Abstract
Males typically outperform females on spatial tasks, beginning early in life and continuing into adulthood. This study aimed to characterize age and sex differences in human spatial ability using a virtual Water Maze Task (vWMT), which is based on the classic Morris water maze spatial navigation task used in rodents. Performance on the vWMT and on a task assessing visuospatial perception, Mental Rotations Test (MRT), was examined in 33 adolescents and 39 emerging adults. For the vWMT, significant effects of age and sex were observed for path length in the target region (narrower spatial sampling), and heading error, with emerging adults performing better than adolescents, and an overall male advantage. For the MRT, males scored higher than females, but only in emerging adulthood. Overall, sex differences in visuospatial perception (MRT) emerge differently from those observed on a classic navigation task, with age and sex-specific superior vWMT performance likely related to the use of more efficient strategies. Importantly, these results extend the developmental timeline of spatial ability characterization to include adolescent males and females performing a virtual version of the classic vWMT.
Keywords: adolescence, emerging adulthood, spatial ability, sex differences, hippocampus
1. Introduction
As early as infancy, children have the ability to localize spatial information based on the relationship between an event and environmental features (Keating et al., 1986). There are ample data available from cohorts studied in early childhood (5 years of age and younger) demonstrating the development of place learning (Hupbach et al., 2011; Ribordy et al., 2013; Sluzenski et al., 2004) and scene recognition (Chai et al., 2010) abilities. In general, the ability to process spatial and contextual information appears to mature around age eight on a variety of large-environment spatial tasks (Overman et al., 1996) and around age 9 on a computerized spatial navigation task (Laurance et al., 2003). Not surprisingly, dynamic and rapid structural and functional brain changes beyond childhood and into adolescence (Blakemore, 2012; Paus, 2005; Spear, 2000) underlie significant improvements across a variety of cognitive domains (Casey et al., 2005; Paus, 2005), including spatial ability (Klingberg, 2006; Piper et al., 2011; Sowell et al., 2001). Results from a functional magnetic resonance imaging (fMRI) study of scene complexity demonstrated greater activation of a brain region implicated in spatial processing, the parahippocampal gyrus (PHG), for high-complexity versus low-complexity scenes in healthy participants ages 8 to 24 years (Chai et al., 2010). Differences in brain activation not only increased with age, but also were associated with superior memory formation.
The Morris Water Maze Task (WMT) was developed in 1981 as an alternative to the radial arm maze to study spatial learning in rodents (Wenk, 2004). The Morris WMT has since been used extensively in animal research to probe spatial memory ability (Morris, 1984), with many variants of this task being used over the past 30 years to study the neurobiology (Clark et al., 2013) and neuropharmacology (Cha et al., 2007; Markwiese et al., 1998; Swartzwelder et al., 2014) of spatial learning, as well as for developing neurobiological models of neurocognitive disorders (D'Hooge et al., 2001). Successful spatial learning in the WMT is characterized by execution of (more or less) direct trajectories to a hidden, submerged escape platform from multiple starting locations and persistence in searching at the platform location when it is removed for a probe trial. It is generally accepted that optimal performance in the WMT is based on the use of spatial information provided from visual environmental cues, for example, based on the fixed spatial relationship between the platform and the available visual cues (O'Keefe et al., 1978) or the contributions of visual cues to the selection of trajectories to the platform location (Knierim et al., 2011). The hippocampus plays an integral role in memory function; it is critical for processing spatial and contextual information and for generating and maintaining representations utilized for navigating in space (Jarrard, 1993; O'Keefe et al., 1978; Wegman et al., 2014). To this end, rodents with hippocampal lesions have been shown to exhibit deficits in spatial learning on this task, as evidenced by an inability to efficiently navigate to the hidden platform (Morris et al., 1982; Sutherland et al., 1982). Prominent sex differences also have been reported in rodents on the WMT, with adult male rats demonstrating better navigation to the hidden platform than female counterparts (Keeley et al., 2013; Perrot-Sinal et al., 1996; Roof et al., 1999; Saucier et al., 2008), although some studies report no differences (Bucci et al., 1995). While most examination of developmental differences in performance of the WMT in rodents has been within the context of alcohol or drug challenges (Cha et al., 2007; Markwiese et al., 1998; Sircar et al., 2005), or hormone manipulations (Roof, 1993), there is some limited evidence of age-related improvements in acquisition (reduced escape latency and shorter swim distances) and retention (more time spent in goal quadrant) from adolescence to adulthood (Markwiese et al., 1998).
It has recently become possible to translate WMT findings from preclinical to clinical populations (Hamilton et al., 2009), given the development of virtual versions of the WMT (vWMT) to assess spatial ability in humans (Hamilton et al., 2009; Astur et al., 2002; Driscoll et al., 2005; Herting et al., 2012; Moffat et al., 2002; Newhouse et al., 2007; Sneider et al., 2013; Sneider et al., 2011; Woolley et al., 2010). Although to date no human developmental data are available to assess age-related improvements in performance on the vWMT, a cross sectional comparison of healthy volunteers across a large age span (6 to 67 years of age) demonstrates superior performance in adolescents and younger-adults relative to children and relative to older individuals on a variant of a spatial memory task, the Memory Island task (Piper et al., 2011). Furthermore, effects of age and sex examined in a modified version of the WMT in young adults and older adults (mid-50’s) indicated that young adults performed better than older adults, again with a male advantage being evident in both age groups (Schoenfeld et al., 2010). In a virtual version of the WMT, a study of 8–10 year old prepubertal children likewise showed a male advantage in spatial ability, with boys performing better on the retention trial (probe) than girls (Newhouse et al., 2007). On the other end of the age spectrum, WMT performance declines with aging (Driscoll et al., 2005). In addition, WMT performance examined in combination with functional magnetic resonance imaging (fMRI) has been useful for elucidating underlying neurobiology associated with performance differences in healthy adults and in substance abusers, confirming that the hippocampus shows significant activation during task performance (Sneider et al., 2013). From a translational perspective, impaired spatial navigation on the virtual WMT has been observed in patients with unilateral hippocampal resections (Astur et al., 2002) and in amnesic participants with hippocampal damage (Goodrich-Hunsaker et al., 2010), which is similar to observations found in animal hippocampal lesion studies. Thus, applications of the virtual WMT have been invaluable for investigating spatial learning and memory and for validating rodent models of neurocognitive disorders and treatments for memory disorders in humans (D'Hooge et al., 2001). While new tasks and quantitative statistical approaches for assessing place learning have become available and have been applied to study participants age 18 and older (Furman et al., 2014; Nardi et al., 2011), to date, the virtual WMT has been underemployed for elucidating the neurobiological role of the hippocampus during adolescence, a time when the brain is rapidly developing and when the hippocampus is particularly vulnerable to insults such as alcohol and drug use (Chin et al., 2010), and is structurally and functionally altered in adolescents with depression and other psychiatric conditions (Whittle et al., 2014).
Accordingly, the purpose of the present study is to fill a critical gap in the literature regarding healthy developmental changes in spatial ability, along with an examination of sex differences, using a virtual version of the classic WMT. Given reports that visual perception may influence spatial learning on tests such as the vWMT (Astur et al., 2004), the Mental Rotations Test (MRT) also was administered to assess visuospatial ability. Performance on mental rotation tests likewise demonstrate robust sex differences, with men demonstrating better performance than women (Astur et al., 2004; Linn et al., 1985; Parsons et al., 2004), and sex differences in children as early as kindergarten, with kindergarten boys outperforming age-matched girls on rotational and translational elements of a spatial transformation task (transforming two separate halves of a shape into a whole shape) (Levine et al., 1999) and in preadolescents on house plans, mirror images and 3D object rotation tasks (Kerns et al., 1991). Inclusion of the MRT permits an ability to dissociate whether hypothesized developmental improvements and male-related advantages in spatial ability reflect better memory-related function or better visuospatial perception, or both.
2. Method
2.1. Participants
The study sample consisted of 33 healthy adolescents (ranging in age from 12.3 – 15.0 years, mean age 13.6 ± 0.9 years) and 39 healthy emerging adults (ranging in age from 18.5 – 25.5 years, mean age 21.6 ± 1.7 years). Demographic details are provided in Table 1, including socioeconomic status (SES) (Barratt, 2006) and handedness. Participants were recruited through local advertisement and screened by telephone interview to ensure they met criteria for inclusion in the study. All aspects of the clinical research protocol were reviewed and approved by the Institutional Review Board of McLean Hospital (Belmont, MA, USA). After a complete description of the study, all participants and their parent(s) or guardian(s) (adolescent group only) provided written informed assent or consent. All participants received monetary compensation for study completion.
Table 1.
Participant Demographics
| Adolescents (n= 33) | Young Adults (n= 39) | p | |
|---|---|---|---|
| Age (years) | 13.6 ± 0.9 (range 12.3–15.0) | 21.6 ± 1.7 (range 18.5–25.5) | <.001 |
| Education (years) | 7.5 ± 1.48 | 14.7 ± 1.5 | <.001 |
| SESa | 50.1 ± 11.1 | 50.6 ± 11.0 | .87 |
| Handedness | 28R, 4L, 1A | 37R, 2L | - |
| Female | 52% | 44% | - |
| Ethnicityb | 97% Non-Hispanic | 97% Non-Hispanic | - |
| Racec | 85% Caucasian | 64% Caucasian | - |
Data represent mean values ± SD.
Abbreviations: SES,Socioeconomic status.
SES unavailable for n=1 Adolescent and n=1 Emerging Adult.
Ethnicity: Hispanic vs. Non-Hispanic;
Race: Caucasian vs. Non-Caucasian (classification consisted of Asian, African American, and Other)
2.2. Clinical Assessment
A trained psychologist conducted diagnostic clinical interviews in adolescents using the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS-E) (Puig-Antich et al., 1980) and in emerging adults using the Structured Clinical Interview for the Diagnostic and Statistical Manual (DSM IV) Non-Patient Edition (SCID-I/NP) (First et al., 1997). All participants were free of Axis-I psychiatric diagnoses, neurological illness, and severe medical problems. Participants also completed urine screening to rule out current psychoactive substance use (Triage® Drugs of Abuse Panel: Immediate Response Diagnostics, Biosite, San Diego, CA), as negative urine toxicology results were required for study inclusion in the concurrent neuroimaging study (Silveri et al., 2013). Adolescent participants reported no current or < 3 lifetime alcohol drinking bouts. Emerging adults reported consumption of 1.8 ± 1.2 alcoholic beverages per drinking bout (averaged over the three months prior to study participation). Given that menstrual cycle phase has been reported to influence memory performance in females (McCormick et al., 2001), menstrual cycle phase was recorded for all females participants using self-report: 50.0% of adolescent and emerging adult females were in their follicular phase (cycle days 3–13), 14.7% were in their non-follicular phase (days 14–28), 17.6% of adolescent females had not yet begun cycling or reported less than one month since first menses, and menstrual cycle phase information was unavailable for the remaining female participants.
2.3. Spatial Ability: Virtual Water Maze Task
A PC-compatible laptop (screen resolution: 800 x 600) was used to control the presentation of the virtual environment and vWMT data collection. The vWMT program (Hamilton et al., 2009) was written in C (DH), with a field of view = 50 degrees (first-person perspective). Distances in virtual space were measured in arbitrary units: virtual room 16 (width) x 3 (height) units. Distal room walls only differed by placement of four unique abstract pictures (3 x 3 units) on each wall, which served as landmarks. The circular pool was located in the center of the square room and had a diameter of 3.2 units, with a perimeter wall that extended approximately 0.66 units above the surface of the water. The platform was either hidden or visible as specified in the experiment and was located in the northeast (NE) quadrant for all trials. Navigation was controlled using a joystick, which allowed right, left and forward movement but not backward movement. The fixed full rotation duration and time to traverse the pool at its widest point in a straight line is approximately 2.5s and 4s, respectively. A visual message was displayed on the screen when the platform was located: “Platform found”. If the platform was not located within 60 sec, the platform became visible and the following message was displayed on the screen: “The platform is visible, swim to it”. The inter-trial interval (ITI) was one second (1 sec).
Participants completed a training phase that consisted of four visible platform trials in a virtual environment featuring distinct spatial cues from the experimental virtual environment. Subsequently, participants completed three experimental conditions: Learning (Hidden trials), Retention (Probe trial), and Motor Control (Visible trials). The Learning condition included 16 hidden platform trials (1 trial starting in each quadrant (NW, NE, SW, SE), 4 trials per 4 blocks). The Probe trial lasted for 30 seconds, during which time the platform was removed from the pool, unbeknownst to participants. The Motor Control condition included 8 visible platform trials (1 trial starting in each quadrant (NW, NE, SW, SE), 4 trials per 2 blocks). For additional task details, see (Sneider et al., 2013) .
2.3.1. Dependent Measures: Platform Trials
For both Learning (hidden) and Motor (visible) platform trials, time to enter the platform area and path length were recorded for each trial, 4 trials per 4 blocks (escape latency, sec). Path length of the pool diameter was recorded for each trial block.
2.3.2. Dependent Measures: No-Platform Probe Trial
For analysis purposes, a notional circular region (the critical region) with a diameter equal to 25% of the pool diameter was centered on the platform location used during training (Figure 1). The path length to first enter this region and the total percentage of the overall path length within this region were calculated. Heading error (angular deviation from a direct path from the release point to the platform) was determined at the point where the cumulative path length first exceeded an amount equal to 25% of the pool diameter. The path length to enter and percentage of total path length spent within the quadrant containing the escape platform were also calculated. Collectively, these dependent measures provide information about initial trajectory to the platform location and search persistence at this location on different spatial scales. Chance values for percent of total path length in the quadrant and percent of total path length in the region were approximately 25% and 5%, respectively.
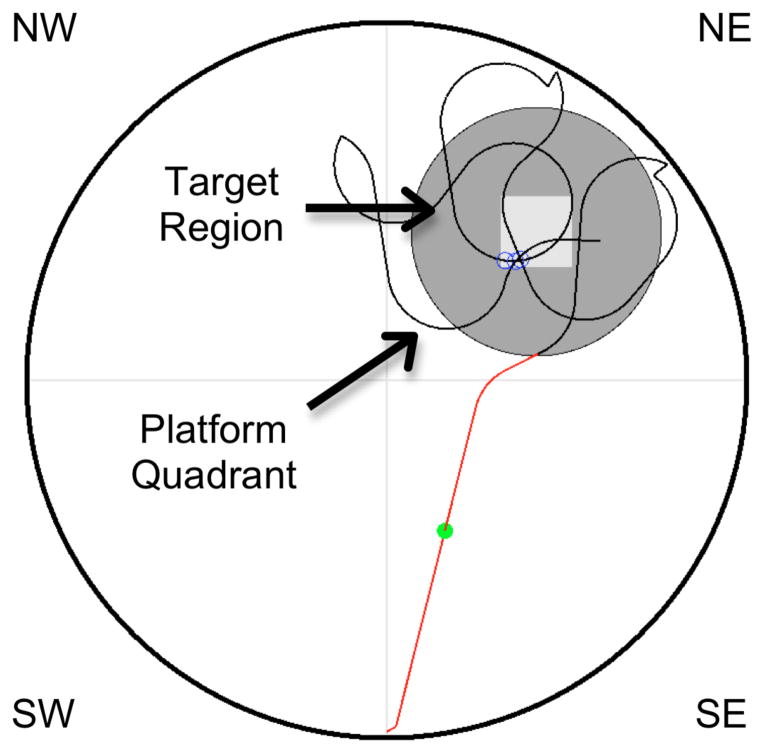
Figure 1.
Schematic representation of the virtual pool, denoting four quadrants (light gray lines: NW, NE, SW, SE). The large gray circle represents the target region, which is 25% of the pool diameter centered on the platform (white square), located in the NE quadrant.
Independent raters blind to participant age and sex determined navigation strategies during performance on the Probe trial to be either: 1) a direct strategy, where participants navigated directly to the platform location from the starting location, or 2) a non-direct strategy, where participants navigated in a circuitous or random route that was not in the direction of the platform quadrant (NE) (Astur et al., 2004). While there are several types of non-direct strategies: zigzag, circuitous, random, landmark, these strategies were coded as “non-direct”. Two coders viewed individual output files of navigation maps produced during task performance to classify strategy. The intraclass correlation coefficient between raters for strategy coding was ICC = 0.95, p < .001.
2.4. Visuospatial Perception: Mental Rotations Test
A paper pencil version of the MRT was administered to assess visuospatial perception (Vandenberg et al., 1978). In this 12-item task, participants had a time limit of four-minutes to match target 3-D cube figures to two of four rotated versions of the reference item. For each item, participants were asked to select the two correct figures from four options. One point is given for each correctly identified rotated figure, with a maximum total score of 24 points. This timed task required participants to visualize how an object would look if it were rotated.
3. Statistical Analyses
For the vWMT, Learning trial data were analyzed using three-way (Age x Sex x Block (4 trials)) mixed-model repeated measures analyses of variance (ANOVAs), with Age and Sex as between-subject variables and Block as a within-subject variable. The primary outcome variables of interest for Learning trial and Motor Control trial analyses were escape latency and path length (expressed as the ratio to the pool diameter). To evaluate Probe trial performance, two-way (Age x Sex) ANOVAs were conducted for path length to enter the target quadrant, path length to enter the target region, the percent path length traveled in the target quadrant and target region, and the initial heading error. Planned analyses were conducted for each “persistence” measure (% path length in region and quadrant) to evaluate whether each group (combination of sex and age) performed significantly above these approximate chance thresholds (25% and 5%) using one sample t-tests for each age/sex, with test values set to 25% and 5%, respectively. Chi-square non-parametric analyses were conducted to compare navigation strategies employed (i.e., direct strategy versus the non-direct strategy) between groups. For the MRT, Total score was analyzed using a two-way (Age x Sex) ANOVA. Follow-up analyses were conducted using paired t tests for repeated measures and ANOVAs for univariate analyses to determine sources of differences when interactions were statistically significant. Effect size (ES) F was calculated for significant main effects and interactions using G*power (Version 3.0.6). Based on previous work (Astur et al., 2004), correlations between age and vWMT and MRT performance were conducted separately for each sex using Pearson’s correlation coefficients. SPSS 18.0 (SPSS, Chicago, IL) was used for all statistical analyses (α=.05).
4. Results
4.1. Demographic and Clinical Variables
No age or sex differences (within each age epoch) were observed for demographic variables (Table 1).
4.2. Spatial Ability: Virtual Water Maze Task
4.2.1. Learning Hidden Platform Trials
Mean performance over trial blocks for latency (sec) and path length/diameter measures for hidden platform trial blocks are presented in Figure 2. A significant main effect of Block was observed for latency (F(3,204) = 25.7, p < .001; ES = .61), with shorter swim latencies observed by Block 4 (H4) on Learning trials (Figure 2, left), as well as an Age x Block interaction for latency (F(3,204) = 2.9, p = .04; ES = .21). Follow-up paired t tests for the interaction demonstrated that while all groups exhibited significant learning, adolescents exhibited shorter latencies between Block 2 and Block 3 (t(32) = 3.74, p=.001), whereas emerging adults exhibited shorter latencies between Block 1 and Block 2 (t(38) = 3.06, p=.004). A significant main effect of Block also was observed for path length/diameter (F(3,204) = 17.1, p < .001; ES = .50), with shorter path length/diameter being observed by Block 3 (t(71)= 3.4, p= .001) (Figure 2, right). No significant main effects of Age or any interactions were observed for path length/diameter, nor were there significant main effects of Sex for either measure.
Figure 2.
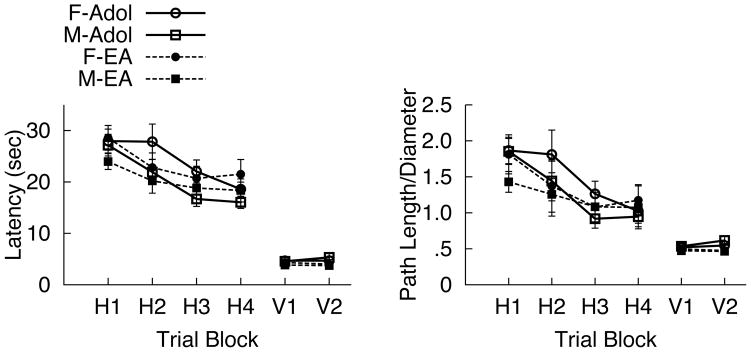
Mean (+SEM) vWMT measures during the training (left) and visible (right) trials for males and females at each age (adolescent v. emerging adult (EA)): A) Latency (sec) across hidden trial blocks (left) and visible trial blocks (right) and B) Path length to enter the platform region across 4 hidden trial blocks (left) and visible trial blocks, (right). Adolescent females and males represented with solid lines and open circles/squares, respectively, and emerging adult females and males represented with dashed lines and closed circles/squares, respectively.
4.2.2. Motor Visible Platform Trials
Mean performance over trial blocks for latency (sec) and path length/diameter measures for visible platform trial blocks are presented in Figure 2. Significant main effects of Age were observed for latency to reach the platform [Adolescent > Emerging Adult; F(1, 67) = 11.66, p = 0.001; ES= 0.42] and path length [Adolescent > Emerging Adult; F(1, 67) = 18.42, p < 0.001; ES=0.53] (Figure 2, left). A significant Age X Block interaction also was observed for path length/diameter [F(1, 67) = 6.03, p = 0.017; ES=0.29]. Post hoc analyses indicated that adolescents showed a trend towards shorter path length/diameter Block 2 (V2) compared to Block 1 (V1) [F(1, 32) = 3.50, p = 0.07; ES=0.33], whereas the main effect of Block reached significance in emerging adults, with significantly shorter path length/diameter observed on Block 2 (V2) versus Block 1 (V1) [F(1, 37) = 5.14, p = 0.029; ES=0.36] (Figure 2, right). No significant main effects of or interactions with Sex were observed.
4.2.3. No-Platform Probe Trial
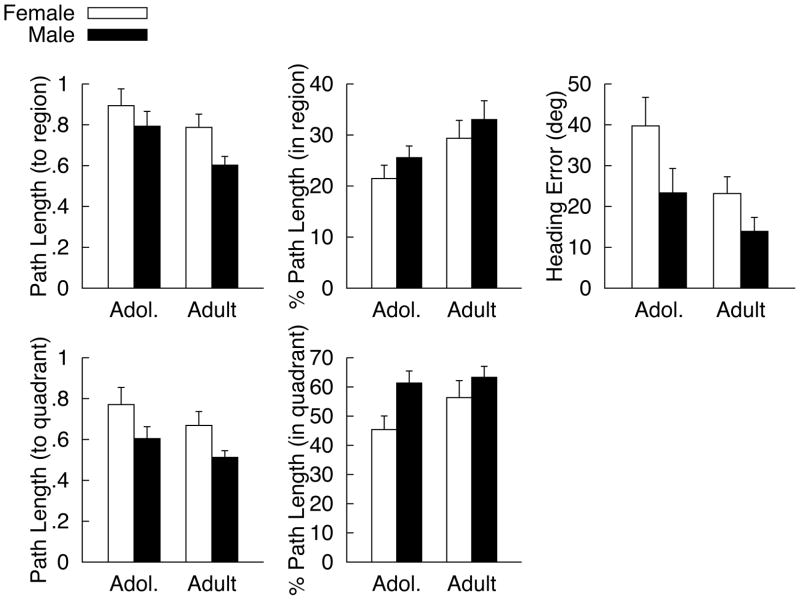
Mean performance for Probe trial dependent measures are presented in Figure 3. There were significant effects of Age for path length to enter the target region [Emerging Adult < Adolescent; F(1, 68) = 5.19, p = 0.026; ES= 0.27], percentage of total path length in the target region [Emerging Adult > Adolescent; F(1, 68) = 5.56, p = 0.021; ES= 0.28], and heading error [Emerging Adult < Adolescent; F(1, 68) = 6.45, p = 0.013; ES=0.30]. Although similar trends were observed for the path length to enter the platform quadrant (Emerging Adult < Adolescent) and percentage of overall path length in the platform quadrant (Emerging Adult > Adolescent), neither reached statistical significance. There were significant Sex effects for path length to enter the target region [Males < Females; F(1, 68) = 4.78, p = 0.032; ES=0.27], heading error [Males < Females; F(1, 68) = 6.26, p = 0.015; ES=0.30], path length to enter the platform quadrant [Males < Females; F(1, 68) = 6.95, p = 0.01; ES=0.32], and percentage of overall path length in the platform quadrant [Males > Females; F(1, 68) = 6.18, p = 0.015; ES=0.30]. Males demonstrated a higher percentage of overall path length in the target region, however, this effect did not reach statistical significance. There also were no significant Age X Sex interactions observed for any measure (all ps > 0.333). Percentage of overall path length was significantly different than chance for all participants for both the region (5%) and the quadrant (25%) analyses (p=.000, for all one sample t-tests).
Figure 3.
Mean (+SEM) vWMT measures during the no-platform probe trial for males and females at each age (adolescent v. emerging adult (EA)): A) path length to enter the platform region, B) percentage of over path length in the platform region, C) heading error, D) path length to enter the platform quadrant, and E) percentage of overall path length spent in the platform quadrant.
4.2.4. Navigation Strategies
No significant Strategy preference was observed on the Probe Trial for adolescents: 52% (n = 17) of adolescents utilized a direct strategy and 48% (n = 16) of adolescents utilized a non-direct strategy to reach the platform (χ2(1,33) = .03, ns). In contrast, emerging adults demonstrated a significant Strategy preference, with a greater percentage of emerging adults using a direct strategy (74%, n = 29) than a non-direct strategy (26%, n = 10) (χ2(1,39) = 9.3, p = .002). In addition, significantly more emerging adult males (69%, n = 20) used a direct strategy relative to emerging adult females (31%, n = 9) (χ2(1,29) = 4.2, p = .04; p=.38). This pattern of sex differences was not observed between male and female adolescents (Figure 4).
Figure 4.
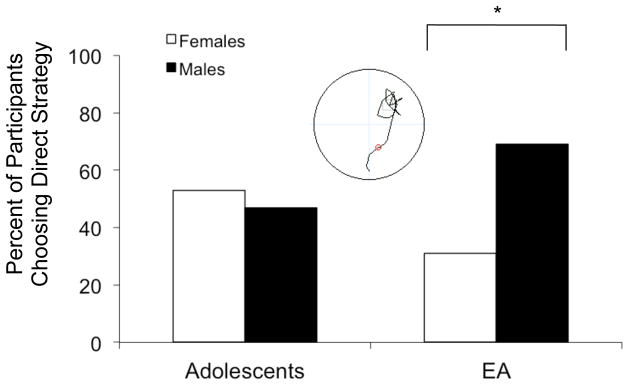
Percent of participants using a direct strategy on the Probe trials, displayed by age group and broken down by sex, adolescent group (left panel) and emerging adults (right panel), *p < .05.
4.3. Spatial Perception: Mental Rotations Test
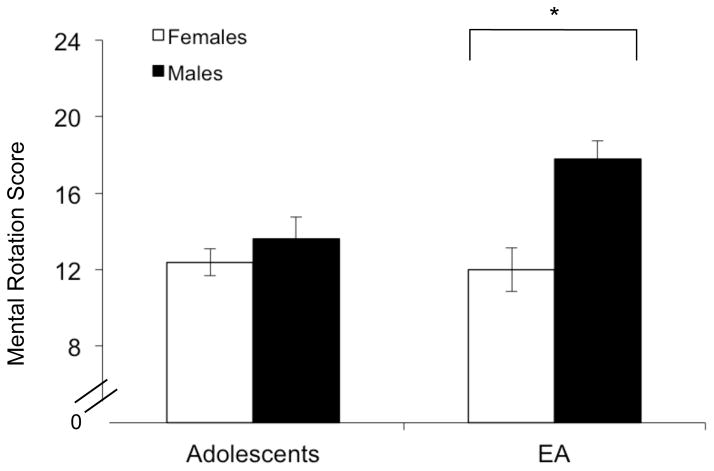
There was a significant main effect of Sex (F (1, 63) = 12.0, p = .001; ES =0.44), and a significant Age x Sex interaction (F (1, 63) = 7.01, p = .01; ES = .33) for total score on the MRT. However, the Age main effect did not reach significance (F(1,63) = 3.3, p = .07). Follow-up analyses revealed that emerging adult males exhibited higher scores than emerging adult females (F (1, 34) = 17.1, p < .001; ES = .72), whereas no differences were observed between adolescent males and females (Figure 5).
Figure 5.
Mean (+SEM) MRT total score in adolescent females and males (left panel, open and closed bars, respectively) and emerging adult females and males (right panel, open and closed bars, respectively), *p < .05.
4.4. Correlations: Age and Performance
Age was significantly correlated with heading error on the vWMT in females (r(34) = −.329, p = .029), but not with any other vWMT measure or with MRT total score. In contrast, in males, Age was significantly correlated with path length to enter the target region (r(38) = −.369, p = .011) and percent path length in the target region (r(38) = .279, p = .045) on the vWMT, and total score on the MRT (r(35) = .521, p = .001). Further, MRT total score was significantly correlated with path length to enter the target region (r(35) = −.333, p = .025), percent path length in the target region (r(35) = .411, p = .007) and heading error (r(35) = −.333, p = .025), and percentage of overall path length in the platform quadrant (r(35) = .292, p = .044) again only in males. However, based on these correlations, when MRT total score was included in mediation analyses as a covariate, it was found not to be significant for any vWMT measure.
5. Discussion
To date, there have been a number of reports examining vWMT performance in adults, as well as a growing number of studies conducted in children. The results of the present study, however, are the first to demonstrate human evidence for age-related improvements and sex-specific differences in spatial performance on the WMT specific to adolescence compared with emerging adults.
All participants demonstrated significant evidence of learning on the vWMT, with a steeper learning curve being observed in emerging adults than in adolescents, i.e., emerging adults reached asymptote at an earlier Block than adolescents during learning/hidden platform trials). Emerging adults also demonstrated better retention of the platform location than adolescents, as indicated on the Probe trial. Collectively, vWMT Probe outcome measures indicate that emerging adults outperformed adolescents in searching near the platform location, on both initial trajectory (path length to enter the target region) and persistence (percent path length within the target region). The pattern of significant results also suggests that measures derived from more narrow spatial scale at the target location are more sensitive to age effects within the sampled range of ages (i.e., measures taken at the level of the entire platform quadrant may not be sufficiently sensitive to detect age effects).
With regard to sex differences, there were no significant differences on learning trials, nor were there significant interactions with sex. However, most measures of navigation during the Probe trial revealed better performance in male participants, albeit the effect for persistence in searching at the target region (a more narrow spatial sampling) failed to reach significance. This observation suggests that measures derived from a broader spatial sampling may be more sensitive to sex differences, although it is noted that the difference between males and females in spatial search was similar in magnitude for both narrow (target region) and broader (target quadrant) spatial scales. Correlational analyses corroborated age and sex differences in performance of the vWMT, suggesting that spatial ability improves with age, to a larger extent in males (older age associated with shorter path length to enter and higher percent path length in the target region) than females (older age associated only with smaller heading error).
Performance on a test isolating visuospatial perception, the MRT, also was assessed, again revealing age-related improvements that were consistent with previous studies (Andreano et al., 2009; Astur et al., 2004; Voyer, 2011). Importantly, for males in the present study, better vWMT performance (shorter path length to enter and higher percent path length in the target region, and smaller heading error) was correlated with higher MRT total score. A similar correlation has also been reported between performance on these tasks in young adults (Astur et al., 2004). Despite the observed male vWMT/MRT relationship in this study, MRT total score included as a covariate was not significant in a mediation analysis of vWMT performance, suggesting that mental rotation ability does not simply account for sex differences in spatial navigation.
The present findings are also consistent with the rodent literature demonstrating age-related improvements in acquisition (reduced escape latency and shorter swim distances) and retention (more time spent in goal quadrant), observed from adolescence to adulthood (Akers et al., 2007; Carman et al., 2001). Specifically, spatial navigation abilities emerge very early in rat development (i.e., generally around postnatal days 19–22) (Carman et al., 2001; Carman et al., 2002; Castro et al., 1987; Akers et al., 2007; Brown et al., 2000). Overall, preweanling rats have been shown to successfully learn to navigate to the platform in place and cued versions of the WMT. An age-related improvement in human spatial ability also was observed in participants between ages 6 and 67 on the Memory Island task. In that task, adolescents and younger-adults outperformed children and older ages (Piper et al., 2011). The overall male advantage on spatial ability on the vWMT, observed in the present study regardless of age, also is consistent with previous work in adults (Astur et al., 2004) and in prepubertal children (Newhouse et al., 2007), extending the literature to now include adolescents. Similarly, a comparison of twenty- and fifty-year-olds on a vWMT and on the MRT demonstrated that emerging adults outperformed older adults, and males outperformed females, although no age or sex differences were observed for mental rotation ability (Schoenfeld et al., 2010). Taken together, age-related changes in spatial abilities across the lifespan appear to follow an inverted U-shape pattern, in which adolescents improve significantly in ability by emerging adulthood, and overall, emerging adults exhibit superior WMT performance relative to younger and older counterparts.
Differences in spatial processing on the vWMT may be related to differences in the strategy employed to solve the task (Liu et al., 2011; Rodgers et al., 2012) and possibly greater confidence in navigating toward and identifying the platform location when the platform is not visible. That is, significantly more emerging adult males employed a direct navigation route, which relies on a more efficient spatial strategy that is a more efficient means to solve the task. For adolescents and emerging adult females, however, approximately half used a direct approach and the other half used a non-direct approach to complete the task. This finding is consistent with prior work (Astur et al., 2004). Notably, unlike emerging adults, this study documents that the superior performance observed in adolescent males versus females was not due to differences in the strategy employed to solve the task. In a study combining the vWMT with eye tracking during task performance, young adults who employed direct navigation routes during training were found to primarily look at the distal room environment early in the trial, and at the pool later in the trial (Hamilton et al., 2009). In contrast, participants employing less efficient, non-direct routes were found to transiently look at distal cues, focusing more on the pool throughout the task. Thus, age and sex differences in establishing directionality of the initial trajectory, relative to distal room cues, and the ability to interpret relative distance to the platform within the pool (Hamilton et al., 2007) may also contribute to the observed differences in adolescent versus emerging adult males and females.
It is plausible that females, regardless of age, navigate away from the correct platform quadrant during the Probe trial in the absence of confirmation that they have successfully reached the platform, as the platform is removed in this condition unbeknownst to participants, thereby reducing reinforcement of choosing a direct strategy. To this end, in adults, sex differences in wayfinding strategies have been reported, with men reporting greater preference for an orientation strategy (e.g., maintain sense of own position relative to geographical reference points) relative to women who reported greater preference for a route strategy (e.g., use of signs or directions from another person) (Lawton, 1996; Lawton et al., 2002). Further, women reported greater wayfinding anxiety (i.e., spatial anxiety) than men, which was attributed to personal safety in environments related to choosing a more familiar route which may not be the most direct per se (Lawton, 1996; Lawton et al., 2002). Orientation strategy was significantly correlated with spatial ability, whereas the route strategy was anti-correlated with spatial anxiety. Thus, age-related changes in spatial ability could potentially be attributed to differences in anxiety related to wayfinding. In contrast, generational changes in gender roles could provide more opportunities for exposure to wayfinding across sexes, which could perhaps offset negative influences of anxiety on spatial ability in today’s younger generations (Lawton, 1994).
From a neurobiological perspective, fMRI studies in adults provide evidence that women show a pattern of hippocampal activation that is unique from men during vWMT performance: women activate right hippocampus, bilateral parahippocampal area, and right mid-cingulate, whereas men activate left hippocampus, left parahippocampal area, left mid-cingulate, and right anterior cingulate during task performance (Sneider et al., 2011). An fMRI vWMT investigation to probe neural correlates of spatial ability in adolescents is therefore warranted to better understand neurobiological underpinnings of age and sex differences in task performance, particularly since evidence from structural MRI studies point to differences in hippocampal tissue volumes between girls and boys (ages 8–15 years) that likely influence spatial ability (Neufang et al., 2009). Moreover, a recent review of neurobiological differences between virtual reality navigation and real-world navigation tasks, suggests additional involvement of vestibular, motor and proprioceptive systems during virtual navigation (Taube et al., 2013). Thus, potential age and sex differences in activation of regional (hippocampal) and multi-regional networks need to be taken into account when investigating neural contributions to spatial cognition.
One limitation that should be considered when interpreting the current study results is that joystick use/video game experience was not directly assessed in the study participants, which could contribute to performance differences between groups. This is unlikely, however, given that adolescents typically have more video game exposure than young adults (Rideout et al., 2010), which would result in better, not worse performance. Further, Schoenfeld and colleagues (Schoenfeld et al., 2010) reported that vWMT performance was not significantly correlated with computer game experience. Nonetheless, future studies of spatial ability in adolescent cohorts must account for joystick experience. A limitation to consider for the MRT is that the time limit imposed in the test (4 minute limit for task completion) could be a potential factor contributing to sex-related performance differences, or lack thereof (Voyer, 2011), and accordingly, could be empirically examined in future studies. Finally, the influence of hormone fluctuations associated with menstrual cycle phase was not directly assessed in the present study. Cycle phase is a relevant factor, as better spatial ability has been reported during the follicular phase of the menstrual cycle in adult women (McCormick et al., 2001). However, exploratory analyses based on self-report in a subset of female participants in the present study yielded evidence of similar task performance across follicular and non-follicular phases. It will be necessary in future studies to empirically investigate age-related differences in memory performance relative to sex hormone levels in adolescence.
The current findings have important practical applications. Previous studies conducted in first grade boys and girls demonstrate that interventions (e.g., skill-building) that encourage the use of spatial strategies for processing visuospatial information can improve spatial ability, thereby mitigating performance differences between sexes (Tzuriel et al., 2010). Thus, intervention and education efforts that encourage the use of direct navigation strategies could help improve performance via identification of important visuospatial cues, and accordingly, improve spatial ability at any age, but particularly in those that have suboptimal task performance. To this end, Uttal and colleagues (Uttal et al., 2013) recently conducted a meta-analysis demonstrating that training can enhance spatial reasoning in male and female children and adults. Other training methods that can enhance spatial ability include exposure to video games geared towards spatial attention and mental rotation, which also have been shown to reduce sex differences in spatial skills (Feng et al., 2007).
In conclusion, this is the first study to establish sex-specific performance differences in adolescents relative to emerging adults on a vWMT. Significant sex differences were evident in both age groups, with males performing better than females on the vWMT regardless of age, but no sex differences were apparent in the adolescent group on mental rotation ability. Furthermore, measures derived from a more narrow spatial scale at the target location were shown to be more sensitive for identifying age effects. Utilization of a more efficient strategy for locating the platform, the direct strategy, yielded better vWMT performance in emerging adult males, although strategy choice did not account for sex differences in the adolescent cohort, nor did visuospatial perception ability. Overall, the present study provides important information regarding the developmental profile of spatial ability in adolescents as compared to a slightly older cohort, emerging adults. The WMT is one the most frequently used tools to assess spatial memory in animal models, and virtual analogues of this classic task have provided invaluable applications for translational research on the study of learning and memory. Future studies should address four major areas to advance the literature on developmental improvements in spatial ability: 1) how other types of memory ability relate to spatial ability; 2) the influence of motoric ability, such as joystick use or keyboard manipulation, on performance; 3) the role of developmental maturation of the neural circuitry underlying learning and memory on vWMT performance; and 4) the impact of biological influences, such as sex-related hormones, on spatial navigation. Future work should also address whether spatial abilities are vulnerable to environmental influences during adolescence, such as exposure to alcohol or drugs (Sneider et al., 2013), and whether spatial abilities can be enhanced across the lifespan.
Highlights.
Adolescents and emerging adults were tested on two spatial tasks.
Emerging adults performed better than adolescents on both tasks.
Male performance was better on water maze and mental rotation in emerging adults.
Strategy use and visuospatial perception in emerging adults enhance performance.
Acknowledgments
This work was supported by R03 DA022482 (JTS), and K01 AA014651 and R01 AA018153 (MMS).
Footnotes
List Poster Presentations:
These data were presented at the Society for the Study of Human Development (2011) Providence, RI.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akers KG, Hamilton DA. Comparison of developmental trajectories for place and cued navigation in the Morris water task. Dev Psychobiol. 2007;49:553–64. doi: 10.1002/dev.20227. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–66. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res. 2004;151:103–15. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Barratt W. Barratt simplified measure of social status (BSMSS) Indiana State University; 2006. [Google Scholar]
- Blakemore SJ. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Brown RW, Whishaw IQ. Similarities in the development of place and cue navigation by rats in a swimming pool. Dev Psychobiol. 2000;37:238–45. doi: 10.1002/1098-2302(2000)37:4<238::aid-dev4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Chiba AA, Gallagher M. Spatial learning in male and female Long-Evans rats. Behav Neurosci. 1995;109:180–3. doi: 10.1037//0735-7044.109.1.180. [DOI] [PubMed] [Google Scholar]
- Carman HM, Booze RM, Mactutus CF. Long-term retention of spatial navigation by preweanling rats. Dev Psychobiol. 2002;40:68–77. doi: 10.1002/dev.10014. [DOI] [PubMed] [Google Scholar]
- Carman HM, Mactutus CF. Ontogeny of spatial navigation in rats: a role for response requirements? Behav Neurosci. 2001;115:870–9. [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Sciences. 2005;9:104–10. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Castro CA, Paylor R, Rudy JW. A developmental analysis of the learning and short-term-memory processes mediating performance in conditional- spatial discrimination problems. Psychobiology. 1987;15:308–316. [Google Scholar]
- Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS. Sex differences in the effects of delta9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav Pharmacol. 2007;18:563–9. doi: 10.1097/FBP.0b013e3282ee7b7e. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Ofen N, Jacobs LF, Gabrieli JD. Scene complexity: influence on perception, memory, and development in the medial temporal lobe. Front Hum Neurosci. 2010;4:21. doi: 10.3389/fnhum.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin VS, Van Skike CE, Matthews DB. Effects of ethanol on hippocampal function during adolescence: a look at the past and thoughts on the future. Alcohol. 2010;44:3–14. doi: 10.1016/j.alcohol.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Rice JP, Akers KG, Candelaria-Cook FT, Taube JS, Hamilton DA. Lesions of the dorsal tegmental nuclei disrupt control of navigation by distal landmarks in cued, directional, and place variants of the Morris water task. Behav Neurosci. 2013;127:566–81. doi: 10.1037/a0033087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm Behav. 2005;47:326–35. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Feng J, Spence I, Pratt J. Playing an action video game reduces gender differences in spatial cognition. Psychol Sci. 2007;18:850–5. doi: 10.1111/j.1467-9280.2007.01990.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (Clinical Version) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Furman AJ, Clements-Stephens AM, Marchette SA, Shelton AL. Persistent and stable biases in spatial learning mechanisms predict navigational style. Cogn Affect Behav Neurosci. 2014 doi: 10.3758/s13415-014-0279-6. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Livingstone SA, Skelton RW, Hopkins RO. Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus. 2010;20:481–91. doi: 10.1002/hipo.20651. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Johnson TE, Redhead ES, Verney SP. Control of rodent and human spatial navigation by room and apparatus cues. Behav Processes. 2009;81:154–69. doi: 10.1016/j.beproc.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Herting MM, Nagel BJ. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav Brain Res. 2012;233:517–25. doi: 10.1016/j.bbr.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Nadel L. Episodic memory updating: the role of context familiarity. Psychon Bull Rev. 2011;18:787–97. doi: 10.3758/s13423-011-0117-6. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Keating MB, McKenzie BE, Day RH. Spatial localization in infancy: position constancy in a square and circular room with and without a landmark. Child Dev. 1986;57:115–24. [PubMed] [Google Scholar]
- Keeley RJ, Tyndall AV, Scott GA, Saucier DM. Sex difference in cue strategy in a modified version of the Morris water task: correlations between brain and behaviour. PLoS One. 2013;8:e69727. doi: 10.1371/journal.pone.0069727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns KA, Berenbaum SA. Sex differences in spatial ability in children. Behav Genet. 1991;21:383–96. doi: 10.1007/BF01065974. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44:2171–7. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Hamilton DA. Framing spatial cognition: neural representations of proximal and distal frames of reference and their roles in navigation. Physiol Rev. 2011;91:1245–79. doi: 10.1152/physrev.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurance HE, Learmonth AE, Nadel L, Jacobs WJ. Maturation of spatial navigation strategies: Convergent findings from computerized spatial environments and self-report. Journal of Cognition and Development. 2003;4:211–238. [Google Scholar]
- Lawton CA. Gender differences in way-finding strategies: Relationship to spatial ability and spatial anxiety. Sex Roles. 1994;30:765–779. [Google Scholar]
- Lawton CA. Strategies for indoor wayfinding: The role of orientation. Journal of Environmental Psychology. 1996;16:137–145. [Google Scholar]
- Lawton CA, Kallai J. Gender differences in wayfinding strategies and anxiety about wayfinding: A cross-cultural comparison. Sex Roles. 2002;47:389–401. [Google Scholar]
- Levine SC, Huttenlocher J, Taylor A, Langrock A. Early sex differences in spatial skill. Development Psychology. 1999;35:940–9. doi: 10.1037//0012-1649.35.4.940. [DOI] [PubMed] [Google Scholar]
- Linn MC, Petersen AC. Emergence and characterization of sex differences in spatial ability: a meta-analysis. Child Dev. 1985;56:1479–98. [PubMed] [Google Scholar]
- Liu I, Levy RM, Barton JJ, Iaria G. Age and gender differences in various topographical orientation strategies. Brain Res. 2011;1410:112–9. doi: 10.1016/j.brainres.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–21. [PubMed] [Google Scholar]
- McCormick CM, Teillon SM. Menstrual cycle variation in spatial ability: relation to salivary cortisol levels. Horm Behav. 2001;39:29–38. doi: 10.1006/hbeh.2000.1636. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Resnick SM. Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behav Neurosci. 2002;116:851–9. doi: 10.1037//0735-7044.116.5.851. [DOI] [PubMed] [Google Scholar]
- Morris RG. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nardi D, Newcombe NS, Shipley TF. The world is not flat: can people reorient using slope? J Exp Psychol Learn Mem Cogn. 2011;37:354–67. doi: 10.1037/a0021614. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19:464–73. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Newhouse C, Astur RS. Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav Brain Res. 2007;183:1–7. doi: 10.1016/j.bbr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a cognitive map. Oxford University Press; Oxford: 1978. [Google Scholar]
- Overman WH, Pate BJ, Moore K, Peuster A. Ontogeny of place learning in children as measured in the radial arm maze, Morris search task, and open field task. Behav Neurosci. 1996;110:1205–28. doi: 10.1037//0735-7044.110.6.1205. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Larson P, Kratz K, Thiebaux M, Bluestein B, Buckwalter JG, Rizzo AA. Sex differences in mental rotation and spatial rotation in a virtual environment. Neuropsychologia. 2004;42:555–62. doi: 10.1016/j.neuropsychologia.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9:60–8. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav Neurosci. 1996;110:1309–20. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Acevedo SF, Edwards KR, Curtiss AB, McGinnis GJ, Raber J. Age, sex, and handedness differentially contribute to neurospatial function on the Memory Island and Novel-Image Novel-Location tests. Physiol Behav. 2011;103:513–22. doi: 10.1016/j.physbeh.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Antich J, Orvaschel H, Tabrizi M, Chambers W. The Schedule for Affective Disorders and Schizophrenia for School-Aged Children - Epidemiologic Version (Kiddie-SADS-E) New York State Psychiatric Institute and Yale University School of Medicine; New York: 1980. [Google Scholar]
- Ribordy F, Jabes A, Banta Lavenex P, Lavenex P. Development of allocentric spatial memory abilities in children from 18 months to 5 years of age. Cogn Psychol. 2013;66:1–29. doi: 10.1016/j.cogpsych.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Rideout VJ, Foehr GU, Roberts DF. Generation M2 Media in the lives of 8-to 18- year-olds. A Kaiser Family Foundation Study; 2010. [Google Scholar]
- Rodgers MK, Sindone JA, Moffat SD. Effects of age on navigation strategy. Neurobiol Aging. 2012;33:202.e15–202.e22. doi: 10.1016/j.neurobiolaging.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof RL. Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze performance in prepubescent and adult rats. Behav Brain Res. 1993;53:1–10. doi: 10.1016/s0166-4328(05)80261-x. [DOI] [PubMed] [Google Scholar]
- Roof RL, Stein DG. Gender differences in Morris water maze performance depend on task parameters. Physiol Behav. 1999;68:81–6. doi: 10.1016/s0031-9384(99)00162-6. [DOI] [PubMed] [Google Scholar]
- Saucier DM, Shultz SR, Keller AJ, Cook CM, Binsted G. Sex differences in object location memory and spatial navigation in Long-Evans rats. Anim Cogn. 2008;11:129–37. doi: 10.1007/s10071-007-0096-1. [DOI] [PubMed] [Google Scholar]
- Schoenfeld R, Lehmann W, Leplow B. Effects of age and sex and mental rotation and spatial learning from virtual environments. Journal of Individual Differences. 2010;31:78–82. [Google Scholar]
- Silveri MM, Sneider JT, Crowley DJ, Covell MJ, Acharya D, Rosso IM, Jensen JE. Frontal lobe gamma-aminobutyric acid levels during adolescence: associations with impulsivity and response inhibition. Biol Psychiatry. 2013;74:296–304. doi: 10.1016/j.biopsych.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol Clin Exp Res. 2005;29:1402–10. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- Sluzenski J, Newcombe NS, Satlow E. Knowing where things are in the second year of life: implications for hippocampal development. J Cogn Neurosci. 2004;16:1443–51. doi: 10.1162/0898929042304804. [DOI] [PubMed] [Google Scholar]
- Sneider JT, Cohen-Gilbert JE, Crowley DJ, Paul MD, Silveri MM. Differential effects of binge drinking on learning and memory in emerging adults. J Addict Res Ther. 2013;(Suppl 7) doi: 10.4172/2155-6105.S7-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneider JT, Gruber SA, Rogowska J, Silveri MM, Yurgelun-Todd DA. A preliminary study of functional brain activation among marijuana users during performance of a virtual water maze task. J Addict. 2013;2013:461029. doi: 10.1155/2013/461029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneider JT, Sava S, Rogowska J, Yurgelun-Todd DA. A preliminary study of sex differences in brain activation during a spatial navigation task in healthy adults. Percept Mot Skills. 2011;113:461–80. doi: 10.2466/04.22.24.27.PMS.113.5.461-480. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. Journal of the International Neuropsychological Society. 2001;7:312–22. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci Lett. 1982;31:271–6. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Hogan A, Risher ML, Swartzwelder RA, Wilson WA, Acheson SK. Effect of sub-chronic intermittent ethanol exposure on spatial learning and ethanol sensitivity in adolescent and adult rats. Alcohol. 2014;48:353–60. doi: 10.1016/j.alcohol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Valerio S, Yoder RM. Is navigation in virtual reality with FMRI really navigation? J Cogn Neurosci. 2013;25:1008–19. doi: 10.1162/jocn_a_00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzuriel D, Egozi G. Gender differences in spatial ability of young children: the effects of training and processing strategies. Child Dev. 2010;81:1417–30. doi: 10.1111/j.1467-8624.2010.01482.x. [DOI] [PubMed] [Google Scholar]
- Uttal DH, Meadow NG, Tipton E, Hand LL, Alden AR, Warren C, Newcombe NS. The malleability of spatial skills: a meta-analysis of training studies. Psychol Bull. 2013;139:352–402. doi: 10.1037/a0028446. [DOI] [PubMed] [Google Scholar]
- Vandenberg SG, Kuse AR. Mental rotations, a group test of three-dimensional spatial visualization. Percept Mot Skills. 1978;47:599–604. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- Voyer D. Time limits and gender differences on paper-and-pencil tests of mental rotation: a meta-analysis. Psychon Bull Rev. 2011;18:267–77. doi: 10.3758/s13423-010-0042-0. [DOI] [PubMed] [Google Scholar]
- Wegman J, Tyborowska A, Janzen G. Hippocampus. 2014. Encoding and retrieval of landmark-related spatial cues during navigation: An fMRI study. [DOI] [PubMed] [Google Scholar]
- Wenk G. Assessment of spatial memory using the radial arm maze and Morris water maze. In: Gerfen C, Holmes A, Sibley D, Skolnick, Wray S, editors. Current Protocols in Neuroscience. Wiley & Sons; New York: 2004. [DOI] [PubMed] [Google Scholar]
- Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, Simmons JG, Yucel M, Pantelis C, McGorry P, Allen NB. Structural brain development and depression onset during adolescence: a prospective longitudinal study. Am J Psychiatry. 2014;171:564–71. doi: 10.1176/appi.ajp.2013.13070920. [DOI] [PubMed] [Google Scholar]
- Woolley DG, Vermaercke B, Op de Beeck H, Wagemans J, Gantois I, D'Hooge R, Swinnen SP, Wenderoth N. Sex differences in human virtual water maze performance: novel measures reveal the relative contribution of directional responding and spatial knowledge. Behav Brain Res. 2010;208:408–14. doi: 10.1016/j.bbr.2009.12.019. [DOI] [PubMed] [Google Scholar]