Abstract
Background
Patients with Ewing sarcoma require local primary tumor control with surgery, radiation, or both. Optimal choice of local control for overall and local disease control remains unclear.
Methods
Patients with localized Ewing sarcoma of bone treated on three consecutive protocols with standard dose 5-drug chemotherapy every 3 weeks were included (n = 465). We used propensity scores to control for differences between local control groups by constructing multivariate models to assess the impact of local control type on clinical endpoints [event-free survival (EFS); overall survival; local failure; distant failure] independent of differences in propensity to receive each local control type.
Results
Patients treated with surgery were younger (p=0.02) and had more appendicular tumors (p<0.001). Radiation, compared to surgery, had a higher unadjusted risk of any event (HR 1.70; 95% CI 1.18–2.44), death (HR 1.84; 95% CI 1.18–2.85), or local failure (HR 2.57; 95% CI 1.37–4.83). On multivariate analysis, radiation, compared to surgery, had a higher risk of local failure (2.41; 95% CI 1.24–4.68), though there were no significant differences in EFS (HR 1.42; 95% CI 0.94–2.14), overall survival (HR 1.37; 95% CI 0.83–2.26), or distant failure (HR 1.13; 95% CI 0.70–1.84) between local control groups.
Conclusions
In this large group of similarly treated patients, choice of local control was not significantly related to EFS, overall survival, or distant failure, though risk of local failure was greater for radiation compared to surgery. These data support surgical resection when appropriate, while radiotherapy remains a reasonable alternative in selected patients.
Keywords: Ewing sarcoma, local control, surgery, radiation, propensity score
Introduction
Patients with Ewing sarcoma require a multimodal treatment approach, including chemotherapy and local control of the primary tumor. Ewing sarcoma is radiosensitive and, historically, local control typically involved definitive radiation therapy alone.1 Several factors have increased the use of surgical local control for these tumors, including increased awareness of late effects of radiotherapy and advances in imaging and limb-sparing surgery.2 Current local control options consist of radiation alone, surgery alone, or a combination of surgery with radiation.
The optimal mode of local control in Ewing sarcoma remains unclear. No prospective studies have compared surgery to radiotherapy in a randomized trial and numerous barriers to such a trial exist. Instead, clinical trials in Ewing sarcoma have made recommendations regarding local control strategies, but the choice is individualized and depends on factors such as tumor location, tumor size, age, patient preference, and institutional practice. Many of these factors also influence prognosis. As a result, analyses comparing local control strategies without adjustment for other prognostic factors have generally shown that patients treated with definitive radiotherapy have lower rates of both local control and overall survival than patients treated with definitive surgery.
We used a large cohort of patients treated with a similar chemotherapy regimen on three consecutive clinical trials to evaluate the optimal mode of local control for patients with localized osseous Ewing sarcoma. We controlled for known confounding factors influencing choice of local control and prognosis using both a propensity score method3 and conventional multivariate methods.
Patients and Methods
Patients
The cohort included eligible patients treated on the experimental arm of study INT-00914 and on the standard arms of study INT-01545 and AEWS0031.6 Patients were < 30 (<50 for AEWS0031) years of age before enrollment and had not received prior therapy. Only patients with nonmetastatic Ewing sarcoma or primitive neuroectodermal tumor (PNET) of bone were eligible for this analysis. Patients with tumors arising in the head were excluded due to multiple deviations from local control guidelines in this unusual site. Only patients with complete local control data and who received local control after neoadjuvant chemotherapy were included. Only patients starting local control ≥ 2 months and ≤ 6 months from randomization were included.
Patients were primarily treated at Children’s Oncology Group centers located in the United States and Canada. Each center’s Institutional Review Board approved the treatment protocols. Written informed consent was obtained for all patients at enrollment.
Treatment
A detailed summary of chemotherapy and local control is provided in the Supplemental Text and in the primary manuscripts derived from INT-0091, INT-0154, and AEWS0031.4–6
Statistical Methods
Tumors were classified by site using the following categories: spine; chest wall (rib, clavicle, sternum, and scapula); pelvis (pelvis and sacrum); proximal extremity (humerus and femur); and distal extremity (radius, ulna, tibia, fibula, and bones of the hands and feet). Tumors were classified as small (< 8 cm in maximum diameter) or large (≥ 8 cm) according to initial imaging. Tumor size data were not collected on AEWS0031.
We compared categorical patient characteristics between local control groups using chi-square tests. Continuous variables were compared between local control groups using analysis of variance (ANOVA).
We used logistic regression to generate propensity scores indicating the probability each patient treated on one of the included trials would have been selected to receive each of three modes of local control: radiation; surgery; or surgery plus radiation. The logistic regression model included covariates thought to influence individual physician and patient choice of local control, but importantly did not include as potential predictive covariates the actual mode of local control received or any of the clinical outcomes of interest described below. Potential covariates assessed were: age; sex; tumor site; tumor size; clinical trial; and year of study entry. By comparing clinical outcomes among patients with similar likelihood (or propensity) to receive the same mode of local control who were nevertheless selected for different modes of local control, one can take advantage of variation in clinical practice to control for confounding by comparing similar groups of patients. For a comprenhensive description of this approach, the reader is directed to the review article by Rubin.3
The primary outcome was event-free survival (EFS), with events defined as disease progression, death from any cause, or second malignant neoplasm. Secondary outcomes included overall survival, local failure, and distant failure. All survival outcomes were determined from the start of the first local control intervention occurring after initial neoadjuvant chemotherapy. EFS and overall survival were estimated using Kaplan-Meier methods, with patients without events censored at the time of last follow-up.7 We determined the cumulative incidence of local failure using a competing risks approach in which patients experiencing distant failure, death, or second malignant neoplasm prior to local failure were censored at the time of that event. We used an analogous approach to determine the cumulative incidence of distant failure.
We constructed Cox proportional hazards models to evaluate differences in EFS and overall survival according to mode of local control. We used competing risk regression analysis to evaluate differences in local failure and distant failure according to mode of local control.8 Definitive surgery was chosen as the reference group. Analyses performed with definitive radiation as the reference group yielded similar results. The proportional hazards assumption was tested using time-dependent covariates and satisfied in all models.
Unadjusted models included mode of local control as the sole covariate. The primary adjusted models also included surgical propensity score and radiation propensity scores with and without age and tumor site as additional covariates. We performed sensitivity analyses that separately controlled for clinical trial and tumor size for the subset of patients with available size data. We also constructed conventional Cox and competing risk regression models that did not rely on propensity score methods, but rather used each variable as covariates.
All statistical analyses were performed using SAS, version 9.2 and STATA, version 12.
Results
Patient Selection
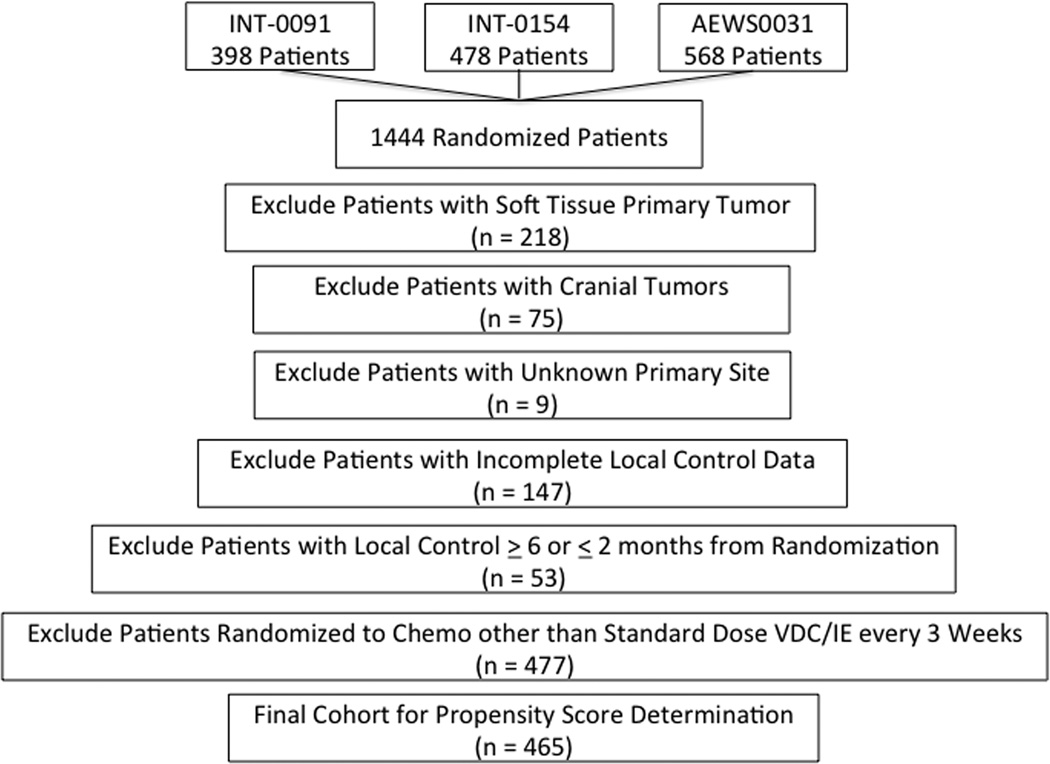
Of 1444 patients with localized Ewing sarcoma randomized as part of INT-0091, INT-0154, and AEWS0031, 979 patients were excluded (Figure 1). The most common reasons for exclusion were randomization to other chemotherapy regimens (n = 477) and soft tissue Ewing sarcoma (n = 218). Data from the remaining 465 patients were used to compare characteristics by local control groups and to generate propensity scores.
Figure 1.
CONSORT diagram of patient selection for analytic cohort.
Patient Characteristics Differ by Local Control Group
Patient characteristics differed according to mode of local control (Table 1). Patients treated with definitive radiation were more likely to have pelvic tumors, while patients treated with surgery were more likely to have extremity tumors. Patients treated with surgery were more likely to be younger compared to patients treated with definitive radiation or surgery plus radiation. Tumor size <8 cm vs. ≥ 8 cm was not associated with choice of local control (p = 0.24). Lower rates of definitive radiotherapy were seen in more recent years (p < 0.001). Of the 103 patients treated with surgery plus radiation, 17 (16.5%) received pre-operative radiotherapy and 86 (83.5%) received post-operative radiotherapy.
Table 1.
Clinical features of 465 patients with Ewing sarcoma of bone according to mode of local control received.
| Entire Cohort (n = 465) |
Radiation Alone (n = 121) |
Surgery Alone (n = 241) |
Radiation plus Surgery (n = 103) |
p-value* | |
|---|---|---|---|---|---|
| Males | 253 (54.4%) | 64 (52.9%) | 132 (54.8%) | 57 (55.3%) | 0.92 |
| Mean age at diagnosis (range) | 12.4 years (0.7–33.0) |
13.3 years (2.5–28.6) |
11.7 years (0.7–33.0) |
13.1 years (1–28.0) |
0.006 |
| Age < 12 years | 203 (43.7%) | 46 (38.0%) | 120 (49.8%) | 37 (35.9%) | 0.021 |
|
Tumor site Distal extremity Proximal extremity Pelvis Chest wall Spine |
124 (26.7%) 123 (26.5%) 98 (21.1%) 95 (20.4%) 25 (5.4%) |
16 (13.2%) 22 (18.2%) 50 (41.3%) 15 (12.4%) 18 (14.9%) |
89 (36.9%) 80 (33.2%) 26 (10.8%) 45 (18.7%) 1 (0.4%) |
19 (18.5%) 21 (20.4%) 22 (21.4%) 35 (34.0%) 6 (5.8%) |
< 0.001 |
|
Tumor size < 8 cm ≥ 8 cm |
77 (35.5%) 140 (64.5%) |
28 (41.2%) 40 (58.8%) |
39 (35.8%) 70 (64.2%) |
10 (25%) 30 (75%) |
0.24 |
|
Study INT-0091 INT-0154 AEWS0031 |
152 (32.7%) 120 (25.8%) 193 (41.5%) |
62 (51.2%) 22 (18.2%) 37 (30.6%) |
59 (24.5%) 81 (33.6%) 101 (41.9%) |
31 (30.1%) 17 (16.5%) 55 (53.4%) |
< 0.001 |
|
Year on Study 1988–1990 1991–1993 1994–1996 1997–1999 2000–2002 2003–2005 |
80 (17.2%) 72 (15.5%) 49 (10.5%) 71 (15.3%) 64 (13.8%) 129 (27.7%) |
36 (29.8%) 26 (21.5%) 8 (6.6%) 14 (11.6%) 16 (13.2%) 21 (17.4%) |
29 (12.0%) 30 (12.5%) 32 (13.3%) 49 (20.3%) 33 (13.7%) 68 (28.2%) |
15 (14.6%) 16 (15.5%) 9 (8.7%) 8 (7.8%) 15 (14.6%) 40 (38.8%) |
< 0.001 |
Unadjusted p-value. See Supplemental Table comparisons of baseline characteristics according to quintiles of surgical propensity score.
Local Control Propensity Scores
We constructed a series of logistic regression models of choice of local control based on the potential covariates detailed in Statistical Methods. Age, tumor site, clinical trial, and year of study entry were significant predictors of mode of local control on univariate and multivariate analysis. Since clinical trial and year of study entry were correlated, we excluded clinical trial from the final model, and only age, tumor site, and year of study entry were used. Models generated with and without tumor size data from patients with complete tumor size data yielded nearly identical propensity scores (Supplemental Figure). Tumor size was not included in the final model, since data were unavailable for patients treated on AEWS0031 and some patients from the other trials.
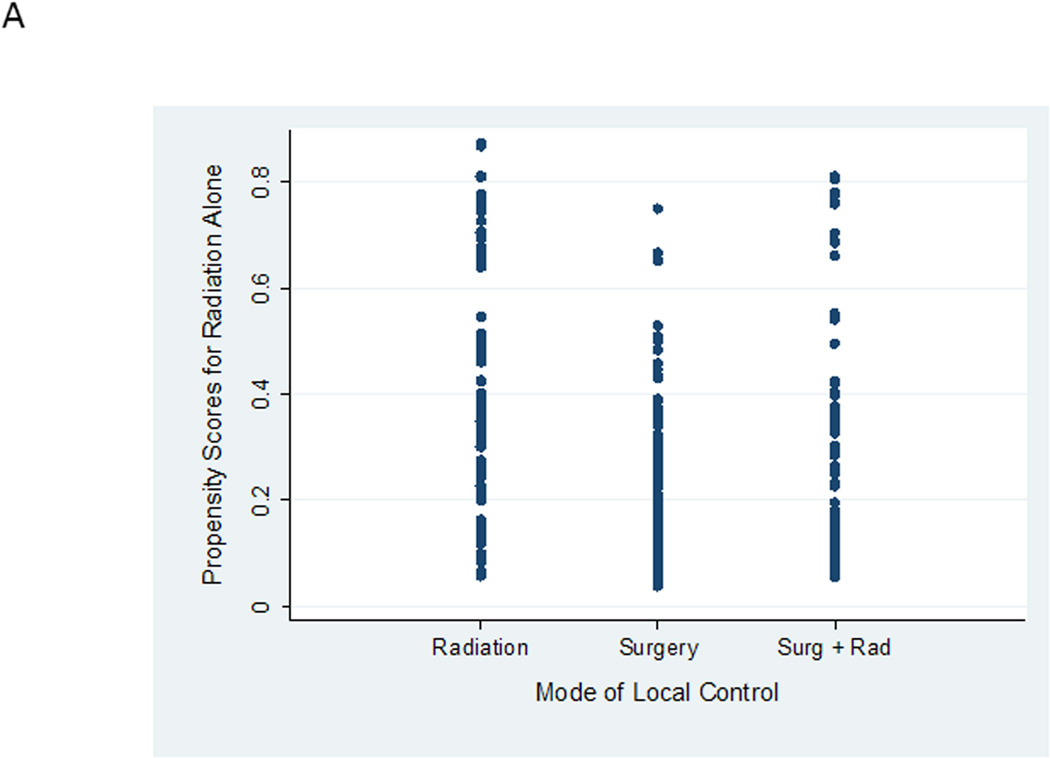
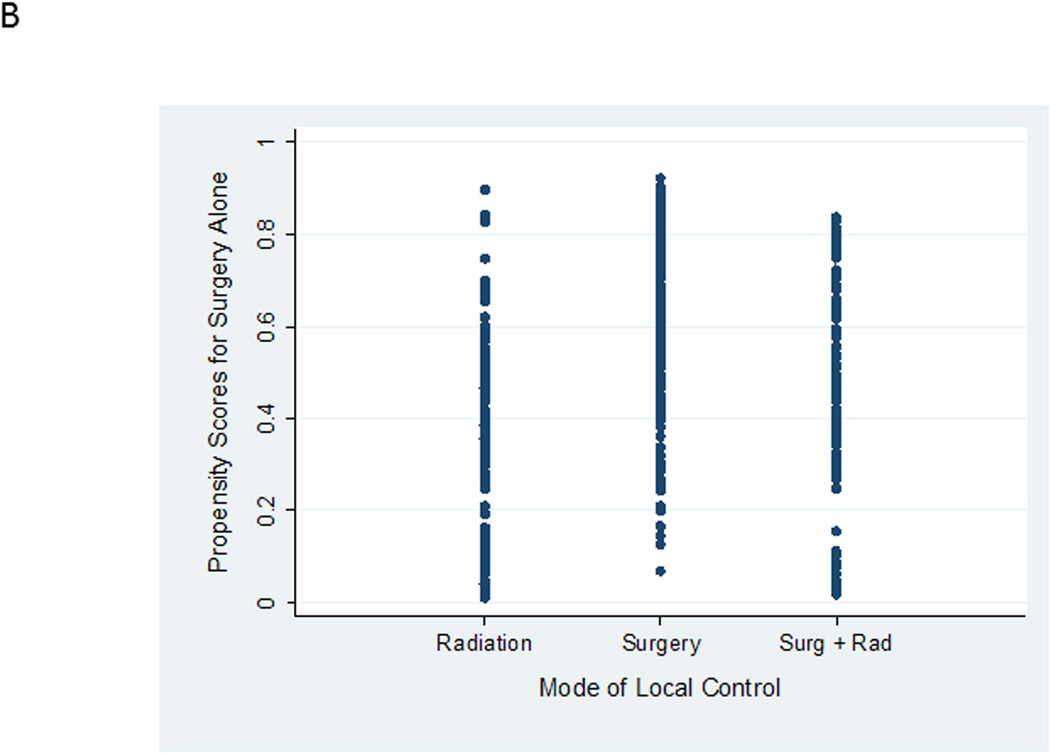
Both surgical propensity scores and radiation propensity scores were well distributed between local control groups (Figure 2). This distribution indicates variation in clinical practice in the choice of local control for similar patients. For example, in Figure 2A, there is a group of patients who are predicted based upon characteristics of the entire cohort of having < 20% likelihood (or propensity) of being selected for definitive radiation who were nevertheless selected for definitive radiation by their treating physicians. Likewise, in Figure 2B, there are patients predicted to have a high propensity for receiving definitive surgery who nevertheless were treated with definitive radiation. This overlap in propensity scores between groups is critical for this type of analysis as it allows outcomes for similar patients treated with different approaches to be compared. However, 7 of 9 patients with radiation propensity scores > 0.8 were nearly uniformly selected for radiation therapy. These 9 patients were excluded from future analyses. Re-evaluating patient characteristics according to quintiles of surgical propensity score demonstrated improved balance between local control groups (Supplemental Table 1).
Figure 2.
A. Propensity scores for definitive radiation alone according to actual mode of local control received. B. Propensity scores for definitive surgery alone according to actual mode of local control received.
Impact of Mode of Local Control on Event-Free Survival
One-hundred fifty-eight analytic events were observed in the cohort (70 with definitive surgery; 49 with definitive radiation; 39 with surgery plus radiation). Patients treated with definitive radiation, compared to definitive surgery, had higher unadjusted risk of any event (HR 1.70; 95% CI 1.18 – 2.44; p = 0.004; Table 2; see Supplemental Table 2 for unadjusted survival rates according to mode of local control). Unadjusted risk of any event was not significantly different for patients treated with surgery plus radiation compared to surgery.
Table 2.
Results of Cox proportional hazards models of event-free survival according to mode of local control.
| Covariates in Addition to Mode of Local Control |
Hazard Ratio for Patients Treated with Definitive Surgery |
Hazard Ratio for Patients Treated with Definitive Radiation (95% Confidence Interval) |
Hazard Ratio for Patients Treated with Surgery plus Radiation (95% Confidence Interval) |
|---|---|---|---|
| None (unadjusted model) | 1 | 1.70 (1.18 – 2.44) p = 0.004 |
1.42 (0.96 – 2.10) p = 0.08 |
| Surgical and radiation propensity scores | 1 | 1.42 (0.94 – 2.14) p = 0.10 |
1.16 (0.77 – 1.75) p = 0.47 |
| Surgical and radiation propensity scores, age, and tumor site | 1 | 1.38 (0.91 – 2.07) p = 0.13 |
1.15 (0.76 – 1.73) p = 0.51 |
| Surgical and radiation propensity scores, age, tumor site, and tumor size | 1 | 1.59 (0.87 – 2.94) p = 0.14 |
1.35 (0.70 – 2.59) p = 0.37 |
| Surgical and radiation propensity scores, age, tumor site, and clinical trial | 1 | 1.40 (0.93 – 2.12) p = 0.11 |
1.13 (0.75 – 1.71) p = 0.55 |
| Age, tumor site, and year of diagnosis | 1 | 1.39 (0.92 – 2.10) p = 0.12 |
1.14 (0.75 – 1.72) p = 0.54 |
| Age, tumor site, and clinical trial | 1 | 1.40 (0.92 – 2.11) p = 0.11 |
1.14 (0.76 – 1.72) p = 0.53 |
| Age, tumor site, year of diagnosis, and tumor size | 1 | 1.56 (0.84 – 2.88) p = 0.16 |
1.30 (0.67 – 2.50) p = 0.43 |
After adjusting for surgical propensity scores and radiation propensity scores, risk for any event was not significantly different for patients treated with either definitive radiation or surgery plus radiation compared to surgery (HR 1.42; 95% CI 0.94 – 2.14; p = 0.10 for definitive radiation and HR 1.16; 95% CI 0.77 – 1.75; p = 0.47 for surgery plus radiation).
While age and tumor site were used to generate surgical and radiation propensity scores, it is possible that differences in these characteristics between local control groups could lead to residual confounding. Therefore, we constructed additional models that controlled for these variables in addition to propensity scores and yielded similar results. Separate models that also controlled for either clinical trial or maximum tumor dimension among the 212 patients with available tumor size data yielded similar conclusions.
To further evaluate the robustness of these findings, we constructed two additional models that did not rely upon propensity scores (Table 2). The first model used the variables utilized to construct the propensity scores (age, tumor site, and year of diagnosis) as covariates in the model. The second model used these same covariates and maximum tumor dimension. Results were similar to findings obtained using propensity score methods.
Impact of Mode of Local Control on Secondary Clinical Endpoints
We applied these same methods to evaluate risk of death according to mode of local control (Table 3). Patients treated with either definitive radiation or surgery plus radiation, compared to surgery alone, had higher unadjusted risk of death (HR 1.84; 95% CI 1.18 – 2.85; p = 0.006 for definitive radiation and HR 1.75; 95% CI 1.10 – 2.76; p = 0.02 for surgery plus radiation). After adjusting for surgical propensity scores and radiation propensity scores, risk for death was not statistically significantly different for patients treated with either definitive radiation or surgery plus radiation compared to surgery. Similar results were obtained in the additional models in Table 3.
Table 3.
Results of Cox proportional hazards models of overall survival and competing risks regression models of local failure and distant failure according to mode of local control. Patients treated with definitive surgery form the reference group in all models and therefore have hazard ratios of 1.
| Covariates in Addition to Mode of Local Control |
Hazard Ratio for Death for Patients Treated with Definitive Radiation (95% Confidence Interval) |
Hazard Ratio for Death for Patients Treated with Surgery plus Radiation (95% Confidence Interval) |
Hazard Ratio for Distant Failure for Patients Treated with Definitive Radiation (95% Confidence Interval) |
Hazard Ratio for Distant Failure for Patients Treated with Surgery plus Radiation (95% Confidence Interval) |
Hazard Ratio for Local Failure for Patients Treated with Definitive Radiation (95% Confidence Interval) |
Hazard Ratio for Local Failure for Patients Treated with Surgery plus Radiation (95% Confidence Interval) |
|---|---|---|---|---|---|---|
| None (unadjusted models) | 1.84 (1.19 – 2.85) p = 0.006 |
1.75 (1.10 – 2.76) p = 0.02 |
1.41 (0.89 – 2.22) p = 0.14 |
1.22 (0.76 – 1.97) p = 0.40 |
2.57 (1.37 – 4.83) p = 0.003 |
1.33 (0.62 – 2.89) p = 0.47 |
| Surgical and radiation propensity scores | 1.37 (0.83 – 2.26) p = 0.21 |
1.39 (0.86 – 2.25) p = 0.18 |
1.13 (0.70 – 1.84) p = 0.61 |
0.98 (0.60 – 1.58) p = 0.93 |
2.41 (1.24 – 4.68) p = 0.01 |
1.04 (0.46 – 2.35) p = 0.93 |
| Surgical and radiation propensity scores, age, and tumor site | 1.37 (0.84 – 2.23) p = 0.21 |
1.36 (0.85 – 2.20) p = 0.20 |
1.11 (0.69 – 1.78) p = 0.66 |
0.99 (0.61 – 1.60) p = 0.96 |
2.33 (1.17 – 4.62) p = 0.02 |
0.97 (0.41 – 2.29) p = 0.95 |
| Surgical and radiation propensity scores, age, tumor site, and clinical trial | 1.38 (0.84 – 2.27) p = 0.20 |
1.35 (0.83 – 2.19) p = 0.22 |
1.15 (0.71 – 1.86) p = 0.57 |
0.99 (0.61 – 1.61) p = 0.96 |
2.39 (1.20 – 4.74) p = 0.01 |
0.96 (0.40 – 2.27) p = 0.92 |
| Surgical and radiation propensity scores, age, tumor site, and tumor size | 1.65 (0.82 – 3.32) p = 0.16 |
1.58 (0.75 – 3.30) p = 0.23 |
1.27 (0.60 – 2.65) p = 0.50 |
1.45 (0.71 – 2.95) p = 0.33 |
4.28 (1.63 – 11.24) p = 0.003 |
1.43 (0.26 – 7.92) p = 0.67 |
| Age, tumor site, and year of diagnosis | 1.38 (0.84 – 2.27) p = 0.20 |
1.34 (0.83 – 2.18) p = 0.23 |
1.16 (0.71 – 1.89) p = 0.55 |
0.97 (0.60 – 1.58) p = 0.91 |
2.39 (1.17 – 4.88) p = 0.02 |
1.01 (0.43 – 2.38) p = 0.98 |
| Age, tumor site, and clinical trial | 1.39 (0.85 – 2.28) p = 0.19 |
1.35 (0.84 – 2.19) p = 0.22 |
1.16 (0.71 – 1.90) p = 0.54 |
0.98 (0.61 – 1.60) p = 0.95 |
2.32 (1.14 – 4.70) p = 0.02 |
1.02 (0.43 – 2.39) p = 0.96 |
| Age, tumor site, year of diagnosis, and tumor size | 1.61 (0.80 – 3.26) p = 0.18 |
1.50 (0.72 – 3.15) p = 0.28 |
1.26 (0.59 – 2.71) p = 0.52 |
1.45 (0.71 – 2.94) p = 0.33 |
4.17 (1.53 – 11.39) p = 0.006 |
1.43 (0.26 – 7.92) p = 0.68 |
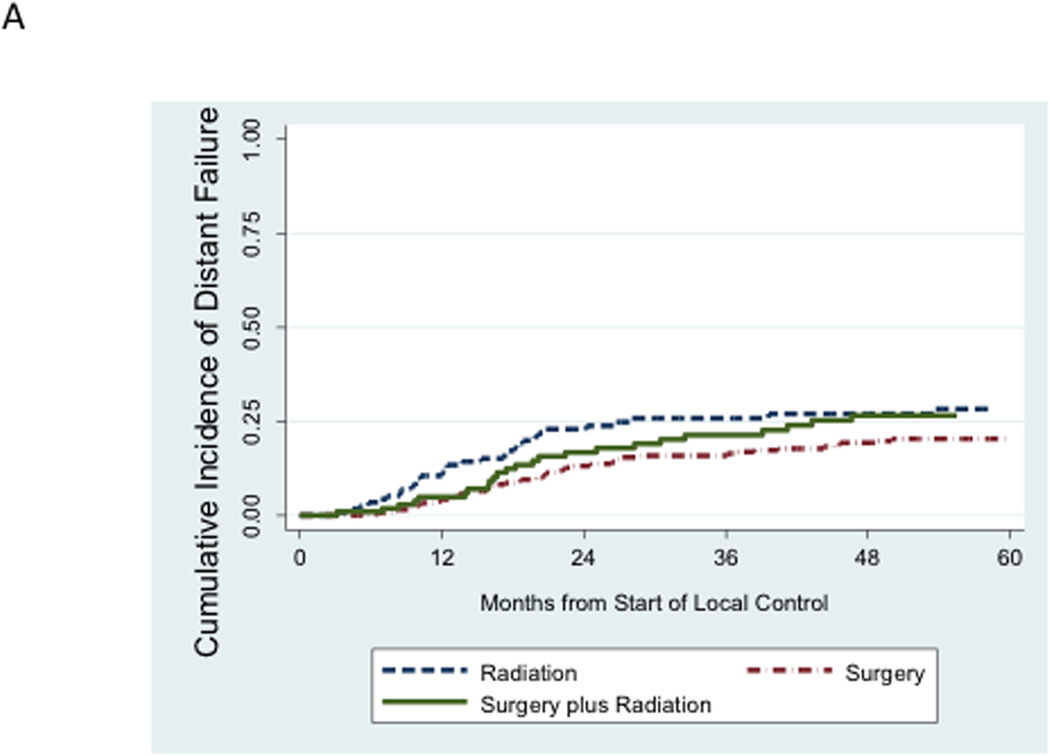
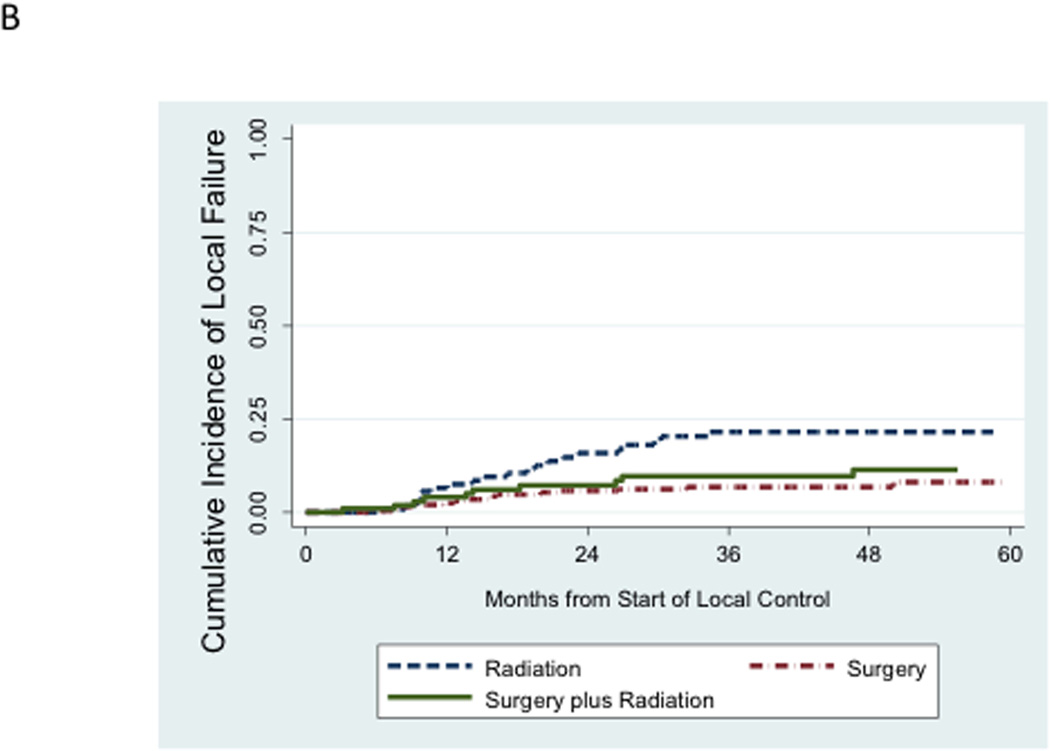
We next evaluated the incidence of distant failure and local failure in this cohort. Distant failure as a component of a first event (isolated distant failure or combined distant + local failure) accounted for 106 of 131 disease failures. Local failure as a component of a first event accounted for 49 of 131 disease failures. There were only 25 cases of isolated local failure as a first episode of disease failure. While the unadjusted cumulative incidence of distant failure appeared relatively similar between local control groups (Figure 3A), the unadjusted cumulative incidence of local failure was higher among patients treated with definitive radiation compared to the other two local control groups (Figure 3B).
Figure 3.
A. Unadjusted cumulative incidence of distant failure (either isolated distant failure or combined local plus distant failure) as a first event according to mode of local control. B. Unadjusted cumulative incidence of local failure (either isolated local failure or combined local plus distant failure) as a first event according to mode of local control.
These findings were recapitulated in competing risk regression analyses (Table 3). On univariate analysis, the risk of distant failure was not significantly different for patients treated with either definitive radiation or surgery plus radiation compared to surgery. Similar results were obtained in multivariate models of distant failure, using either propensity score methods or conventional methods incorporating raw covariates into the models.
In contrast, the risk of local failure was significantly higher among patients treated with definitive radiation compared to surgery (Table 3). On univariate analysis, the hazard ratio for local failure was 2.57 (95% CI 1.37 – 4.83; p = 0.003) for patients treated with radiation compared to surgery. After controlling for propensity scores, this difference remained significant (hazard ratio 2.41; 95% CI 1.24 – 4.68; p = 0.01). Secondary models that added additional covariates or relied solely on the raw covariates to control for confounding confirmed this difference.
Discussion
In this large cohort of patients receiving similar chemotherapy, patient and tumor characteristics differed according to chosen mode of local control. We observed significant heterogeneity in clinical decision making around local control. We noted a broad overlap in surgical propensity scores among patients who received either definitive surgery or definitive radiation. We were able to exploit this heterogeneity to compare outcomes among patients with similar propensity to receive a specific local control modality. Overall disease control and overall survival did not differ according to mode of local control. Definitive radiation was associated with higher risk of local failure. The somewhat paradoxical finding of higher risk of local failure for patients treated with definitive radiotherapy but similar EFS and overall survival reflects the relatively low contribution of local failure to overall disease failure in Ewing sarcoma. Nevertheless, when planning for a specific patient’s local therapy, our findings as well as the risk of radiation-induced second malignancy support current practice of surgical resection when appropriate, while validating radiotherapy as a reasonable alternative in selected patients, depending upon individual factors.
Patients treated with surgery plus radiation had similar outcomes to patients treated with surgery alone. This group was heterogeneous, comprising patients receiving post-operative radiotherapy for positive margins, post-operative radiotherapy for gross residual disease after debulking surgery, or pre-operative radiotherapy due to institutional practice. Similar outcomes in this group compared to definitive surgery alone may reflect the contribution of the patients in that group treated with preoperative radiotherapy who may have been able to achieve complete resection without it. Moreover, this group of was the smallest of the three local control cohorts studied. Due to the size and heterogeneity of this group, it is difficult to draw firm conclusions about the role of combined surgery plus radiation. Current North American standard practice discourages “debulking” surgeries in which gross residual tumor is anticipated. Instead, combined surgery plus radiotherapy is currently reserved for cases in which margins are unexpectedly positive. Our results suggest that disease control is acceptable with this approach.
Several other large analyses have compared surgery and radiation as local control for Ewing sarcoma. In studies from the Rizzoli Institute, radiotherapy for local control has been associated with inferior EFS, overall survival, and local control.9–11 Cox models that controlled for differences in age and tumor site between local control groups abrogated this difference in EFS.9, 10 An analysis of 191 patients with nonmetastatic Ewing sarcoma treated with an ifosfamide-containing chemotherapy regimen demonstrated that the rate of local failure did not differ between patients treated with surgery or radiation.12 An unadjusted analysis from the CESS-81 study indicated that the rate of overall disease recurrence was greatest for patients treated with radiation alone13, though this finding was not observed in the CESS-86 trial in which the risk of overall disease recurrence did not differ according to mode of local control.14, 15 In a pooled analysis of CESS studies, patients treated with definitive radiotherapy had the highest rate of local failure.16 The overall rate of distant failure did not differ between patients treated with radiotherapy and patients whose local control included surgery. In a second pooled CESS analysis, patients treated with definitive radiation had inferior local control and EFS.17 None of the CESS analyses used formal statistical methods to control for differences in patient characteristics and patients received heterogeneous chemotherapy regimens.
Our study has a number of strengths. Our sample size is among the largest used in local control analyses. The use of a similar chemotherapy regimen is a major strength. This homogeneity is critical for this type of analysis since improved systemic therapy improves not only distant disease control, but also local tumor control.18 We utilized two complementary methods to control for the confounding by indication that has led to uncertainty about the optimal mode of local control in this disease. Both methods yielded similar results, indicating that our findings are not due solely to an artifact of our novel propensity score approach. Finally, our results are consistent with previous analyses demonstrating inferior local control with definitive radiotherapy.
We acknowledge limitations of our analysis that highlight potential areas for future study. In an effort to study a homogeneous group of patients receiving similar chemotherapy, we excluded patients with metastatic disease, soft tissue tumors, and patients treated with interval-compressed chemotherapy. The extent to which our findings will generalize to these groups will require further investigation. Detailed data on surgical margins, radiographic and pathologic response to neoadjuvant chemotherapy, and level of experience of treating physicians were not routinely available and therefore not included in this analysis. The clinical endpoints used in this study focused on oncologic control of Ewing sarcoma, largely ignoring other clinical endpoints impacted by local control modalities. Risk of second malignancy, particularly solid cancers, would be expected to be higher in patients treated with radiation.19 While second malignancy was included as an analytic event for the purposes of EFS estimation, these data are not yet fully mature for this cohort as AEWS0031 completed accrual in 2005. Functional outcomes, need for repeat surgical repair, and quality of life are important factors included in local control decision-making. These data are not available for these patients, though they are being collected on new patients treated on Children’s Oncology Group trials. In addition, our cohort was treated over a 17-year period during which certain aspects of surgery, radiotherapy, and chemotherapy evolved. For example, the duration of chemotherapy decreased from 18 cycles in INT-0091 to 14 cycles in AEWS0031. As with any observational study, there is the risk of residual confounding by variables not available for this analysis, including inherent selection bias for radiotherapy in patients with more locally advanced tumors. Finally, although this is one of the largest analyses on this topic, it is possible that some of our negative findings reflect low event rates.
In conclusion, overall disease control in Ewing sarcoma is comparable for patients treated with surgery or definitive radiation. Risk of local failure is higher for patients treated with definitive radiation. Therefore, definitive surgery when appropriate remains the preferred mode of local control, with radiotherapy an acceptable alternative. Distant failures account for the majority of relapses in this disease. Since better systemic therapies improve both local and distant disease control, our findings highlight the need for improved systemic therapies in this disease.
Supplementary Material
Supplemental Figure. Dot plot of surgical propensity scores obtained with tumor size data (y-axis) and without tumor size data (x-axis) in patients from INT-0091 and INT-0154 all of whom had tumor size data available.
Acknowledgments
Support: Supported by the Frank A. Campini Foundation, Nick Currey Fund, ASCO Young Investigator Grant, the WWWW Foundation, the Daniel P. Sullivan Fund, NIH/NCI 1K23CA154530, and NIH/NCI U10 CA98543. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclosures: None
References
- 1.Donaldson SS. Ewing sarcoma: radiation dose and target volume. Pediatr Blood Cancer. 2004;42:471–476. doi: 10.1002/pbc.10472. [DOI] [PubMed] [Google Scholar]
- 2.Hosalkar HS, Dormans JP. Limb sparing surgery for pediatric musculoskeletal tumors. Pediatr Blood Cancer. 2004;42:295–310. doi: 10.1002/pbc.10406. [DOI] [PubMed] [Google Scholar]
- 3.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 4.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 5.Granowetter L, Womer R, Devidas M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group Study. J Clin Oncol. 2009;27:2536–2541. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 8.Fine J, Gray R. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 9.Bacci G, Ferrari S, Bertoni F, et al. Prognostic factors in nonmetastatic Ewing's sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol. 2000;18:4–11. doi: 10.1200/JCO.2000.18.1.4. [DOI] [PubMed] [Google Scholar]
- 10.Bacci G, Forni C, Longhi A, et al. Long-term outcome for patients with non-metastatic Ewing's sarcoma treated with adjuvant and neoadjuvant chemotherapies. 402 patients treated at Rizzoli between 1972 and 1992. Eur J Cancer. 2004;40:73–83. doi: 10.1016/j.ejca.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Bacci G, Longhi A, Briccoli A, Bertoni F, Versari M, Picci P. The role of surgical margins in treatment of Ewing's sarcoma family tumors: experience of a single institution with 512 patients treated with adjuvant and neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:766–772. doi: 10.1016/j.ijrobp.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Shankar AG, Pinkerton CR, Atra A, et al. Local therapy and other factors influencing site of relapse in patients with localised Ewing's sarcoma. United Kingdom Children's Cancer Study Group (UKCCSG) Eur J Cancer. 1999;35:1698–1704. doi: 10.1016/s0959-8049(99)00144-6. [DOI] [PubMed] [Google Scholar]
- 13.Dunst J, Sauer R, Burgers JM, et al. Radiation therapy as local treatment in Ewing's sarcoma. Results of the Cooperative Ewing's Sarcoma Studies CESS 81 and CESS 86. Cancer. 1991;67:2818–2825. doi: 10.1002/1097-0142(19910601)67:11<2818::aid-cncr2820671118>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Ahrens S, Hoffmann C, Jabar S, et al. Evaluation of prognostic factors in a tumor volume-adapted treatment strategy for localized Ewing sarcoma of bone: the CESS 86 experience. Cooperative Ewing Sarcoma Study. Med Pediatr Oncol. 1999;32:186–195. doi: 10.1002/(sici)1096-911x(199903)32:3<186::aid-mpo5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Dunst J, Jurgens H, Sauer R, et al. Radiation therapy in Ewing's sarcoma: an update of the CESS 86 trial. Int J Radiat Oncol Biol Phys. 1995;32:919–930. doi: 10.1016/0360-3016(95)00016-r. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki T, Hillmann A, Hoffmann C, et al. Significance of surgical margin on the prognosis of patients with Ewing's sarcoma. A report from the Cooperative Ewing's Sarcoma Study. Cancer. 1996;78:892–900. doi: 10.1002/(SICI)1097-0142(19960815)78:4<892::AID-CNCR29>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 17.Schuck A, Ahrens S, Paulussen M, et al. Local therapy in localized Ewing tumors: results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int J Radiat Oncol Biol Phys. 2003;55:168–177. doi: 10.1016/s0360-3016(02)03797-5. [DOI] [PubMed] [Google Scholar]
- 18.Yock TI, Krailo M, Fryer CJ, et al. Local control in pelvic Ewing sarcoma: analysis from INT-0091--a report from the Children's Oncology Group. J Clin Oncol. 2006;24:3838–3843. doi: 10.1200/JCO.2006.05.9188. [DOI] [PubMed] [Google Scholar]
- 19.Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children's Oncology Group cohort. Cancer. 2008;113:2597–2604. doi: 10.1002/cncr.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Dot plot of surgical propensity scores obtained with tumor size data (y-axis) and without tumor size data (x-axis) in patients from INT-0091 and INT-0154 all of whom had tumor size data available.