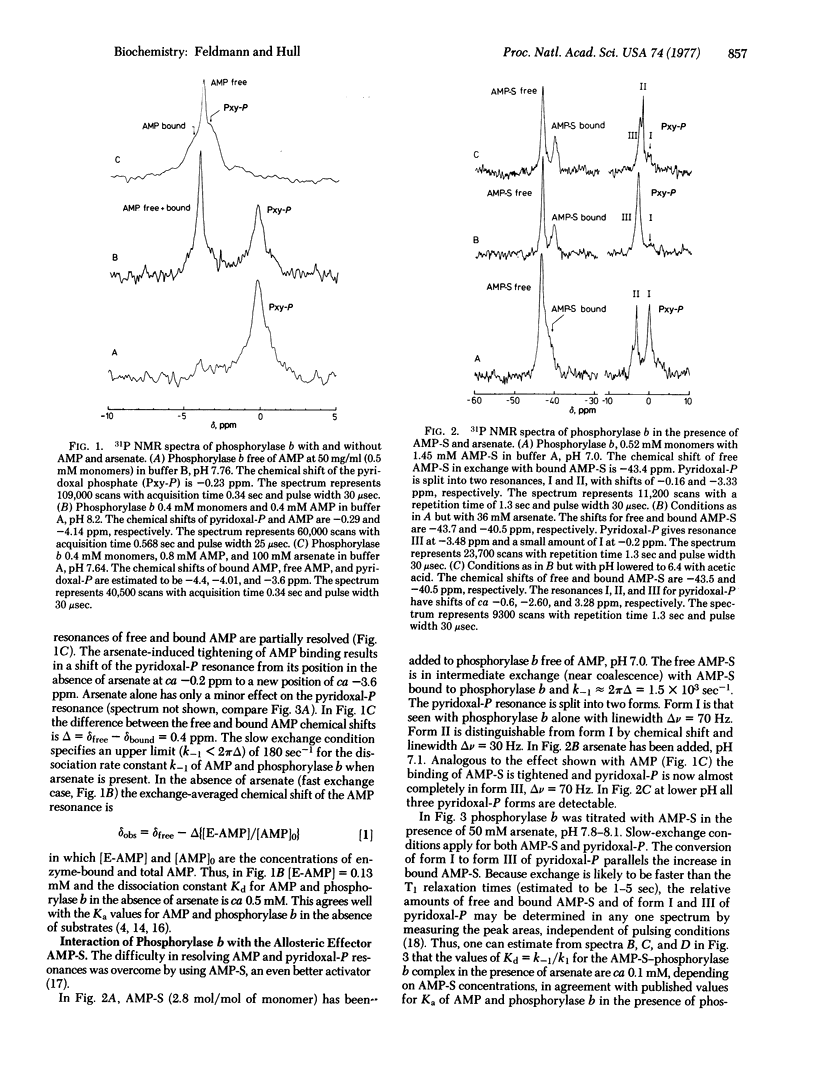
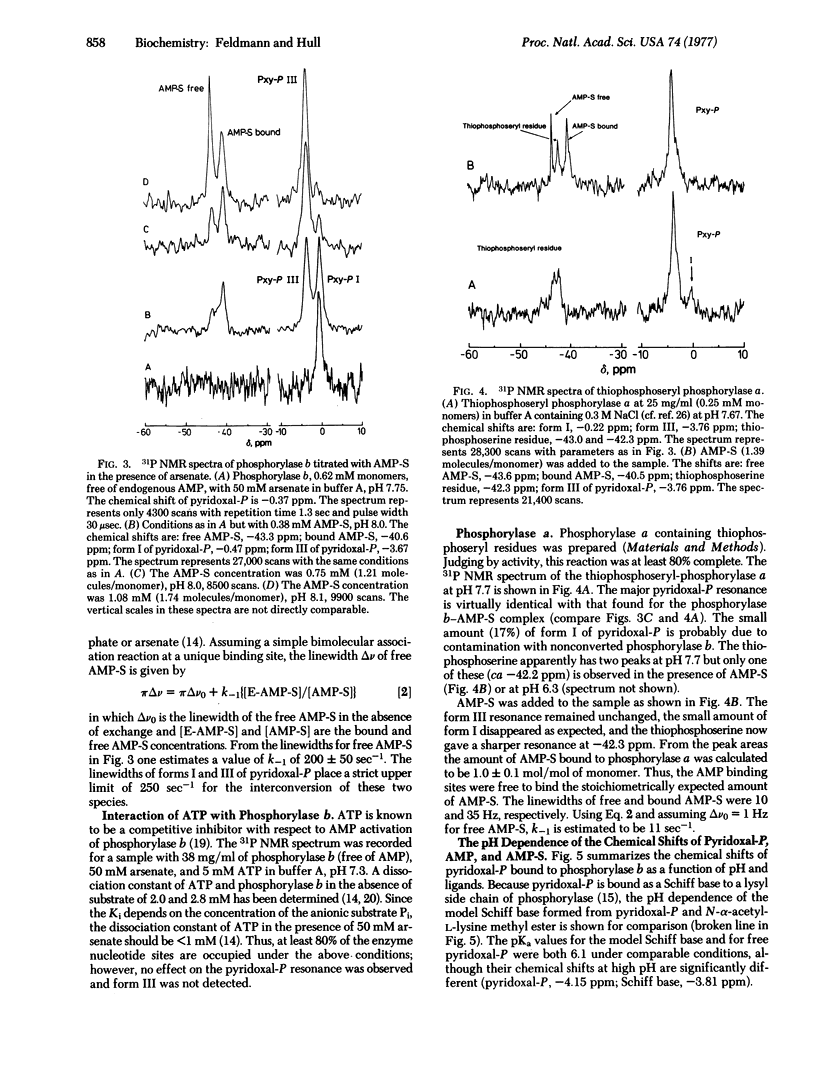
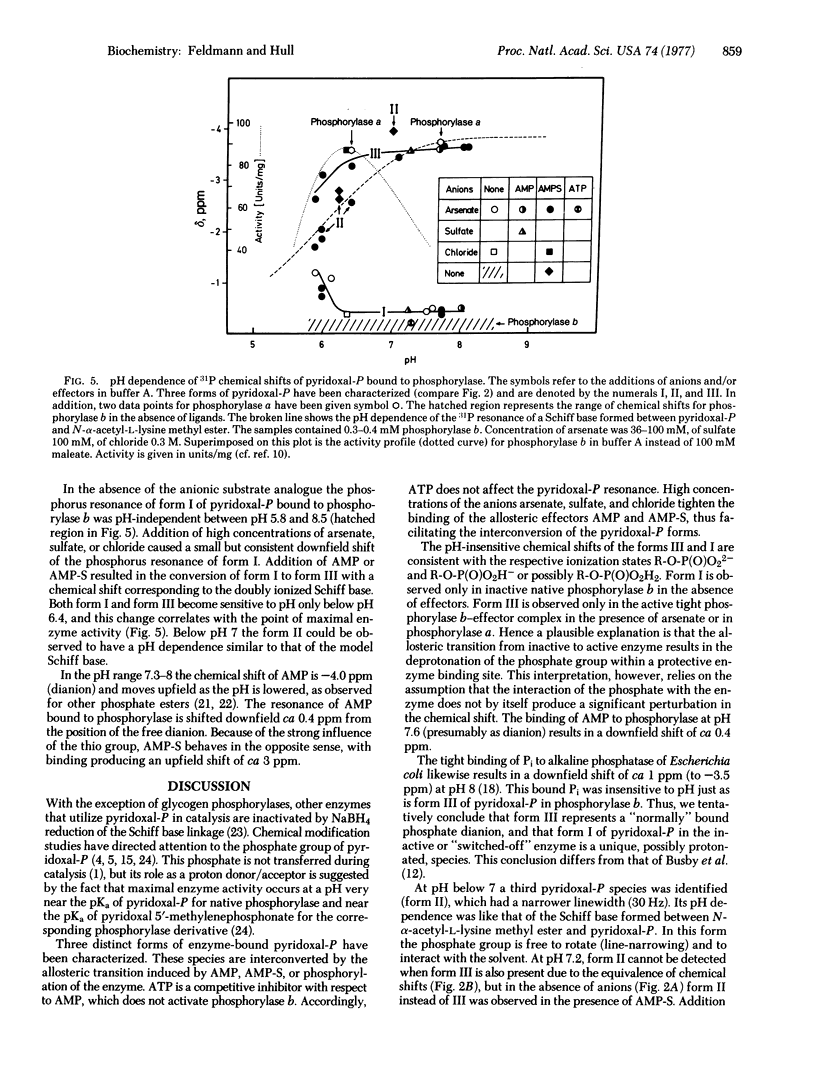
Abstract
31P nuclear magnetic resonance (NMR) at 72.8 MHZ has been used to study glycogen phosphorylase from rabbit muscle (1,4-alpha-D-glucan:orthophosphate alpha-glucosyltransferase, EC 2.4.1.1) at concentrations as low as 25 mg/ml, using a WH-180 wide-bore superconducting spectrometer. The use of a thio analogue for 5'-AMP and arsenate for inorganic phosphate allowed the observation of three distinct forms of enzyme-bound pyridoxal 5'-phosphate at --0.2 ppm (Form I), --2 to --3 ppm (Form II), and --3.5 ppm (Form III) relative to triethylphosphate. Conversion of I to III occurs by activation of phosphorylase either by formation of a ternary complex of phosphorylase b with effector and arsenate or, more efficiently, by direct phosphorylation to give the a form of the enzyme. The ionization state and exposure to solvent of each of the three forms is inferred from the 31P NMR data.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Pyridoxal phosphate in glycogen phosphorylase: a phosphorus NMR study. FEBS Lett. 1975 Jul 15;55(1):14–17. doi: 10.1016/0014-5793(75)80945-8. [DOI] [PubMed] [Google Scholar]
- COHN M., HUGHES T. R., Jr Phosphorus magnetic resonance spectra of adenosine di- and triphosphate. I. Effect of pH. J Biol Chem. 1960 Nov;235:3250–3253. [PubMed] [Google Scholar]
- Cohen P., Duewer T., Fischer E. H. Phosphorylase from dogfish skeletal muscle. Purification and a comparison of its physical properties to those of rabbit muscle phosphorylase. Biochemistry. 1971 Jul 6;10(14):2683–2694. doi: 10.1021/bi00790a005. [DOI] [PubMed] [Google Scholar]
- FISCHER E. H., KREBS E. G. The isolation and crystallization of rabbit skeletal muscle phosphorylase b. J Biol Chem. 1958 Mar;231(1):65–71. [PubMed] [Google Scholar]
- Feldmann K., Helmreich E. J. The pyridoxal 5' -phosphate site in rabbit skeletal muscle glycogen phosphorylase b: an ultraviolet and 1H and 31P nuclear magnetic resonance spectroscopic study. Biochemistry. 1976 Jun 1;15(11):2394–2401. doi: 10.1021/bi00656a023. [DOI] [PubMed] [Google Scholar]
- Feldmann K., Zeisel H. J., Helmreich E. J. Complementation of subunits from glycogen phosphorylases of frog and rabbit skeletal muscle and rabbit liver. Eur J Biochem. 1976 May 17;65(1):285–291. doi: 10.1111/j.1432-1033.1976.tb10416.x. [DOI] [PubMed] [Google Scholar]
- Gratecos D., Fischer E. H. Adenosine 5'-O(3-thiotriphosphate) in the control of phosphorylase activity. Biochem Biophys Res Commun. 1974 Jun 18;58(4):960–967. doi: 10.1016/s0006-291x(74)80237-8. [DOI] [PubMed] [Google Scholar]
- HELMREICH E., CORI C. F. THE ROLE OF ADENYLIC ACID IN THE ACTIVATION OF PHOSPHORYLASE. Proc Natl Acad Sci U S A. 1964 Jan;51:131–138. doi: 10.1073/pnas.51.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar W., Thompson J. C., Maurer W., Rüterjans H. Investigation of nucleotide-ribonuclease-A complexes with high-resolution 31P-nuclear-magnetic-resonance spectroscopy. Eur J Biochem. 1973 Dec 3;40(1):259–266. doi: 10.1111/j.1432-1033.1973.tb03193.x. [DOI] [PubMed] [Google Scholar]
- Helmreich E., Michaelides M. C., Cori C. F. Effects of substrates and a substrate analog on the binding of 5'-adenylic acid to muscle phosphorylase a. Biochemistry. 1967 Dec;6(12):3695–3710. doi: 10.1021/bi00864a012. [DOI] [PubMed] [Google Scholar]
- Hull W. E., Halford S. E., Gutfreund H., Sykes B. D. 31P nuclear magnetic resonance study of alkaline phosphatase: the role of inorganic phosphate in limiting the enzyme turnover rate at alkaline pH. Biochemistry. 1976 Apr 6;15(7):1547–1561. doi: 10.1021/bi00652a028. [DOI] [PubMed] [Google Scholar]
- Illingworth B., Jansz H. S., Brown D. H., Cori C. F. OBSERVATIONS ON THE FUNCTION OF PYRIDOXAL-5-PHOSPHATE IN PHOSPHORYLASE. Proc Natl Acad Sci U S A. 1958 Dec 15;44(12):1180–1191. doi: 10.1073/pnas.44.12.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenschmidt L. L., Kastenschmidt J., Helmreich E. Subunit interactions and their relationship to the allosteric properties of rabbit skeletal muscle phosphorylase b. Biochemistry. 1968 Oct;7(10):3590–3608. doi: 10.1021/bi00850a037. [DOI] [PubMed] [Google Scholar]
- Kastenschmidt L. L., Kastenschmidt J., Helmreich E. The effect of temperature on the allosteric transitions of rabbit skeletal muscle phosphorylase b. Biochemistry. 1968 Dec;7(12):4543–4556. doi: 10.1021/bi00852a051. [DOI] [PubMed] [Google Scholar]
- MORGAN H. E., PARMEGGIANI A. REGULATION OF GLYCOGENOLYSIS IN MUSCLE. II. CONTROL OF GLYCOGEN PHOSPHORYLASE REACTION IN ISOLATED PERFUSED HEART. J Biol Chem. 1964 Aug;239:2435–2439. [PubMed] [Google Scholar]
- Martinez-Carrion M. 31P nuclear-magnetic-resonance studies of pyridoxal and pyridoxamine phosphates. Interaction with cytoplasmic aspartate transaminase. Eur J Biochem. 1975 May;54(1):39–43. doi: 10.1111/j.1432-1033.1975.tb04111.x. [DOI] [PubMed] [Google Scholar]
- Mott D. M., Bieber A. L. Structural specificity of the adenosine 5'-phosphate site on glycogen phosphorylase b. J Biol Chem. 1970 Aug 25;245(16):4058–4066. [PubMed] [Google Scholar]
- Murray A. W., Atkinson M. R. Adenosine 5'-phosphorothioate. A nucleotide analog that is a substrate, competitive inhibitor, or regulator of some enzymes that interact with adenosine 5'-phosphate. Biochemistry. 1968 Nov;7(11):4023–4029. doi: 10.1021/bi00851a032. [DOI] [PubMed] [Google Scholar]
- Pfeuffer T., Ehrlich J., Helmreich E. Role of pyridoxal 5'-phosphate in glycogen phosphorylase. II. Mode of binding of pyridoxal 5'-phosphate and analogs of pyridoxal 5'-phosphate to apophosphorylase b and the aggregation state of the reconstituted phosphorylase proteins. Biochemistry. 1972 May 23;11(11):2136–2145. doi: 10.1021/bi00761a021. [DOI] [PubMed] [Google Scholar]
- Shaltiel S., Hedrick J. L., Pocker A., Fischer E. H. Reconstitution of apophosphorylase with pyridoxal 5'-phosphate analogs. Biochemistry. 1969 Dec;8(12):5189–5196. doi: 10.1021/bi00840a073. [DOI] [PubMed] [Google Scholar]
- Vidgoff J. M., Pocker A., Hullar T. L., Fischer E. H. Interaction of muscle glycogen phosphorylase with pyridoxal 5'-methylenephosphonate. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1166–1174. doi: 10.1016/0006-291x(74)90819-5. [DOI] [PubMed] [Google Scholar]
- WANG J. H., GRAVES D. J. THE RELATIONSHIP OF THE DISSOCIATION TO THE CATALYTIC ACTIVITY OF GLYCOGEN PHOSPHORYLASE A. Biochemistry. 1964 Oct;3:1437–1445. doi: 10.1021/bi00898a008. [DOI] [PubMed] [Google Scholar]



