Abstract
Objectives/Hypothesis
Most patients with unilateral vocal fold paralysis experience some degree of spontaneous reinnervation, which depends upon the type and severity of recurrent laryngeal nerve (RLN) injury. After partial recovery, the paretic vocal fold may or may not adduct adequately to allow glottic closure, which in turn affects phonatory and swallowing outcomes. This process was studied in a series of canine laryngeal nerve injury models.
Study Design
Animal (canine) experiments.
Methods
Maximum stimulable laryngeal adductor pressure (LAP) was measured pre-treatment (baseline) and at 6 months following experimental RLN injuries (total n=59). The 9 study groups were designed to simulate a range of severities of RLN injury.
Results
The greatest LAP recovery, at 108% of original baseline, was seen in a 50% transection model; the least recovery was seen when the RLN underwent complete transection with repair, at 56% with precise alignment and 50% with alignment reversed. Intermediate models (partial RLN injuries) gave intermediate results. Crush models recovered 105% of LAP, while a half-transection, half-crush injury recovered 72% and cautery injuries recovered 61%. Controls (complete transection without repair) had no measurable recovery.
Conclusions
The injured RLN has a strong tendency to recover. Restoration of adductor strength, as determined by the LAP, was predictably related to the severity of RLN injury. The model RLN injuries studied provide a range of expected outcomes that can be used for future experiments exploring interventions that may improve post-injury adductor function.
Keywords: vocal cord, paralysis, synkinesis, reinnervation, canine
Introduction
Most injuries of the recurrent laryngeal nerve (RLN) are due to trauma during neck or chest surgeries.{1-3} Such nerve injuries include partial or complete transection, crush, stretch, electrocautery, and combinations of these. The degree of adductor function following RLN recovery from these injuries depends on the site and severity of the lesion. We sought to study this process in an animal model, which could then be used to test interventions that might improve nerve recovery and adductor function.
Animal models are commonly used to study RLN injuries. Rat models were used by Kupfer et al{4} to study sources of reinnervating axons following laryngeal denervation, and by Toya et al{5} to look at post-injury myelination. Porcine models were reported by Wu et al.{6} to evaluate the role of intraoperative nerve monitoring, and by Björck et al{7} to determine the contribution of the superior laryngeal nerve. A canine model was utilized by Lee et al{8} to investigate the effects of injury by a harmonic scalpel, and by Scott et al{9} to follow EMG after transection and crush injuries. There are many more examples, but none of these studies reported measures of adductor strength following recovery from a model RLN injury.
There are well-described differences between the laryngeal adductor (thyroarytenoid, TA, and lateral cricoarytenoid, LCA) and abductor (posterior cricoarytenoid, PCA) muscles. As a respiratory muscle, the PCA has a majority of slow-twitch, type I muscle fibers which respond optimally at lower stimulation frequencies.{10} The adductor muscles, for their protective glottic closure reflex function, have a majority of fast-twitch, type II muscle fibers.{11,12} At low stimulation frequencies (20-30 Hz), the slow-twitch abductor PCA muscle predominates, and there is little or no net adduction. As the frequency is increased, the fast-twitch adductor muscles take over, and the vocal fold begins to adduct, reaching a force plateau at 70-100 Hz.{13}
As an experimental construct, the strength of adduction can be measured by stimulating the RLN while resisting the motion of the vocal fold toward the midline. The force measurement is obtained using the inflated cuff of an endotracheal tube, inserted between the vocal folds, and measuring the squeezing pressure (with a transducer) during adduction; hence we refer to this as “laryngeal adductor pressure” (LAP).{13} A plot of these measurements against the stimulation frequency results in a characteristic curve, which has the same general shape in all of the dogs studied (shown below). Supramaximal stimulation of the main RLN trunk will result in non-selective, simultaneous stimulation of all of its axons, clearly a non-physiologic, artificial construct, but one that can be reliably repeated at different time intervals (since it is maximal) for comparison. Sub-maximal stimuli cannot be reliably compared on different dates, as the experimental conditions surrounding the stimulating electrode (e.g., blood and fluid surrounding the electrode) cannot be standardized between dates; but supramaximal stimulation reliably gives the same LAP values on different dates. It was not our goal to simulate physiologic behavior, but instead to develop a reliable method for assessing laryngeal reinnervation. We have been using this method for a variety of experimental preparations over the last 20 years, and it has proved highly reproducible (e.g., reference 14).
In this study, LAP measurements were made before nerve injury (baseline) and at 6 months post-op in a series of RLN injury models. We have found that reinnervation is essentially complete by 4 months post-operative, but we wait until 6 months for final measurements to eliminate any uncertainty of full recovery.
Methods and Materials
59 hemilaryngeal preparations were performed on purpose-bred, conditioned, female mongrel dogs weighing 20 to 25 kg. The animals were maintained in a facility approved by the American Association for Accreditation of Laboratory Animal Care, and National Institutes of Health guidelines for animal care were followed strictly. All experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee of the Washington University School of Medicine. The following protocol was followed for each dog:
Initial procedure
General anesthesia was induced with intravenous thiopental sodium and maintained with 2% isoflurane inhalant. A permanent tracheostomy through rings 10 to 13 was performed according to our previously described method.{15} The RLN and SLN were identified, and the laryngeal adductory pressure (LAP) was measured during stimulation of the left or right RLN in the manner previously described.{13} Briefly, a Harvard electrode is placed around the RLN, and an endotracheal tube is placed retrograde through the stoma so that the cuff lies just inferior to the vocal folds. The cuff is inflated and a pressure transducer is attached to the pilot. A variable frequency, constant current nerve stimulator is applied to the electrode, causing the vocal fold to adduct; the balloon is passed superiorly through the glottis, and the pressure on the balloon is recorded. The stimulating current is adjusted to just achieve the maximum pressure. The LAP is the difference between the stimulated maximum and the unstimulated baseline measurement. Measurements are made at each stimulating frequency from 20 Hz to 100 Hz at 10 Hz intervals in random sequence. This initial set of measurements constitutes the dog's control (untreated baseline) data for later comparison. The endotracheal tube is labeled with the dog's ID and saved for use in post-treatment data collection.
Intervention
The choices of experimental group assignment, and of left side versus right side, were made randomly. Following the data collection and experimental intervention, the neck wound was closed with sutures placed to mature the permanent stoma. Postoperative care included daily wound care and antibiotics for one week. This method of canine tracheostomy does not require placement of a tracheostomy tube.{15} The following experimental groups were studied:
Complete transection model, with precise repair. The RLN main trunk was divided 4 cm inferior to the cricothyroid joint, and re-anastomosed with the both ends oriented as closely as possible to their original orientation, using an operating microscope and 9-0 nylon epineural sutures. This is the “standard” model of acute transection with repair. (n=8)
Complete transection model, with reverse orientation repair. The RLN main trunk was divided as in group C, but the distal portion was rotated 180° from its original orientation before performing anastomosis. (n=6)
Complete transection control. RLN main trunk was transected 4 cm inferior to the cricothyroid joint, and 6 cm of the nerve was removed inferior to this point. Both nerve endings were ligated with suture, and no repair was performed. (n=5)
Half transection model. The RLN main trunk was divided through 50% of its diameter, then repaired. This simulates a partial transection injury. (n=6)
Nerve crush model. The RLN main trunk was crushed 4 cm inferior to the cricothyroid joint. To create the nerve crush, a small hemostat was applied to one click, held for 20 seconds, and released. An obvious depression at the crush site was evident in all cases. (n=7)
Partial transection model with crush. The RLN main trunk was divided through 50% of its diameter as in group C. The other 50% was subjected to a crush injury as in group D. This simulates a more severe, but still incomplete, partial RLN trauma. (n9)
Nerve cautery model. The RLN main trunk was cauterized 4 cm inferior to the cricothyroid joint. A small bipolar electrocautery tip was applied across the nerve (without completely squeezing the tips together), with the cautery set at 20 watts, and applied for 0.2 seconds. A discoloration at the cautery site, but not a complete burn, was evident in all cases. (n=6)
Minimum synkinesis model. The RLN adductor (AD) and abductor (AB) divisions were transected, then repaired by end-to-end anastomosis. This model simulates the result that would occur if there were a complete transection, and then in recovery all abductor axons found their way back into distal abductor axons, and vice-versa (i.e., best possible luck). (n=9)
Maximum synkinesis model. The RLN AD and AB divisions were transected, their positions reversed, then sutured together by end-to-end anastomosis. This model simulated the result that would occur if there was a complete transection, then in recovery all of the abductor axons ended up reinnervating adductor muscles, and vice-versa (i.e., worst possible luck). (n=8).
The injury groups were chosen to hypothetically result in a range of recovery patterns. Groups D-G are models of partial RLN injuries. Group C is a complete transection control. Groups H and I represent the extremes of possible synkinesis recovery patterns.{13}
Infraglottic exams
At 1-2 months postoperative, vocal fold movement was assessed visually to confirm complete unilateral paralysis had been achieved by the RLN intervention. With the dog sitting comfortably on an exam table, a 0° telescope was passed through the tracheostomy into the subglottis, where an infraglottal view of the vocal folds was obtained. The dog was given 1-2cc water by mouth to stimulate a swallow and glottic closure reflex.{16}
Terminal procedure
At 6 months post-operative, each dog was returned to the operating room and general anesthesia induced as before. The neck was explored, the RLNs were identified at a site inferior to the previous intervention (typically just inferolateral to the stoma), and Harvard electrodes were applied. The same endotracheal tube used 6 months earlier was inserted and used to collect LAP measurements as before, with the same volume of air in the cuff. When data collection was complete, the animal was euthanized.
LAP data processing
each set of baseline (pre-treatment) measurements was corrected for the nonstimulated offset, then normalized to the 80 Hz value. The 6-month post-treatment measurements were corrected for offset, then expressed as a percentage of the 80 Hz pre-treatment value. This approach cancels any systemic change in stimulation parameters between the two data intervals. The adductor plateau LAP values from 70 to 100 Hz were averaged to give a single adductor measure for each animal. The group means were compared with a t-test. Differences were considered statistically significant at the p<0.05 level.
Results
During the several years of this project, there were 7 dogs that expired prior to their scheduled completion date, usually due to tracheostomy obstruction from crusting. These are excluded from the data below, as no final data was obtained from them.
Baseline LAP Data
The pooled results for all 59 baseline, pre-treatment LAP curves are shown in Figure 1. There is a plateau at 20-30 Hz, then a fairly linear increase in LAP to a higher plateau at 70-100 Hz. At low frequencies, the predominantly slow-twitch PCA muscle predominates. As the frequency is increased above 30 Hz, the predominantly fast-twitch adductor muscles begin an orderly recruitment of fibers until they are all contracting maximally at the plateau frequencies. This characteristic curve was found in all of the canine larynges, thus, the error bars (± one standard deviation) in Figure 1 are small.
Figure 1.
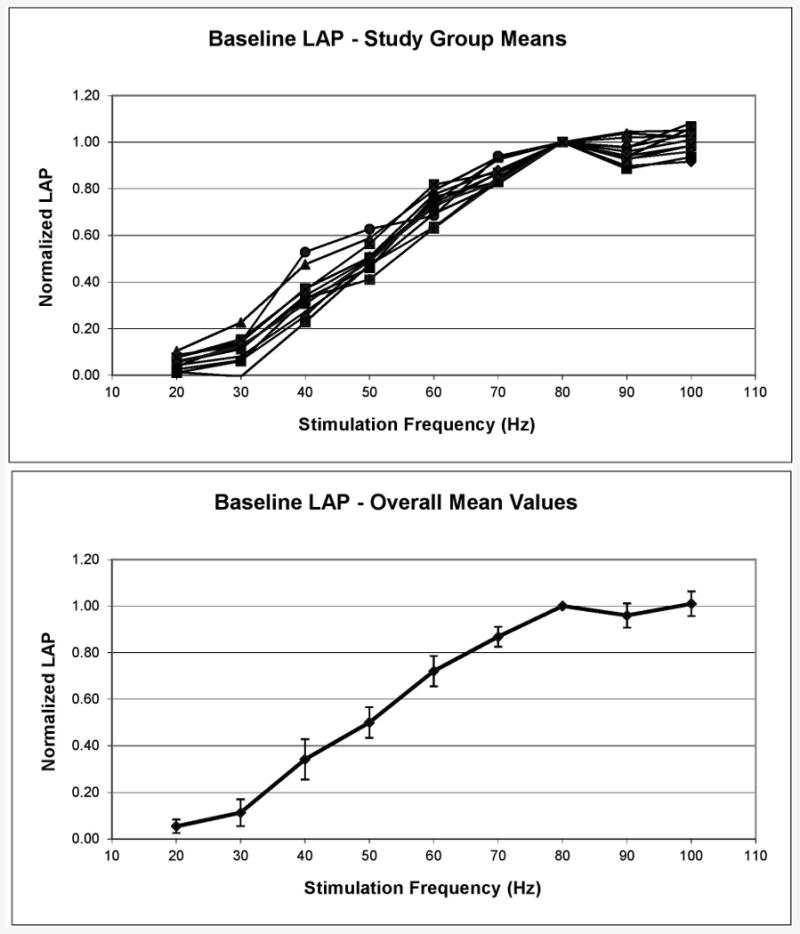
Pre-treatment (baseline) laryngeal adductor pressure (LAP) values for all 59 experiments. Top, mean LAPs for each study group. Bottom, combined mean for entire group. Error bars are ± one standard deviation.
Experimental Groups – 6 month LAP Data
The 70-100 Hz mean LAP values for all study groups are given in Table 1. Groups D, E and H had 6-month LAP values that returned to the pre-treatment baseline, while the remainder were significantly less. Except for the complete transection group (C), all study groups showed evidence of reinnervation, regaining at least 50% of their adductor strength by 6 months postoperative.
Table 1.
6-month postop mean laryngeal adductory pressure (LAP) values at 70-100 Hz for model RLN injury study groups. p-values based on comparison with pre-treatment baseline using t-test. NS, not significant; transx, transection; rot, rotation.
| Group | Model | N | LAP | ±SD | p |
|---|---|---|---|---|---|
| A | Full transx, 0° rot | 8 | 0.56 | 0.11 | <0.001 |
| B | Full transx, 180° rot | 6 | 0.50 | 0.04 | <0.001 |
| C | Full transx, no repair | 5 | 0.00 | 0.00 | <0.001 |
| D | ½ transx | 6 | 1.08 | 0.11 | NS |
| E | Full crush | 7 | 1.05 | 0.10 | NS |
| F | ½ transx, ½ crush | 4 | 0.72 | 0.13 | 0.036 |
| G | Cautery | 6 | 0.61 | 0.15 | 0.001 |
| H | AD/AB divisions, 0° rot | 9 | 0.99 | 0.05 | NS |
| I | AD/AB divisions, 180° rot | 8 | 0.70 | 0.04 | <0.001 |
| Baseline | Pre-Rx | 59 | 0.96 | 0.06 |
The LAP curves for the study groups with transection injuries (A-D) are shown in Figure 2. Error bars were omitted for clarity, but the standard deviations are given in Table 1. The complete transection injury with precise repair group (A) recovered 56% of its LAP. Interestingly, when the distal nerve segment was rotated 180° (group B), the result was essentially the same at 50% (p=0.096). This may reflect the randomness involved in the reinnervation process; Gacek et al. demonstrated the AD and AB axons are not organized into fascicles but are intermingled throughout the nerve until it splits distally into adductor and abductor divisions.{17} The half-transection group (D) recovered to a normal LAP. This may reflect a dominant activity of the uninjured axons in the other 50% of the nerve. The five dogs in the complete transection control group (C) had no measurable LAP at any frequency. Vocal fold movement could not be elicited with a nerve stimulator probe, and neck exploration showed no evidence of nerve re-growth between the cut ends of the RLN.
Figure 2.
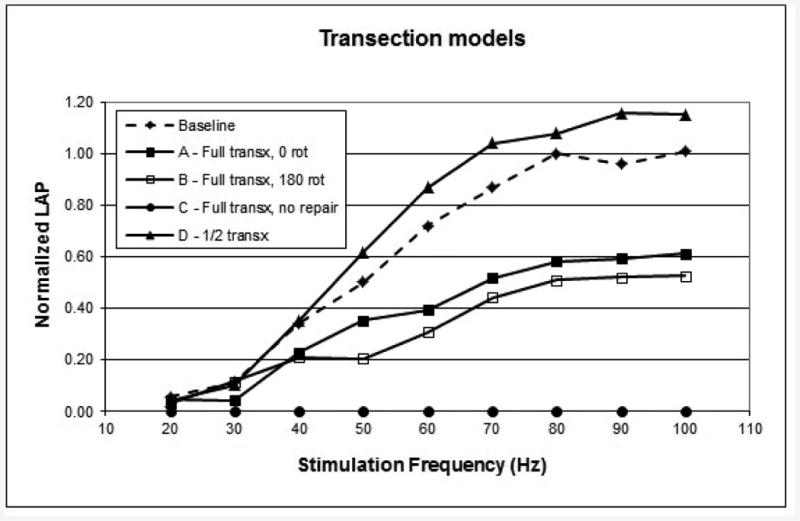
6-month LAP measurements for the transection (transx) models. Each curve is the composite mean curve for the group.
The LAP curves from study groups that represent partial RLN injuries (D-G) are shown in Figure 3. The half-transection and the crush groups (D-E) both recovered to normal LAP, but the combination of these injuries (group F) was more severe and only recovered 72%. The cautery injury (group G), which affects an unknown portion of the axons, had less recovery than group F with only 61% return of LAP.
Figure 3.
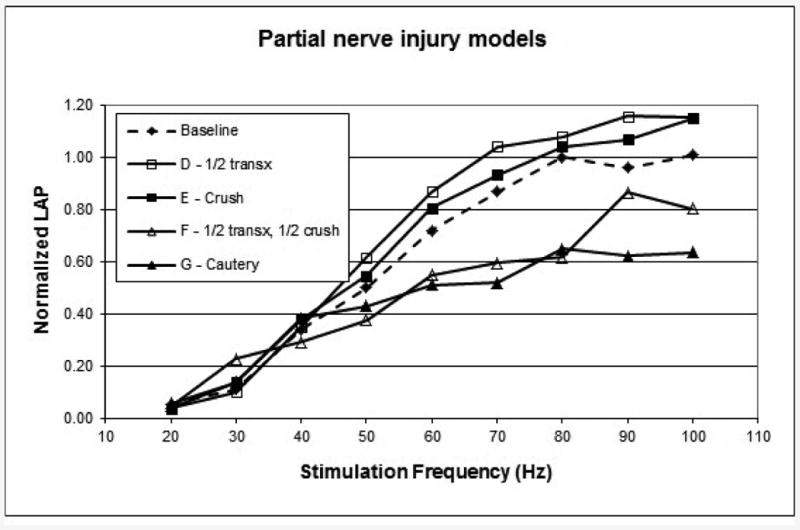
6-month LAP measurements for the partial nerve injury models. Each curve is the composite mean curve for the group. Transx, transection.
Data from the experimental synkinesis extremes are plotted in Figure 4. The minimal synkinesis model (group H) recovered 99% of baseline LAP, while the maximal synkinesis model (group I) recovered 70% (p<0.001), a bit higher than expected (see Discussion).
Figure 4.
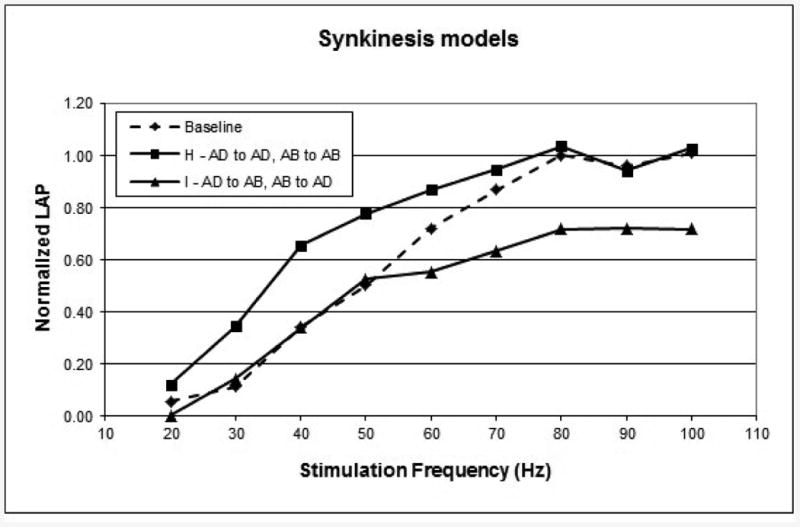
6-month LAP measurements for the extremes of synkinesis models. Each curve is the composite mean curve for the group. AD, adductor trunk; AB, abductor trunk.
The results of t-tests comparing all nine study groups are shown in Figure 5. It can be seen that the LAPs in the partial recovery groups (A, B, F and G) were not significantly different from each other due to the small N, but were all significantly lower than the complete recovery groups (D and E).
Figure 5.
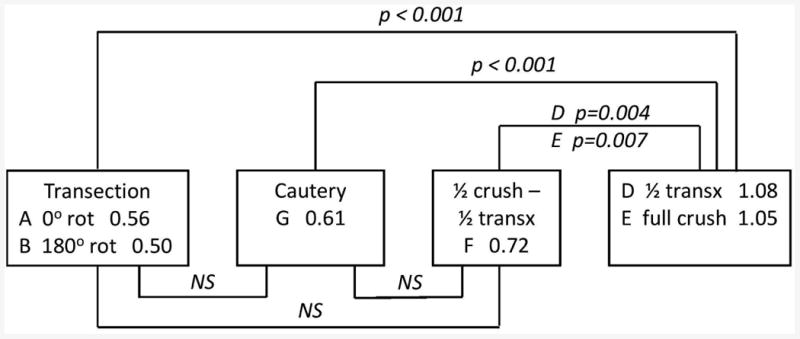
Significance diagram showing group names, LAP values, and t-test p-values for each comparison. NS, not significant (p >0.05). A vs. B, and D vs. E, are NS. Group C (not shown) is p <0.001 vs. all other groups. Groups H and I (not shown) are similar to groups E and F, respectively, with slightly lower p- values due to higher N.
Discussion
The injured RLNs in this study showed a wide range of recovery patterns. It must be emphasized that the curves in Figures 2-4 are the composite results for each study group, and that within each group there was also a range of results (e.g., baseline values in Figure 1).
The LAP measure used for this study is clearly non-physiological; simultaneous maximal co-contraction of adductor and abductor muscles does not normally occur. For example, EMG studies by Hillel showed that during phonation, the TA and LCA muscles have an initial burst followed by low-level activity, while the PCA is generally quiet except during unvoiced consonants.{18} Similarly, the adductor muscles are inactive during quiet respiration. The advantage of the LAP for research is that is repeatable, since it uses supramaximal stimulation parameters that do not vary based on local factors at the site of the stimulating electrode (nature of scar tissue, presence of blood or other fluid, etc.). The LAP measures the net result of this artificial co-contraction of adductor and abductor muscles. Thus, changes in the LAP represent quantitative differences in the degree of reinnervation, as well as shifts in the agonist-antagonist balance.
Further, measurable LAP does not directly translate into vocal fold movement in the awake animal. Many of the dogs in this study had limited spontaneous vocal fold movement, but could be electrically stimulated to strong adductory pressures. The LAP results in this study should not be interpreted as indicators of vocal fold movement, but simply as experimental measures. Laryngeal EMG studies often show signficant neuromotor activity in TA muscles of patients with clinical vocal fold paralysis.{19} However, laryngeal reinnervation procedures, such as ansa cervicalis to RLN anastomosis, provide significant voice improvement without generating significant vocal fold movement;{20-22} the benefit seems to come from improved laryngeal muscle “tone.” Thus, it is unknown how these canine LAP measurements might translate into human vocal fold activity.
The LAP, as a measure of functional reinnervation, is advantageous over histologic measures such as axon counting. Due to the variable size of a motor unit (group of muscle fibers innervated by a single axon), each axon may induce contraction of a single muscle fiber or many fibers, depending on the degree of intramuscular axon sprouting. Simple axon counting cannot distinguish between axons that innervate large or small motor units. Peterson et al.{23} reported that the average size of canine laryngeal motor units increases following denervation/reinnervation to become larger than it was pre-injury. The LAP measures the combined strength of contraction of functioning motor units of all sizes.
The nerve injuries modeled in this study were intended to represent potential iatrogenic trauma that may occur during surgery involving the RLN, such as thyroidectomies, carotid endarterectomies, and others. Transection injuries, typically from sharp dissection, may occur at any severity; we chose 50% and 100% transections to provide a range of possible recoveries. Interestingly, the half-transection group recovered to full LAP. This may indicate that there is some redundancy within the RLN, and not all of the axons are needed to achieve maximal adductor strength. This may also reflect the tendency for adductor axons to recover more quickly and more completely than abductors (“Semon's law”), such that reduced antagonism from the PCA results in higher measured LAP. A crush injury (Sunderland class I-II), such as occurs with retractor pressure or hemostat clamping, is generally expected to have a near-complete recovery, as demonstrated in these data. The more severe injuries had lesser degrees of recovery. These experiments provide a sample of expected recoveries from potential intraoperative injuries, but also provide experimental RLN injury models that may be used in future research on improving laryngeal function following such injuries.
The findings in the synkinesis study groups (H and I) are intriguing. All RLN axons were transected when the adductor and abductor divisions were divided; yet, the correctly aligned group (H) had complete recovery of LAP to normal. Synkinesis of one adductor axon into an alternate adductor muscle likely occurred, but would not likely be reflected in the LAP measure since all adductor muscles would still contract maximally. When the AD trunk was anastomosed to the distal AB division, and vice-versa, an LAP of 70% was still generated. It is likely that the PCA was maximally reinnervated by adductor axons, which in turn generated maximal abductor contraction when stimulated. The PCA axons that reinnervated the adductor muscles must have been capable of generating significant adductor strength as well, despite the lower number of total axons present. Together this finding suggests that the maximum synkinetic antagonism, in which the PCA pulls against the adductors, is about 30%. Interventions that could block PCA reinnervation could thus potentially increase adductor strength by this amount.
However, the LAP measure does not provide a means of quantifying synkinesis, because there are too many simultaneous variables that cannot be controlled. The measured LAP reflects 1) the number of RLN axons that have reinnervated the laryngeal muscles (i.e., all other factors equal, more RLN axons will result in higher LAP); 2) the appropriateness of the reinnervation (adductor axons into adductor muscles, etc.)(less synkinesis results in less PCA antagonism); 3) the completeness of histochemical transformation of muscle fibers that have been reinnervated by axons of a different type (e.g., slow axons into a fast muscle)(manifested by the differential frequency response of the LAP);{24-25} and 4) the balance or net effect of these factors on the agonist and antagonist muscles. Quantifying synkinesis will remain a research challenge.
It is worth considering alternate sources of reinnervating axons following these model nerve injuries. Kupfer et al.{26} showed superior laryngeal nerve (SLN) axons involved in reinnervating the denervated rat larynx, with the RLN completely transected and not repaired. Björk et al.{27} found a “surprisingly high” amount of motor input from the SLN in a series of dissections of normal pig larynges. Hydman et al.{28} found SLN fibers responsible for reinnervating the PCA following RLN transection in the rat larynx, but concluded this innervation was due to sprouting of existing SLN fibers in the PCA. These studies raise the question of whether non-RLN reinnervation may be responsible for some of the LAP measured in the present study. Although this cannot be completely excluded, we note that 1) the LAP is measured while directly stimulating the RLN, other nerves such as the SLN are not depolarized by this; 2) the LAP measurement drops to zero if the RLN is divided between the stimulating electrode and the larynx; and 3) the non-repaired transection control group (C) had no measurable LAP. These findings suggest that any alternate reinnervation is minimal in this model. It is also possible that alternate reinnervation could block potential reinnervation by the RLN by occupying motor endplates. The returns to normal LAP in the crush and half-transection groups (D and E) suggest that this effect is also minimal; and, we would expect any such blocking to affect all of our models similarly. Thus, alternate reinnervation, such as by SLN axons, was not a likely confounder of our data.
Conclusion
Several models of laryngeal injury and repair were found to give a range of adductor recoveries when studied 6 months post-RLN injury and repair. The degree of recovery does appear to be related to the severity of the nerve injury. Three models of RLN injury that typify intraoperative recurrent laryngeal nerve injuries showed intermediate levels of functional recovery. These models of RLN injury may be useful for future studies of interventions to improve functional adductor recovery.
Acknowledgments
The authors would like to thank other former residents and fellows who helped with this project over the years: Drs. Steve West, Sid Khosla, Patty Lee, and Dave Dahm. We would also like to thank Dr. Mike Talcott, Angie Lewis, and Julie Long, of the Division of Comparative Medicine, for their diligent care of our dogs.
This work was supported by a VA Merit Review grant, and NIH grants R03DC003859 and R01DC010884.
Footnotes
Presented at the annual meeting of the American Laryngological Association, May 28, 2009, in Phoenix, AZ. Recipient of the Casselberry Award.
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committees (IACUC) of Washington University School of Medicine and the St. Louis VA Medical Center.
The authors have no conflicts of interest or financial interests to disclose.
References
- 1.Rosenthal LH, Benninger MS, Deeb RH. Vocal fold immobility: a longitudinal analysis of etiology over 20 years. Laryngoscope. 2007;117:1864–70. doi: 10.1097/MLG.0b013e3180de4d49. [DOI] [PubMed] [Google Scholar]
- 2.Takano S, Nito T, Tamaruya N, et al. Single institutional analysis of trends over 45 years in etiology of vocal fold paralysis. Auris Nasus Larynx. 2012;39:597–600. doi: 10.1016/j.anl.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Spataro EA, Grindler DJ, Paniello RC. Etiology and time to presentation of unilateral vocal fold paralysis. Otolaryngol. Head Neck Surg. 2014;151:286–294. doi: 10.1177/0194599814531733. [DOI] [PubMed] [Google Scholar]
- 4.Kupfer RA, Old MO, Oh SS, et al. Spontaneous laryngeal reinnervation following chronic recurrent laryngeal nerve injury. Laryngoscope. 2013;123:2216–27. doi: 10.1002/lary.24049. [DOI] [PubMed] [Google Scholar]
- 5.Toya Y, Kumai Y, Minoda R, Yumoto E. Modulation of nerve fibers in the rat thyroarytenoid muscle following recurrent laryngeal nerve injury. Acta Otolaryngol. 2012;132:305–13. doi: 10.3109/00016489.2011.637176. [DOI] [PubMed] [Google Scholar]
- 6.Wu CW, Dionigi G, Sun H, et al. Intraoperative neuromonitoring for the early detection and prevention of RLN traction injury in thyroid surgery: a porcine model. Surgery. 2014;155:329–30. doi: 10.1016/j.surg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Björck G, Margolin G, Måbäck GM, et al. New animal model for assessment of functional laryngeal motor innervation. Ann Otol Rhinol Laryngol. 2012;121:695–9. doi: 10.1177/000348941212101013. [DOI] [PubMed] [Google Scholar]
- 8.Lee KE, Jee HG, Kim HY, et al. Development of a canine model for recurrent laryngeal injury by harmonic scalpel. Lab Anim Res. 2012;28:223–8. doi: 10.5625/lar.2012.28.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott AR, Chong PS, Hartnick CJ, Randolph GW. Spontaneous and evoked laryngeal electromyography of the thyroarytenoid muscles: a canine model for intraoperative recurrent laryngeal nerve monitoring. Ann Otol Rhinol Laryngol. 2010;119:54–63. doi: 10.1177/000348941011900111. [DOI] [PubMed] [Google Scholar]
- 10.Malmgren LT, Gacek RR. Histochemical characteristics of muscle fiber types in the posterior cricoarytenoid muscle. Ann Otolaryngol. 1981;90:423–429. doi: 10.1177/000348948109000503. [DOI] [PubMed] [Google Scholar]
- 11.Martensson A, Skoglund CR. Contraction properties of intrinsic laryngeal muscles. Acta Physiol Scand. 1964;60:318–336. doi: 10.1111/j.1748-1716.1964.tb02895.x. [DOI] [PubMed] [Google Scholar]
- 12.Braund KG, Steiss JE, Marshall AE, et al. Morphologic and morphometric studies of the intrinsic laryngeal muscles in clinically normal adult dogs. Am J Vet Res. 1988;49:2105–2110. [PubMed] [Google Scholar]
- 13.Paniello RC, West SE. Laryngeal adductory pressure as a measure of post-reinnervation synkinesis. Laryngoscope. 2000;109:447–451. doi: 10.1177/000348940010900502. [DOI] [PubMed] [Google Scholar]
- 14.Lee P, Paniello RC. Laryngeal chemodenervation: effects of injection site, dose, and volume. Ann Otol Rhinol Laryngol. 1999;108:1140–1145. doi: 10.1177/000348949910801208. [DOI] [PubMed] [Google Scholar]
- 15.Dahm JD, Paniello RC. Tracheotomy for long-term laryngeal experimentation. Otolaryngol. Head Neck Surg. 1998;118:376–80. doi: 10.1016/S0194-59989870318-3. [DOI] [PubMed] [Google Scholar]
- 16.Paniello RC, Dahm JD. Long-term model of induced canine phonation. Otolaryngol Head Neck Surg. 1998;118:512–522. doi: 10.1177/019459989811800413. [DOI] [PubMed] [Google Scholar]
- 17.Gacek RR, Malmgren LT, Lyon MJ. Localization of adductor and abductor motor nerve fibers to the larynx. Ann Otol Rhinol Laryngol. 1977;86:771–776. [Google Scholar]
- 18.Hillel AD. The study of laryngeal muscle activity in normal human subjects and in patients with laryngeal dystonia using multiple fine-wire electromyography. Laryngoscope. 2001;111:1–47. doi: 10.1097/00005537-200104001-00001. [DOI] [PubMed] [Google Scholar]
- 19.Grosheva M, Wittekindt C, Pototschnig C, et al. Evaluation of peripheral vocal cord paralysis by electromyography. Laryngoscope. 2008;118:987–90. doi: 10.1097/MLG.0b013e3181671b2d. [DOI] [PubMed] [Google Scholar]
- 20.Crumley RL. Update: ansa cervicalis to recurrent laryngeal nerve anastomosis for unilateral laryngeal paralysis. Laryngoscope. 1991;101:384–388. doi: 10.1002/lary.1991.101.4.384. [DOI] [PubMed] [Google Scholar]
- 21.Olson DEL, Goding GS, Michael DD. Acoustic and perceptual evaluation of laryngeal reinnervation by ansa cervicalis transfer. Laryngoscope. 1998;108:1767–1772. doi: 10.1097/00005537-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Paniello RC, Edgar JD, Kallogjeri D, Piccirillo JF. Medialization versus reinnervation for unilateral vocal fold paralysis: a multicenter randomized clinical trial. Laryngoscope. 2011;121:2172–9. doi: 10.1002/lary.21754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson KL, Graves M, Berke GS, Ye M, Wallace R, Bell T, Sercarz JA. Role of motor unit number estimate electromyography in experimental canine laryngeal reinnervation. Otolaryngol Head Neck Surg. 1999;121:180–4. doi: 10.1016/S0194-5998(99)70168-3. [DOI] [PubMed] [Google Scholar]
- 24.Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato F, Hisa Y. Contraction properties and histochemical study of cross-innervated intrinsic laryngeal muscles. In: Hirano M, editor. Neurolaryngology: Recent Advances. College-Hill; Boston: 1987. pp. 120–129. [Google Scholar]
- 26.Kupfer RA, Old MO, Oh SS, Feldman EL, Hogikyan ND. Spontaneous laryngeal reinnervation following chronic recurrent laryngeal nerve injury. Laryngoscope. 2013;123:2216–27. doi: 10.1002/lary.24049. [DOI] [PubMed] [Google Scholar]
- 27.Björck G, Margolin G, Måbäck GM, Persson JK, Mattsson P, Hydman J. New animal model for assessment of functional laryngeal motor innervation. Ann Otol Rhinol. Laryngol. 2012;121:695–9. doi: 10.1177/000348941212101013. [DOI] [PubMed] [Google Scholar]
- 28.Hydman J, Mattsson P. Collateral reinnervation by the superior laryngeal nerve after recurrent laryngeal nerve injury. Muscle Nerve. 2008;38:1280–9. doi: 10.1002/mus.21124. [DOI] [PubMed] [Google Scholar]


