Abstract
Objective
Role of intra-articular calcium crystals in osteoarthritis (OA) is unclear. Imaging modalities used to date for its evaluation have limitations in their ability to fully characterize intra-articular crystal deposition. Since Computed Tomography (CT) imaging provides excellent visualization of bones and calcified tissue, in this pilot project we evaluated the utility of CT scan in describing intra-articular calcium crystal deposition in the knees.
Method
We included 12 subjects with and 4 subjects without radiographic chondrocalcinosis in the most recent visit from the Multicenter Osteoarthritis (MOST) study, which is a longitudinal cohort of community-dwelling older adults with or at risk for knee OA. All subjects underwent CT scans of bilateral knees. Each knee was divided into 25 subregions and each subregion was read for presence of calcium crystals by a musculoskeletal radiologist. To assess reliability, readings were repeated 4 weeks later.
Results
CT images permitted visualization of 25 subregions with calcification within and around the tibio-femoral and patello-femoral joints in all 24 knees with radiographic chondrocalcinosis. Intra-articular calcification was seen universally including meniscal cartilage (most common site involved in 21/24 knees), hyaline cartilage, cruciate ligaments, medial collateral ligament and joint capsule. Readings showed good agreement for specific tissues involved with calcium deposition (kappa: 0.70, 95% CI 0.62–0.80).
Conclusion
We found CT scan to be a useful and reliable tool for describing calcium crystal deposition in the knee and therefore potentially for studying role of calcium crystals in OA. We also confirmed that “chondrocalcinosis” is a misnomer because calcification is present ubiquitously.
Keywords: CT imaging, Chondrocalcinosis, Calcium crystals, Knee, Osteoarthritis
INTRODUCTION
Despite knee osteoarthritis (OA) being the most common form of joint disease affecting older adults and a leading cause of lower-extremity disability globally,1,2 no effective disease-modifying pharmacologic therapies are available at this time. This reflects, in part, an incomplete understanding of the underlying pathogenesis of OA. A relatively understudied potential contributor to the disease pathogenesis is intra-articular calcium crystals, which often co-exists with knee OA although their exact role is unclear.3 There are two main types of calcium crystals i.e. calcium pyrophosphate (CPP) and basic calcium apatite (BCP) crystals, which differ in chemical properties, appearance and presentation and potentially in their role in OA.4,5 While one school of thought considers these crystals as “innocent bystanders” or the natural consequence of the joint damage,6 others posit that these crystals play an active role in cartilage destruction by induction of “oxidative stress” through release of inflammatory cytokines and matrix metalloproteases.7 If calcium crystals do in fact contribute to cartilage degeneration in OA, then it would cast them as a novel target for the treatment and prevention of OA.
A major challenge in assessing the role of calcium crystals in OA to date has been the practical imaging modality that provides accurate visualization of intra-articular calcium crystals. Conventional radiography is the most commonly used imaging method for visualization of intra-articular calcium. However, its use is limited because of its poor sensitivity, limiting insights into burden and localization.8 While ultrasonography has been found to be a sensitive tool in identifying cartilage calcification,9 its inability to visualize tissues deep to bone surfaces, and thus inability to visualize some cartilage and soft tissue surfaces (including the cruciate ligaments) is a limitation.10,11 There are also questions about specificity of ultrasound for calcium pyrophosphate crystals.8 Traditional MRI pulse sequences are also limited in sensitivity and in their ability to distinctly identify calcified cartilage from other abnormalities, such as meniscal tears12,13 which may be overcome by using ultrashort-echo-time MR sequence, as shown in a feasibility study.14 To that end, computed tomography (CT) is of interest because it provides highly spatial, 3- dimensional information to identify presence of pathologic processes within scanned anatomical regions with excellent visualization of bones and calcifiedtissues, yet it has not been studied extensively for calcium crystal deposition.15,16
As the burden of OA is rising, the need for understanding the role of depositions of calcium crystals as part of a larger effort to understand underlying pathophysiology of OA is becoming crucial. We therefore undertook this pilot project to examine the utility of CT scans in describing intra-articular calcium crystal deposition, particularly in evaluating the precise tissues involved, among subjects with and without radiographic presence of calcium crystals (chondrocalcinosis).
METHODS
Study sample
All participants in this study were recruited from the Multicenter Osteoarthritis (MOST) Study, a NIH-funded multicenter, longitudinal, observational study of 3026 individuals at baseline, who had or were at high risk for knee OA, recruited from two US centers, Iowa City Iowa and Birmingham, Alabama. Details of the study population have been published elsewhere.17 All participants in the MOST Study had fixed-flexion bilateral knee x-rays at baseline and at each follow-up study visit (30-, 60- and 84-month visits). We included 16 subjects from the Iowa study site who had x-rays obtained at the 84-month (most recent) follow-up visit (5/5/11 to 12/17/12), 12 subjects read by the MOST x-ray readers as having chondrocalcinosis in both knees and 4 with no evidence of chondrocalcinosis in either knee.
Because cartilage loss and consequent joint space narrowing can compromise assessment of intra-articular calcification, we preferentially selected subjects who did not have radiographic OA (Kellgren and Lawrence grade <2)18 to ensure that the joint space was preserved to optimize visualization of calcium deposition of intra-articular tissues. In fact, in a recent study evaluating sensitivity and specificity of CT scans for diagnosing gout, all false positives had advanced OA.19 In our study, out of 32 knees, 6 had definite radiographic OA (KL grade ≥2) and none had a KL grade of 4.
CT scanning
An advance to conventional CT, Dual Energy CT (DECT) was obtained given its availability at the research facility (Department of Radiology, University of Iowa, Iowa city, Iowa) and plans for a future study of comparison of intra-articular calcium crystal vs. urate crystal deposition. All 16 participants underwent CT scanning using a Definition Flash scanner Siemens Healthcare of bilateral knees. For this study, we utilized only the images from 1 of the 2 x-ray guns in the DECT acquisition mode. The scan protocol from the x-ray gun selected used an effective mAs of 45, kV of 140, 0.8 pitch and a rotation speed of 0.285 seconds. The raw projection data were reconstructed using a slice thickness of 0.6mm and a slice interval of 0.3mm with a standard 512 × 512 imaging matirx. Reconstruction diameters (DFOV) were standardized to approximately 15cm for each respective knee data set. The DFOV provided a in-plane resolution 0.3mm (x plane) × 0.3mm (y plane) which corresponded to an isotrophic voxel dimension of 0.3mm × 0.3mm × 0.3mm when using a slice interval of 0.3mm in the z-plane. A high spatial reconstruction kernel of B70 was used to increase the CNR of the anatomical structures. The radiation dose for knee joint for DECT is the same as single energy CT scans, which is 0.15 mSv for one knee,20 compared to 0.001–0.005mSv for knee x-ray, but less than the average annual natural background radiation dose (2.4 mSv).21
A two-step scoring system was devised. First, each knee was divided into 25 subregions. Then, each subregion within a knee was read by a board certified musculoskeletal radiologist with 15 years of experience in semi-quantitative scoring of knee OA features (AG) for presence (yes/no) of calcium crystals separately. The location of the calcium deposition and the shape of the structure in which calcium was deposited made it possible to identify the tissue affected. Readings were repeated after 4 weeks for calculation of intra-rater reliability. We used the axial images but also the coronal and sagittal reformatted images when semi-quantitatively scoring the knees. We performed multiplanar reformats (MPR) in sagittal and coronal planes and smooth reconstruction kernels in order to reduce the image noise. We also created maximum intensity projection (MIP) images.
Statistical Analysis
The intra-rater reliability for presence (yes/no) of intra-articular calcium crystals overall and in specific subregions (hyaline cartilage and meniscal cartilage) between readings 1 and 2 was measured by calculating kappa statistics (95% confidence interval) using SAS 9.3 (SAS Inc., NC).
This study was approved by Institutional Review Board as well as the Radiation Safety committee at University of Iowa, Iowa city, Iowa where subjects were scanned.
RESULTS
In comparison with the participants without radiographic chondrocalcinosis (n=4), those with radiographic chondrocalcinosis (n=12) in this study were older (mean age 72 vs. 64 years) and had lower mean BMI (26kg/m2 vs. 32kg/m2). Seven out of 12 subjects and 3 out of 4 subjects were women in the chondrocalcinosis and non-chondrocalcinosis, respectively. While female predominace has been noted in prior studies of chondrocalcinosis,22 in our study that is not the case likely due to small number of total participants. A history of knee injury was present among 2 subjects with radiographic chondrocalcinosis.
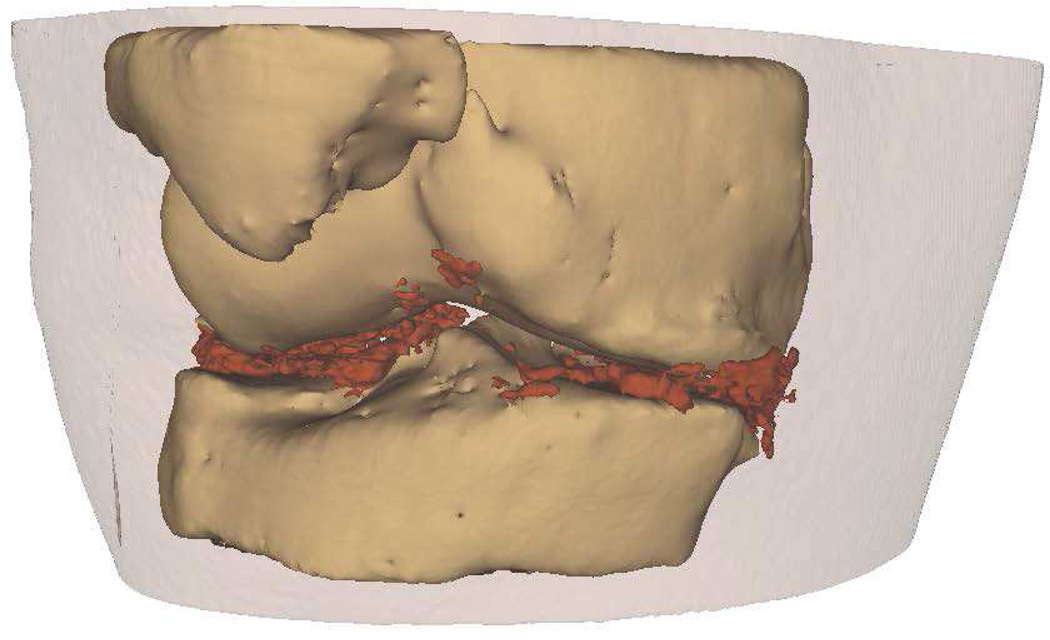
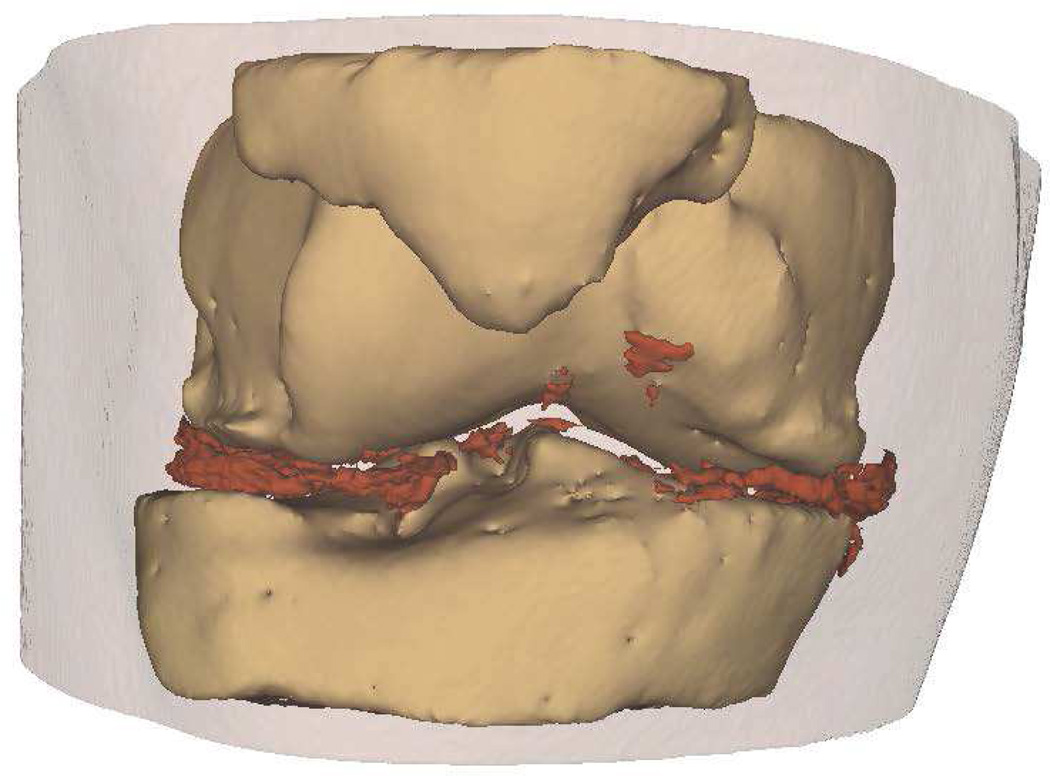
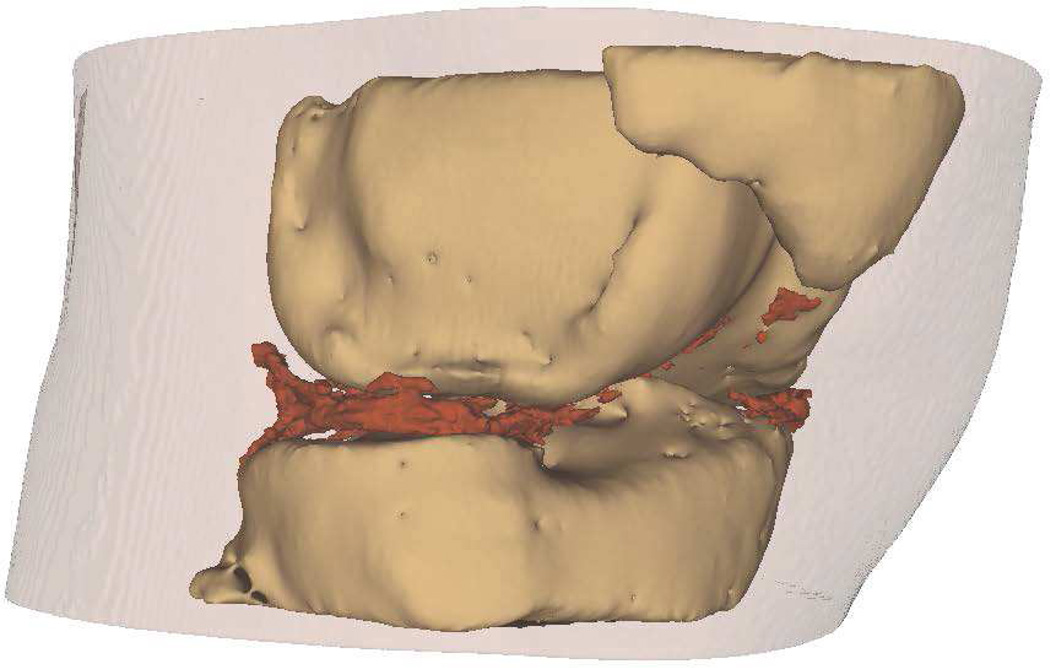
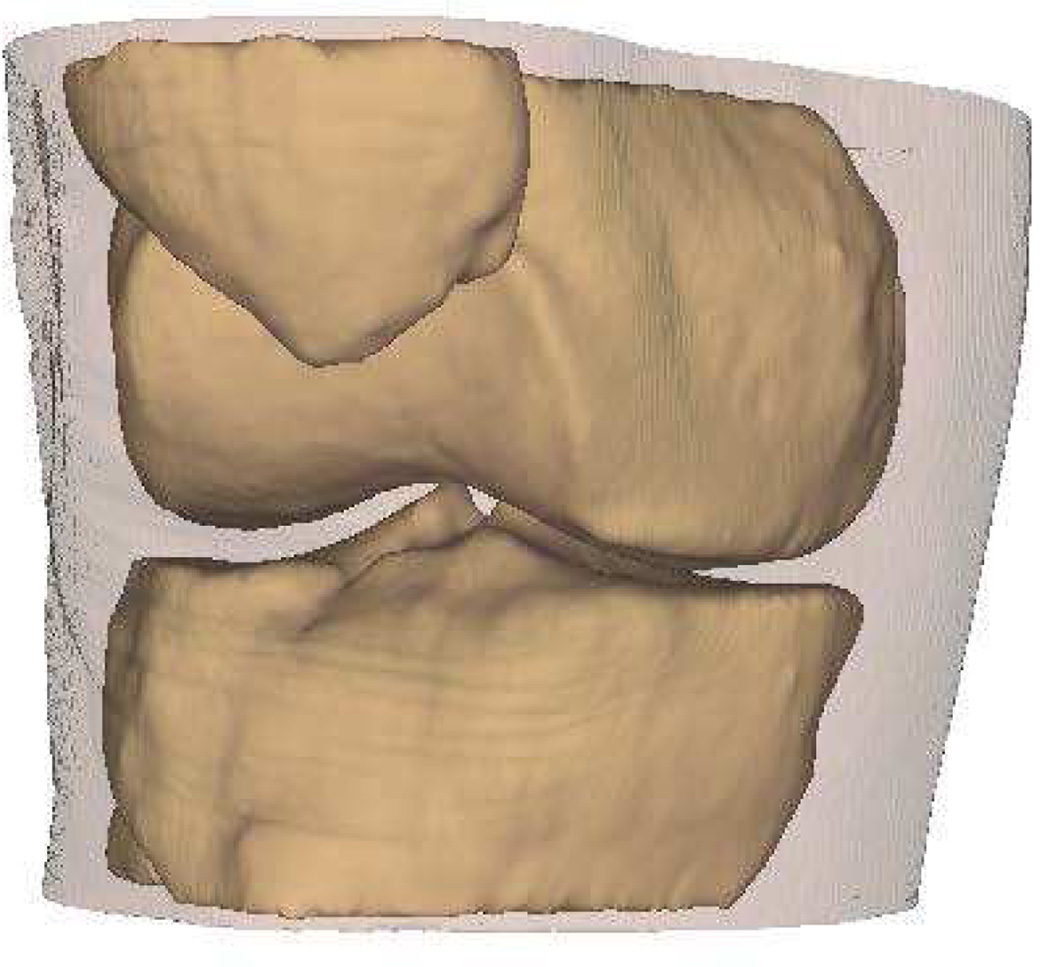
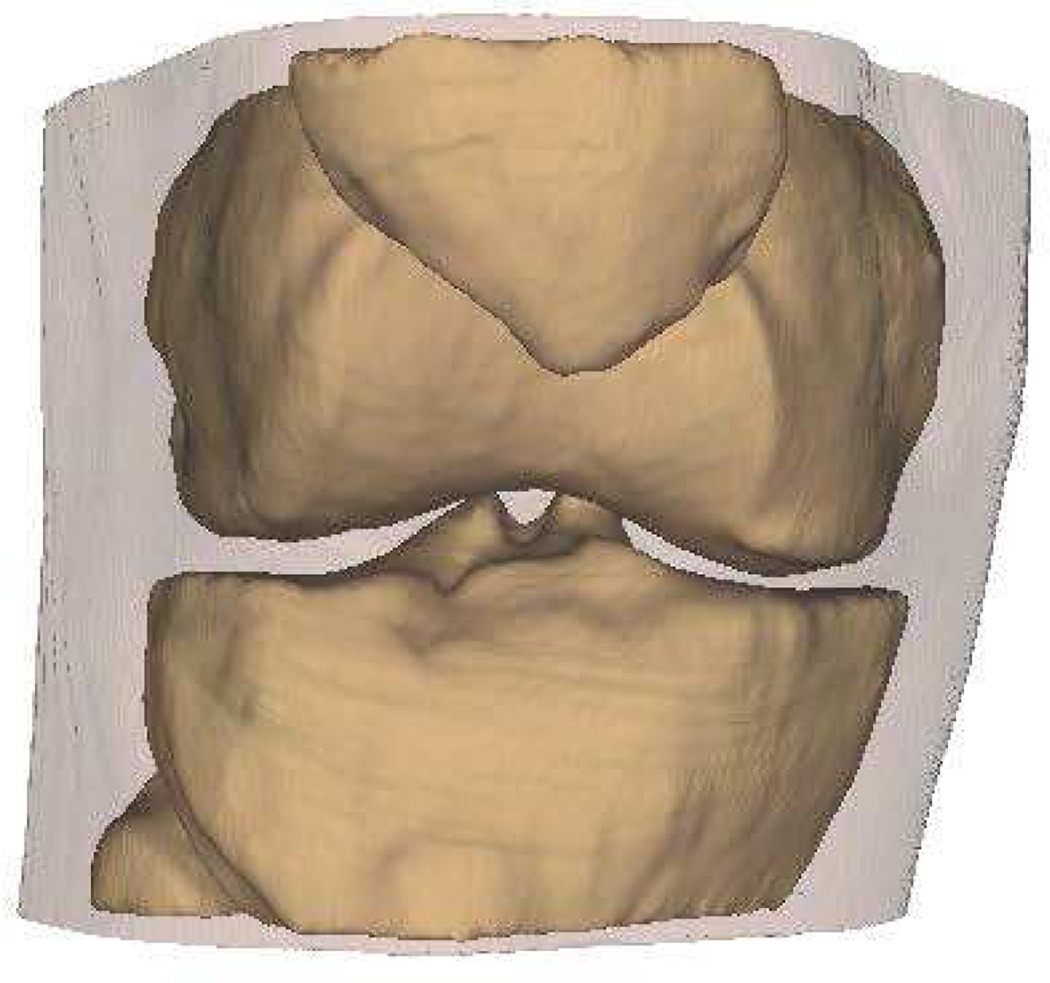
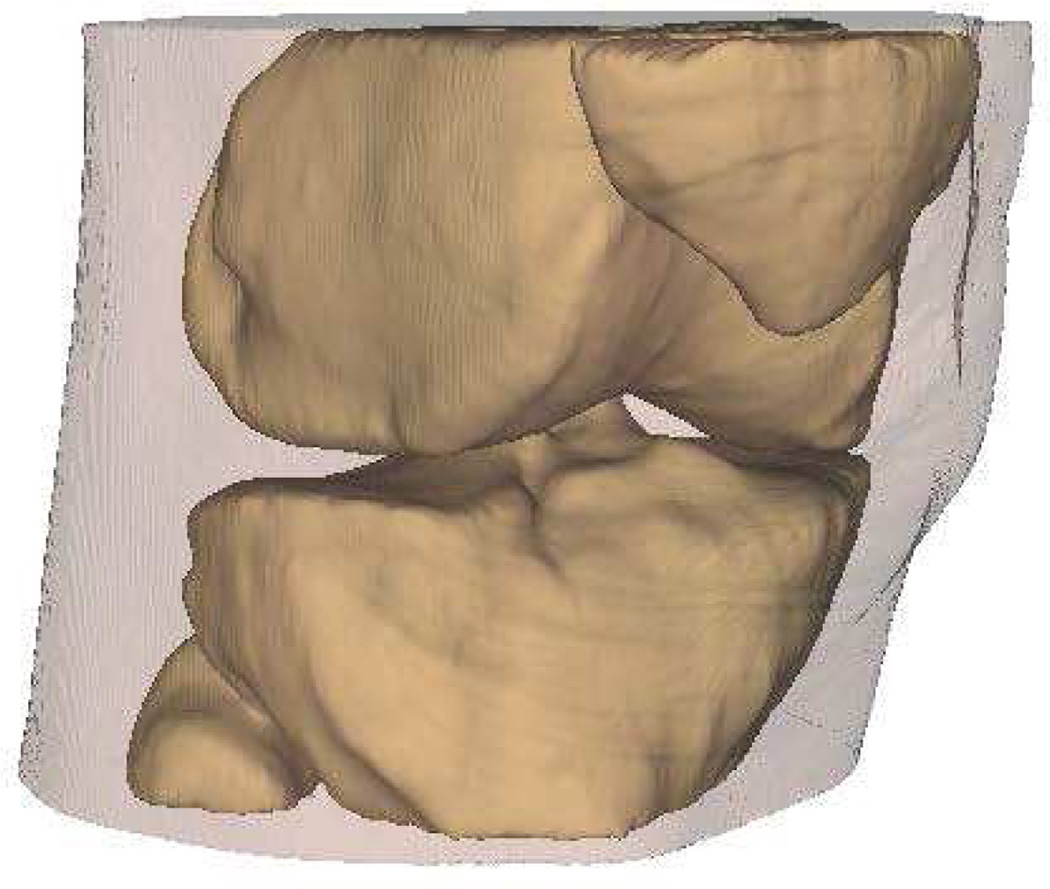
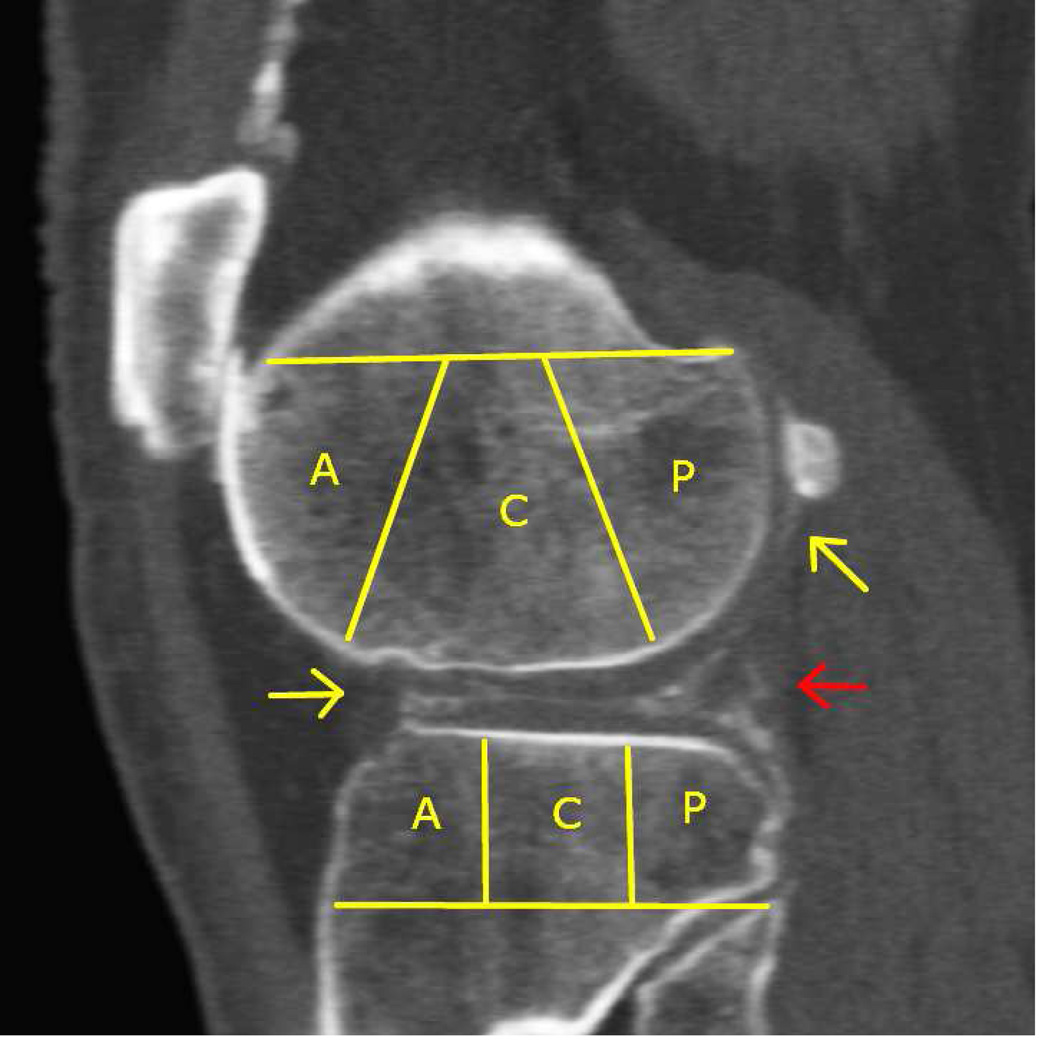
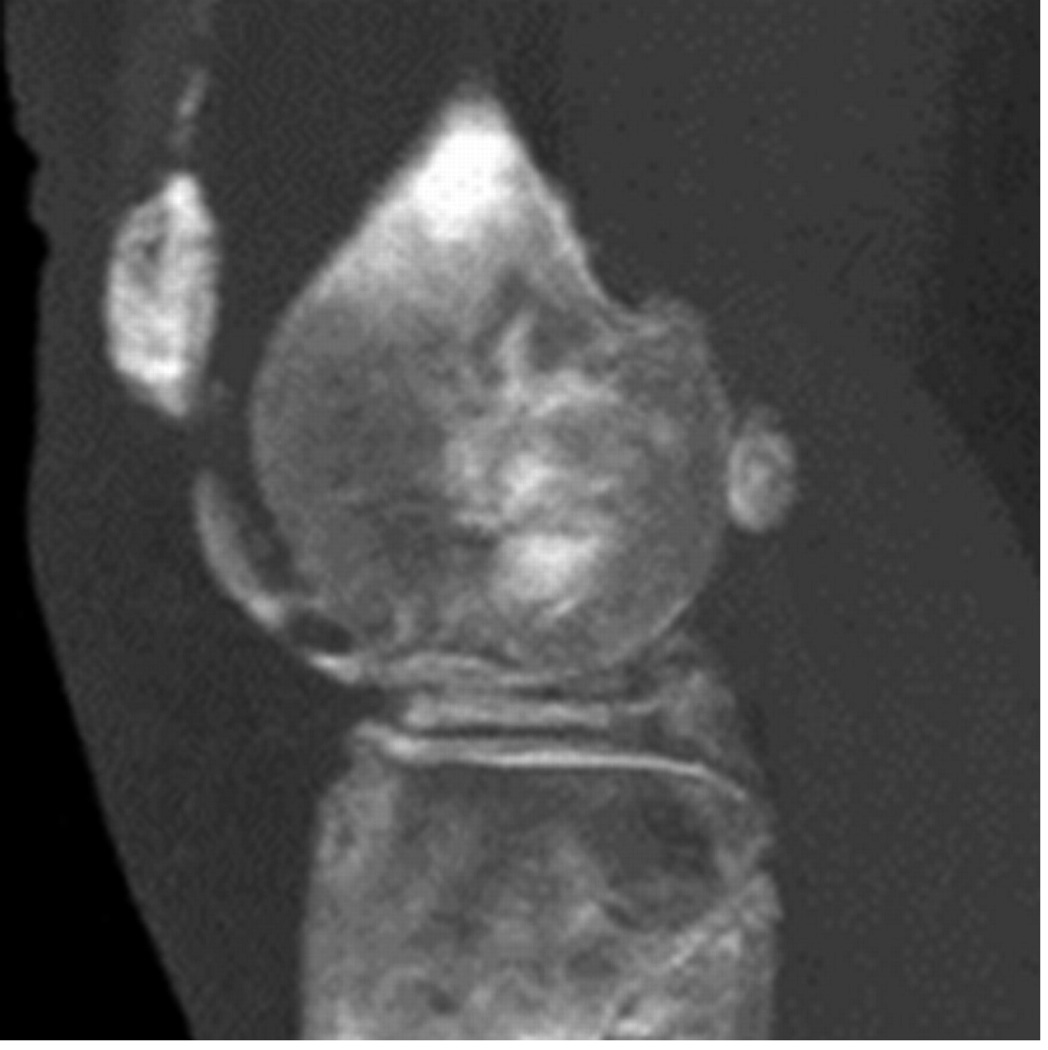
Intra-articular calcium crystals were detected on CT images of all 24 knees (12 subjects) with bilateral radiographic chondrocalcinosis and not detected in any of the 8 knees (4 subjects) without radiographic chondrocalcinosis. CT images provided excellent visualization of intra-articular tissue structures and calcium crystal deposition was noted not only in meniscal and hyaline cartilage but also in deeper structures including cruciate ligaments and joint capsules. In Figure 1, 3-D CT images of knee joint with and without intra-articular calcium deposition is shown. Figure 2 demonstrates calcium crystal deposition in femoral hyaline cartilage, meniscal cartilage and joint capsule in a subject with radiographic chondrocalcinosis. Additional images demonstrating distribution of calcium crystals in cartilage, ligaments and joint capsule are presented in a video as supplementary data.
Figure 1.
[A–C] 3-D CT images of the knee of a subject with chondrocalcinosis show meniscal and chondral calcifications.
[D–F] 3-D CT images of the knee of a subject without chondrocalcinosis show no calcification at the tibio-femoral joint.
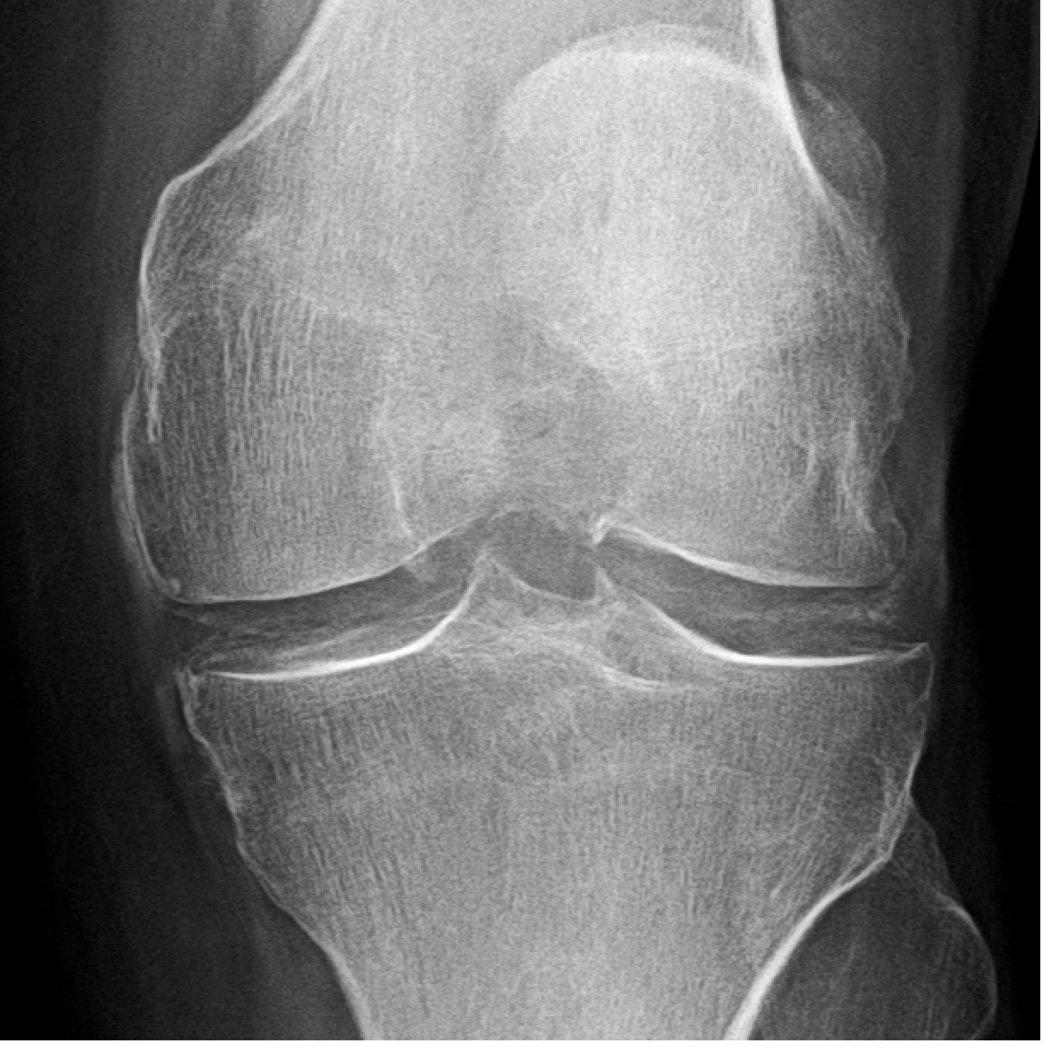
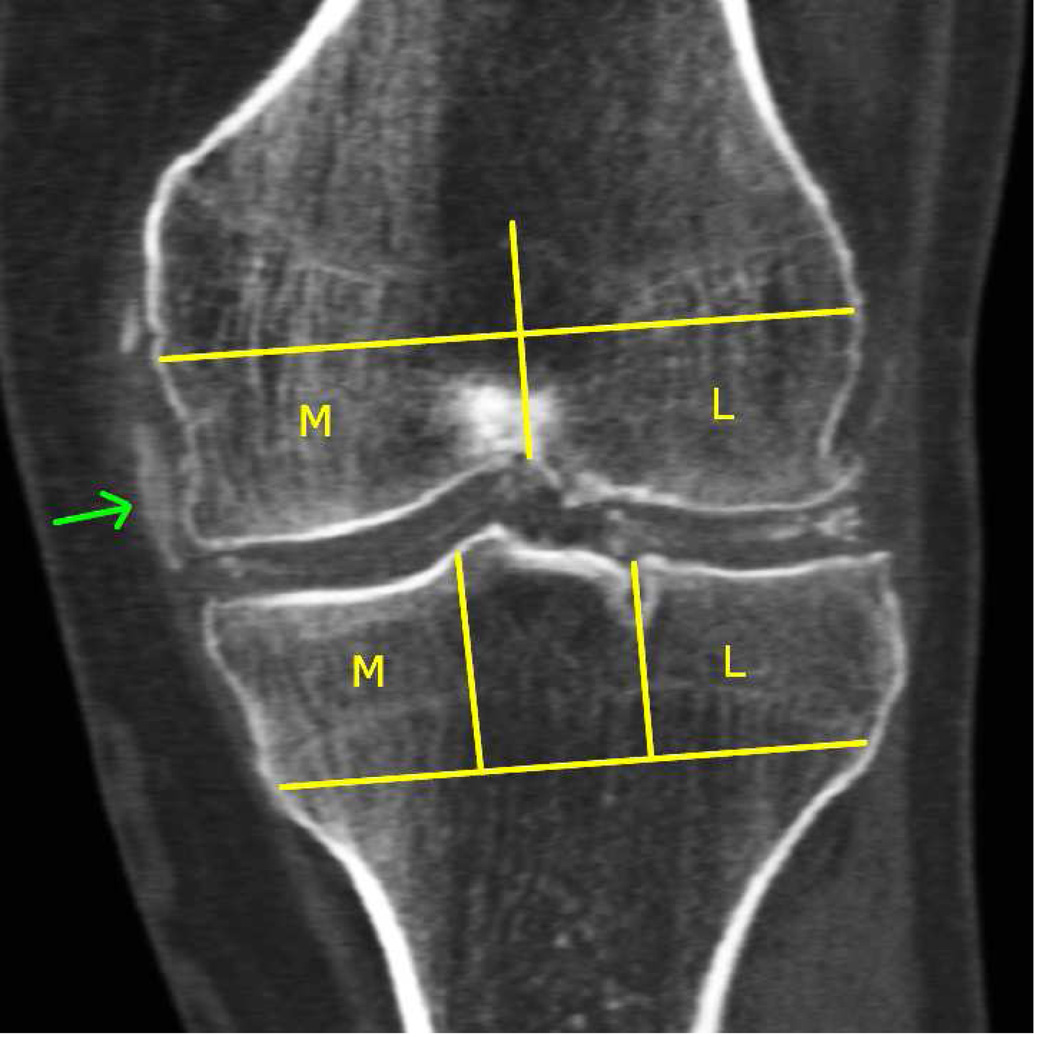
Figure 2.
X-ray depicting chondrocalcinosis in the knee as medial and lateral meniscal calcifications [A]. Coronal [B] and sagittal [C] CT image reformats of the same knee (with segmentation) demonstrating distribution of calcium crystals in the menisci and cartilage. Note the PA x-ray and coronal CT reformat (although a slice) show similar depositions except for deposition in medial capsule and collateral ligament (green arrow). The sagittal CT reformat [C] and maximum-intensity projection [D] provide a more comprehensive picture of deposition location. Arrows show meniscal deposition (red arrow) and hyaline cartilage deposition (yellow arrow) but also in the supra patellar articular capsule.
Menisci were the most commonly involved structures however, hyaline cartilage, cruciate ligaments and joint capsule were also frequently involved (Table1). The intra-rater reliability for overall presence of intra-articular calcium crystals in specific subregions between readings 1 and 2 was excellent (kappa: 0.70, 95% CI 0.62–0.80).
Table 1.
Intra-articular calcium crystal deposition involving specific subregions on CT images among knees with radiographic chondrocalcinosis (N=24)
| Number of knees with presence of calcification at each specific soft tissue site |
|||
|---|---|---|---|
| Tissue Subregions | Reading 1 | Reading 2 | |
| Femur Hyaline Cartilage | |||
| Medial: | |||
| Anterior | 12 | 14 | |
| Central | 14 | 12 | |
| Posterior | 22 | 22 | |
| Lateral: | |||
| Anterior | 8 | 10 | |
| Central | 16 | 14 | |
| Posterior | 13 | 13 | |
| Tibia Hyaline Cartilage | |||
| Medial: | |||
| Anterior | 3 | 2 | |
| Central | 6 | 6 | |
| Posterior | 8 | 5 | |
| Lateral: | |||
| Anterior | 8 | 4 | |
| Central | 12 | 7 | |
| Posterior | 13 | 13 | |
| Patellar Hyaline Cartilage: | |||
| Medial | 12 | 11 | |
| Lateral | 9 | 11 | |
| Meniscus Fibrocartilage | |||
| Medial: | |||
| Anterior | 16 | 17 | |
| Body | 20 | 23 | |
| Posterior | 22 | 23 | |
| Lateral: | |||
| Anterior | 21 | 22 | |
| Body | 22 | 23 | |
| Posterior | 22 | 22 | |
| Ligaments | |||
| ACL | 10 | 10 | |
| PCL | 13 | 19 | |
| MCL | 3 | 3 | |
| LCL | 0 | 0 | |
| Capsule | 15 | 14 | |
DISCUSSION
In this study, we found that CT scan can provide comprehensive assessment of intra articular calcium crystal deposition owing to its MPR ability.23 While there was no discrepancy between x-ray and CT scan in terms of detection of calcification, due to 3-D images, CT enabled detection of calcium crystals in deeper intra-articular structures such as cruciate ligaments and joint capsule which was otherwise not possible by conventional radiography. Further, our study demonstrated excellent intra-rater reliability for detection of calcium crystal deposition at specific subregions within the knee joint.
Our findings have implications. Firstly, our study provides new insight with respect to calcium crystal deposition in subjects with radiographic chondrocalcinosis. We found that in subjects with radiographic chondrocalcinosis, calcification is not limited to cartilage, but rather ubiquitously present, involving ligaments and joint capsules. In that sense, our findings confirm that the term chondrocalcinosis, which refers to cartilage calcification on radiographs, is a misnomer. Secondly, our CT imaging can potentially be used to detect intra-articular calcium crystal deposition in early stages. This is possible in two scenarios: 1) calcification may be present in other intra-articular structures without yet involvement of cartilage; or 2) calcification may have local effects on cartilage without being deposited in sufficient quantity to be detected by plain radiography; and 3) presence of less prolific deposition of calcium crystals on x-ray may appear as osteophytes. Thus, CT may be the ideal imaging method to study role of calcium crystals in OA pathogenesis.
Further, our study extends observations from a recent study using CT imaging for detection of calcium crystal deposition in the knee and its correlation with knee OA in 68 non-embalmed cadaveric knees (mean age 84 years).24 Calcification in meniscus (34% knees) and hyaline cartilage (21% knees) were noted to be common and significantly correlated.24 Since these knees were not selected in advance to have chondrocalcinosis, the extent of calcium deposition was less than our study knees. Unlike our study, calcification of other intra-articular structures was not reported in that study.
While we found advantages of CT scan in describing calcium crystals in and around the joints, we also acknowledge some limitations. One limitation of conventional CT scan is that it may not be able to distinguish between the different types of calcium crystals. Second, when using ionizing radation such as in CT, there is concern for radiation exposure. However, as demonstrated by Biswas et al, radiation exposure in the knee area is not a as much of a concern.20 Sticking with the ALARA principle (As Low As Reasonably Achievable), there are radiation reduction techniques, mainly used for more radiation sensitive areas, are available options in the OA knee protocols as well. Since bone marrow in the knee is not weighted as sensitive as main tissue organs of the body, longitudinal scanning to follow progression of disease can be achieved well below the natural background levels, even considered within the range of standard x-ray. This allows CT to be a safe and powerful modality with the ability to provide comprehensive detection intra-articular calcium crystal deposition. New advances in CT such as automatic exposure control and Iterative reconstruction, have been shown to reduce CT radiation exposure significantly.25 Typically, radiation dose studies like the one by Shin at el. are more focused on organs, i.e. cells with a greater likely hood of cancer development such as breast, orbits, and genital tissues.25 Another study of interest, by Becce at el, compared the use of standard dose using filtered back projection reconstruction, versus a lower dose interative reconstruction method in 40 patient’s having cervical spine CT’s.26 Their findings, similar to Shin, demonstrated a 40% lower exposure savings when using an iterative reconstruction technique over conventional filtered back projection.26
Few limitations of this study need to be acknowledged. First, because this small pilot project is an initial effort to evaluate utility of CT scans in charcatrizing calcium crystal deposition, images were read by a single radiology reader. Thus, while we have demonstrated very good intra-rater reliability, having one more reader would have improved reliability by providing inter-rater reliability. Secondly, we were unable to quantitate the burden of calcium crystals in the knee because given prolific deposition involving multiple tissue structures within the joint, accurate assessment of the volume was difficult with the semiquantitative method used to read the CT scans.
In conclusion, despite the small numbers and other limitations as mentioned above, this pilot study provides valuable insight into potential utility of CT imaging in evaluation of intra-articular calcium crystal deposition and potentially studying role of calcium crystals in OA pathogenesis. Larger studies are needed to confirm our findings, test reliability of the scoring system as well as determine whether quantification of the burden of calcium crystals within the joints is possible using CT scans. Further, advances in CT such as DECT should be evaluated to distinguish different types of calcium crystals in the knee joint, which is not possible by conventional CT scans.
Supplementary Material
ACKNOWLEDGEMENT
We acknowledge Ms. Patricia Feldick at University of Iowa, Iowa City for her help in recruiting subjects for this study; Dr. Sanjay Mudigonda (Radiologist) at Newton-Wellsley hospital, for his help in selecting parameters for the CT scans; and Felix Liu at University of California, San Francisco for segmentation of the CT scan images of knee with and without calcium crystal deposition.
Funding: Dr. Misra is supported by Rheumatology Research Foundation Investigator Award. Support for the Multicenter Osteoarthritis (MOST) Study is by NIA U01-AG18820 (Felson, PI), Nevitt – UO1 AG19069and U01-AG18832 (Torner, PI). This study is also supported by AR47785 (MRCR P60). Dr. Neogi is supported by AF Innovative Research grant, K23 AR055127 and R01AR062506-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: All authors included on the paper as coauthors fulfil the criteria of authorship.
Competing interests: Ali Guermazi is shareholder of Boston Imaging Core Lab, LLC and Consultant to TissueGene, MerckSerono and Sanofi-Aventis. Others have no conflict of interest.
Contributor Information
Devyani Misra, Email: demisra@bu.edu.
Ali Guermazi, Email: guermazi@bu.edu.
Jered P. Sieren, Email: jered-sieren@uiowa.edu.
John Lynch, Email: JLynch@psg.ucsf.edu.
James Torner, Email: Torner@uiowa.edu.
Tuhina Neogi, Email: tneogi@bu.edu.
David T. Felson, Email: DFelson@bu.edu.
References
- 1.Centers for Disease Control and Prevention. Prevalence of disabilities and associated health conditions among adults: United States, 1999. MMWR Morb Mortal Wkly Rep. 2001:120–125. [PubMed] [Google Scholar]
- 2.Vos T, Flaxman A, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derfus BA, Kurian JB, Butler JJ, Daft LJ, Carrera GF, Ryan LM, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29:570–574. [PubMed] [Google Scholar]
- 4.Ea H, Liote F. Advances in understanding calcium-containing crystal disease. Current Opinion in Rheumatology. 2009;21:150–157. doi: 10.1097/BOR.0b013e3283257ba9. [DOI] [PubMed] [Google Scholar]
- 5.Wise C. Crystal-associated arthritis in the elderly. Rheum Dis Clin North Am. 2007;33:33–55. doi: 10.1016/j.rdc.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Mitsuyama H, Healey RM, Terkeltaub RA, Coutts RD, Amiel D. Calcification of human articular knee cartilage is primarily an effect of aging rather than osteoarthritis. Osteoarthritis Cartilage. 2007;15:559–565. doi: 10.1016/j.joca.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ea HK, Nguyen C, Bazin D, Bianchi A, Guicheux J, Reboul P, et al. Articular cartilage calcification in osteoarthritis: insights into crystal-induced stress. Arthritis Rheum. 2011;63:10–18. doi: 10.1002/art.27761. [DOI] [PubMed] [Google Scholar]
- 8.Ellabban AS, Kamel SR, Omar HA, El-Sherif AM, Abdel-Magied RA. Ultrasonographic diagnosis of articular chondrocalcinosis. Rheumatol Int. 2012;32:3863–3868. doi: 10.1007/s00296-011-2320-1. [DOI] [PubMed] [Google Scholar]
- 9.Barskova VG, Kudaeva FM, Bozhieva LA, Smirnov AV, Volkov AV, Nasonov EL. Comparison of three imaging techniques in diagnosis of chondrocalcinosis of the knees in calcium pyrophosphate deposition disease. Rheumatology (Oxford) 2013 doi: 10.1093/rheumatology/kes433. [DOI] [PubMed] [Google Scholar]
- 10.Foldes K. Knee chondrocalcinosis: an ultrasonographic study of the hyalin cartilage. Clin Imaging. 2002;26:194–196. doi: 10.1016/s0899-7071(01)00385-0. [DOI] [PubMed] [Google Scholar]
- 11.Sofka CM, Adler RS, Cordasco FA. Ultrasound diagnosis of chondrocalcinosis in the knee. Skeletal Radiol. 2002;31:43–45. doi: 10.1007/s002560100434. [DOI] [PubMed] [Google Scholar]
- 12.Disler DG, McCauley TR, Kelman CG, Fuchs MD, Ratner LM, Wirth CR, et al. Fat-suppressed three-dimensional spoiled gradient-echo MR imaging of hyaline cartilage defects in the knee: comparison with standard MR imaging and arthroscopy. AJR Am J Roentgenol. 1996;167:127–132. doi: 10.2214/ajr.167.1.8659356. [DOI] [PubMed] [Google Scholar]
- 13.Burke BJ, Escobedo EM, Wilson AJ, Hunter JC. Chondrocalcinosis mimicking a meniscal tear on MR imaging. AJR Am J Roentgenol. 1998;170:69–70. doi: 10.2214/ajr.170.1.9423602. [DOI] [PubMed] [Google Scholar]
- 14.Omoumi P, Bae W, Du J, Diaz E, Statum S, Bydder G, et al. Meniscal Calcifications: Morphologic and Quantitative Evaluation by using 2D Inversion-Recovery Ultrashort Echo Time and 3D Ultrashort Echo Time 3.0-T MR Imaging Techniques—Feasibility Study. Radiology. 2012;264:260–268. doi: 10.1148/radiol.12111439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saparin P, Thomsen JS, Kurths J, Beller G, Gowin W. Segmentation of bone CT images and assessment of bone structure using measures of complexity. Med Phys. 2006;33:3857–3873. doi: 10.1118/1.2336501. [DOI] [PubMed] [Google Scholar]
- 16.Subhawong TK, Fishman EK, Swart JE, Carrino JA, Attar S, Fayad LM. Soft-tissue masses and masslike conditions: what does CT add to diagnosis and management? AJR Am J Roentgenol. 2010;194:1559–1567. doi: 10.2214/AJR.09.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felson DT, Niu J, Yang T, Torner J, Lewis CE, Aliabadi P, et al. Physical activity, alignment and knee osteoarthritis: data from MOST and the OAI. Osteoarthritis Cartilage. 2013;21:789–795. doi: 10.1016/j.joca.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bongartz T, Glazebrook KN, Kavros SJ, Murthy NS, Merry SP, Franz WB, 3rd, et al. Dual-energy CT for the diagnosis of gout: an accuracy and diagnostic yield study. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-205095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91:1882–1889. doi: 10.2106/JBJS.H.01199. [DOI] [PubMed] [Google Scholar]
- 21.Report of the United Nations Scientific Committee on the Effects of Atomic Radiation to the General Assembly. [accessed 4 August 2008];2000 http://www.unscear.org/docs/reports/gareport.pdf. [Google Scholar]
- 22.Choi MH, MacKenzie JD, Dalinka MK. Imaging features of crystal-induced arthropathy. Rheum Dis Clin North Am. 2006;32:427–446. viii. doi: 10.1016/j.rdc.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Freire V, Becce F, Feydy A, Guerini H, Campagna R, Allanore Y, et al. MDCT imaging of calcinosis in systemic sclerosis. Clin Radiol. 2013;68:302–309. doi: 10.1016/j.crad.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Touraine S, Ea HK, Bousson V, Cohen-Solal M, Laouisset L, Chappard C, et al. Chondrocalcinosis of femoro-tibial and proximal tibio-fibular joints in cadaveric specimens: a high-resolution CT imaging study of the calcification distribution. PLoS One. 2013;8:e54955. doi: 10.1371/journal.pone.0054955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin HJ, Chung YE, Lee YH, Choi JY, Park MS, Kim MJ, et al. Radiation dose reduction via sinogram affirmed iterative reconstruction and automatic tube voltage modulation (CARE kV) in abdominal CT. Korean J Radiol. 2013;14:886–893. doi: 10.3348/kjr.2013.14.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becce F, Ben Salah Y, Verdun FR, Vande Berg BC, Lecouvet FE, Meuli R, et al. Computed tomography of the cervical spine: comparison of image quality between a standard-dose and a low-dose protocol using filtered back-projection and iterative reconstruction. Skeletal Radiol. 2013;42:937–945. doi: 10.1007/s00256-013-1576-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.