Abstract
Background
Aberrant Notch activation confers a proliferative advantage onto many human tumors, including melanoma. This phase II trial assessed the antitumor activity of RO4929097, a gamma-secretase inhibitor of Notch signaling, on the progression-free and overall survival of patients with advanced melanoma.
Methods
Chemotherapy-naïve patients with metastatic melanoma of cutaneous or unknown origin were treated with RO4929097 at a dose of 20 mg orally daily, 3 consecutive days per week. A two-step accrual design was used, with an interim analysis on the first 32 patients, and continuation of enrollment if ≥4/32 patients responded.
Results
Thirty-six patients from 23 institutions were enrolled; 32 patients were evaluable. RO4929097 was well-tolerated, and most toxicities were grade 1 or 2. The most common toxicities were nausea (53%), fatigue (41%), and anemia (22%). There was 1 confirmed partial response lasting 7 months, and 8 patients with stable disease lasting at least through week 12, with one of these continuing for 31 months. The 6-month PFS was 9% (95% CI: 2–22%), and 1-year OS was 50% (95% CI: 32–66%). Peripheral blood T cell assays showed no significant inhibition of IL-2 production, a surrogate pharmacodynamic marker of Notch inhibition, suggesting that the drug levels were insufficient to achieve Notch target inhibition.
Conclusions
RO4929097 showed minimal clinical activity against metastatic melanoma in this phase II trial, possibly due to inadequate exposure to therapeutic drug levels. While Notch inhibition remains a compelling target in melanoma, our results do not support further investigation of RO4929097 at this dose and schedule.
Keywords: metastatic melanoma, notch, gamma-secretase inhibitor, RO4929097
INTRODUCTION
The Notch pathway is a highly conserved signaling cascade that plays an essential role in the normal development of a variety of human tissues through the regulation of gene expression that controls stem cell homeostasis and differentiation, cell survival and apoptosis 1. In oncogenesis, dysregulation of the Notch pathway confers on many human tumors a proliferative advantage, resistance to apoptosis, and the ability to maintain a stem-cell-like phenotype 2, 3.
The role of aberrant Notch signaling in melanoma has garnered a great deal of interest in recent years. Melanoma is a particularly aggressive cancer, with the ability to metastasize at a relatively small primary tumor size. Two well-established steps of melanoma invasion and metastasis include the loss of cell adhesion molecule E-cadherin and the gain of cell adhesion molecule MCAM 4, 5. Multiple groups have demonstrated that amplified Notch signaling contributes to melanoma growth in vitro and in vivo and promotes a more aggressive phenotype, at least in part by inhibiting E-cadherin expression and upregulating MCAM 6–8.
Notch signaling relies on the intramembrane cleavage of the Notch receptor by a gamma-secretase complex to release a Notch intracellular domain that translocates to the nucleus to activate the transcription of target genes, including Hey1 and Hes1, involved in cell fate determination, tissue differentiation, and vasculogenesis. Understanding of this pathway has fueled the investigation of gamma-secretase inhibitors as a therapeutic strategy to inhibit Notch signaling in melanoma as well as other cancers.
In addition to a role for Notch signaling in melanoma progression, the Notch pathway has also been shown to be critical for normal T cell development and function 10, 11. Therefore, given the importance of T cell immunity in the control of melanoma in particular, it is critical to assess the effect of these agents on T cell function in patients, as pharmacologic strategies for Notch inhibition in cancer therapy are developed. Analysis of the effects on T cells, using the production of interleukin-2 (IL-2), a potent T cell growth factor, as a measure of T cell function, could also serve as an indirect pharmacodynamic biomarker of Notch inhibition12.
RO4929097 is a small-molecule inhibitor of gamma-secretase (γ-secretase) with high oral bioavailability and is a potent and selective inhibitor of γ-secretase, leading to the blockade of Notch signaling in tumor cells. A Phase I dose-escalation study in 110 patients with refractory metastatic or locally advanced solid tumors demonstrated that RO4929097 was well-tolerated, with the majority (95%) of toxicities being grade 1 or 2 fatigue and mucocutaneous effects 13. Most toxicities, including all that were considered dose-limiting, were more common in a 7-days-on/14-days-off schedule compared to a 3-days-on/4-days-off schedule for 2 out of 3 weeks. Antitumor activity was seen in 26 of 96 evaluable patients (27%) with 1 partial response in a colonic adeno/neuroendocrine tumor, 1 mixed response in epithelioid sarcoma, 1 minor response and 1 near-complete PET response of cutaneous metastases in melanoma, and 22 other patients with stable disease for at least 3 to 6 months (most frequently in melanoma, sarcoma, and ovarian carcinoma). The clinical outcomes from this Phase I trial provided the rationale to continue the investigation of RO4929097 in patients with metastatic melanoma, at the 3-days-on/4-days-off schedule, using a 20mg dose level, which demonstrated less autoinduction of drug metabolism and potential for drug-drug interactions than the other dose and schedule. We therefore conducted a Phase II clinical trial of RO4929097 in 32 patients with metastatic melanoma. The study objectives were to assess the 6-month progression-free survival (PFS) and 1-year overall survival (OS) in advanced treatment-naïve melanoma patients, using the pooled data from a well-accepted advanced melanoma meta-analysis to set the levels of activity that would support further study of this regimen. We also wished to further assess the safety and tolerability of the regimen and to evaluate the effects of the study drug on T cell function and Notch target genes.
PATIENTS AND METHODS
The trial was performed by SWOG, and the investigational agent was provided by the Cancer Therapy Evaluation Program of the National Cancer Institute under an agreement with Roche/Genentech (ClinicalTrials.gov identifier: NCT01120275). All study subjects provided voluntary, written informed consent using a document approved by the institutions' human subject protection committee. The protocol and all amendments were also approved by SWOG and by the regulatory committees at the participating institutions.
Patient Selection
Eligible patients had stage IV, histologically confirmed, melanoma of cutaneous or unknown origin (ocular and mucosal excluded), with measurable disease as defined by RECIST 1.1. Study subjects were not preselected for the expression of known oncogenic pathways or for any marker of Notch pathway activation, however patients were required to have archival or fresh tissue available from pre-study for laboratory correlates. Patients must have had no prior cytotoxic chemotherapy for stage IV disease (prior immunotherapy and adjuvant therapy were allowed), and no history of CNS metastasis. They were required to have a Zubrod performance status of 0–1, adequate hematologic, hepatic, cardiac and renal function, with a leukocyte count ≥ 3,000/mcL, absolute neutrophil count ≥ 1,500/mcL, platelets ≥ 100,000/mcL, hemoglobin ≥ 9 g/dL, creatinine clearance ≥ 60 mL/min, total bilirubin ≤ institutional upper limit of normal (IULN), AST and ALT ≤ 2.5 × IULN, and QTcF ≤ 500 msec. Women of childbearing potential were required to have a negative serum pregnancy test, and subjects of both genders were required to practice adequate birth control during protocol participation.
Treatment and Monitoring
The study drug RO4929097 was given orally on an empty stomach at 20mg daily on days 1–3, 8–10, and 15–17 of every 3-week cycle of therapy. This was the recommended phase 2 dose (RP2D), based on the phase I dose escalation study. Treatment was given on a continuous schedule with dose adjustments and brief breaks from therapy specified in the protocol for treatment-related toxicities. Drug compliance was recorded by patients on an Intake Calendar that was submitted to the research team, along with all unused tablets, at each study visit. Prior to dispensing RO4929097, the investigator confirmed and documented the patient's use of two contraceptive methods, dates of negative pregnancy test, and confirmed the patient's understanding of the teratogenic potential of the study drug. Patients were removed from study for disease progression, symptomatic deterioration, unacceptable toxicity, treatment delay for any reason>14 days, or patient request.
Additional patient consent (optional) was requested for fresh tumor samples pre-study (if the patient had not already undergone a pre-study biopsy) and at Week 3 of Cycle 1. The tumor tissue could be obtained by surgical excision, surgical core biopsy, or CT-guided core biopsy.
Patients were evaluated with a history and physical, laboratory analyses (complete blood count, metabolic panel, pregnancy test, thyroid stimulating hormone), ECG, toxicity assessment, and drug compliance assessment at least every 3 weeks at the beginning of each cycle. Imaging studies for disease assessment were performed pre-study, week 7, week 13, and then as clinically indicated until progression. Specific guidance for dose modifications was provided for the management of hematologic toxicities, hypertension, electrolyte abnormalities, diarrhea, and other non-hematologic toxicities.
Statistical Methods
The primary objectives of this Phase II trial were to assess six-month PFS and one-year OS, using historical benchmarks established by a large meta-analysis of Phase II cooperative group clinical trials by Korn et al. 14 Our objective was to distinguish between a true 6-month PFS probability < 15% versus > 30% and a true 1-year OS probability < 35% versus > 50%. The results of this study would be considered evidence that this agent warranted further study if at least 17 of 72 eligible patients survived and were progression-free for at least 6 months, or if 31 or more eligible patients survived at least one year. A two-step accrual design was used, which required an interim analysis on the first 32 patients evaluable for response. The criteria for continuation of enrollment to 72 patients would be the observation of 4 or more clinical responses in the first 32 patients, or 9 or more of the first 32 patients evaluable for 6-month PFS were alive and progression-free at that milestone. Objective response was used in lieu of the primary study objectives of PFS and OS as criteria for trial continuation, because the prolonged time to reach PFS and OS endpoints would have obviated their utility as an interim checkpoint for this actively accruing trial. The secondary objectives were to investigate the relationship between Notch activation status, Notch target gene expression in the tumor, and clinical outcome; to study the effects of the drug on T cell function; to assess the objective response rate (ORR) and disease control rate (DCR), defined as the number of patients with a best response of stable disease or better at 12 weeks following the initiation of therapy; and to assess toxicity. PFS and OS estimates were calculated using the method of Kaplan-Meier 15. Confidence intervals for the medians were constructed using the method of Brookmeyer and Crowley 16, and confidence intervals for point estimates (e.g. 6-month PFS) were calculated using the log-log transformation. Clopper-Pearson confidence intervals were calculated for binary outcomes (e.g., ORR). An exploratory analysis of the relationship between biomarkers values and clinical outcomes was performed. The biomarker values were treated two ways, first as continuous variables, using a log transformation if the values were skewed, and by dichotomizing at the observed median. Cox regression was used to analyze the relationship of biomarker values with PFS and OS. Logistic regression was used to evaluate the relationships with OR and DCR. Fisher's exact test was used to evaluate the dichotomized variables. To explore the relationship between the change in T cell IL-2 production values from baseline to Week 3 and OS and PFS, a landmark analysis was performed, with OS and PFS measured starting at Week 3. All analyses were performed using SAS version 9.2.
Laboratory Correlates
Secondary objectives of this study included the evaluation of Notch1 by immunohistochemistry (IHC), and real-time RT-PCR for Hey1 and Hes1 on pre-treatment patient samples (and on-treatment samples, if available), and to explore potential indicators of Notch activity in on-treatment biopsies and their association with clinical response to the study drug. IHC was performed on pre-treatment paraffin tissue. The sections were prepared at SWOG and shipped to the University of Chicago for analysis. The slides were stained with antibodies specific for total Notch1 (Santa Cruz Biotechnology, #sc-6014) versus a secondary antibody alone. H&E staining was performed in parallel. A semi-quantitative scoring was used to determine the IHC results, which were manually evaluated and scored as negative or +, ++, +++ by a pathologist. For qRT-PCR, total RNA was extracted from formalin fixed, paraffin embedded tissue slides using RNeasy FFPE kit (Qiagen, Cat# 74404) according to manufacturer's procedure. cDNAs were prepared using the High Capacity cDNA Reverse Transcription Kits (Applied Biosystems, #4368814). qRT-PCR was performed using primer/probe sets specific for Hey1 (Applied Biosystems, Hs01114113_m1) and Hes1 (Applied Biosystems, Hs00172878_m1). Human ACTB (actin, beta) (Life Technologies Crop, 4352935E_3614263566) was used as an internal standard.
The effect of the study drug on T cell function was also investigated by evaluating IL-2 production by pre-treatment and on-treatment patient peripheral blood mononuclear cells (PBMCs) stimulated with the superantigen Staphylococcal enterotoxin A (SEA). This assay also served as a pharmacodynamic biomarker. Cryopreserved PBMCs were thawed, counted, and resuspended at 1 × 106 /ml in Iscove's modified Dulbecco medium (IMDM) and seeded at 100,000 PBMCs per well in a 96-well flat-bottom tissue culture plate. Cells were treated with medium alone, the superantigen SEA at 100ng/ml, or SEA (100ng/ml) plus a nonclinical gamma secretase inhibitor (InSolution γ-Secretase Inhibitor X, Calbiochem, Cat# 565771, 10μM) for 24 hours. IL-2 levels in the supernatant were measured by ELISA.
RESULTS
Patient Characteristics
Thirty-six patients from 23 SWOG institutions were registered on the first stage of this study between January 2011 and November 2011. The study was then closed after not showing sufficient activity to warrant opening the second stage of accrual. Three patients were ineligible: two had no measurable disease at baseline per RECIST, and one had inadequate renal function. In addition, one eligible patient refused protocol treatment after giving initial consent. Of the 32 evaluable patients, the median age was 60 (range 32–85), 69% were male, and 41% had a serum LDH over the institutional upper limits of normal. Sites of metastasis were node/soft tissue/skin (53%), lung (53%), liver (38%), and bone (25%). Nine patients (28%) received prior systemic therapies, including adjuvant interferon alfa (16%), interleukin-2 (6%), denileukin diftitox (3%), and sargramostim (3%). Mutational testing was not required for enrollment, and the BRAF mutation status of the study patients was not recorded. The patient characteristics are listed in Table 1.
Table 1.
Baseline Patient Characteristics
| Patient Characteristics | (n=32) |
|
|---|---|---|
| AGE | ||
| Median | 60.9 | |
| Minimum | 32.8 | |
| Maximum | 85.9 | |
| SEX | ||
| Male | 22 | 69% |
| Female | 10 | 31% |
| PERFORMANCE STATUS | ||
| 0 | 24 | 75% |
| 1 | 8 | 25% |
| PRIMARY TYPE | ||
| Cutaneous | 22 | 69% |
| Unknown primary | 10 | 31% |
| SITE(S) OF METASTASES | ||
| Bone | 8 | 25% |
| Liver | 12 | 38% |
| Lymph node, skin, soft tissue | 17 | 53% |
| Lung | 17 | 53% |
| Other non-visceral | 1 | 3% |
| Other visceral | 9 | 28% |
| ELEVATED LDH (Lactate dehydrogenase) | ||
| No | 19 | 59% |
| Yes | 13 | 41% |
| PRIOR SYSTEMIC THERAPY | ||
| No | 23 | 72% |
| Yes | 9 | 28% |
| TYPE OF PRIOR SYSTEMIC THERAPY | ||
| Adjuvant interferon alfa | 5 | 16% |
| lnterleukin-2 | 2 | 6% |
| Other (Denileukin diftitox, sargramostim) | 2 | 6% |
Toxicities
The majority of the toxicities attributed to RO4929097 were Grade 1 and 2. The most common adverse events (across all grades) were nausea (53%), fatigue (41%), anemia (22%), anorexia (19%), headache (13%), constipation (13%), and diarrhea (13%). Six patients experienced Grade 3 events (see Table 2). There were no Grade 4 or 5 toxicities.
Table 2.
Toxicities of therapy, number and percent of patients (%)
| Adverse Event (AE) | Any Grade | Grade 3 | ||
|---|---|---|---|---|
| Nausea | 17 | 53% | 1 | 3% |
| Fatigue | 13 | 41% | 1 | 3% |
| Anemia | 7 | 22% | 1 | 3% |
| Anorexia | 6 | 19% | 0 | 0% |
| Diarrhea | 4 | 13% | 0 | 0% |
| Constipation | 4 | 13% | 0 | 0% |
| Headache | 4 | 13% | 0 | 0% |
| Hypophosphatemia | 3 | 9% | 2 | 6% |
| Vomiting | 3 | 9% | 0 | 0% |
| Hyponatremia | 3 | 9% | 0 | 0% |
| Dysgeusia | 3 | 9% | 0 | 0% |
| Transaminase elevation | 2 | 6% | 1 | 3% |
| Pain in extremity | 2 | 6% | 1 | 3% |
| Abdominal pain | 2 | 6% | 1 | 3% |
| Lymphocyte count decreased | 2 | 6% | 1 | 3% |
| QTc prolongation | 2 | 6% | 1 | 3% |
| Small intestinal obstruction | 1 | 3% | 1 | 3% |
| Stroke | 1 | 3% | 1 | 3% |
|
Patients with Any AE
|
30
|
94%
|
6
|
19%
|
Clinical Responses
A two-stage accrual design was applied as detailed above, and after accrual of the first 32 evaluable patients, the clinical outcomes did not meet criteria for accrual of additional patients. There was one confirmed partial response lasting 7 months (ORR 3%, 95% CI 0–16%); this patient was taken off of protocol therapy for progression at 10 months, but remains alive at 28+ months following protocol entry. Eight additional patients had stable disease lasting at least through the week 12 assessment as their best response to therapy. Among these patients with stable disease, one patient with BRAF wild-type melanoma remained on protocol treatment for 31 months before stopping due to disease progression. This patient had not received any additional therapy other than the study drug. This patient and the one patient with a partial response both received adjuvant interferon alfa as their only prior systemic treatment.
The DCR at 12 weeks was 31% (95% CI: 16–50%). The median PFS was 1.5 months (95% CI: 1.3–2.6 months) and the median overall survival was 13 months (95% CI: 8–20 months). Using the model proposed by Korn et al.14, predicted values for six-month PFS and one-year OS were calculated based on the observed distributions of gender, performance status, and visceral metastases. A one-sided exact binomial test was used to test the hypothesis that the observed six-month PFS and/or one-year OS were superior to these predicted values. The 6-month PFS was 9% (95% CI: 2–22%), which was not superior to the predicted value of 17% (p=0.91). The 1-year overall survival (OS) was 50% (95% CI: 32–66), which was not superior to the predicted value of 44% (p=0.32). The Kaplan-Meier estimates for PFS and OS are shown in Figure 1 and 2, respectively.
Figure 1.
Progression Free Survival
Figure 2.
Overall Survival
Analysis of Peripheral T cell Function
Notch pathway inhibition has been shown to inhibit T cell function in vitro 17, and Notch signaling is required for T cell development 18, 19. Therefore, we analyzed effects of RO4929097 administration on the activation of peripheral T cells, both to assess potential effects on immune function and also as a potential pharmacodynamic biomarker for drug effect. Cryopreserved peripheral blood mononuclear cell preparations were available from 23 patients. Production of IL-2 was evaluated in response to the polyclonal T cell stimulus provided by SEA, pre-study versus week 3 on treatment. However, no significant difference was observed using this ex vivo assay. In contrast, all patients showed inhibition of T cell cytokine production when a non-clinical gamma-secretase inhibitor was included during the in vitro stimulation (Figure 3). Therefore, these data suggest that the Notch pathway was likely not adequately inhibited at week 3 of administration of RO4929097 in treated patients. We investigated in a preliminary manner the relationship between clinical outcome and baseline IL-2 levels as well as the change in IL-2 levels at Week 3 from 20 eligible patients. There was no significant association found between baseline IL-2 and PFS (p=0.21), OS, (p=0.58), ORR, (p=0.74), or DCR. (p=0.51) or the change at Week 3 and PFS (p=0.48), OS (p=0.88), ORR (p=0.23), or DCR (p=0.64). However, the one patient who achieved a partial response on study drug (patient #229180 in Figure 3) also experienced a 65% drop in IL-2 production, compared to a median 0% change in IL-2 production of all study subjects.
Figure 3. T cell functional studies.
SEA-stimulated IL-2 production by peripheral blood lymphocytes (panel A), compared to control inhibition using a non-clinical gamma secretase inhibitor (panel B).
Notch Gene Correlates in Tumor Tissue
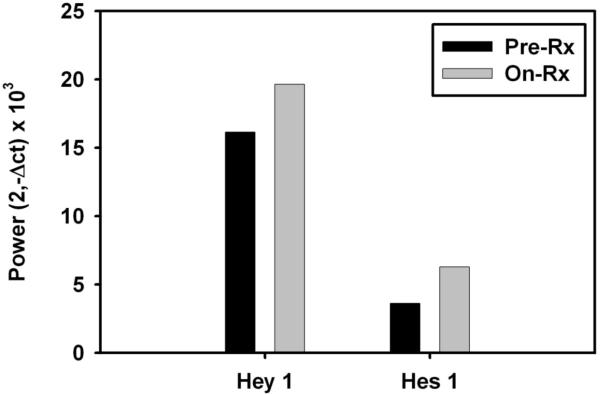
Fresh pre-treatment and 3 week on-treatment tumor biopsies for gene expression profiling were obtained from one patient. When the known Notch target genes Hey1 and Hes 1 were interrogated, no decrease was seen at the on-treatment time point (Figure 4). Although these data were obtained from only one patient, they are consistent with the peripheral blood T cell surrogate tissue analysis and suggest that stable inhibition of the Notch pathway might not have been achieved in tumor tissue.
Figure 4.
Pretreatment and on treatment tumor tissue from one patient showed a lack of inhibition of Notch target genes Hey1 and Hes1 by real-time RT-PCR.
Archived formalin-fixed, paraffin-embedded tumor tissue was available from 12 eligible patients and analyzed for baseline parameters indicative of Notch pathway activation. All samples showed some degree of staining for Notch1 by immunohistochemistry (Figure 5), suggesting availability of Notch for engagement. To further explore whether the Notch pathway was activated, quantitative RT-PCR was performed for expression of the Notch target genes Hes1 and Hey1. The signal for Hes1 was more robust and was chosen as the reference for activation. All samples showed detectable expression of Hes1 mRNA above background at baseline; most of these showed expression of Hey1 as well (Figure 6). Hey1 expression alone was detected in one additional sample. These results confirm that the Notch pathway is indeed activated in a major fraction of melanoma patients. When the patient samples were divided into “high” and “low” expression of Hes1 and Hey1 based on whether the expression was above or below the median for all the samples, there was no significant association found between “high” expression of Hes1 and PFS, (p=0.83), OS, (p=0.70), ORR (p=1.00), DCR (p=0.55), or between “high” expression of Hey1 and PFS (p=0.48), OS (p=0.59), ORR (p=0.45), DCR (p=1.00). However, the sample size was small and not powered to evaluate a correlation between target gene expression and clinical outcome. Since tumors in our study were not required to be molecularly characterized, we were unable to study a potential relationship between Notch 1 activation and the presence of other oncogenic or related pathways, such as BRAF activation.
Figure 5.
Notch1 expression was seen by IHC in all 12 of available patient tumor samples (7 had moderate expression and 5 had strong expression). Two representative samples are shown here.
Figure 6.
Notch1 activation was demonstrated by increased expression of Hes1 (Panel A) and Hey1 mRNA (Panel B) at baseline.
DISCUSSION
This Phase II clinical trial evaluated the safety, anti-tumor activity and laboratory correlates of the gamma-secretase inhibitor RO4929097 in patients with stage IV cutaneous melanoma. Although well-tolerated, RO4929097 demonstrated minimal activity at the recommended phase 2 dose and schedule in these molecularly unselected patients with advanced melanoma. One of the known downstream effects of Notch inhibition is the impairment of T cell function, perhaps best demonstrated by the ability of Notch inhibitors to block graft-versus-host-disease in animal models 20, 21. The absence of T cell functional impairment, as measured by change in IL-2 production, in most patients after treatment with study drug, in addition to the lack of downregulation of Notch target genes Hey1 and Hes1 after 3 weeks on study drug in one patient, suggests that sustained target inhibition may not have been achieved in most subjects. A definitive analysis would require serial biopsies on a greater number of patients. Although a firm conclusion cannot be drawn from a single data point, it is of interest that the only patient who experienced a partial response to the study drug also demonstrated a 65% drop in IL-2 production, compared to a median 0% change in IL-2 production of the study patients overall, suggesting the possibility that more effective Notch inhibition may have been achieved in this patient.
One possible explanation for why responses to RO4929097 were higher in the Phase I trial may be that the more intense dosing schedules investigated in that study, while causing more toxicity, may have also achieved better target inhibition. All four patients who experienced tumor regression on the Phase I study had been treated on the 7-days-on/14-days-off schedule, and the best responder who had an objective partial response on that trial was treated at a dose of 40mg, compared to the 20mg dose and 3-days-on/4-days-off schedule used in the present study. The reported stable disease rate was also slightly higher (32%) on the 7-days-on/14-days-off schedule compared to the 3-days-on/4-days-off schedule (25%). A Phase II study of RO4929097 in metastatic colorectal cancer, treated with at least two prior lines of systemic chemotherapy, also used the 20mg dose on a 3-days-on/4-days-off schedule and observed no objective responses out of 33 evaluable patients, despite IHC evidence of expression of Notch receptor, intracellular Notch and transcriptional target HES1 in the majority of patient tumors 22. However, repeated dosing higher than this 20mg, a 3-days-on/4-days-off, schedule also led to significant CYP3A4 autoinduction in the Phase I trial, which poses an additional pharmacokinetic ceiling further limiting the narrow therapeutic index of this drug. Notably, in T-cell acute lymphoblastic leukemia (T-ALL), a tumor with greater than 50% incidence of activating mutations in Notch1, the use of GSIs also led to disappointing clinical results, largely due to an unfavorable therapeutic index 23. Thus, although we did not perform pharmacokinetic analyses in this study, we believe that the use of recommended Phase II dose from Phase I development could have underexposed patients to drug and/or resulted in a gradual fall in peak and/or steady-state drug exposures.
It remains to be seen whether the degree and nature of T cell impairment induced by optimal Notch inhibition will cause a clinical impact on the patient's endogenous anti-tumor T cell responses to melanoma, and how this would affect the timing of Notch therapy in relation to immune-based therapies in a patient's treatment course. The parallel development of both immunotherapies and targeted signal transduction inhibitors for melanoma, the latter which could also adversely affect T cell function, is a potential challenge that may require logical adjustments in scheduling to maximize therapeutic synergy in combination. The downstream effects on T cell function by Notch inhibitors remain an important area of investigation in other tumor types as well, as the landscape of immunotherapy continues to unfold and T cell checkpoint blockade with anti-CTLA-4 and/or anti-PD-1 is rapidly becoming a mainstay of treatment for multiple cancers.
Given the autoinduction of metabolism of RO4929097 and the GI toxicities that have limited higher dosing of this and other gamma secretase inhibitors (GSI) 24–26, the use of GSIs to target the Notch pathway in tumors may not be the best direction for continued future drug development. However, our confirmatory data that the pathway is active in most melanomas, combined with laboratory data indicating that Notch inhibition has major anti-tumor activity against melanoma both in vitro and in vivo, suggests that alternative strategies to target the Notch pathway are warranted. A monoclonal antibody to a Notch ligand has been shown in preclinical studies to be a promising mechanism for achieving Notch inhibition 20, 27, and is currently being studied in a Phase I clinical trial (NCT01577745). As these and other more effective Notch inhibitors are studied as a cancer therapeutic, more detailed analysis of effects on T cell subsets will be warranted. Aberrant Notch signaling is a critical pathway in tumorigenesis and remains a promising therapeutic target for cancer treatment, however this Phase II trial does not support the continued development of the GSI RO4929097 in unselected patients with metastatic melanoma.
ACKNOWLEDGMENTS
We would like to thank Terri Li from the Immunohistochemistry Core Facility of the University of Chicago for performing the Notch1 staining. This work was supported in part by PHS Cooperative Agreements, NCI, DHHS CA3210, CA38926, CA27057, CA35261, CA20319, CA46282, CA67575, CA45808, CA35128, CA22433, CA95860, CA95860, CA35090, CA35178, CA52654, CA35176, CA128567, CA35421, CA13612, CA12644, CA35119, CA35431, CA68183 and CA11083.
FUNDING: This work was supported in part by PHS Cooperative Agreements, NCI, DHHS CA32102, CA38926, CA27057, CA35261, CA20319, CA46282, CA67575, CA45808, CA35128, CA22433, CA95860, CA95860, CA35090, CA35178, CA52654, CA35176, CA128567, CA35421, CA13612, CA12644, CA35119, CA35431, CA68183 and CA11083.
FINANCIAL DISCLOSURES: Thomas F. Gajewski served on an advisory board for Roche/Genentech in 2013 for a drug that was not used in this study and received grants/personal fees. Vernon K. Sondak reports personal fees from Merck (speaker's bureau and consultant/advisory board), Amgen (consultant/advisory board), OncoSec (consultant/advisory board), MabVax (consultant/advisory board), Polynoma (consultant/advisory board), Bristol-Myers Squibb (data safety monitoring board), Glaxo Smith-Kline (data safety monitoring board), and Novartis (data safety monitoring board) outside the submitted work.
Footnotes
Presented in part at the 48th Annual Meeting of the American Society of Clinical Oncology (June 4, 2012, Chicago, IL)
REFERENCES
- 1.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999 Apr 30;284(5415):770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004 Oct 8;306(5694):269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 3.Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006 Mar 15;107(6):2223–2233. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- 4.Li G, Schaider H, Satyamoorthy K, Hanakawa Y, Hashimoto K, Herlyn M. Downregulation of E-cadherin and Desmoglein 1 by autocrine hepatocyte growth factor during melanoma development. Oncogene. 2001 Dec 6;20(56):8125–8135. doi: 10.1038/sj.onc.1205034. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann JM, Holzmann B, Breitbart EW, Schmiegelow P, Riethmuller G, Johnson JP. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Res. 1987 Feb 1;47(3):841–845. [PubMed] [Google Scholar]
- 6.Liu ZJ, Xiao M, Balint K, et al. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006 Apr 15;66(8):4182–4190. doi: 10.1158/0008-5472.CAN-05-3589. [DOI] [PubMed] [Google Scholar]
- 7.Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB. Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest. 2008 Nov;118(11):3660–3670. doi: 10.1172/JCI36157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinnix CC, Lee JT, Liu ZJ, et al. Active Notch1 confers a transformed phenotype to primary human melanocytes. Cancer Res. 2009 Jul 1;69(13):5312–5320. doi: 10.1158/0008-5472.CAN-08-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massi D, Tarantini F, Franchi A, et al. Evidence for differential expression of Notch receptors and their ligands in melanocytic nevi and cutaneous malignant melanoma. Mod Pathol. 2006 Feb;19(2):246–254. doi: 10.1038/modpathol.3800526. [DOI] [PubMed] [Google Scholar]
- 10.Tsukumo S, Yasutomo K. Notch governing mature T cell differentiation. J Immunol. 2004 Dec 15;173(12):7109–7113. doi: 10.4049/jimmunol.173.12.7109. [DOI] [PubMed] [Google Scholar]
- 11.Kuijk LM, Verstege MI, Rekers NV, et al. Notch controls generation and function of human effector CD8+ T cells. Blood. 2013 Apr 4;121(14):2638–2646. doi: 10.1182/blood-2012-07-442962. [DOI] [PubMed] [Google Scholar]
- 12.Bohler T, Nolting J, Kamar N, et al. Validation of immunological biomarkers for the pharmacodynamic monitoring of immunosuppressive drugs in humans. Ther Drug Monit. 2007 Feb;29(1):77–86. doi: 10.1097/FTD.0b013e318030a40b. [DOI] [PubMed] [Google Scholar]
- 13.Tolcher AW, Messersmith WA, Mikulski SM, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol. 2012 Jul 1;30(19):2348–2353. doi: 10.1200/JCO.2011.36.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008 Feb 1;26(4):527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 16.Brookmeyer R, Crowley J. A k-sample median test for censored data. Journal of the American Statistical Association. 1982;77(378):433–440. [Google Scholar]
- 17.Palaga T, Miele L, Golde TE, Osborne BA. TCR-mediated Notch signaling regulates proliferation and IFN-gamma production in peripheral T cells. J Immunol. 2003 Sep 15;171(6):3019–3024. doi: 10.4049/jimmunol.171.6.3019. [DOI] [PubMed] [Google Scholar]
- 18.Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999 May;10(5):547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 19.Radtke F, Wilson A, Mancini SJ, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol. 2004 Mar;5(3):247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 20.Tran IT, Sandy AR, Carulli AJ, et al. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest. 2013 Apr 1;123(4):1590–1604. doi: 10.1172/JCI65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandy AR, Chung J, Toubai T, et al. T Cell-Specific Notch Inhibition Blocks Graft-versus-Host Disease by Inducing a Hyporesponsive Program in Alloreactive CD4+ and CD8+ T Cells. J Immunol. 2013 Jun 1;190(11):5818–5828. doi: 10.4049/jimmunol.1203452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strosberg JR, Yeatman T, Weber J, et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur J Cancer. 2012 May;48(7):997–1003. doi: 10.1016/j.ejca.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paganin M, Ferrando A. Molecular pathogenesis and targeted therapies for NOTCH1-induced T-cell acute lymphoblastic leukemia. Blood Rev. 2011 Mar;25(2):83–90. doi: 10.1016/j.blre.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milano J, McKay J, Dagenais C, et al. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004 Nov;82(1):341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 25.Wong GT, Manfra D, Poulet FM, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004 Mar 26;279(13):12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 26.van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005 Jun 16;435(7044):959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins DW, Ross S, Veldman-Jones M, et al. MEDI0639: a novel therapeutic antibody targeting Dll4 modulates endothelial cell function and angiogenesis in vivo. Mol Cancer Ther. 2012 Aug;11(8):1650–1660. doi: 10.1158/1535-7163.MCT-11-1027. [DOI] [PubMed] [Google Scholar]