Abstract
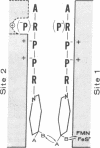
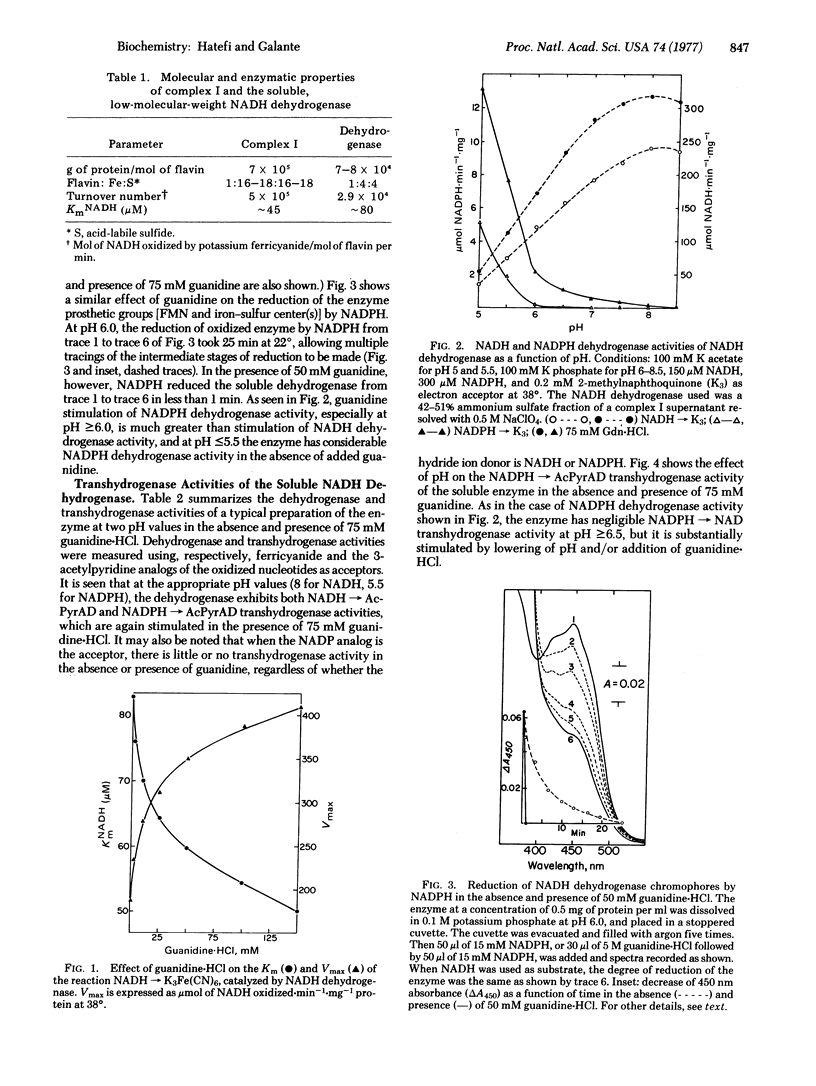
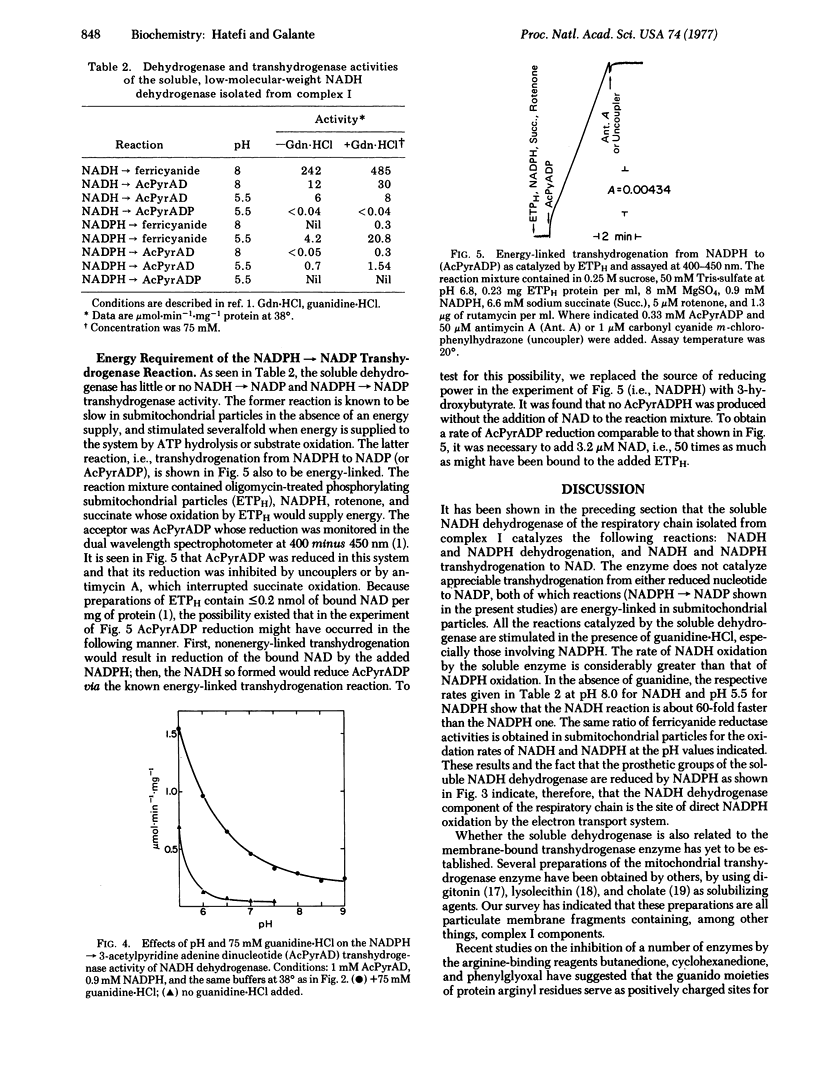
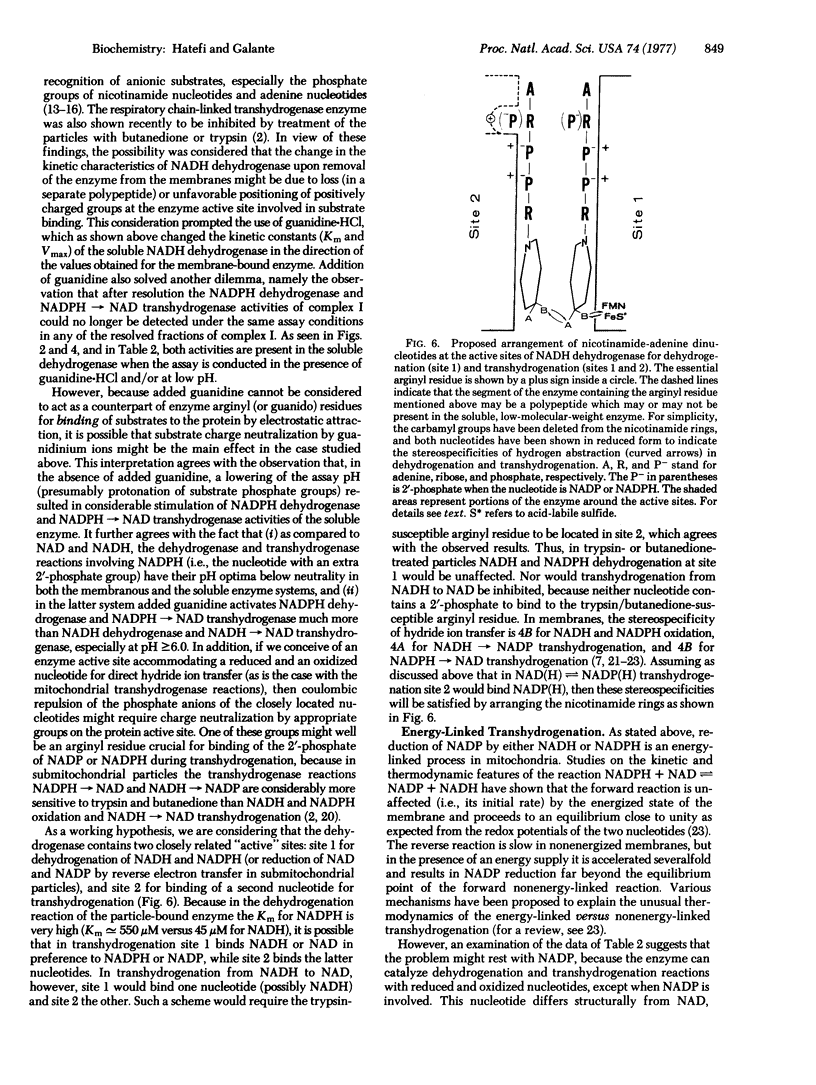
The soluble NADH dehydrogenase of low molecular weight, isolated from complex I (NADH:ubiquinone oxidoreductase, EC 1.6.5.3) of the respiratory chain, has been shown to have NADPH dehydrogenase and NADPH leads to NAD transhydrogenase activities. Both activities are greatly increased in the presence of added guanidine-HCl and at pH values less than 6.5. The chromophores of the soluble enzyme (flavin and iron--sulfur centers) are reduced by NADH and NADPH to the same extent. The latter reduction is extremely slow, and is considerably stimulated in the presence of guanidine-HCl. The soluble dehydrogenase has little or no NADH leads to NADP and NADPH leads to NADP transhydrogenase activity. The former reaction is known to be energy-linked in submitochondrial particles; the latter was shown in the present studies also to be energy-linked. In view of the above and earlier results, possible mechanisms for dehydrogenation and transhydrogenation (nonenergy-linked and energy-linked) involving reduced and oxidized NAD and NADP are proposed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borders C. L., Jr, Riordan J. F. An essential arginyl residue at the nucleotide binding site of creatine kinase. Biochemistry. 1975 Oct 21;14(21):4699–4704. doi: 10.1021/bi00692a021. [DOI] [PubMed] [Google Scholar]
- DANIELSON L., ERNSTER L. Demonstration of a mitochondrial energy-dependent pyridine nucleotide transhydrogenase reaction. Biochem Biophys Res Commun. 1963 Jan 18;10:91–96. doi: 10.1016/0006-291x(63)90274-2. [DOI] [PubMed] [Google Scholar]
- Djavadi-Ohaniance L., Hatefi H. Oxidation of NADPH by submitochondrial particles from beef heart in complete absence of transhydrogenase activity from NADPH to NAD. J Biol Chem. 1975 Dec 25;250(24):9397–9403. [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., GRIFFITHS D. E. Studies on the electron transfer system. XL. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. J Biol Chem. 1962 May;237:1676–1680. [PubMed] [Google Scholar]
- Hatefi Y., Bearden A. J. Electron paramagnetic resonance studies on the reduction of the components of complex I and transhydrogenase-inhibited complex I by NADH and NADPH. Biochem Biophys Res Commun. 1976 Apr 19;69(4):1032–1038. doi: 10.1016/0006-291x(76)90476-9. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Interactions of reduced and oxidized triphosphopyridine nucleotides with the electron-transport system of bovine heart mitochondria. Biochemistry. 1973 Aug 28;12(18):3515–3522. doi: 10.1021/bi00742a026. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E., Hanstein W. G. Inhibitors and activators of the mitochondrial reduced diphosphopyridine nucleotide dehydrogenase. J Biol Chem. 1969 May 10;244(9):2358–2365. [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Isolation and enzymatic properties of the mitochondrial reduced diphosphopyridine nucleotide dehydrogenase. J Biol Chem. 1969 May 10;244(9):2350–2357. [PubMed] [Google Scholar]
- Lange L. G., 3rd, Riordan J. F., Vallee B. L. Functional arginyl residues as NADH binding sites of alcohol dehydrogenases. Biochemistry. 1974 Oct 8;13(21):4361–4370. doi: 10.1021/bi00718a019. [DOI] [PubMed] [Google Scholar]
- Lee C. P., Simard-Duquesne N., Ernster L., Hoberman H. D. Stereochemistry of hydrogen-transfer in the energy-linked pyridine nucleotide transhydrogenase and related reactions. Biochim Biophys Acta. 1965 Sep 20;105(3):397–409. doi: 10.1016/s0926-6593(65)80226-0. [DOI] [PubMed] [Google Scholar]
- Marcus F., Schuster S. M., Lardy H. A. Essential arginyl residues in mitochondrial adenosine triphosphatase. J Biol Chem. 1976 Mar 25;251(6):1775–1780. [PubMed] [Google Scholar]
- Miles D. W., Urry D. W. Reciprocal relations and proximity of bases in pyridine dinucleotides. J Biol Chem. 1968 Aug 25;243(16):4181–4188. [PubMed] [Google Scholar]
- Ragan C. I. The structure and subunit composition of the particulate NADH-ubiquinone reductase of bovine heart mitochondria. Biochem J. 1976 Feb 15;154(2):295–305. doi: 10.1042/bj1540295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydström J., Da Cruz A. T., Ernster L. Steady-state kinetics of mitochondrial nicotinamide nucleotide transhydrogenase. 2. The energy-linked reaction. Eur J Biochem. 1971 Nov 11;23(2):212–219. doi: 10.1111/j.1432-1033.1971.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Rydström J. Evidence for a proton-dependent regulation of mitochondrial nicotinamide-nucleotide transhydrogenase. Eur J Biochem. 1974 Jun 1;45(1):67–76. doi: 10.1111/j.1432-1033.1974.tb03530.x. [DOI] [PubMed] [Google Scholar]
- Rydström J., Hoek J. B., Hundal T. Selective solubilization of nicotinamide nucleotide transhydrogenase from the mitochondrial inner membrane. Biochem Biophys Res Commun. 1974 Sep 9;60(1):448–455. doi: 10.1016/0006-291x(74)90224-1. [DOI] [PubMed] [Google Scholar]
- Rydström J., Kanner N., Racker E. Resolution and reconstitution of mitochondrial nicotinamide nucleotide transhydrogenase. Biochem Biophys Res Commun. 1975 Nov 17;67(2):831–839. doi: 10.1016/0006-291x(75)90888-8. [DOI] [PubMed] [Google Scholar]
- Vehar G. A., Freisheim J. H. Functional arginine residues involved in coenzyme binding by dihydrofolate reductase. Biochem Biophys Res Commun. 1976 Feb 9;68(3):937–941. doi: 10.1016/0006-291x(76)91235-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]