Abstract
Objective
Identify and interrupt the vascular supply to portions of the distal femoral articularepiphyseal cartilage complex (AECC) in goat kids to induce cartilage necrosis, characteristic of early lesions of osteochondrosis; then utilize MRI to identify necrotic areas of cartilage.
Design
Distal femora were perfused and cleared in goat kids of various ages to visualize the vascular supply to the distal femoral AECC. Vessels located on the axial aspect of the medial femoral condyle and on the abaxial side of the lateral trochlear ridge were transected in eight 4-day-old goats to induce cartilage necrosis. Goats were euthanized 1, 2, 3, 4, 5, 6, 9, and 10 weeks post operatively and operated stifles were harvested. Adiabatic T1ρ relaxation time maps of the harvested distal femora were generated using a 9.4T MR scanner, after which samples were evaluated histologically.
Results
Interruption of the vascular supply to the medial femoral condyle caused lesions of cartilage necrosis in 6/8 goat kids that were demonstrated histologically. Adiabatic T1ρ relaxation time mapping identified these areas of cartilage necrosis in 5/6 cases. No significant findings were detected after transection of perichondrial vessels supplying the lateral trochlear ridge.
Conclusions
Cartilage necrosis, characteristic of early osteochondrosis, can be induced by interrupting the vascular supply to the distal femoral AECC in goat kids. The ability of high field MRI to identify these areas of cartilage necrosis in the AECC using the adiabatic T1ρ sequence suggests that this technique may be useful in the future for the early diagnosis of osteochondrosis.
Keywords: cartilage necrosis, adiabatic T1ρ, MRI, osteochondrosis, goat
Introduction
Osteochondrosis (OC), or osteochondritis dissecans as it is often referred to in human medicine, is a developmental orthopedic disease that affects juvenile humans[1] and animals[2, 3]. In the veterinary medical field OC is defined as a focal failure of enchondral ossification[3], whereas in human medicine it is often considered an acquired, idiopathic lesion of the subchondral bone resulting in delamination and sequestration with or without articular cartilage involvement and instability[1]. In spite of differences in disease definition, a recent review focusing on the clinical aspects of the disease presents compelling evidence that OC affecting animals and humans is the same disease[4]. Predilection sites are often shared among species, e.g., human OC most frequently affects the femoral condyles, which are also commonly affected sites in pigs[5].
The etiology and pathogenesis of OC affecting humans are poorly understood; genetic factors, ischemic events, (repetitive micro) trauma, and ossification abnormalities all are considerations[1, 6, 7]. These uncertainties likely arise from the fact that OC in humans is rarely recognized before clinical signs become apparent. Thus, inferences regarding the etiology and pathogenesis of OC in humans are largely based on histological evaluation of osteochondral fragments removed from symptomatic joints[8]. Although these fragments accurately represent the end stage disease, they provide little information regarding its pathogenesis. This limited understanding of the pathogenesis of OC makes treatment selection and disease prognostication in humans challenging[6].
In children with open physes, stable OC lesions are known to heal in 50% of the cases by simply altering activity levels[9]. However, predicting which lesions will heal and which will progress and require surgical treatment is difficult, and the disease is rarely detected before the onset of clinical signs. The ability of conservative treatment to succeed, if applied early during the course of the disease, provides a strong argument for development of diagnostic imaging methods capable of identifying these lesions during the early stages of their development. Additionally, novel imaging techniques capable of identifying and monitoring the development of early OC lesions will likely yield insight into the disease pathogenesis.
In contrast to humans, the pathogenesis of OC affecting animals has been described in detail[3]. Histological studies using samples obtained from predilection sites of OC in young, asymptomatic animals have identified changes characteristic of specific stages of the disease. First, ischemic necrosis of the epiphyseal cartilage subjacent to the articular cartilage (OC latens); followed by the associated delay in the progression of the ossification front (OC manifesta) after it reaches the area of necrotic cartilage. Finally, cleft formation through the area of necrosis (OC dissecans)[10, 11]. The presence of necrotic vascular channels located within or adjacent to the areas of necrosis provides strong evidence that vascular failure plays a central role in the development of cartilage necrosis, a hallmark of subclinical disease. However, the upstream events triggering ischemia in the epiphyseal cartilage in naturally occurring disease are yet to be identified. Recent work performed in horses[12] and pigs[13] demonstrated that surgical interruption of vascular supply to a portion of the articular-epiphyseal cartilage complex (AECC, immature joint cartilage at the ends of growing long bones) results in development of lesions identical to those present in naturally occurring OC. This ability to surgically induce OC-like lesions in animals suggests that development of a clinically relevant animal model of the disease is feasible. However, the high incidence of naturally occurring disease, costs associated with their housing, and their large size are challenges in using horses and pigs as animal models. Goats have the potential to be an ideal model for surgically-induced OC because of their low cost, long life span, absence of naturally occurring disease, and relatively small size that allows evaluation of lesions by in vivo magnetic resonance imaging (MRI).
Magnetic resonance imaging has the potential to play a central role in the early diagnosis of OC by allowing noninvasive, in vivo investigations. Our recent studies demonstrate that the vasculature of developing joints in pigs can be visualized using susceptibility weighted imaging [14, 15]. Thus, this technique might be utilized to image failure of vascular supply associated with OC. Relaxometry, a quantitative parametric MRI technique, is another method increasingly applied in the evaluation of cartilage[16, 17]. T1ρ and adiabatic T1ρ, two of these quantitative parameters, have received attention lately due to their sensitivity to changes in cartilage proteoglycan content[18-23]. Results from these studies suggest that cartilage necrosis and the associated decrease in proteoglycan content, characteristic features of early OC, might be utilized for early diagnosis of this disease using MRI.
In the present study, goat kids were used to study surgical induction of OC-like lesions. The objectives of the study reported here were to: 1) assess the vascular supply to the AECC of the distal femur in goat kids to identify vessels for surgical interruption, 2) induce cartilage necrosis in the AECC of the distal femur by surgically interrupting the blood supply to a specific area, and 3) image early changes within the AECC after interruption of the vascular supply using high field MRI and validate these MRI findings by histological examination of the affected site.
Materials and methods
The study was conducted in two stages, which were approved by the University of Minnesota Institutional Animal Care and Use Committee (IACUC # 1207A18021).
First stage – vascular perfusion and clearing
Seven male dairy goat kids aged 4, 7, 14, 21, 28, 35, and 42 days were used to assess the vascular supply of the AECC of the distal femora. The goats were sedated with an intramuscular injection of 0.1 mg/kg xylazine, followed by 500 IU/kg heparin administered intravenously. Immediately after heparin administration the goats were euthanized and then eviscerated via a median laparotomy. The left external iliac artery was identified and catheterized with a red rubber catheter. The limb was then perfused with approximately 150 mL radiographic contrast media (200 g BaSO4 [Sol-O-Pake] diluted to 1000 mL with 10% neutral buffered formalin). After completion of the perfusion, left distal femora were harvested, then cleared using the modified Spalteholz technique [24]. Briefly, samples were fixed in 10% neutral buffered formalin for 48 h then dehydrated by immersing them in sequentially increasing concentrations of ethyl alcohol (70%, 80%, 90%, 95% then 100%). Dehydrated samples were cleared by placing them into concentrated methyl-salicylate solution. Cleared specimens were visually inspected and then photographed to identify specific vessels as well as the overall vascular architecture supplying the AECC.
Second stage – surgical interruption of vascular supply
During the second stage of the study we sought to surgically interrupt the vascular supply to portions of the distal femur to induce OC-like lesions. Initially, perichondrial vessels were transected in six 14-day-old goats using the techniques described below; however, this did not result in OC-like lesion development identifiable histologically or by MRI over a period of 12 weeks. A review of the results obtained in the first stage of the study revealed that, by the age of 14 days, large numbers of vessels bridged the ossification front in the distal femoral epiphysis, providing an alternative blood supply to the perichondrial vessels that were surgically interrupted. Assuming that failure to induce necrosis in the 14-day-old goats was due to this alternative blood supply, we elected to repeat the experiment using 4- to 5-day-old goats, which had the most extensive perichondrial blood supply with only a limited number of vessels bridging the ossification front.
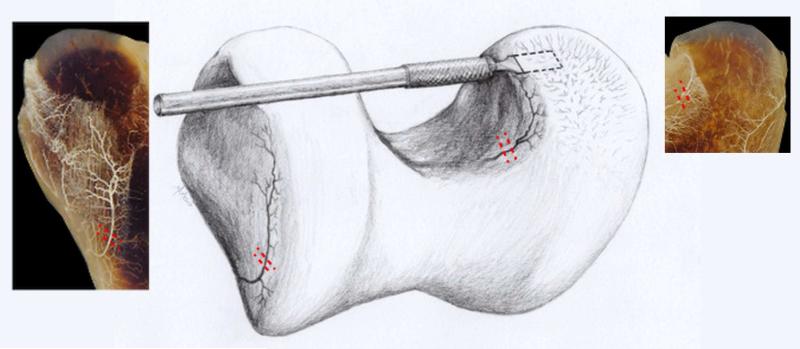
Eight 4- to 5-day-old male dairy goat kids were selected for surgical intervention. Briefly, goats were anesthetized with a combination of midazolam and ketamine administered intravenously and anesthesia was maintained by inhalation of sevoflurane vaporized in oxygen. The right pelvic limb was prepared for aseptic surgery and the stifle joint was approached via a lateral parapatellar arthrotomy. The patella was luxated medially, the femorotibial joint was brought into complete flexion, and the infrapatellar fat pad was retracted, exposing the intercondylar region of the distal femur cranial to the attachment of the cranial cruciate ligament. After transecting the perichondrial vessel that was consistently present on the axial aspect of the medial femoral condyle (MFC) (figure 1) an additional ~3 x 4 mm (beaver blade, goats euthanized at 1, 2, 4, 6, and 10 weeks post operatively) or 5 x 5 mm (custom designed blade, goats euthanized 3, 5, and 9 weeks post operatively) incision extending into the AECC at the cranio-axial aspect of the MFC was created (figure 1) parallel to the articular surface to interrupt any blood supply to the AECC traversing the ossification front. We also attempted to induce OC-like lesions in the proximal portion of the femoral trochlea by transecting perichondrial vessels that were consistently present on the proximo-abaxial aspect of the lateral trochlear ridge (figure 1). These vessels were transected in two locations 3-4 mm apart. Upon completing the procedure, the joint capsule, subcutaneous tissues, and skin were closed in layers. After assisted recovery, goats were housed together in an approximately 6 x 6 m pen until euthanasia. Goats were euthanized 1, 2, 3, 4, 5, 6, 9, and 10 weeks post operatively and distal femora were harvested and photographed.
Figure 1.
Drawing demonstrating the surgical procedure used to interrupt the vascular supply to the distal femoral AECC. Dashed lines indicate location of incisions used to interrupt perichondrial vessels on the axial aspect of the medial femoral condyle (right) and lateral trochlear ridge (left). The incision extending into the epiphyseal cartilage is indicated by the beaver blade entering the medial femoral condyle. Perichondrial vessels interrupted in the axial aspect of the medial femoral condyle (right inset) and the abaxial aspect of the lateral trochlear ridge (left inset) are also shown in perfused, cleared specimens obtained from a 2-week-old goat.
MRI
MR imaging of the specimens was conducted on a 9.4 Tesla scanner driven with VnmrJ software (VnmrJ, version 3.2A, Agilent Technologies, Santa Clara, CA, USA), using a quadrature volume transceiver coil (Millipede, Varian NMR Systems, Palo Alto, CA) within six hours of euthanasia. The specimens were placed in flexible latex containers for optimal fitting in the coil and immersed in perfluoropolyether for clean and susceptibility matched background. Three-dimensional gradient recalled echo images were acquired to localize the operated areas within the MFC and the lateral trochlear ridge. Subsequently, adiabatic T1ρ relaxation time[23, 25] of the specimens was measured in a single axial slice covering the operated area as determined from 3-D gradient recalled echo images. The relaxation time was measured using a magnetization preparation block consisting of 0, 4, 8, 12 and 16 adiabatic full passage pulses from the hyperbolic secant family[18] of 6 ms duration at peak power γB1,max = 2.5 kHz coupled to a fast spin echo readout sequence[23, 25]. Slice thickness was 1 mm with imaging matrix of 256 x 256; echo train length was 8; echo spacing was set to minimum (~5 ms) and a repetition time of 5 s was used. The final imaging resolution was dependent on the sample size and was approximately 150 – 165 m in the image plane. The adiabatic T1ρ relaxation time was calculated using monoexponential 2-parameter fit to the measured data in Matlab (MATLAB R2011b, The MathWorks, Natick, MA, USA).
Histology
At the conclusion of the MRI procedures, distal femora from each goat were placed in 10% neutral buffered formalin for 48 hours followed by decalcification using 10% EDTA. Decalcified samples were divided to separate the femoral trochlea from the condyles and then were trimmed into 2 to 3mm thick slabs in the coronal plane, yielding 5 slabs from each condyle and trochlea. Slabs were routinely processed into paraffin blocks and five m thick sections were obtained from the surface of each slab and stained with hematoxylin and eosin. Blocks containing the surgical incision were thoroughly explored. If necrotic cartilage canals and/or areas of cartilage necrosis were identified in any of the sections, additional sections were obtained in 200-500 m intervals adjacent to the original section until the lesion was no longer present. Selected sections containing necrotic cartilage or cartilage canals were also stained with safranin O and toluidine blue to demonstrate changes in the extracellular matrix. Histological sections corresponding with the MRI slice in which the adiabatic T1ρ relaxation time was measured were identified and changes in the cartilage maps corresponding with changes noted in histological sections were compared. For both histological and MRI evaluations, corresponding areas of the lateral femoral condyle (LFC) and the medial trochlear ridge within the same stifle joint served as control sites.
Results
During the first stage of the study, perfusion and subsequent clearing of the left pelvic limb in seven goats enabled successful visualization of the vascular supply to the AECC. Perichondrial vessels that were consistently present at the axial aspect of the MFC (figure 1) and the abaxial aspect of the proximolateral femoral trochlea (figure 1) were designated for transection in the next stage of the study. The most abundant perichondrial blood supply, including small branches extending into the distal femoral AECC, was noted in the 4-day-old goat. Although perichondrial blood supply remained apparent in older goats, vessels crossing the ossification front became more prominent as the animals aged.
Surgical procedures to interrupt blood supply to the distal femoral AECC were completed in eight 4- to 5-day-old goats without any complications. After recovery, all animals exhibited non-weightbearing lameness on the operated limb for 48 to 72 hours which was treated with an NSAID (Flunixin meglumine 1.1 mg/kg sc) for 3 days and gradually resolved by the 10th post operative day. The goat that was euthanized 1 week post-operatively developed a seroma on the lateral side on the operated femorotibial joint which persisted until euthanasia. The goat that was euthanized 2 weeks post-operatively developed a hard, fibrous, pea-sized swelling on the lateral side of the femorotibial joint involving the periarticular soft tissues. The remaining six goats exhibited no complications prior to euthanasia at 3, 4, 5, 6, 9, and 10 weeks post-operatively.
Histological and MRI findings
Histological changes (Table 1) in the goat euthanized 1 week post-operatively were restricted to the AECC immediately adjacent and distal to the surgical incision created in the axial aspect of the MFC. In the hematoxylin and eosin stained sections, the surgical incision extending into the AECC was clearly visible and a thin zone of necrotic chondrocytes was present on either side of the incision. Cartilage canals located between the incision and the articular surface appeared necrotic, characterized by loss of endothelium, absence of erythrocytes, and a lumen filled with amorphous eosinophilic material. Conversely, cartilage canals located outside this region lacked these features and appeared to be viable (supplementary figure 1). Due to a mistake during setup, the MRI study missed the area of this lesion; thus, no MRI data were available for this sample. No pathological changes were seen in the corresponding control sections from the LFC.
Table 1.
Histological and MRI findings obtained from eight goat kids after interruption of the vascular supply to portions of the distal femur.
| Goat age at the time of euthanasia | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 week | 2 week | 3 week | 4 week | 5 week | 6 week# | 9 week | 10 week | ||
| Incision | Original size (mm) |
~3 × 4 | ~3 × 4 | 5 × 5 | ~3 × 4 | 5 × 5 | ~3 × 4 | 5 × 5 | ~3 × 4 |
| Healing | No | Deep portion is forming granulation tissue |
Deep ~50% is healed |
Deep ~75% is healed |
Deep ~90% is healed |
Deep portion is forming granulation tissue |
100% healed | 100% healed | |
|
Cartilage canals |
Necrosis distal to incision |
Necrosis in axial ~50% of condyle |
Absent in axial ~50% of condyle |
Absent in axial ~20% of condyle |
Absent in axial ~50% of condyle |
Absent in axial ~50% of condyle |
Low numbers in abaxial 50% of condyle |
Absent or chondrified throughout |
|
| Chondrocyte | Extent of necrosis |
Adjacent to incision |
1.9 mm2 area in AECC |
1.8 mm2 area in AECC |
0.5 mm2 area in AECC |
1.2 mm2 area in AECC |
Not apparent | 5.3 mm2 area a ossification front |
0.6 mm2 area in subchondral bone |
| Morphology | Shrinkage, pyknosis |
Shrinkage, pyknosis |
Shrinkage, pyknosis |
Shrinkage, pyknosis |
Shrinkage, pyknosis |
Normal | Shrinkage, pyknosis |
Shrinkage, pyknosis |
|
| ECM$ staining |
Retained | Mildly decreased |
Markedly decreased |
Mildly decreased |
Markedly decreased |
Retained | Markedly decreased |
Nearly absent | |
|
T1rho relaxation time in necrotic cartilage |
N/A* | Mildly increased (~250 ms) |
Moderately increased (~300 ms) |
No changes noted (~150 ms) |
Markedly increased (~350 ms) |
No changes noted (~150 ms) |
Markedly increased (~350 ms) |
Mildly increased (~250 ms) |
|
|
OC classification |
N/A | OC latens | OC latens | OC latens | OC latens | N/A | OC manifesta | Healing OC manifesta |
|
In the 6-week-old goat the incision into the articular-epiphyseal cartilage-complex was created too superficially relative to the rest of the animals.
ECM = extracellular matrix
MRI study missed the area of lesion
More extensive histological changes were noted in the goats euthanized 2, 3, 4 and 5 weeks post-operatively (table 1). A portion of the epiphyseal cartilage that was located between the incision and the axial articular surface of the MFC was necrotic in these animals, characterized by shrunken chondrocytes with pyknotic nuclei. Pallor of the extracellular matrix was also apparent in multiple adjacent sections (both hematoxylin and eosin and special stains). These findings are consistent with OC latens[26]. In the MRI images, a mild (~250 ms) to moderate (~300 ms) focal increase in adiabatic T1ρ relaxation time was observed in the epiphyseal cartilage corresponding with the area of cartilage necrosis in goats euthanized 2 (figure 2), 3, and 5 (figure 3) weeks post operatively. In the remainder of the MFC adiabatic T1ρ relaxation time was similar to that observed in the LFC. In the goat euthanized 4 weeks post operatively adiabatic T1ρ relaxation times of the lateral and medial femoral condyles appeared similar (~150 ms) (supplementary figure 2).
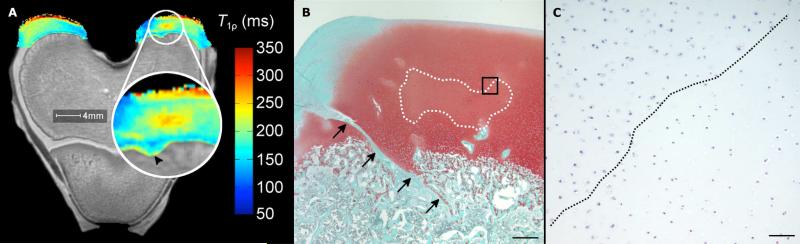
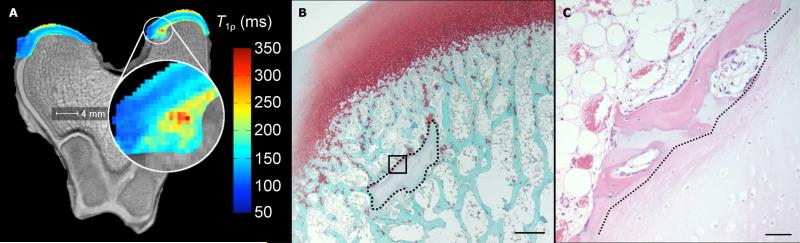
Figure 2.
Adiabatic T1ρ relaxation time map (panel A) obtained from the distal femur of a goat 2 weeks post operatively with the affected area enlarged, demonstrating mildly increased relaxation time in the area corresponding with the area of decreased proteoglycan content in panel B. Arrowhead indicates minor alterations in the advancement of the ossification front. Photomicrograph of a safranin-O stained section (panel B) obtained from the axial aspect of the medial femoral condyle of the same specimen (scale bar = 500 m). The surgical incision is partially healed (black arrows in panel A). Faint staining marked by a dotted line indicates decreased proteoglycan content in the area of cartilage necrosis. Panel C depicts high magnification image (scale bar = 50 m) of the area marked by the black box in panel B in a hematoxylin and eosin stained ssection. Chondrocytes to the right of the dotted diagonal line are necrotic, characterized by pyknotic nuclei and shrinkage.
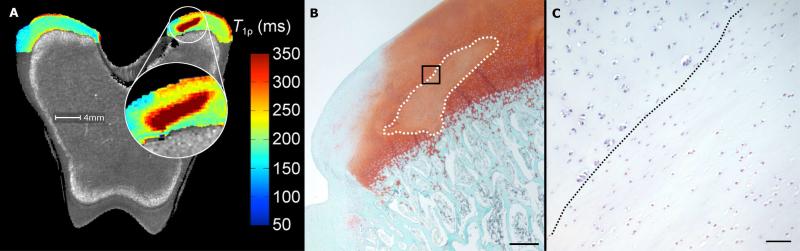
Figure 3.
Adiabatic T1ρ relaxation time map (panel A) obtained from the distal femur of a goat 5 weeks post operatively with the affected area enlarged, demonstrating markedly increased relaxation time in the area corresponding with the area of decreased proteoglycan content in panel B. Photomicrograph of a safranin-O stained section (panel B) obtained from the axial aspect of the medial femoral condyle of the same specimen (scale bar = 500 m). Faint staining marked by a dotted line indicates decreased proteoglycan content in the area of cartilage necrosis. Panel C depicts high magnification image (scale bar = 50 m) of the area marked by the black box in panel B in a hematoxylin and eosin stained section. Chondrocytes to the right of the dotted diagonal line are necrotic, characterized by pyknotic nuclei and shrinkage.
Histological results of the MFC in the goat euthanized 6 weeks post-operatively indicated that the surgical incision in this animal was created too close to the articular surface, entering approximately at the border between the articular and epiphyseal cartilage. Deeper portions of the surgical incision were filled with granulation tissue delaying the advancement of the surrounding ossification front. The superficial portion of the incision persisted with no or minimal signs of healing, and appeared similar to that present in the goat euthanized 1 week post-operatively. In the MRI images of the MFC an irregularity at the cartilage-bone interface (ossification front) was clearly apparent, but adiabatic T1ρ relaxation times were no different in the AECC between the MFC and LFC (~150 ms)(supplementary figure 3).
Histological findings obtained from the goat euthanized 9 weeks post operatively were consistent with an OC manifesta lesion. Progression of the ossification front in the axial portion of the MFC was delayed by the presence of a necrotic island (5.3 mm2) of epiphyseal cartilage. This island of necrotic epiphyseal cartilage was clearly present in the MRI image as a focal area of markedly (~350ms) increased adiabatic T1ρ relaxation time adjacent to the ossification front (figure 4). No pathologic changes were noted in the control LFC.
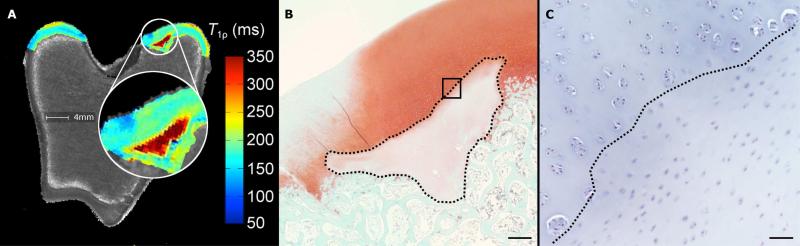
Figure 4.
Adiabatic T1ρ relaxation time map (panel A) obtained from the distal femur of a goat 9 weeks post operatively with the affected area enlarged, demonstrating markedly increased relaxation time in the area corresponding with the area of decreased proteoglycan content in panel B. Photomicrograph of a safranin-O stained section (panel B) obtained from the axial aspect of the medial femoral condyle of the same specimen (scale bar = 500 m). Faint staining marked by a dotted line indicates decreased proteoglycan content in the area of cartilage necrosis. Delayed progression of the ossification front in the area of necrotic cartilage is also apparent, which is consistent with an OC manifesta lesion. Panel C depicts high magnification image (scale bar = 50 m) of the area marked by the black box in panel B in a hematoxylin and eosin stained section. Chondrocytes to the right of the dotted diagonal line are necrotic, characterized by pyknotic nuclei and shrinkage. Chondrocyte clones are present in the viable cartilage to the left of the dotted diagonal line.
An island composed of necrotic cartilage was identified within the subchondral bone in multiple adjacent sections obtained from the MFC of the goat euthanized 10 weeks postoperatively. The appearance and location of this lesion, apparently bypassed by the ossification front, was consistent with a healing OC manifesta lesion. In the MRI images the epiphyseal cartilage appeared to have nearly identical adiabatic T1ρ relaxation times in the lateral and medial femoral condyles. In the subchondral bone of the MFC, however, there was a well-defined area with mildly (~250 ms) increased adiabatic T1ρ relaxation time corresponding with the island of necrotic cartilage seen histologically (figure 5). No pathologic changes were noted in the control LFC.
Figure 5.
Adiabatic T1ρ relaxation time map (panel A) obtained from the distal femur of a goat 10 weeks post operatively with the affected area enlarged, demonstrating moderately increased relaxation time in the area corresponding with the area of necrotic cartilage in panel B. Photomicrograph of a safranin-O stained section (panel B) obtained from the axial aspect of the medial femoral condyle of the same specimen (scale bar = 500 m). An island composed of necrotic cartilage (marked by the dotted line), characterized by markedly decreased staining relative to the epiphyseal cartilage, is retained in the subchondral bone. Necrotic cartilage that was initially surrounded by epiphyseal cartilage (as shown in Figure 3) has become incorporated in subchondral bone as the animal aged and the ossification front progressed towards the articular surface. Panel C depicts high magnification image (scale bar = 50 m) of the area marked by the black box in panel B in a hematoxylin and eosin stained slide. Tissue to the right of the dotted diagonal line is composed of necrotic cartilage.
No significant lesions were noted in any of the sections obtained from the trochlear ridges in any of the goats.
Discussion
In the study reported here, surgical interruption of the vascular supply to a portion of the axial aspect of the MFC resulted in focal necrosis of the epiphyseal cartilage in 6/7 goats operated at the age of 4 to 5 days. In the remaining goat, euthanized 1 week after the surgery, cartilage canal necrosis, a precursor of cartilage necrosis, was noted. Necrotic islands of epiphyseal cartilage were differentiated from surrounding normal tissues ex vivo using adiabatic T1ρ relaxation time maps generated using a 9.4 T magnet in 5/6 cases.
Vascular perfusion and clearing allowed visualization of the vasculature supplying the distal femoral AECC in goats, enabling identification of perichondrial vessels for surgical interruption. Interestingly, the vessels designated for transection on the abaxial aspect of the lateral trochlear ridge had a nearly identical appearance and location to those which were interrupted in horses in another study[12] in which OC-like lesions of the lateral trochlear ridge were successfully induced. Conversely, interruption of these vessels in goats failed to produce OC-like lesions in any of the operated animals. This difference between species is likely explained by variations in the blood supply to the distal femoral AECC. Indeed, re-evaluation of the perfused specimens obtained from goats ranging from 1 to 6 weeks old demonstrated a large number of small vessels bridging the ossification front, with some of these vessels already present in the 4-day-old goat. These bridging vessels are particularly important, because they provide an alternative blood supply to the AECC, thereby decreasing its dependence on the perichondrial vessels.
This apparent difference in blood supply prompted a minor alteration in the surgical technique employed in the 4- to 5-day-old goats whereby, in addition to interrupting the perichondrial vasculature (the only procedure performed in the 14-day-old goats), a horizontal incision extending into the AECC of the MFC was also created to interrupt the vessels bridging the ossification front. It is likely that this additional step was critical in the successful induction of cartilage necrosis in six of the goats, as none of these animals developed a lesion in the trochlear ridges where only the perichondrial vessels were transected. These findings are different from those obtained in 2-week-old horses[12] and pigs up to 6 weeks old[13], where cartilage necrosis was consistently induced by interruption of the perichondrial blood supply. This species difference in dependence on perichondrial blood supply of the AECC at older ages might provide a partial explanation for the high incidence of OC in pigs[27] and horses[20] and the lack of reports in the literature describing OC in goats.
Our results, along with data obtained from studies using imaging and clearing techniques of the distal femora in pigs[11] and horses[28, 29], indicate that changes in the vascular architecture of the AECC occur at different times in various species. It is possible that goats have already passed a ‘threshold’ in joint development at the time of birth, and the vascular architecture of their AECC both makes them resistant to naturally-occurring OC and hampers surgical induction of OC-like lesions. This is in contrast to humans, where development of joints is still ongoing a decade after birth, and may explain in part why OC lesions are diagnosed at a more advanced age in humans than in animals[6, 30]. It would be ideal to time the surgical intervention to induce OC-like lesions in any species based on the degree of skeletal development as determined by the degree of closure of the epiphyseal growth cartilage, however this information is currently unavailable. Based on the volume ratio of the epiphyseal cartilage to the secondary ossification center (unpublished data) joints of goats appear to be more developed (increased volume of secondary ossification center compared to epiphyseal cartilage) than pigs or humans at the time of birth, which might also contribute to their apparent resistance to OC.
Another important finding of the present study is the remarkable ability of extensive lesions affecting the epiphyseal cartilage in goats to heal, demonstrated by the fact that none of the goats developed clinically apparent disease (osteochondrosis dissecans). Indeed, in the goat euthanized 10 weeks post-operatively, the ossification front had completely bypassed the necrotic cartilage, leaving it surrounded by bone. Rapid replacement/bypass of the necrotic portion of the epiphyseal cartilage by bone appears to be an effective strategy in preventing the progression of OC manifesta lesions. Studies of spontaneous disease in swine suggest that areas of necrotic cartilage surrounded by bone gradually decrease in size and eventually disappear [3, 13].
Quantitative mapping of adiabatic T1ρ relaxation time identified areas of cartilage necrosis within the AECC or the subchondral bone in 5/6 cases. One plausible explanation for missing the lesion in the goat examined 4 weeks post-operatively in the MRI study is the substantial difference in average slice thickness between histology (5 m) and the MRI (1 mm). Due to the thicker slices, MRI may occasionally miss (“average out”) a lesion that is only a couple hundred m in size. Alternatively, a small lesion could also be missed due to operator error upon slice selection for adiabatic T1ρ imaging, as was demonstrated by our unfortunate failure to identify the correct region for MRI in the goat euthanized 1 week post-operatively. Nevertheless, results obtained from the remaining 5 goats suggest that MRI allows identification of lesions within cartilage. Longitudinal monitoring of the developing OC lesions might also become possible after the above sequences are tested in vivo at a clinically applicable field strength.
An important limitation of our study is its inability to assess biological variability due to our results being derived from single observation per time point. Also, it appears that goats receiving an incision measuring exactly 5 x 5 mm (created with the custom designed blade) developed more uniform lesions than those operated using the beaver blade; therefore, future studies would ideally use this larger, more standardized incision.
While initial results are promising, further refinement of the surgical technique presented here, as well as evaluation of additional joints, should be performed before recommending the use of goats as a model of surgically-induced OC. In its current stage the model is best suited to evaluate repair mechanism of ischemic cartilage necrosis rather than studying clinically apparent disease, i.e., OC dissecans. Our findings do suggest an explanation as to why goats appear to be free of naturally occurring OC, and may provide further insight into general disease pathogenesis. The ability of ultra high field MRI using the adiabatic T1ρ sequence to identify areas of necrotic cartilage within the AECC is an important finding, which is currently being evaluated at clinically relevant field strengths.
Supplementary Material
Supplementary figure 1: Photomicrographs of a hematoxylin and eosin stained section obtained from the medial femoral condyle of a goat one week after surgical interruption of the vascular supply to the area (panels A and B; scale bars = 500μm). Necrotic cartilage canals (dotted circles) are apparent between the incision extending into the epiphyseal cartilage (black arrowheads) and the articular surface, whereas cartilage canals located abaxially to the incision appear normal (black arrows).
Supplementary figure 2: Adiabatic T1ρ relaxation time map (panel A) obtained from the distal femur of a goat 4 weeks post operatively with the operated area enlarged. Lateral and medial femoral condyles appear similar except for subtle overall increase of adiabatic T1ρ relaxation time in the axial aspect of the medial femoral condyle corresponding with the site of surgical incision (black arrowheads). Photomicrograph of a safranin-O stained section obtained from the axial aspect of the medial femoral condyle of the same specimen (Panel B; scale bar = 500μm). The surgical incision is nearly healed (black arrows). An island composed of necrotic cartilage (marked by dotted line) characterized by decreased staining relative to surrounding epiphyseal cartilage is present at the axial most aspect of the condyle. Panel C depicts high magnification image (scale bar = 50μm) of the area marked by the black box in panel B in a hematoxylin and eosin stained slide. Chondrocytes to the left of the dotted diagonal line are necrotic, characterized by pyknotic nuclei and shrinkage.
Supplementary figure 3: Adiabatic T1ρ relaxation time map (panel A) obtained from the distal femur of a goat 6 weeks post operatively with the operated area enlarged. An irregularity at the cartilage bone interface (ossification front) is evident, corresponding with the surgical incision. Photomicrograph of a safranin-O stained section obtained from the axial aspect of the medial femoral of the same specimen (Panel B; scale bar = 500μm). The superficial portion of the incision (black arrows) persists with no or minimal signs of healing. Deeper portions of the surgical incision are filled with granulation tissue (black arrowheads) delaying the advancement of the subjacent ossification front.
Acknowledgments
The authors are grateful to Lindsey Harper, Paula Overn, Drs. Annette McCoy, Elizabeth Pluhar, and Lindsey Mathews for their assistance with the histologic, anesthetic, and surgical procedures.
Role of the funding source:
Support from grants T32OD010993, K18OD010468, P41 EB015894, R21AR065385, WM KECK Foundation, and University of Minnesota College of Veterinary Medicine Comparative Medicine Faculty Grant were used during the completion of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
All authors contributed significantly to the article formulation. FT drafted the primary manuscript with input from CSC. All authors were involved in critical revision of the manuscript and approved the final version to be published. Surgical procedures and histological evaluation were performed by FT and CSC. MRI was performed and interpreted by MJN, LW, and JME. Responsibility for the integrity of this work as a whole is assumed by Tóth (ftoth@umn.edu), and Carlson (carls099@umn.edu).
Conflict of interest
There are no conflicting interests to report.
Contributor Information
Ferenc Tóth, Diplomate American College of Veterinary Surgeons; Postdoctoral Fellow, Veterinary Population Medicine Department, University of Minnesota, St. Paul, MN, USA.
Mikko J. Nissi, Research Associate, Center for Magnetic Resonance Research, Department of Radiology, University of Minnesota, 2021 Sixth Street SE, Minneapolis, MN 55455, United States; Department of Orthopaedic Surgery, University of Minnesota, Minneapolis, MN, USA; nissi@cmrr.umn.edu.
Luning Wang, Postdoctoral Fellow, Center for Magnetic Resonance Research, Department of Radiology, University of Minnesota, Minneapolis, MN, United States; lnwang1222@gmail.com.
Jutta M. Ellermann, Assistant Professor, Department of Radiology, Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, USA; eller001@umn.edu.
Cathy S Carlson, Diplomate American College of Veterinary Pathologists; Professor, Veterinary Population Medicine Department, University of Minnesota, St. Paul, MN, USA; carls099@umn.edu.
References
- 1.Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current concepts review. The American journal of sports medicine. 2006;34:1181–1191. doi: 10.1177/0363546506290127. [DOI] [PubMed] [Google Scholar]
- 2.Foland JW, McIlwraith CW, Trotter GW. Arthroscopic surgery for osteochondritis dissecans of the femoropatellar joint of the horse. Equine veterinary journal. 1992;24:419–423. doi: 10.1111/j.2042-3306.1992.tb02870.x. [DOI] [PubMed] [Google Scholar]
- 3.Ytrehus B, Carlson CS, Ekman S. Etiology and pathogenesis of osteochondrosis. Veterinary pathology. 2007;44:429–448. doi: 10.1354/vp.44-4-429. [DOI] [PubMed] [Google Scholar]
- 4.McCoy AM, Toth F, Dolvik NI, Ekman S, Ellermann J, Olstad K, et al. Articular osteochondrosis: a comparison of naturally-occurring human and animal disease. Osteoarthritis Cartilage. 2013;21:1638–1647. doi: 10.1016/j.joca.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher DR, De Smet AA. Radiologic analysis of osteochondritis dissecans and related osteochondral lesions. Contemp Diag Rad. 1993;16:1–5. [Google Scholar]
- 6.Crawford DC, Safran MR. Osteochondritis dissecans of the knee. The Journal of the American Academy of Orthopaedic Surgeons. 2006;14:90–100. doi: 10.5435/00124635-200602000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Kocher MS, Czarnecki JJ, Andersen JS, Micheli LJ. Internal fixation of juvenile osteochondritis dissecans lesions of the knee. The American journal of sports medicine. 2007;35:712–718. doi: 10.1177/0363546506296608. [DOI] [PubMed] [Google Scholar]
- 8.Yonetani Y, Nakamura N, Natsuume T, Shiozaki Y, Tanaka Y, Horibe S. Histological evaluation of juvenile osteochondritis dissecans of the knee: a case series. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2010;18:723–730. doi: 10.1007/s00167-009-0898-6. [DOI] [PubMed] [Google Scholar]
- 9.Cahill BR, Phillips MR, Navarro R. The results of conservative management of juvenile osteochondritis dissecans using joint scintigraphy. A prospective study. Am J Sports Med. 1989;17:601–605. doi: 10.1177/036354658901700502. discussion 605-606. [DOI] [PubMed] [Google Scholar]
- 10.Carlson CS, Meuten DJ, Richardson DC. Ischemic necrosis of cartilage in spontaneous and experimental lesions of osteochondrosis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1991;9:317–329. doi: 10.1002/jor.1100090303. [DOI] [PubMed] [Google Scholar]
- 11.Ytrehus B, Carlson CS, Lundeheim N, Mathisen L, Reinholt FP, Teige J, et al. Vascularisation and osteochondrosis of the epiphyseal growth cartilage of the distal femur in pigs--development with age, growth rate, weight and joint shape. Bone. 2004;34:454–465. doi: 10.1016/j.bone.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Olstad K, Hendrickson EH, Carlson CS, Ekman S, Dolvik NI. Transection of vessels in epiphyseal cartilage canals leads to osteochondrosis and osteochondrosis dissecans in the femoro-patellar joint of foals; a potential model of juvenile osteochondritis dissecans. Osteoarthritis Cartilage. 2013;21:730–738. doi: 10.1016/j.joca.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Ytrehus B, Andreas Haga H, Mellum CN, Mathisen L, Carlson CS, Ekman S, et al. Experimental ischemia of porcine growth cartilage produces lesions of osteochondrosis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2004;22:1201–1209. doi: 10.1016/j.orthres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Nissi MJ, Toth F, Zhang J, Schmitter S, Benson M, Carlson CS, et al. Susceptibility weighted imaging of cartilage canals in porcine epiphyseal growth cartilage ex vivo and in vivo. Magn Reson Med. 2013 doi: 10.1002/mrm.24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toth F, Nissi MJ, Zhang J, Benson M, Schmitter S, Ellermann JM, et al. Histological confirmation and biological significance of cartilage canals demonstrated using high field MRI in swine at predilection sites of osteochondrosis. J Orthop Res. 2013 doi: 10.1002/jor.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold GE, Burstein D, Dardzinski B, Lang P, Boada F, Mosher T. MRI of articular cartilage in OA: novel pulse sequences and compositional/functional markers. Osteoarthritis Cartilage. 2006;14(Suppl A):A76–86. doi: 10.1016/j.joca.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Nieminen MT, Nissi MJ, Mattila L, Kiviranta I. Evaluation of chondral repair using quantitative MRI. J Magn Reson Imaging. 2012;36:1287–1299. doi: 10.1002/jmri.23644. [DOI] [PubMed] [Google Scholar]
- 18.Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153:155–177. doi: 10.1006/jmre.2001.2340. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9:1388–1394. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 21.Wheaton AJ, Dodge GR, Borthakur A, Kneeland JB, Schumacher HR, Reddy R. Detection of changes in articular cartilage proteoglycan by T(1rho) magnetic resonance imaging. J Orthop Res. 2005;23:102–108. doi: 10.1016/j.orthres.2004.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salo E-N, Liimatainen T, Michaeli S, Mangia S, Ellermann J, Nieminen MT, et al. Intl. Soc. Magn. Reson. Med. Vol. 20. Melbourne: 2012. T1rho dispersion in constituent-specific degradation models of articular cartilage with correlation to biomechanical properties. p. 51. [Google Scholar]
- 23.Ellermann J, Ling W, Nissi MJ, Arendt E, Carlson CS, Garwood M, et al. MRI rotating frame relaxation measurements for articular cartilage assessment. Magn Reson Imaging. 2013;31:1537–1543. doi: 10.1016/j.mri.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyer MF. Practical Exercises in Zoological Micro-Technique. University of Chicago Press; Chicago: 1953. Objects of general interest. In: Animal Micrology. pp. 110–113. [Google Scholar]
- 25.Michaeli S, Sorce DJ, Springer CS, Jr., Ugurbil K, Garwood M. T1rho MRI contrast in the human brain: modulation of the longitudinal rotating frame relaxation shutter-speed during an adiabatic RF pulse. Journal of magnetic resonance. 2006;181:135–147. doi: 10.1016/j.jmr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Ytrehus B, Ekman S, Carlson CS, Teige J, Reinholt FP. Focal changes in blood supply during normal epiphyseal growth are central in the pathogenesis of osteochondrosis in pigs. Bone. 2004;35:1294–1306. doi: 10.1016/j.bone.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Ytrehus B, Grindflek E, Teige J, Stubsjoen E, Grondalen T, Carlson CS, et al. The effect of parentage on the prevalence, severity and location of lesions of osteochondrosis in swine. Journal of veterinary medicine. A, Physiology, pathology, clinical medicine. 2004;51:188–195. doi: 10.1111/j.1439-0442.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 28.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Epiphyseal cartilage canal blood supply to the distal femur of foals. Equine veterinary journal. 2008;40:433–439. doi: 10.2746/042516408X300269. [DOI] [PubMed] [Google Scholar]
- 29.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Early lesions of articular osteochondrosis in the distal femur of foals. Veterinary pathology. 2011;48:1165–1175. doi: 10.1177/0300985811398250. [DOI] [PubMed] [Google Scholar]
- 30.Hixon AL, Gibbs LM. Osteochondritis dissecans: a diagnosis not to miss. American family physician. 2000;61:151–156. 158. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Photomicrographs of a hematoxylin and eosin stained section obtained from the medial femoral condyle of a goat one week after surgical interruption of the vascular supply to the area (panels A and B; scale bars = 500μm). Necrotic cartilage canals (dotted circles) are apparent between the incision extending into the epiphyseal cartilage (black arrowheads) and the articular surface, whereas cartilage canals located abaxially to the incision appear normal (black arrows).
Supplementary figure 2: Adiabatic T1ρ relaxation time map (panel A) obtained from the distal femur of a goat 4 weeks post operatively with the operated area enlarged. Lateral and medial femoral condyles appear similar except for subtle overall increase of adiabatic T1ρ relaxation time in the axial aspect of the medial femoral condyle corresponding with the site of surgical incision (black arrowheads). Photomicrograph of a safranin-O stained section obtained from the axial aspect of the medial femoral condyle of the same specimen (Panel B; scale bar = 500μm). The surgical incision is nearly healed (black arrows). An island composed of necrotic cartilage (marked by dotted line) characterized by decreased staining relative to surrounding epiphyseal cartilage is present at the axial most aspect of the condyle. Panel C depicts high magnification image (scale bar = 50μm) of the area marked by the black box in panel B in a hematoxylin and eosin stained slide. Chondrocytes to the left of the dotted diagonal line are necrotic, characterized by pyknotic nuclei and shrinkage.
Supplementary figure 3: Adiabatic T1ρ relaxation time map (panel A) obtained from the distal femur of a goat 6 weeks post operatively with the operated area enlarged. An irregularity at the cartilage bone interface (ossification front) is evident, corresponding with the surgical incision. Photomicrograph of a safranin-O stained section obtained from the axial aspect of the medial femoral of the same specimen (Panel B; scale bar = 500μm). The superficial portion of the incision (black arrows) persists with no or minimal signs of healing. Deeper portions of the surgical incision are filled with granulation tissue (black arrowheads) delaying the advancement of the subjacent ossification front.