Abstract
Relapse represents one of the most significant problems in the long-term treatment of drug addiction. Cocaine blocks plasma membrane monoamine transporters and increases dopamine (DA) overflow in the brain, and DA is critical for the motivational and primary reinforcing effects of the drug as well as cocaine-primed reinstatement of cocaine seeking in rats, a model of relapse. Thus, modulators of the DA system may be effective for the treatment of cocaine dependence. The endogenous neuropeptide galanin inhibits DA transmission, and both galanin and the synthetic galanin receptor agonist, galnon, interfere with some rewarding properties of cocaine. The purpose of this study was to further assess the effects of galnon on cocaine-induced behaviors and neurochemistry in rats. We found that galnon attenuated cocaine-induced motor activity, reinstatement, and DA overflow in the frontal cortex at a dose that did not reduce baseline motor activity, stable self-administration of cocaine, baseline extracellular DA levels, or cocaine-induced DA overflow in the nucleus accumbens (NAc). Similar to cocaine, galnon had no effect on stable food self-administration but reduced food-primed reinstatement. These results indicate that galnon can diminish cocaine-induced hyperactivity and relapse-like behavior, possibly in part by modulating DA transmission in the frontal cortex.
Keywords: cocaine, cortex, dopamine, galanin, galnon, reinstatement
Introduction
Cocaine addiction, defined as intense craving and compulsive use of cocaine despite negative consequences, is a major problem in our society. Current treatment options (e.g. behavioral therapy) are not very effective, and there are currently no FDA-approved or generally accepted pharmacotherapies for cocaine dependence. Because relapse is a major obstacle in the treatment of drug addiction, one promising strategy is to develop therapies that block the ability of triggers such as the drug itself, drug-associated cues, or stress to precipitate relapse (Bossert et al., 2013; Sinha, 2009). Cocaine blocks plasma membrane monoamine transporters, which in turn increases extracellular levels of DA, norepinephrine (NE), and serotonin (5-HT) in the brain. It is well established that DA is critical for mediating the motivational and reinforcing effects of cocaine, and blocking its transmission attenuates drug-seeking behavior during reinstatement, a model of relapse (Bossert et al., 2013; Schmidt et al., 2005). NE and 5-HT play modulatory roles and are also implicated in reinstatement.
One intriguing molecule that modulates DA transmission and behavioral responses to addictive drugs is the neuropeptide galanin. Galanin and its G protein-coupled receptors (GalR1-3) are expressed within the mesocorticolimbic circuit implicated in drug addiction (Hawes and Picciotto, 2004; Melander et al., 1986). Galanin receptors can also be activated by galnon, a synthetic non-peptide agonist that crosses the blood-brain barrier and binds to GalR1 and GalR2 (Saar et al., 2002). In general, galanin reduces DA release (Jansson et al., 1989; Melander et al., 1987; Nordstrom et al., 1987; Tsuda et al., 1998), and both galanin and galnon attenuate responses to drugs of abuse (Picciotto, 2008). For example, intracerebroventricular administration of galanin attenuates morphine conditioned place preference (Zachariou et al., 1999), and opiate withdrawal is decreased by galanin overexpression or galnon and exacerbated by genetic knockout of galanin or GalR1 (Holmes et al., 2012; Zachariou et al., 2003). Moreover, galanin knockout mice are hypersensitive to morphine and cocaine conditioned place preference, and these phenotypes are abolished by galnon administration (Narasimhaiah et al., 2009). By contrast, complete knockout of galanin has minimal effect on cocaine self-administration in mice using several doses and schedules of reinforcement (Brabant et al., 2010; Narasimhaiah et al., 2009). However, several important aspects of drug responses have not been examined after galanin receptor activation, including relapse-like behavior and DA transmission. In this study, we examined the consequences of galnon administration on drug seeking during the maintenance and reinstatement phases of operant cocaine self-administration, as well as on cocaine-induced changes in DA overflow in the frontal cortex and the nucleus accumbens (NAc).
Materials and methods
Subjects
Male Sprague-Dawley rats (151–175 g) were used for all experiments. Self-administration experiments were conducted at Emory University (N=54) rats purchased from Charles River, Wilmington, MA) and motor activity and microdialysis experiments were conducted at the University of Georgia (N=107 rats purchased from Harlan, Prattville, AL). Rats were individually housed in clear polycarbonate cages (50 × 30 × 30 cm) and given ad libitum access to food and water unless otherwise specified in a temperature and humidity controlled animal facility and maintained on a 12-hour reverse light/dark cycle. Testing occurred during the dark phase with background noise emitted by a white noise generator. Animals were allowed to acclimate to the vivarium for one week prior to surgery. Rats were treated in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. Experiments were approved by Emory and University of Georgia IACUCs.
Drugs
Cocaine was obtained from NIDA and dissolved in 0.9% physiological saline. Galnon (Bachem, Torrance, CA, USA) was sonicated in 1% DMSO. Galnon is a non-selective galanin receptor agonist (Kd =1 μM) with a half-life of approximately 60 min (Bartfai and Wang, 2013). Galnon was injected 20–30 min before cocaine based on effective pretreatment schedules in rodents (Jackson et al., 2011; Lu et al., 2005a; Narasimhaiah et al., 2009; Rajarao et al., 2007). All injections were performed in a volume of 1 ml/kg.
Stereotaxic cannulation surgery
Rats were anesthetized with isoflurane administered by vaporizer with oxygen delivered through a nose cone, and the surgical site was shaved and cleaned with Betadine. Rats were positioned in a stereotaxic apparatus, a longitudinal incision was made along the scalp, and 3 screws were anchored to the skull. Unilateral guide cannulae (MAB 6.6.IC; SciPro, Sanborn, NY) were implanted targeting the frontal cortex (3.2 mm anterior, 2.2 mm lateral, −1.5 mm ventral, relative to bregma) or NAc shell (1.7 mm anterior, 0.6 mm lateral, −6.5 mm ventral) according to the atlas of Paxinos and Watson (1998). Cannulae and a plastic guard were fixed to the skull using fast-drying epoxy. Rats received banamine (2.5 mg/kg, s.c.) immediately and 24 h after surgery. At the end of experiments, 4 μl of dye (2 mg/ml India ink) was injected to verify cannulae placement, determined by inspection of dye and termination of the cannulae track in 12 μm Nissl-stained coronal cryosections.
Motor activity
Rats were placed in the center of an open arena (44.5 × 44 × 30 cm; ENV 515–16, Med Associates, St. Albans, VT) that contained fresh bedding as described (Sciolino et al., 2012). Infrared beam breaks were used to track the coordinate position and movement of the rat (ENV-520, Med Associates) every 50 ms using default settings (SOF-810). Behavior was recorded continuously during the 140 min test, pausing in 20 min intervals for all rats to collect a dialysis sample or to inject drug. Behavior was sampled for 40 min before treatment with vehicle or galnon (2, 5, or 10 mg/kg i.p.) (Phase I, “habituation”), collected for another 20 min before cocaine (10 mg/kg i.p.) (Phase II, “pretreatment”), and recorded for 60 min post-cocaine (Phase III, “cocaine”). Ambulation was initiated after 3 beam breaks and measured as ambulatory distance traveled in continuous (< 500 ms without rest) movement outside a 2×2 area of x-y beams. Non-ambulatory movement was defined as movements that did not achieve criteria for ambulation, and thus was the continuous movement within a 2×2 area of x-y beams. Rats were not habituated to the open field before testing because pilot experiments showed that novelty-induced increases in ambulation diminished steadily across baseline testing, after which time locomotor exploration was nearly zero (i.e., see Fig. 1A).
Figure 1. Galnon reduces cocaine-induced hyperactivity.
Rats were placed in an open field, administered vehicle or galnon (2, 5, or 10 mg/kg, i.p.), and injected with cocaine (10 mg/kg, i.p.) 20 min later. Shown are the mean ± SEM for (A) ambulatory distance across all time points, (B) AUC for ambulatory distance during the second half of post-cocaine time, (C) frequency of non-ambulatory movement across all time points, and (D) AUC for non-ambulatory movement during the second half of post-cocaine time. N = 46 (vehicle), 32 (galnon 2 mg/kg), 23 (galnon 5 mg/kg), and 5 (galnon 10 mg/kg). **p<0.01, *p<0.05 compared to vehicle. Hab, habituation (phase I). Gal, galnon (phase II). Coc, cocaine (phase III). Veh, vehicle.
In vivo microdialysis
Microdialysis was performed in all rats during the motor activity test (see above), as we described previously (Soares et al., 1999). A microdialysis probe was inserted into cannulae targeting the frontal cortex (MAB 9.6.2, 0.6 mm OD, 6 kDa cutoff PES membrane; SciPro, Sanborn, NY) or NAc (S-8020, 0.36 mm OD, 20 kDA cutoff PAN membrane; Synaptech, Marquette, MI) the night before testing and extended 2 mm beyond the guide cannulae. Probes were connected to tubing (PE50, VWR, Westchester, PA) before experimentation. Rats were allowed to freely explore an open field while artificial CSF (pH 7.4; 0.13 M NaCl, 3.1 mM KHCO3, 1 mM MgCl2, 2 mM NaHCO3, 2.5 mM dextrose, 1.2 mM CaCl2; Sigma Chemicals, St. Louis, MO) was delivered through the probe at 2 μl/min. Dialysate was collected every 20 min during the 140 min open field test, including before galnon (2, 5, or 10 mg/kg i.p.) or vehicle injection at time −40, before cocaine at time 0, and for 60 min post-cocaine. These procedures were employed to allow DA levels to equilibrate and become Ca++-dependent (i.e., probes inserted 24 hours before testing, baseline sampling lasted 40 min before drug manipulation), as shown previously (Santiago and Westerink, 1990). Dialysate was transferred to sterile microcentrifuge vials (Fischer Scientific, Suwanee, GA) pre-filled with 0.1% phosphoric acid and DHBA (10% sample volume, 0.08 ng/ul) or 0.1 M perchloric acid in EDTA (1 mM of final solution volume). Different preservatives were used in a counterbalanced manner to determine if analyte detection could be improved, but preservatives did not alter DA content in our study (data not shown). Samples were transported in an opaque container on ice and stored at −80° C. Tubing was flushed with 70% EtOH, distilled H20, air, and aCSF between rats.
High performance liquid chromatography
Samples were thawed and injected into the HPLC system that consisted of two ESA 584 pumps (ESA, Chelmsfor, MA) with a pre-column filter (Synergi Max-RP 4u Security Guard, 150 × 4.6 mm, Phenomenex Inc., Torrance, CA) and Max-RP cartridges (Phenomenex). Mobile phase, containing 100 mM sodium phosphate monobasic (Fisher), 0.1 mM EDTA (Sigma), 0.25 mM octanesulfonic acid (Sigma), and 5% acetonitrile (JT Baker) was delivered at 1 ml/min. Samples and standards were housed and sampled using an ESA 542 autosampler maintained at 4° C. Dialysate injection was 20 μl, and a duplicate set of standards was run every 12 samples. Peaks were detected over 30 min using an ESA CoulArray electrochemical detector (−150 mV on initial electrode, 200 mV on a subsequent electrode). The position and height of peaks for DA were compared with reference standard solutions (Sigma; diluted in aCSF). Peak areas from chromatograms were integrated and analyzed by CoulArray Data Station Software 3.05.
Food training
To facilitate acquisition of drug self-administration, rats were first trained to lever press for food (45 mg pellets) in an operant chamber (Med Associates, St Albans, VT) prior to surgery. Each chamber was equipped with a house light and two retractable levers with a stimulus light above each lever. Animals were trained on a fixed ratio 1 (FR1) schedule with a 20-s time out. One lever press on the active lever resulted in the delivery of one food pellet. Presses on the inactive lever had no programmed consequences. Food training sessions lasted 8 h, or until the animal met criteria, defined as at least 70% active lever selection and at least 100 food pellets obtained. Most rats met criteria on the first day of food training, but a few required 2–3 days.
Jugular catheter surgery
All instruments and implants were sterilized prior to surgery. Rats were surgically implanted with a catheter into the right jugular vein after food training, as described (Schroeder et al., 2010; Schroeder et al., 2013). Rats were anesthetized with isoflurane administered by vaporizer with oxygen delivered through a nose cone, and the surgical site was shaved and cleaned with Betadine. Catheter tubing was threaded subcutaneously from the back and guided over the shoulder into the right jugular vein, and tubing was sutured down. Rats received meloxicam (1 mg/kg, s.c.) immediately following surgery, and allowed to recover for 1 week prior to cocaine self-administration. Catheters were flushed daily with 0.05 ml gentamicin (3 mg/ml) and 0.1 ml heparinized saline (30 ml in sterile saline) to help maintain patency. Catheter patency was verified prior to cocaine self-administration by administering 0.08–0.12 ml of the short-acting barbiturate methohexital sodium (10 mg/ml, IV; Eli Lilly, Indianapolis, IN, USA), which rapidly produces moderate sedation.
Cocaine self-administration
Daily cocaine self-administration sessions were run for 2 h on a FR1 schedule. At the start of each session, both active and inactive levers were extended, and rats received a non-contingent infusion of cocaine (0.5 mg/kg). During training, each press of the active lever resulted in a cocaine infusion (0.5 mg/kg, 167 μl/kg) accompanied by a discrete flashing light above the lever. Following a 20-s timeout period (during which time active lever presses were recorded but did not result in drug infusion), the stimulus light was extinguished and responses were again reinforced. Responses on the inactive lever had no programmed consequences. To prevent overdose, the session was terminated early if the number of cocaine infusions exceeded 40. The effects of galnon were assessed once rats reached a stable level of responding (number of drug infusions varied by <20% of the mean and preference for the active lever was at least 75% for 3 consecutive days, with a minimum of 5 total days of cocaine self-administration). Rats received an injection of vehicle or galnon (2 mg/kg, i.p.) 30 min prior to the self-administration session. Each rat received both pretreatments in a counterbalanced fashion.
Extinction
Following completion of the maintenance phase of cocaine self-administration, lever pressing was extinguished in daily 2-h sessions during which presses on the previously active lever no longer resulted in delivery of cocaine or presentation of cocaine-paired cues. Behavior was considered extinguished when active lever presses over 3 consecutive days was <30% of the average number of active lever presses during the last 3 days of maintenance.
Reinstatement
Rats were pretreated with vehicle or galnon (2 mg/kg, i.p.) the day after extinction criteria were met. Thirty min later, they were given a non-contingent priming injection of saline or cocaine (10 mg/kg, i.p.) and placed in operant chambers under extinction conditions (i.e., presses on the “active” lever had no programmed consequences) for 2 h. Each rat received both pretreatments in a counterbalanced fashion, separated by extinction sessions until they met criteria (described above).
Food self-administration
Separate groups of rats were used for the food self-administration and reinstatement experiments. Rats were maintained on a restricted diet of 16 g of normal rat chow per day, given in the evening at least 1 h after self-administration sessions ended. Parameters of food self-administration were identical to the cocaine self-administration experiments, except that rats received a food pellet instead of a cocaine infusion for each active lever press, and sessions lasted 1 h and were terminated if the number of reinforcers obtained exceeded 60. Once rats reached maintenance criteria, rats received an injection of vehicle or galnon (2 mg/kg, i.p.) 30 min prior to the self-administration session. Each rat received both pretreatments in a counterbalanced fashion.
Extinction and food-primed reinstatement
After extinction training was completed (extinction criteria were identical to those used for cocaine-primed reinstatement), one group of rats was pretreated with vehicle or galnon (2 mg/kg, i.p.). Thirty min later, they were placed in the operant chambers for either another extinction session or a food-primed reinstatement session. For the “food-primed reinstatement” group of rats, three food pellets were delivered non-contingently in the first 10 sec of the session and levers were presented. Responses on either of the levers had no programmed consequence. Throughout the 60 min food reinstatement session, a food pellet was delivered every 3 min non-contingently, and responses upon the formerly active and inactive levers were recorded. Each rat received both pretreatments in a counterbalanced fashion, separated by extinction sessions as described above.
Statistics
Self-administration data were analyzed by ANOVA followed by Newman-Keuls post hoc tests or by Chi-square (for the fraction of rats that obtained the maximum number of rewards). Motor activity was combined from rats implanted with cannulae in the cortex and NAc because there were no differences between these groups. Motor activity were analyzed using repeated measures ANOVA, and area under the curve (AUC) was performed as follow-up tests for significant interaction effects to detect the source of group differences during relevant experimental phases, specifically during habituation (Phase I: −60 to −20 min), pretreatment (Phase II: −20 to 0 min), and cocaine phases (Phase III: 0 to 60 min). Analyte levels are reported in nmol/ml for descriptive purposes. Repeated measures ANOVA was used to analyze % DA overflow (post-cocaine analyte at 0, 20, 40, or 60 min/lowest baseline analyte at −40 or −20 min X 100) to reduce within-subject variability. To test a priori hypotheses and minimize a type II error, AUC for % DA was performed to assess the effects of galnon during the cocaine phase and t-tests were performed to assess the effects of galnon at baseline (time 0). Based on standard criteria (2 standard deviations ± mean), occasional outliers (e.g., <1% of values) were removed and missing values (e.g., ~6% of values due to loss of the microdialysis sample during transfer or collection) were replaced by the group mean to prevent loss of statistical power. In addition, one rat in the galnon 10 mg/kg group was removed entirely from locomotor activity analyses because this subject achieved outlier criteria. The number of subjects per group differed in the behavioral and dialysis studies because HPLC data were not available for all subjects and we focused our analysis on the vehicle and 2 mg/kg galnon dose. Data were analyzed using SPSS (IBM PAWS Statistical Software, Chicago, IL) and graphed using Prism 5.0 (GraphPad, La Jolla, CA).
Results
Galnon reduces cocaine-induced motor activity
The effect of galnon (2, 5, or 10 mg/kg) on baseline and cocaine-induced motor activity were assessed in an open field. Ambulatory distance changed across time in a cubic fashion (F1,100=130.97, p<0.01; Cubic) (Fig. 1A). Distance traveled reduced across time compared to initial exploration (−40 vs. 0 min; p<0.01), was stimulated immediately after cocaine administration (10 min; p<0.01), and then steadily declined at 40 min (p<0.01), 50 min (p<0.01), and 60 min post-cocaine (p<0.01) (Fig. 1A) compared to the initial effects of cocaine at 10 min. Although the main effect of galnon was not significant for ambulatory distance (F3,102=1.08, p=0.36), there was a significant drug x time interaction (F3,102=3.94, p<0.01) (Fig. 1A). Follow-up AUC analyses reveled that ambulatory distance was no different across groups during habituation in phase I (−60 to −20 min; F3,102=0.39, p=0.76), after pretreatment with vehicle or galnon in phase II (−20 to 0 min; F3,102=0.92, p=0.44), overall post-cocaine in phase III (1 to 60 min; F3,102=1.56, p=0.21), or initially after cocaine administration in phase IIIa (1 to 30 min; F3,102=0.43, p=0.73). However galnon reduced cocaine-induced ambulatory distance later in phase IIIb compared to vehicle (31 to 60 min; F3,102=3.08, p<0.05). Posthoc tests revealed a significant effect of the 2 mg/kg (p<0.05) and 5 mg/kg (p<0.05) doses. The 10 mg/kg dose also tended to reduce the locomotor effects of cocaine during phase IIIb, but it was not significant (p=0.13) (Fig. 1B).
Non-ambulatory movement was also different across time (F1,102=150.30, p<0.01; Cubic) (Fig. 1C). Non-ambulation was less frequent across time compared to initial ambulation (−40 vs. 0 min; p<0.01), more frequent immediately after cocaine administration (10 vs. 0 min; p<0.01), and then became less frequent at 40 min (p<0.01), 50 min (p=0.01), and 60 min post-cocaine (p<0.01) (Fig. 1C) compared to initial effects of cocaine at 10 min. Although the main effect of galnon was not significant for non-ambulatory movement (F3,102=0.74, p=0.53), there was a significant drug x time interaction (F3,102=4.02, p<0.01) (Fig. 1C). Follow-up AUC analyses revealed that non-ambulatory movement was no different across groups during habituation in phase I (−60 to −20 min; F3,102=0.94, p=0.43), after pretreatment with vehicle or galnon in phase II (−20 to 0 min; F3,102=0.07, p=0.97), overall post-cocaine in phase III (1 to 60 min; F3,102=1.36, p=0.26), or initially after cocaine administration in phase IIIa (1 to 30 min; F3,102=0.34, p=0.80). However, galnon reduced cocaine-induced ambulatory distance later in phase IIIb compared to vehicle (31 to 60 min; F3,102=2.90, p<0.05). Posthoc tests showed a significant effect of the 5 mg/kg dose (p<0.01), but not at the 2 mg/kg (p=0.26) or 10 mg/kg doses (p=0.15) (Fig. 1D). Based on these results and other data showing galnon suppresses general motor activity and consummatory behavior at higher doses (Abramov et al., 2004; our unpublished data), we chose the 2 mg/kg dose for the remainder of the experiments.
Galnon attenuates cocaine-induced dopamine overflow in the frontal cortex but not the nucleus accumbens
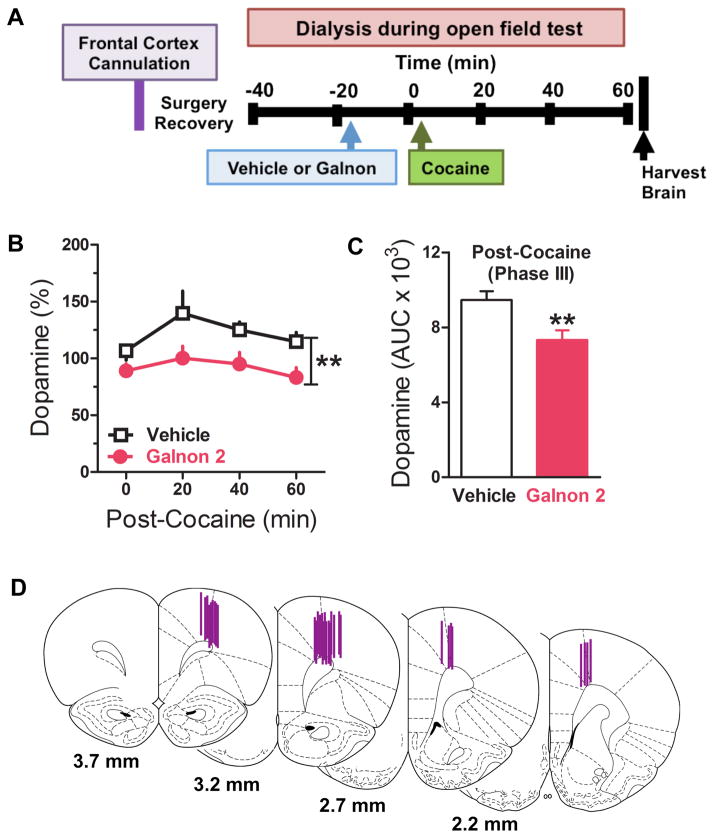
DA in the frontal cortex was measured in rats treated with vehicle and galnon (2 mg/kg) during motor activity testing, as shown in the timeline (Fig. 2A). Extracellular DA levels were not different between groups at baseline (vehicle 77.81±18.87 nmol/ml, galnon 73.39±22.33 nmol/ml, p>0.05) or following galnon pretreatment relative to vehicle (Time 0; t22=1.59, p=0.11) (Fig. 2B). DA overflow increased following cocaine administration, and was attenuated by galnon (Fig. 2B). Two-way repeated measures ANOVA revealed a significant effect of time (F1,22=5.09, p<0.05; Quadratic) and galnon (F1,22=7.27, p=0.01), but not a galnon x time interaction (F1,22=0.76, p=0.39) (Fig. 2B). AUC analyses revealed that galnon significantly reduced post-cocaine DA overflow relative to vehicle (t22=3.03, p<0.01) (Fig. 2C).
Figure 2. Galnon attenuates cocaine-induced increases in dopamine overflow in the frontal cortex.
Microdialysis samples were collected through probes targeting the frontal cortex from rats administered vehicle or galnon (2, 5, or 10 mg/kg, i.p.), and injected with cocaine (10 mg/kg, i.p.) during behavioral testing (see Fig. 1). Shown are (A) experimental timeline, (B) mean ± SEM extracellular DA levels (% baseline) for vehicle (n=14) and galnon 2 mg/kg (n=9) across all time points, (C) mean ± SEM AUC for total post-cocaine extracellular DA levels, and (D) probe placements. **p<0.01, *p<0.05 compared to vehicle.
DA in the NAc was measured in a separate group of rats treated with vehicle or galnon (2 mg/kg) during motor activity testing (Fig. 3A). Extracellular DA levels were not different between groups at baseline (vehicle 30.60±3.31 nm/ml, galnon 28.12±3.54 nmol/ml, p>0.05) or following galnon pretreatment relative to vehicle (Time 0; t13=−1.13, p=0.28) (Fig. 3B). Cocaine increased DA overflow (F1,13=15.26, p<0.01; Quadratic), but there was no main effect of galnon (F1,13=1.85, p=0.20). There was a significant galnon x time interaction (F1,13=10.36, p<0.01; Cubic) (Fig. 3B). Post hoc tests revealed that extracellular DA levels were not different 20 min post-cocaine (p=0.29), but were significantly different 40 (p<0.05) and 60 min post-cocaine (p<0.05) (Fig. 3B). Total post-cocaine DA overflow, as assessed by AUC, was not significantly different in the vehicle and galnon groups (t13=−1.65, p=0.12) (Fig. 3C).
Figure 3. Galnon does not alter cocaine-induced increases in dopamine overflow in the nucleus accumbens.
Microdialysis samples were collected through probes targeting the NAc shell from rats administered vehicle or galnon (2, 5, or 10 mg/kg, i.p.), and injected with cocaine (10 mg/kg, i.p.) during behavioral testing (see Fig. 1). Shown are (A) experimental timeline, (B) mean ± SEM extracellular DA levels (% baseline) for vehicle (n=8) and galnon 2 mg/kg (n=7) across all time points, (C) mean ± SEM AUC for total post-cocaine extracellular DA levels, and (D) probe placements.
Galnon has minimal effect on cocaine self-administration but blocks cocaine-primed reinstatement of cocaine seeking
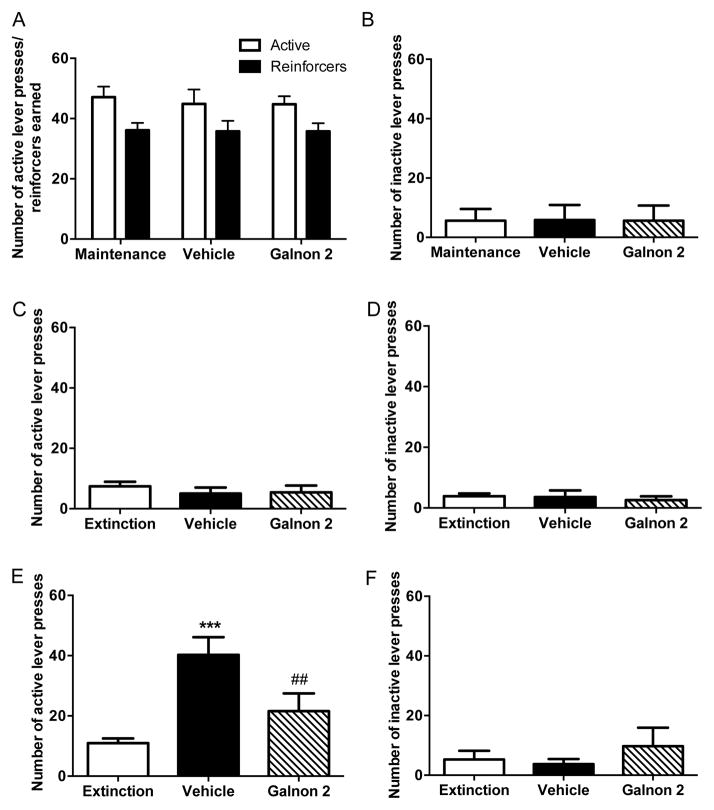
After reaching maintenance criteria for cocaine or food self-administration, rats were pretreated with vehicle or galnon (2 mg/kg, i.p.) 30 min prior to a self-administration session, and we found that galnon had no effect on operant responding for drug over the 2-hour session (one way repeated measures ANOVA: active lever, F2,14=0.24, p=0.79; reinforcers earned, F2,14=0.01, p=0.99; inactive lever, F2,14=0.03, p=0.97; Chi-square test for fraction of rats that obtained the maximum number of reinforcers = 1.07, p=0.59) (Fig. 4A, 4B). However, breaking down the 2-hour session into 30-min bins revealed a modest but significant difference in the pattern of cocaine infusions. Vehicle-treated rats tended to obtain most of their infusions during the first 30 min and then tapered off, while infusions were more stable in galnon-treated rats, resulting in fewer infusions during the first 30 min and more infusions during the final 30 min compared to vehicle (Fig. S1). A two-way repeated measures ANOVA showed a main effect of time (F3,21=10.08, p<0.001) and a time x treatment interaction (F3,21=3.38, p<0.05), although post hoc tests did not reveal significant pairwise differences for any individual time bin.
Figure 4. Galnon has no effect on cocaine self-administration but attenuates cocaine-primed reinstatement.
Following the establishment of stable maintenance responding for cocaine on a FR1 schedule (maintenance values reflect an average of the last 3 days of maintenance sessions), subjects (n=8) were treated with vehicle or galnon (2 mg/kg, i.p.) 30 min prior to a self-administration session. Shown are the mean ± SEM of (A) active lever presses and reinforcers earned and (B) inactive lever presses during the 2-h test session. Lever pressing was then extinguished (extinction values reflect an average of the last 3 days of extinction), and rats were pretreated with vehicle or galnon (2 mg/kg, i.p.) 30 min prior to a cocaine-primed (10 mg/kg, i.p., n=7) or saline-primed (n=5) reinstatement test. Shown are the mean ± SEM active and inactive lever responses during the 2-h saline-primed (C and D) and cocaine-primed (E and F) sessions. ***p<0.001 compared to extinction, ##p<0.01 compared to vehicle.
Rats were next subjected to non-reinforced sessions until meeting extinction criteria, and were then pretreated with vehicle or galnon (2 mg/kg, i.p.) 30 min prior to a reinstatement test following a non-contingent priming injection of saline or cocaine (10 mg/kg, i.p.). A saline prime did not reinstate cocaine-seeking behavior, and no differences were seen between pretreatment groups (active lever, F2,8=1.77, p=0.23; inactive lever, F2,8=0.31, p=0.74) (Fig. 4C, 4D). In contrast to its inability to alter cocaine self-administration, we found that galnon attenuated cocaine-primed reinstatement of cocaine seeking (Fig. 4E). One way repeated measures ANOVA showed a main effect of treatment on active lever presses (F2,12=16.07, p<0.001). Post hoc tests revealed that vehicle-pretreated rats robustly reinstated following cocaine prime compared to extinction (p<0.001), while galnon-pretreated rats did not significantly reinstate (p>0.05). Galnon-pretreated rats also displayed significantly fewer active lever presses than vehicle-pretreated animals (p<0.01). Inactive lever presses were low and did not differ between groups (F2,12=1.35, p=0.30) (Fig. 4F).
Galnon attenuates food-primed reinstatement of food seeking
To determine whether the effects of galnon on cocaine-primed reinstatement were specific to drug-induced relapse-like behavior, we also evaluated the consequences of galnon on food self-administration and reinstatement. Similar to what we found with cocaine, galnon (2 mg/kg, i.p.) did not significantly affect reinforced food self-administration (one way repeated measures ANOVA: active lever, F2,18=0.12, p=0.88; all rats obtained the maximum number of reinforcers except for one animal on one day that obtained 57 instead of the 61 possible; inactive lever, F2,18=3.45, p=0.054) (Fig. 5A, 5B). In contrast to our cocaine self-administration data, breaking down the 1-h session into 15-min bins did not reveal a difference in the pattern of food reinforcement; all animals obtained a majority of their food rewards during the first 15 min, and all rats except for one obtained the maximum number of food rewards in the first 30 min (Fig. S2). Galnon also had no effect on extinction responding (active lever, F2,10=0.1, p=0.91; inactive lever, F2,10=4.28, p=0.05) (Fig. 5C, 5D). However, galnon did partially attenuate food-primed reinstatement of food seeking (active lever: F2,18=12, p<0.001) (Fig. 5E). Posthoc tests revealed that, while both vehicle- (p<0.001) and galnon- (p<0.05) pretreated rats significantly reinstated following a food prime compared to extinction, galnon-pretreated animals displayed significantly less active lever pressing than vehicle-pretreated animals (p<0.05). Inactive lever presses were low and did not differ between groups (F2,18=0.93, p=0.41) (Fig. 5F).
Figure 5. Galnon has no effect on food self-administration but attenuates food-primed reinstatement.
Following the establishment of stable maintenance responding for food pellets on a FR1 schedule (maintenance values reflect an average of the last 3 days of maintenance sessions), subjects (n=10) were treated with vehicle or galnon (2 mg/kg, i.p.) 30 min prior to a self-administration session. Shown are the mean ± SEM of (A) active lever presses and reinforcers earned and (B) inactive lever presses during the 1-h test session. Lever pressing was then extinguished (extinction values reflect an average of the last 3 days of extinction), and rats were pretreated with vehicle or galnon (2 mg/kg, i.p.) 30 min prior to another extinction session (n=6) or a food-primed reinstatement test (n=10). Shown are the mean ± SEM active and inactive lever responses during the 1-h extinction (C and D) and food-primed reinstatement (E and F) sessions. *p<0.05 compared to extinction, ***p<0.001 compared to extinction, #p<0.05 compared to vehicle.
Discussion
Our study is the first to report that galanin receptor activation decreases relapse-like behavior at a dose that had no impact on locomotion following habituation to a novel environment or reinforced cocaine/food self-administration. The behavioral effects of galnon were accompanied by a complete suppression of cocaine-evoked DA overflow in the frontal cortex, but not the NAc. These results are consistent with, and extend, previous reports showing that galanin receptor signaling diminishes the rewarding properties of cocaine and other drugs of abuse, and support targeting the galanin system as a potential treatment for addiction.
The effects of galnon on motor activity
Galanin signaling has little impact on basal motor activity and attenuates drug-induced ambulation under some conditions. For instance, galnon fails to alter general locomotion at doses below 5 mg/kg (Abramov et al., 2004; Brabant et al., 2010; Hawes et al., 2008), but at high doses (e.g. 10 mg/kg and above) it impairs motor activity and food consumption under some conditions (Abramov et al., 2004) (our unpublished data). Consistent with these data, we found that doses of galnon ranging from 2–10 mg/kg had no effect on motor activity before cocaine administration. We also found that galnon decreased cocaine-induced motor activity during the second half of the time course examined, consistent its ability to suppresses morphine-induced locomotion in galanin knockout mice (Hawes et al., 2008). In contrast to our present findings in rats, locomotor activity following cocaine was not altered in mice pretreated with galnon (1–4 mg/kg) or in galanin knockout mice (Brabant et al., 2010), suggesting the existence of species differences. Combined, these data indicate that, at low doses that do not reliably alter baseline motor activity, galnon modestly attenuates hyperlocomotion following acute administration of cocaine or morphine. To avoid a general locomotor effect of galnon, we chose the lowest dose (2 mg/kg) for the remaining experiments in this study, which aimed to examine the effects of galnon on reward-related behaviors and neurochemistry.
The effects of galnon on cocaine and food self-administration and reinstatement
In general, galanin receptor signaling opposes the rewarding properties of cocaine and other drugs of abuse. For example, conditioned place preference to cocaine and morphine is facilitated in galanin knockout mice, while galanin or galnon suppresses the rewarding effects of these drugs (Brabant et al., 2010; Hawes et al., 2008; Narasimhaiah et al., 2009; Picciotto, 2008; Zachariou et al., 1999). However, nicotine conditioned place preference is attenuated in galanin knockout mice (Neugebauer et al., 2011), suggesting the nature of the drug influences the valence of galanin on reward. The effect of galnon on conditioned place preference likely does not extend to the reinforcing properties of cocaine as measured by operant self-administration; neither galanin knockout nor galnon altered cocaine self-administration in mice (Brabant et al., 2010; Narasimhaiah et al., 2009). Our data showing that galnon did not profoundly alter stable operant responding for cocaine during the maintenance phase are consistent with these results, although we only examined FR1 responding, and it has been reported that galanin or a GalR1 agonist can alter operant reward responding under higher contingency schedules of reinforcement (Anderson et al., 2013; McNamara and Robinson, 2010). There was modest but significant change in the pattern of cocaine infusions between groups. The significance of this result is not clear, but it may be related to the delay in peak accumbal DA overflow in response to cocaine we observed in galnon-treated rats.
The effect of galanin receptor activation on relapse-like behavior has not been studied, and we employed the reinstatement paradigm to address this gap in the literature. It is important to note that reinstatement responding differs from the maintenance phase of self-administration sessions because it is run under extinction conditions (i.e., active lever presses have no programmed consequences), and represents non-reinforced drug-seeking behavior thought to model aspects of relapse (Bossert et al., 2013). We found that galnon abolished cocaine-primed reinstatement. There was no significant difference between extinction and reinstatement responding following galnon pretreatment, while robust reinstatement was observed following vehicle pretreatment. This reduction in drug seeking appears to be specific for reinstatement as opposed to a general suppression of motor activity or operant behavior because the dose of galnon used had no effect on baseline motor activity, cocaine self-administration, food self-administration, or inactive lever presses during any phase. Moreover, galnon only modestly attenuated cocaine-induced motor activity, which unlikely accounts for the robust loss of reinstatement behavior because reinstatement was also reduced for a non-drug reinforcer (food) that does not produce hyperactivity.
The effects of galnon on cocaine-induced changes in dopamine overflow
Cocaine-primed reinstatement of cocaine seeking requires DA transmission in the prefrontal cortex, but not the NAc (Cornish and Kalivas, 2000; McFarland and Kalivas, 2001; McFarland et al., 2003; Schmidt et al., 2005; Sun and Rebec, 2005). Galanin can reduce DA release in some brain regions (Jansson et al., 1989; Melander et al., 1987; Nordstrom et al., 1987; Tsuda et al., 1998), but the consequences of galanin receptor activation on cocaine-induced DA overflow have not been investigated. We employed in vivo microdialysis in the frontal cortex and NAc to determine whether changes in extracellular DA accompanied galnon’s ability to attenuate the behavioral effects of cocaine. The region of frontal cortex selected for microdialysis sampling primarily represents motor cortex. This area receives dense dopaminergic innervation from ventral tegmental area neurons (Lindvall et al., 1978) and was selected because it is a reliable target for catecholamine recovery based on our previous microdialysis experiments (Soares et al., 1999). The region is also highly sensitive to acute cocaine challenge as indicated by magnetic resonance imaging of regional cerebral blood volume in rats (Chen et al., 2011). In these studies, cocaine elicited equivalent increases in peak blood volume in motor cortex, dorsal striatum, and nucleus accumbens. DA overflow in the motor cortex thus serves as a proxy for cocaine-induced activation of the mesocortical system. We found that galnon had no immediate impact (e.g., 20 min later) on baseline DA levels following habituation to a novel environment. We also found that galnon prevented cocaine-induced increases in locomotor activity and DA overflow in the frontal cortex. These data suggest that attenuated cocaine-induced DA overflow in the frontal cortex may contribute to the ability of galnon to reduce cocaine-induced locomotor activity and possibly aspects of relapse-like behavior, although the latter hypothesis needs to be directly evaluated by collecting dialysate in the prefrontal cortex during reinstatement testing. Our results are consistent with data from slice preparations showing that galanin reduces DA release (Melander et al., 1987; Nordstrom et al., 1987; Tsuda et al., 1998), although one study found increased DA utilization in the striatum following galanin administration (Jansson et al., 1989).
By contrast, we show that galnon does not reduce DA overflow in the NAc following cocaine administration. A simple explanation is that mesocortical DA neurons are more sensitive to inhibition by galanin receptor activation than mesolimbic DA neurons. Relative to other areas (e.g., NAc, ventral tegmental area), all three galanin receptors are highly expressed in the prefrontal cortex (Hawes and Picciotto, 2004), and this region is changed substantially in both structure and function by cocaine administration (Kalivas, 2007; Morales and Pickel, 2012; Tritsch and Sabatini, 2012). Alternatively, the failure of galnon to alter DA overflow in the NAc may result from complex interactions within mesolimbic circuitry. For example, because cortical DA transmission opposes subcortical DA transmission (Doherty and Gratton, 1996; King et al., 1997; Pycock et al., 1980; Ventura et al., 2004), suppression of cortical DA overflow by galnon could help preserve normal accumbal DA levels. In support of this idea, our finding that the peak increase in cocaine-induced DA overflow in the NAc following galnon pretreatment is delayed (~40 min post-cocaine) compared with vehicle-pretreated animals (~20 min post-cocaine) implicates a secondary circuit rather than a direct excitatory influence of galnon on mesolimbic DA neurons.
The exact mechanisms underlying the changes in cortical DA transmission in the present study are not clear. One possibility is that galnon modulates cocaine-induced DA release by suppressing the activity of ventral tegmental area DA neurons that project to the frontal cortex or by acting directly on mesocortical DA terminals. Indeed, galanin typically inhibits neuronal activity (Xu et al., 2005), and galanin receptors are present in both brain regions (Hawes and Picciotto, 2004). Alternatively, galnon may act indirectly by altering the activity of other brain nuclei (i.e., locus coeruleus) that, in turn, project to and control the activity of mesocortical DA neurons (Picciotto, 2008). In support of this hypothesis, galnon suppresses morphine-induced neuronal activity in the locus coeruleus, and transgenic overexpression of galanin in noradrenergic neurons reduces behavior associated with morphine withdrawal (Zachariou et al., 2003). Although future experiments are needed to identify the loci and mechanisms of action that mediate the benefit of galnon on cocaine-induced behavior, these data collectively suggest that galnon is well positioned to alter catecholamine transmission across circuits targeted by drugs of abuse and support a model in which galnon primarily affects mesocoritcal DA neurons that drive cocaine-primed reinstatement.
Conclusions
In summary, we report that galnon reduces reward-seeking behavior during reinstatement and cocaine-induced DA overflow in the frontal cortex. However, the results should be interpreted with caution, and further studies are required to define the underlying mechanisms. Galnon is a synthetic non-peptide galanin receptor agonist that crosses the blood brain barrier and has equal binding affinity for GalR1 and GalR2 (Lu et al., 2005b; Saar et al., 2002). It is important to note that high concentrations of galnon (e.g., 10 uM) in vitro also produce agonist activity at other GPCRs and activate intracellular G-proteins independent of receptor activation (Floren et al., 2005). However, at doses comparable to those used in the present study, the behavioral effects of galnon are similar to galanin (Sollenberg et al., 2005) and blocked by co-administration of a galanin receptor antagonist (Abramov et al., 2004; Saar et al., 2002; Wu et al., 2003). Thus, the effects of galnon in the present study are in all likelihood mediated, at least in part, by galanin receptor signaling. The modulatory action of galanin on other drug-induced behaviors appears to involve GalR1 and GalR2 receptor subtypes (Einstein et al., 2013; Holmes et al., 2012). Future experiments using selective antagonists for GalR1 and GalR2 will be necessary to assess the contribution of each receptor subtype. In addition, although all existing evidence points to a central effect of galanin and galnon (Zachariou et al., 2003; Zachariou et al., 1999), peripheral galanin receptors cannot be ruled out due to our systemic route of galnon administration. Although further experiments are required to identify the galanin receptor subtype and neuroanatomical substrates involved, the data presented here suggest that the galaninergic system is a candidate target for anti-relapse therapies.
Supplementary Material
Acknowledgments
These studies were funded by the National Institutes of Health (NIDA Grants DA027535 to DW and PVH, and DA033091 and DA015040 to YEO). DW is co-inventor on a patent concerning the use of selective dopamine β-hydroxylase inhibitors for the treatment of cocaine dependence (US-2010-0105748-A1; “Methods and Compositions for Treatment of Drug Addiction”).
Footnotes
Authorship Contributions. YEO, NRS, PVH, and DW participated in the research design, YEO conducted the reinstatement experiments, NRS and JLGC conducted the motor activity and microdialysis experiments, KGF conducted the HPLC experiments, GLE contributed analytic tools, and YEO, NRS, PVH, and DW wrote the manuscript.
The other authors declare no conflicts of interest.
References
- Abramov U, Floren A, Echevarria DJ, Brewer A, Manuzon H, Robinson JK, Bartfai T, Vasar E, Langel U. Regulation of feeding by galnon. Neuropeptides. 2004;38:55–61. doi: 10.1016/j.npep.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Runesson J, Saar I, Langel U, Robinson JK. Galanin, through GalR1 but not GalR2 receptors, decreases motivation at times of high appetitive behavior. Behavioural brain research. 2013;239:90–93. doi: 10.1016/j.bbr.2012.10.045. [DOI] [PubMed] [Google Scholar]
- Bartfai T, Wang MW. Positive allosteric modulators to peptide GPCRs: a promising class of drugs. Acta pharmacologica Sinica. 2013;34:880–885. doi: 10.1038/aps.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant C, Kuschpel AS, Picciotto MR. Locomotion and self-administration induced by cocaine in 129/OlaHsd mice lacking galanin. Behavioral neuroscience. 2010;124:828–838. doi: 10.1037/a0021221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YI, Famous K, Xu H, Choi JK, Mandeville JB, Schmidt HD, Pierce RC, Jenkins BG. Cocaine self-administration leads to alterations in temporal responses to cocaine challenge in limbic and motor circuitry. Eur J Neurosci. 2011;34:800–815. doi: 10.1111/j.1460-9568.2011.07806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty MD, Gratton A. Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain research. 1996;715:86–97. doi: 10.1016/0006-8993(95)01557-4. [DOI] [PubMed] [Google Scholar]
- Einstein EB, Asaka Y, Yeckel MF, Higley MJ, Picciotto MR. Galanin-induced decreases in nucleus accumbens/striatum excitatory postsynaptic potentials and morphine conditioned place preference require both galanin receptor 1 and galanin receptor 2. The European journal of neuroscience. 2013;37:1541–1549. doi: 10.1111/ejn.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floren A, Sollenberg U, Lundstrom L, Zorko M, Stojan J, Budihna M, Wheatley M, Martin NP, Kilk K, Mazarati A, Bartfai T, Lindgren M, Langel U. Multiple interaction sites of galnon trigger its biological effects. Neuropeptides. 2005;39:547–558. doi: 10.1016/j.npep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Brunzell DH, Narasimhaiah R, Langel U, Wynick D, Picciotto MR. Galanin protects against behavioral and neurochemical correlates of opiate reward. Neuropsychopharmacology. 2008;33:1864–1873. doi: 10.1038/sj.npp.1301579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes JJ, Picciotto MR. Characterization of GalR1, GalR2, and GalR3 immunoreactivity in catecholaminergic nuclei of the mouse brain. The Journal of comparative neurology. 2004;479:410–423. doi: 10.1002/cne.20329. [DOI] [PubMed] [Google Scholar]
- Holmes FE, Armenaki A, Iismaa TP, Einstein EB, Shine J, Picciotto MR, Wynick D, Zachariou V. Galanin negatively modulates opiate withdrawal via galanin receptor 1. Psychopharmacology. 2012;220:619–625. doi: 10.1007/s00213-011-2515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Chen X, Miles MF, Harenza J, Damaj MI. The neuropeptide galanin and variants in the GalR1 gene are associated with nicotine dependence. Neuropsychopharmacology. 2011;36:2339–2348. doi: 10.1038/npp.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson A, Fuxe K, Eneroth P, Agnati LF. Centrally administered galanin reduces dopamine utilization in the median eminence and increases dopamine utilization in the medial neostriatum of the male rat. Acta Physiol Scand. 1989;135:199–200. doi: 10.1111/j.1748-1716.1989.tb08568.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Cocaine and amphetamine-like psychostimulants: neurocircuitry and glutamate neuroplasticity. Dialogues Clin Neurosci. 2007;9:389–397. doi: 10.31887/DCNS.2007.9.4/pkalivas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D, Zigmond MJ, Finlay JM. Effects of dopamine depletion in the medial prefrontal cortex on the stress-induced increase in extracellular dopamine in the nucleus accumbens core and shell. Neuroscience. 1997;77:141–153. doi: 10.1016/s0306-4522(96)00421-6. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A, Divac I. Organization of catecholamine neurons projecting to the frontal cortex in the rat. Brain Res. 1978;142:1–24. doi: 10.1016/0006-8993(78)90173-7. [DOI] [PubMed] [Google Scholar]
- Lu X, Barr AM, Kinney JW, Sanna P, Conti B, Behrens MM, Bartfai T. A role for galanin in antidepressant actions with a focus on the dorsal raphe nucleus. Proc Natl Acad Sci U S A. 2005a;102:874–879. doi: 10.1073/pnas.0408891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Lundstrom L, Langel U, Bartfai T. Galanin receptor ligands. Neuropeptides. 2005b;39:143–146. doi: 10.1016/j.npep.2004.12.012. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara IM, Robinson JK. Conditional stimulation by galanin of saccharin and ethanol consumption under free and response contingent access. Neuropeptides. 2010;44:445–451. doi: 10.1016/j.npep.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Melander T, Fuxe K, Harfstrand A, Eneroth P, Hokfelt T. Effects of intraventricular injections of galanin on neuroendocrine functions in the male rat. Possible involvement of hypothalamic catecholamine neuronal systems. Acta Physiol Scand. 1987;131:25–32. doi: 10.1111/j.1748-1716.1987.tb08201.x. [DOI] [PubMed] [Google Scholar]
- Melander T, Hokfelt T, Rokaeus A, Cuello AC, Oertel WH, Verhofstad A, Goldstein M. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1986;6:3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Pickel VM. Insights to drug addiction derived from ultrastructural views of the mesocorticolimbic system. Ann N Y Acad Sci. 2012;1248:71–88. doi: 10.1111/j.1749-6632.2011.06299.x. [DOI] [PubMed] [Google Scholar]
- Narasimhaiah R, Kamens HM, Picciotto MR. Effects of galanin on cocaine-mediated conditioned place preference and ERK signaling in mice. Psychopharmacology. 2009;204:95–102. doi: 10.1007/s00213-008-1438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Henehan RM, Hales CA, Picciotto MR. Mice lacking the galanin gene show decreased sensitivity to nicotine conditioned place preference. Pharmacology, biochemistry, and behavior. 2011;98:87–93. doi: 10.1016/j.pbb.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom O, Melander T, Hokfelt T, Bartfai T, Goldstein M. Evidence for an inhibitory effect of the peptide galanin on dopamine release from the rat median eminence. Neurosci Lett. 1987;73:21–26. doi: 10.1016/0304-3940(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. 4. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Picciotto MR. Galanin and addiction. Cell Mol Life Sci. 2008;65:1872–1879. doi: 10.1007/s00018-008-8151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycock CJ, Carter CJ, Kerwin RW. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on neurotransmitter systems in subcortical sites in the rat. Journal of neurochemistry. 1980;34:91–99. doi: 10.1111/j.1471-4159.1980.tb04625.x. [DOI] [PubMed] [Google Scholar]
- Rajarao SJ, Platt B, Sukoff SJ, Lin Q, Bender CN, Nieuwenhuijsen BW, Ring RH, Schechter LE, Rosenzweig-Lipson S, Beyer CE. Anxiolytic-like activity of the non-selective galanin receptor agonist, galnon. Neuropeptides. 2007;41:307–320. doi: 10.1016/j.npep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Saar K, Mazarati AM, Mahlapuu R, Hallnemo G, Soomets U, Kilk K, Hellberg S, Pooga M, Tolf BR, Shi TS, Hokfelt T, Wasterlain C, Bartfai T, Langel U. Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7136–7141. doi: 10.1073/pnas.102163499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago M, Westerink BH. Characterization of the in vivo release of dopamine as recorded by different types of intracerebral microdialysis probes. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:407–414. doi: 10.1007/BF00169457. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. European journal of pharmacology. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, Pozdeyev N, Freeman KG, Iuvone PM, Edwards GL, Holmes PV, Weinshenker D. Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine beta-hydroxylase. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2440–2449. doi: 10.1038/npp.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Epps SA, Grice TW, Weinshenker D. The selective dopamine beta-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1032–1038. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Dishman RK, Holmes PV. Voluntary exercise offers anxiolytic potential and amplifies galanin gene expression in the locus coeruleus of the rat. Behavioural Brain Research. 2012;233:191–200. doi: 10.1016/j.bbr.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction biology. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J, Holmes PV, Renner KJ, Edwards GL, Bunnell BN, Dishman RK. Brain noradrenergic responses to footshock after chronic activity-wheel running. Behavioral Neuroscience. 1999;113:558–566. doi: 10.1037//0735-7044.113.3.558. [DOI] [PubMed] [Google Scholar]
- Sollenberg U, Bartfai T, Langel U. Galnon--a low-molecular weight ligand of the galanin receptors. Neuropeptides. 2005;39:161–163. doi: 10.1016/j.npep.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology. 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Tsuda S, Nishio I, Masuyama Y, Goldstein M. Effects of galanin on dopamine release in the central nervous system of normotensive and spontaneously hypertensive rats. Am J Hypertens. 1998;11:1475–1479. doi: 10.1016/s0895-7061(98)00168-x. [DOI] [PubMed] [Google Scholar]
- Ventura R, Alcaro A, Cabib S, Conversi D, Mandolesi L, Puglisi-Allegra S. Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:72–80. doi: 10.1038/sj.npp.1300300. [DOI] [PubMed] [Google Scholar]
- Wu WP, Hao JX, Lundstrom L, Wiesenfeld-Hallin Z, Langel U, Bartfai T, Xu XJ. Systemic galnon, a low-molecular weight galanin receptor agonist, reduces heat hyperalgesia in rats with nerve injury. Eur J Pharmacol. 2003;482:133–137. doi: 10.1016/j.ejphar.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Zheng K, Hokfelt T. Electrophysiological studies on galanin effects in brain--progress during the last six years. Neuropeptides. 2005;39:269–275. doi: 10.1016/j.npep.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Brunzell DH, Hawes J, Stedman DR, Bartfai T, Steiner RA, Wynick D, Langel U, Picciotto MR. The neuropeptide galanin modulates behavioral and neurochemical signs of opiate withdrawal. Proc Natl Acad Sci U S A. 2003;100:9028–9033. doi: 10.1073/pnas.1533224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Parikh K, Picciotto MR. Centrally administered galanin blocks morphine place preference in the mouse. Brain Res. 1999;831:33–42. doi: 10.1016/s0006-8993(99)01476-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.