Summary
Natural killer (NK) large granular lymphocyte (LGL) leukaemia features a clonal proliferation of CD3− NK cells that can be classified into either aggressive or chronic categories. The NKL cell line, derived from an aggressive Asian NK cell leukaemia, and patient samples from chronic NK-LGL leukaemia were used in our study to probe for synergistic efficacy of the epigenetic drugs vorinostat (SAHA) and cladribine in this disease. We demonstrate that histone deacetylases (HDACs) are over-expressed in both aggressive and chronic NK leukaemia. Administration of the HDAC inhibitor SAHA reduces class I and II HDAC expression and enhances histone acetylation in leukaemic NK cells. In vitro combination treatment with SAHA and cladribine dose-dependently exerts synergistic cytotoxic and apoptotic effects on leukaemic NK cells. Expression profiling of apoptotic regulatory genes suggests that both compounds led to caspase-dependent apoptosis through activation of intrinsic mitochondrial and extrinsic death receptor pathways. Collectively, these data show that combined epigenetic therapy, using HDAC and DNA methyltransferase inhibitors, may be a promising therapeutic approach for NK-LGL leukaemia.
Keywords: natural killer large granular lymphocyte leukaemia, vorinostat (SAHA), cladribine, histone deacetylation, combined epigenetic therapy
Introduction
Large granular lymphocyte (LGL) leukaemia is a rare haematological malignancy characterized by excessive proliferation of cytotoxic CD3+ T cells or CD3− natural killer (NK) cells (Lamy & Loughran, 1998). In 2008, the World Health Organization (WHO) classification included a new provisional entity to make a clear distinction between aggressive and chronic NK-cell leukaemias (Swerdlow et al 2008). Though both types display a CD3−CD56+ immunophenotype, the chronic lymphoproliferative disorder of NK cells (also known as chronic NK-LGL leukaemia) is characterized by a more indolent course and is more common in the West (Lim et al, 2009). There is no known curative therapy for patients with LGL leukaemia, making development of novel therapeutics essential to improve the outcome of this disease.
Epigenetic modifications control gene expression without altering the underlying DNA sequence and have been implicated in aberrant oncogene expression in multiple cancers (Marks et al, 2000; Sharma et al, 2009). A previous study showed that the demethylating agent 5-aza-2'-deoxycytidine (DAC) interferes with activation of the JAK/STAT signalling pathway, which plays a central pathogenic role in LGL leukaemia (Teramo et al, 2013). Such data suggest a potential role for epigenetic modulators of chromatin structure, including histone modifications and DNA methylation, in the treatment of LGL leukaemia.
The histone deacetylases (HDACs) are a class of enzymes that deacetylate the ε-amino group of histones to promote a transcriptionally inactive chromatin configuration (Kalyaanamoorthy & Chen, 2013) and have been shown to play an integral role in cancer progression through a wide variety of signalling pathways (Chowdhury et al, 2011). HDAC inhibitors (HDACi) promote cell differentiation, apoptosis and cell cycle arrest in multiple transformed cell types (Marks et al, 2000). SAHA (suberoylanilide hydroxamic acid, Vorinostat) was the first U.S. Food and Drug Administration (FDA)-approved pan-HDACi, for use in cutaneous T-cell lymphoma. HDACi decrease histone-DNA interactions by promoting acetylation of histones. Acetylation neutralizes the positive charges on histones, thus leading to less compact and more transcriptionally active chromatin (Cohen et al, 2011). Numerous in vitro and in vivo studies have been reported in which various HDACi exhibit anti-cancer activities against several tumour types through changes in transcriptional gene regulation (Bolden et al, 2006; Butler et al, 2000; Thurn et al, 2011; Wang et al, 2013). In addition, emerging data suggest that SAHA has little toxicity against normal tissues (Blattmann et al, 2012; Chen et al, 2013; Dong et al, 2008), making it a promising therapeutic agent for human cancer.
Another drug that utilizes a different mechanism to exert epigenetic regulation is cladribine (2-chlorodeoxyadenosine, 2-CdA). Cladribine received FDA approval in the late 1980s and has been used to treat a range of different leukaemias (Robak & Robak, 2012), including LGL leukaemia (Edelman et al, 1997; O'Brien et al, 1994). Its main mechanism as an anti-cancer agent is through chain termination during DNA synthesis. Therefore, it exerts the strongest effect on rapidly dividing tissues, including tumours and immune cells. At lower doses though, cladribine possesses hypomethylating and epigenetic activities, especially in haematological malignancies. It inhibits histone and DNA methylation, leading to de-repression of silenced genes in tumour cells (Byrd et al, 2003; Fenaux et al, 2009; Spurgeon et al, 2011). These epigenetic effects occur at low doses and can avert much of the toxicity that is observed at high doses, such as death of normal lymphocytes, suppression of bone marrow and irreversible neurological damage as well as permanent kidney damage and debilitating cytopenias (Betticher et al, 1998; Cheson et al, 1994; Kluin-Nelemans et al, 2003; Romine et al, 1997; Sigal et al, 2010). These severe side effects strictly limit dosing in patients, making treatment with low dose cladribine as an epigenetic adjuvant to other therapies that much more appealing.
Deacetylated histone lysine tails and hypermethylated CpG islands are correlated with transcriptionally inactive or condensed chromatin, while acetylated histone lysine tails and unmethylated CpG islands are indicators of transcriptionally active or open chromatin. Thus, DNA methyltransferase inhibitors (DNMTi) and HDACi facilitate changes between these chromatin conformations (Griffiths & Gore, 2008; Leone et al, 2008). In order to take advantage of the epigenetic effects of cladribine without invoking its significant side effect profile, SAHA was used in combination with low dose cladribine to affect chromatin remodelling and un-silence repressed genes in NK-LGL cells. The aim of our study was to determine the efficacy of these epigenetic drugs in NK-LGL. We investigated the individual and combined effects of SAHA and cladribine on cell viability and apoptosis, and evaluated differences in apoptotic gene expression, HDAC expression and histone acetylation status in leukaemic NK cells. Here we show for the first time that the combination of SAHA and cladribine had synergistic efficacy in inducing cell death through apoptosis in leukaemic NK cells.
Methods and Materials
Cell culture and therapeutics
The human NK-LGL cell line, NKL, was kindly provided by Dr. Howard Young [National Cancer Institute (NCI), Frederick, MD]. Cells were cultured in RPMI 1640 medium with 15% fetal bovine serum (FBS; Atlanta Biologicals, Altanta, GA) and 10 u/ml human recombinant interleukin 2 (IL2; Allergy and Infectious Diseases [AIDS] Research and Reference Reagent Program, National Institutes of Health [NIH], Bethesda, MD) at 37°C and 5% CO2. Cells were harvested during exponential growth phase. SAHA was purchased from Selleckchem (Houston, TX) and cladribine from Sigma-Aldrich (St Louis, MO).
Patient characteristics and preparation of peripheral blood mononuclear cells (PBMCs)
All patients met the clinical criteria for NK-LGL leukaemia, mainly increased counts (>80%) of CD3−, CD16+/CD56+ NK cells in the peripheral blood. All patients were diagnosed with clinically stable chronic NK-LGL leukaemia (n=8) and were treatment-naïve during sample acquisition. Peripheral blood specimens were obtained with informed consent, in accordance with protocols approved by the Institutional Review Board of the Penn State Hershey Cancer Institute (Hershey, PA). White blood cell buffy coats from anonymous normal donors were obtained from the blood bank of Milton S. Hershey Medical Center (Hershey, PA).
PBMCs were isolated by Ficoll-hypaque gradient separation as described previously (Lamy et al, 1998). Cell viability was determined by Trypan blue exclusion assay. A cut-off of >95% viability was used as a quality control. Normal NK cells from anonymous age- and gender-matched healthy donors were isolated by a negative selection process (StemCell Technologies, Vancouver, Canada) as described previously (Epling-Burnette et al, 2004). The purity of freshly isolated CD3−CD56+ cells (2 × 105/sample, in triplicate) was determined by flow cytometry for positive staining of surface CD56+ on NK cells. The purity for normal purified NK cells was 85–90%.
HDAC gene expression: real-time quantitative RT-PCR
Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using primer sets specific for 11 human HDACs and an internal standard, RPS18 rRNA, in an ABI PRISM 7900 sequence detector (Applied Biosystems, Foster City, CA) as described previously (Liu et al, 2010). Total RNA was isolated from PBMCs (5×106) from NK-LGL leukaemia patients and healthy donors, and SAHA- or dimethylsulphoxide (DMSO)-treated NKL cells, as described previously (Liu et al, 2010). Primer sequences are shown in Supplemental Table S1 and were purchased from RealTimePrimers.com (Elkins Park, PA).
In vitro cell viability and apoptosis assay
Cell viability was determined using the CellTiter 96 Aqueous One Solution assay kit (Promega, Madison, WI). Colour change (490 nm) was detected with the Synergy HT Multi-Detection Microplate Reader (Bio-TEK, Winooski, VT). Apoptosis was determined by 2-colour flow cytometry with annexin V (5 µl/sample) and 7-amino-actinomycin D (7-AAD; 10 µl/sample, BD Pharmingen, San Jose, CA) staining using 5 × 105 cells/sample on a BD Biosciences FACS Calibur instrument in the flow cytometry core at Penn State Hershey College of Medicine. The percentage of specific apoptosis was calculated using the following formula: Apoptosis (%) = ((% annexinV–fluorescein isothiocyanate [FITC] conjugate positive in assay well - % annexinV–FITC positive in control well)/(100 - % annexinV–FITC positive in control well))×100.
Apoptosis RT2 profiler PCR microarray
Human apoptosis PCR array (Cat. PAHS-012ZA; SABiosciences, Valencia, CA) was performed to analyse the mRNA expression of 84 key genes. NKL cells were treated with SAHA (5 µM), cladribine (0.125 µM), both or DMSO vehicle for 24 h. Total RNA was extracted and reverse transcribed into cDNA by the RT2 First Strand Kit (SABiosciences). Equal aliquots of the mixture (10 µl) were added into wells of the PCR Array plate, which contains pre-dispensed primer sets. RT2 SYBR Green/ROX PCR Master Mix (SABiosciences) was used to monitor the fluorescent signal during each cycle of PCR reactions as described above.
Western blot analysis
NKL cells (107) were treated with various concentrations of SAHA and cladribine for 24 h and then lysed in radioimmunoprecipitation assay buffer (Sigma-Aldrich) with protease inhibitor cocktail P8340 (Sigma-Aldrich) and phosphatase inhibitor cocktail 2 (Sigma-Aldrich). Proteins (30 µg) were electrophoresed and transferred to polyvinylidene difluoride membrane, blocked, probed with primary antibodies diluted in 5% non-fat dry milk overnight, then washed, incubated with secondary antibody and visualized using enhanced chemiluminescence (ECL; Thermo Scientific, West Palm Beach, FL). Protein bands were subjected to densitometric scanning and were quantified using the ImageJ software (NIH). All primary and secondary antibodies were purchased from Cell Signaling Technology (Beverly, MA).
Statistical Analysis
All data are presented as mean ± standard error of the mean (SEM). Two-tailed paired Student t test and two-way analysis of variance tests were used to determine statistical significance. P < 0.05 was considered statistically significant. Synergy was analysed with CalcuSyn software (Biosoft, Cambridge, UK) using median-effect methods of Chou and Talalay (Chou & Talalay, 1984). Combination index (CI) values determined the combination effect as synergistic (<1), additive (=1), or antagonistic (>1).
Results
HDACs are highly expressed in leukaemic NK cells
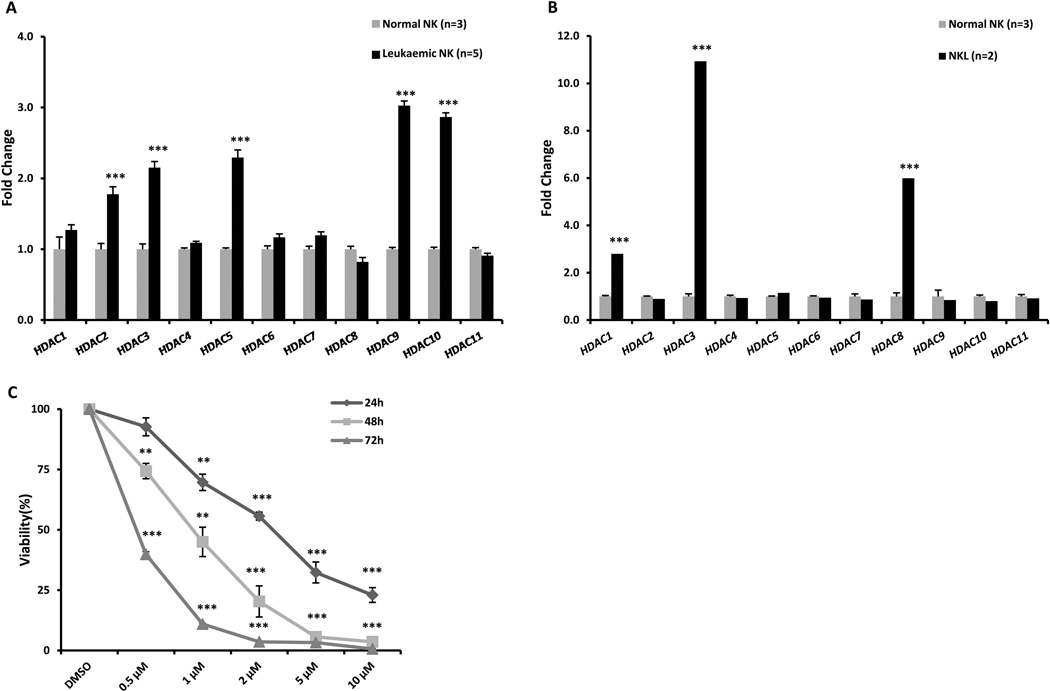
We characterized the mRNA expression levels of the 11 HDACs in purified chronic NK-LGL patient samples and normal NK cells by quantitative RT-PCR. The mRNA levels of HDAC2, HDAC3, HDAC5, HDAC9 and HDAC10 are significantly over-expressed in leukaemic cells compared to normal NK cells (Figure 1A). Aggressive NK-LGL leukaemia is rare in the West and access to primary patient materials is limited. Therefore, we used NKL cells (an aggressive human NK-LGL leukaemia cell line) (Robertson et al, 1996) for further mechanistic studies. Quantitative RT-PCR results demonstrate that HDAC1, HDAC3 and HDAC8, belonging to HDAC class I, are significantly up-regulated in NKL cells compared to normal NK cells (Figure 1B). Collectively, we found that HDAC transcripts are significantly up-regulated in chronic leukaemic NK samples as well as the NKL cell line. To evaluate the effects of HDACi in leukaemic NK cells, cell survival was determined in SAHA-treated NKL cells (Figure 1C). SAHA inhibits cell viability in a time and dose-dependent manner, with a 50% inhibition concentration (IC50) of 2.00 µM, 0.93 µM and 0.44 µM at 24, 48 and 72 h.
Figure 1.
HDACs are over-expressed in leukaemic NK cells. (A) Real-time quantitative PCR was performed to measure mRNA levels in NK cells from five patients with chronic NK large granular lymphocyte (LGL) leukaemia (NK-LGL) (CD3−CD56+ NK cells > 80%) and three normal donors. Data are representative of two independent experiments performed in triplicate and normalized against 18S as a control gene. ***P < 0.0005 vs. normal NK cells. (B) Real-time quantitative PCR was performed to measure mRNA levels in the NKL cell line and three normal donors. Data are representative of three independent experiments performed in triplicate and normalized against RPS18 as a control gene. ***P < 0.0005 vs. normal NK cells. (C) NKL cells were treated with SAHA (1, 2, 5 and 10 µM) for 24, 48 and 72 h and cell survival was determined by MTS assay. Results are expressed as the average of three independent experiments performed in triplicate. *P < 0.05, **P < 0.005, ***P < 0.0005 vs. dimethylsulphoxide (DMSO) control.
SAHA and combination treatment with low-dose cladribine induces dose-dependent cytotoxic effects in leukaemic NK cells
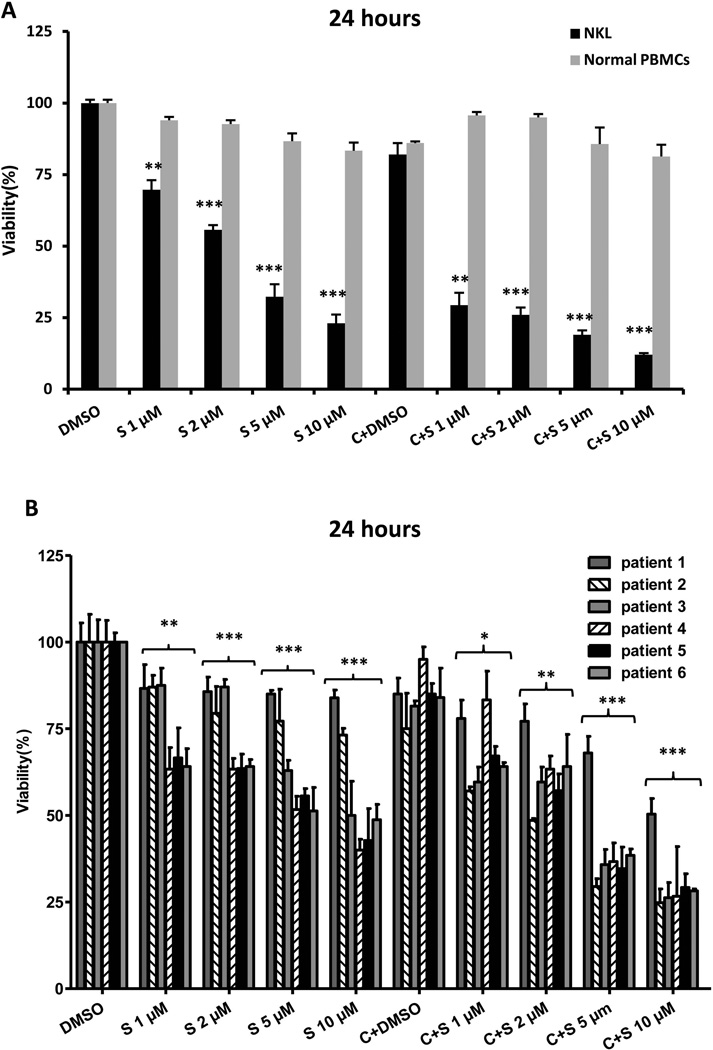
Due to the potential benefit of DNA hypomethylation concurrent with histone acetylation, cells were co-treated with cladribine and SAHA in order to evaluate synergistic effectiveness. NKL cells were treated with SAHA and cladribine, and cell survival was determined from 24 to 72 h (single viability inhibition curves for cladribine are shown in Supplemental Figure 1). Based upon the significant relationship that cell viability has with time and dose, we chose to stratify using these two covariates in our analysis. Due to the strong cytotoxicity of SAHA and cladribine at 72 h, data from 24 and 48 h was selected for analysis. NKL cells were exposed to combinations of the indicated concentrations of SAHA (1, 2, 5 and 10 µM) and cladribine (0.125 µM). This dose of cladribine reduces cell viability by 18% and 74% in NKL cells when applied alone for 24 and 48 h, respectively. Combination therapy induces significantly greater cytotoxicity than SAHA and cladribine administered alone (Figure 2A, Supplemental Figure 2A). To extend these findings to clinically relevant samples, experiments were repeated using PBMCs from six chronic NK-LGL leukaemia patients. Both SAHA and combination treatment result in dose-dependent growth inhibitory effects in NK-LGL leukaemic cells. The combination treatment causes greater decreased viability compared to SAHA alone at 24 and 48 h (Figure 2B, Supplemental Figure 2B). SAHA and combination treatment also exerts no detectable cytotoxic effect on PBMCs from healthy donors (Figure 2A, Supplemental Figure 2A). Overall, our data show that cellular growth inhibition is significantly increased in a dose-dependent manner in leukaemic NK cells treated with combined SAHA and cladribine when compared to normal controls.
Figure 2.
SAHA treatment alone and in combination with cladribine induces dose-dependent cytotoxic effects in leukaemic NK cells. (A) NKL cells and normal peripheral blood mononuclear cells (PBMCs) from three healthy donors were treated with SAHA (1, 2, 5 and 10 µM) and 0.125 µM cladribine for 24 h and cell survival was determined by MTS assay. Results are expressed as the average of three independent experiments performed in triplicate. (B) Leukaemic NK cells from six chronic NK-LGL patients (CD3−CD56+ NK cells > 80%) were treated with SAHA (1, 2, 5 and 10 µM) and 0.125 µM cladribine for 24 h and cell survival was determined by MTS assay. Each sample was assayed in triplicate. S: SAHA, C: cladribine and C+S: cladribine + SAHA. *P < 0.05, **P < 0.005, ***P < 0.0005 vs. dimethylsulphoxide (DMSO) control.
SAHA and combination treatment with low-dose cladribine induce dose-dependent apoptotic cell death in leukaemic NK cells
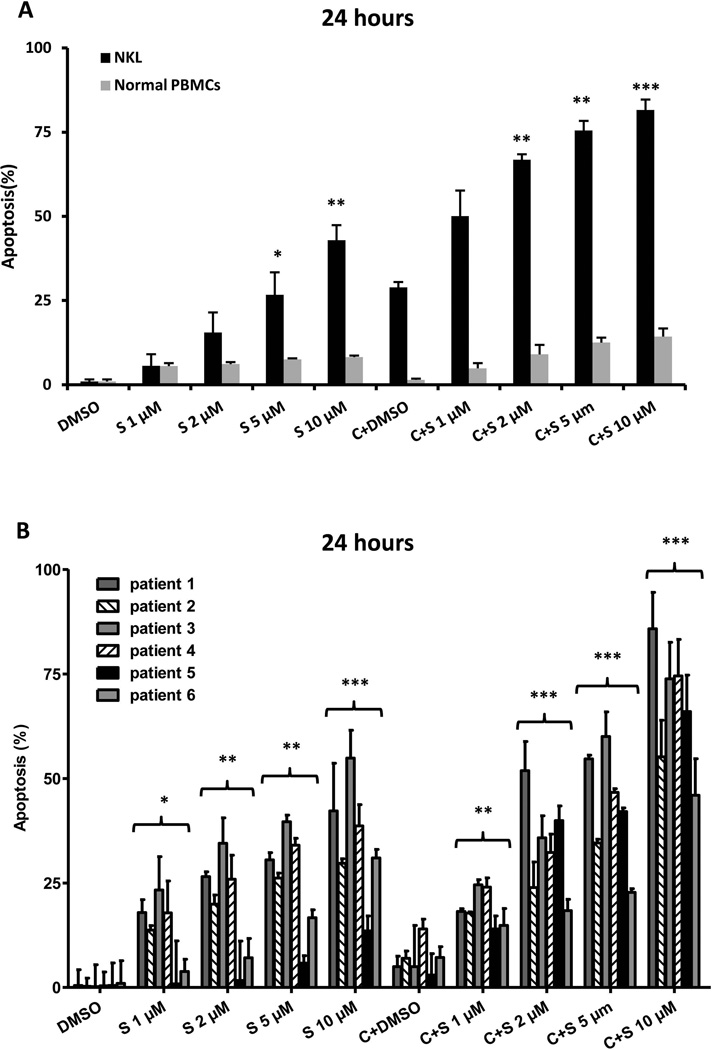
To explore whether the effects of SAHA and cladribine were associated with cellular apoptosis, NKL cells were treated with SAHA alone or in combination with cladribine for 24 and 48 h. A dose-dependent increase in apoptosis was detected with SAHA treatment (1, 2, 5 and 10 µM) alone and in combination with 0.125 µM cladribine at both 24 and 48 h. Combination therapy significantly increases apoptosis compared to SAHA alone (Figure 3A, Supplemental Figure 3A), and synergistic effects were seen all doses of SAHA combined with 0.125 µM cladribine at both 24 and 48 h (Figure 4). Both SAHA and combination treatment induce apoptotic cell death in patient-derived NK-LGL leukaemic cells in a dose-dependent manner, with combination therapy showing greater effects (Figure 3B, Supplemental Figure 3B). Minimal apoptosis is seen in PBMCs from healthy controls with the same treatment (Figure 3A, Supplemental Figure 3A). These results demonstrate a strong correlation between the degree of apoptosis and viability inhibition and indicate that cell death induced by these compounds is mostly apoptotic in nature.
Figure 3.
SAHA treatment alone and in combination with cladribine induces apoptotic cell death in leukaemic NK cells. (A) NKL cells and normal peripheral blood mononuclear cells (PBMCs) from three healthy donors were treated with SAHA (1, 2, 5 and 10 µM) and 0.125 µM cladribine for 24 h and apoptosis was detected as described in Methods and Materials. Results are expressed as the average of three independent experiments performed in triplicate. (B) Leukaemic NK cells from six chronic NK-LGL patients (CD3−CD56+ NK cells > 80%) were treated with SAHA (1, 2, 5 and 10 µM) and 0.125 µM cladribine for 24 h and apoptotic cells were determined as in (A). S: SAHA, C: cladribine and C+S: cladribine + SAHA. *P < 0.05, **P < 0.005, ***P < 0.0005 vs. dimethylsulphoxide (DMSO) control.
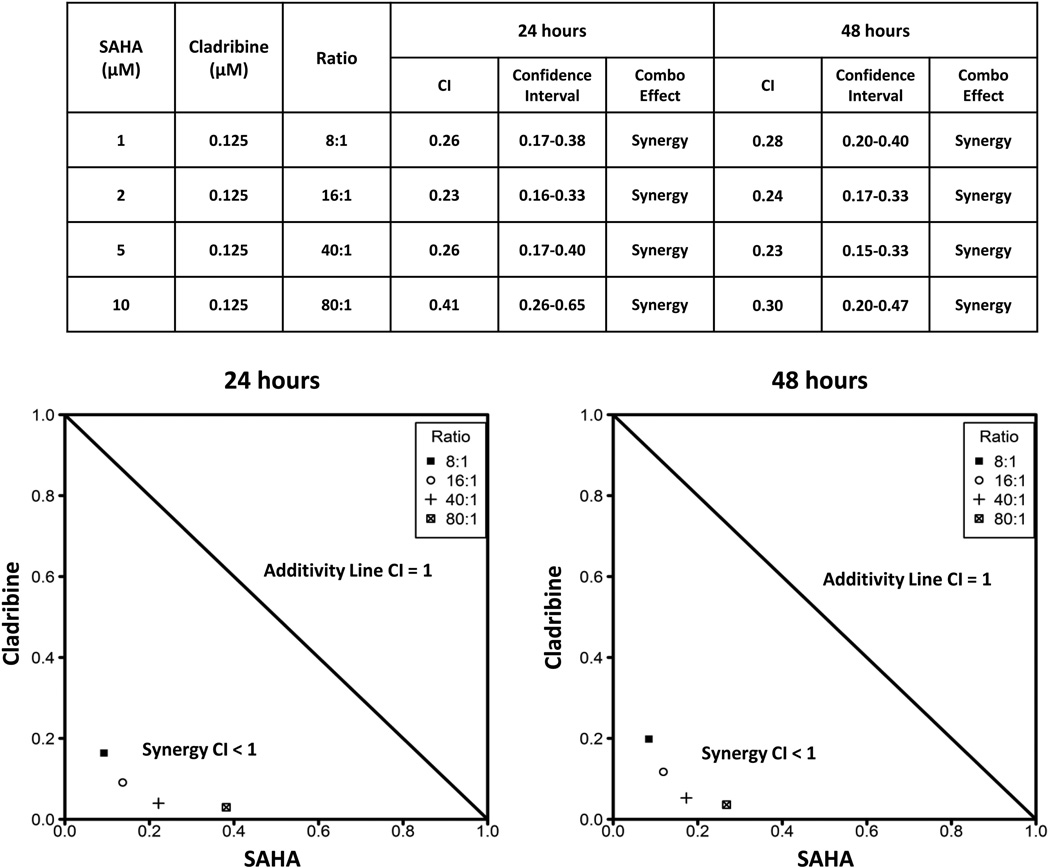
Figure 4.
Combination of SAHA and cladribine synergistically induces apoptosis in leukaemic NK cells. Combination index (CI) values and corresponding 95% confidence intervals represent interaction effects of combination therapy (Combo Effect) in leukaemic NK cells. For a CI < 1 (95% confidence interval < 1), the combination is defined as synergism; a CI = 1 (95% confidence interval includes 1), the combination is defined as additive; a CI > 1 (95% confidence interval > 1), the combination is defined as antagonism. The normalized isobologram analysis presents a graphical depiction of the combination index analyses. The analysis was performed with CalcuSyn software, which conducts the drug dose-effect calculation with the median effect method of Chou and Talalay. Data below the additivity line in the isobologram indicate synergy, whereas data above the additivity line indicate antagonism. All combination groups were below the additivity line, indicating synergism between SAHA and cladribine in NK-LGL cells.
SAHA inhibits HDAC expression and enhances histone acetylation in leukaemic NK cells
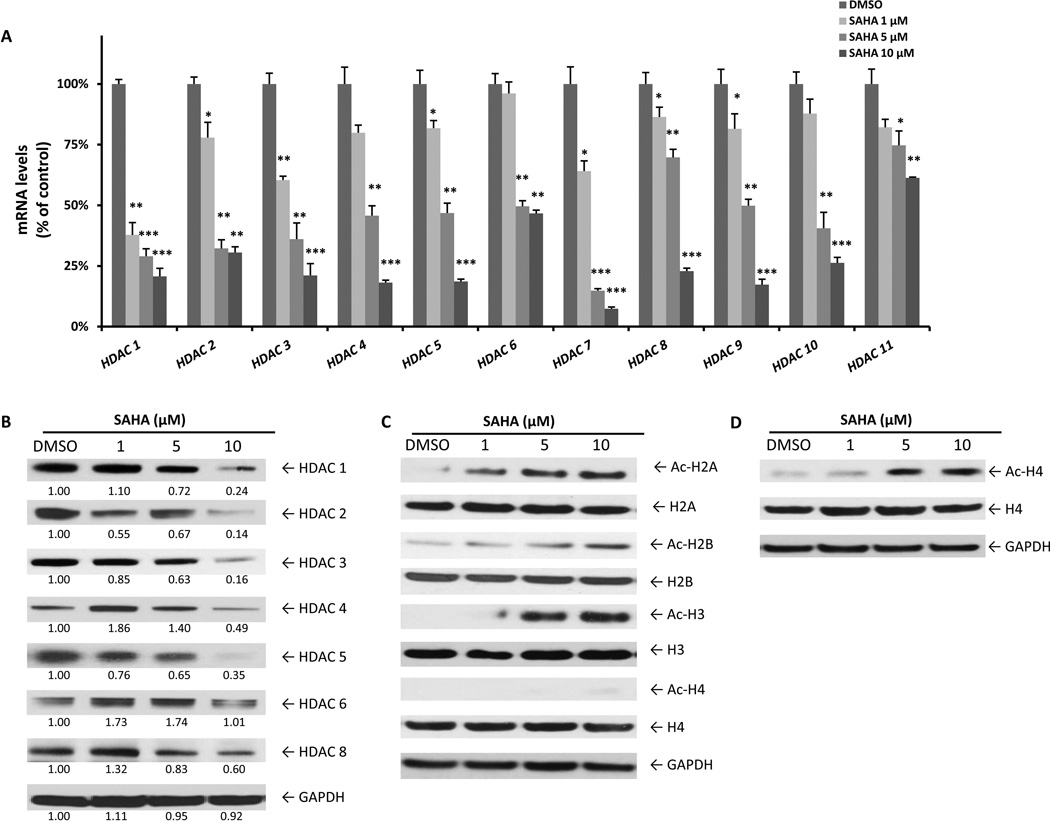
Next we determined the effect of HDACi treatment on HDAC mRNA levels in NKL cells. After incubation with SAHA (1, 5 and 10 µM) for 24 h, relative mRNA levels of nearly all HDACs, including isoforms that are highly expressed in NKL cells (HDAC1, HDAC3 and HDAC8), are significantly decreased by SAHA in a dose-dependent manner (Figure 5A). Importantly, HDAC mRNA levels were also reduced after 12 or 18 h of SAHA treatment (10 µM), which demonstrates that these changes precede the induction of apoptosis (Supplemental Figure 4). Class I (HDACs 1, 2, 3 and 8) and the extensively studied class II HDAC enzymes (HDACs 4, 5 and 6) were selected for Western blot analysis. As predicted by mRNA expression results, SAHA also reduces the protein levels of class I and II HDAC enzymes dose-dependently (Figure 5B). Moreover, all four of the core histones become hyper-acetylated and show a dose-dependent increase apparent at 24 h of SAHA treatment for H2A, H2B and H3 (Figure 5C) and 48 h for H4 (Figure 5D).
Figure 5.
SAHA reduces HDAC expression and enhances histone acetylation in leukaemic NK cells. (A) NKL cells were treated with SAHA (1, 5 and 10 µM) for 24 h and RNA was extracted for qRT-PCR analysis. Data are representative of two independent experiments performed in triplicate and normalized against 18S as a control gene. *P < 0.05, **P < 0.005, ***P < 0.0005 vs. dimethylsulphoxide (DMSO) control. (B) Western blot analysis was performed for HDACs after treatment with SAHA (1, 5 and 10 µM) for 24 h. Data were quantified by densitometry and ratios of HDAC/GAPDH are shown under each band. Results are representative of three independent experiments. (C) Western blot analysis was performed for acetyl-Histone after treatment with SAHA (1, 5 and 10 µM) for 24 h or (D) 48 h. Results are representative of three independent experiments. Equal loading of protein lysates was confirmed by re-probing membranes for GAPDH.
Effects of SAHA and/or cladribine treatment on apoptotic gene expression in leukaemic NK cells
To investigate effects of SAHA and cladribine treatment on apoptotic signalling events and regulatory proteins, PCR microarray and Western blot analyses were carried out in NKL cells after incubation with 5 µM SAHA or/and 0.125 µM cladribine for 24 h. Our data suggest that either single or combination therapy with SAHA and cladribine triggers apoptosis through activation of both intrinsic mitochondrial and extrinsic death receptor pathways in NK-LGL. As illustrated in Table I, SAHA and cladribine treatment leads to increases in expression of AIFM1 (apoptosis inducing factor), CYCS (cytochrome complex) and APAF1 (apoptotic protease activating factor-1). Increases in pro-apoptotic regulators including BBC2, BAX, BAK1 and BCL2L11 accompanied by decreases in anti-apoptotic genes like BCL2 and BCL2L1 also suggest activation of the intrinsic apoptotic pathway (Table I). Moreover, gene expression levels of FAS, FASLG, FADD, TNFSF10, TNFRSF1A, TNFRSF10B, initiator and effector CASP (caspases) are greatly enhanced in response to SAHA and/or cladribine (Table I), suggesting that epigenetic combination therapy may also activate the extrinsic pathway. Additionally, combination therapy shows strong synergistic effects in gene expression of CYCS, ABL1, BCL2L11, CASP4, CASP8, CD40L, CD70, FAS, FASLG, TNFSF10, NOL3 and RIPK2 compared to SAHA or cladribine administered alone (Table I).
Table I.
Analysis of gene expression for apoptotic regulators in SAHA-and cladribine-treated leukaemic NK cells
| Symbol* | Previous/alternative symbols | Fold change |
||
|---|---|---|---|---|
| SAHA | Cladribine | Combination | ||
| ABL1 | ABL, JTK7, bcr, abl, c-ABL, p150, v-abl | 1.68 | 1.82 | 7.16 |
| APAF1 | APAF-1, CED4, DKFZp781B1145 | 3.41 | 2.26 | 2.71 |
| AIFM1 | AIF, COXPD6, MGC111425 | 6.10 | 2.12 | 5.26 |
| BAD | BBC2, BCL2L8 | 1.3 | 2.32 | 1.16 |
| BAK1 | BAK, BAK-LIKE, BCL2L7, CDN1 | 2.59 | 1.59 | 3.5 |
| BAX | BCL2L4 | 2.05 | 1.78 | 1.47 |
| BCL2 | Bcl-2 | 1.02 | −2.28 | −1.86 |
| BCL2L1 | BCL-XL, BCL2L, BCLX, BCLXS, Bcl-X | 1.45 | −5 | −5.41 |
| BCL2L11 | BAM, BIM, BimEL, BimL | 34.86 | 12.79 | 38.1 |
| BCL2L2 | BCL-W, BCL2-L-2, BCLW | 2.09 | −3.49 | −1.25 |
| CASP1 | ICE, IL1BC, P45 | 4.37 | 1.42 | 6.75 |
| CASP3 | CPP32, CPP32B, SCA-1 | 1.95 | 2.93 | 1.65 |
| CASP4 | ICE(rel)II, ICEREL-II, ICH-2, Mih1, TX | 1.65 | 1.85 | 4.87 |
| CASP8 | ALPS2B, CAP4, Casp-8, FLICE | 2.62 | 1.49 | 5.02 |
| CASP9 | APAF-3, APAF3, CASPASE-9c | 2.71 | 1.91 | 3.78 |
| CD40LG | CD154, CD40L, HIGM1, IGM, IMD3 | 1.11 | 1.15 | 4.19 |
| CD70 | CD27L, CD27LG, TNFSF7 | 6.58 | 2.08 | 19.45 |
| CYCS | CYC, HCS, THC4 | 2.72 | 10.32 | 15.06 |
| FADD | MGC8528, MORT1 | 1.61 | 1.85 | 2.76 |
| FAS | APO-1, APT1, CD95, FAS1, TNFRSF6 | 2.77 | 1.35 | 5.04 |
| FASLG | CD178, CD95-L, CD95L, FASL, TNFSF6 | 6.84 | 5.13 | 31.72 |
| NOL3 | ARC, FLJ35304, MYP, NOP, NOP30 | 1.47 | 1.11 | 7.01 |
| RIPK2 | CARD3, CCK, GIG30, RICK | 4.26 | 1.18 | 9.93 |
| TNFRSF10B | CD262, DR5, KILLER, TRAILR2, TRICK2 | 4.3 | 2.04 | 2.69 |
| TNFRSF1A | TNF-R, TNFR1, TNFR55, TNFR60, p55, p60 | 1.13 | 2.05 | 2.09 |
| TNFSF10 | APO2L, Apo-2L, CD253, TL2, TRAIL | 3.73 | 2.74 | 7.63 |
Gene expression levels in NKL cells cultured with 5 µM SAHA or/and 0.125 µM cladribine for 24 h are illustrated as fold changes in reference with the control samples (without drugs). Samples were quantified in duplicate and all duplicates agreed within ± 10%.
HGNC (Human Genome Organization [HUGO] Gene Nomenclature Committee symbol (http://www.genenames.org)
Consistent with this mRNA expression data, either single or combination therapy of SAHA and cladribine for 24 h alters protein levels of key apoptosis regulators in NKL cells (Figure 6A). Combination therapy increased expression of the pro-apoptotic proteins BCL2L11, BAX and BAK1 and suppressed anti-apoptotic proteins like BCL2, BCL2L1 and MCL1. Protein quantification data shows that combination therapy exerts significantly greater effects on some apoptotic regulators, such as BCL2, BCL2L1 and BAX, compared to SAHA alone. There are significant differences between combination therapy and cladribine treatment on BCL2, BCL2L1, MCL1 and BCL2L11 expression (Figure 6A). Both compounds and their combination subsequently induced activation of CASP3 and poly(ADP-ribose) polymerase (PARP) cleavage in leukaemic NK cells (Figure 6B). Combination therapy also reduced activation of the ERK and STAT3 cellular signalling pathways (Figure 6C), both of which are strongly implicated in the pathogenesis and cell survival of LGL leukaemia (Epling-Burnette et al, 2001; Yue & Turkson, 2009). Collectively, these results show that SAHA and SAHA/cladribine combination treatment alters the aberrant survival signalling pathways in LGL leukaemia.
Figure 6.
Effects of SAHA and cladribine on apoptotic regulatory proteins and signalling pathways. (A) Western blot analysis was performed for the indicated apoptotic regulatory proteins after treatment with SAHA (1, 2 and 5 µM), cladribine (0.125, 0.25 and 0.5 µM) or both for 24 h. Upper panels show representative Western blots, and the bar graph indicates average relative protein expression determined by densitometric analysis of three independent experiments. *P < 0.05, **P < 0.005, ***P < 0.0005 vs. C+S. S: SAHA, C: cladribine and C+S: cladribine + SAHA. (B) Apoptosis induction was detected by Western blot analysis of caspase-3 and PARP cleavage after treatment as described in (A). Results are representative of three independent experiments. (C) Western blot analysis was performed for phosphorylated (p)-ERK, total ERK, p-STAT3 and total STAT3 after treatment as described in (A). Results are representative of three independent experiments. Equal loading of protein lysates was confirmed by re-probing membranes for GAPDH.
Discussion
Here we find dramatic synergistic effects in induction of apotosis in leukaemic NK cells using low doses of cladribine (0.125 uM) in combination with SAHA in vitro. Cell viability and cell death assays reveal that both agents exert cytotoxic and apoptotic effects dose-dependently in NK-LGL leukaemia patient samples and NKL cells, while sparing normal NK cells. The mechanisms underlying the responses to combination therapy with DNMTi and HDACi in cancer are not yet fully understood but appear to involve global epigenetic changes that affect many cellular pathways. Recent reports have highlighted that both DNMTi and HDACi can synergistically create a more transcriptionally active chromatin configuration through demethylation of CpG islands and acetylation of histone lysine tails, resulting in global alterations in the expression of genes responsible for cell proliferation, differentiation and death (Griffiths & Gore, 2008; Leone et al, 2008; Maes et al, 2013). Additionally, Arzenani et al. (2001) revealed that synergistic effects of DNMTi and HDACi are achieved through a common target, DNMT1. Moreover, DNMT recruits both methyl-binding proteins and HDACs to promoter regions, leading to chromatin condensation and suppressed gene expression (Jones et al, 1998; Nan et al, 1998; Sarkar et al, 2011; Sharma et al, 2009). The role of DNMT in NK-LGL responses to DNMTi and HDACi will be further explored in future studies. Collectively, these data suggest that combined epigenetic therapy with HDACi and DNMTi, especially SAHA and cladribine, should be considered as investigational therapy for NK-LGL leukaemia.
We also find a general up-regulation of HDAC gene expression in leukaemic NK cells. The HDAC family plays an essential role in the control of gene expression and is aberrantly expressed in multiple human cancers (Arzenani et al, 2011; Jones et al, 1998; Sarkar et al, 2011). We show significant over-expression of HDAC2, HDAC3, HDAC5, HDAC9 and HDAC10 in patient samples and HDAC1, HDAC3 and HDAC8 in NKL cells. Differences between the patient samples and cell line are probably caused by varying isoform over-expression in the NK-LGL patient population (n = 5) as opposed to very specific changes in the clonal NKL cell line. Collectively, these results suggest that HDAC over-expression is a common feature in NK-LGL leukaemia, and provide a strong rationale for targeting HDAC as a therapeutic strategy. We also demonstrate that the HDACi SAHA induces hyperacetylation of core histones and dose-dependently inhibits gene expression and reduces protein levels of class I and II HDAC in leukaemic NK cells. Modest differences between HDAC mRNA and HDAC protein levels, such as for HDAC1, are probably related to the additional time required for transcriptional changes to be reflected in altered protein content. Of note, selective inhibition of HDAC3 activity, the isoform most highly expressed relative to normal NK cells, was unable to recapitulate the downregulation of HDAC proteins observed with SAHA treatment (data not shown). More specific characterization of HDAC activity in NK-LGL leukaemia will require fluorometric activity assays that are selective for the individual HDAC isoforms.
Our data suggest that both intrinsic and extrinsic apoptotic pathways may be involved in the robust and synergistic death response induced by cladribine and SAHA treatment of leukaemic NK cells. The mechanisms responsible for HDACi and DNMTi-induced apoptosis seem to be complex and vary among different cell types. Activation of caspases and over-expression of pro-apoptotic genes accompanied by suppression of anti-apoptotic genes has been commonly observed following treatment with SAHA or cladribine in various tissues (Bosanquet et al, 2002; Ceruti et al, 2003; Fandy et al, 2005; Henderson et al, 2003; Perez-Galan et al, 2002; Zhang et al, 2005). Indeed we observe similar findings in leukaemic NK cells treated with combination therapy. Using PCR microarray, we find up-regulation of pro-apoptotic BCL2L11, BAX and BAK1 while BCL2 and BCL2L1 are down-regulated (Table I). Additionally, Western blot data show greater alternations caused by combination therapy than either drug alone, indicating synergistic effects of HDACi and DNMTi in regulating expression of key apoptotic proteins (Figure 6A). Of interest, SAHA alone minimally alters the mRNA expression of BCL2 and BCL2L1, while Western blot analysis shows SAHA suppresses protein levels of these factors. Possible explanations for the differential expression may be the post-transcriptional regulation of the BCL2 family after HDACi treatment in NK-LGL. HDACi have been reported to regulate gene expression at transcriptional and post-transcriptional levels (Hirsch et al, 2010; Kretzner et al, 2011). Similar results were seen in multiple myeloma and acute lymphoblastic leukaemia, suggesting that expression of apoptosis-related proteins may be down-regulated by SAHA at a post-transcriptional level (Liu et al, 2013; Willimott & Wagner, 2010).
It is noteworthy that the BH3-only pro-apoptotic gene, BCL2L11, was enhanced 35-fold following SAHA treatment at the mRNA level, and similar results were observed at the protein level. Recent studies show that BCL2L11 is involved in the regulation of programmed cell death of various cancer types (Akiyama et al, 2009; Gillings et al, 2009; Rahmani et al, 2009), and over-expression of BCL2L11 and enhanced BCL2L11-mediated apoptosis are commonly found in response to oncogene-targeted therapeutics (Gillings et al, 2009). Our data also suggest an important role of BCL2L11 in HDACi-induced intrinsic apoptosis in leukaemic NK cells.
We observed synergistic effects of SAHA and cladribine on gene expression of CD40LG, CD70, FASLG, FAS and CASP8. CD40LG and CD70, both inflammatory cytokines of the tumour necrosis factor (TNF) superfamily, play important roles in the cytotoxic function of NK cells (Carbone et al, 1997; Yang et al, 1996). FASLG and FAS are important in regulating lymphocyte homeostasis and in mediating extrinsic pathway apoptosis through the recruitment of FADD and the activation of CASP8 and effector caspases (Holler et al, 2000). Over-expression of FASLG, FAS and FADD is reported to enhance FAS-mediated apoptosis (De Paepe et al, 2008; Huang et al, 1999; Kim et al, 2003; Roger et al, 1999). Here, the 32-fold up-regulation of FASL expression suggests that FAS-mediated apoptosis may contribute to the synergistic efficacy of SAHA and cladribine in inducing cell death in NK-LGL. Interestingly, one of our previous studies demonstrated that leukaemic NK cells are relatively resistant to FAS-mediated apoptosis, but MEK inhibition increased the sensitivity to FAS-induced apoptosis in NK-LGL cells (Epling-Burnette et al, 2004). Similar to MEK inhibition increasing sensitivity, we find reduced ERK pathway activation in SAHA and combination therapy in our study (Figure 6C). Whether the observed synergistic apoptosis is due to the enhanced FAS-sensitization by epigenetic therapy with HDACi and DNMTi in NK-LGL cells needs to be further explored in future studies.
In summary, we show that the HDAC family is a key regulator of cell survival in NK-LGL leukaemia. The pan-HDACi SAHA targets all HDAC enzymes and efficiently reduces expression of HDAC class I and II and increases histone acetylation, thereby leading to apoptotic cell death. We also demonstrate that combination therapy with SAHA and cladribine exerts synergistic cytotoxic and apoptotic effects, potentially through both the intrinsic and extrinsic-mediated apoptotic pathways, and that these effects were selective for leukaemic rather than normal NK cells. Collectively, SAHA in combination with low-dose cladribine may be a promising therapeutic strategy for NK-LGL leukaemia.
Supplementary Material
Acknowledgments
We thank Nate Sheaffer of the Cell Science/Flow Cytometry Core Facility and Robert Brucklacher of the Functional Genomics Core Facility at the Penn State College of Medicine for their technical assistance, as well as Kendall Baab, Andy Awwad and Alden Dewey for processing patient samples. The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division AIDS, NIAID, NIH: Human rIL2 from Dr. Maurice Gately, Hoffmann-La Roche Inc. The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number R01CA098472 (to T.P.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Authorship Contributions
X.L., Z.S.H, D.J.F., H.W., X.S., D.Z., T.P.L. and K.X. designed the experiments; X.S. performed and analysed the experiments; A.C. performed the statistic studies; and T.P.L., X.S., Z.S.H. and D.J.F. wrote the manuscript.
Conflict of Interest
The authors declare no competing financial interest.
Supporting information
Additional Supporting information may be found in the online version of this article:
References
- Akiyama T, Dass CR, Choong PF. Bim-targeted cancer therapy: a link between drug action and underlying molecular changes. Molecular Cancer Therapeutics. 2009;8:3173–3180. doi: 10.1158/1535-7163.MCT-09-0685. [DOI] [PubMed] [Google Scholar]
- Arzenani MK, Zade AE, Ming Y, Vijverberg SJ, Zhang Z, Khan Z, Sadique S, Kallenbach L, Hu L, Vukojevic V, Ekstrom TJ. Genomic DNA hypomethylation by histone deacetylase inhibition implicates DNMT1 nuclear dynamics. Molecular and Cellular Biology. 2011;31:4119–4128. doi: 10.1128/MCB.01304-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betticher DC, von Rohr A, Ratschiller D, Schmitz SF, Egger T, Sonderegger T, Herrmann R, Kroner T, Zulian GB, Cavalli F, Fey MF, Cerny T. Fewer infections, but maintained antitumor activity with lower-dose versus standard-dose cladribine in pretreated low-grade non-Hodgkin's lymphoma. Journal of Clinical Oncology. 1998;16:850–858. doi: 10.1200/JCO.1998.16.3.850. [DOI] [PubMed] [Google Scholar]
- Blattmann C, Oertel S, Thiemann M, Weber KJ, Schmezer P, Zelezny O, Lopez Perez R, Kulozik AE, Debus J, Ehemann V. Suberoylanilide hydroxamic acid affects gammaH2AX expression in osteosarcoma, atypical teratoid rhabdoid tumor and normal tissue cell lines after irradiation. Strahlentherapie und Onkologie. 2012;188:168–176. doi: 10.1007/s00066-011-0028-5. [DOI] [PubMed] [Google Scholar]
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nature Reviews. Drug Discovery. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- Bosanquet AG, Sturm I, Wieder T, Essmann F, Bosanquet MI, Head DJ, Dorken B, Daniel PT. Bax expression correlates with cellular drug sensitivity to doxorubicin, cyclophosphamide and chlorambucil but not fludarabine, cladribine or corticosteroids in B cell chronic lymphocytic leukemia. Leukemia. 2002;16:1035–1044. doi: 10.1038/sj.leu.2402539. [DOI] [PubMed] [Google Scholar]
- Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, Thaler HT, Rifkind RA, Marks PA, Richon VM. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Research. 2000;60:5165–5170. [PubMed] [Google Scholar]
- Byrd JC, Peterson B, Piro L, Saven A, Vardiman JW, Larson RA, Schiffer C. A phase II study of cladribine treatment for fludarabine refractory B cell chronic lymphocytic leukemia: results from CALGB Study, 9211. Leukemia. 2003;17:323–327. doi: 10.1038/sj.leu.2402752. [DOI] [PubMed] [Google Scholar]
- Carbone E, Ruggiero G, Terrazzano G, Palomba C, Manzo C, Fontana S, Spits H, Karre K, Zappacosta S. A new mechanism of NK cell cytotoxicity activation: the CD40-CD40 ligand interaction. Journal of Experimental Medicine. 1997;185:2053–2060. doi: 10.1084/jem.185.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceruti S, Beltrami E, Matarrese P, Mazzola A, Cattabeni F, Malorni W, Abbracchio MP. A key role for caspase-2 and caspase-3 in the apoptosis induced by 2-chloro-2'-deoxy-adenosine (cladribine) and 2-chloro-adenosine in human astrocytoma cells. Molecular Pharmacology. 2003;63:1437–1447. doi: 10.1124/mol.63.6.1437. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhao Y, Gou WF, Zhao S, Takano Y, Zheng HC. The anti-tumor effects and molecular mechanisms of suberoylanilide hydroxamic acid (SAHA) on the aggressive phenotypes of ovarian carcinoma cells. PLoS ONE. 2013;8:e79781. doi: 10.1371/journal.pone.0079781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Vena DA, Foss FM, Sorensen JM. Neurotoxicity of purine analogs: a review. Journal of Clinical Oncology. 1994;12:2216–2228. doi: 10.1200/JCO.1994.12.10.2216. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Howell GM, Teggart CA, Chowdhury A, Person JJ, Bowers DM, Brattain MG. Histone deacetylase inhibitor belinostat represses survivin expression through reactivation of transforming growth factor beta (TGFbeta) receptor II leading to cancer cell death. Journal of Biological Chemistry. 2011;286:30937–30948. doi: 10.1074/jbc.M110.212035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I, Poreba E, Kamieniarz K, Schneider R. Histone modifiers in cancer: friends or foes? Genes Cancer. 2011;2:631–647. doi: 10.1177/1947601911417176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paepe ME, Gundavarapu S, Tantravahi U, Pepperell JR, Haley SA, Luks FI, Mao Q. Fas-ligand-induced apoptosis of respiratory epithelial cells causes disruption of postcanalicular alveolar development. American Journal of Pathology. 2008;173:42–56. doi: 10.2353/ajpath.2008.071123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Wang L, Wang CY, Yang T, Kumar MV, Dong Z. Induction of apoptosis in renal tubular cells by histone deacetylase inhibitors, a family of anticancer agents. Journal of Pharmacology and Experimental Therapeutics. 2008;325:978–984. doi: 10.1124/jpet.108.137398. [DOI] [PubMed] [Google Scholar]
- Edelman MJ, O'Donnell RT, Meadows I. Treatment of refractory large granular lymphocytic leukemia with 2-chlorodeoxyadenosine. American Journal of Hematology. 1997;54:329–331. doi: 10.1002/(sici)1096-8652(199704)54:4<329::aid-ajh13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang JM, Yang-Yen HF, Karras J, Jove R, Loughran TP., Jr Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. Journal of Clinical Investigation. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epling-Burnette PK, Bai F, Wei S, Chaurasia P, Painter JS, Olashaw N, Hamilton A, Sebti S, Djeu JY, Loughran TP. ERK couples chronic survival of NK cells to constitutively activated Ras in lymphoproliferative disease of granular lymphocytes (LDGL) Oncogene. 2004;23:9220–9229. doi: 10.1038/sj.onc.1208122. [DOI] [PubMed] [Google Scholar]
- Fandy TE, Shankar S, Ross DD, Sausville E, Srivastava RK. Interactive effects of HDAC inhibitors and TRAIL on apoptosis are associated with changes in mitochondrial functions and expressions of cell cycle regulatory genes in multiple myeloma. Neoplasia. 2005;7:646–657. doi: 10.1593/neo.04655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach C, Silverman LR. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncology. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillings AS, Balmanno K, Wiggins CM, Johnson M, Cook SJ. Apoptosis and autophagy: BIM as a mediator of tumour cell death in response to oncogene-targeted therapeutics. FEBS Journal. 2009;276:6050–6062. doi: 10.1111/j.1742-4658.2009.07329.x. [DOI] [PubMed] [Google Scholar]
- Griffiths EA, Gore SD. DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Seminars in Hematology. 2008;45:23–30. doi: 10.1053/j.seminhematol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson C, Mizzau M, Paroni G, Maestro R, Schneider C, Brancolini C. Role of caspases Bid p53 in the apoptotic response triggered by histone deacetylase inhibitors trichostatin-A (TSA) and suberoylanilide hydroxamic acid (SAHA) Journal of Biological Chemistry. 2003;278:12579–12589. doi: 10.1074/jbc.M213093200. [DOI] [PubMed] [Google Scholar]
- Hirsch CL, Ellis DJ, Bonham K. Histone deacetylase inhibitors mediate post-transcriptional regulation of p21WAF1 through novel cis-acting elements in the 3' untranslated region. Biochemical and Biophysical Research Communications. 2010;402:687–692. doi: 10.1016/j.bbrc.2010.10.085. [DOI] [PubMed] [Google Scholar]
- Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature Immunology. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- Huang DC, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, Newton K, Tschopp J, Strasser A. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x(L) Proceedings of the National Academy of Sciences of the United States of America. 1999;96:14871–14876. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genetics. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- Kalyaanamoorthy S, Chen YP. Energy based pharmacophore mapping of HDAC inhibitors against class I HDAC enzymes. Biochimica et Biophysica Acta. 2013;1834:317–328. doi: 10.1016/j.bbapap.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Kim PK, Park SY, Koty PP, Hua Y, Luketich JD, Billiar TR. Fas-associating death domain protein overexpression induces apoptosis in lung cancer cells. Journal of Thoracic and Cardiovascular Surgery. 2003;125:1336–1342. doi: 10.1016/s0022-5223(02)73227-3. [DOI] [PubMed] [Google Scholar]
- Kluin-Nelemans HC, Oldhoff JM, Van Doormaal JJ, Van 't Wout JW, Verhoef G, Gerrits WB, van Dobbenburgh OA, Pasmans SG, Fijnheer R. Cladribine therapy for systemic mastocytosis. Blood. 2003;102:4270–4276. doi: 10.1182/blood-2003-05-1699. [DOI] [PubMed] [Google Scholar]
- Kretzner L, Scuto A, Dino PM, Kowolik CM, Wu J, Ventura P, Jove R, Forman SJ, Yen Y, Kirschbaum MH. Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-Myc, hTERT, and microRNA levels. Cancer Research. 2011;71:3912–3920. doi: 10.1158/0008-5472.CAN-10-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy T, Loughran TP. Large Granular Lymphocyte Leukemia. Cancer Control. 1998;5:25–33. doi: 10.1177/107327489800500103. [DOI] [PubMed] [Google Scholar]
- Lamy T, Liu JH, Landowski TH, Dalton WS, Loughran TP., Jr Dysregulation of CD95/CD95 ligand-apoptotic pathway in CD3(+) large granular lymphocyte leukemia. Blood. 1998;92:4771–4777. [PubMed] [Google Scholar]
- Leone G, D'Alo F, Zardo G, Voso MT, Nervi C. Epigenetic treatment of myelodysplastic syndromes and acute myeloid leukemias. Current Medicinal Chemistry. 2008;15:1274–1287. doi: 10.2174/092986708784534947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MS, de Leval L, Quintanilla-Martinez L. Commentary on the 2008 WHO classification of mature T- and NK-cell neoplasms. Journal of Hematopathology. 2009;2:65–73. doi: 10.1007/s12308-009-0034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang X, Liu A, Liu S, Zhang L, Wu B, Hu Q. Berberine Induces Apoptosis in p53-Null Leukemia Cells by Down-Regulating XIAP at the Post-Transcriptional Level. Cellular Physiology and Biochemistry. 2013;32:1213–1224. doi: 10.1159/000354520. [DOI] [PubMed] [Google Scholar]
- Liu X, Ryland L, Yang J, Liao A, Aliaga C, Watts R, Tan SF, Kaiser J, Shanmugavelandy SS, Rogers A, Loughran K, Petersen B, Yuen J, Meng F, Baab KT, Jarbadan NR, Broeg K, Zhang R, Liao J, Sayers TJ, Kester M, Loughran TP., Jr Targeting of survivin by nanoliposomal ceramide induces complete remission in a rat model of NK-LGL leukemia. Blood. 2010;116:4192–4201. doi: 10.1182/blood-2010-02-271080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes K, Menu E, Van Valckenborgh E, Van Riet I, Vanderkerken K, De Bruyne E. Epigenetic modulating agents as a new therapeutic approach in multiple myeloma. Cancers (Basel) 2013;5:430–461. doi: 10.3390/cancers5020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. Journal of the National Cancer Institute. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- O'Brien S, Kurzrock R, Duvic M, Kantarjian H, Stass S, Robertson LE, Estey E, Pierce S, Keating MJ. 2-Chlorodeoxyadenosine therapy in patients with T-cell lymphoproliferative disorders. Blood. 1994;84:733–738. [PubMed] [Google Scholar]
- Perez-Galan P, Marzo I, Giraldo P, Rubio-Felix D, Lasierra P, Larrad L, Anel A, Naval J. Role of caspases and apoptosis-inducing factor (AIF) in cladribine-induced apoptosis of B cell chronic lymphocytic leukemia. Leukemia. 2002;16:2106–2114. doi: 10.1038/sj.leu.2402650. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Anderson A, Habibi JR, Crabtree TR, Mayo M, Harada H, Ferreira-Gonzalez A, Dent P, Grant S. The BH3-only protein Bim plays a critical role in leukemia cell death triggered by concomitant inhibition of the PI3K/Akt and MEK/ERK1/2 pathways. Blood. 2009;114:4507–4516. doi: 10.1182/blood-2008-09-177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robak T, Robak P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Current Pharmaceutical Design. 2012;18:3373–3388. doi: 10.2174/138161212801227005. [DOI] [PubMed] [Google Scholar]
- Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R, Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Experimental Hematology. 1996;24:406–415. [PubMed] [Google Scholar]
- Roger PM, Bernard-Pomier G, Counillon E, Breittmayer JP, Bernard A, Dellamonica P. Overexpression of Fas/CD95 and Fas-induced apoptosis in a patient with idiopathic CD4+ T lymphocytopenia. Clinical Infectious Diseases. 1999;28:1012–1016. doi: 10.1086/514739. [DOI] [PubMed] [Google Scholar]
- Romine JS, Sipe JC, Koziol JA, Zyroff J, McMillan R, Beutler E. Cladribine. BioDrugs. 1997;7:386–393. doi: 10.2165/00063030-199707050-00006. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Abujamra AL, Loew JE, Forman LW, Perrine SP, Faller DV. Histone deacetylase inhibitors reverse CpG methylation by regulating DNMT1 through ERK signaling. Anticancer Research. 2011;31:2723–2732. [PubMed] [Google Scholar]
- Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2009;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal DS, Miller HJ, Schram ED, Saven A. Beyond hairy cell: the activity of cladribine in other hematologic malignancies. Blood. 2010;116:2884–2896. doi: 10.1182/blood-2010-02-246140. [DOI] [PubMed] [Google Scholar]
- Spurgeon SE, Pindyck T, Okada C, Chen Y, Chen Z, Mater E, Abbi K, Epner EM. Cladribine plus rituximab is an effective therapy for newly diagnosed mantle cell lymphoma. Leukemia and Lymphoma. 2011;52:1488–1494. doi: 10.3109/10428194.2011.575489. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer Press; 2008. [Google Scholar]
- Teramo A, Gattazzo C, Passeri F, Lico A, Tasca G, Cabrelle A, Martini V, Frezzato F, Trimarco V, Ave E, Boscaro E, Piazza F, Facco M, Trentin L, Semenzato G, Zambello R. Intrinsic and extrinsic mechanisms contribute to maintain the JAK/STAT pathway aberrantly activated in T-type large granular lymphocyte leukemia. Blood. 2013;121:3843–3854. S3841. doi: 10.1182/blood-2012-07-441378. [DOI] [PubMed] [Google Scholar]
- Thurn KT, Thomas S, Moore A, Munster PN. Rational therapeutic combinations with histone deacetylase inhibitors for the treatment of cancer. Future Oncology. 2011;7:263–283. doi: 10.2217/fon.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Jing Y, Ouyang S, Liu B, Zhu T, Niu H, Tian Y. Inhibitory effect of valproic acid on bladder cancer in combination with chemotherapeutic agents in vitro and in vivo. Oncology Letters. 2013;6:1492–1498. doi: 10.3892/ol.2013.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willimott S, Wagner SD. Post-transcriptional and post-translational regulation of Bcl2. Biochemical Society Transactions. 2010;38:1571–1575. doi: 10.1042/BST0381571. [DOI] [PubMed] [Google Scholar]
- Yang FC, Agematsu K, Nakazawa T, Mori T, Ito S, Kobata T, Morimoto C, Komiyama A. CD27/CD70 interaction directly induces natural killer cell killing activity. Immunology. 1996;88:289–293. doi: 10.1111/j.1365-2567.1996.tb00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert Opinion on Investigational Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Richon V, Ni X, Talpur R, Duvic M. Selective induction of apoptosis by histone deacetylase inhibitor SAHA in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. Journal of Investigative Dermatology. 2005;125:1045–1052. doi: 10.1111/j.0022-202X.2005.23925.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.