of Cx.quinquefasciatus, and the mature miR-278-3p sequence were identified using the RNAhybrid program. Among these P450s, only the 3′UTR of CYP6AG11 match the seed region of miR-278-3p, and the free energies is low [Fig 1]. So, we investigated whether miR-278-3p could involve in pyrethroid resistance by regulating the expression level of CYP6AG11.
Fig 1.
Complementary sequence diagram of miR-278-3p and the 3′UTR of CYP6AG11. Red: 3′UTR of CYP6AG11, Green: miR-278-3p. mfe: −24.7 kcal/mol, calculated by RNAhybrid 2.2.
Materials and methods
Insects and cells
DS-strain of C. pipiens pallens was obtained from the Tangkou of Shandong Province and was maintained in our laboratory which was reared at 28 °C with 70–80% humidity and a constant light/dark cycle (14:10). DR-strain was selected with deltamethrin from DS-strain of early 4th instar larvae for more than 60 generations to reach a 146 fold resistance (LC50 of deltamethrin, 7.30 vs 0.04 mg/l).
HEK293T cells were supplemented with 10% fetal bovine serum at 37 °C, and Ae. albopictus C6/36 (ATCC no. CRL-1660) cells (offered by China Center for Type Culture Collection, CCTCC) was supplemented with 10% (v/v) fetal bovine serum (GIBCO, USA) at 28 °C.
Reverse transcription and quantitative PCR (qRT-PCR)
Total RNA was extracted from five female mosquitoes, which were one day post emergence, with TRIzol (Invitrogen, USA) according to the manufacturer’s instructions. Reverse transcription to make cDNA was done using the miScript Reverse Transcription kit (Qiagen, China). Quantitative real-time PCR was performed on the ABI PRISM 7300 (CA, USA) using Light Cycler FastStart DNA Master SYBR Green I (ABI, USA) according to the manufacturer’s instructions. The sequences of primer for CYP6AG11 were listed in Table 1. MiRNA quantification was calculated by the Bulge-loop™ miRNA qRT-PCR method (Dedeoglu 2014; Fulci et al. 2007) and primer sets (one RT primer and a pair of qPCR primers) specific for miR-278-3p were designed and listed in Table 1. The relative expression level of CYP6AG11 was normalized to the internal control β-actin, and U6 small nuclear (U6) was used to normalize the expression level of miR-278-3p. The expression level in DS-strain was considered as 1. The qRT-PCR was repeated three times with independent samples.
Table 1.
PCR primers used in this study
| Primers | Primer sequence |
|---|---|
| For real-time PCR | |
| QmiR-278-3p-F | 5′ACACTCCAGCTGGGTCGGTGGGACTTTCGT3′ |
| QmiR-278-3p-R | 5′TGGTGTCGTGGAGTCG3′ |
| QCYP6AG11-F | 5′CCCTACCTAGAGCAAGTTATCAA3′ |
| QCYP6AG11-R | 5′ACGCCCTTTTCAATGTGGA3′ |
| QACTIN-F | 5′AGCGTGAACTGACGGCTCTG3′ |
| QACTIN-R | 5′ACTCGTCGTACTCCTGCTTGG3′ |
| QU6-F | 5′GCTTCGGCTGGACATATACTAAAAT3′ |
| QU6-R | 5′GAACGCTTCACGATTTTGCG3′ |
| For vector constructs | |
| 293-CYP6AG11-F1 | 5′CGAGCTCGAGGCATCAGCGTGCTTACAA3′ |
| 293-CYP6AG11-R1 | 5′CCAAGCTTATTCAACCCCCGGTGGGT3′ |
| 293-CYP6AG11-R2 | 5′CCAAGCTTATTCAACCCCGGCTCGCT3′ |
| C6/36-CYP6AG11-F | 5′GGACTAGTGAGATGGAAATGATTCTAACCATAACTCTCGTCG3′ |
| C6/36-CYP6AG11-R | 5′CCCTCGAGCGTTTAGTTATCGCCTTATAGGTCAACC3′ |
F: Forward; R: Reverse
Amplification of 3′UTR/MUT and ORF sequence of CYP6AG11
Two pairs of primers (Table 1) were designed based on the similar cDNA sequences from Culex quinquefasciatus to amplify 3′UTR/MUT of CYP6AG11 (3′UTR was a fragment sequence which included complementary sequence of seed region of miR-278-3p, and the other one changed four bases in the seed region). The first PCR was performed with primers F1 and R1 using the following conditions: denaturing at 95 °C for 30 s followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s and a final extension at 72 °C for 10 min. PCR for the mutation of the 3′UTR was carried out using the primers F1 and R2 using the same conditions as for the first PCR. Amplified fragments had been sequenced (Additional material). The ORF of CYP6AG11 was amplified using a pair of specific primers (Table 1). ATC was added before ATGG to formed Kozak sequence in the forward primer, and for the later Western blot identification. The amplified product was separated by agarose gel electrophoresis and purified using the MiniBEST Agarose Gel DNA Extraction Kit Ver.4.0 (TaKaRa, Japan). The purified DNA was ligated into the pMD19-T vector (TaKaRa, Japan).
Plasmid constructs and transient transfection
Reporter constructs for expression in 293T cells were generated by amplifying the 3′UTR/MUT sequences, followed by cloning the PCR product into a plasmid downstream of the Renilla luciferase coding region using the Sac I and Hind III restriction sites (pMIR-REPORT™ miRNA Expression Reporter Vector, ABI, US). Plasmid constructs for expression in C6/36 cells were generated by integrating the ORF sequences into the pIB/V5-His vector, using the Spe I and Xho I restriction site (Invitrogen, CA, USA).The PCR primers were listed in Table 1.
The 293T Cells were seeded at 4×105 cells per well in 6-well plates 12 h before transfection. The cells were transfected with 0.4 μg of reporter construct and 100 nM miRNA mimics (microRNA mimics are double-stranded RNA molecules intended to “mimic” native microRNAs) (GenePharma, Germany) per well. The cells were lysed after 48 h in 1× Passive Lysis Buffer (Promega, USA), and the firefly and Renilla luciferase activities were determined with the Dual-Luciferase Assay System (Promega, USA). The C6/36 cells were seeded at 5×105 cells/well in a six-well plate 24 h before transfection. For each well, plasmid DNA was diluted to a concentration of 2 μg/100μl (0.02 μg/μl). 72 hours post-transfection, cells were selected for Western blotting. At the same time, pIB/Ctrl empty vector (Invitrogen, CA, USA) as a negative control was also established using the same protocol described above. DNA transfection was performed with the Fugene HD (Roche, USA) Transfection Reagent according to the manufacturer’s protocols.
Western blotting
The C6/36 cells (5×106) were washed twice with PBS, and protein was extracted with lysis buffer (KeyGen Biotech, China) for 30 min on ice. To determine the total protein amount in samples, the bicinchoninic acid protein assay kit and protocol were used (Perbio Science, UK). Forty micrograms of protein was electrophoresed on 15% SDS–polyacrylamide gels and transferred to a polyvinylidene difluoride membrane (Millipore Corporation, USA). The fusion protein was detected using an anti-His antibody (1:1,000, Bioworld Technology, USA) and a peroxidase-conjugated rabbit antimouse secondary antibody (1:2,000, Bioworld Technology, USA) at room temperature. Detection was done with the enhanced chemiluminescence light detecting kit (Bioworld Technology, USA) according to the manufacturer’s instruction.
Cytotoxicity assay
Cell Counting Kit-8 (CCK-8, Dojindo, Japan) was used to determine the deltamethrin resistance of transient C6/36 transfectants under deltamethrin treatments. Cell suspension (100 μL) was distributed (5000 cells/well) in a 96-well plate and plates were pre-incubated for 24 h in a 5% CO2-humidified incubator at 28 °C. Wells were then treated with 100 μL of various concentrations of deltamethrin (final concentrations: 0, 100.5 μg/mL, 101.0 μg/mL, 101.5 μg/mL, 102.0 μg/mL, and 102.5μg/mL). After another 72 h, 10 μL of CCK-8 solution were added to each well. Plates were incubated for 4 h in the incubator, then the absorbance was measured at 450 nm using a microplate reader. Deltamethrin was dissolved in DMSO (Sigma, USA) and the wells of various concentrations of deltamethrin had same final concentration of DMSO. Three independent experiments were done.
Microinjection and CDC Bottle Bioassay
The one day post emergence female mosquitoes of DR-strain were used for the injection experiment. Five hundred nanoliters miRNA mimics (0.1 ng/nL) was injected into the side of the protocoele of the mosquito using microinjection. Two controls were performed in which an equivalent volume of DEPC or miRNA mimics negative control (NC) (GenePharma, Germany). 3 days post-injection, mosquitoes were selected for qRT-PCR and subsequent experiments.
The Centers for Disease Control and Prevention (CDC) bottle was used as a surveillance tool for detecting resistance to insecticides in vector populations (Aizoun et al. 2013). One milliliter deltamethrin (4mg/ml) dissolved in acetone was smeared evenly in each bottle, and one empty bottle performed in which an equivalent volume of acetone was used as control. About 20 female mosquitoes after 3 days injection were placed in each bottles, then the mortality was counted in the next two hours. The experiment was repeated three times.
Statistics
Data were analyzed by Student’s t test. All values represent the mean ± SEM. Any p value of <0.05 is considered statistically significant.
Results
Identification of miR-278-3p that target CYP6AG11
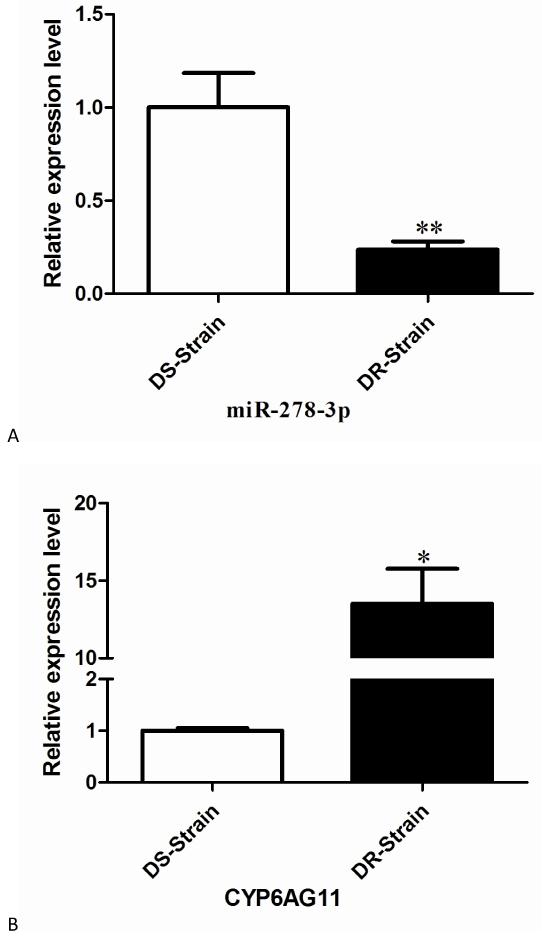
To validate miR-278-3p and the predicted CYP6AG11 potential function in C. pipiens pallens, the expression levels of miR-278-3p and CYP6AG11 were detected by quantitative real-time PCR. The result showed that the expression level of miR-278-3p was 4.2-fold lower in the DR-strain compared with the DS-strain (Fig. 2A), while the result of gene targeted by miR-278-3p showed an opposite mode that the expression level was 13.5-fold higher in the DR-strain (Fig. 2B), suggesting that CYP6AG11 might be under the regulation of miR-278-3p related to deltamethrin resistance.
Fig. 2.
Expression levels of miR-278-3p and CYP6AG11 in DR-strain and DS-strain of C. pipiens pallens. (A) miR-278-3p. (B) CYP6AG11. The expression level of U6/β-actin in the same template was considered as background level or 1, and the mRNA expression of miR-278-3p/CYP6AG11 is shown as the relative value against U6/β-actin. Results are expressed as mean ± standard error (SE) of three independent experiments. *p<0.05, ** p<0.01.
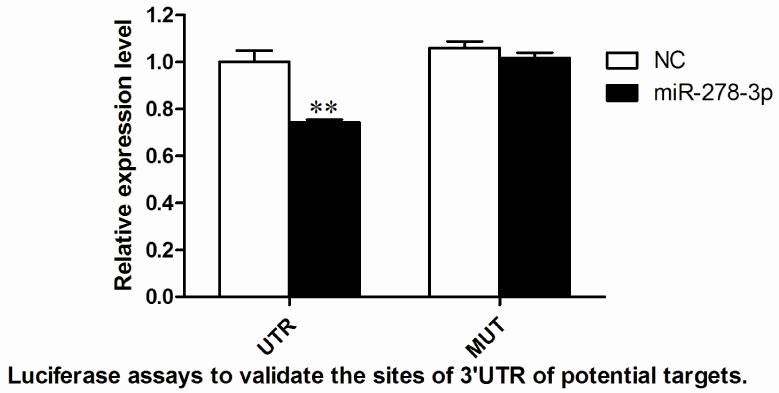
The most common method to test the interaction between a miRNA and its predicted target is a reporter assay in which the putative target sequence and its mutation sequence are fused to a reporter gene and the level of reporter gene expression is measured in the different situations (Chen et al. 2013). HEK293T cells were transfected with the recombinant plasmid (pMIR-UTR/pMIR-MUT) (which encodes both the luciferase genes and target sequences) and pGL4.74 (control reporter) vectors. In addition, miRNA mimics and NC (unrelated small RNA sequence as a control) were co-transfected into cells to measure and normalize the luciferase expression levels. Transfection of miRNA-278-3p decreased the expression of luciferase from the constructs with the 3′UTR sequences of CYP6AG11 by approximately 25% in 293T cell (Fig. 3).
Fig. 3.
Target identification of miR-278-3p in cells. Luciferase assays to validate the sites of 3′UTR of potential targets. NC represents an unrelated small RNA sequence and blank represents a reporter without 3′UTR sequence, which were used as controls. *p<0.05, ** p<0.01.
CYP6AG11 decreased the sensitivity of mosquito cells
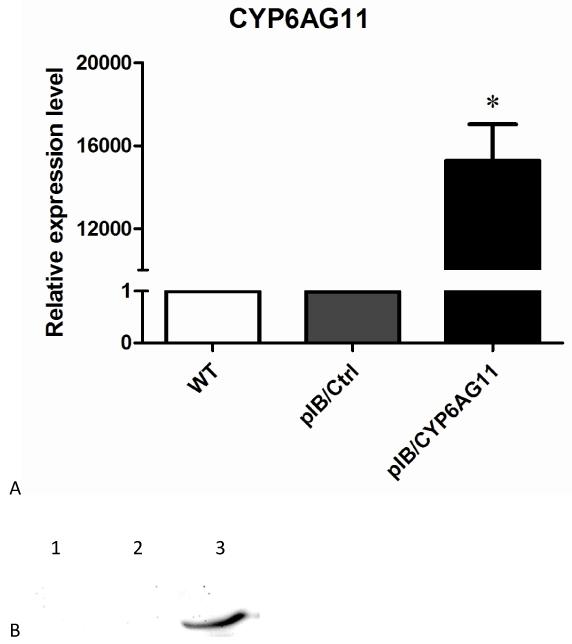
To confirm transfection and expression efficiency of exogenous CYP6AG11, qRT–PCR and Western blotting analysis were conducted. QRT–PCR results showed that the level of CYP6AG11 expression was more than 16000 folds higher in cells that were transfected with CYP6AG11 (Fig. 4A), confirming that CYP6AG11 had been transcribed in the transfected cells. Western blotting analysis using anti-His antibody identified a protein of 56 kDa in the cells transfected with CYP6AG11 (Fig. 4B).
Fig. 4.
Characterization of transient transfection of pIB/CYP6AG11-His plasmid. (A) QRT-PCR characterization of over-expression of CYP6AG11 in C6/36 cells. (B) Western blot analysis of CYP6AG11 expression in cells. The His-tagged CYP6AG11 was detected with a mouse anti-His tag antibody followed by a horseradish peroxidase conjugated goat anti-mouse secondary antibody. 1 wild-type cells, 2 pIB/Ctrl transfected cells, 3 pIB/CYP6AG11-His transfected cells.
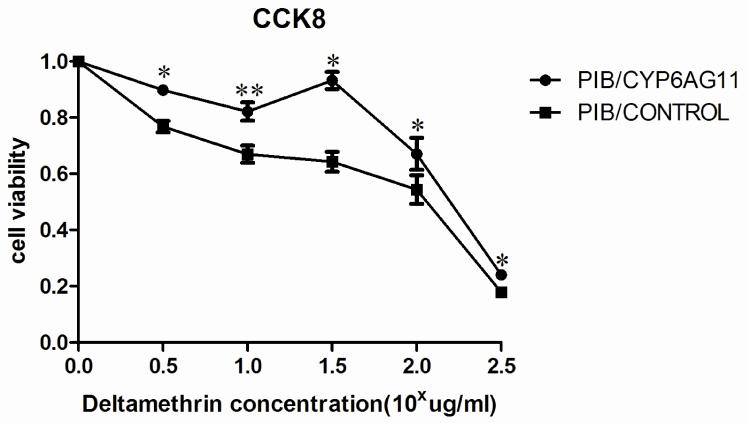
To investigate the CYP6AG11 overexpression in relation to deltamethrin resistance, C6/36 cell line transient transfected with pIB/V5-his-CYP6AG11 and pIB/V5-his were used for this assay. The dose response of cell viability over a wide range of concentrations of deltamethrin was measured based on the cytotoxicity assay using CCK-8 kit. As shown in Fig. 5, C6/36-CYP6AG11 cells were relatively lower susceptible to deltamethrin at all concentrations (100.5 μg/mL, 101.0 μg/mL, 101.5 μg/mL, 102.0 μg/mL, and 102.5 μg/mL) (p<0.05).
Fig. 5.
Overexpression of CYP6AG11 enhances deltamethrin resistance in C6/36 cells. C6/36–CYP6AG11 cells were treated with deltamethrin at the indicated concentrations, and viable cells were measured after 72 h of treatment. The percentage of viable cells is shown relative to the control. Results are expressed as mean ± standard deviation (SD) of triplicate wells from one representative experiment out of three. *p<0.05, ** p<0.01.
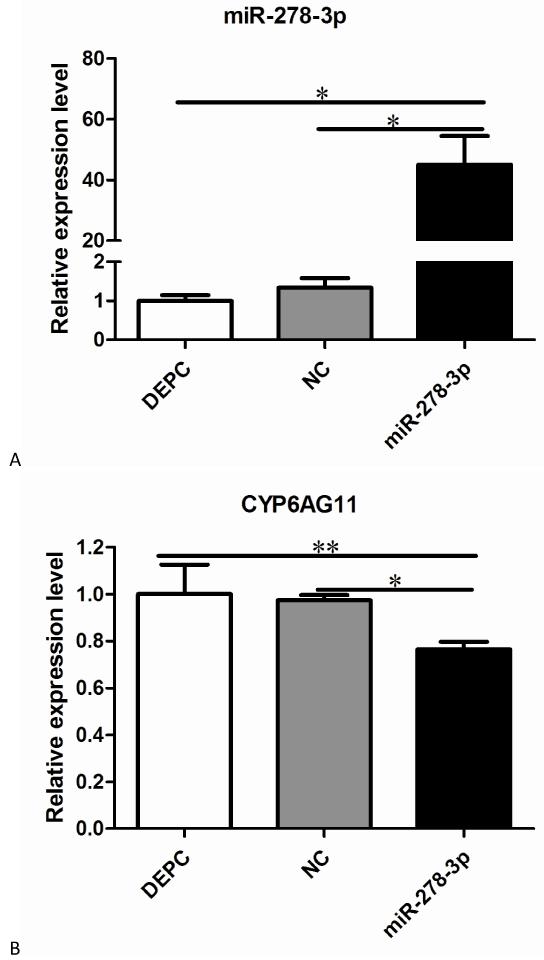
miR-278-3p could regulate the deltamethrin resistance of mosquitos
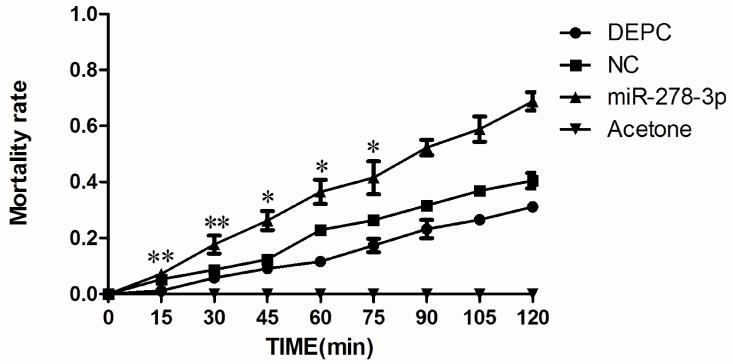
To confirm the functions of miR-278-3p in the mosquitos, qRT–PCR and CDC Bottle Bioassay were conducted after injection with miRNA mimics at 72 h post-injection. QRT–PCR results showed the level of miR-278-3p expression was 60-fold higher compared with the control group (Fig. 6A). In contrast, the level of CYP6AG11 expression was significantly lower (Fig. 6B). And the CDC Bottle Bioassay showed that the mosquitoes were more susceptible to deltamethrin compared with other groups when exposed to deltamethrin in two hours (Fig. 7).
Fig. 6.
Expression levels of miR-278-3p and CYP6AG11 post-injection. (A) miR-278-3p. (B) CYP6AG11. All the levels are relative to the level of NC and DEPC. *p<0.05, ** p<0.01.
Fig. 7.
Mortality rates with the miRNA mimics, NC and DEPC injecting of miR-278-3p. Five hundred nanoliters miRNA mimics (0.1 ng/nL) was injected into the side of the protocoele of the mosquito using microinjection. Two controls were performed in which an equivalent volume of DEPC or NC. All mosquitoes in the Acetone bottles were alive. *p<0.05, ** p<0.01.
Discussion
MiRNAs are estimated to comprise 1%–5% of animal genes (Chang et al. 2010; Yu et al. 2012), and predicted to regulate more than 30% of protein coding genes (Cai et al. 2013; Liu et al. 2010; Truini et al. 2014). MiRNA-278, as a conserved microRNA, plays different roles among insects. In Drosophila, miR-278-3p has been implicated in tissue growth and InR signaling (Teleman et al. 2006). While, miR-278-3p was also associated with kinase, neural function, synaptotagmin and energy, and might play important roles in regulating dancing behaviors in honey bees (Li et al. 2012). In our previous study, the miR-278-3p injected mosquitos showed higher susceptibility to deltamecthrin, indicating that miR-278-3p might participated in pyrethroid resistance.
P450s, especial CYP6 subfamily, have been proved to be associated with pyrethroid resistance (Edi et al. 2014; Kotewong et al. 2014). In this paper, qRT-PCR analysis demonstrated that CYP6AG11 exhibited a 13.5-fold higher level of transcription in the DR-strain. Cytotoxicity assay showed that the viability of C6/36–CYP6AG11 was higher than that of the C6/36–pIB/V5-His control cells. These findings suggested that increased expression of CYP6AG11 might play some role in the development of deltamethrin resistance in Cx.pipiens pallens. As miR-8-5p and miR-2a-3p could modulate chitin biosynthesis through membrane-bound trehalase (Tre-2) and phosphoacetylglucosamine mutase (PAGM) in Nilaparvata lugens (Chen et al. 2013), miRNAs often play a role by regulating target genes. Here, Luciferase assay showed that miR-278-3p decreased the expression of luciferase by approximately 25% in 293T cell. QRT-PCR results after microinjection also demonstrated that the expression of CYP6AG11 was 1.3-fold lower in mosquitos which had injected miR-278-3p mimics. Thus, miR-278-3p might regulate CYP6AG11 in vitro and in vivo.
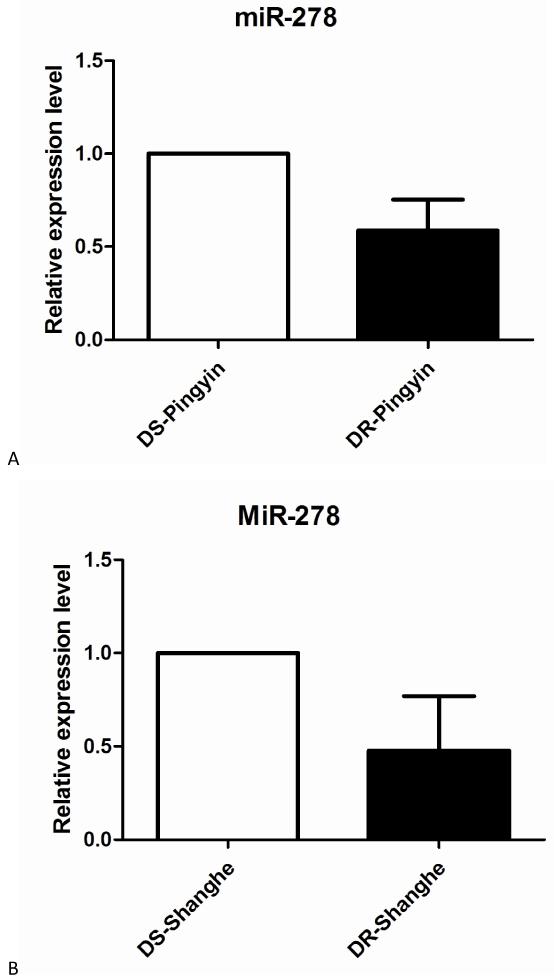
MiRNAs are often used as some diagnostic indicators, such as miR-23a is a potential biomarker for diagnosis of pre-diabetes and type 2 diabetes (Yang et al. 2014), and miR-296-5p may be a prognostic indicator in prostate cancer therapy (Lee et al. 2014). In our study, we also detected the expression level of miR-278-3p in the field mosquito populations, which were obtained in public area of Pinyin (36°17′48.062″N ; 116°25′21.298″E) and Shanghe (37° 18′52.124″N ; 117°09′25.955″E), and were described according to the standard WHO testing protocol (WHO 2013) and previous studies (Bonizzoni et al. 2012). QRT-PCR analysis demonstrated that the expression level of miR-278-3p was respectively 2.1-fold and 1.7-fold higher in the DS-strain in SH and PY population (Fig. 8), which suggested that miR-278-3p might be used as a potential genetic marker to monitor and predict the pyrethroid resistance level of field mosquito populations.
Fig. 8.
Expression levels of miR-278-3p in the field populations. (A) Pingyin. (B) Shanghe. The expression level of U6 in the same template was considered as background level or 1, and the mRNA expression of miR-278-3p is shown as the relative value against U6.
In conclusion, our present showed that miR-278-3p might regulate the pyrethroid resistance of mosquitos by decreasing the expression level of CYP6AG11. This study is the first to predict target genes and to establish a microinjection technique to study the functions of miRNAs in C. pipiens pallens. In addition, the roles of miR-278-3p in pyrethroid resistance provide a novel thinking to illuminate the mechanism of insecticide resistance.
Acknowledgement
This work was supported by the National Institutes of Health of US (NIH) (Grant No.2R01AI075746), the National Natural Science Foundation of China (Grant No.81171900, 81101279 and 81301458), the National S&T Major Program (Grant No.2012ZX10004-219 and 2012ZX10004-220), Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant No.20113234120007), and Natural Science Foundation of Jiangsu Province (Grant No.81101279).
References
- Aizoun N, et al. Comparison of the standard WHO susceptibility tests and the CDC bottle bioassay for the determination of insecticide susceptibility in malaria vectors and their correlation with biochemical and molecular biology assays in Benin, West Africa. Parasit Vectors. 2013;6:147. doi: 10.1186/1756-3305-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizullah A, et al. Chlorophyll derivatives can be an efficient weapon in the fight against dengue. Parasitol Res. 2014 doi: 10.1007/s00436-014-4175-3. doi:10.1007/s00436-014-4175-3. [DOI] [PubMed] [Google Scholar]
- Bonizzoni M, et al. Comparative transcriptome analyses of deltamethrin-resistant and -susceptible Anopheles gambiae mosquitoes from Kenya by RNA-Seq. PLoS One. 2012;7(9):e44607. doi: 10.1371/journal.pone.0044607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier JC, Boivin T, Beslay D, Sauphanor B. Age-dependent response to insecticides and enzymatic variation in susceptible and resistant codling moth larvae. Arch Insect Biochem Physiol. 2002;51(2):55–66. doi: 10.1002/arch.10052. [DOI] [PubMed] [Google Scholar]
- Bouzid M, Colon-Gonzalez FJ, Lung T, Lake IR, Hunter PR. Climate change and the emergence of vector-borne diseases in Europe: case study of dengue fever. BMC Public Health. 2014;14(1):781. doi: 10.1186/1471-2458-14-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai ZG, Zhang SM, Zhang H, Zhou YY, Wu HB, Xu XP. Aberrant expression of microRNAs involved in epithelial-mesenchymal transition of HT-29 cell line. Cell Biol Int. 2013;37(7):669–74. doi: 10.1002/cbin.10087. [DOI] [PubMed] [Google Scholar]
- Chandor-Proust A, et al. The central role of mosquito cytochrome P450 CYP6Zs in insecticide detoxification revealed by functional expression and structural modelling. The Biochemical journal. 2013;455(1):75–85. doi: 10.1042/BJ20130577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, et al. Hepatic stellate cell-specific gene silencing induced by an artificial microRNA for antifibrosis in vitro. Dig Dis Sci. 2010;55(3):642–53. doi: 10.1007/s10620-009-1021-z. [DOI] [PubMed] [Google Scholar]
- Chen J, Liang Z, Liang Y, Pang R, Zhang W. Conserved microRNAs miR-8-5p and miR-2a-3p modulate chitin biosynthesis in response to 20-hydroxyecdysone signaling in the brown planthopper, Nilaparvata lugens. Insect Biochem Mol Biol. 2013;43(9):839–48. doi: 10.1016/j.ibmb.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Dedeoglu BG. High-throughput approaches for microRNA expression analysis. Methods Mol Biol. 2014;1107:91–103. doi: 10.1007/978-1-62703-748-8_6. [DOI] [PubMed] [Google Scholar]
- Edi CV, et al. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 2014;10(3):e1004236. doi: 10.1371/journal.pgen.1004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulci V, et al. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood. 2007;109(11):4944–51. doi: 10.1182/blood-2006-12-062398. [DOI] [PubMed] [Google Scholar]
- Govindarajan M, Sivakumar R. Larvicidal, ovicidal, and adulticidal efficacy of Erythrina indica (Lam.) (Family: Fabaceae) against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus (Diptera: Culicidae) Parasitol Res. 2014;113(2):777–91. doi: 10.1007/s00436-013-3709-4. [DOI] [PubMed] [Google Scholar]
- Hewezi T, Maier TR, Nettleton D, Baum TJ. The Arabidopsis microRNA396-GRF1/GRF3 regulatory module acts as a developmental regulator in the reprogramming of root cells during cyst nematode infection. Plant Physiol. 2012;159(1):321–35. doi: 10.1104/pp.112.193649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XM, et al. Cloning and identification of microRNAs in earthworm (Eisenia fetida) Biochem Genet. 2012;50(1-2):1–11. doi: 10.1007/s10528-011-9452-6. [DOI] [PubMed] [Google Scholar]
- Huber K, et al. Distribution and genetic structure of Aedes japonicus japonicus populations (Diptera: Culicidae) in Germany. Parasitol Res. 2014;113(9):3201–10. doi: 10.1007/s00436-014-4000-z. [DOI] [PubMed] [Google Scholar]
- Kotewong R, Duangkaew P, Srisook E, Sarapusit S, Rongnoparut P. Structure-function relationships of inhibition of mosquito cytochrome P450 enzymes by flavonoids of Andrographis paniculata. Parasitol Res. 2014;113(9):3381–92. doi: 10.1007/s00436-014-4003-9. [DOI] [PubMed] [Google Scholar]
- Lee KH, et al. MicroRNA-296-5p (miR-296-5p) functions as a tumor suppressor in prostate cancer by directly targeting Pin1. Biochim Biophys Acta. 2014;1843(9):2055–66. doi: 10.1016/j.bbamcr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Lertkiatmongkol P, Jenwitheesuk E, Rongnoparut P. Homology modeling of mosquito cytochrome P450 enzymes involved in pyrethroid metabolism: insights into differences in substrate selectivity. BMC Res Notes. 2011;4:321. doi: 10.1186/1756-0500-4-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. Differences in microRNAs and their expressions between foraging and dancing honey bees, Apis mellifera L. J Insect Physiol. 2012;58(11):1438–43. doi: 10.1016/j.jinsphys.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tuo W, Gao H, Zhu XQ. MicroRNAs of parasites: current status and future perspectives. Parasitol Res. 2010;107(3):501–7. doi: 10.1007/s00436-010-1927-6. [DOI] [PubMed] [Google Scholar]
- Marimuthu G, Rajamohan S, Mohan R, Krishnamoorthy Y. Larvicidal and ovicidal properties of leaf and seed extracts of Delonix elata (L.) Gamble (family: Fabaceae) against malaria (Anopheles stephensi Liston) and dengue (Aedes aegypti Linn.) (Diptera: Culicidae) vector mosquitoes. Parasitol Res. 2012;111(1):65–77. doi: 10.1007/s00436-011-2802-9. [DOI] [PubMed] [Google Scholar]
- Pio G, Malerba D, D’Elia D, Ceci M. Integrating microRNA target predictions for the discovery of gene regulatory networks: a semi-supervised ensemble learning approach. BMC Bioinformatics. 2014;15(Suppl 1):S4. doi: 10.1186/1471-2105-15-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Wang H, Xia X, Zhou H, Xu Z. A construct with fluorescent indicators for conditional expression of miRNA. BMC Biotechnol. 2008;8:77. doi: 10.1186/1472-6750-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaraweera L, Grandinetti KB, Huang R, Spengler BA, Ross RA. MicroRNAs define distinct human neuroblastoma cell phenotypes and regulate their differentiation and tumorigenicity. BMC Cancer. 2014;14:309. doi: 10.1186/1471-2407-14-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somwang P, et al. Enzymes-based resistant mechanism in pyrethroid resistant and susceptible Aedes aegypti strains from northern Thailand. Parasitol Res. 2011;109(3):531–7. doi: 10.1007/s00436-011-2280-0. [DOI] [PubMed] [Google Scholar]
- Teleman AA, Maitra S, Cohen SM. Drosophila lacking microRNA miR-278 are defective in energy homeostasis. Genes Dev. 2006;20(4):417–22. doi: 10.1101/gad.374406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini A, et al. Role of microRNAs in malignant mesothelioma. Cell Mol Life Sci. 2014;71(15):2865–78. doi: 10.1007/s00018-014-1584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MT, et al. Negative cross resistance mediated by co-treated bed nets: a potential means of restoring pyrethroid-susceptibility to malaria vectors. PLoS One. 2014;9(5):e95640. doi: 10.1371/journal.pone.0095640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. 2013. [Google Scholar]
- Yang Z, et al. Serum miR-23a, a potential biomarker for diagnosis of pre-diabetes and type 2 diabetes. Acta Diabetol. 2014 doi: 10.1007/s00592-014-0617-8. doi:10.1007/s00592-014-0617-8. [DOI] [PubMed] [Google Scholar]
- Yu H, et al. Epstein-Barr virus downregulates microRNA 203 through the oncoprotein latent membrane protein 1: a contribution to increased tumor incidence in epithelial cells. J Virol. 2012;86(6):3088–99. doi: 10.1128/JVI.05901-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger RM, Miller R, Eddy WE, White LJ, Johnston RE, Shresta S. Role of humoral versus cellular responses induced by a protective dengue vaccine candidate. PLoS Pathog. 2013;9(10):e1003723. doi: 10.1371/journal.ppat.1003723. [DOI] [PMC free article] [PubMed] [Google Scholar]