Abstract
Introduction
Women at high risk for ovarian cancer due to BRCA1 or BRCA2 mutation or family history are recommended to undergo risk-reducing salpingo-oophorectomy (RRSO) after age 35 or completion of childbearing. This potentially life-saving surgery leads to premature menopause, frequently resulting in distressing and unaddressed sexual dysfunction.
Aim
To pilot a novel sexual health intervention for women with BRCA1/2 mutations who previously underwent RRSO a using a single-arm trial. Feasibility and primary outcomes including sexual dysfunction and psychological distress were assessed.
Methods
This single-arm trial included a one-time, half-day educational session comprised of targeted sexual health education, body awareness and relaxation training, and mindfulness-based cognitive therapy strategies, followed by two sessions of tailored telephone counseling. Assessments were completed at baseline and two months post-intervention.
Main Outcome Measure
Study endpoints include feasibility and effectiveness as reported by the participant.
Results
Thirty-seven women completed baseline and post-intervention assessments. At baseline, participants had a mean age of 44.4 (SD=3.9) years and mean duration of 3.8 (SD=2.7) years since RRSO. Overall sexual functioning (P=.018), as well as desire (P=.003), arousal (P=.003), satisfaction (P=.028), and pain (P=.018) improved significantly. There were significant reductions in somatization (P=.029) and anxiety scores (P<.001), and, overall, for the Global Severity Index (P<.001) of the BSI. Sexual self-efficacy and sexual knowledge also improved significantly from baseline to post-intervention (both P<.001). Women were highly satisfied with the intervention content and reported utilizing new skills to manage sexual dysfunction.
Conclusions
This intervention integrates elements of cognitive-behavioral therapy with sexual health education to address a much-neglected problem after RRSO. Results from this promising single-arm study provide preliminary data to move toward conducting a randomized-controlled trial.
Keywords: Risk-reducing salpingo-oophorectomy and sexual dysfunction, Sexual health and cancer, quality of life and cancer
INTRODUCTION
Women with a deleterious mutation in BRCA1/2 are at significantly increased risk for ovarian cancer and effective screening to detect ovarian cancer at an early stage is not available [1]. High risk women are thus advised to undergo risk-reducing salpingo-oophorectomy (RRSO) between ages 35 and 40 years or after completion of childbearing [2]. RRSO leads to an 85% reduction in BRCA1-associated gynecological cancer risk and 72% reduction in BRCA2-associated breast cancer risk [3].
However, RRSO results in surgically-induced menopause, which is related to significantly compromised sexual function [4–6]. Symptoms include decreased desire, sexual discomfort, and diminished sexual satisfaction [7]. Sexual side effects are the most frequently cited post-RRSO concern of mutation carriers [8, 9], and women are surprised by the magnitude of the impact of RRSO on quality of life [10]. Surgically-induced menopause and sexual dysfunction are also related to changes in self-image [10] decreased self-efficacy [9, 11] and increased depressive symptoms [5]. Evidence also suggests that time since RRSO may not lead to diminution of sexual problems [4].
Unfortunately, effective interventions to manage sexual dysfunction after RRSO are lacking [12]. Although hormone replacement therapy (HRT) has been shown to mitigate menopause-related vasomotor symptoms [6], it does not appear that HRT similarly alleviates sexual problems post-RRSO [13]. It has been shown that both women who take and do not take HRT post-RRSO report comparable levels of diminished sexual functioning [6].
Although there are no established treatments for these distressing sexual problems post-RRSO, recent psychoeducational interventions that have successfully improved women’s sexual problems after cancer support the development of a psychosexual intervention for this population [14–16]. Psychosexual counseling paired with an educational intervention has been shown to improve overall sexual function in female cancer survivors [16] and a mindfulness-based cognitive intervention improved sexual desire/arousal in women treated for gynecological cancer [14].
AIMS
Guided by an integrative treatment model for addressing sexual dysfunction after cancer [17], our aim was to bring together targeted sexual health education [16, 18] with elements of mindfulness-based cognitive therapy [19] in order to improve sexual function and decrease distress in women who had previously undergone RRSO. Of note, previous validated psychosexual interventions have been high in intensity, ranging from 5 to 13 sessions [14, 20, 21], which can limit dissemination and availability in non-research settings. Building upon the utility and efficacy of group education [22], an additional aim was to develop a brief, low-intensity intervention for these women.
METHODS
Participants and Recruitment
Potential participants were identified through clinics for women at high risk for ovarian and breast cancer from three Boston-area hospitals as well as gynecological surgeons and patient databases at these institutions. Recruitment letters were sent to 259 women; letters contained a toll-free opt-out telephone number and a participation form. Study information was also disseminated through community newsletters from FORCE (Facing Our Risk of Cancer Empowered), a national advocacy group for BRCA1/2 women. Through Boston, Washington, DC, and Philadelphia FORCE chapter newsletters, an additional 31 women contacted study investigators for more information. Ninety-six women who expressed interest in the study or did not opt-out were contacted by telephone and screened for eligibility. Of 96 women screened, 77 were eligible and 43 enrolled in the study. In order to meet study eligibility, women needed to be <50, English-speaking, have undergone RRSO for ovarian cancer risk reduction prior to enrollment and endorse at least one distressing symptom of sexual dysfunction on the Sexual Problem Subscale of the Sexual Function Questionnaire [23]. Exclusion criteria included history of ovarian cancer, pelvic radiation, or chemotherapy within the previous year. Women with history of other cancers were not excluded unless active treatment had ended less than one year prior. Primary reasons for declining participation included inconvenient timing, being too busy and distance. The study response rate was comparable to other interventions for female sexual dysfunction after cancer [14, 16].
Intervention Design and Content
The trial was designed to: (1) test feasibility of the intervention; and (2) improve subject satisfaction with sexual function, increase knowledge about sexual function after RRSO, increase perceived self-efficacy to manage sexual side effects, and decrease psychological distress. Content was composed of sexual health education after RRSO, body awareness and relaxation training, and instruction in mindfulness-based cognitive therapy strategies, e.g., managing automatic negative thoughts and maladaptive changes in self image. This intervention was designed to be useful regardless of partner status.
The intervention was comprised of a single, half-day psychoeducational group session, take-home educational materials, and two follow-up tailored telephone counseling calls. Patient-reported assessments of sexual health and psychological functioning were completed in-person immediately prior to the psychoeducational session (baseline) and by mail two months following the session (post-intervention). Upon completion of the post-intervention measures, participants received a $20 gift certificate. The group sessions were conducted in two metropolitan areas, with three groups conducted in Boston and one group conducted in Philadelphia. Written informed consent was obtained from all participants at the group sessions. All procedures were approved by the institutional review board.
Psychoeducational Session
The 3.5-hour group session was structured around three modules facilitated by the lead investigator (S.B), a clinical psychologist and expert in sexual rehabilitation after cancer treatment. Module 1 focused on pyschoeducation about RRSO-related sexual problems, including improving vaginal health. Module 2 focused on relaxation training and body awareness; key components of sexual rehabilitation therapy. Module 3 was guided by principles of mindfulness-based cognitive therapy, such as addressing negative assumptions related to sexual self-esteem. In the final portion of the group session participants create an individual action plan. Women completed a “Next Steps” worksheet which identified the problems they wanted to address, such as decreasing vaginal discomfort or improving desire and at least two actionable steps which they could take based on what they had learned, such as starting regular use of a vaginal moisturizer or practicing a body scan relaxation exercise. It was explained that the worksheet would be reviewed during the upcoming phone call.
Take-Home Educational Materials
Educational materials, distributed during the group session, were selected to help consolidate information from the group session. Materials included instructions for mindfulness-based body scan [24] and muscle relaxation exercises [25], and information about vaginal dilators, personal products, and resources for sexual health websites and books.
Telephone Counseling
At approximately two weeks and four weeks after the psychoeducational session, tailored, individual telephone counseling was provided to each participant by the lead investigator of the study (S.B.). During the first telephone counseling session, remaining questions from the psychoeducational group were addressed and women’s’ action plans were reviewed. Counseling addressed any challenges or barriers and modified the action plan if needed. The aim of the first telephone session was to help women move their personalized goals forward; the aim of the second, “booster” telephone session was to help women review and consolidate their progress, as well as to plan for maintenance moving ahead.
Feasibility and Program Evaluation
Feasibility of the intervention was assessed by rates of: (1) recruitment (at least 45% proportion of eligible women enrolled); and (2) completion of group intervention (at least 70%). Participants’ program evaluation was collected to provide a measure of acceptability of the intervention.
MAIN OUTCOME MEASURES
Demographic and Medical Information
At baseline, patients completed a questionnaire about sociodemographics and medical history.
Female Sexual Function Index (FSFI)
The FSFI measures sexual functioning over the past four weeks. The 19-item FSFI comprises five domains: (1) desire; (2) lubrication; (3) orgasm; (4) pain; and (5) satisfaction. Items are rated on a Likert Scale, and scores range from 2 to 36, where a higher score indicates better sexual function. Studies have supported the reliability and validity of the FSFI total and domain scores in both non-cancer [26] and cancer survivor populations [27]. A total score under 26.55 is the cutoff score indicating clinically significant sexual dysfunction [28].
Brief Symptom Inventory-18 (BSI-18)
The BSI-18 is a well-validated brief screen of psychological distress [29]. The 18 items form a Global Severity Index, as well three subscales: (1) somatization; (2) depression; and (3) anxiety. Each item contains a 5-point scale. A higher score indicates increased psychological distress.
Sexual Self-Efficacy Scale
The sexual self-efficacy scale used in the present study was adapted from the Painful Intercourse Self-Efficacy Scale [30] to measure sexual self-efficacy after RRSO. Participants rated their perceived certainty or confidence to address sexual side effects of RRSO. Scores range from 10 to 100, with higher scores indicating greater self-efficacy.
Sexual Knowledge Scale
Ten true/false items developed for this study assessed sexual knowledge after RRSO. Items reflect information necessary for successful management of sexual dysfunction post-RRSO. Items were modeled from a previous 12-item sexual knowledge questionnaire used to assess successful adjustment after gynecological cancer [31]. A total summary score is derived from the number of items answered correctly with higher scores indicating greater knowledge.
Participant Satisfaction
Participants completed a 12-item satisfaction rating form about the group session and its components following the group session and at two-month post-intervention. Participants also rated the intervention content in terms of understandability and overall helpfulness. Items were rated on a 5-point Likert-scale.
Statistical Analyses
Descriptive statistics were calculated for participants’ demographic and medical information, FSFI, BSI-18, sexual self-efficacy scale, and sexual knowledge scale, as well as for participant satisfaction measures. Differences in baseline and post-intervention scores of the FSFI, BSI-18, sexual self-efficacy scale, and sexual knowledge scale were examined using the paired samples t-test. Analyses were repeated with the non-parametric Wilcoxon signed rank test. Results from non-parametric analyses were consistent with t-tests, and therefore, not included in this report. All P-values were 2-sided and a P-value ≤.05 was considered statistically significant. For outcome measures in which changes were statistically significant, percentage change from baseline to post-intervention was calculated. Magnitude of change between baseline and post-intervention was examined by calculating Cohen’s d as a measure of effect size. An effect size of .20 is considered small, .50 considered moderate, and ≥.80 is considered large [32]. SPSS software (version 20) was used for all analyses.
RESULTS
Recruitment and Study Participation
Of the 96 women who were screened, 77 were interested and met eligibility criteria. Of these 77 potential participants, 34 expressed interest but were unable to attend one of the scheduled group times. Forty-three women enrolled in the study and attended one of the scheduled groups (56% of eligible women screened). Six women did not return the post-intervention assessment, despite reminders, yielding an evaluable sample of 37 women (86% completion rate). Participants who returned the post-intervention assessment did so at an average of 2.3 (SD=.6) months following the psychoeducational session.
Study Sample Characteristics
Demographic and medical characteristics of the 37 women who completed the study are shown in Table 1. At baseline, participants had an average age of 44.4 (SD=3.9; range, 36.8–49.7) years and were on average, 3.8 (SD=2.7; range, .8–12.4) years since RRSO. Table 2 shows participants’ mean scores for the FSFI, BSI-18, sexual self-efficacy scale, and sexual knowledge scale at baseline and post-intervention. At baseline, all women met FSFI classification criteria for having sexual dysfunction (FSFI total score <26.55) [28]. Fourteen women (37%) in our sample had a history of breast cancer. Women with a history of breast cancer were compared with women without a history breast cancer on variables of interest, including sexual function, anxiety, depression sexual self-efficacy, and sexual knowledge at both baseline and follow-up. No differences were detected between groups at either timepoint.
Table 1.
Demographic and medical characteristics of participants (N=37)
| Participant characteristics | M | SD | n | % |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (n=35) | 44.42 | 3.94 | ||
| Race/ethnicity (n=37) | ||||
| White, non-Hispanic | 34 | 91.9 | ||
| Hispanic/Latino | 2 | 5.4 | ||
| Native American/Alaskan Native | 1 | 2.7 | ||
| Marital status (n=37) | ||||
| Married | 30 | 81.1 | ||
| Living as married | 1 | 2.7 | ||
| Single, never married | 1 | 2.7 | ||
| Divorced | 5 | 13.5 | ||
| Education (n=37) | ||||
| High school graduate | 1 | 2.7 | ||
| Completed some college | 3 | 8.1 | ||
| College graduate | 18 | 48.6 | ||
| Postgraduate level training | 15 | 40.5 | ||
| Employment status (n=37) | ||||
| Working full-time | 24 | 64.9 | ||
| Working part-time | 10 | 27.0 | ||
| Full-time homemaker | 3 | 8.1 | ||
| Medical characteristics | ||||
| Years since RRSO (n=36) | 3.84 | 2.69 | ||
| Currently taking hormonal therapy (n=36) | 14 | 38.9 | ||
| Currently taking medication for depression, anxiety, pain, or hot flashes (n=37) | 15 | 40.5 | ||
| History of breast cancer (n=37) | 14 | 37.8 | ||
| Years since breast cancer diagnosis (n=13) | 6.13 | 4.20 | ||
Note. RRSO, risk-reducing bilateral salpingo-oophorectomy.
Table 2.
Change in psychosexual adjustment from baseline to post-intervention (N=37)
| Measure | Baseline
|
Post-Intervention
|
Changea
|
t | P | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ΔM | ΔSD | ||||
| FSFI totalb | 15.63 | 10.23 | 19.54 | 9.47 | 3.91 | 9.12 | 2.50 | .018 | .43 |
| Desire | 1.98 | 1.14 | 2.42 | 1.09 | .44 | .83 | 3.20 | .003 | .53 |
| Arousalc | 2.69 | 1.98 | 3.68 | 1.84 | .98 | 1.86 | 3.17 | .003 | .53 |
| Lubricationc | 2.38 | 2.09 | 2.97 | 2.05 | .58 | 2.03 | 1.72 | .093 | .29 |
| Orgasmc | 2.86 | 2.39 | 3.59 | 2.23 | .73 | 2.32 | 1.90 | .066 | .32 |
| Satisfaction | 2.70 | 1.73 | 3.23 | 1.67 | .53 | 1.40 | 2.29 | .028 | .38 |
| Pain | 2.35 | 2.36 | 3.28 | 2.45 | .93 | 2.28 | .73 | .018 | .41 |
| BSI-18 GSI | 50.89 | 7.99 | 46.97 | 7.52 | 3.92 | 5.94 | 4.01 | <.001 | .66 |
| Somatization | 50.89 | 8.22 | 48.14 | 8.20 | 2.76 | 7.35 | 2.28 | .029 | .38 |
| Depression | 49.76 | 8.48 | 47.54 | 7.31 | 2.22 | 7.72 | 1.75 | .090 | .29 |
| Anxiety | 51.16 | 7.85 | 47.41 | 6.36 | 3.76 | 5.89 | 3.88 | <.001 | .64 |
| Sexual self-efficacy scale | 63.54 | 24.98 | 75.68 | 18.38 | 12.14 | 20.56 | 3.59 | <.001 | .59 |
| Sexual knowledge scalec | 7.97 | 1.63 | 9.06 | 1.17 | 1.08 | 1.50 | 4.33 | <.001 | .72 |
Note. FSFI, Female Sexual Function Index; BSI-18, Brief Symptom Inventory-18; GSI, Global Severity Index.
Change scores are expressed in absolute value;
n=34;
n=36.
Impact of Intervention on Sexual Function and Psychological Distress
As shown in Table 2, participants showed significant improvement in sexual function, as evidenced by increased scores on the FSFI total score (P=.018) and its subscales, including desire (P=.003), arousal (P=.003), satisfaction (P=.028), and pain (P=.018) from baseline to post-intervention. Mean scores on the BSI-18 decreased significantly for the Global Severity Index (P<.001), as well as the somatization (P=.029) and anxiety (P<.001) subscales, indicating significant improvement in psychological distress. Women’s perceived self-efficacy to manage sexual side effects of RRSO, as measured by the sexual self-efficacy scale (P<.001), and knowledge about sexual side effects of RRSO (P<.001) also significantly improved from baseline to post-intervention.
Magnitude of change between baseline and post-intervention varied with moderate to moderately large effect sizes observed on the desire and arousal subscales, the Global Severity Index and anxiety subscale, the sexual self-efficacy scale, and the sexual knowledge scale (d=.53–.72). Small to moderate effect sizes were observed for the FSFI total score, its satisfaction and pain subscales, and the somatization subscale (d=.38–.43). There was improvement in lubrication and orgasm subscale scores with small effect sizes observed (d=.29, .32), though these changes were not statistically significant.
For measures in which baseline and post-intervention scores differed significantly, percentage of participants who improved, stayed the same or worsened between time points is depicted in Figure 1. In order to explore factors associated with lack of response to the intervention, women who improved on the FSFI subscales or total score (n=29) were compared to those who did not improve (n=8) using Mann-Whitney and Chi-square tests. No differences were found on age, time since treatment, history of breast cancer, baseline sexual function, depression, and anxiety scores (all P>.05; results not shown).
Figure 1.
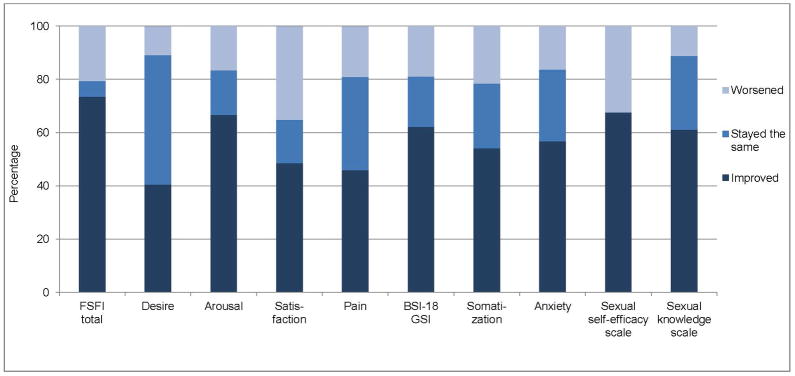
Percentage of participants reporting change from baseline to post-intervention.
Participant Satisfaction
All 37 participants reported by questionnaire that they enjoyed participating in the psychoeducation group session and 100% felt “certain” or “very certain” that they had learned new skills to help cope with sexual side effects of RRSO. Ninety-eight percent of participants felt more empowered to address sexual problems, and 95% of participants reported feeling satisfied with the content. Additional qualitative feedback about the group session reflected that women felt less isolated after the group experience and that the majority of participants began regularly utilizing at least one new strategy identified in their action plan.
CONCLUSIONS
This pilot study demonstrated that the psychosexual intervention significantly improved overall sexual function, reduced psychological distress, and perceived sexual self-efficacy and increased knowledge about sexual side effects and in women with sexual dysfunction who had undergone RRSO following identification as a BRCA1/2 mutation carrier. Considering the practical and logistical needs of younger women, most of whom are working, brevity of the intervention was emphasized. It is also notable that this brief, low intensity intervention produced change with moderate to moderately large effect sizes in several domains including desire, arousal, and anxiety. The brevity of the intervention also contrasts with previous sexual health interventions that are much more time and labor intensive for participants [33] and staff. Promising results from this intervention also support the hypothesis that a brief, multi-modal educational intervention could provide an acceptable format for sexual rehabilitation for women after RRSO. In order to bolster the impact of such a condensed intervention, women were asked to set concrete goals with a viable action plan, which were then addressed during the follow-up telephone calls. Telephone contact provided ‘booster’ reinforcement as well as practical help in addressing ongoing challenges.
Results also showed that the intervention had beneficial impact of reducing psychological distress, most notably, overall distress as well as anxiety. This finding is consistent with the growing evidence that improved sexual function after cancer is correlated with gain in quality of life more generally [34]. Although psychological distress was not the primary focus of the intervention, our data show that women reported a reduction in anxiety and they experienced improvement in both perceived self-efficacy and sexual knowledge. There is a striking dyssynchrony between the psychological experience of “choosing” to have potentially lifesaving prophylactic surgery, which can be very empowering [13, 35], with the subsequent experience of struggling with sexual health consequences that go unaddressed and may seem beyond help [8]. Results from this intervention suggest that when women are given information, skill-based education, and practical strategies for addressing these issues, it is likely that the impact can enhance not only sexual health but quality of life more broadly.
The magnitude of effect sizes that we detected from this single session intervention were comparable to the small to moderate effect sizes that have previously been observed in other more intensive behavioral interventions that also used the FSFI as a primary outcome measure [36]. In the present study, the small effect size of the FSFI total score was likely driven by small effect sizes for particular areas of physical function (e.g., lubrication, orgasm, and pain) in contrast to other domains (e.g., desire and arousal) where moderate effect sizes were found. To understand why women showed greater gains regarding desire and arousal compared to lubrication, orgasm, and pain it is helpful to draw upon the perspective of mindfulness-based cognitive therapy. It has been argued that the essence of mindfulness-based cognitive theory is to help individuals focus on changing the function of psychological events that are experienced, rather than changing the events themselves [37, 38]. In this sample of women who have undergone RRSO, it is unlikely that women would be able to completely ameliorate the physical impact of surgically-induced menopause (e.g., diminished intensity of orgasm, loss of lubrication, subsequent discomfort); however, our results suggest that women can learn to adapt to and positively cope with changes so that other aspects of their sexual experience may significantly improve. Additional exploratory analyses did not identify any variables that were associated with improving sexual function, though these analyses were limited by small sample size.
Limitations of this intervention included the use of a relatively small sample and the lack of a control group. In addition, the sample was mainly composed of white, college-educated women; therefore, it is not possible to generalize findings to other more diverse groups of women. In addition, assessment at two months post-intervention does not allow us to know how the impact of this intervention might be maintained over time. Another limitation was that almost half of the prospective participants who expressed interest were not able to attend one of the scheduled groups. Next steps should be to explore how this intervention may be developed into a more portable and/or accessible platform, such as administration via online format (e.g., webinar).
Nonetheless, our concise intervention produced significant changes in sexual function, psychological distress and anxiety, self-efficacy, and knowledge in women who completed the intervention. Strengths of our study include an innovative approach to addressing sexual dysfunction in a group educational setting that is brief and easily replicable. This study also has other important clinical implications. It is possible that development of an intervention to successfully manage sexual problems post-RRSO may also encourage uptake of RRSO among high risk women whose plans for surgery are delayed or dropped due to concerns about post-surgical sexual dysfunction. If high risk women believe that post-RRSO sexual problems are amenable to intervention, uptake of RRSO might increase, or occur earlier or be less conflicted. In addition, the availability of brief and effective treatment for RRSO-related sexual problems may also serve to encourage providers to address these issues, which are typically not discussed [8]. When providers feel that they have resources to offer patients suffering from treatment-related sexual dysfunction, they are more likely to broach this topic [39]. More immediately, success of this single-arm study provides strong initial evidence for an intervention that can reduce adverse effects in the important area of sexual functioning for younger women who undergo RRSO.
Acknowledgments
Funding: Supported by grant from NIH 1R03 CA153815-01A2.
Footnotes
Conflict of interest statement: The authors report no conflicts of interest.
References
- 1.American Cancer Society. Cancer facts & figures 2013. Atlanta, GA: American Cancer Society, Inc; 2013. [Google Scholar]
- 2.National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian cancer (v 1.2014) National Comprehensive Cancer Network; 2014. [DOI] [PubMed] [Google Scholar]
- 3.Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26:1331–7. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robson M, Hensley M, Barakat R, Brown C, Chi D, Poynor E, et al. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol Oncol. 2003;89:281–7. doi: 10.1016/s0090-8258(03)00072-6. S0090825803000726 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Fang CY, Cherry C, Devarajan K, Li T, Malick J, Daly MB. A prospective study of quality of life among women undergoing risk-reducing salpingo-oophorectomy versus gynecologic screening for ovarian cancer. Gynecol Oncol. 2009;112:594–600. doi: 10.1016/j.ygyno.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finch A, Metcalfe KA, Chiang JK, Elit L, McLaughlin J, Springate C, et al. The impact of prophylactic salpingo-oophorectomy on menopausal symptoms and sexual function in women who carry a BRCA mutation. Gynecol Oncol. 2011;121:163–8. doi: 10.1016/j.ygyno.2010.12.326. [DOI] [PubMed] [Google Scholar]
- 7.Vanchieri C. Risk reduction works for BRCA mutation carriers--with heavy costs. J Natl Cancer Inst. 2005;97:1032–3. doi: 10.1093/jnci/dji228. [DOI] [PubMed] [Google Scholar]
- 8.Campfield Bonadies D, Moyer A, Matloff ET. What I wish I’d known before surgery: BRCA carriers’ perspectives after bilateral salipingo-oophorectomy. Fam Cancer. 2011;10:79–85. doi: 10.1007/s10689-010-9384-z. [DOI] [PubMed] [Google Scholar]
- 9.Matloff ET, Barnett RE, Bober SL. Unraveling the next chapter: sexual development, body image, and sexual functioning in female BRCA carriers. Cancer J. 2009;15:15–8. doi: 10.1097/PPO.0b013e31819585f1. [DOI] [PubMed] [Google Scholar]
- 10.Hallowell N, Baylock B, Heiniger L, Butow PN, Patel D, Meiser B, et al. Looking different, feeling different: women’s reactions to risk-reducing breast and ovarian surgery. Fam Cancer. 2012;11:215–24. doi: 10.1007/s10689-011-9504-4. [DOI] [PubMed] [Google Scholar]
- 11.Davis CS, Zinkand JE, Fitch MI. Cancer treatment-induced menopause: meaning for breast and gynecological cancer survivors. Can Oncol Nurs J. 2000;10:14–21. doi: 10.5737/1181912x1011421. [DOI] [PubMed] [Google Scholar]
- 12.Abbott-Anderson K, Kwekkeboom KL. A systematic review of sexual concerns reported by gynecological cancer survivors. Gynecol Oncol. 2012;124:477–89. doi: 10.1016/j.ygyno.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Finch A, Narod SA. Quality of life and health status after prophylactic salpingo-oophorectomy in women who carry a BRCA mutation: A review. Maturitas. 2011;70:261–5. doi: 10.1016/j.maturitas.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Brotto LA, Erskine Y, Carey M, Ehlen T, Finlayson S, Heywood M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320–5. doi: 10.1016/j.ygyno.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canada AL, Schover LR, Li Y. A pilot intervention to enhance psychosexual development in adolescents and young adults with cancer. Pediatr Blood Cancer. 2007;49:824–8. doi: 10.1002/pbc.21130. [DOI] [PubMed] [Google Scholar]
- 16.Schover LR, Yuan Y, Fellman BM, Odensky E, Lewis PE, Martinetti P. Efficacy trial of an internet-based intervention for cancer-related female sexual dysfunction. J Natl Compr Canc Netw. 2013;11:1389–97. doi: 10.6004/jnccn.2013.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bober SL, Varela VS. Sexuality in adult cancer survivors: challenges and intervention. J Clin Oncol. 2012;30:3712–9. doi: 10.1200/JCO.2012.41.7915. [DOI] [PubMed] [Google Scholar]
- 18.Bober SL, Carter J, Falk S. Addressing female sexual function after cancer by internists and primary care providers. J Sex Med. 2013;10 (Suppl 1):112–9. doi: 10.1111/jsm.12027. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann SG, Sawyer AT, Fang A. The empirical status of the “new wave” of cognitive behavioral therapy. Psychiatr Clin North Am. 2010;33:701–10. doi: 10.1016/j.psc.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JL, Kayser K. A review of couple-based interventions for enhancing women’s sexual adjustment and body image after cancer. Cancer J. 2009;15:48–56. doi: 10.1097/PPO.0b013e31819585df. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell R, Classen C, McGarvey E, Lagana L, Baum L, Duenke SD, et al. Changes in sexual functioning and mood among women treated for gynecological cancer who receive group therapy: a pilot study. J Clin Psychol Med Settings. 2003;10:149–56. [Google Scholar]
- 22.Meyer TJ, Mark MM. Effects of psychosocial interventions with adult cancer patients: a meta-analysis of randomized experiments. Health Psychol. 1995;14:101–8. doi: 10.1037//0278-6133.14.2.101. [DOI] [PubMed] [Google Scholar]
- 23.Syrjala KL, Schroeder TC, Abrams JR, Atkins TZ, Brown WS, Sanders JE, et al. Sexual function measurement and outcomes in cancer survivors and matched controls. J Sex Res. 2000;37:213–25. doi: 10.1080/00224490009552042. [DOI] [Google Scholar]
- 24.Kabat-Zinn J. Full catastrophe living: Using the wisdom of your mind and body to face stress, pain, and illness. New York: Delta Trade Paperbacks; 1990. [Google Scholar]
- 25.Heiman JR, LoPiccolo J. Becoming orgasmic: a sexual and personal growth program for women. New York: Prentice Hall; 1998. [Google Scholar]
- 26.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 27.Baser RE, Li Y, Carter J. Psychometric validation of the Female Sexual Function Index (FSFI) in cancer survivors. Cancer. 2012;118:4606–18. doi: 10.1002/cncr.26739. [DOI] [PubMed] [Google Scholar]
- 28.Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 29.Derogatis LR. BSI 18, Brief Symptom Inventory 18: Administration, Scoring and Procedure Manual. Minneapolis, MN: NCS Pearson, Incorporated; 2001. [Google Scholar]
- 30.Desrochers G, Bergeron S, Khalife S, Dupuis MJ, Jodoin M. Provoked vestibulodynia: psychological predictors of topical and cognitive-behavioral treatment outcome. Behav Res Ther. 2010;48:106–15. doi: 10.1016/j.brat.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Robinson JW, Faris PD, Scott CB. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynecological carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys. 1999;44:497–506. doi: 10.1016/s0360-3016(99)00048-6. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 33.Fruhauf S, Gerger H, Schmidt HM, Munder T, Barth J. Efficacy of psychological interventions for sexual dysfunction: a systematic review and meta-analysis. Arch Sex Behav. 2013;42:915–33. doi: 10.1007/s10508-012-0062-0. [DOI] [PubMed] [Google Scholar]
- 34.Schover LR, Jenkins R, Sui D, Adams JH, Marion MS, Jackson KE. Randomized trial of peer counseling on reproductive health in African American breast cancer survivors. J Clin Oncol. 2006;24:1620–6. doi: 10.1200/JCO.2005.04.7159. [DOI] [PubMed] [Google Scholar]
- 35.Litton JK, Westin SN, Ready K, Sun CC, Peterson SK, Meric-Bernstam F, et al. Perception of screening and risk reduction surgeries in patients tested for a BRCA deleterious mutation. Cancer. 2009;115:1598–604. doi: 10.1002/cncr.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reese JB, Porter LS, Somers TJ, Keefe FJ. Pilot feasibility study of a telephone-based couples intervention for physical intimacy and sexual concerns in colorectal cancer. J Sex Marital Ther. 2012;38:402–17. doi: 10.1080/0092623X.2011.606886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44:1–25. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Segal ZV, Williams JG, Teasdale JD. Mindfulness-based cognitive therapy for depression. 2. New York: Guilford Press; 2012. [Google Scholar]
- 39.Stead ML, Brown JM, Fallowfield L, Selby P. Lack of communication between healthcare professionals and women with ovarian cancer about sexual issues. Br J Cancer. 2003;88:666–71. doi: 10.1038/sj.bjc.6600799. [DOI] [PMC free article] [PubMed] [Google Scholar]


