Abstract
Background
Obesity is a well documented problem associated with childhood acute lymphoblastic leukemia (ALL) with increasing body mass index often observed during therapy. This study aims to evaluate if weight gain, early in therapy, is predictive of obesity at the end of treatment.
Procedure
In this secondary analysis, data from 1,017 high-risk ALL patients previously treated on a Children’s Oncology Group protocol (CCG study 1961) were reviewed. Logistic regression was used to examine whether change in BMI z-score at Induction or Delayed Intensification (DI) 1 were predictive of obesity at the end of therapy.
Results
The BMI z-score at the beginning of Induction and the change in BMI z-score during Induction were both significant predictors of obesity at the end of therapy. The change in BMI z-score during cycle 1 of Delayed Intensification was not found to be associated with obesity.
Conclusions
It is well know that obesity at the beginning of therapy is predictive of obesity at the end of ALL therapy. The new, and more important, finding from this study is that even after adjusting for baseline weight, the increase in BMI z-scores during induction was an independent predictor of obesity at the end of therapy. Most researchers agree that prevention is the best form of treatment for obesity as it is difficult to reverse once it is present. This study suggests that monitoring weight trends during Induction may be useful in guiding healthcare practitioners in identifying which patients are at highest risk for obesity development so that early intervention may occur.
Keywords: obesity, childhood, acute lymphoblastic leukemia, weight gain
Introduction
Acute lymphoblastic leukemia (ALL) is the most common form of cancer in children, accounting for roughly one third of all new cancer cases. This once fatal disease is now highly treatable with overall 5 year survival rates currently at 90% [1]. These improved survival rates are encouraging and are due to advances in treatment, but these same treatments carry risks for the development of adverse health events [2]. Obesity is an example of one treatment related adverse condition as its presence is well documented both during and after ALL therapy [3–5].
Children undergoing treatment for ALL are already at risk for late effects of cancer therapy and obesity can add to the risks for health conditions such as type 2 diabetes, metabolic syndrome, lipid abnormalities, cardiovascular disease, hypertension, secondary cancers, low self-esteem, depression and lower quality of life [6–8]. In adults, the presence of obesity has been shown to contribute to the development of cancer [9], including leukemia [10,11], and has been shown to worsen survival outcomes for cancer patients [12]. The impact of obesity development during treatment on survival outcomes is yet unknown in childhood ALL, but the presence of obesity at diagnosis has been showed to be linked to an increased risk of relapse in ALL patients 10 years of age or older [13]. These findings demonstrate the need for better understanding the causes of obesity during and after childhood ALL therapy.
Prior studies have identified that significant weight gain often occurs in ALL patients between diagnosis and the end of therapy [4,5,14–16]. The purpose of this study was to explore early weight gain during childhood ALL treatment as a predictor of obesity at the end of therapy. This study explored weight gain during Induction (the first cycle of ALL therapy) and Delayed Intensification 1 (the fourth cycle of chemotherapy), to determine if a change in BMI z-scores, during either of these cycles, was predictive of obesity at the end of therapy. These two cycles of therapy were selected as they occur early in treatment and because they both utilize corticosteroids, which have been shown to influence weight gain [4,17,18].
Methods
Data Collection
All data from the 1,017 patients utilized in this study were previously collected through Children’s Cancer Group (CCG) therapeutic trial number CCG 1961. Of the 2078 children enrolled on the CCG 1961 trial, 1089 completed therapy and 1017 had heights/weights at baseline and at the beginning of each chemotherapy cycle. Patients who did not complete the treatment study, or who were missing height and weight data (n=72), were excluded from this analysis. Results of the primary treatment study have been published [19]. Parental or subject consent for treatment and data collection were obtained at the time of original diagnosis through the patients’ local institutions. CCG 1961 was open to accrual from November 1996 to May 2002. This secondary data analysis research study was reviewed by the University of Arizona’s Institutional Review Board and deemed exempt.
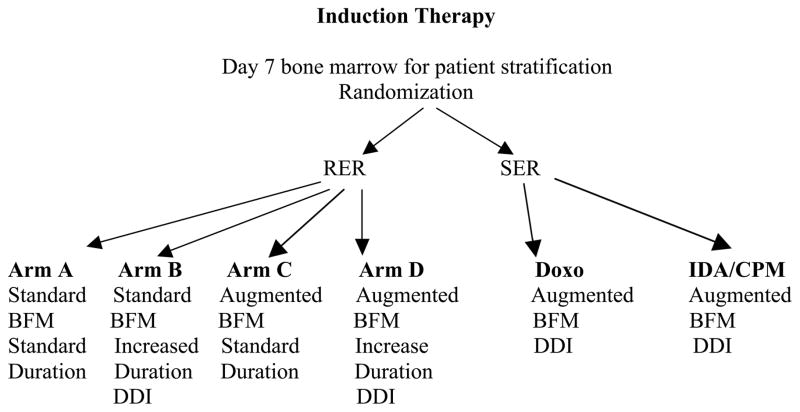
Patients were initially treated on CCG 1961 if they had newly diagnosed, previously untreated, ALL with unfavorable features (age 1 to 9 years with initial WBC > 50,000/μL or age 10 to 21 years inclusive with any white blood cell count). Figure 1 displays the schema for the original treatment study. A larger percentage of patients responded rapidly to the first 7 days of treatment and were therefore randomized on the initial treatment study to receive either arm A, B, C or D. Patients who responded slower to the first 7 days of therapy were randomized between Augmented BFM DDI with Doxorubicin (DOXO) or Augmented BFM DDI utilizing Idarubicin and Cyclophosphamide (IDA/CPM).
Figure 1.
CCG 1961 study schema. RER: rapid early response, SER: slow early response, Standard: standard chemotherapy, Augmented: intensified chemotherapy, DDI: double delayed intensification, DOXO: Doxorubicin, IDA: Idarubicin, CPM: Cyclophosphamide.
Patients treated on all study arms as rapid early responders (RER) or slow early responders (SER) were included in this analysis provided that they were between the ages of 2 to 20 years, had completed all cycles of chemotherapy and had height / weight measures available for BMI calculations. Children less than 2 years of age or older than 20 years were excluded as BMI percentiles could not be calculated for these age groups using the U.S. Centers for Disease Control (CDC) growth charts.
All SER patients received 1800 cGy cranial prophylaxis during the Consolidation phase of therapy. Although cranial radiation has been previously associated with obesity in ALL survivors, it was not found to be a risk factor for obesity, by the end of therapy, for patients treated on CCG study 1961 and was therefore not included in this analysis [14]. Per the treatment protocol, girls ended therapy 2 years from the beginning of interim maintenance (IM) 1 and boys ended therapy 3 years from the beginning of IM 1. Girls therefore completed therapy between Maintenance cycles 4 and 8, while boys completed therapy between Maintenance cycles 7 to 12.
Body Mass Measurements
Height and weight were measured at the beginning of each new cycle of chemotherapy. Body mass index (BMI) was calculated as weight (kg) / height (m2). For patients aged 2–20, percentiles for BMI were calculated with respect to the United States mean for sex and age. Percentiles for BMI and z-scores for BMI were found using the U.S. Centers for Disease Control SAS macro (http://www.cdc.gov/nccdphp/dnpa/growthcharts/sas.htm). BMI percentiles for age and sex were categorized as follow: 1) < 5th percentile classified as underweight; 2) 5th to 84.9th percentile classified as normal weight; 3) 85th to 94.9th classified as overweight; and 4) ≥95th percentile classified as obese.
The use of BMI percentiles to measure weight and growth patterns in children within the United States is a common clinical practice. BMI z-scores are less used in clinical practice; however, they are often used in research and are considered to be more accurate than BMI percentiles when measuring adiposity in children across wide age spans [20,21].
Data Analysis
Demographic and clinical variables were summarized using descriptive statistics. Logistic regression was used to examine whether change in BMI z-score at Induction or DI 1 were predictive of obesity (BMI ≥95th percentile) at the end of therapy. Univariate and multivariate models were utilized.
The change in BMI z-score during Induction was calculated as BMI z-score at the beginning of consolidation minus the BMI z-score at the beginning of Induction. The change in BMI z-score during DI was calculated using the BMI z-score at the beginning of the next cycle of therapy after DI 1, minus the BMI z-score at the beginning of DI 1. The calculated age and gender-specific BMI z-score values were used as a continuous variable in the developed statistical models.
Univariate Analysis
Univariate analysis was used to examine the relationship between obesity at the end of therapy and the individual variables of the difference in BMI z-score during Induction, the difference in BMI z-score during DI 1, the BMI z-score at the beginning of Induction (baseline), age, race, and treatment arm. Age was categorized and divided into ranges so that trends within age groups could be identified.
Multivariate Analysis
Multivariate logistic regression analyses were utilized to determine if the change in BMI z-score during Induction or DI (cycle I) were associated with obesity at the end of therapy. Since the male patients ended therapy a year after the female patients (per therapy protocol), the two genders were analyzed separately utilizing two models. The Induction Model (Model 1) examined the change in BMI z-score during Induction in relationship to obesity at the end of therapy, while the DI Model (Model 2) examined the change in BMI z-score during DI 1 in relationship to obesity at the end of therapy. Outlier cases were detected in both males and females for the Induction model. The regression models were re-run without the outliers and the models did not significantly change (p-values and odds ratio were approximately the same as in the original model). The decision was therefore made to keep the outliers in the presented models. Goodness of fit for the logistic regression models was assessed with influence plots and DFBetas plots [22].
Multivariate logistic regression included change in BMI z-score at Induction or DI 1 after adjusting for covariates including: BMI z-score at Induction, age, race, therapy arm, and the maintenance course (cycle number) in which therapy was completed. Some arms of therapy included two courses of Delayed Intensification (DDI). Only the change in BMI z-score during DI cycle 1was utilized for this study as all patients received this cycle of therapy.
Results
Baseline Characteristics
Of the eligible patients who completed therapy on CCG1961, the required height and weight information was available for review on 1,017 patients. Table I shows the patient demographics. There were 572 (56%) males and 445 (44%) females. The median age for the males was 11.3 years (ranging from 2 to 17). The median age for the females was similar at 11.4 years (ranging from 2 to 18). The majority of the participants were white (68%), but females had a higher proportion of Hispanics when compared to males. At the beginning of therapy, 27% of the children were overweight (14%) or obese (13%). Table II displays the weight distribution pre and post treatment.
Table I.
Patient demographics.
| Total 1,017 | Males 572 | Females 445 | |
|---|---|---|---|
| Age in years at diagnosis, median (range) | 11.3 (2.0 – 17.8) | 11.3 (2.0–16.9) | 11.4 (2.0–17.8) |
| Race | |||
| White | 689 (68%) | 429 (75%) | 260 (58%) |
| Black | 61 (6%) | 31 (5%) | 30 (7%) |
| Hispanic | 201 (20%) | 78 (14%) | 123 (28%) |
| Other/Unknown | 66 (6%) | 34 (6%) | 32 (7%) |
| Treatment Arm | |||
| Arm A Standard BFM standard duration | 198 (19%) | 121 (21%) | 77 (17%) |
| Arm B Standard BFM increased duration | 186 (18%) | 106 (19%) | 80 (18%) |
| Arm C Augmented BFM standard duration | 199 (20%) | 107 (19%) | 92 (21%) |
| Arm D Augmented BFM increased duration | 189 (19%) | 104 (18%) | 85 (19%) |
| Augmented BFM DDI Doxo | 129 (13%) | 69 (12%) | 60 (13%) |
| Augmented BFM DDI IDA/CPM | 116 (11%) | 65 (11%) | 51 (11%) |
BFM: Berlin-Frankfurt-Munster, DDI: double delayed intensification, DOXO: Doxorubicin, IDA: Idarubicin, CPM: Cyclophosphamide.
Table II.
BMI distribution before and after therapy.
| Total 1,017 | Males 572 | Females 445 | |
|---|---|---|---|
| Pre-treatment | |||
| Underweight - BMI < 5th percentile | 54 (5%) | 30 (5%) | 24 (5%) |
| Healthy Weight - BMI 5th – 84th percentile | 685 (67%) | 383 (67%) | 302 (68%) |
| Overweight - BMI 85th – 94th percentile | 146 (14%) | 83 (15%) | 63 (14%) |
| Obese - BMI >= 95th percentile | 132 (13%) | 76 (13%) | 56 (13%) |
| At last Maintenance cycle | |||
| Underweight - BMI < 5th percentile | 31 (3%) | 21 (4%) | 10 (2%) |
| Healthy Weight - BMI 5th – 84th percentile | 562 (55%) | 318 (56%) | 244 (55%) |
| Overweight - BMI 85th – 94th percentile | 189 (19%) | 102 (18%) | 87 (20%) |
| Obese - BMI >= 95th percentile | 235 (23%) | 131 (23%) | 104 (23%) |
BMI: Body Mass Index
It was found that 62 subjects (3.9%) experienced greater than or equal to a 20% increase in kilograms (weight) during Induction. Of the patients who experienced this rapid and excessive weight gain, the majority (93%) were not obese at the beginning of therapy.
Univariate Analysis
Of the variables included in the univariate analysis (tables not shown), only BMI z-score at the beginning of Induction was significant in predicting obesity at the end of therapy in both males and females (p = <0.0001). Among males, race was significant (p = 0.049) with Hispanics being most likely to be obese at the end of therapy (OR=1.97) as compared to the reference group of white males. Black males also demonstrated an increased risk for obesity at the end of therapy (OR=1.87). The change in BMI z-score during Induction or during DI cycle 1 was not predictive of obesity at the end of therapy in a univariate analysis (p = 0.98 for Induction and 0.37 for DI).
Multivariate Analysis for Males
The results of the multivariate analysis for the difference in BMI z-score for males using the Induction Model, Table III, show that BMI z-score at the beginning of Induction and the change in BMI z-score during Induction were significant predictors of obesity at the end of therapy after adjusting for the other covariates (age, race, treatment arm, and course when patient finished therapy). The interaction between BMI z-score at the beginning of Induction and the change in BMI z-score during Induction was considered but was not statistically significant (p=0.077). When all other variables were held fixed a one unit increase in the BMI z-score at the beginning of Induction results in a 7.7 fold increased risk of obesity at the end of therapy. When all other variables are held fixed, a one unit increase in the difference in BMI z-score during Induction results in a 3.0 fold increased risk of obesity at the end of therapy (95% confidence interval (CI): 1.90 to 4.84, p<0.0001).
Table III.
Multivariate analysis Induction Model - Males.
| OR | Lower 95% CI | Upper 95% CI | P-value | |
|---|---|---|---|---|
| Age | ||||
| 2–4 years | 1.64 | 0.65 | 4.18 | 0.20 |
| 5–9 years | 2.08 | 0.85 | 5.08 | |
| 10–14 years | 2.35 | 1.06 | 5.24 | |
| 15–20 years | Ref. | - | - | |
| Race | ||||
| Black | 1.06 | 0.37 | 3.01 | 0.53 |
| Hispanic | 1.64 | 0.79 | 3.40 | |
| Other/Unknown | 0.76 | 0.26 | 2.22 | |
| White | Ref. | - | - | |
| Treatment Arm | ||||
| Arm A | 1.64 | 0.41 | 6.48 | 0.85 |
| Arm B | 1.37 | 0.51 | 3.70 | |
| Arm C | 1.96 | 0.49 | 7.91 | |
| Arm D | 1.16 | 0.43 | 3.10 | |
| Augmented BFM DDI Doxo | 1.83 | 0.64 | 5.24 | |
| Augmented BFM DDI IDA/CPM | Ref. | - | - | |
| Completed Therapy | ||||
| Maintenance 10 | 0.87 | 0.27 | 2.76 | 0.86 |
| Maintenance 11 | 0.62 | 0.17 | 2.35 | |
| Maintenance 12 | 0.74 | 0.16 | 3.55 | |
| Maintenance 7–9 | Ref. | - | - | |
| BMI Z-score at beginning of Induction | ||||
| 1 unit increase | 7.69 | 5.23 | 11.3 | <0.0001 |
| Difference BMI Z-score during Induction | ||||
| 1 unit increase | 3.03 | 1.90 | 4.84 | <0.0001 |
OR: Odds Ratio, CI: Confidence Interval, BFM: Berlin-Frankfurt-Munster, DDI: double delayed intensification, DOXO: Doxorubicin, IDA: Idarubicin, CPM: Cyclophosphamide, BMI: Body Mass Index
A second Delayed Intensification model was utilized to predict obesity at the end of therapy in relationship to a change in BMI z-score during cycle 1 of DI, in the context of the covariates. The interaction between BMI z-score at the beginning of Induction and the difference in BMI z-score during DI cycle 1 was considered but not statistically significant (p=0.22). BMI z-score at the beginning of Induction was a significant predictor of obesity at the end of therapy after adjusting for all other covariates. The change in BMI z-score during cycle 1 of DI was not associated with obesity at the end of therapy for males (p = 0.82).
Multivariate Analysis for Females
The Induction Model was then utilized in females to examine obesity at the end of therapy using change in BMI z-score during Induction as a predictor in the context of other covariates. The covariates for the female Induction Model were age, race, treatment arm, BMI z-score at the beginning of Induction and the difference in BMI z-score during Induction. The course when the female patients completed therapy could not be included in the model due to multicollinearity with treatment regimen. The interaction between BMI z-score at the beginning of Induction and the change in BMI z-score during Induction was considered, but was not statistically significant (p=0.51).
The results of the multivariate analysis for females, using the Induction Model, indicate that both BMI z-score at the beginning of Induction and the change in BMI z-score during Induction were significant predictors of obesity at the end of therapy after adjusting for other covariates in the model, Table IV. When all other variables are held fixed a one unit increase in the BMI z-score at the beginning of Induction resulted in a 14.6 fold increased risk of obesity at the end of therapy. When all other variables are held fixed a one unit increase in the difference in BMI z-score during Induction, resulted in a 4.1 (95% CI: 2.32 to 7.43, p<0.0001) fold increased risk of obesity in females at the end of Maintenance.
Table IV.
Multivariate analysis Induction Model - Females.
| OR | Lower 95% CI | Upper 95% CI | P-value | |
|---|---|---|---|---|
| Age | ||||
| 2–4 years | 0.98 | 0.33 | 2.91 | 0.96 |
| 5–9 years | 0.93 | 0.28 | 3.14 | |
| 10–14 years | 0.81 | 0.31 | 2.12 | |
| 15–20 years | Ref. | - | - | |
| Race | ||||
| Black | 2.13 | 0.63 | 7.18 | 0.13 |
| Hispanic | 2.33 | 1.13 | 4.78 | |
| Other/Unknown | 1.39 | 0.40 | 4.76 | |
| White | Ref. | - | - | |
| Treatment Arm | ||||
| Arm A | 1.56 | 0.47 | 5.17 | 0.58 |
| Arm B | 1.29 | 0.36 | 4.66 | |
| Arm C | 2.79 | 0.86 | 9.03 | |
| Arm D | 1.67 | 0.51 | 5.45 | |
| Augmented BFM DDI Doxo | 1.57 | 0.44 | 5.55 | |
| Augmented BFM DDI IDA/CPM | Ref. | - | - | |
| BMI Z-score at beginning of Induction | ||||
| 1 unit increase | 14.62 | 8.38 | 25.53 | <0.0001 |
| Difference BMI Z-score during Induction | ||||
| 1 unit increase | 4.15 | 2.32 | 7.43 | <0.0001 |
OR: Odds Ratio, CI: Confidence Interval, BFM: Berlin-Frankfurt-Munster, DDI: double delayed intensification, DOXO: Doxorubicin, IDA: Idarubicin, CPM: Cyclophosphamide, BMI: Body Mass Index
A second multivariate model, the DI model, was used to predict obesity at the end of therapy in female patients to determine if the change in BMI z-score during cycle 1 of DI was predictive of obesity in the context of the covariates. The interaction between BMI z-score at the beginning of Induction and the change in BMI z-score during DI cycle 1 were also considered, but not statistically significant (p=0.78). The change in BMI z-score during cycle 1 of DI was not associated with obesity at the end of therapy for females (p = 0.32).
Discussion
The primary aim of this study was to determine if early weight gain in children undergoing treatment for ALL was associated with obesity at the end of therapy. The important finding is that weight gain during the first four weeks of ALL therapy is predictive of obesity at the end of therapy in high risk ALL patients. This finding held true for both males and females. These findings extend prior work using a similar patient dataset from COG study 1961 [14]. This prior research demonstrated the change in weight patterns during therapy and identified factors (age, race, and baseline weight) that were predictive of obesity at the end of therapy. This current study differs in that it focused on early weight gain, described as change in BMI z-scores, during two cycles of ALL therapy (Induction and DI cycle 1) as predictors of obesity at the end of therapy.
Rapid weight gain is often observed clinically during ALL Induction therapy and is most likely related to the glucocorticoids that are administered. For protocol 1961, this entailed a 28 day course of Prednisone (60 mg/m2 per day) during Induction, followed by a 10 day taper. Although this was an older treatment protocol, it is important to note that current childhood ALL studies for higher risk patients utilize similar Induction strategies with either 28 days of 60 mg/m2 per day of Prednisone (for patients ≥ 10 years of age) or 14 days of 10 mg/m2 per day of Dexamethasone (for patients < 10 years of age). Current COG standard risk ALL studies, utilize Dexamethasone at 6mg/m2 per day on days 1–28 of Induction.
Although there are numerous other studies that describe weight gain during ALL therapy as well as the presence of obesity in ALL survivors, this is the first known study that identifies weight gain during Induction as a potential predictor for later obesity. Other studies have previously identified early medication related weight gain as a predictor of later weight status. For example, early weight gain with Risperidone use in children and adolescents has been shown to be predictive of weight status at six months [23]. Another study found that early weight gain, in adolescents on depot medroxprogesterone acetate, predicted increased BMI percentile at 18 months [24].
Within the present study, it was found that 3.9% of patients experienced greater than or equal to a 20% increase in body weight during Induction. Of the patients who experienced this rapid and excessive weight gain, the majority (93%) were not obese at the beginning of therapy. These findings suggest that non-obese patients at the initiation of treatment are at greatest risk for excessive weight gain early in therapy.
The exact mechanisms by which rapid weight gain predisposes to higher BMI status later in life are still unknown. In younger children, this finding may be related to adiposity rebound [25]. For older children and adolescents, the underlying mechanism for additional weight gain may be related to genetics or perhaps to systemic changes secondary to adipocyte cytokine production and systemic inflammation [26].
Another aim for this study was to determine if change in BMI z-scores during cycle 1 of DI ALL therapy was associated with obesity at the end of treatment. The DI cycle was selected because Dexamethasone was administered for 14 days during this course of therapy (10 mg/m2 per day on days 0–6 and 14–20). Dexamethasone has been shown, in some studies, to be more strongly associated with weight gain than Prednisone [27,28]. The hypothesis that weight gain during the DI cycle of therapy would be predictive of obesity at the end of Maintenance was not supported. Weight gain, during DI, may have been offset by the treatment related toxicities (such as gastrointestinal toxicities). Subjects on arms A & C of CCG 1961 did not receive a second cycle of DI, while the other treatment arms did. This was considered during the analysis and no statistical differences were observed.
The overall significance of this study is that it offers evidence that monitoring weight trends during Induction, at least in the high risk ALL population, may help predict the presence of obesity at the end of therapy. Patients’ heights and weights are routinely measured during therapy. These measurements, along with the patient’s age and gender, can be entered into a free on line calculator (such as http://stokes.chop.edu/web/zscore/) for easy determination of BMI percentile scores and BMI z-scores. These numbers can then be tracked during therapy to quickly identify changes and weight trends.
Additionally, this study reinforces findings from previous studies in which increased BMI at the beginning of therapy is a strong predictor of increased BMI at the end of therapy [5,14,15,29–31]. Obesity that is present at the onset of therapy should be used to initiate early family intervention aimed at assisting the child to normalize weight gain during treatment. Family counseling regarding nutrition and physical activity should begin soon after diagnosis and continue throughout therapy.
This study is primarily limited by virtue of being a secondary data analysis. The inherent limitation with these studies is that not all confounding variables can be controlled, which may weaken internal validity. In this study, the diets and physical activity patterns of the child were not captured, nor were family history of obesity or parental BMI. Elevated maternal BMI has been noted as a risk factor for obesity in ALL survivors [30, 32] while other researchers report that an elevated BMI status of both parents is associated with an increased BMI in the child at the time of ALL diagnosis [5]. It remains to be determined if these correlations in weight between parent and child are genetic or behavioral in nature. Regardless, the unreported parental BMI status for this analysis is a study limitation.
Additionally, the data collection forms used for this study were completed at the end of each cycle of therapy. As a retrospective study, there is no means available to verify that the correct heights and weights were entered or that they were entered from the appropriate time points. Although this study had significant patient numbers, from geographically varied institutions, it is limited in that it only included those treated for higher-risk ALL.
Conclusion
Obesity is a health problem that is common within children treated for ALL. Within this study, obesity was present in 23% of childhood leukemia patients by the end of therapy while an additional 20% ended therapy overweight. This means that nearly half of ALL patients ended therapy with an unhealthy weight status. Although obesity is prevalent in ALL patients, there are few studies that offer insight regarding how to best intervene or treat.
Most researchers agree that prevention is the best form of treatment for obesity as it is difficult to “cure” or reverse once it is present. Findings from this current study suggest that monitoring weight trends during Induction, through changes in BMI z-scores, may be useful in guiding healthcare practitioners to identify which patients are at increased risk for obesity development during therapy. This may be particularly important for children who do not begin therapy obese. Monitoring early changes in weight may assist in intervening sooner to reduce morbidity and mortality associated with obesity in the pediatric ALL population.
Acknowledgments
Financial support of this study was received from American Cancer Society Doctoral Degree Scholarship in Cancer Nursing (Grant 119073-DSCN-10-090-01-SCN) and National Cancer Institute - Children’s Oncology Group Chair’s Grant (U10CA098543).
Footnotes
Conflict of Interest: No competing financial or other conflicts of interest exist for any of the authors.
References
- 1.Howlader NN, Krapcho AM, Garshell M, Neyman J, Altekruse N, Kosary SF, Yu CL, Ruhl M, Tatalovich J, Cho Z, Mariotto H, Lewis A, Chen DR, Feuer HS, Cronin EJ, KA . SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; 2013. http://seer.cancer.gov/csr/1975_2010/results_merged/sect_28_childhood_cancer.pdf. [Google Scholar]
- 2.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood. 2008;111(12):5515–5523. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers PC, Meacham LR, Oeffinger KC, et al. Obesity in pediatric oncology. Pediatr Blood Cancer. 2005;45(7):881–891. doi: 10.1002/pbc.20451. [DOI] [PubMed] [Google Scholar]
- 4.Chow EJ, Pihoker C, Hunt K, et al. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110(10):2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 5.Esbenshade AJ, Simmons JH, Koyama T, et al. Obesity and insulin resistance in pediatric acute lymphoblastic leukemia worsens during maintenance therapy. Pediatr Blood Cancer. 2013;60(8):1287–1291. doi: 10.1002/pbc.24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reilly JJ, Methven E, McDowell ZC, et al. Health consequences of obesity. Arch Dis Child. 2003;88(9):748–752. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berenson GS Bogalusa Heart Study g. Health consequences of obesity. Pediatr Blood Cancer. 2012;58(1):117–121. doi: 10.1002/pbc.23373. [DOI] [PubMed] [Google Scholar]
- 8.Kanellopoulos A, Hamre HM, Dahl AA, et al. Factors associated with poor quality of life in survivors of childhood acute lymphoblastic leukemia and lymphoma. Pediatr Blood Cancer. 2013;60(5):849–855. doi: 10.1002/pbc.24375. [DOI] [PubMed] [Google Scholar]
- 9.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 10.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008;122(6):1418–1421. doi: 10.1002/ijc.23176. [DOI] [PubMed] [Google Scholar]
- 11.Castillo JJ, Reagan JL, Ingham RR, et al. Obesity but not overweight increases the incidence and mortality of leukemia in adults: a meta-analysis of prospective cohort studies. Leukemia research. 2012;36(7):868–875. doi: 10.1016/j.leukres.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1244–1259. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butturini AM, Dorey FJ, Lange BJ, et al. Obesity and outcome in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2007;25(15):2063–2069. doi: 10.1200/JCO.2006.07.7792. [DOI] [PubMed] [Google Scholar]
- 14.Withycombe JS, Post-White JE, Meza JL, et al. Weight patterns in children with higher risk ALL: A report from the Children’s Oncology Group (COG) for CCG 1961. Pediatr Blood Cancer. 2009;53(7):1249–1254. doi: 10.1002/pbc.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan M, Lin CH, Torno L. Risk factors for obesity and time frame of weight gain in non-irradiated survivors of pediatric acute lymphoblastic leukemia. Journal of Cancer Therapy. 2013;4:124–132. [Google Scholar]
- 16.Fuemmeler BF, Pendzich MK, Clark K, et al. Diet, physical activity, and body composition changes during the first year of treatment for childhood acute leukemia and lymphoma. J Pediatr Hematol Oncol. 2013;35(6):437–443. doi: 10.1097/MPH.0b013e318279cd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy AJ, Wells JC, Williams JE, et al. Body composition in children in remission from acute lymphoblastic leukemia. Am J Clin Nutr. 2006;83(1):70–74. doi: 10.1093/ajcn/83.1.70. [DOI] [PubMed] [Google Scholar]
- 18.Reilly JJ, Brougham M, Montgomery C, et al. Effect of glucocorticoid therapy on energy intake in children treated for acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2001;86(8):3742–3745. doi: 10.1210/jcem.86.8.7764. [DOI] [PubMed] [Google Scholar]
- 19.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111(5):2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Field AE, Laird N, Steinberg E, et al. Which metric of relative weight best captures body fatness in children? Obes Res. 2003;11(11):1345–1352. doi: 10.1038/oby.2003.182. [DOI] [PubMed] [Google Scholar]
- 21.Must A, Anderson SE. Body mass index in children and adolescents: considerations for population-based applications. International Journal of Obesity. 2006;30(4):590–594. doi: 10.1038/sj.ijo.0803300. [DOI] [PubMed] [Google Scholar]
- 22.SAS Institute. SAS/STAT 9.1 User’s Guide. Cary, N.C: SAS Institute, Inc; 2004. [Google Scholar]
- 23.Martin A, Landau J, Leebens P, et al. Risperidone-associated weight gain in children and adolescents: a retrospective chart review. J Child Adolesc Psychopharmacol. 2000;10(4):259–268. doi: 10.1089/cap.2000.10.259. [DOI] [PubMed] [Google Scholar]
- 24.Bonny AE, Secic M, Cromer B. Early weight gain related to later weight gain in adolescents on depot medroxyprogesterone acetate. Obstet Gynecol. 2011;117(4):793–797. doi: 10.1097/AOG.0b013e31820f387c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor RW, Williams SM, Carter PJ, et al. Changes in fat mass and fat-free mass during the adiposity rebound: FLAME study. IJPO. 2011;6(2–2):e243–251. doi: 10.3109/17477166.2010.549488. [DOI] [PubMed] [Google Scholar]
- 26.Power C, Miller SK, Alpert PT. Promising new causal explanations for obesity and obesity-related diseases. Biological Research for Nursing. 2007;8(3):223–233. doi: 10.1177/1099800406292674. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed SF, Tucker P, Mushtaq T, et al. Short-term effects on linear growth and bone turnover in children randomized to receive prednisolone or dexamethasone. Clin Endocrinol. 2002;57(2):185–191. doi: 10.1046/j.1365-2265.2002.01580.x. [DOI] [PubMed] [Google Scholar]
- 28.Van Dongen-Melman JE, Hokken-Koelega AC, Hahlen K, et al. Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatr Res. 1995;38(1):86–90. doi: 10.1203/00006450-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Razzouk BI, Rose SR, Hongeng S, et al. Obesity in survivors of childhood acute lymphoblastic leukemia and lymphoma. J Clin Oncol. 2007;25(10):1183–1189. doi: 10.1200/JCO.2006.07.8709. [DOI] [PubMed] [Google Scholar]
- 30.Asner S, Ammann RA, Ozsahin H, et al. Obesity in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51(1):118–122. doi: 10.1002/pbc.21496. [DOI] [PubMed] [Google Scholar]
- 31.Love E, Schneiderman JE, Stephens D, et al. A cross-sectional study offoverweight in pediatric survivors of acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2011;57(7):1204–1209. doi: 10.1002/pbc.23010. [DOI] [PubMed] [Google Scholar]
- 32.Shaw MP, Bath LE, Duff J, Kelnar CJ, Wallace WH. Obesity in leukemia survivors: the familial contribution. Pediatric Hematology and Oncology. 2000;17(3):231–237. doi: 10.1080/088800100276406. [DOI] [PubMed] [Google Scholar]