Abstract
Objective
This study aimed to determine the incidence of major complications following both primary and revision transsphenoidal pituitary surgery. Major complications included endocrinopathic, skull base, orbital, hemorrhagic and thromboembolic complications, respiratory failure, and death. Secondarily, this study aimed to examine factors associated with the occurrence of complications.
Study Design
Retrospective cohort analysis of California and Florida all-payer databases from 2005-2008.
Methods
The major complication rate following both primary and revision transsphenoidal pituitary surgery was calculated. Bivariate analyses were performed to investigate the relationship of patient characteristics with complication occurrence, and a multivariate model was constructed to determine risk factors associated with these complications.
Results
5,277 primary cases and 192 revision cases met inclusion criteria. There was a non-significant absolute difference of 3.09% (95% CI −11.00 to 16.14) between the rate of complications following primary (n=443; 8.39%) and revision (n=22; 11.46%) surgeries. Multivariate analyses showed that patients with Medicare (OR=1.74; 95% CI 1.17 to 2.61), Medicaid (OR=2.13; 95% CI 1.59 to 2.86), or a malignant neoplasm (OR=3.10; 95% CI 1.62 to 5.93) were more likely to have complications.
Conclusions
The rate of major complications following transsphenoidal pituitary surgery is lower than earlier retrospective reports. The overall complication rate following revision surgery was not significantly different from primary surgery. Insurance status and a diagnosis of a malignant neoplasm were associated with a higher rate of complications.
Keywords: transsphenoidal surgery, complications, pituitary adenomas, CSF leak, panhypopituitarism, hemorrhage, diabetes insipidus, orbital hematoma, vision loss, diplopia
INTRODUCTION
Pituitary tumors (PT) are a diverse group of neoplasms of the pituitary with an estimated prevalence of approximately 15%.1 The vast majority of PTs involve adenohypophyseal cells of the pituitary, and are histologically proven to be benign pituitary adenomas. While most PTs are asymptomatic, they may compress surrounding structures and secrete abnormal levels of pituitary hormones causing various endocrinologic symptoms. Although pharmacologic2 and radiation therapies3, 4 are used, surgical resection is the primary treatment of choice for most patients with symptomatic PTs.5, 6
Today the most common surgical treatment of PTs is resection using an endonasal transsphenoidal approach. This approach has been used since 19067 with continued refinement and adoption of microsurgical8, 9 and, more recently, endoscopic techniques.10 The introduction of the endoscope to endonasal pituitary surgery in the last 20 years has been an evolutionary milestone in the field and led to the increased role of the otolaryngologist in PT resection surgeries.11 Despite its long history and widespread use, reports of the incidence of transphenoidal pituitary surgery-related complications have primarily come from experiences at single institutions with varying characteristics and patient populations. As a result, the risk of major complications from transphenoidal pituitary surgery is unknown with reported rates ranging from 0% to 20%.12-27 The major complications described include cerebrospinal fluid (CSF) leak, dural tears, bacterial meningitis, perioperative hemorrhage, carotid artery injuries, orbital injuries, permanent diabetes insipidus (DI), and permanent panhypopituitarism.
Although rare, recurrence of PTs may warrant revision surgical resection.28 Revision surgery is theoretically more challenging due to distorted anatomy and scarring. Additionally, revision surgery often occurs in the context of residual tumors that were either missed or not easily resected on primary surgery. Therefore, these cases may require more aggressive and technically challenging dissection.29 While intuitively believed to be more risky than primary surgery, to date no study has examined the rate of complications specifically following revision pituitary surgery.
The goal of this study was to determine the rate of major complications following both primary and revision transsphenoidal PT resection using a large population-based database containing nearly 6,000 cases. Major complications queried included endocrine, skull base, orbital, hemorrhagic and thromboembolic complications, respiratory failure, and death. In addition, using multivariate analysis this study aimed to examine factors that may be associated with the occurrence of major surgical complications including patient and clinical characteristics.
METHODS
Study Design
This study is a retrospective analysis of patients who underwent either a primary or revision transsphenoidal resection of a PT using large population-based databases from 2005-2008. The primary outcome of interest was the rate of major surgical complications. The Institutional Review Board of Washington University in St. Louis School of Medicine approved this study.
Data Sources
Using the Healthcare Cost and Utilization Project (HCUP) state databases from California and Florida, a cohort of patients who underwent transsphenoidal PT resection between 2005 and 2008 was identified. The HCUP state databases contain information abstracted from hospital discharge records regardless of primary payer (including Medicare, Medicaid, private insurance, and no insurance). In the HCUP state databases, the State Inpatient Database (SID) provides information from inpatient hospital visits,30 the State Ambulatory Surgery Database (SASD) provides records from ambulatory surgery visits at both hospitals and free-standing ambulatory surgical centers,31 and the State Emergency Department Database(SEDD) provides records from emergency department visits.32
The Agency for Healthcare Research and Quality (AHRQ) revisit files allow individual patients to be linked across all three databases longitudinally using date of birth, gender, and an encrypted patient identifier. Additionally, the revisit files provide information about the time period from one visit to another for each patient while keeping exact dates encrypted to protect patient confidentiality. As a result, these databases offer researchers the ability to examine surgical complications that occur at the time of surgery or after hospital discharge. Taken together, the California and Florida HCUP databases offer access to a combined population of over 56 million with information from more than 90% of community hospitals contained in the databases.33
Study Population
Cases of transsphenoidal surgery of PTs were identified from the SID from January 2005 through December 2008. Current Procedural Terminology (CPT) codes are not tracked within the SID. Therefore, all cases were identified using International Classification of Diseases Ninth Revision (ICD-9) Volume 3 procedure codes for transsphenoidal pituitary surgery (07.14, 07.62, or 07.65). All cases were required to have a concurrent ICD-9 diagnosis code for a benign, uncertain, or malignant pituitary neoplasm (227.3, 237.0, 239.7, or 194.3), acromegaly (253.0), or Cushing’s syndrome (255.0).
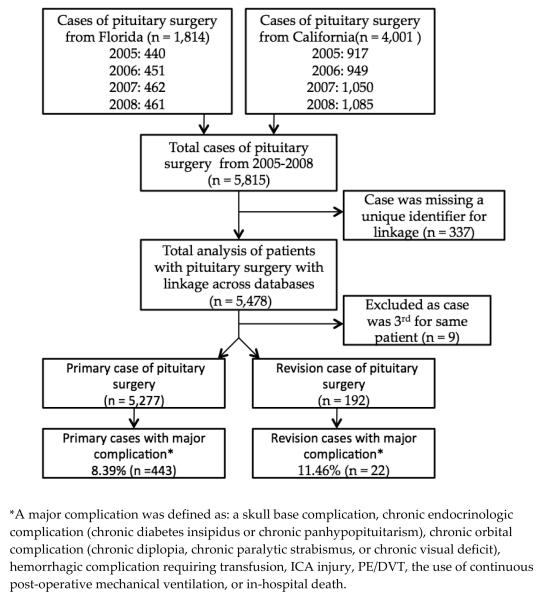
Primary cases were defined as the first case of transsphenoidal pituitary surgery for a PT identified in the SID database from 2005-2008. If a patient had a previously documented pituitary surgery in the database, the next case meeting the aforementioned criteria was defined as the revision case. Subsequent cases (third or more surgery for the same patient) were excluded from all analyses as repeat revision surgeries were believed to be exceedingly rare and these results would have limited widespread clinical applicability. After identification, primary and revision cases were linked to SASD and SEDD using AHRQ revisit files. Cases were linked to the other databases to extract information on complications that may have occurred outside of the initial hospitalization. If a case was missing the encrypted patient identifier used for linkage, it was excluded from this study since these cases could not be examined for future complications (Figure 1).
Figure 1.
Study flow diagram
Measures
Primary Outcome
The primary outcome of this study was the occurrence of a major complication following transsphenoidal pituitary surgery. All potential complications were identified based on either ICD-9 diagnosis codes or ICD-9 procedure codes related to a complication (Table 1).
Table 1.
ICD-9 diagnosis and procedure codes for included complications
| Complication or Procedure | ICD-9 Code(s) | ICD-9 Procedure Code(s) |
|---|---|---|
| Skull Base: | ||
| CSF rhinorrhea | 349.81 | |
| Bacterial meningitis | 320.x | |
| Orbital: | ||
| Chronic diplopia* | 368.2 | |
| Chronic paralytic strabismus* | 378.5x | |
| Optic nerve injury | 950 | |
| Chronic visual deficit/blindness* | 369.x | |
| Orbital hemorrhage | 376.32 | |
| Endocrinologic: | ||
| Chronic diabetes insipidus* | 253.5 | |
| Chronic panhypopituitarism* | 253.2, 253.7 | |
| Hemorrhagic: | ||
| Transfusion | 99.04 | |
| Internal carotid artery injury | 900.03 | |
| Other: | ||
| Pulmonary embolism or DVT | 415.1-415.19; 453.8-453.9 | |
| Continuous Mechanical Ventilation | 96.70-96.72 |
ICD: International Classification of Diseases; CPT: Current Procedural Terminology; DVT: Deep venous thrombosis; “x” denotes any numerical value.
Chronic complications are defined based on a new diagnosis of the complication after surgery that persists for >90 days.
Complications were divided into the following categories: skull base, orbital, endocrinologic, hemorrhagic, other (including pulmonary embolism (PE)/Deep Venous Thrombosis (DVT) or use of post-operative continuous mechanical ventilation), or death. Cases were considered to have a skull base complication if they were diagnosed with a CSF leak or bacterial meningitis within 90 days after the time of surgery. Cases were considered to have a chronic endocrinologic or orbital complication, if they were diagnosed with diabetes insipidus, panhypopituitarism, diplopia, paralytic strabismus, or visual disturbance within 30 days of surgery with documentation that the complication persisted >90 days after surgery. Cases were considered to have a hemorrhagic complication if injury to the internal carotid artery (ICA) was documented or the patient required a blood transfusion within 30 days of surgery. Additionally, patients were considered to have an “other” major complication if they required continuous mechanical ventilation after surgery, were diagnosed with a DVT, PE, or underwent inferior vena cava filter placement within 30 days of surgery. For all complications, records one year prior to surgery were examined in an effort to assure that a complication was not present prior to the time of surgery.
Covariates Analyzed
All covariates were defined based on information at the time of surgery. Age was divided into four categories: <18 years, 18-40 years, 41-65 years, and >65 years. Race was divided into four categories: white, black, Hispanic, and other. The category of other race included Asian, Pacific Islander, as well as Native American. Primary expected payer was collapsed into three categories: private insurance, Medicare, and Medicaid/other.
Statistical Analysis
Standard descriptive statistics were used to describe the study population, and the rate of major complications following transsphenoidal pituitary surgery was then calculated. For primary surgical cases, the associations between available demographic and clinical characteristics and the occurrence of complications were evaluated using chi-squared tests. The rate of complications following revision transsphenoidal pituitary surgery was then examined. The rate of complications following primary surgery and revision surgery was initially compared using standard chi-squared tests.
All covariates that achieved a level of significance less than 0.10 on univariate analyses, as well as gender and revision status, were entered into a logistic regression analysis to determine potential predictors of major complications. In order to determine the risk of revision surgery as compared to primary surgery the PROC SURVEY LOGISTIC command in SAS® was applied to the logistic regression model. Diagnostic tests including tests of multicolinearity were performed to test the assumptions of the final model. In addition, potential interactions between predictive covariates were checked. SAS® 9.2 (SAS Institute, Cary, NC) was used for database management and statistical analyses. In accordance with rules described by HCUP, all results based on tabulated data of ≤10 individuals were reported as “≤ 10” in the tables to protect patient confidentiality.
RESULTS
Patient Characteristics
Among the 5,815 cases of transsphenoidal surgery for PT identified, 5,277 primary cases and 192 revision cases met inclusion criteria (Figure 1). The mean (SD) age of the included population was 51.3 (16.8). Approximately twice as many cases came from the California database as compared to Florida. Nearly half of the cases were white (n = 2,574; 49%) with the majority having a primary payer of private insurance (n = 3,304; 63%). The most common primary transsphenoidal procedure performed in this cohort was a partial excision (n = 3,951; 75%) of the pituitary for a diagnosis of a benign neoplasm (n = 5,168; 98%).
Characteristics of Primary Complications
The distribution of characteristics for patients experiencing a skull base complication or any major complication is described in Table 2. Among the 5,277 primary transsphenoidal pituitary surgery cases, a total of 443 major complications were identified representing a major complication rate of 8.39% (95% CI 7.67–9.17%). Of the major complications following primary surgery, 189 skull base complications (complication rate of 3.58%), 25 orbital complications (complication rate of 0.47%), 84 endocrinologic complications (complication rate of 1.59%), and 110 hemorrhagic complications (complication rate of 2.08%) were identified. Additionally, 113 other complications (complication rate of 2.14%) involving occurrence of a DVT/PE or the use of continuous mechanical ventilation post-operatively occurred. Finally 23 cases resulted in a death within the hospitalization (death rate of 0.13%).
Table 2.
Demographics and clinical characteristics of complications after primary transsphenoidal surgery
| Characteristic | Patients | Patients with skull base complication, n (%) |
Unadjusted OR (95% CI) |
Patients with any major complication, n (%) |
Unadjusted OR (95% CI) |
|---|---|---|---|---|---|
| Total | 5,277 | 189 (3.58) | -- | 443 (8.39) | -- |
| State | |||||
| California | 3,587 | 123 (3.43) | Ref | 294 (8.20) | Ref |
| Florida | 1,690 | 66 (3.91) | 1.15 (0.84-1.55) | 149 (8.82) | 1.08 (0.88-1.33) |
| Age at surgery (yrs) | |||||
| ≤18 | 134 | ≤10 (<7.47)† | -- | 13 (9.70) | 1.48 (0.80-2.73) |
| 19-40 | 1,164 | 46 (3.95) | Ref | 79 (6.79) | Ref |
| 41-65 | 2,576 | 97 (3.77) | 0.95 (0.67-1.36) | 202 (7.84) | 1.17 (0.89-1.53) |
| >65 | 1,248 | 40 (3.21) | 0.81 (0.52-1.24) | 148 (11.86) | 1.85 (1.39-2.46)* |
| Gender | |||||
| Female | 2,508 | 101 (4.03) | Ref | 214 (8.53) | Ref |
| Male | 2,358 | 86 (3.65) | 0.90 (0.67-1.21) | 225 (9.54) | 1.13 (0.93-1.38) |
| Race | |||||
| White | 2,574 | 101 (3.92) | Ref | 218 (8.47) | Ref |
| Black | 602 | 18 (2.99) | 0.76 (0.45-1.26) | 58 (9.63) | 1.15 (0.85-1.56) |
| Hispanic | 995 | 39 (3.92) | 1.00 (0.69-1.46) | 96 (9.65) | 1.15 (0.90-1.49) |
| Other | 441 | 24 (5.44) | 1.41 (0.89-2.23) | 53 (12.02) | 1.48 (1.07-2.03)* |
| Insurance Status | |||||
| Private insurance | 3,304 | 117 (3.54) | Ref | 210 (6.36) | Ref |
| Medicare | 1,279 | 44 (3.44) | 0.97 (0.65-1.38) | 152 (11.89) | 1.99 (1.60-2.48)* |
| Medicaid | 648 | 27 (4.17) | 1.18 (0.77-1.82) | 77 (11.88) | 1.99 (1.51-2.62)* |
| Procedure | |||||
| Biopsy of pituitary | 109 | ≤10 (<9.18)† | -- | 12 (11.01) | 1.36 (0.74-2.50) |
| Excision of pituitary | 5,168 | 188 (3.64) | Ref | 431 (8.34) | Ref |
| Diagnosis | |||||
| Benign neoplasm of pituitary |
4,881 | 167 (3.42) | Ref | 384 (7.87) | Ref |
| Uncertain neoplasm | 74 | ≤10 (<13.52)† | -- | ≤10 (<13.52)† | -- |
| Malignant neoplasm | 56 | ≤10 (<17.86)† | -- | 13 (23.2) | 3.54 (1.89-6.64)* |
OR: odds ratio; CI: confidence interval;
p <0 .05;
Exact numbers not reported in accordance with the HCUP data user agreement that prohibits reporting of cells with ≤10 observations.
On bivariate analysis, patients aged >65 had an increased rate of complications (11.86%; OR 1.85; 95% CI 1.39-2.46) as compared to younger adults (complication rate of 6.79%). Furthermore, patients with a primary payer of Medicare (11.89%; OR 1.99; 95% CI 1.60-2.48) or Medicaid (11.88%; OR 1.99; 95% CI 1.51-2.62) were at an increased risk of complications when compared to those with private insurance (6.36%). Finally, patients with a diagnosis of a malignant neoplasm (23.2%) as opposed to a benign neoplasm (7.87%) were more likely to have a major complication (OR 3.54; 95% CI 1.89-6.64).
Comparison of risk of complications following primary vs. revision surgery
The rate of each individual complication was described and compared between primary and revision cases in Table 3. The rate of major complications following revision surgery was not significantly higher than primary surgery (11.46% vs. 8.39; OR 1.41; 95% CI 0.89 to 2.25). However, this did represent a non-significant absolute increase of 3.09% (95% CI −11.00 to 16.14) in complications following revision surgery. The rate of skull base complications was nearly twice as high following revision cases as compared to primary cases (6.25% vs. 3.58%; OR 1.80; 95% CI 0.98 to 3.28). Finally, the rate of chronic endocrinologic complications, orbital complications, hemorrhagic complications, and other complications (PE/DVT, use of mechanical ventilation, or death) was similar between primary and revision cases.
Table 3.
Complications after primary and revision transspheonidal pituitary surgery
| Primary Cases (n=5,277) |
Revision Cases (n=192) |
|||
|---|---|---|---|---|
| Complication | Patients, n (%) |
Patients, n (%) | Unadjusted OR (95% CI) |
P value |
| All major complications † | 443 (8.39) | 22 (11.46) | 1.41 (0.89 to 2.23) | 0.135 |
| Skull base complications | 189 (3.58) | 12 (6.25) | 1.80 (0.98 to 3.28) | 0.056 |
| Orbital complications | 25 (0.47) | ≤10 (<5.21)* | -- | |
| Endocrinologic complications | 84 (1.59) | ≤10 (<5.21)* | -- | |
| Hemorrhagic complications | 110 (2.08) | ≤10 (<5.21)* | -- | |
| Other complications† | 113 (2.14) | ≤10 (<5.21)* | -- | |
| Death | 23 (0.44) | ≤10 (<5.21)* | -- |
OR: odds ratio; CI: confidence interval;
Exact numbers not reported in accordance with the HCUP data user agreement that prohibits reporting of cells with ≤10 observations.
Other complications defined as the occurrence of a DVT or PE or the use of continuous mechanical ventilation post-operatively.
Predictors of major complications (multivariate model)
Patient age, primary payer, type of neoplasm (benign, uncertain, or malignant), and type of case (primary or revision) were entered into a logistic regression model to examine predictors of major complications following surgery. In this model (Table 4), the rate of major complications was significantly increased in patients with a primary payer of Medicare (OR 1.74; 95% CI 1.17-2.61) or Medicaid (OR 2.13; 95% CI 1.59 to 2.86) as compared to patients with private insurance. In addition, major surgical complications were more common in cases with a diagnosis of a malignant neoplasm as compared to a benign neoplasm (OR 3.10; 95% CI 1.62 to 5.93). In the multivariate model, cases involving a revision surgery were not significantly more likely to have a major complication as compared to cases involving a primary surgery (OR 1.41; 95% CI 0.85 to 2.32).
Table 4.
Multivariate analysis of characteristics associated with major complications following transspheonidal pituitary surgery
| Skull Base Complications |
All Major Complications |
|
|---|---|---|
|
| ||
| Characteristic | Adjusted OR (95% CI) | Adjusted OR (95% CI) |
|
| ||
| Age at surgery (yrs) | ||
| ≤18 | 1.14 (0.44 to 2.96) | 1.21 (0.59 to 2.47) |
| 19-40 | Ref | Ref |
| 41-65 | 0.91 (0.63 to 1.33) | 1.18 (0.88 to 1.58) |
| >65 | 0.67 (0.37 to 1.20) | 1.36 (0.86 to 2.17) |
| Insurance Status | ||
| Private insurance | Ref | Ref |
| Medicare | 1.29 (0.78 to 2.14) | 1.74 (1.17 to 2.61)* |
| Medicaid | 1.35 (0.88 to 2.08) | 2.13 (1.59 to 2.86)* |
| Diagnosis | ||
| Benign neoplasm of pituitary | Ref | Ref |
| Uncertain neoplasm | 0.72 (0.18 to 2.93) | 1.19 (0.54 to 2.62) |
| Malignant neoplasm | 0.96 (0.23 to 4.03) | 3.10 (1.62 to 5.93)* |
| Case | ||
| Primary case | Ref | Ref |
| Revision case | 1.69 (0.88 to 3.24) | 1.41 (0.85 to 2.32) |
OR: odds ratio; CI: confidence interval;
p <0 .05
DISCUSSION
Using a population based cohort, we examined the rate of major complications following 5,277 primary and 192 revision cases of transsphenoidal pituitary surgery. The overall rate of major complications following primary surgeries was 8.39%, while 11.46% of revision cases resulted in a major complication. Among primary cases and revision cases, approximately 0.44% and 0.52% of cases respectively resulted in a death during the hospitalization. Of note, the rate of skull base complications was nearly twice as high after revision cases as compared to primary cases. However, the rate of chronic endocrinologic, orbital, hemorrhagic, and other complications was similar following both primary and revision surgeries.
Prior reports of major complications following transsphenoidal pituitary surgery have ranged significantly and have consisted primarily of experiences at single institutions with varying characteristics and patient populations. A recent retrospective study of a single surgeon’s endoscopic experience in 570 patients with pituitary adenomas reported an overall complication rate of 12.1%. In this study, Beker et al34 noted that major complications included post-operative CSF leak in 1.3%, meningitis in 0.8%, permanent DI in 0.4% and death in 0% of patients. Somewhat higher rates of complications were shown in a meta-analysis published in 2012 containing 38 studies with 2125 endoscopic and 3518 microscopic approaches. In this study, major complication rates were similar when comparing endoscopic to microscopic approaches, with only the rate of vascular complications being statistically significantly higher in the endoscopic group. In the endoscopic group, this study noted a death rate of 0.49% with complications including CSF leak in 7.0%, meningitis in 1.1%, vascular complication in 1.6%, visual loss in 0.7%, transient DI in 9.1%, and permanent DI in 2.31% of patients.35 Unfortunately, using this cohort, we were not able to specifically compare microscopic vs. endoscopic approaches.
As compared to these and earlier studies,12-27 the rate of complications following transsphenoidal pituitary surgery in this cohort was comparatively low with 8.39% of primary cases resulting in major complications. The lower rate of complications in this study could be due to a number of factors including improved imaging, more extensive preoperative planning, increased experience with the surgery, or improved instrumentation and surgical training that may have occurred since publication of these earlier studies. One major advance in endoscopic skull base surgery has been the development, mastery, and increasing use of the pedicled nasoseptal flap described in 2006,36 which has proven to significantly improve the success of CSF leak repair and outcomes following endoscopic skull base surgery. This would have been increasingly used in the later half of our years of data collection, and may explain the lower numbers of CSF leak complications found in our dataset. Additionally, earlier reports on complications came primarily from experiences at single institutions. These reports generally represented cases from tertiary facilities that were potentially treating patients with comparatively more challenging anatomy and more co-morbidities. Our cohort contained cases from tertiary centers and greater than 90% of community hospitals, thus potentially skewing our data to show a lower complication rate. However, the possibility exists that the comparatively low rate of complications found in this study may be partly attributable to the study’s design, which was reliant on administrative coding and may have systematically missed complications
Reports of complications specifically following revision transsphenoidal pituitary surgery are limited. One retrospective report from the UK looked at complications in 35 revision cases performed at a single center over 18 years and noted a high rate of complications.37 This study demonstrated that revision cases involved a CSF leak rate of 17.1%, bacterial meningitis rate of 8.6%, posterior pituitary insufficiency rate of 14.3%, and anterior pituitary insufficiency rate of 14.3%. There were no incidences of ICA injury, blindness, or death in this study. Of note, this experience spanned nearly two decades, during a time period when instrumentation, technique, and expertise significantly changed, possibly confounding the results.37 In our cohort, patients receiving revision surgery were not significantly more likely to have a major complication as compared to patients receiving primary surgery. However there was a more than 3% absolute increased risk of complications in revision cases. Furthermore, revision cases were nearly twice as likely to result in a skull base complication in this cohort.
In our dataset, the complication rate did not vary by age; however, it did vary significantly by insurance status. To our knowledge no one has investigated how complications following pituitary tumor surgery vary by insurance status. One recent study by Momin et al.38 did note that patients undergoing a craniotomy for a brain tumor who were uninsured or had a primary payer of Medicaid were more likely to have in-hospital postoperative death. This finding is similar to other studies, involving numerous surgeries, that have found worse outcomes and increased complication rates in patients that are not privately insured.39-43 There are several potential explanations for this finding. First, patients without private insurance have been shown to have decreased access to specialty care44 which may lead to delayed treatment, presentation with more advanced disease, and presentation with more co-morbidities. Unfortunately, this is only postulation since the presence of co-morbidities and extent of disease could not be specifically evaluated in this database. In addition, Medicaid specifically could serve as a marker for other socioeconomic factors that could be associated with decreased health awareness, worse post-operative compliance, and consequently a higher risk of complications.45
This study does have several limitations worthy of note. First, all information collected in this population-based analysis was collected retrospectively from an administrative dataset. As a result, the possibility exists that complications may be systematically under-coded in this dataset. Furthermore, all information from these datasets is collected and linked at the state level using encrypted patient identifiers. Therefore, if a patient has a surgery in one state and his or her complication is noted in a different state, that complication will not be contained in the dataset and lost for analysis. In addition, it is possible that a patient had a pituitary tumor surgery prior to the beginning of the dataset, thus falsely increasing the number of primary cases and decreasing the number of revision cases in our cohort. This would likely result in an overestimation of complications in the primary group and a Type II error in comparing primary to revision cases. Finally, the possibility exists that complications were only temporally related to the patient’s pituitary surgery and were not truly a complication from the surgery itself. We tried to mitigate this possibility by reviewing patient records for up to a year prior to surgery to see if a complication existed prior to surgery.
CONCLUSIONS
This study suggests that the rate of major complications following transsphenoidal pituitary surgery is comparably lower than earlier retrospective reports. The overall complication rate following revision surgery was higher than that from primary surgery; however, this difference was not statistically significant. Predictors of increased major complication rates included Medicare and Medicaid insurance status and a diagnosis of a malignant neoplasm. These associations are worthy of further consideration and investigation.
Acknowledgments
This research was supported by a grant from the Council of the Triological Society, the Doris Duke Clinical Research Fellowship, and the Stanford Medical Scholars Fellowship. The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ), and Grant Number KM1CA156708 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH).
Footnotes
Conflict of interest: none
Research presented at the Triological Society’s 117th Annual Meeting at the Combined Otolaryngology Spring Meetings (COSM) – #566
References
- (1).Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004 Aug 1;101(3):613–9. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- (2).Colao A, Pivonello R, Di SC, Savastano S, Grasso LF, Lombardi G. Medical therapy of pituitary adenomas: effects on tumor shrinkage. Rev Endocr Metab Disord. 2009 Jun;10(2):111–23. doi: 10.1007/s11154-008-9107-z. [DOI] [PubMed] [Google Scholar]
- (3).Sheplan Olsen LJ, Robles IL, Chao ST, et al. Radiotherapy for prolactin-secreting pituitary tumors. Pituitary. 2011 Sep 27; doi: 10.1007/s11102-011-0348-6. [DOI] [PubMed] [Google Scholar]
- (4).Minniti G, Scaringi C, Enrici RM. Radiation techniques for acromegaly. Radiat Oncol. 2011;6:167. doi: 10.1186/1748-717X-6-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Petrovich Z, Jozsef G, Yu C, Apuzzo ML. Radiotherapy and stereotactic radiosurgery for pituitary tumors. Neurosurg Clin N Am. 2003 Jan;14(1):147–66. doi: 10.1016/s1042-3680(02)00031-1. [DOI] [PubMed] [Google Scholar]
- (6).Chanson P, Salenave S. Diagnosis and treatment of pituitary adenomas. Minerva Endocrinol. 2004 Dec;29(4):241–75. [PubMed] [Google Scholar]
- (7).Schloffer H. Erfolreiche Operation eines Hypophysentumors auf nasalem Wege. Wien Klein Wochnschr. 1907;20:621–4. [Google Scholar]
- (8).Hardy J. Surgery of the pituitary gland, using the open trans-sphenoidal approach. Comparative study of 2 technical methods. Ann Chir. 1967 Aug;21(15):1011–22. [PubMed] [Google Scholar]
- (9).Fatemi N, Dusick JR, de Paiva Neto MA, Kelly DF. The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: a 10-year experience. Neurosurgery. 2008 Oct;63(4 Suppl 2):244–56. doi: 10.1227/01.NEU.0000327025.03975.BA. [DOI] [PubMed] [Google Scholar]
- (10).Jankowski R, Auque J, Simon C, Marchal JC, Hepner H, Wayoff M. Endoscopic pituitary tumor surgery. Laryngoscope. 1992 Feb;102(2):198–202. doi: 10.1288/00005537-199202000-00016. [DOI] [PubMed] [Google Scholar]
- (11).Senior BA, Dubin MG, Sonnenburg RE, Melroy CT, Ewend MG. Increased role of the otolaryngologist in endoscopic pituitary surgery: endoscopic hydroscopy of the sella. Am J Rhinol. 2005 Mar;19(2):181–4. [PubMed] [Google Scholar]
- (12).Cappabianca P, Cavallo LM, Colao A, et al. Endoscopic endonasal transsphenoidal approach: outcome analysis of 100 consecutive procedures. Minim Invasive Neurosurg. 2002 Dec;45(4):193–200. doi: 10.1055/s-2002-36197. [DOI] [PubMed] [Google Scholar]
- (13).Casler JD, Doolittle AM, Mair EA. Endoscopic surgery of the anterior skull base. Laryngoscope. 2005 Jan;115(1):16–24. doi: 10.1097/01.mlg.0000150681.68355.85. [DOI] [PubMed] [Google Scholar]
- (14).Charalampaki P, Ayyad A, Kockro RA, Perneczky A. Surgical complications after endoscopic transsphenoidal pituitary surgery. J Clin Neurosci. 2009 Jun;16(6):786–9. doi: 10.1016/j.jocn.2008.09.002. [DOI] [PubMed] [Google Scholar]
- (15).D’Haens J, Van RK, Stadnik T, Haentjens P, Poppe K, Velkeniers B. Fully endoscopic transsphenoidal surgery for functioning pituitary adenomas: a retrospective comparison with traditional transsphenoidal microsurgery in the same institution. Surg Neurol. 2009 Oct;72(4):336–40. doi: 10.1016/j.surneu.2009.04.012. [DOI] [PubMed] [Google Scholar]
- (16).Dehdashti AR, Ganna A, Karabatsou K, Gentili F. Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series. Neurosurgery. 2008 May;62(5):1006–15. doi: 10.1227/01.neu.0000325862.83961.12. [DOI] [PubMed] [Google Scholar]
- (17).Frank G, Pasquini E, Farneti G, et al. The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology. 2006;83(3-4):240–8. doi: 10.1159/000095534. [DOI] [PubMed] [Google Scholar]
- (18).Kabil MS, Eby JB, Shahinian HK. Fully endoscopic endonasal vs. transseptal transsphenoidal pituitary surgery. Minim Invasive Neurosurg. 2005 Dec;48(6):348–54. doi: 10.1055/s-2005-915635. [DOI] [PubMed] [Google Scholar]
- (19).Rudnik A, Zawadzki T, Wojtacha M, et al. Endoscopic transnasal transsphenoidal treatment of pathology of the sellar region. Minim Invasive Neurosurg. 2005 Apr;48(2):101–7. doi: 10.1055/s-2004-830185. [DOI] [PubMed] [Google Scholar]
- (20).Zhang Y, Wang Z, Liu Y, et al. Endoscopic transsphenoidal treatment of pituitary adenomas. Neurol Res. 2008 Jul;30(6):581–6. doi: 10.1179/174313208X298110. [DOI] [PubMed] [Google Scholar]
- (21).Abosch A, Tyrrell JB, Lamborn KR, Hannegan LT, Applebury CB, Wilson CB. Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. J Clin Endocrinol Metab. 1998 Oct;83(10):3411–8. doi: 10.1210/jcem.83.10.5111. [DOI] [PubMed] [Google Scholar]
- (22).Chen JC, Amar AP, Choi S, Singer P, Couldwell WT, Weiss MH. Transsphenoidal microsurgical treatment of Cushing disease: postoperative assessment of surgical efficacy by application of an overnight low-dose dexamethasone suppression test. J Neurosurg. 2003 May;98(5):967–73. doi: 10.3171/jns.2003.98.5.0967. [DOI] [PubMed] [Google Scholar]
- (23).Eisele DW, Flint PW, Janas JD, Kelly WA, Weymuller EA, Jr., Cummings CW. The sublabial transseptal transsphenoidal approach to sellar and parasellar lesions. Laryngoscope. 1988 Dec;98(12):1301–8. doi: 10.1288/00005537-198812000-00005. [DOI] [PubMed] [Google Scholar]
- (24).Mortini P, Losa M, Barzaghi R, Boari N, Giovanelli M. Results of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery. 2005 Jun;56(6):1222–33. doi: 10.1227/01.neu.0000159647.64275.9d. [DOI] [PubMed] [Google Scholar]
- (25).Choe JH, Lee KS, Jeun SS, Cho JH, Hong YK. Endocrine outcome of endoscopic endonasal transsphenoidal surgery in functioning pituitary adenomas. J Korean Neurosurg Soc. 2008 Sep;44(3):151–5. doi: 10.3340/jkns.2008.44.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Sharma K, Tyagi I, Banerjee D, Chhabra DK, Kaur A, Taneja HK. Rhinological complications of sublabial transseptal transsphenoidal surgery for sellar and suprasellar lesions: prevention and management. Neurosurg Rev. 1996;19(3):163–7. doi: 10.1007/BF00512046. [DOI] [PubMed] [Google Scholar]
- (27).Urquhart AC, Bersalona FB, Ejercito VS, Holt JJ. Nasal septum after sublabial transseptal transsphenoidal pituitary surgery. Otolaryngol Head Neck Surg. 1996 Jul;115(1):64–9. doi: 10.1016/S0194-5998(96)70138-9. [DOI] [PubMed] [Google Scholar]
- (28).Kollen K, Senior B. Revision Endoscopic Transsphenoidal Hypophysectomy. In: Kountakis S, Jacobs J, Gosepath J, editors. Revision sinus surgery. Berlin: 2008. pp. 245–50. [Google Scholar]
- (29).Shiley SG, Limonadi F, Delashaw JB, et al. Incidence, etiology, and management of cerebrospinal fluid leaks following trans-sphenoidal surgery. Laryngoscope. 2003 Aug;113(8):1283–8. doi: 10.1097/00005537-200308000-00003. [DOI] [PubMed] [Google Scholar]
- (30).Overview of the State Inpatient Database [AccessedAugust 15, 2011];Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. 2012 Available at: URL: http://www.hcup-us.ahrq.gov/sidoverview.
- (31).Overview of the State Ambulatory Surgery Database [AccessedAugust 15, 2011];Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. 2012 Aug 15; Available at: URL: http://www.hcupus.ahrq.gov/sasdoverview.jsp.
- (32).Overview of the State Emergency Department Database [AccessedAugust 15, 2011];Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. 2012 Available at: URL: http://www.hcup-us.ahrq.gov/seddoverview.
- (33).State & County QuickFacts [AccessedAugust 15, 2011];U S Bureau of the Census. 2012 Available at: URL: http://quickfacts.census.gov/qfd/index.html.
- (34).Berker M, Hazer DB, Yucel T, et al. Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary. 2012 Sep;15(3):288–300. doi: 10.1007/s11102-011-0368-2. [DOI] [PubMed] [Google Scholar]
- (35).DeKlotz TR, Chia SH, Lu W, Makambi KH, Aulisi E, Deeb Z. Meta-analysis of endoscopic versus sublabial pituitary surgery. Laryngoscope. 2012 Mar;122(3):511–8. doi: 10.1002/lary.22479. [DOI] [PubMed] [Google Scholar]
- (36).Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006 Oct;116(10):1882–6. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- (37).Kumar S, Darr A, Hobbs CG, Carlin WV. Endoscopic, endonasal, trans sphenoidal hypophysectomy: retrospective analysis of 171 procedures. J Laryngol Otol. 2012 Oct;126(10):1033–40. doi: 10.1017/S0022215112001223. [DOI] [PubMed] [Google Scholar]
- (38).Momin EN, Adams H, Shinohara RT, Frangakis C, Brem H, Quinones-Hinojosa A. Postoperative mortality after surgery for brain tumors by patient insurance status in the United States. Arch Surg. 2012 Nov 1;147(11):1017–24. doi: 10.1001/archsurg.2012.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Trinh QD, Schmitges J, Sun M, et al. Morbidity and mortality of radical prostatectomy differs by insurance status. Cancer. 2012 Apr 1;118(7):1803–10. doi: 10.1002/cncr.26475. [DOI] [PubMed] [Google Scholar]
- (40).Abdo A, Trinh QD, Sun M, et al. The effect of insurance status on outcomes after partial nephrectomy. Int Urol Nephrol. 2012 Apr;44(2):343–51. doi: 10.1007/s11255-011-0056-1. [DOI] [PubMed] [Google Scholar]
- (41).Kelz RR, Gimotty PA, Polsky D, Norman S, Fraker D, DeMichele A. Morbidity and mortality of colorectal carcinoma surgery differs by insurance status. Cancer. 2004 Nov 15;101(10):2187–94. doi: 10.1002/cncr.20624. [DOI] [PubMed] [Google Scholar]
- (42).Parikh PB, Gruberg L, Jeremias A, et al. Association of health insurance status with presentation and outcomes of coronary artery disease among nonelderly adults undergoing percutaneous coronary intervention. Am Heart J. 2011 Sep;162(3):512–7. doi: 10.1016/j.ahj.2011.06.002. [DOI] [PubMed] [Google Scholar]
- (43).Hacquebord J, Cizik AM, Malempati SH, et al. Medicaid status is associated with higher complication rates after spine surgery. Spine (Phila Pa 1976 ) 2013 Jul 15;38(16):1393–400. doi: 10.1097/BRS.0b013e3182959b68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Pollock SG. Access of Medicaid recipients to outpatient care. N Engl J Med. 1994 Sep 29;331(13):878. [PubMed] [Google Scholar]
- (45).Chung KC, Kotsis SV, Kim HM. Predictors of functional outcomes after surgical treatment of distal radius fractures. J Hand Surg Am. 2007 Jan;32(1):76–83. doi: 10.1016/j.jhsa.2006.10.010. [DOI] [PubMed] [Google Scholar]