Abstract
The Wnt signaling pathways are a group of signal transduction pathways that play an important role in cell fate specification, cell proliferation and cell migration. Aberrant signaling in these pathways has been implicated in the development and progression of multiple cancers by allowing increased proliferation, angiogenesis, survival and metastasis. Activation of the Wnt pathway also contributes to the tumorigenicity of cancer stem cells (CSCs). Therefore, inhibiting this pathway has been a recent focus for cancer research with multiple targetable candidates in development. OMP-54F28 is a fusion protein that combines the cysteine-rich domain of frizzled family receptor 8 (Fzd8) with the immunoglobulin Fc domain that competes with the native Fzd8 receptor for its ligands and antagonizes Wnt signaling. Preclinical models with OMP-54F28 have shown reduced tumor growth and decreased CSC frequency as a single agent and in combination with other chemotherapeutic agents. Due to these findings, a phase 1a study is nearing completion with OMP-54F28 in advanced solid tumors and 3 phase 1b studies have been opened with OMP-54F28 in combination with standard of care chemotherapy backbones in ovarian, pancreatic and hepatocellular cancers. This article will review the Wnt signaling pathway, preclinical data on OMP-54F28 and other Wnt pathway inhibitors and ongoing clinical trials.
Keywords: OMP-54F28, Wnt inhibitor, Frizzled family receptor 8
1. Introduction
Wnt genes, defined for their sequence homology to Integration 1 (INT-1) in mice (Nusse and Varmus, 1982) and its homologue Wingless (Wg) in Drosophila (Cabrera et al., 1987), were shown early on to be imperative for cell fate determination and cell polarity during development. The vital role of Wnt signaling in development was largely elucidated in Drosophila, where loss of Wg led to segment polarity defects in mutant embryos (Nusslein-Volhard and Wieschaus, 1980). Since then, Wnt signaling has been shown to be important in development and axis formation in many organisms, including nematodes, frogs, mice and humans (Bodmer et al., 1987; McMahon and Moon, 1989; Moon et al., 1993; Nusse and Varmus, 1982; Rocheleau et al., 1997; Thorpe et al., 1997).
Wnt signaling pathway
Three Wnt signaling pathways have been defined, including the canonical, non-canonical planar cell polarity pathway and the non-canonical Wnt/Ca2+ pathway. Of the three, the canonical Wnt pathway is the best described. Here, a cysteine-rich Wnt ligand binds the extracellular cysteine-rich domain (CRD) at the amino terminus of a seven pass transmembrane receptor termed Frizzled (FZ/Fzd [Bhanot et al., 1996; Vinson et al., 1989]) and low-density lipoprotein (LDL) receptor-related protein 5/6 (LRP5/6) that acts as a co-receptor (Pinson et al., 2000; Tamai et al., 2000; Wehrli et al., 2000) to start the activation of the canonical Wnt signaling pathway. Nineteen Wnt ligands have been identified along with 10 Fzd receptors (Huang and Klein, 2004). Various Wnt ligands have been shown to bind to particular Fzd receptors, but this interaction is promiscuous wherein one Wnt can bind multiple Fzd receptors (Bhanot et al., 1996).
Wnt glycoproteins are relatively hydrophobic and insoluble possibly due to cysteine palmitoylation by Porcupine (PORC [Willert et al., 2003; Zhai et al., 2004]). However, PORC is required for Wnt signaling, suggesting that palmitoylation is essential in Wnt ligand secretion and pathway activation. Wnt ligands can activate signaling by both autocrine and paracrine signaling (Bafico et al., 2004). Wnt signaling can be inhibited through the binding of soluble Dickkopf (DKK) to LRP5/6 (Glinka et al., 1998) or secreted Frizzled-related protein (SFRP) binding to Wnt ligands due to their sequence homology to the CRD domain of Fzd (Hoang et al., 1996). Wnt inhibitor factor (WIF) proteins, due to their similarity to the extracellular domain of derailed/RYK Wnt transmembrane receptors, can also regulate Wnt signaling by interacting with Wnt ligands (Hsieh et al., 1999a).
When there is no Wnt ligand present, β-catenin levels are limited by the destruction complex that includes Adenomatous Polyposis Coli (APC) and AXIN. With Wnt signaling “off,” AXIN facilitates the phosphorylation of β-catenin by casein kinase 1 (CK1) and glycogen synthase kinase 3 (GSK-3 [Liu et al., 2002; Peifer et al., 1994; Sakanaka et al., 1999; Yost et al., 1996]). These phosphorylated ser/thr sites are recognized by an E3 ubiquitin ligase complex, and β-catenin is subsequently targeted for proteasomal degradation (Aberle et al., 1997). Therefore, β-catenin is maintained at low cytoplasmic and nuclear levels. In the “on” state, Wnt ligand binds the extracellular CRD of the amino terminus of Fzd and the LRP5/6 co-receptor (Dann et al., 2001; Pinson et al., 2000; Tamai et al., 2000). Dishevelled (Dsh/Dvl) is activated and recruited along with the destruction complex to the plasma membrane (Lee et al., 1999; Rothbacher et al., 2000). AXIN also interacts with the plasma membrane, possibly by binding the cytoplasmic tail of LRP5/6 (Mao et al., 2001). This binding is promoted by phosphorylation of LRP5/6 by GSK-3 and CK1 (Davidson et al., 2005; Zeng et al., 2005). AXIN is degraded, and GSK-3 is thus prevented from phosphorylating β-catenin. This leads to the accumulation of β-catenin in the nucleus and its interaction with T-cell factor (TCF) and lymphoid enhancer-binding protein (LEF) transcription factors to activated downstream targets (Behrens et al., 1996; Huber et al., 1996).
Wnt pathway and cancer
Aberrant Wnt signaling was first implicated in cancer in mouse studies, where mouse mammary tumor virus (MMTV) was found to be virally inserted into the promoter region of Int-1, promoting mammary tumors (Nusse and Varmus, 1982; Tsukamoto et al., 1988). It was later found that Int-1 was a homologue to Wg, and thus renamed Wnt (Nusse et al., 1991; Rijsewijk et al., 1987). Since this time, the Wnt pathway has been shown to be aberrantly regulated in many cancers. Abnormal β-catenin activation has been well characterized in colon cancer, where mutations in APC, or less frequently in β-catenin, results in constitutively active β-catenin and consequently active downstream effectors (Morin et al., 1997). While APC and β-catenin mutations are rare in lung cancer, overexpression of Dvl, Wnt-1 and Wnt-2 have all been correlated with non-small cell lung cancer (NSCLC) (He et al., 2004; Pongracz and Stockley, 2006; Ueda et al., 2001; Uematsu et al., 2003; You et al., 2004c). Moreover, increased tumor relapse was associated with a TCF4 Wnt gene signature in lung adenocarcinomas (Nguyen et al., 2009b). Together, this data provide strong evidence for the role of Wnt signaling in lung cancers. Wnt-5a has also been shown to be increased in breast cancer (Lejeune et al., 1995). Several of Fzds that have been shown to be overexpressed in cancers and/or cancer cell migration include Fzd4, Fzd7, Fzd8 and Fzd10 (Fukukawa et al., 2009; Jin et al., 2011; Ueno et al., 2009; Wang et al., 2012b; Yang et al., 2011). These have been shown to activate the canonical and/or non-canonical Wnt pathway. However, these are just a few of the studies linking Fzd overexpression with cancer, and an extensive list was previously covered by Ueno et al. (Ueno et al., 2013).
Wnt expression has also been associated with metastasis and tumor microenvironment. Inhibition of Wnt signaling by RNAi targeting LEF1 and HOXB9 reduced brain and bone metastasis using a mouse model of lung adenocarcinoma (Nguyen et al., 2009b). The mechanism of LEF1 and HOXB9 metastasis promotion was not elucidated in this study although Wnt signaling, specifically Wnt-1 and Wnt-5a, has been shown to increase proliferation and survival of endothelial cells (Masckauchan et al., 2006; Masckauchan et al., 2005). β-catenin was also shown to correlate with VEGF expression in colon cancer (Easwaran et al., 2003; Zhang et al., 2001), suggesting a role for Wnt signaling in angiogenesis. Moreover, Wnt-5a expression has recently been shown to be increased in NSCLC; its expression in patient tissue was correlated with expression of angiogenesis related proteins such as vascular endothelial cadherin and matrix metalloprotease 2, microvessel density and vasculogenic mimicry, all of which suggest a role for Wnt-5a in promoting angiogenesis (Yao et al., 2014).
Decreased expression of Wnt pathway inhibitors (WIF-1, DKKs, and SFRPs) “allow” for the activation of Wnt signaling and have also been observed in various cancers. For example, the down-regulation of associated Wnt antagonist, WIF-1, has been implicated in breast, prostate, lung and bladder cancer (Wissmann et al., 2003). Furthermore, WIF-1 has been shown to be epigenetically silenced in lung and bladder cancer (Mazieres et al., 2004; Urakami et al., 2006). Epigenetic silencing of DKK-1 has been shown in colorectal cancer (Aguilera et al., 2006) and SFRP in NSCLC, hepatocellular carcinoma and colorectal cancer (Fukui et al., 2005; Shih et al., 2006; Suzuki et al., 2004). Recent studies suggest that Wnt inhibitors may also play a pro-apoptotic role, where reduced apoptosis and p53 expression were observed in mammary glands isolated from SFRP−/− mice following induction of DNA damage by γ-irradiation (Gauger and Schneider, 2014). In addition, another study suggests that WIF-1 may inhibit angiogenesis. DKK-1 and WIF-1 directly interact and together may act as co-regulators in promoting apoptosis in the human umbilical vein endothelial cell (HUVEC) system (Ko et al., 2014).
Although Wnt signaling is not as well correlated with head and neck squamous cell carcinoma (HNSCC) as with other cancers, such as colon cancer, recent studies provide evidence that Wnt signaling is an attractive target in HNSCC. Wnt pathway activation has been shown in HPV positive HNSCC, possibly driven by E6 and E7 (Rampias et al., 2010). β-catenin nuclear accumulation was also observed in the majority of patient HNSCC tumor samples (Wend et al., 2013). Up-regulation of several Fzd receptors was observed in HNSCC, including Fzd1, Fzd7a, Fzd10b, Fzd2 and Fzd13 (Rhee et al., 2002). Furthermore, Wnt expression may affect radiosensitivity in HNSCC cell lines, where β-catenin nuclear accumulation was correlated with radiation-resistance (Chang et al., 2008). Similarly, radiation-resistant mouse mammary progenitor cells were associated with active Wnt signaling (Chen et al., 2007; Woodward et al., 2007). Wnt expression has been correlated with therapy resistance in prostate cancer, where Wnt16B increased following therapy and lessened DNA damage following treatment with a topoisomerase inhibitor (Sun et al., 2012). In this study, Wnt16B increased growth and proliferation. Taken together, these studies suggest that Wnt expression not only promotes cancer cell proliferation, but may also affect treatment efficacy. Furthermore, the up-regulation of Wnt16B originating specifically in the stroma compartment, and through tumor-stroma interactions promoting therapy resistance in the tumor compartment, suggests that the stroma is a favorable target for therapy. Consistent with this, human ovarian fibroblasts released Wnt16B into the stroma compartment following DNA damage by radiation or chemotherapy (Shen et al., 2014). Interestingly stromal Wnt16B activated the Wnt signaling pathway in dendritic cells (DCs), causing the release of interleukin-10 ( IL-10) and tumor growth factor-β (TGF-β) and regulatory T cell differentiation. Thus, Wnt16B may not only confer therapy resistance, but also alter the tumor microenvironment and as the authors suggest, possibly promote immune evasion.
Wnt signaling and cancer stem cells (CSCs)
Wnt signaling is important in stem cell homeostasis. In the intestinal villi Wnt signaling is particularly important in stem cell maintenance as well as determining stem cell fate (Batlle et al., 2002; Korinek et al., 1998). Wnt signaling has also been shown to be essential in stem cell proliferation and hair follicle development and may function to activate stem cells in the bulge to more proliferative progenitor cells, as well as determining cell fate (Andl et al., 2002; Choi et al., 2013; Lien et al., 2014; Lowry et al., 2005). Similarly, Wnt overexpression in hematopoietic stem cells lead to the expansion of progenitor cells, suggesting that Wnt signaling is also important in hematopoiesis (Austin et al., 1997).
Aberrant Wnt signaling in the stem cell compartment has been shown to contribute to tumorigenesis. Here, it is important to note that while some authors appropriately choose conservative terminology in the definition of CSCs, for the purpose of coherency in this review, we loosely combine tumor-initiating, tumor propagating and CSCs into one term, as CSCs. Loss of APC, consequently leading to the accumulation of β-catenin, in colorectal cells resulted in cells maintaining a phenotype similar to progenitor cells of the crypt (Sansom et al., 2004). In another approach, high levels of Wnt expression were observed in CSCs from colon cancer grown as spheroids (Vermeulen et al., 2010). Similarly, Wnt-1, -3 and -5a all promoted murine mammosphere growth, a method that enriches for stem cells, and results suggested both canonical and non-canonical Wnt signaling could promote growth (Many and Brown, 2014). Furthermore, hair follicle tumors were observed to have stable expression of β-catenin in mice (Gat et al., 1998). A recent study found hair follicle stem cells (HFSC) treated with dimethylbenzanthracene (DMBA) and 12-O-Tetradecanoylphorbol-13-acetate (TPA) induced sebaceous neoplasms in C57BL/6 mice, as well as increased Wnt10b expression in basal cells via immunostaining (Qiu et al., 2014). Here the authors propose a model wherein increased Wnt10b results in proliferation and differentiation of HFSCs and thus promoting sebaceous neoplasms. High levels of Wnt expression was also observed in granulocyte-macrophage progenitors isolated from chronic myeloid leukemia (CML) patients and correlated with increased self-renewal (Jamieson et al., 2004). Fzd4 was suggested to regulate “stemness” of cancer cells and promote invasiveness in glioma cells (Jin et al., 2011). In HNSCC cell lines, side populations sorted by Hoechst efflux, a functional assay for enriching stem cells, were more invasive and tumorigenic in nude mice, and importantly these populations exhibited higher Wnt signaling (Song et al., 2010). Together, the data suggest that the same Wnt signaling mechanisms that regulate stem cells, when abnormal, may contribute to the tumorigenic potential of CSCs.
2. Targeting the Wnt pathway
Wnt pathway components are often difficult to target due to their redundancy in other functions. β-catenin, for example, also interacts with E-cadherin, an interaction that is essential for cell adhesion, as well as interacting with APC and TCF competitively within the same armadillo repeat domain (Behrens et al., 1996; Hulsken et al., 1994; Ozawa et al., 1989). In order to circumvent this, specific inhibitors that disrupt the β-catenin and TCF interaction have been widely explored, as well as RNAi approaches. However, even with utmost specificity, due to the essential role of the Wnt pathway in stem cell maintenance, tissue homeostasis and cell fate determination, targeting this signaling pathway has potential pitfalls. A potential concern is that toxicity, specifically to the GI tract, as well as anemia and immune suppression, might be too great for obtaining an adequate therapeutic index. In spite of these potential hurdles, research toward identifying potent Wnt pathway antagonists for cancer treatment has been promising.
Natural compounds
Non-steroidal anti-inflammatory drugs (NSAIDS), vitamins A and D, and polyphenols, such as curcumin and resveratrol, have all been shown to inhibit the Wnt pathway, and these have been elegantly reviewed (Table 1 and Figure 1 [Barker and Clevers, 2006; Takahashi-Yanaga and Kahn, 2010; Takahashi-Yanaga and Sasaguri, 2007]). These compounds, although promising, have shown insufficient efficacy and thus may prove ineffectual as single-agent treatments. For example, the use of NSAIDS, specifically Sulindac, in patients diagnosed with Familial Adenomatous Polyposis (FAP) reduced the number of polyps by only ~44% (Giardiello et al., 1993). Quercetin, a polyphenol and dietary flavonoid, has also been shown to decrease β-catenin and TCF protein levels (Figure 1) and inhibit colon cancer cell growth in vitro via decreased cyclin D1 and survivin levels (Park et al., 2005; Shan et al., 2009). Quercetin was also shown to inhibit murine mammary cancer cell growth and target the Wnt pathway through DKK1,2,3 and 4 upregulation (Kim et al., 2013). Salinomycin, an antibacterial potassium ionophore, was first identified by high throughput screening and was shown to inhibit breast CSCs (Gupta et al., 2009). Its mechanism was later elucidated and was shown to inhibit LRP5/6 phosphorylation, causing its degradation (Figure 1 [Lu et al., 2011a]). Salinomycin has recently been shown to inhibit breast and prostate cancer cell proliferation and induce apoptosis, targeting Wnt signaling by decreased LRP5/6 expression, but also by targeting mTORC (Lu and Li, 2014), suggesting it may function in targeting multiple pathways. Salinomycin has also been shown to have anti-tumorigenic effects in hepatocellular carcinoma, osteosarcoma, gastric cancer, NSCLC and nasopharygeal carcinoma; studies suggest that is specifically targets CSCs by inhibiting cell proliferation, inducing apoptosis and limiting cell migration (Arafat et al., 2013; Mao et al., 2014; Tang et al., 2011; Wang et al., 2012a; Wu et al., 2014). COX-2 inhibitors may target the Wnt pathway by inhibiting prostaglandin E2 (PGE2), the product of COX-2, which acts to phosphorylate GSK-3 (Figure 1 [Fujino et al., 2002]). Celecoxib, a NSAID and a COX-2 inhibitor, has been shown to decrease CD133 expression, a surface marker of prostate CSCs, by targeting the Wnt pathway, and this effect was observed to be independent of its COX-2 inhibiting activity (Deng et al., 2013). In order to circumvent the toxicities associated with long term COX-2 inhibition, one group suggests using synthetic derivatives of sulindac, another NSAID that was previously mentioned, that do not target COX-2 and were successful in limiting colon cancer cell growth and promoting apoptosis in vitro (Li et al., 2013; Whitt et al., 2012). Resveratrol has recently been shown to inhibit the growth of breast CSCs both in in vitro and when implanted in NOD/SCID mice by targeting the canonical Wnt pathway and inducing autophagy (Fu et al., 2014). Resveratrol also limited growth of cervical cancer cells by causing cell cycle arrest and inducing apoptosis (Zhang et al., 2014b). This study found resveratrol not only disrupted Wnt signaling, but also abrogated Notch and STAT3 signaling. Although resveratrol inhibits the Wnt pathway, possibly by disrupting the β-catenin/TCF interaction (Figure 1 [Chen et al., 2012]), its mechanism may not be specific to cancer cells. When ingested by patients, resveratrol appeared to primarily target the normal colon mucosa (Nguyen et al., 2009a). In this scenario, it is obvious that the effectiveness of these compounds may depend on the innovation of researchers to deliver the drug directly and specifically to the tumor.
Table 1.
Investigational Wnt inhibitors tested in pre-clinical models
| Wnt inhibitor | Target | Endpoint | Experimental cancer targets | References |
|---|---|---|---|---|
| Natural Compounds | ||||
| NSAIDS (e.g. Sulindac and Celecoxib) | Cyclooxygenase | In vitro cell proliferation, in vitro cell death | Colorectal (CRC) | Li et al. 2013, Whitt et al. 2013 |
| Polyphenols (e.g Resveratrol and Quercetin) | B-catenin/TCF interaction | In vitro cell proliferation, In vitro cell death, in vivo tumor growth | CRC, breast, cervical | Fu et al. 2014, Zhang et al. 2014b, Chen et al. 2012, Nguyen et al. 2009a, Park et al. 2005, Kim et al. 2013 |
| Salinomycin | LRP5/6 | In vitro cell proliferation, in vitro cell death, in vivo tumor growth, migration/invasion | CRC, breast, prostate, NSCLC, gastric, osteosarcoma, hepatocellular | Shan et al. 2009, Gupta et al. 2009, Lu and Li 2014, Arafat et al. 2013, Mao et al. 2013, Tang et al. 2011, Wang et al. 2012, Lu et al. 2014 |
| PKF115–584, PKF222–815 and CPG049090 | B-catenin/TCF interaction | In vitro cell proliferation, in vitro cell death | CRC | Lepourcelet et al., 2004, Mologni et al. 2012 |
| Rabdoternin B and Maoecrystal I | In vitro cell proliferation, in vitro cell death | CRC | Zhang 2014a | |
| Small molecule inhibitors | ||||
| PNU74654 | B-catenin/TCF interaction | Trosset et al., 2006 | ||
| ICG-001 | B-catenin/CBP interaction | In vitro cell proliferation, in vitro cell death, in vivo tumor growth | CRC, HNSCC, breast, pancreatic | Emami et al. 2004 Wend et al. 2013, Holland et al. 2013, Arensman et al. 2014 |
| 2,4-diamino-quinazoline | B-catenin/TCF interaction | In vivo tumor growth | CRC | Chen et al. 2009c |
| XAV939 | Tankyrase inhibition/Axin stabilization | In vitro cell proliferation, in vitro cell death, migration | CRC, neuroblastoma, Breast | Huang et al. 2009, Bao et al. 2012, Tian et al. 2013 |
| IWR-1 | Tankyrase inhibition/Axin stabilization | In vitro cell proliferation | CRC prostate | Chen et al. 2009a |
| JW55 | Tankyrase inhibition/Axin stabilization | In vitro cell proliferation, In vivo tumor growth | CRC | Waaler et al. 2012 |
| LGK974 | PORC/Wnt ligand palmitoylation | In vitro cell proliferation, in vivo tumor growth | Breast, HNSCC | Liu et al. 2013 |
| NSC668036 | Dvl | Shan et al. 2005 | ||
| FJ9 | Dvl | In vitro cell death, in vivo tumor growth | Melanoma, NSCLC | Fujii et al. 2007 |
| 3289–8625 | Dvl | In vitro cell proliferation | Prostate | Grandy et al. 2009 |
| Pyrivinium | CK1 and Pygopus | In vitro cell proliferation, in vitro cell death, in vivo tumor growth, migration | CRC | Thorne et al. 2010, Wiegering et al. 2014 |
| Niclosamide | Fz-1 and Dvl-2 and/or LRP6 | In vitro cell proliferation, in vitro cell death, in vivo tumor growth, migration | Osteosarcoma, breast, ovarian, prostate, hepatocellular, glioblastoma | Chen et al. 2009b, Wang et al. 2013, Ye et al. 2014, Yo et al. 2012, Londono-Joshi et al. 2014, Lu et al. 2011b, Arend et al. 2014, Tomizawa et al. 2013, Wieland et al. 2013 |
| Viral based inhibitors | ||||
| Recombinant adenoviruses | Promote TCF dependent cell killing | In vitro cell proliferation, in vitro cell death, in vivo tumor growth | CRC, lung, hepatocellular | Chen and McCormick 2001, Lipinkski et al. 2004, Kwong et al. 2002, Brunori et al. 2001, Fuerer et al. 2002, Toth et al. 2004, Lukashev et al. 2005, Fuerer and Iggo 2004., Peerlinck et al. 2009 |
| Antibody-based inhibitors | ||||
| Wnt-1 antibody | Wnt-1 | In vitro cell proliferation, in vitro cell death, in vivo tumor growth | NSCLC, CRC, sarcoma, mesothelioma, HNSCC | He et al. 2004, He et al. 2005, Mikami et al. 2005, You et al. 2004a, Rhee et al. 2002 |
| Wnt-2 antibody | Wnt-2 | In vitro cell death, in vivo tumor growth | Melanoma, NSCLC | You et al. 2004b, You et al. 2004c |
| Wnt-10b antibody | Wnt-10b | In vitro cell proliferation | HNSCC | Rhee et al. 2002 |
| OMP-18R5 | Fzd1,2,5,7, and 8 | In vivo tumor growth | CRC, breast, pancreatic, NSCLC, teratocarcinoma | Gurney et al. 2012 |
| WIF1-Fc and sFRP-Fc | Wnt ligands | In vitro cell proliferation, in vitro cell death, in vivo tumor growth | Hepatocellular | Hu et al. 2009 |
| Fzd8(1–173)hFc | Wnt ligands | Hsieh et al. 1999b, Reya et al. 2003 | ||
| F8CRDhFc | Wnt ligands | In vivo tumor growth | Breast, teratoma | DeAlmeida et al. 2007 |
| OMP-54F28 | Wnt ligands | In vivo tumor growth | Breast, pancreatic | Hoey 2013 |
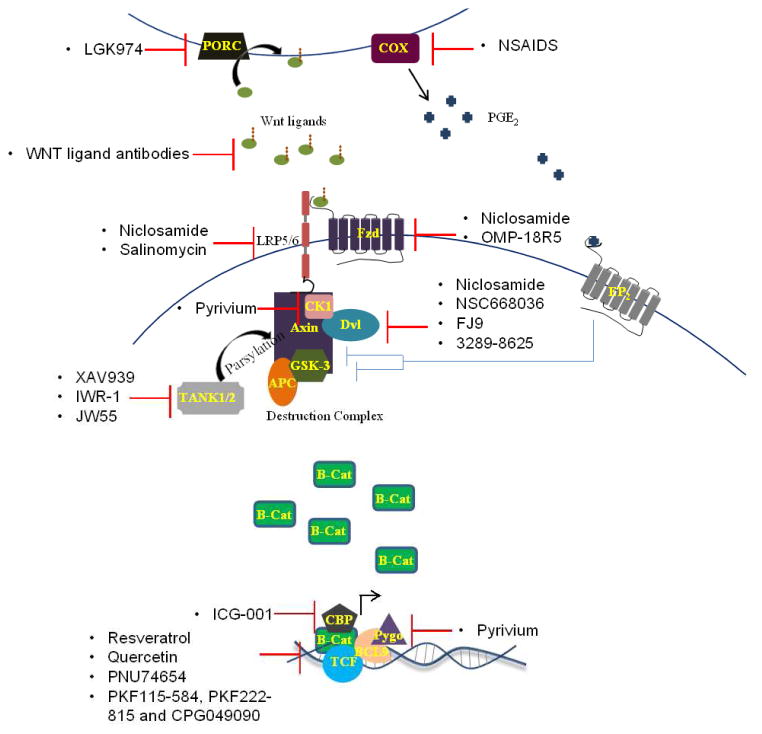
Figure 1. Mechanisms of inhibitors within the Wnt pathway.
Wnt inhibitors act at various points within the active Wnt pathway. Common targets include Wnt ligands, including sequestration by OMP-54F28, and the β-catenin/TCF interaction. LGK974 is unique in that it inhibits pathway activation by preventing Wnt ligand secretion by inhibiting palmitoylation by PORC. COX inhibition by NSAIDS prevents PGE2 from blocking the function of GSK-3 and Axin. Other targets are the Wnt receptor, Fzd, and co-receptor LRP5/6. Several inhibitors act to stabilize the destruction complex, thus preventing the accumulation of β-catenin and transcription of downstream effectors. Alternatively, others prevent transcription by inhibiting transcriptional co-factors.
Similarly, natural compounds that disrupt the β-catenin/TCF interaction have been identified using high-throughput ELISAs, including PKF115–584, PKF222–815 and CPG049090 (Lepourcelet et al., 2004). Although these compounds were shown to decrease colon cancer cell proliferation with minimal disruption to the β-catenin/E-cadherin interaction, they abolished the β-catenin/APC interaction suggesting that targeting specific β-catenin interactions is problematic. One group showed synergistic effects in growth inhibition when combining PKF115–584 or pyrivium, a Wnt small molecule inhibitor mentioned below, with a KRAS inhibitor in colorectal cancer cells that were Wnt driven and/or carrying an activating KRAS mutation, making an argument for Wnt inhibitors in combination therapy (Mologni et al., 2012).
Two natural compounds, rabdoternin B and maoecrystal I, have recently been identified by using a dual-luciferase reporter assay to screen compounds that target the Wnt pathway (Zhang et al., 2014a). In this study, cytotoxicity was observed when colon cancer cells were treated with either compound compared to limited cell killing in treated normal colon cells, emphasizing the promise of newly discovered and unexplored compounds.
Small molecule inhibitors
There are many inhibitors that specifically disrupt the interaction of β-catenin with other key components of the Wnt pathway. PNU74654 was discovered by high-throughput screening, and was shown to inhibit the interaction of β-catenin and TCF (Figure 1 [Trosset et al., 2006]). Treatment with certain 2,4-diamino-quinazoline derivatives, another compound that disrupts the β-catenin/TCF interaction, resulted in 20–35% tumor growth inhibition when colorectal cells were implanted in nude mice (Chen et al., 2009c).
Another approach is to disrupt a different β-catenin/activator interaction. Emami et al. identified a small molecule inhibitor using a cell-based screen that specifically bound to CREB binding protein (CBP), a TCF co-activator, termed ICG-001 (Figure 1 [Emami et al., 2004]). This inhibitor has been experimentally explored in other diseases with aberrant Wnt signaling including kidney disease and pulmonary fibrosis with promising success (Hao et al., 2011; Henderson et al., 2010; Sasaki et al., 2013). ICG-001, at higher doses, was found to induce apoptosis in colon cancer cells with minimal effects on normal colon cells in vitro (Emami et al., 2004). Using ICG-001 in combination with a Met inhibitor and a CXCR4 inhibitor delayed tumor onset in a breast cancer mouse model (Holland et al., 2013). Tumors that arose from CSCs (CD24+ CD29+) isolated from salivary gland tumors grown in NOD/SCID mice and passed into NOD/SCID mice had decreased tumor volume when treated with ICG-001 (Wend et al., 2013). These salivary gland tumors were originally grown in mice that were double-mutants, wherein mice had a gain of function mutation in β-catenin and a loss of function mutation in BMPR1A, a receptor in bone morphogenetic proteins (BMP) signaling which has been shown to inhibit CSCs proliferation in glioblastomas (Piccirillo et al., 2006). These were subsequently implanted into NOD/SCID mice, suggesting that Wnt inhibition with ICG-001 is effective in inhibiting tumor growth where Wnt activation is one of the key drivers. ICG-001 has also been shown to inhibit cell proliferation in pancreatic ductal adenocarcinoma (PDAC) by causing a G1 cell cycle arrest; however this effect appeared to be independent of Wnt signaling inhibition, suggesting ICG-001 may also target other pathways (Arensman et al., 2014).
Several small molecule inhibitors, including XAV939, JW55 and IWR-1 promote β-catenin degradation by inhibiting PARsylation by Tankyrase 1 and Tankyrase 2 and thereby stabilizing axin (Figure 1 [Chen et al., 2009a; Huang et al., 2009; Waaler et al., 2012]). XAV939 promoted apoptosis in neuroblastoma cells and inhibited proliferation under serum-deprivation in breast and colorectal cancer cells (Bao et al., 2012; Tian et al., 2013). JW55 inhibited in vivo tumor growth in APC mutant mice using colorectal carcinoma cells (Waaler et al., 2012). Similarly, IWR-1 inhibited colon and prostate cancer cell growth (Chen et al., 2009a).
Other small molecule inhibitors target Dvl or PORC (Figure 1). LGK974 inhibits PORC, an O-acyltransferase that is required for the palmitoylation of Wnt ligands and ligand secretion (Zhai et al., 2004) and induced tumor regression in vivo using a mouse model for Wnt-driven breast cancer and HNSCC (Liu et al., 2013). Interestingly exome sequencing a panel of 40 HNSCC cell lines showed a strong correlation between LGK974 sensitivity and Notch1 mutations although the significance of this has yet to be elucidated. Several small molecule inhibitors target the Wnt pathway by interacting with Dvl and thereby the destruction complex, ultimately leading to decreased β-catenin. NSC668036, FJ9 and 3289–8625 were shown to inhibit Wnt signaling by directly binding the PDZ domain of Dvl (Fujii et al., 2007; Grandy et al., 2009; Shan et al., 2005). 3289–8625 was shown to inhibit PC3 prostate cancer cells growth (Grandy et al., 2009), and FJ9 was shown to induce apoptosis in both melanoma and NSCLC cell lines (Fujii et al., 2007). FJ9 also inhibited tumor growth using implanted NSCLC cells in a mouse xenograft model.
Two FDA-approved anthelmintics effectively inhibit the pathway by targeting several factors. Using a high-throughput small molecule screen, Pyrivinium was identified as a Wnt antagonist (Thorne et al., 2010). Pyrivinium, classically used in the treatment for pinworm infection (Royer and Berdnikoff, 1962), inhibits Wnt signaling at multiple points in the pathway (Figure 1). It binds and induces a conformational change in CK1, promoting its kinase activity, and thus stabilizing axin and retaining β-catenin in the cytoplasm. Furthermore, it promoted the degradation of pygopus, a nuclear factor that is required by β-catenin for transcription of downstream Wnt targets (Thorne et al., 2010). Pyrivinium has recently been shown to target Wnt signaling in colon cancer cells, resulting in increased cell death, inhibition of cell migration and delaying liver metastasis growth in vivo (Wiegering et al., 2014). Another FDA-approved drug termed niclosamide, routinely used in the treatment of tapeworm, inhibited Wnt signaling by causing Fzd1 receptor internalization and decreased Dvl2 protein levels in human osteosarcoma cells (Figure 1 [Chen et al., 2009b]). In contrast, another study suggests that niclosamide acts through targeting LRP6, both decreasing its phosphorylation and overall protein expression. In this study, Dvl2 was unperturbed, and decreased cell proliferation and apoptosis induction was observed in prostate and breast cancer cells (Lu et al., 2011b). These findings suggest the mechanisms are dependent on cell type, warranting more studies on this compound. Niclosamide was found to decrease spheroid growth, increase apoptosis and inhibit tumor growth in NOD/SCID mice when using the side population sorted from breast cancer cells (Wang et al., 2013). Both spheroid growth and side population highly enrich for CSCs, indicating that niclosamide may function in target CSCs. Ye et al. used breast cancer cells and observed decreased proliferation, migration and invasion, as well as increased apoptosis and decreased tumor growth in an in vivo mouse model (Ye et al., 2014). Niclosamide has also been used to target basal-like breast, liver, brain and ovarian cancer (Arend et al., 2014; Londono-Joshi et al., 2014; Tomizawa et al., 2013; Wieland et al., 2013; Yo et al., 2012). It is important to note that these in vivo studies, as well as the ones stated earlier, have shown little to limited levels of toxicity, providing hopeful optimism for Wnt inhibition in human cancer therapy.
Viral-based inhibitors
Numerous studies have used viral-based targeting with recombinant adenoviruses (Barker and Clevers, 2006). This is accomplished by integrating TCF binding sites into robust promoters and thus achieving cell killing specific to cells with active Wnt signaling. Cancer cell killing was attained through manipulation of adenoviruses with E1 and E2 promoters, and these effectively targeted cancers with aberrant Wnt signaling (Brunori et al., 2001; Fuerer and Iggo, 2002). Surprisingly, Brunori et al. observed cell killing in lung cancer cells, and little effect in colon cancer cells. However, the reason for this was largely unknown. Variations of this have been done, and in one study, the addition of ADP cytosolic protein boosted the ability of the virus to spread from cell to cell (Toth et al., 2004). In addition, viral expression and effectiveness in tumor growth inhibition using mouse xenograft models was specific to colon cancer cells and non-effective in lung cancer cells. This suggests specificity to those cancers with greater levels of Wnt activity. In another approach, using TCF-driven E1 and E4 promoters, the Na/I symporter (hNIS) gene was included in the recombinant adenovirus (Peerlinck et al., 2009). This resulted in the enhancement of 131I− radiotherapy and allowed for imaging and tracking the spread of the adenovirus using computed tomography (CT) imaging when injected with 99mTcO4−.
Similarly, other studies combine cytotoxic gene expression with specificity to the Wnt pathway by the integration of promoters that are under the control of TCF. Using this technique and various promoters, apoptosis promoting Fas-associated via death domain (fadd), diphtheria toxin A (DTA) and herpes simplex virus thymidine kinase (HSV TK) genes have all been expressed and shown to be effective in targeting cancer cells with active Wnt signaling (Chen and McCormick, 2001; Kwong et al., 2002; Lipinski et al., 2004). Alternatively, others have added a gene that enhances cytotoxicity of prodrugs in order to increase therapeutic efficacy (Fuerer and Iggo, 2004; Lukashev et al., 2005).
Antibody-based inhibitors
As stated earlier, because of the overexpression of Wnt ligands and/or receptors in many cancers, antibody-based inhibitors have been developed to bind and sequester either free ligand or Fzd receptors (Figure 1). Several antibodies toward Wnt ligands have been produced. A monoclonal Wnt-1 antibody was shown to induce apoptosis in NSCLC and breast cancer cells by Wnt inhibition and activating cytochrome c and caspase 3, as well as decreasing survivin expression (He et al., 2004). Furthermore, the Wnt-1 antibody inhibited tumor growth in nude mice with NSCLC cells implanted subcutaneously, independent of whether the antibody was administered at implantation or once tumors were established, suggesting the timing for antibody administration was irrelevant for tumor control. Similar results were observed in colon cancer and sarcoma using the Wnt-1 antibody (He et al., 2005; Mikami et al., 2005). The Wnt-1 antibody also induced apoptosis in mesothelioma cells that were deficient in β-catenin, suggesting non-canonical Wnt signaling inhibition was possible as well (You et al., 2004a). By the same token, a monoclonal Wnt-2 antibody induced apoptosis in NSCLC and melanoma, as well as inhibited tumor growth in a melanoma xenograft model (You et al., 2004b; You et al., 2004c). In Wnt-activated HNSCC cells, both Wnt-1 and Wnt-10b antibodies effectively blocked Wnt signaling, induced apoptosis and inhibited cell proliferation (Rhee et al., 2002).
OMP-18R5 is a monoclonal antibody that was initially identified for its ability to bind Fzd7. Since then, OMP-18R5 has been found to bind Fzd1, Fzd2, Fzd5, Fzd7 and Fzd8 and block β-catenin signaling in response to Wnt3a ligand (Gurney et al., 2012). In the same study, using human tumor xenografts, OMP-18R5 inhibited tumor growth in several tumor types, including colon, breast, pancreatic and lung cancer. Tumor recurrence was also delayed. Furthermore, the addition of OMP-18R5 to standard-of-care chemotherapies, such as paclitaxel, increased efficacy in tumor growth inhibition in a synergistic manner. Although off-target effects were suggested in that several Wnt genes were inhibited in the mouse liver, at effective doses there was little toxicity observed to the GI tract.
In another approach, peptides with complementary sequences interacting with either the Wnt ligand or Fzd receptor are fused with the immunoglobulin Fc domain. Using this approach, for example, WIF1-Fc and SFRP-Fc were expressed in cancer cells using recombinant adenoviruses (Hu et al., 2009). Wnt signaling inhibition by these antagonists inhibited tumor growth and prolonged survival in hepatocellular xenografts.
3. OMP-54F28: Preclinical Data
Effective Wnt targeting has been accomplished using an immunoglobin Fc fused to Fzd8, Fzd8(1–173)hFc (Figure 1 [Hsieh et al., 1999b; Reya et al., 2003]). Others improved upon this, and constructed a minimal Fzd8 protein (residues 1–155), wherein possible protease cleavage sites were removed (DeAlmeida et al., 2007). The fusion of the CRD domain of Fzd8 with Fc (F8CRDhFc) exhibited an extended half-life in vivo in comparison to Fzd8(1–173)hFc and successfully inhibited growth in human teratoma tumor xenografts with very limited toxicity to regenerating tissues.
OMP-54F28 is a truncated Fzd8 receptor fused to the IgG1 Fc region. This inhibitor has been shown to block Wnt signaling and block tumor growth using a MMTV-Wnt1 induced tumor model (Hoey, 2013). Furthermore, OMP-54F28 was shown to synergize with chemotherapeutic agents. When a patient-derived pancreatic cancer xenograft model was treated with gemcitabine and OMP-54F28, OMP-54F28 alone reduced tumor growth to a greater extent than gemcitabine alone and a combination of the two gave a slight advantage over single agent OMP-54F28. OMP-54F28 also reduced the frequency of CSCs as quantitated by the number of tumors that regrew when serially passaged (30, 90 or 270 cells) into NOD/SCID for 82 days. Similar to tumor growth inhibition, the greatest reduction in CSC frequency occurred in combination, and this was slightly greater than OMP-54F28 alone. However, with gemcitabine alone the frequency of CSCs increased when compared to control. The percent of cells expressing CD44+, a marker for CSCs, decreased from ~12.7% to 1.9% with OMP-54F28 treatment alone as compared to an increase from ~12.7% to 13.9% when treated with gemcitabine alone and from ~12.7 % to 1.7% when a combination of OMP-54F28 and gemcitabine was used. Using luciferase-labelled pancreatic tumor cells implanted orthotopically, tumors were grown for 30 days, treated with OMP-54F28 and imaged in vivo for metastases. A decrease in both liver and lung metastasis was observed (Hoey, 2013).
Although cancers, including pancreatic cancers, are initially sensitive to gemcitabine, they can become resistant with treatment. A gemcitabine resistant pancreatic tumor model was created by continuously passing cells in increasing concentration of gemcitabine. Using this gemcitabine resistant model, tumor growth was inhibited with a combination of 5FU and irinotecan or OMP-54F28 alone in comparison to control. However when all three are combined, there is a greater effect in tumor growth inhibition. Epithelial specific antigen (ESA)+CD201+, a marker of pancreatic CSCs, was assessed and a substantial decrease was observed with a combination of the three compounds, while treatment with OMP-54F28 alone showed the greatest decrease in ESA+CD201+. Treatment of gemcitabine resistant xenografts with OMP-54F28, gemcitabine and Abraxane resulted in tumor growth inhibition, and this growth inhibition was greater than that with the combination of gemcitabine and Abraxane (Hoey, 2013). Together the data suggest that OMP-54F28 inhibits tumor growth, limits CSC frequency and tumor recurrence and is active in gemcitabine resistant tumors. It is effective as a single-agent, but also in combination with chemotherapeutic agents.
4. OMP-54F28: First-in-human Clinical Data
With the efficacy seen in preclinical solid tumor models, OMP-54F28 has been recently investigated in a first-in-human Phase 1a study with advanced solid tumors (Jimeno, 2014). The primary objective of this study was to determine the safety and toxicity profile of the drug in patients with advanced solid tumors. Secondary objectives included pharmacokinetics, immunogenicity, and preliminary efficacy of OMP-54F28. The study was designed as a 3+3 dose escalation trial with dose levels between 0.5 and 20 mg/kg given intravenously every 3 weeks. Dose limiting toxicities (DLTs) were assessed every 28 days, and tumor assessment was done every eight weeks.
At the time of submission of this review, only preliminary data from the phase 1 study has been reported. The main adverse effects seen with OMP-54F28 included dysgeusia, fatigue, muscle spasms, decreased appetite, nausea and vomiting. Modulation of the WNT pathway has been shown to have effects in the bone including bone remodeling (Goldring and Goldring, 2007). In the present study, β-C-terminal telopeptide (β-CTX), a marker of increased bone turnover, was closely assessed, and it was recommended that zoledronic acid should be given to patients with doubling of their β-CTX levels.
5. Ongoing Studies with OMP-54F28
There are 3 ongoing phase 1b studies combining OMP-54F28 with other drugs in solid tumors based on preclinical data and the safety and tolerability found in the phase 1a trial (Table 2 [OncoMed Pharmaceuticals Inc, 2014]). The first trial is combining OMP-54F28 with sorafenib in patients with hepatocellular cancer. Patients included must have locally advanced or metastatic hepatocellular cancer with no prior systemic therapies. Patients will receive sorafenib 400 mg orally twice daily with OMP-54F28 intravenously on day 1 of a 21-day cycle. Initially doses of 5 mg/kg or 10 mg/kg will be used, and based on safety data higher or lower doses may be evaluated. The primary objectives are to evaluate the safety and tolerability of OMP-54F28 in combination with sorafenib, to identify dose-limiting toxicities (DLTs) and maximum tolerated dose (MTD) and to determine the recommended phase 2 dose. Secondary objectives include characterization of the pharmacokinetics of OMP-54F28 in combination with sorafenib, characterization of the immunogenicity of OMP-54F28 and preliminary assessment of efficacy of the two drugs combined.
Table 2.
List of Ongoing Clinical Trials with OMP-54F28
| Clinical trials.gov number | Drug(s) studied | Phase | Malignancy studied | Status | Estimated completion date |
|---|---|---|---|---|---|
| NCT01608867 | OMP-54F28 | I | Solid tumors | Recruiting | July 2015 |
| NCT02069145 | OMP-54F28 + Sorafenib | Ib | Hepatocellular Cancer | Recruiting | January 2016 |
| NCT02092363 | OMP-54F28 + Paclitaxel | Ib | Recurrent Platinum-Sensitive Ovarian Cancer | Recruiting | August 2015 |
| NCT02050178 | OMP-54F28 + Nab-Paclitaxel + Gemcitabine | Ib | Metastatic Pancreatic Cancer | Recruiting | December 2015 |
Another phase 1b study will be evaluating the combination of OMP-54F28, nab-paclitaxel, and gemcitabine in patients with pancreatic cancer. Patients enrolled must have previously untreated stage IV ductal adenocarcinoma of the pancreas. They will receive nab-paclitaxel 125 mg/m2 and gemcitabine 1,000 mg/m2 intravenously on days 1, 8 and 15 of a 28-day cycle. OMP-54F28 will be given at either 3.5 mg/kg or 7.0 mg/kg intravenously on days 1 and 15. Depending on emerging safety data, higher doses may also be evaluated. The primary objectives of this study will include evaluation of the safety and tolerability of the drug combinations, identification of the DLTs and MTD and identification of the recommended phase 2 dose for OMP-54F28 in combination with nab-paclitaxel and gemcitabine. Secondary objectives will be characterization of the pharmacokinetics and immunogenicity of the drug combinations and preliminary assessment of the efficacy of these drugs for metastatic pancreatic cancer.
The third phase 1b trial open is studying OMP-54F28 in combination with paclitaxel and carboplatin in ovarian cancer. The patients included should have recurrent, platinum-sensitive ovarian cancer, defined as disease progression greater than 6 months after completing a minimum of 4 cycles of a platinum-containing chemotherapy regimen. Patients who have received prior treatment with paclitaxel and carboplatin for recurrent disease will be excluded. Paclitaxel 175 mg/m2 and carboplatin AUC 5 will be given intravenously on day 1 of a 21-day cycle. OMP-54F28 will be given at 5 mg/kg or 10 mg/kg intravenously on day 1 with potential further dose escalation based on safety data. The paclitaxel and carboplatin will be given for a maximum total of 6 cycles with OMP-54F28 continuing until disease progression. The primary objectives are safety and tolerability of OMP-54F28 in combination with paclitaxel and carboplatin, determination of any DLTs and the MTD and planned phase 2 dose of OMP-54F28. Secondary objectives will include pharmacokinetics, pharmacodynamics and efficacy of the drug combination.
6. Conclusions
The Wnt pathway has been shown to be an important target in many cancers, promoting tumor growth potentially through CSC proliferation, creating a favorable microenvironment for tumor growth and metastasis and contributing to therapy resistance. Many approaches have been taken in targeting this pathway in human cancer, including the use of natural compounds, small molecule inhibitors, viral-based inhibitors and antibody-based inhibitors with encouraging results. OMP-54F28 is a novel fusion protein targeting Fzd8-interacting Wnt ligands that has been shown to decrease activity in the Wnt signaling pathway, causing decreased tumor growth. Preclinical studies show activity as a single agent and a synergistic effect when added to various chemotherapeutic and targeted agents in various solid tumors. The phase 1a study with single agent OMP-54F28 given to patients with advanced solid tumors has nearly been completed with preliminary data released and more to come. The three phase 1b studies opened in ovarian, pancreatic and hepatocellular cancers will describe the safety of the drug in combination with standard regimens and define the acceptable dose for phase 2 testing of efficacy. Wnt inhibition and targeting CSCs is an exciting area of research and could become an important addition to traditional treatment regimens if these studies prove to be efficacious.
Acknowledgments
Dr. Jimeno is supported by R21 DE019712, and R01 CA149456 and is the principal investigator of clinical trials sponsored by OncoMed Pharmaceuticals, and the University of Colorado manages this research support. Dr. Le is supported by T32 CA174648.
Abbreviations
- AE
adverse effect
- APC
Adenomatous Polyposis Coli
- CSC
cancer stem cell
- DLT
dose limiting toxicities
- Fzd
Frizzled
- Fzd8
frizzled family receptor 8
- HNSCC
head and neck squamous cell cancer
- MTD
maximum tolerated dose
- NSCLC
non-small cell lung cancer
- PORC
porcupine
Footnotes
Conflict of Interest:
The other authors declare that they have no conflicts of interest.”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. The EMBO journal. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, Espada J, Garcia JM, Munoz A, Esteller M, Gonzalez-Sancho JM. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006;25:4116–4121. doi: 10.1038/sj.onc.1209439. [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Developmental cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Arafat K, Iratni R, Takahashi T, Parekh K, Al Dhaheri Y, Adrian TE, Attoub S. Inhibitory Effects of Salinomycin on Cell Survival, Colony Growth, Migration, and Invasion of Human Non-Small Cell Lung Cancer A549 and LNM35: Involvement of NAG-1. PloS one. 2013;8:e66931. doi: 10.1371/journal.pone.0066931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend RC, Londono-Joshi AI, Samant RS, Li Y, Conner M, Hidalgo B, Alvarez RD, Landen CN, Straughn JM, Buchsbaum DJ. Inhibition of Wnt/beta-catenin pathway by niclosamide: a therapeutic target for ovarian cancer. Gynecologic oncology. 2014;134:112–120. doi: 10.1016/j.ygyno.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Arensman MD, Telesca D, Lay AR, Kershaw KM, Wu N, Donahue TR, Dawson DW. The CREB binding protein inhibitor ICG-001 suppresses pancreatic cancer growth. Molecular cancer therapeutics. 2014 doi: 10.1158/1535-7163.MCT-13-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin TW, Solar GP, Ziegler FC, Liem L, Matthews W. A role for the Wnt gene family in hematopoiesis: expansion of multilineage progenitor cells. Blood. 1997;89:3624–3635. [PubMed] [Google Scholar]
- Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Bao R, Christova T, Song S, Angers S, Yan X, Attisano L. Inhibition of tankyrases induces Axin stabilization and blocks Wnt signalling in breast cancer cells. PloS one. 2012;7:e48670. doi: 10.1371/journal.pone.0048670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nature reviews Drug discovery. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Bodmer WF, Bailey CJ, Bodmer J, Bussey HJ, Ellis A, Gorman P, Lucibello FC, Murday VA, Rider SH, Scambler P, et al. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987;328:614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- Brunori M, Malerba M, Kashiwazaki H, Iggo R. Replicating adenoviruses that target tumors with constitutive activation of the wnt signaling pathway. Journal of virology. 2001;75:2857–2865. doi: 10.1128/JVI.75.6.2857-2865.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera CV, Alonso MC, Johnston P, Phillips RG, Lawrence PA. Phenocopies induced with antisense RNA identify the wingless gene. Cell. 1987;50:659–663. doi: 10.1016/0092-8674(87)90039-0. [DOI] [PubMed] [Google Scholar]
- Chang HW, Roh JL, Jeong EJ, Lee SW, Kim SW, Choi SH, Park SK, Kim SY. Wnt signaling controls radiosensitivity via cyclooxygenase-2-mediated Ku expression in head and neck cancer. International journal of cancer Journal international du cancer. 2008;122:100–107. doi: 10.1002/ijc.23069. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature chemical biology. 2009a;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Hsu LS, Shia YT, Lin MW, Lin CM. The beta-catenin/TCF complex as a novel target of resveratrol in the Wnt/beta-catenin signaling pathway. Biochemical pharmacology. 2012;84:1143–1153. doi: 10.1016/j.bcp.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Chen M, Wang J, Lu J, Bond MC, Ren XR, Lyerly HK, Barak LS, Chen W. The anti-helminthic niclosamide inhibits Wnt/Frizzled1 signaling. Biochemistry. 2009b;48:10267–10274. doi: 10.1021/bi9009677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Woodward WA, Behbod F, Peddibhotla S, Alfaro MP, Buchholz TA, Rosen JM. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. Journal of cell science. 2007;120:468–477. doi: 10.1242/jcs.03348. [DOI] [PubMed] [Google Scholar]
- Chen RH, McCormick F. Selective targeting to the hyperactive beta-catenin/T-cell factor pathway in colon cancer cells. Cancer research. 2001;61:4445–4449. [PubMed] [Google Scholar]
- Chen Z, Venkatesan AM, Dehnhardt CM, Dos Santos O, Delos Santos E, Ayral-Kaloustian S, Chen L, Geng Y, Arndt KT, Lucas J, et al. 2,4-Diamino-quinazolines as inhibitors of beta-catenin/ Tcf-4 pathway: Potential treatment for colorectal cancer. Bioorganic & medicinal chemistry letters. 2009c;19:4980–4983. doi: 10.1016/j.bmcl.2009.07.070. [DOI] [PubMed] [Google Scholar]
- Choi YS, Zhang Y, Xu M, Yang Y, Ito M, Peng T, Cui Z, Nagy A, Hadjantonakis AK, Lang RA, et al. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell stem cell. 2013;13:720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- DeAlmeida VI, Miao L, Ernst JA, Koeppen H, Polakis P, Rubinfeld B. The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer research. 2007;67:5371–5379. doi: 10.1158/0008-5472.CAN-07-0266. [DOI] [PubMed] [Google Scholar]
- Deng Y, Su Q, Mo J, Fu X, Zhang Y, Lin EH. Celecoxib downregulates CD133 expression through inhibition of the Wnt signaling pathway in colon cancer cells. Cancer investigation. 2013;31:97–102. doi: 10.3109/07357907.2012.754458. [DOI] [PubMed] [Google Scholar]
- Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, et al. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer research. 2003;63:3145–3153. [PubMed] [Google Scholar]
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proc Natl Acad Sci U S A. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Chang H, Peng X, Bai Q, Yi L, Zhou Y, Zhu J, Mi M. Resveratrol Inhibits Breast Cancer Stem-Like Cells and Induces Autophagy via Suppressing Wnt/beta-Catenin Signaling Pathway. PloS one. 2014;9:e102535. doi: 10.1371/journal.pone.0102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerer C, Iggo R. Adenoviruses with Tcf binding sites in multiple early promoters show enhanced selectivity for tumour cells with constitutive activation of the wnt signalling pathway. Gene therapy. 2002;9:270–281. doi: 10.1038/sj.gt.3301651. [DOI] [PubMed] [Google Scholar]
- Fuerer C, Iggo R. 5-Fluorocytosine increases the toxicity of Wnt-targeting replicating adenoviruses that express cytosine deaminase as a late gene. Gene therapy. 2004;11:142–151. doi: 10.1038/sj.gt.3302148. [DOI] [PubMed] [Google Scholar]
- Fujii N, You L, Xu Z, Uematsu K, Shan J, He B, Mikami I, Edmondson LR, Neale G, Zheng J, et al. An antagonist of dishevelled protein-protein interaction suppresses beta-catenin-dependent tumor cell growth. Cancer research. 2007;67:573–579. doi: 10.1158/0008-5472.CAN-06-2726. [DOI] [PubMed] [Google Scholar]
- Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. The Journal of biological chemistry. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- Fukui T, Kondo M, Ito G, Maeda O, Sato N, Yoshioka H, Yokoi K, Ueda Y, Shimokata K, Sekido Y. Transcriptional silencing of secreted frizzled related protein 1 (SFRP 1) by promoter hypermethylation in non-small-cell lung cancer. Oncogene. 2005;24:6323–6327. doi: 10.1038/sj.onc.1208777. [DOI] [PubMed] [Google Scholar]
- Fukukawa C, Nagayama S, Tsunoda T, Toguchida J, Nakamura Y, Katagiri T. Activation of the non-canonical Dvl-Rac1-JNK pathway by Frizzled homologue 10 in human synovial sarcoma. Oncogene. 2009;28:1110–1120. doi: 10.1038/onc.2008.467. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Gauger KJ, Schneider SS. Tumour supressor secreted frizzled related protein 1 regulates p53-mediated apoptosis. Cell biology international. 2014;38:124–130. doi: 10.1002/cbin.10176. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. The New England journal of medicine. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Goldring SR, Goldring MB. Eating bone or adding it: the Wnt pathway decides. Nature medicine. 2007;13:133–134. doi: 10.1038/nm0207-133. [DOI] [PubMed] [Google Scholar]
- Grandy D, Shan J, Zhang X, Rao S, Akunuru S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, et al. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. The Journal of biological chemistry. 2009;284:16256–16263. doi: 10.1074/jbc.M109.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y. Targeted inhibition of beta-catenin/CBP signaling ameliorates renal interstitial fibrosis. Journal of the American Society of Nephrology : JASN. 2011;22:1642–1653. doi: 10.1681/ASN.2010101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005;24:3054–3058. doi: 10.1038/sj.onc.1208511. [DOI] [PubMed] [Google Scholar]
- He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, McCormick F, Jablons DM. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia. 2004;6:7–14. doi: 10.1016/s1476-5586(04)80048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang B, Moos M, Jr, Vukicevic S, Luyten FP. Primary structure and tissue distribution of FRZB, a novel protein related to Drosophila frizzled, suggest a role in skeletal morphogenesis. The Journal of biological chemistry. 1996;271:26131–26137. doi: 10.1074/jbc.271.42.26131. [DOI] [PubMed] [Google Scholar]
- Hoey T. Development of FZD8-Fc (OMP-54F28), a Wnt signaling antagonist that inhibits tumor growth and reduces tumor initiating cell frequency. Presented at: AACR Annual Meeting; 2013 April; Washington DC. 2013. [Google Scholar]
- Holland JD, Gyorffy B, Vogel R, Eckert K, Valenti G, Fang L, Lohneis P, Elezkurtaj S, Ziebold U, Birchmeier W. Combined Wnt/beta-catenin, Met, and CXCL12/CXCR4 signals characterize basal breast cancer and predict disease outcome. Cell reports. 2013;5:1214–1227. doi: 10.1016/j.celrep.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999a;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc Natl Acad Sci U S A. 1999b;96:3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Dong A, Fernandez-Ruiz V, Shan J, Kawa M, Martinez-Anso E, Prieto J, Qian C. Blockade of Wnt signaling inhibits angiogenesis and tumor growth in hepatocellular carcinoma. Cancer research. 2009;69:6951–6959. doi: 10.1158/0008-5472.CAN-09-0541. [DOI] [PubMed] [Google Scholar]
- Huang HC, Klein PS. The Frizzled family: receptors for multiple signal transduction pathways. Genome biology. 2004;5:234. doi: 10.1186/gb-2004-5-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mechanisms of development. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Hulsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. The Journal of cell biology. 1994;127:2061–2069. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blastcrisis CML. The New England journal of medicine. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Jimeno A, Gordon M, Messersmith WA, Chugh R, Mendelson D, Dupont J, Stagg R, Kapoun AM, Xu L, Brachmann RK, Smith DA. A first-in-human Phase 1 study of anti-cancer stem cell (CSC) agent OMP-54F28 (FZD8-Fc) targeting the WNT pathway in patients with advanced solid tumors. American Society of Clinical Oncology (ASCO) Annual Meeting, Oral Presentation; June 2014.p. abstr 3505. [Google Scholar]
- Jin X, Jeon HY, Joo KM, Kim JK, Jin J, Kim SH, Kang BG, Beck S, Lee SJ, Kim JK, et al. Frizzled 4 regulates stemness and invasiveness of migrating glioma cells established by serial intracranial transplantation. Cancer research. 2011;71:3066–3075. doi: 10.1158/0008-5472.CAN-10-1495. [DOI] [PubMed] [Google Scholar]
- Kim H, Seo EM, Sharma AR, Ganbold B, Park J, Sharma G, Kang YH, Song DK, Lee SS, Nam JS. Regulation of Wnt signaling activity for growth suppression induced by quercetin in 4T1 murine mammary cancer cells. International journal of oncology. 2013;43:1319–1325. doi: 10.3892/ijo.2013.2036. [DOI] [PubMed] [Google Scholar]
- Ko YB, Kim BR, Yoon K, Choi EK, Seo SH, Lee Y, Lee MA, Yang JB, Park MS, Rho SB. WIF1 can effectively co-regulate pro-apoptotic activity through the combination with DKK1. Cellular signalling. 2014 doi: 10.1016/j.cellsig.2014.07.026. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature genetics. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Kwong KY, Zou Y, Day CP, Hung MC. The suppression of colon cancer cell growth in nude mice by targeting beta-catenin/TCF pathway. Oncogene. 2002;21:8340–8346. doi: 10.1038/sj.onc.1206050. [DOI] [PubMed] [Google Scholar]
- Lee JS, Ishimoto A, Yanagawa S. Characterization of mouse dishevelled (Dvl) proteins in Wnt/Wingless signaling pathway. The Journal of biological chemistry. 1999;274:21464–21470. doi: 10.1074/jbc.274.30.21464. [DOI] [PubMed] [Google Scholar]
- Lejeune S, Huguet EL, Hamby A, Poulsom R, Harris AL. Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. 1995;1:215–222. [PubMed] [Google Scholar]
- Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- Li N, Xi Y, Tinsley HN, Gurpinar E, Gary BD, Zhu B, Li Y, Chen X, Keeton AB, Abadi AH, et al. Sulindac selectively inhibits colon tumor cell growth by activating the cGMP/PKG pathway to suppress Wnt/beta-catenin signaling. Molecular cancer therapeutics. 2013;12:1848–1859. doi: 10.1158/1535-7163.MCT-13-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Polak L, Lin M, Lay K, Zheng D, Fuchs E. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nature cell biology. 2014;16:179–190. doi: 10.1038/ncb2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski KS, Djeha HA, Gawn J, Cliffe S, Maitland NJ, Palmer DH, Mountain A, Irvine AS, Wrighton CJ. Optimization of a synthetic beta-catenin-dependent promoter for tumor-specific cancer gene therapy. Molecular therapy : the journal of the American Society of Gene Therapy. 2004;10:150–161. doi: 10.1016/j.ymthe.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110:20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londono-Joshi AI, Arend RC, Aristizabal L, Lu W, Samant RS, Metge BJ, Hidalgo B, Grizzle WE, Conner M, Forero-Torres A, et al. Effect of Niclosamide on Basal-like Breast Cancers. Molecular cancer therapeutics. 2014 doi: 10.1158/1535-7163.MCT-13-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes & development. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci U S A. 2011a;108:13253–13257. doi: 10.1073/pnas.1110431108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Li Y. Salinomycin suppresses LRP6 expression and inhibits both Wnt/beta-catenin and mTORC1 signaling in breast and prostate cancer cells. Journal of cellular biochemistry. 2014 doi: 10.1002/jcb.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Lin C, Roberts MJ, Waud WR, Piazza GA, Li Y. Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/beta-catenin pathway. PloS one. 2011b;6:e29290. doi: 10.1371/journal.pone.0029290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev AN, Fuerer C, Chen MJ, Searle P, Iggo R. Late expression of nitroreductase in an oncolytic adenovirus sensitizes colon cancer cells to the prodrug CB1954. Human gene therapy. 2005;16:1473–1483. doi: 10.1089/hum.2005.16.1473. [DOI] [PubMed] [Google Scholar]
- Many AM, Brown AM. Both canonical and non-canonical wnt signaling independently promote stem cell growth in mammospheres. PloS one. 2014;9:e101800. doi: 10.1371/journal.pone.0101800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, Wang H, Tang B, Zhang Q, Yu X, et al. Roles of Wnt/beta-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell death & disease. 2014;5:e1039. doi: 10.1038/cddis.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Molecular cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Masckauchan TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, Khoo A, Tycko B, Brown AM, Kitajewski J. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Molecular biology of the cell. 2006;17:5163–5172. doi: 10.1091/mbc.E06-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- Mazieres J, He B, You L, Xu Z, Lee AY, Mikami I, Reguart N, Rosell R, McCormick F, Jablons DM. Wnt inhibitory factor-1 is silenced by promoter hypermethylation in human lung cancer. Cancer research. 2004;64:4717–4720. doi: 10.1158/0008-5472.CAN-04-1389. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989;58:1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Mikami I, You L, He B, Xu Z, Batra S, Lee AY, Mazieres J, Reguart N, Uematsu K, Koizumi K, et al. Efficacy of Wnt-1 monoclonal antibody in sarcoma cells. BMC cancer. 2005;5:53. doi: 10.1186/1471-2407-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mologni L, Brussolo S, Ceccon M, Gambacorti-Passerini C. Synergistic effects of combined Wnt/KRAS inhibition in colorectal cancer cells. PloS one. 2012;7:e51449. doi: 10.1371/journal.pone.0051449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Campbell RM, Christian JL, McGrew LL, Shih J, Fraser S. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development. 1993;119:97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Nguyen AV, Martinez M, Stamos MJ, Moyer MP, Planutis K, Hope C, Holcombe RF. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer management and research. 2009a;1:25–37. [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, Gerald WL, Massague J. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009b;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, Moon R, Varmus H. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991;64:231. doi: 10.1016/0092-8674(91)90633-a. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- OncoMed Pharmaceuticals Inc. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); Jan, 2014. Available from: http://clinicaltrials.gov/ct2/results?term=omp-54f28&Search=Search. [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. The EMBO journal. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Chang JY, Hahm ER, Park S, Kim HK, Yang CH. Quercetin, a potent inhibitor against beta-catenin/Tcf signaling in SW480 colon cancer cells. Biochemical and biophysical research communications. 2005;328:227–234. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- Peerlinck I, Merron A, Baril P, Conchon S, Martin-Duque P, Hindorf C, Burnet J, Quintanilla M, Hingorani M, Iggo R, et al. Targeted radionuclide therapy using a Wnt-targeted replicating adenovirus encoding the Na/I symporter. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:6595–6601. doi: 10.1158/1078-0432.CCR-09-0262. [DOI] [PubMed] [Google Scholar]
- Peifer M, Pai LM, Casey M. Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Developmental biology. 1994;166:543–556. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Pongracz JE, Stockley RA. Wnt signalling in lung development and diseases. Respiratory research. 2006;7:15. doi: 10.1186/1465-9921-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Lei M, Li J, Wang N, Lian X. Activated Hair Follicle Stem Cells and Wnt/beta-catenin Signaling Involve in Pathnogenesis of Sebaceous Neoplasms. International journal of medical sciences. 2014;11:1022–1028. doi: 10.7150/ijms.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampias T, Boutati E, Pectasides E, Sasaki C, Kountourakis P, Weinberger P, Psyrri A. Activation of Wnt signaling pathway by human papillomavirus E6 and E7 oncogenes in HPV16-positive oropharyngeal squamous carcinoma cells. Molecular cancer research : MCR. 2010;8:433–443. doi: 10.1158/1541-7786.MCR-09-0345. [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, Corr M, Carson DA. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002;21:6598–6605. doi: 10.1038/sj.onc.1205920. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell. 1997;90:707–716. doi: 10.1016/s0092-8674(00)80531-0. [DOI] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. The EMBO journal. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer A, Berdnikoff K. Pinworm infestation in children: the problem and its treatment. Canadian Medical Association journal. 1962;86:60–65. [PMC free article] [PubMed] [Google Scholar]
- Sakanaka C, Leong P, Xu LC, Harrison SD, Williams LT. Casein kinase I epsilon in the Wnt pathway: Regulation of beta-catenin function. P Natl Acad Sci USA. 1999;96:12548–12552. doi: 10.1073/pnas.96.22.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes & development. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Hwang H, Nguyen C, Kloner RA, Kahn M. The small molecule Wnt signaling modulator ICG-001 improves contractile function in chronically infarcted rat myocardium. PloS one. 2013;8:e75010. doi: 10.1371/journal.pone.0075010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan BE, Wang MX, Li RQ. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/beta-catenin signaling pathway. Cancer investigation. 2009;27:604–612. doi: 10.1080/07357900802337191. [DOI] [PubMed] [Google Scholar]
- Shan J, Shi DL, Wang J, Zheng J. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry. 2005;44:15495–15503. doi: 10.1021/bi0512602. [DOI] [PubMed] [Google Scholar]
- Shen CC, Kang YH, Zhao M, He Y, Cui DD, Fu YY, Yang LL, Gou LT. WNT16B from Ovarian Fibroblasts Induces Differentiation of Regulatory T Cells through beta-Catenin Signal in Dendritic Cells. International journal of molecular sciences. 2014;15:12928–12939. doi: 10.3390/ijms150712928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YL, Shyu RY, Hsieh CB, Lai HC, Liu KY, Chu TY, Lin YW. Promoter methylation of the secreted frizzled-related protein 1 gene SFRP1 is frequent in hepatocellular carcinoma. Cancer. 2006;107:579–590. doi: 10.1002/cncr.22023. [DOI] [PubMed] [Google Scholar]
- Song J, Chang I, Chen Z, Kang M, Wang CY. Characterization of side populations in HNSCC: highly invasive, chemoresistant and abnormal Wnt signaling. PloS one. 2010;5:e11456. doi: 10.1371/journal.pone.0011456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nature medicine. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nature genetics. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- Takahashi-Yanaga F, Sasaguri T. The Wnt/beta-catenin signaling pathway as a target in drug discovery. Journal of pharmacological sciences. 2007;104:293–302. doi: 10.1254/jphs.cr0070024. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ, Zou CY, Xie XB, Zeng YX, Shen JN, Kang T, et al. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer letters. 2011;311:113–121. doi: 10.1016/j.canlet.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nature chemical biology. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CJ, Schlesinger A, Carter JC, Bowerman B. Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell. 1997;90:695–705. doi: 10.1016/s0092-8674(00)80530-9. [DOI] [PubMed] [Google Scholar]
- Tian XH, Hou WJ, Fang Y, Fan J, Tong H, Bai SL, Chen Q, Xu H, Li Y. XAV939, a tankyrase 1 inhibitior, promotes cell apoptosis in neuroblastoma cell lines by inhibiting Wnt/beta-catenin signaling pathway. Journal of experimental & clinical cancer research : CR. 2013;32:100. doi: 10.1186/1756-9966-32-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Sueishi M, Yoshida T. Niclosamide suppresses Hepatoma cell proliferation via the Wnt pathway. OncoTargets and therapy. 2013;6:1685–1693. doi: 10.2147/OTT.S50065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Djeha H, Ying B, Tollefson AE, Kuppuswamy M, Doronin K, Krajcsi P, Lipinski K, Wrighton CJ, Wold WS. An oncolytic adenovirus vector combining enhanced cell-to-cell spreading, mediated by the ADP cytolytic protein, with selective replication in cancer cells with deregulated wnt signaling. Cancer research. 2004;64:3638–3644. doi: 10.1158/0008-5472.CAN-03-3882. [DOI] [PubMed] [Google Scholar]
- Trosset JY, Dalvit C, Knapp S, Fasolini M, Veronesi M, Mantegani S, Gianellini LM, Catana C, Sundstrom M, Stouten PF, et al. Inhibition of protein-protein interactions: the discovery of druglike beta-catenin inhibitors by combining virtual and biophysical screening. Proteins. 2006;64:60–67. doi: 10.1002/prot.20955. [DOI] [PubMed] [Google Scholar]
- Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- Ueda M, Gemmill RM, West J, Winn R, Sugita M, Tanaka N, Ueki M, Drabkin HA. Mutations of the beta- and gamma-catenin genes are uncommon in human lung, breast, kidney, cervical and ovarian carcinomas. British journal of cancer. 2001;85:64–68. doi: 10.1054/bjoc.2001.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu K, He B, You L, Xu Z, McCormick F, Jablons DM. Activation of the Wnt pathway in non small cell lung cancer: evidence of dishevelled overexpression. Oncogene. 2003;22:7218–7221. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- Ueno K, Hazama S, Mitomori S, Nishioka M, Suehiro Y, Hirata H, Oka M, Imai K, Dahiya R, Hinoda Y. Down-regulation of frizzled-7 expression decreases survival, invasion and metastatic capabilities of colon cancer cells. British journal of cancer. 2009;101:1374–1381. doi: 10.1038/sj.bjc.6605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Hirata H, Hinoda Y, Dahiya R. Frizzled homolog proteins, microRNAs and Wnt signaling in cancer. International journal of cancer Journal international du cancer. 2013;132:1731–1740. doi: 10.1002/ijc.27746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakami S, Shiina H, Enokida H, Kawakami T, Tokizane T, Ogishima T, Tanaka Y, Li LC, Ribeiro-Filho LA, Terashima M, et al. Epigenetic inactivation of Wnt inhibitory factor-1 plays an important role in bladder cancer through aberrant canonical Wnt/beta-catenin signaling pathway. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:383–391. doi: 10.1158/1078-0432.CCR-05-1344. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature cell biology. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- Waaler J, Machon O, Tumova L, Dinh H, Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova O, et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer research. 2012;72:2822–2832. doi: 10.1158/0008-5472.CAN-11-3336. [DOI] [PubMed] [Google Scholar]
- Wang F, He L, Dai WQ, Xu YP, Wu D, Lin CL, Wu SM, Cheng P, Zhang Y, Shen M, et al. Salinomycin inhibits proliferation and induces apoptosis of human hepatocellular carcinoma cells in vitro and in vivo. PloS one. 2012a;7:e50638. doi: 10.1371/journal.pone.0050638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HQ, Xu ML, Ma J, Zhang Y, Xie CH. Frizzled-8 as a putative therapeutic target in human lung cancer. Biochemical and biophysical research communications. 2012b;417:62–66. doi: 10.1016/j.bbrc.2011.11.055. [DOI] [PubMed] [Google Scholar]
- Wang YC, Chao TK, Chang CC, Yo YT, Yu MH, Lai HC. Drug screening identifies niclosamide as an inhibitor of breast cancer stem-like cells. PloS one. 2013;8:e74538. doi: 10.1371/journal.pone.0074538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wend P, Fang L, Zhu Q, Schipper JH, Loddenkemper C, Kosel F, Brinkmann V, Eckert K, Hindersin S, Holland JD, et al. Wnt/beta-catenin signalling induces MLL to create epigenetic changes in salivary gland tumours. The EMBO journal. 2013;32:1977–1989. doi: 10.1038/emboj.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt JD, Li N, Tinsley HN, Chen X, Zhang W, Li Y, Gary BD, Keeton AB, Xi Y, Abadi AH, et al. A novel sulindac derivative that potently suppresses colon tumor cell growth by inhibiting cGMP phosphodiesterase and beta-catenin transcriptional activity. Cancer prevention research. 2012;5:822–833. doi: 10.1158/1940-6207.CAPR-11-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegering A, Uthe FW, Huttenrauch M, Muhling B, Linnebacher M, Krummenast F, Germer CT, Thalheimer A, Otto C. The impact of pyrvinium pamoate on colon cancer cell viability. International journal of colorectal disease. 2014 doi: 10.1007/s00384-014-1975-y. [DOI] [PubMed] [Google Scholar]
- Wieland A, Trageser D, Gogolok S, Reinartz R, Hofer H, Keller M, Leinhaas A, Schelle R, Normann S, Klaas L, et al. Anticancer effects of niclosamide in human glioblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:4124–4136. doi: 10.1158/1078-0432.CCR-12-2895. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M, Kristiansen G, Hsieh JC, Hofstaedter F, Hartmann A, et al. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. The Journal of pathology. 2003;201:204–212. doi: 10.1002/path.1449. [DOI] [PubMed] [Google Scholar]
- Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Zhang Y, Huang J, Fan Z, Shi F, Wang S. Salinomycin inhibits proliferation and induces apoptosis of human nasopharyngeal carcinoma cell in vitro and suppresses tumor growth in vivo. Biochemical and biophysical research communications. 2014;443:712–717. doi: 10.1016/j.bbrc.2013.12.032. [DOI] [PubMed] [Google Scholar]
- Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, Deng X, Chen L, Kim CC, Lau S, et al. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene. 2011;30:4437–4446. doi: 10.1038/onc.2011.145. [DOI] [PubMed] [Google Scholar]
- Yao L, Sun B, Zhao X, Zhao X, Gu Q, Dong X, Zheng Y, Sun J, Cheng R, Qi H, et al. Overexpression of Wnt5a Promotes Angiogenesis in NSCLC. BioMed research international. 2014;2014:832562. doi: 10.1155/2014/832562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T, Xiong Y, Yan Y, Xia Y, Song X, Liu L, Li D, Wang N, Zhang L, Zhu Y, et al. The anthelmintic drug niclosamide induces apoptosis, impairs metastasis and reduces immunosuppressive cells in breast cancer model. PloS one. 2014;9:e85887. doi: 10.1371/journal.pone.0085887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yo YT, Lin YW, Wang YC, Balch C, Huang RL, Chan MW, Sytwu HK, Chen CK, Chang CC, Nephew KP, et al. Growth inhibition of ovarian tumor-initiating cells by niclosamide. Molecular cancer therapeutics. 2012;11:1703–1712. doi: 10.1158/1535-7163.MCT-12-0002. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes & development. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- You L, He B, Uematsu K, Xu Z, Mazieres J, Lee A, McCormick F, Jablons DM. Inhibition of Wnt-1 signaling induces apoptosis in beta-catenin-deficient mesothelioma cells. Cancer research. 2004a;64:3474–3478. doi: 10.1158/0008-5472.CAN-04-0115. [DOI] [PubMed] [Google Scholar]
- You L, He B, Xu Z, Uematsu K, Mazieres J, Fujii N, Mikami I, Reguart N, McIntosh JK, Kashani-Sabet M, et al. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer research. 2004b;64:5385–5389. doi: 10.1158/0008-5472.CAN-04-1227. [DOI] [PubMed] [Google Scholar]
- You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, Reguart N, Moody TW, Kitajewski J, McCormick F, et al. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004c;23:6170–6174. doi: 10.1038/sj.onc.1207844. [DOI] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L, Chaturvedi D, Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. The Journal of biological chemistry. 2004;279:33220–33227. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kong LM, Zhan R, Ye ZN, Pu JX, Sun HD, Li Y. Two Natural -kauranoids as Novel Wnt Signaling Inhibitors. Natural products and bioprospecting. 2014a;4:135–140. doi: 10.1007/s13659-014-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Li H, Yang B, Yang F, Zhang LL, Kong QY, Chen XY, Wu ML, Liu J. Biological significance and therapeutic implication of resveratrol-inhibited Wnt, Notch and STAT3 signaling in cervical cancer cells. Genes & cancer. 2014b;5:154–164. doi: 10.18632/genesandcancer.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer research. 2001;61:6050–6054. [PubMed] [Google Scholar]