Abstract
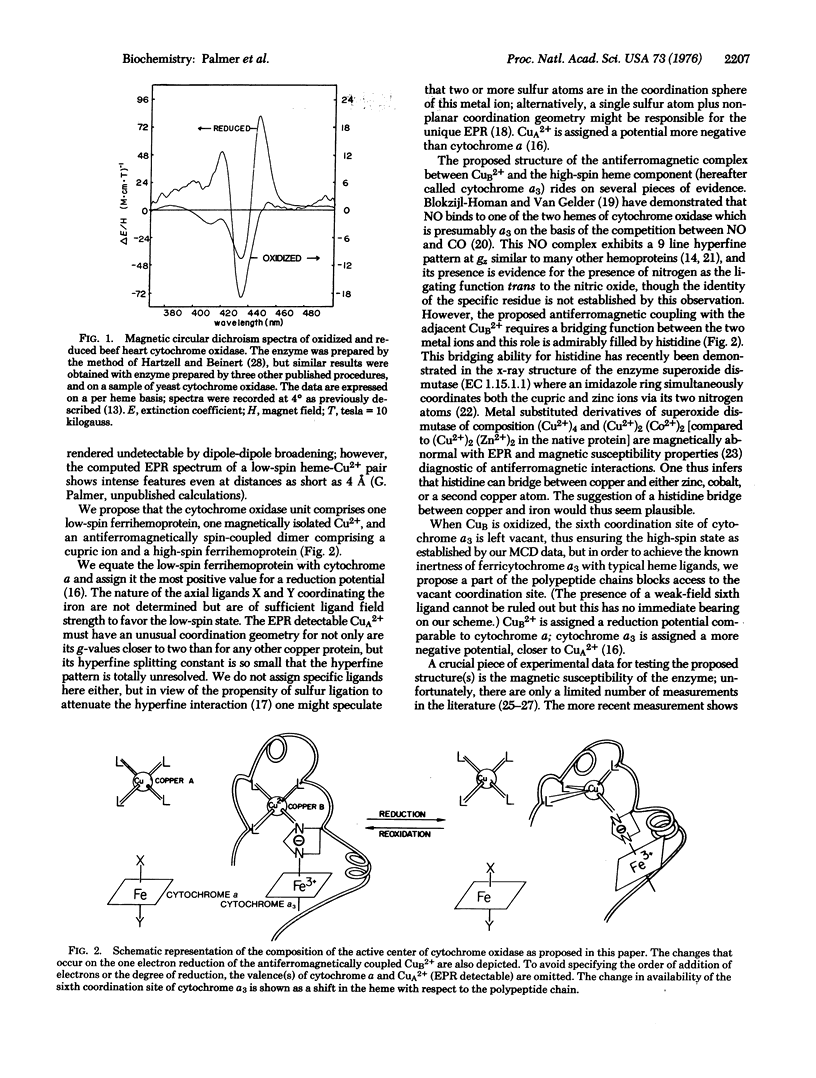
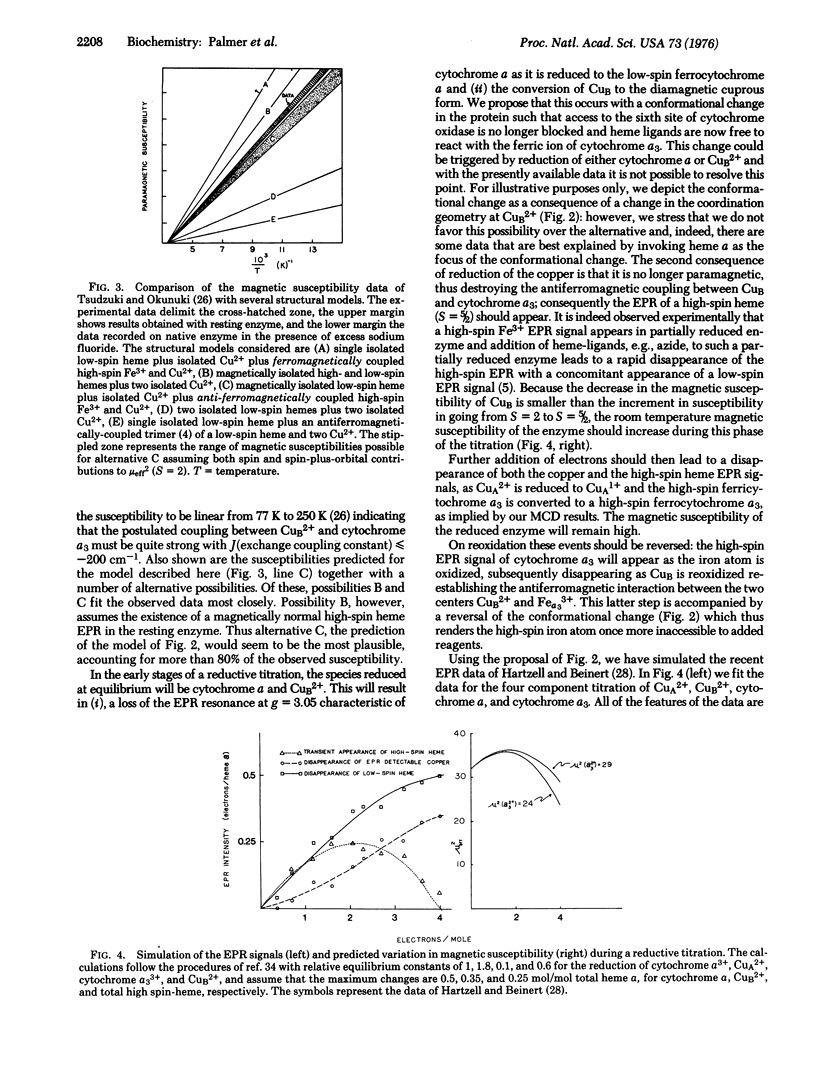
A model is proposed for the active center of cytochrome c oxidase (ferrocytochrome c:oxygen oxidoreductase, EC 1.9.3.1) in which cytochrome a is a low-spin ferrihemoprotein and cytochrome a3 is a high-spin ferrihemoprotein antiferromagnetically coupled to one of the two Cu2+ ions present in the enzyme. It is further proposed that reduction is accompanied by a conformational change in the enzyme thus exposing the sixth coordination site of cytochrome a3 to ligands. With this model it is possible to account for a variety of outstanding observations including the results of magnetic circular dichroism, Mossbauer, and electron paramagnetic resonance spectroscopies, as well as magnetic susceptibility measurements.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini E., Brunori M., Greenwood C., Malmström B. G., Rotilio G. C. The interaction of cyanide with cytochrome oxidase. Eur J Biochem. 1971 Nov 11;23(2):396–400. doi: 10.1111/j.1432-1033.1971.tb01633.x. [DOI] [PubMed] [Google Scholar]
- BEINERT H., GRIFFITHS D. E., WHARTON D. C., SANDS R. H. Properties of the copper associated with cytochrome oxidase as studied by paramagnetic resonance spectroscopy. J Biol Chem. 1962 Jul;237:2337–2346. [PubMed] [Google Scholar]
- Beinert H., Hansen R. E., Hartzell C. R. Kinetic studies on cytochrome c oxidase by combined epr and reflectance spectroscopy after rapid freezing. Biochim Biophys Acta. 1976 Feb 16;423(2):339–355. doi: 10.1016/0005-2728(76)90190-0. [DOI] [PubMed] [Google Scholar]
- Blokzijl-Homan M. F., van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . 3. The EPR spectrum of NO-ferrocytochrome a 3 . Biochim Biophys Acta. 1971 Jun 15;234(3):493–498. doi: 10.1016/0005-2728(71)90215-5. [DOI] [PubMed] [Google Scholar]
- Brill A. S., Bryce G. F. Cupric ion in blue proteins. J Chem Phys. 1968 May 15;48(10):4398–4404. doi: 10.1063/1.1668007. [DOI] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- Fee J. A., Briggs R. G. Studies on the reconstitution of bovine erythrocyte superoxide dismutase. V. Preparation and properties of derivatives in which both zinc and copper sites contain copper. Biochim Biophys Acta. 1975 Aug 19;400(2):439–450. doi: 10.1016/0005-2795(75)90200-7. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. Reactions of cytochrome oxidase with oxygen and carbon monoxide. Biochem J. 1963 Mar;86:541–554. doi: 10.1042/bj0860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H. Oxido-reductive titrations of cytochrome c oxidase followed by EPR spectroscopy. Biochim Biophys Acta. 1976 Feb 16;423(2):323–338. doi: 10.1016/0005-2728(76)90189-4. [DOI] [PubMed] [Google Scholar]
- Hartzell C. R., Hansen R. E., Beinert H. Electron carriers of cytochrome oxidase detectable by electron paramagnetic resonance and their relationship to those traditionally recognized in this enzyme. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2477–2481. doi: 10.1073/pnas.70.9.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. S., Jr, Wilson D. F., Owen C. S., King T. E. Heme-heme interaction in cytochrome c oxidase: the cooperativity of the hemes of cytochrome c oxidase as evidenced in the reaction with CO. Arch Biochem Biophys. 1974 Feb;160(2):476–486. doi: 10.1016/0003-9861(74)90424-x. [DOI] [PubMed] [Google Scholar]
- Lindsay J. G., Wilson D. F. Reaction of cytochrome C oxidase with CO: involvement of the invisible copper. FEBS Lett. 1974 Nov 1;48(1):45–49. doi: 10.1016/0014-5793(74)81058-6. [DOI] [PubMed] [Google Scholar]
- Mackey L. N., Kuwana T., Hartzell C. R. Evaluation of the energetics of cytochrome C oxidase in the absense of cytochrome C. FEBS Lett. 1973 Nov 1;36(3):326–329. doi: 10.1016/0014-5793(73)80402-8. [DOI] [PubMed] [Google Scholar]
- Malmström B. G. Cytochrome c oxidase: some current biochemical and biophysical problems. Q Rev Biophys. 1973 Nov;6(4):389–431. doi: 10.1017/s0033583500001578. [DOI] [PubMed] [Google Scholar]
- Moss T. H., Fee J. A. On the magnetic properties of cobalt substituted bovine superoxide dismutase derivatives. Biochem Biophys Res Commun. 1975 Sep 16;66(2):799–808. doi: 10.1016/0006-291x(75)90580-x. [DOI] [PubMed] [Google Scholar]
- Richardson J., Thomas K. A., Rubin B. H., Richardson D. C. Crystal structure of bovine Cu,Zn superoxide dismutase at 3 A resolution: chain tracing and metal ligands. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1349–1353. doi: 10.1073/pnas.72.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal R. L., Merali Z., Kacew S., Sutherland D. J. Persistence of cadmium-induced metabolic changes in liver and kidney. Science. 1974 Mar 15;183(4129):1094–1096. doi: 10.1126/science.183.4129.1094. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Hirayama Y., Tanaka H., Ishizu K. Copper(II) complex of sulfur-containing peptides. Characterization and similarity of electron spin resonance spectrum to the chromophore in blue copper proteins. J Am Chem Soc. 1975 Sep 17;97(19):5577–5581. doi: 10.1021/ja00852a043. [DOI] [PubMed] [Google Scholar]
- Tiesjema R. H., Muijsers A. O., van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . IV. Some properties of oxygenated cytochrome aa 3 . Biochim Biophys Acta. 1972 Jan 21;256(1):32–42. doi: 10.1016/0005-2728(72)90160-0. [DOI] [PubMed] [Google Scholar]
- Tsudzuki T., Okunuki K. Studies on cytochrome a. XIX. On the subunit structure and spin states of cytochrome oxidase from beef heart muscle. J Biochem. 1971 May;69(5):909–922. doi: 10.1093/oxfordjournals.jbchem.a129542. [DOI] [PubMed] [Google Scholar]
- Van Gelder B. F., Beinert H. Studies of the heme components of cytochrome c oxidase by EPR spectroscopy. Biochim Biophys Acta. 1969 Sep 16;189(1):1–24. doi: 10.1016/0005-2728(69)90219-9. [DOI] [PubMed] [Google Scholar]
- WHARTON D. C. VALENCE STATE OF COPPER AFTER INTERACTION OF THE CYTOCHROME OXIDASE-CARBON MONOXIDE COMPLEX WITH FERRICYANIDE. Biochim Biophys Acta. 1964 Dec 23;92:607–609. doi: 10.1016/0926-6569(64)90022-7. [DOI] [PubMed] [Google Scholar]
- Wever R., Muijsers A. O., van Gelder B. F., Bakker E. P., van Buuren K. J. Biochemical and biophysical studies on cytochrome c oxidase. XI. Reaction with azide. Biochim Biophys Acta. 1973 Oct 19;325(1):1–7. doi: 10.1016/0005-2728(73)90144-8. [DOI] [PubMed] [Google Scholar]
- Wever R., van GELDER B. F., Dervartanian D. V. Biochemical and biophysical studies on cytochrome c oxidase. XX. Reaction with sulphide. Biochim Biophys Acta. 1975 May 15;387(2):189–193. doi: 10.1016/0005-2728(75)90102-4. [DOI] [PubMed] [Google Scholar]



