Abstract
Objective
The aim of this study was to investigate the role of CD43, an integral membrane glycoprotein with both pro-adhesive and anti-adhesive activities, in atherosclerosis.
Approach and Results
LDL receptor-deficient mice (Ldlr−/−) were lethally irradiated and reconstituted with either bone marrow (BM) from CD43−/− mice or from wild-type controls. We found that mice lacking the CD43 on their leukocytes had significantly less severe atherosclerosis and that, contrary to our expectation, macrophage infiltration into the vessel wall was not affected by the lack of CD43 in the leukocytes. However, we found that CD43 mediates cholesterol homeostasis in macrophages by facilitating cholesterol efflux. This resulted in a significant reduction in storage of cholesterol in the aorta of mice lacking CD43 in the leukocytes.
Conclusions
CD43 may be an important mediator of macrophage lipid homeostasis, thereby affecting macrophage foam cell formation and ultimately atherosclerotic plaque development.
Keywords: CD43, atherosclerosis, macrophages, cholesterol efflux
Introduction
Macrophages are present at every stage of atherosclerosis from fatty streak lesion to fibrofatty plaque 1. Migration of leukocytes into the vessel wall is a well-orchestrated series of events involving molecules such as selectins, integrins and chemokines produced locally at the arterial sites. Equally important are the receptors on the surfaces of the leukocytes that are necessary for the interaction with their ligands on the endothelium 2.
One such ligand is CD43, an integral membrane glycoprotein that is commonly found on the surface of all hematopoietic cells. Based on its structural characteristics of the extracellular domain, CD43 can potentially have both pro-adhesive properties resulting from extracellular domain being extensively O-glycosylated 3 or anti-adhesive with extensive negatively charged sialic acid residues on its extracellular domain 4. The abundant expression and far-reaching protrusion from cell surface 3 make CD43 a likely candidate to be the first point of contact with other cell surfaces. Adhesion would be facilitated in cells that express one of the known counter-receptors for CD43, ICAM-1, galectin 1, and MHC-1 5–7. Interaction with other cells that do not express a CD43 counter-receptor would likely result in cell repulsion due to steric hindrance and electrostatic repulsion by the negatively charged molecules.
Because of the abundance of CD43 expression on leukocytes and its intriguing pro- as well as anti-adhesive properties we conducted studies to determine if leukocyte CD43 plays a role in atherosclerosis.
Methods
Materials and Methods are available in the online-only Supplement.
Results
To assess whether CD43 plays a role in atherogenesis, Ldlr−/− mice were lethally irradiated and reconstituted with BM isolated from either CD43−/− mice (CD43−/−BMT) or control mice (CD43+/+BMT) and fed a high fat diet for additional 16 weeks to induce atherosclerosis. After 4 weeks post-BMT FACS analysis of peripheral blood revealed that most of the CD11b+ myeloid cells in the CD43−/−BMT mice did not express CD43 whereas the control mice showed abundant expression of CD43 (Figure 1 in the online-only Data Supplement).
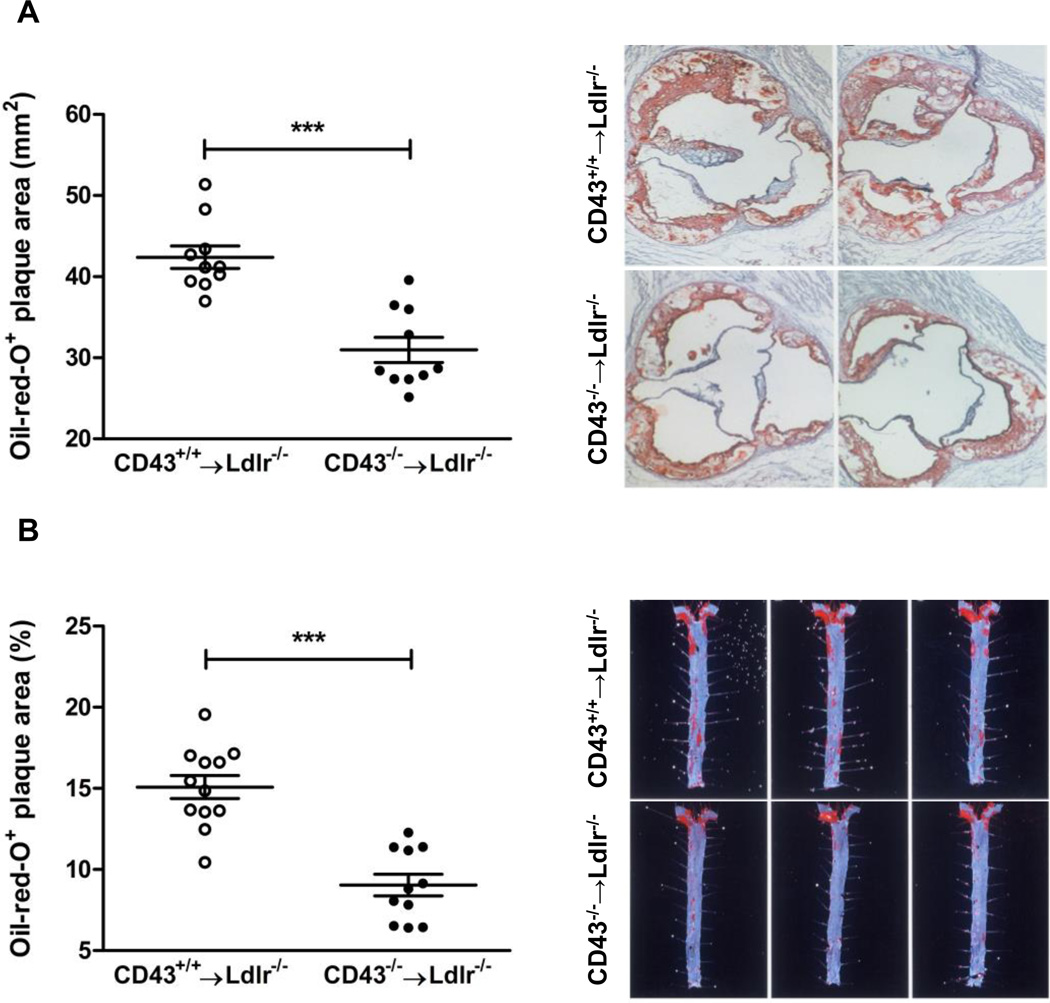
During the 16 weeks on the high fat diet the plasma cholesterol and triglyceride levels were virtually identical between the CD43+/+BMT and CD43−/−BMT mice at all time points (Table 1, Data Supp). After 16 weeks the mice were sacrificed and the aortic valve in the heart was sectioned and stained with Oil red O to quantitate the lesion areas (Figure 1A). The lesions from the CD43−/−BMT mice were appreciably smaller and less advanced compared to lesions from the control CD43+/+BMT mice (571,481 ± 90,110 µm2 vs. 772,980 ± 93,543 µm2, p<0.0001). The aortas from these mice were longitudinally opened and cut into two pieces immediately below the superior mesenteric artery. The top portion was used for quantitating the percent lesion area (Figure 1B). The aortic lesions were much less extensive in the CD43−/−BMT mice compared to the controls (15.1 ± 2.5 % vs. 9.0 ± 2.2 %, p<0.0001). Both measures indicated that leukocyte-specific deficiency of CD43 protected the mice from atherosclerosis.
Figure 1. CD43 deficiency in BM-derived cells reduces atherosclerosis in Ldlr−/− mice.
(A) Atherosclerotic lesions were quantified in the aortic root after staining with oil-red O in CD43+/+BMT and CD43−/−BMT mice on high fat diet for 16 weeks; 3 representative images of the aortic root are shown. (B) Quantification of atherosclerotic lesions in aortas after staining with Sudan IV in CD43+/+BMT and CD43−/−BMT mice; 3 representative images of the aorta are shown.
Quantitative analysis of lesion morphology by immunohistochemistry indicated that macrophage accumulation (Figure 2A, Data Supp) in the plaques were not different in the lesions of CD43−/−BMT mice compared to those in the CD43+/+BMT mice. With n=8 for each group, the MOMA2-stained areas as a percentage of total plaque area were 34.7 ± 5.9 for the CD43+/+BMT mice and 30.3 ± 5.3 for the CD43−/−BMT mice (mean ± SEM). Furthermore, CD43−/− and CD43+/+ macrophages displayed similar propensity to migrate in an M-CSF-mediated transwell migration assay (Figure 2B, Data Supp).
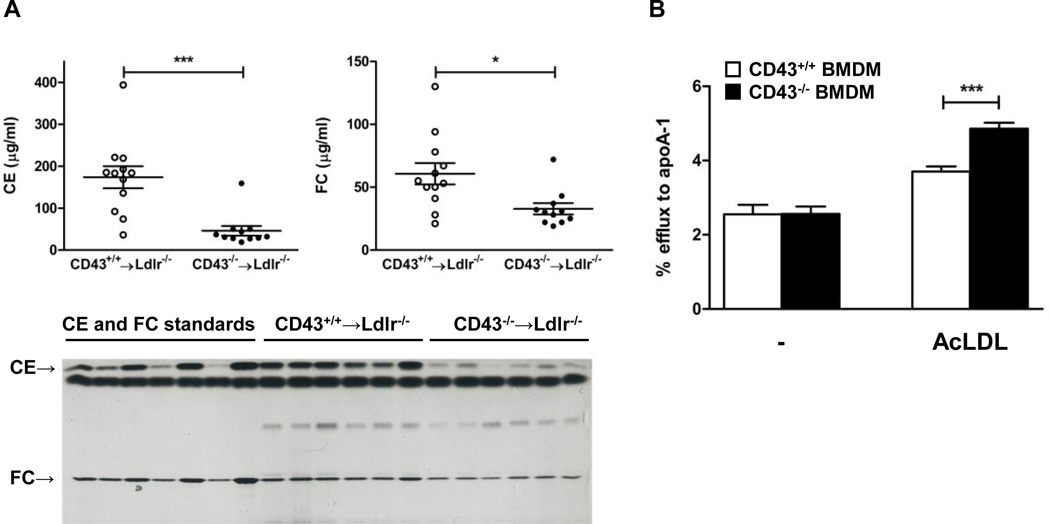
The lower portion of the aorta was used to measure the cholesterol content by extracting the lipids and separating them via thin layer chromatography. There was a dramatic reduction in the cholesteryl ester content of the aortas from CD43−/−BMT mice compared to controls (46 ± 12 vs. 174 ± 26 mg/ml, p<0.001). Free cholesterol was also lower in the CD43−/−BMT mice (33 ± 5 vs. 61 ± 9 mg/ml, p<0.01).
Because our thin layer chromatography results showed far less cholesterol deposited in the lesions of the CD43−/−BMT mice, we performed both cholesterol uptake and efflux experiments to assess if CD43 participates in either of these processes using primary macrophages isolated from CD43−/− and CD43+/+ mice. The results showed that while cholesterol uptake was not affected by CD43 (Figure 3, Data Supp), cholesterol efflux was significantly enhanced in macrophages lacking CD43 (Figure 2B). This suggests that the CD43 on macrophages acts to inhibit cholesterol efflux from foam cells and explains the reduced cholesterol deposits in the aortas of CD43−/−BMT mice. However, expression levels of genes that are known to be involved in both cholesterol uptake (CD36 and SR-A) and efflux (ABCA1 and ABCG1) as well as inflammatory cytokine such as IL-1β were not different between the 2 groups of mice (Figure 4, Data Supp). These results indicate that CD43 participates in lipid homeostasis in macrophages without affecting the cadre of genes known to regulate these processes.
Figure 2. CD43 mediates cholesterol homeostasis.
(A) The bottom half of the aortas (n = 11 in each group) were subjected to lipid extraction by a modified Folch method with chloroform/methanol (2:1). Free (FC) and esterified cholesterol (CE) were separated by high-performance thin layer chromatography and quantified by scanning laser densitometry. FC and CE standards are shown on the left. (B) Cholesterol efflux to apoA-1 in primary control and CD43 deficient macrophages with and without AcLDL stimulation; (n=4 independent experiments). Data are expressed as mean ± SEM. **P<0.005
Discussion
Although CD43 is expressed abundantly on macrophages the role that CD43 has on its function has not been studied carefully. Using an ex vivo assay, McEvoy et al. demonstrated that an anti-CD43 antibody can inhibit the adhesion of macrophages to the aortic endothelium isolated from rabbits fed a cholesterol-rich diet and therefore more prone to atherogenesis 8. Although these results are interesting it is not clear if the results obtained could be extrapolated to the in vivo model. Indeed, using our in vivo model, it was clear that disruption of CD43 on macrophages did not affect the accumulation of these cells in the plaque despite significantly less atherosclerosis in the lesions of CD43−/−BMT mice. This suggests that CD43 deficiency on leukocytes had other effects that led to limited progression of the disease.
Our finding that CD43 mediates cholesterol metabolism is novel and unexpected. We found that the accumulation of cholesteryl ester was reduced by 74% and free cholesterol by 45% in CD43−/−BMT mice. Furthermore, assessing the capabilities of macrophages to metabolize cholesterol we found that cholesterol efflux was markedly enhanced in CD43−/− macrophages compared to controls. These results indicate that CD43 normally inhibits efflux of cholesterol from macrophages without directly affecting the expression of efflux-enhancing membrane proteins, ABCA1 and ABCG1, or the scavenger receptors CD36 and SR-A. It is possible that CD43 may interact in some way with these transporters on the cell membrane to prevent them from shuttling cholesterol out of the cell. It is also possible that CD43 may block the interaction of ABCA1 and/or ABCG1 with potential cholesterol acceptors such as HDL and apoA1. Our findings suggest that inhibiting CD43 may constitute an interesting therapeutic strategy to limit the development of atherosclerosis.
Significance.
CD43 is an integral membrane glycoprotein that is expressed on all leukocytes but whose function has not been clearly elucidated. To assess the role of CD43 in atherogenesis atherosclerosis-prone LDLR−/− mice were lethally irradiated and transplanted with bone marrow from either CD43−/− mice or from the control CD43+/+ mice. The extent of atherosclerosis was less severe in LDLR−/− mice that received CD43−/− marrow than in those that were transplanted with bone marrow from control mice. Further work revealed that CD43 hinders with the process of transporting cholesterol out of lipid-filled macrophages. This study identifies CD43 as a potential target in trying to combat atherosclerosis. It would be relatively easy to inhibit this protein to enhance the reverse cholesterol transport by the macrophages with the ultimate goal of slowing down the development of atherosclerosis.
Acknowledgement
The authors thank Hongwei Wang for technical assistance. This work was performed within the Russian Government Program of Competitive Growth of Kazan Federal University.
Sources of Funding
This work was supported by NIH grants R01HL075677 and R01HL081663 as well as Hawaii Community Foundation grant 10ADVC-47037 to WAB. Core facilities were supported by NIH grants P20GM103516, P20RR016453, G12RR003061, and G12MD007601.
Nonstandard Abbreviations and Acronyms
- ldlr
low density lipoprotein receptor
- BMT
bone marrow transplantation
Footnotes
Disclosures
None.
References
- 1.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 1991;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sackstein R. The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr Opin Hematol. 2005;12:444–450. doi: 10.1097/01.moh.0000177827.78280.79. [DOI] [PubMed] [Google Scholar]
- 3.Cyster JG, Shotton DM, Williams AF. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 1991;10:893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenstein Y, Santana A, Pedraza-Alva G. CD43, a molecule with multiple functions. Immunol Res. 1999;20:89–99. doi: 10.1007/BF02786465. [DOI] [PubMed] [Google Scholar]
- 5.Rosenstein Y, Park JK, Hahn WC, Rosen FS, Bierer BE, Burakoff SJ. CD43, a molecule defective in Wiskott-Aldrich syndrome, binds ICAM-1. Nature. 1991;354:233–235. doi: 10.1038/354233a0. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez JD, Nguyen JT, He J, et al. Galectin-1 binds different CD43 glycoforms to cluster CD43 and regulate T cell death. J Immunol. 2006;177:5328–5336. doi: 10.4049/jimmunol.177.8.5328. [DOI] [PubMed] [Google Scholar]
- 7.Stockl J, Majdic O, Kohl P, Pickl WF, Menzel JE, Knapp W. Leukosialin (CD43)-major histocompatibility class I molecule interactions involved in spontaneous T cell conjugate formation. J Exp Med. 1996;184:1769–1779. doi: 10.1084/jem.184.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEvoy LM, Jutila MA, Tsao PS, Cooke JP, Butcher EC. Anti-CD43 inhibits monocyte-endothelial adhesion in inflammation and atherogenesis. Blood. 1997;90:3587–3594. [PubMed] [Google Scholar]