Abstract
Cadherin plays an important role in the toxicity of Bacillus thuringiensis Cry proteins. We previously cloned a full-length cadherin from Aedes aegypti larvae and reported this protein binds Cry11Aa toxin from B. thuringiensis subsp. israelensis with high affinity, ≈ 16.7 nM. Based on these results, we investigated if Aedes cadherin is involved in the in vivo toxicity of Cry11Aa toxin to Ae. aegypti. We established a mosquito cell line stably expressing the full-length Aedes cadherin and transgenic mosquitoes with silenced Aedes cadherin expression. Cells expressing the Aedes cadherin showed increased sensitivity to Cry11Aa toxin. Cry11Aa toxin at 400 nM killed approximately 37% of the cells in 3 h. Otherwise, transgenic mosquitoes with silenced Aedes cadherin expression showed increased tolerance to Cry11Aa toxin. Furthermore, cells expressing Aedes cadherin triggered Cry11Aa oligomerization. These results show the Aedes cadherin plays a pivotal role in Cry11Aa toxicity to Ae. aegypti larvae by mediating Cry11Aa oligomerization. However, since high toxicity was not obtained in cadherin-expressing cells, an additional receptor may be needed for manifestation of full toxicity. Moreover, cells expressing Aedes cadherin were sensitive to Cry4Aa and Cry11Ba but not Cry4Ba. However transgenic mosquitoes with silenced Aedes cadherin expression showed no tolerance to Cry4Aa, Cry4Ba, and Cry11Ba toxins. These results suggest that while Aedes cadherin may mediate Cry4Aa and Cry11Ba toxicity, this cadherin but is not the main receptor of Cry4Aa, Cry4Ba and Cry11Ba toxin in Ae. aegypti.
Keywords: Bacillus thuringiensis, Cry11Aa toxin, cadherin, C6/36 cell, transgenic mosquito, oligomerization, cytotoxicity
1. Introduction
Unlike other members of the Bacillus cereus group, B. thuringiensis is pathogenic to insects by producing insecticidal proteins, which consists of one or more proteins, called Cry or Cyt toxins [21]. As these proteins are highly selective to the target insect, and harmless to humans and vertebrates, this species has been used for the control of insect pests in agriculture and public health [14].
One subsp., B. thuringiensis subsp. israelensis (Bti), has been used for the control of insect vectors of human diseases, including Simulium damnosum, a vector of onchocerciases, and Aedes and Culex mosquito species, that can be vectors for dengue fever, chikungunya and yellow fever, and filariasis and West Nile fever, respectively [33]. These control programs are possible because Bti, while having high insecticidal activity, has low toxicity to non-target organisms. Consequently, it is an important alternative and environmental-friendly method for control of mosquito and black fly populations.
While Bti has high activity, its mechanism of action is still poorly understood. The bacterium has a megaplasmid, pBtoxis, which encodes a number of toxins (Cry4Aa, Cry4Ba, Cry10Aa, Cry11Aa, Cyt1Aa, Cyt1Ca and Cyt2Ba) [4]. Among them, Cry11Aa is one of the more active toxins towards Ae. aegypti [11]. Fernandez et al. [19] reported domain II of Cry11Aa is important in receptor recognition and binding. Domain II contains four putative loop regions, α-8, 1, 2 and 3. Using competitive binding assays, peptide-displaying phages and mutagenesis, it was revealed that loop α-8 in Cry11Aa was involved in toxicity and receptor binding.
Many putative Cry toxin receptors have been identified in mosquitoes [31]. An aminopeptidase N (APN) from Anopheles quadrimaculatus bound Cry11Ba, and a cadherin receptor from An. gambiae was identified and bound Cry4Ba [1, 26, 39]. In Ae. aegypti, Cry11Aa bound four proteins (200, 100, 65 and 62 kDa) in brush border membrane vesicles (BBMV) isolated from Ae. aegypti midgut epithelia [18]. Among them, the 65 kDa protein was identified as a glycosylphosphatidyl-inositol (GPI)-anchored alkaline phosphatase (ALP) and was a functional receptor of Cry11Aa toxin in Ae. aegypti midgut cells. The 100 kDa protein was identified as an APN and two of these have been characterized [9, 10]. In a previous study, we showed that the Aedes cadherin (AAEL007478 and AAEL007488), which is homologous to the lepidopteran Bt-R1 and mediates Cry1A toxicity in Lepidoptera, bound Cry11Aa with high affinity [8]. This finding suggests that the cadherin is associated with the insecticidal activity of the Cry11Aa toxin.
Based on these results, we investigated further whether Aedes cadherin mediates Cry11Aa toxicity in vivo. We established a cell line expressing Aedes cadherin and a transgenic mosquito line that silences Aedes cadherin. We determined the ability of the Cry11Aa protein to cause cytotoxicity with cells expressing Aedes cadherin, and showed that the transgenic mosquitoes showed increased tolerance to Cry11Aa toxicity.
2. Materials and Methods
2.1 Cell culture
C6/36 (Aedes albopictus) cells were grown and maintained in L15 medium (Gibco, Grand Island, NY) supplemented with 10% FBS (Gibco), 1% L-glutamine (Gibco) and 1% penicillin-streptomycin (Gibco) at 27°C. The cells were grown as a monolayer in T-25 culture flasks (BD Falcon, Franklin Lakes, NJ), 6-well tissue culture plates, or 96-well tissue culture plates (Corning, Tewksbury, MA).
2.2 Construction of cadherin in pACTIN.SV
The pACTIN.SV and pIE1.SV vectors used, including enhanced GFP gene ORF (EGFP) (Fig. 1B), were obtained from Drs. Huynh and Zieler (National Institutes of Health, Bethesda, MD) [27].
Fig. 1.
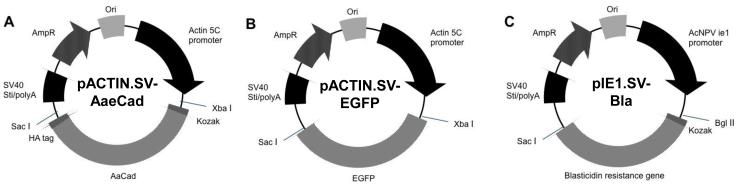
Construction of expression vectors. (A) pACTIN.SV-AaeCad. Modified Aedes cadherin (AaeCad) cDNA from Ae. aegypti larvae of 5301 bp in length was cloned into pACTIN.SV vector. (B) pACTIN.SV-EGFP. The pACTIN.SV vector, including the EGFP gene, was used for selection of a control cell line. (C) pIE1.SV-Bla. A blasticidin-resistance sequence (bla gene) from the pCoBlast vector was cloned into pIE1.SV vector. To make a stable cell line, pACTIN.SV-AaeCad and pIE1.SV-Bla / pACTIN.SV-EGFP and pIE1.SV-Bla were co-transfected into C6/36 (Aedes albopictus) cells and then cells were grown and maintained in fresh medium containing blasticidin.
A full-length Aedes cadherin cDNA (AaeCad) cloned into pCR2.1 vector was previously reported [8]. To remove the 5′ and 3′ UTRs, partial AaeCad fragments (5EM and 3EM) were prepared with a set of primers (Supplementary Table S1). The 5′ end modified fragment (5EM) was amplified using a sense primer (5EM-S), which contained the restriction enzyme sites NotI and StuI, a Kozak sequence (CCACC) and a start codon, and an antisense primer (5EM-A) with a restriction enzyme site, Bstz17I. The 3′ end modified fragment (3EM) was amplified using a sense primer (3EM-S), containing a BlpI restriction enzyme site and an antisense primer (3EMA), which had a HA-tag (TACCCATACGACGTCCCAGACTACGCT), a stop codon, and the restriction sites NheI, PmeI and SacI. All PCR products were cloned into the pCR2.1 vector (Invitrogen, Grand Island, NY) and fully sequenced (Institute for Integrative Genome Biology, University of California, Riverside, CA). To construct the modified AaeCad, the 5EM and AaeCad were each digested with NotI and Bstz17I (New England Biolabs, Ipswich, MA), the fragments then gel-purified and ligated. Next, this construct and the 3EM were digested with BlpI and SacI, gel-purified, and ligated. To construct an expression vector, the modified AaeCad in pCR2.1 vector and pACTIN.SV vector were separately digested with XbaI and SacI, gel-purified and ligated (Fig. 1A).
The blasticidin-resistance gene (bla) from pCoBlast vector (Invitrogen) was amplified using a sense primer (BLA-S), with a restriction enzyme site (BglII), a Kozak sequence, and a start codon, and an antisense primer (BLA-A), which had a stop codon and a restriction enzyme site (PmlI). To construct an antibiotic selection vector to be used for co-expression, the Bla gene and pIE1.SV were separately digested with BglII and PmlI, gel-purified, and then ligated (Fig. 1C).
2.3 Cell transfection
To construct a stable cell line expressing Aedes cadherin, pACTIN.SV containing the cloned AaeCad (5 μg) together with pIE1.SV containing a blasticidin-resistance gene (1 μg) for selection, were co-transfected into C6/36 cells using the FuGENE6 transfection reagent (Roche Applied Science, Madison, WI) following the manufacturer’s protocol. Another stable C6/36 cell line expressing GFP was made using pACTIN.SV containing an EGFP gene ORF (5 μg) together with pIE1.SV containing a blasticidin-resistance gene (1 μg), using identical conditions. In brief, 1.5 × 105 C6/36 cells/well in a 6-well plate were incubated overnight at 27°C. To these cells the plasmid transfection mixtures in FuGENE 6 transfection reagent were added and the cells incubated for an additional 3 days at 27°C. Media was removed and replaced with fresh medium containing blasticidin (15 μg), and a week later, the media was replaced with media with additional blasticidin (7.5 μg). The selecting media was replaced every 3-4 days until cell colonies were observed. Colonies were picked, diluted, and transferred to 96-well plates in order to obtain a single cell in each well. These single cells were grown and maintained in the fresh medium containing blasticidin (7.5 μg) at 27°C. Homogeneous cell lines were analyzed by western blotting to find a cell line highly expressing AaeCad.
2.4 Western blotting with cells
Cells expressing AaeCad or EGFP were harvested, washed with phosphate buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH7.4) twice and resuspended in SDS-PAGE sample buffer. The collected cells were boiled for 10 min and centrifuged at 10,000×g for 10 min to remove insoluble material. The supernatants were loaded in SDS polyacrylamide gel (10%), and then electrotransferred to nitrocellulose membrane. The membrane was blocked with blocking solution (PBS, 5% skim milk and 0.1% Tween-20) for 1 h at room temperature, and washed with PBST (PBS and 0.1% Tween-20). The blocked membrane was incubated with an anti-HA antibody (a rabbit polyclonal IgG, Santa Cruz Biotechnology, Dallas, TX) or an anti-cadherin fragment antibody detecting AaeCad CR7-11 [8] (1:3,000) overnight at 4°C. The membrane was washed with PBST, and then subsequently incubated with anti-rabbit horseradish peroxidase (HRP, 1:5,000) secondary antibody (Sigma, St. Louis, MO) for 90 min at room temperature. After washing with PBST, the HRP activity was revealed with a luminal substrate (Thermo Scientific, Lafayette, CO) and exposed to an X-ray film in a darkroom.
2.5 Immunolocalization of AaeCad
C6/36 cells (1 × 105 cells) were plated in a slide chamber (Fisher, Hampton, NH) and incubated overnight at 27°C, then washed three times with PBS, and then fixed in 4% paraformaldehyde in PBS for 1 h at room temperature. The fixed cells were treated with a blocking solution (PBS, 2% BSA) for 1 h at room temperature. The cells were then incubated with anti-cadherin fragment antibody (1:200 dilution) in PBS containing 1% BSA for 1 h at room temperature, washed three times with washing buffer (PBS, 0.1% BSA, and 2% goat serum), and incubated with Cy3-conjugated goat anti-rabbit IgG (1:1000, Jackson Immuno Research, West Grove, PA) in PBS containing 0.1% BSA and 2% goat serum for 1 h at room temperature in dark condition. The fluorescence was observed using Zeiss 510 confocal microscope (Institute for Integrative Genome Biology).
2.6 Purification of Cry toxins
For crude Cry toxin production, B. thuringiensis strains producing Cry4Aa, Cry4Ba, Cry11Aa, or Cry11Ba [7, 15], were grown in nutrient broth sporulation medium containing erythromycin (25 μg/ml) at 30°C for 4-5 days for cell autolysis to occur [30]. Spores and crystal inclusions were harvested 10,000xg for 10 min at 4°C, washed twice with sterilized water, and stored in water at −80°C until used.
For purified Cry toxins, the inclusion bodies for Cry toxins were isolated as previous reported [13]. The purified inclusions were washed, solubilized in 50 mM Na2CO3 pH 10.5 buffer, and then activated by trypsin (1:20 w/w) at 37°C. Solubilized Cry toxins were quantified using the BCA assay (Pierce, Rockford, IL). Activated Cry toxins were filtered using 0.2 μm syringe filter and stored at −80°C until needed.
2.7 Cytotoxicity test with Cry toxins
The toxicity of Cry toxins to C6/36 cells expressing AaeCad or EGFP was analyzed by using a MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) cytotoxicity assay [12]. In brief, 6 × 104 cells were plated in each well of a 96-well cell culture dish and incubated overnight at 27°C. The plate was centrifuged at 1,000xg for 5 min. The medium was removed and replaced with the fresh medium containing the activated Cry toxins (final concentration 50 – 400 nM) and the cells were incubated for 3 h at 27°C. After centrifugation at 1,000xg for 5 min, the medium was removed and replaced with a mixture of 200 μl fresh medium and 20 μl MTT solution (5 mg/ml MTT in PBS), and the cells were incubated for 2 h at 27°C. The plate was centrifuged at 1,000xg for 5 min and the medium was removed. Isopropanol-HCl-SDS solution (120 μl) was added to each well and the absorbance was read at 570 nm with a microplate reader (Molecular Devices, Sunnyvale, CA).
2.8 Cry11Aa toxin oligomerization
The oligomerization of Cry11Aa was performed by incubation with cells expressing AaeCad or EGFP [34, 35]. The activated Cry11Aa toxin (final concentration 400 nM) or the mixture of Cry11Aa protoxin (final concentration 1,400 nM) and midgut juice from Ae. aegypti larvae were incubated with 1 × 106 cells in 100 μl L15 medium for 1 h at 37°C. The mixture of Cry11Aa and cells was harvested at 500xg for 10 min and washed with PBS twice. The pellets were resuspended in SDS-PAGE sample buffer, boiled for 10 min, and centrifuged at 10,000xg for 10 min. The supernatants were loaded in SDS polyacrylamide gel (10%) and immunoblotted with anti-Cry11Aa antibody as noted above.
2.9 Construction of transformation vector and transgenic mosquitoes
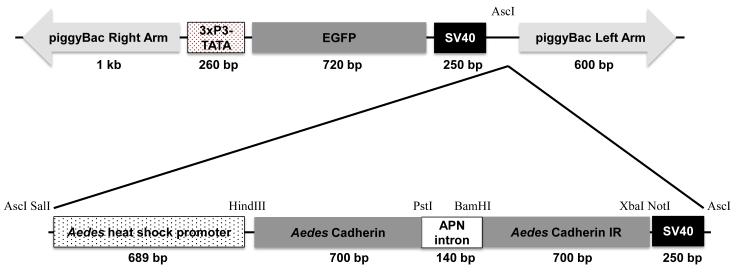
The pBAC[3xP3-EGFP afm] transformation vector containing 3xP3 (eye-specific promoter), TATA box, and enhanced GFP [24] and the phsp-pBac helper plasmid [23] were obtained from Drs. Alexander Raikhel and Vladimir Kokoza, Department of Entomology, University of California, Riverside, CA. The hairpin dsRNA construct of AaeCad was made by using an Aedes heat shock promoter (689 bp), a cadherin (700 bp, AAEL007478) cadherin invert repeat having an APN intron (140 bp, AAEL008155), and SV40 (250 bp), and cloned into pBAC[3xP3-EGFP afm] transformation vector in GenScript company, Piscataway, NJ (Fig. 6).
Fig. 6.
Construction of the mosquito transformation vector. The hairpin dsRNA construct of Aedes cadherin was made by arranging Aedes heat shock promoter, Aedes cadherin, APN, Aedes cadherin inverted repeat (IR), and SV40. This construct was cloned into pBAC[3xP3-EGFP afm] transformation vector containing 3xP3 (eye-specific promoter), TATA box, and enhanced GFP.
The mixture of pBAC[3xP3-EGFP afm, AaeCad dsRNA] and phsp-pBac helper plasmid were injected into Ae. aegypti embryos (Insect Transformation Facility, University of Maryland, Rockville, MD). G0 eggs were hatched and reared with a mixture of dog food and yeast (3:1) in deoxygenated tap water at 29°C, 8:12 h light:dark, and 50% humidity. Adult G0 were backcrossed with wild-type females or males and G1 progeny were screened for green fluorescent eye using fluorescence microscopy (Nikon SMZ1500). Homozygous larvae were obtained by subsequently mating individual male and female showing green-fluorescent eyes until all progeny larvae contained green-fluorescent eyes. To express the hairpin dsRNA of AaeCad regulated by the Aedes heat shock promoter in larvae, 2nd instar wild-type and transgenic larvae were incubated for 1 h at 37°C.
Bioassays were performed with crude Cry4Aa, Cry4Ba, Cry11Aa, and Cry11Ba. In brief, 20 early fourth-instar larvae were transferred to plastic cups containing 200 ml tap water and then fed Cry toxins at different concentration for 24 h. Bioassays were performed twice and the dose-response values were analyzed by probit (EPA) or Origin program (Origin Lab, Northampton, MA).
2.10 Quantitative Real-time PCR (qPCR)
Total RNA was extracted from wild-type or transgenic larvae using TRIzol. cDNA was synthesized from total RNA of each sample with SuperScript III (Invitrogen), diluted, and 5-μl aliquots were used as template for qPCR. Respective primers specific to AaeCad (AaeCad-S and AaeCad-A, AAEL007478) and Actin (Actin-S and Actin-A, AAEL011197) or 40S ribosomal protein S7 (S7-S and S7-A, AAEL009496) as reference genes for quantification were designed to have similar properties in terms of nucleotide length and %GC content (Supplementary Table S1). Our microarray data showed the actin expression (AAEL011197) and 40S ribosomal protein S7 (AAEL009496) were not changed (−0.05, −0.06. or −0.07 / 0.17, −0.08, or 0.09 fold) in Cry11Aa-treated larvae midgut at LC10, LC50, LC90 compared to untreated larvae midgut. PCR conditions, including the template cDNA, primer concentrations and annealing temperatures, were adjusted for amplification efficiencies (Efficiency 90 – 110%) for all genes. Optimized PCR master mix (20 μl) contained the following components: 10 μl iQ SYBR Green supermix (Bio-Rad, Irvine, CA), 5 μl cDNA, 2 μl 10 μM of each primer, and 1 μl water. The qPCR was performed using CFX Real-time PCR (Bio-Rad). Optimized thermal program consisted of: one cycle of 95°C/1 min and 40 cycles of 95°C/1 min, 62°C/1 min, and 72°C/1 min, followed by a final extension of one cycle 72°C /5 min. Following qPCR, the homogeneity of the PCR product was confirmed by melting curve analysis. To check for gDNA contamination in RNA preparation, the melting curve of 40S ribosomal protein S7 using primers that spanned a 114-bp intron was analyzed on every run. Quantification of the transcript levels or relative copy number of the genes was conducted according to the Pfaffl method [37]. Quantitative PCR was performed three times using independently prepared whole body cDNA.
3. Results
3.1 AaeCad are stably expressed in cells
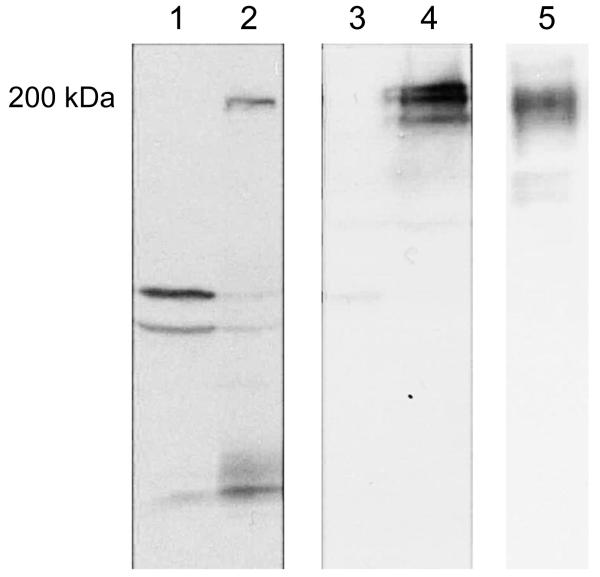
A stable cell line expressing the Aedes cadherin under control of the actin promoter was obtained using a co-expressed selection vector. Analysis of these cells by western using anti-HA antibody and anti-cadherin fragment antibodies showed the presence of a band around 200 kDa in AaeCad-transfected cells. This size band was not detected in control cells expressing only EGFP (Fig. 2). Since the cadherin protein in the midgut of Aedes is also of this size [8] the full length cadherin is expressed in these cells (Fig. 2C).
Fig. 2.
AaeCad is stably expressed in AaeCad-transfected cells. To determine the expression of Aedes cadherin cloned into pACTIN.SV, non-soluble proteins were extracted and detected with an anti-HA antibody (1: EGFP-transfected cells, 2: AaeCad-transfected cells) or an anti-cadherin fragment antibody (3: EGFP-transfected cells, 4: AaeCad-transfected cells, 5: Brush border membrane vesicles from Ae. aegypti midgut). In all cases, we detected a band around 200 kDa in AaeCad-transfected cells. The 200-kDa protein corresponds to the full-length cadherin.
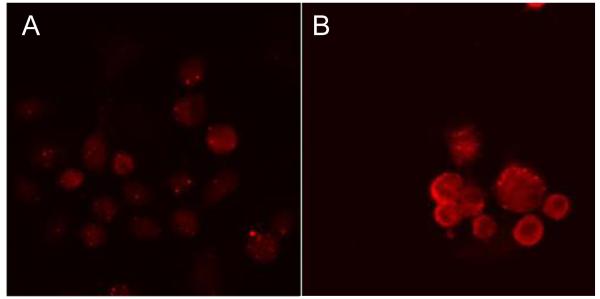
To determine the sub-cellular location of AaeCad the cells were analyzed using an anti-cadherin fragment antibody and a secondary antibody conjugated with Cy3. Cells expressing AaeCad and control cells expressing EGFP were stained without permeabilization to detect AaeCad localized in the plasma membrane. Control cells did not show any specific immunofluorescence staining (Fig. 3A). However, red immunofluorescence was observed in the plasma membrane of AaeCad expressing cells (Fig. 3B).
Fig. 3.
Aedes cadherin is localized in the plasma membrane. To determine the sub-cellular location of Aedes cadherin, the expression was determined by immunofluorescence staining under confocal microscopy. Red immunofluorescence staining without permeabilization showed that Aedes cadherin were localized in the plasma membrane (B). (A) Immunostaining of control cells using anti-cadherin fragment antibody. (B) Immunostaining of cells expressing Aedes cadherin using anti-cadherin fragment antibody.
3.2 Cells expressing AaeCad are more sensitive to Cry11Aa, Cry4Aa, and Cry11Ba toxins
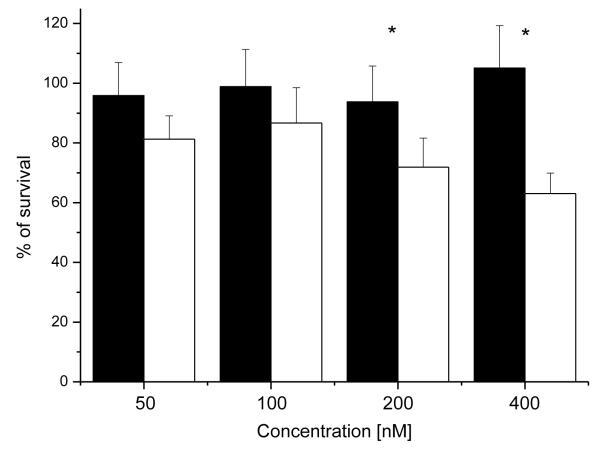
Since Aedes cadherin binds the Cry11Aa and Cry11Ba toxins and hence should mediate Cry toxicity [8, 32], the toxicity of Cry proteins to AaeCad expressing cells was analyzed at final concentrations of 50 - 400 nM. Control cells expressing the EGFP protein were insensitive to all Cry toxins tested even at concentrations of up to 400 nM. However, cells expressing AaeCad showed significant sensitivity to the Cry11Aa toxin from 200 nM (29% death). Cry11Aa toxin at 400 nM killed approximately 37% of the cells in 3 h (Fig. 4). Moreover, these cadherin-expressing cells showed significant sensitivity to Cry4Aa (37.2% death) and Cry11Ba (25.7%) toxins, but were insensitive to Cry4Ba toxin (Table 1).
Fig. 4.
Cells expressing Aedes cadherin are more sensitive to Cry11Aa toxin. The toxicity of Cry11Aa to these cells was analyzed in a MTT cytotoxicity assay using activated Cry11Aa toxin at final concentrations of 50 - 400 nM. After cells were incubated with activated Cry11Aa for 3 hours, live cells were analyzed by measuring reduced MTT. Control cells expressing the EGFP protein (black) were insensitive to Cry11Aa toxin up to 400 nM. However, cells expressing AaeCad (red) showed sensitivity to the toxin and Cry11Aa toxin at 400 nM killed approximately 37% of the cells in 3 hours (n=3, t-test, p < 0.05).
Table 1.
Cells expressing Aedes cadherin are more sensitive to Cry11Aa, Cry4Aa and Cry11Ba toxins.
| EGFP-transfected cell (% survival) |
AaeCad-transfected cell (% survival) |
|
|---|---|---|
| Cry11Aa | 99.7±11.4 | 79.0±6.70 * |
| Cry4Aa | 94.4±3.68 | 62.8±6.32 * |
| Cry4Ba | 114±8.76 | 107±5.67 |
| Cry11Ba | 114±16.5 | 74.3±11.5 * |
The toxicities of Cry4Aa, Cry4Ba, and Cry11Ba to these cells were analyzed in a MTT cytotoxicity assay using activated toxins at a final concentration of 400 nM. After cells were incubated with activated toxins for 3 h, live cells were analyzed by measuring reduced MTT. Control cells expressing the EGFP protein were insensitive to all toxins at 400 nM (94.4 – 114% survival compared to non-treated control cells). However, cells expressing Aedes cadherin showed significantly sensitivity to Cry4Aa (37.2% death) and Cry11Ba (25.7% death) toxins compared to non-treated cells expressing Aedes cadherin (n=3, t-test, p < 0.05). Values given are relative to the Cry11Aa toxicity to EGFP-transfected cells.
3.3 Cells expressing AaeCad trigger Cry11Aa oligomerization
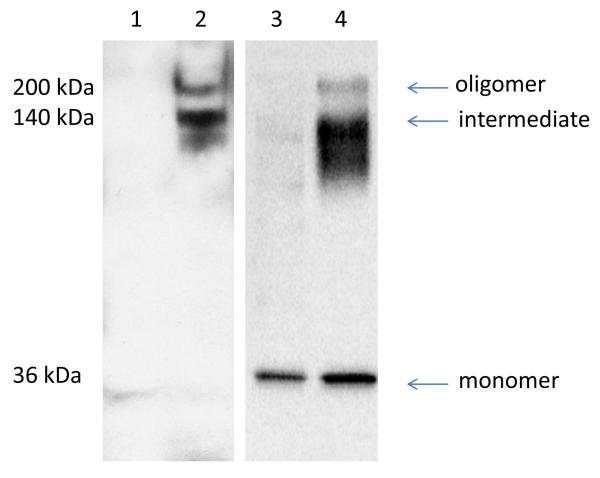
It has been previously demonstrated that the toxicity of Cry toxins depends on the ability of these toxins to oligomerize in the presence of its receptor [22, 28]. Therefore, to determine whether the full-length AaeCad expressed in the C6/36 cells triggers Cry11Aa oligomerization within a cellular context, trypsin activated Cry11Aa toxin at concentrations that causes cytotoxicity was analyzed. Alternatively, a higher concentration of the Cry11Aa protoxin activated with Aedes midgut juice was used as reported [36]. Both of these toxins were incubated with control cells or cells expressing AaeCad. In Cry11Aa incubations with cells expressing AaeCad, 140 kDa (intermediate) and 200 kDa (oligomer) bands from both activated toxin and protoxin were detected (Fig. 5, lane 2 and 4). However, only the Cry11Aa protoxin activated with midgut juice showed a 36 kDa (monomer) band (Fig. 5, lane 3 and 4) in both control and AaeCad expressing cells. Potentially this could be detected in lanes 3 and 4 because of the higher toxin concentrations used. Nevertheless, these data demonstrates that Cry11Aa oligomers are formed in the membranes of viable Aedes cells by both the trypsin activated Cry11Aa and midgut juice activated Cry11Aa protoxin.
Fig. 5.
Cells expressing Aedes cadherin trigger Cry11Aa oligomerization. Control cells expressing EGFP and cells expressing Aedes cadherin were incubated with activated Cry11Aa (final concentration 400 nM) and with trypsin or Cry11Aa protoxin (final concentration 1,400 nM) with midgut juice. After 1 h at 37°C, cells were washed, boiled for 10 min, and separated in 10% SDS-PAGE gel. Cry11Aa oligomerization was detected with an anti-Cry11Aa antibody. Lane 1: EGFP-transfected cells and active Cry11Aa, 2: AaeCad-transfected cells and active Cry11Aa, 3: EGFP-transfected cells and Cry11Aa protoxin with midgut juice, 4: AaeCad-transfected cells and Cry11Aa protoxin with midgut juice.
3.4 Transgenic mosquitoes with silenced AaeCad are tolerant to Cry11Aa toxin
As noted above the Aedes cadherin has been previously shown to bind mosquitocidal Cry toxins [8] and our data above shows that Aedes cells expressing cadherin increased susceptibility to these toxins. To analyze whether the cadherin gene plays a role in the in vivo toxicity of the Cry toxins, we silenced AaeCad expression in Aedes larvae. A hairpin dsRNA construct of the AaeCad was produced under control of the Aedes heat shock promoter and transgenic mosquitoes expressing this construct were obtained using pBAC transformation (Fig. 6, S1).
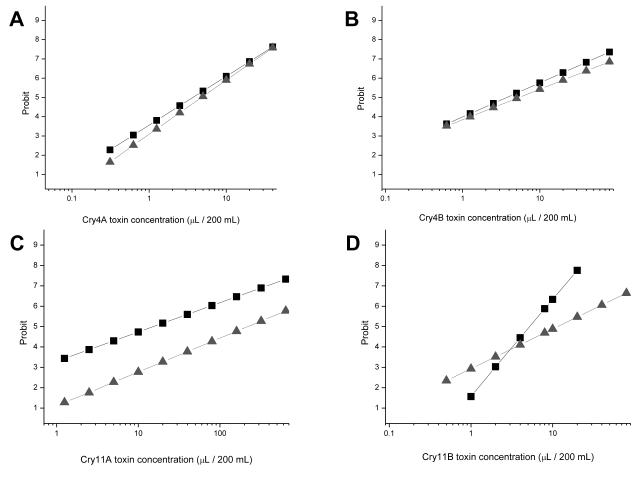
To silence AaeCad expression, wild-type and transgenic larvae were heat-shocked at 2nd instar for 1 h at 37°C. After this heat shock, the larvae were allowed to develop to the 4th instar. The transcript levels of AaeCad in whole larvae and in the midgut were significantly reduced in transgenic larvae compared to wild-type larvae (Table 2). Transgenic larvae with silenced AaeCad showed increased tolerance to Cry11Aa toxicity (Fig. 7C). However, silencing AaeCad did not show any difference in toxicity to Cry4Aa, Cry4Ba, and Cry11Ba (Fig. 7A, 7B, 7D).
Table 2.
Transgenic mosquitoes down-regulate Aedes cadherin expression in whole body.
| Transcript change in whole body | Transcript change in midgut | |
|---|---|---|
| AaeCad | 0.20 ± 0.23 | 0.49 ± 0.31 |
The larval AaeCad transcript levels were determined by qPCR. Transcript levels are relative to the transcript levels in wild-type larvae where the value is 1.0.
Fig. 7.
Transgenic mosquitoes with silenced Aedes cadherin expression show increased tolerance against Cry11Aa toxin. Bioassay was performed with early 4th instar larvae heat shocked at 2nd instar. Wild-type larvae (■) and transgenic larvae ▲ were incubated with Cry4Aa (A), Cry4Ba (B), Cry11Aa (C), and Cry11Ba (D) and mortalities were analyzed in Probit and Origin.
4. Discussion
In Lepidoptera, binding of Cry1 toxins to cadherin causes a mild denaturation of Cry toxin and proteolytic cleavage of helix α-1 which results in a toxin conformational change [22, 28]. This conformational change is thought to cause the formation of oligomers that bind to a secondary receptor, which can be APN or ALP anchored to the membrane by a GPI anchor [6, 17]. Similarly, in Aedes mosquitoes, either Cry11Aa protoxin in the presence of BBMV or proteolytically activated Cry11Aa toxin incubated with AaeCad receptor fragment (CR7-11) alone can trigger toxin oligomerization [36, 38]. Here we also showed that C6/36 cells expressing full-length AaeCad can cause Cry11Aa oligomerization by the use of trypsin-activated Cry11Aa toxin directly or Cry11Aa protoxin in the presence of midgut juice (Fig. 5). There are some differences in the oligomeric forms obtained with these two toxins. Further, oligomerization of the Cry11Aa toxin is observed in cells at toxin concentration levels at which cytotoxicity is observed. Also, it is reported that the partial Tenebrio molitor cadherin (TmCad1) can promote the coleopteran-specific Cry3A toxin oligomerization [16]. Therefore, various cadherin receptors from three major insect pest orders have a conserved function leading to toxin oligomerization, which has been demonstrated as an important step for Cry toxicity.
In this study, AaeCad expressed in cells can not only trigger Cry11Aa oligomerization mentioned afore, but also the cells expressing AaeCad are susceptible to Cry11Aa, Cry4Aa, and Cry11Ba toxin (Fig. 4 and Table 1). Cry11Aa, Cry4Aa, and Cry11Ba toxins at 400 nM can cause about 37%, 37.2% and 25.7% cytotoxicity and Cry4Ba shows no cytotoxicity at the same dose. Our previous toxin binding competition assays showed Cry11Ba readily competes with Cry11Aa binding to AaeCad, while Cry4Aa slightly competes, and Cry4Ba does not compete [8]. In this study, Cry11Aa and Cry11Ba also showed significant cytotoxicity and Cry4Ba caused no cytotoxicity. However, Cry4Aa cytotoxicity is higher than expected since its binding with AaeCad is much weaker than Cry11Aa and Cry11Ba [8]. We continue to investigate this difference between the toxin binding assay and cytotoxicity for Cry4Aa.
In a similar approach, Heliothis cadherin transiently expressed in Drosophila S2 cells showed low cytotoxicity, maximum 20% and 5% cytotoxicity were caused by Cry1Ac and Cry1Ab, respectively at 330 nM [29]. However, only 180 nM Cry1Ab toxin is required to kill most of Trichoplusia High Five cells stably expressing Manduca cadherin [40, 41]. Notably, High Five cells apparently have a much higher level of cadherin expression compared with the AaeCad in C6/36 cells, and with Heliothis cadherin in S2 cells. Thus, it is very likely that the different cadherin expression levels could contribute to the different sensitivities of cell lines to the corresponding toxins. On the other hand, it appears that Cry11Aa and Cry1A toxins shared the same mechanism of action because Cry11Aa can also bind to the same kinds of protein receptors as Cry1A toxin, for example, cadherin, APN and ALP. According to sequential binding model [5], in addition to cadherin, the secondary receptors are essential for the full toxicity in vivo. Thus it is understandable that C6/36 cells expressing only AaeCad show only limited sensitivity to mosquitocidal toxins. To obtain more sensitive cell lines, secondary receptors may have to be introduced into these cells expressing AaeCad.
Although AaeCad RNAi-mediated silencing had been done in Aedes mosquitoes by dsRNA feeding [38], a transgenic Aedes mosquito line with silenced AaeCad was established in order to obtain more homogeneous animals that can be used to screen a number of other toxins. Importantly, these transgenic mosquitoes revealed a higher tolerance to Cry11Aa (about 13 fold) at LC50 value than Aedes larvae fed AaeCad dsRNA, where only a 50% reduction in Cry11Aa toxicity was obtained compared to wild-type mosquitoes [38].
Our bioassay data suggests AaeCad is involved in the toxicity of Cry11Aa but not in the toxicity of Cry4Aa, Cry4Ba, and Cry11Ba (Fig. 7). These bioassay data for Cry11Aa and Cry4Ba are consistent with toxin binding competition assay performed by Chen et al, 2009 [8] and cytotoxicity data from C6/36 cells expressing AaeCad in this study. Thus, while cadherin is critical for Cry11Aa toxicity it is not important for in vivo Cry4Ba toxicity. However, Cry4Aa and Cry11Ba in vivo toxicity in cadherin-silenced mosquiteos is quite different from the in vitro binding assays performed in earlier studies [8, 32] and the cytotoxicity test data reported here. These different results suggest that receptors other than AaeCad could be involved and may be more important for the toxicity of Cry4Aa and Cry11Ba, than they are for Cry11Aa.
Collectively, our data suggest that AaeCad clearly mediates the in vivo Cry11Aa toxicity to Ae. aegypti mosquitoes, and that AaeCad is not involved in Cry4Ba toxicity. In Anopheles gambiae, two Anopheles cadherins, AgCad1 and AgCad2, were identified as putative receptors for Cry4Ba and Cry11Ba toxins, respectively [25, 26]. It is possible a cadherin receptor other than AaeCad is involved in Cry4A or Cry11Ba toxicity. In contrast, Cry4Ba can form oligomer spontaneously in the presence of small unilamellar vesicles [38], and thues it is quite likely that no cadherin receptor is required for Cry4Ba cytotoxicity. Thus, Cry4Ba is a unique toxin and its mechanism of action appears to differ from that of Cry11Aa. Recently an ATP-binding cassette (ABC) transporter was shown to play a vital role in Cry1A toxicity and toxin resistance in lepidopteran insects [2, 3, 20]. It is still unknown if any ABC transporter is also involved in the mechanism of action of mosquitocidal toxins, including Cry4Aa, Cry4Ba and Cry11Ba. We believe novel RNAi technology and genome editing tools could be used for further elucidating the mechanism of action of these toxins
Supplementary Material
Highlights.
Aedes cadherins were stably expressed in a mosquito cell line and silenced in transgenic mosquitoes.
Cells expressing Aedes cadherin are more sensitive to Cry11Aa, Cry4Aa, and Cry11Ba toxins, but not Cry4Ba.
Cells expressing Aedes cadherin trigger Cry11Aa oligomerization just as with lepidopteran cadherins.
Transgenic mosquitoes with silenced Aedes cadherin show increased tolerance to Cry11Aa toxin
Acknowledgments
The technical assistance of Maria Ramirez and Jacqueline Ledezma is gratefully acknowledged. This research was funded in part through grants from the National Institutes of Health, 1R01AI066014 and the University of California Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Abdullah MA, Valaitis AP, Dean DH. Identification of a Bacillus thuringiensis Cry11Ba toxin-binding aminopeptidase from the mosquito, Anopheles quadrimaculatus. BMC Biochem. 2006;7:16. doi: 10.1186/1471-2091-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Atsumi S, Miyamoto K, Yamamoto K, Narukawa J, Kawai S, Sezutsu H, et al. Single amino acid mutation in an ATP-binding cassette transporter gene causes resistance to Bt toxin Cry1Ab in the silkworm, Bombyx mori. Proc Natl Acad Sci. 2012;109:E1591–E8. doi: 10.1073/pnas.1120698109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baxter SW, Badenes-Pérez FR, Morrison A, Vogel H, Crickmore N, Kain W, et al. Parallel evolution of Bacillus thuringiensis toxin resistance in Lepidoptera. Genetics. 2011;189:675–9. doi: 10.1534/genetics.111.130971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Berry C, O’Neil S, Ben-Dov E, Jones AF, Murphy L, Quail MA, et al. Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis. Appl Environ Microbiol. 2002;68:5082–95. doi: 10.1128/AEM.68.10.5082-5095.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bravo A, Gill SS, Soberon M. Bacillus thuringiensis: Mechanisms and Use. In: Gilbert LI, Kostas I, Gill SS, editors. Comprehensive Molecular Insect Science. Elsevier; 2005. pp. 175–205. [Google Scholar]
- [6].Bravo A, Gomez I, Conde J, Munoz-Garay C, Sanchez J, Miranda R, et al. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim Biophys Acta. 2004;1667:38–46. doi: 10.1016/j.bbamem.2004.08.013. [DOI] [PubMed] [Google Scholar]
- [7].Chang C, Yu YM, Dai SM, Law SK, Gill SS. High-level cryIVD and cytA gene expression in Bacillus thuringiensis does not require the 20-kilodalton protein, and the coexpressed gene products are synergistic in their toxicity to mosquitoes. Appl Environ Microbiol. 1993;59:815–21. doi: 10.1128/aem.59.3.815-821.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen J, Aimanova KG, Fernandez LE, Bravo A, Soberon M, Gill SS. Aedes aegypti cadherin serves as a putative receptor of the Cry11Aa toxin from Bacillus thuringiensis subsp. israelensis. Biochem J. 2009;424:191–200. doi: 10.1042/BJ20090730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen J, Aimanova KG, Pan S, Gill SS. Identification and characterization of Aedes aegypti aminopeptidase N as a putative receptor of Bacillus thuringiensis Cry11A toxin. Insect Biochem Mol Biol. 2009;39:688–96. doi: 10.1016/j.ibmb.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen J, Likitvivatanavong S, Aimanova KG, Gill SS. A 104 kDa Aedes aegypti aminopeptidase N is a putative receptor for the Cry11Aa toxin from Bacillus thuringiensis subsp. israelensis. Insect Biochem Mol Biol. 2013;43:1201–8. doi: 10.1016/j.ibmb.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chilcott C, Ellar DJ. Comparative Toxicity of Bacillus thuringiensis var. israelensis Crystal Proteins in vivo and in vitro. J Gen Microbiol. 1988;134:2551–8. doi: 10.1099/00221287-134-9-2551. [DOI] [PubMed] [Google Scholar]
- [12].Chow E, Gill S. A rapid colorimetric assay to evaluate the effects of Bacillus thuringiensis toxins on cultured insect cells. J Tissue Cult Methods. 1989;12:39–42. [Google Scholar]
- [13].Cowles EA, Yunovitz H, Charles JF, Gill SS. Comparison of toxin overlay and solid-phase binding assays to identify diverse CryIA(c) toxin-binding proteins in Heliothis virescens midgut. Appl Environ Microbiol. 1995;61:2738–44. doi: 10.1128/aem.61.7.2738-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Crickmore N, Zeigler DR, Feitelson J, Schnepf E, Van Rie J, Lereclus D, et al. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:807–13. doi: 10.1128/mmbr.62.3.807-813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Delecluse A, Rosso ML, Ragni A. Cloning and expression of a novel toxin gene from Bacillus thuringiensis subsp. jegathesan encoding a highly mosquitocidal protein. Appl Environ Microbiol. 1995;61:4230–5. doi: 10.1128/aem.61.12.4230-4235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fabrick JA, Forlow Jech L, Henneberry TJ. Novel pink bollworm resistance to the Bt toxin Cry 1Ac: effects on mating, oviposition, larval development and survival. J Insect Sci. 2009;9:24. doi: 10.1673/031.009.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fernandez LE, Aimanova KG, Gill SS, Bravo A, Soberon M. A GPI-anchored alkaline phosphatase is a functional midgut receptor of Cry11Aa toxin in Aedes aegypti larvae. Biochemical J. 2006;394:77–84. doi: 10.1042/BJ20051517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fernandez LE, Martinez-Anaya C, Lira E, Chen J, Evans A, Hernandez-Martinez S, et al. Cloning and epitope mapping of Cry11Aa-binding sites in the Cry11Aa-receptor alkaline phosphatase from Aedes aegypti. Biochemistry. 2009;48:8899–907. doi: 10.1021/bi900979b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fernandez LE, Perez C, Segovia L, Todriguez MH, Gill SS, Bravo A, et al. Cry11Aa toxin from Bacillus thuringiensis binds its receptor in Aedes aegypti mosquito larvae through loop α-8 of domain II. FEBS Lett. 2005;579:3508–14. doi: 10.1016/j.febslet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- [20].Gahan LJ, Pauchet Y, Vogel H, Heckel DG. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet. 2010;6:e1001248. doi: 10.1371/journal.pgen.1001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gill SS, Cowles EA, Pietrantonio PV. The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol. 1992;37:615–36. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- [22].Gomez I, Sanchez J, Miranda R, Bravo A, Soberon M. Cadherin-like receptor binding facilitates proteolytic cleavage of helix α-1 in domain I and oligomer pre-pore formation of Bacillus thuringiensis Cry1Ab toxin. FEBS Lett. 2002;513:242–6. doi: 10.1016/s0014-5793(02)02321-9. [DOI] [PubMed] [Google Scholar]
- [23].Handler AM, Harrell RA., 2nd Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect Mol Biol. 1999;8:449–57. doi: 10.1046/j.1365-2583.1999.00139.x. [DOI] [PubMed] [Google Scholar]
- [24].Horn C, Wimmer EA. A versatile vector set for animal transgenesis. Dev Genes Evol. 2000;210:630–7. doi: 10.1007/s004270000110. [DOI] [PubMed] [Google Scholar]
- [25].Hua G, Zhang Q, Zhang R, Abdullah AM, Linser PJ, Adang MJ. AgCad2 cadherin in Anopheles gambiae larvae is a putative receptor of Cry11Ba toxin of Bacillus thuringiensis subsp. jegathesan. Insect Biochem Mol Biol. 2013;43:153–61. doi: 10.1016/j.ibmb.2012.11.007. [DOI] [PubMed] [Google Scholar]
- [26].Hua G, Zhang R, Abdullah MA, Adang MJ. Anopheles gambiae cadherin AgCad1 binds the Cry4Ba toxin of Bacillus thuringiensis israelensis and a fragment of AgCad1 synergizes toxicity. Biochemistry. 2008;47:5101–10. doi: 10.1021/bi7023578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huynh CQ, Zieler H. Construction of modular and versatile plasmid vectors for the high-level expression of single or multiple genes in insects and insect cell lines. J Mol Biol. 1999;288:13–20. doi: 10.1006/jmbi.1999.2674. [DOI] [PubMed] [Google Scholar]
- [28].Jimenez-Juarez N, Munoz-Garay C, Gomez I, Saab-Rincon G, Damian-Almazo JY, Gill SS, et al. Bacillus thuringiensis Cry1Ab mutants affecting oligomer formation are non-toxic to Manduca sexta larvae. J Biol Chem. 2007;282:21222–9. doi: 10.1074/jbc.M701314200. [DOI] [PubMed] [Google Scholar]
- [29].Jurat-Fuentes JL, Adang MJ. The Heliothis virescens cadherin protein expressed in Drosophila S2 cells functions as a receptor for Bacillus thuringiensis Cry1A but not Cry1Fa toxins. Biochemistry. 2006;45:9688–95. doi: 10.1021/bi0606703. [DOI] [PubMed] [Google Scholar]
- [30].Lereclus D, Agaisse H, Gominet M, Chaufaux J. Overproduction of encapsulated insecticidal crystal proteins in a Bacillus thuringiensis spo0A mutant. Biotechnology (N Y) 1995;13:67–71. doi: 10.1038/nbt0195-67. [DOI] [PubMed] [Google Scholar]
- [31].Likirvivatanavong S, Chen J, Evans AM, Bravo A, Soberon M, Gill SS. Multiple receptors as targets of Cry toxins in mosquitoes. J Agric Food Chem. 2011;59:2829–38. doi: 10.1021/jf1036189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Likitvivatanavong SCJ, Bravo A, Soberón M, Gill SS. Cadherin, alkaline phosphatase, and aminopeptidase N as receptors of Cry11Ba toxin from Bacillus thuringiensis subsp. jegathesan in Aedes aegypti. Appl Environ Microbiol. 2011;77:24–31. doi: 10.1128/AEM.01852-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Margalith Y, Ben-Dov E. Biological Control by Bacillus thuringiensis subsp. israelensis. In: Rechicgl JE, Rechcigl NA, editors. Insect Pest Management: Techniques for Environmental Protection. CRC Press; 2000. pp. 243–301. [Google Scholar]
- [34].Munoz-Garay C, Rodriguez-Almazan C, Aguilar JN, Portugal L, Gomez I, Saab-Rincon G, et al. Oligomerization of Cry11Aa from Bacillus thuringiensis has an important role in toxicity against Aedes aegypti. Appl Environ Microbiol. 2009;75:7548–50. doi: 10.1128/AEM.01303-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Obata F, Kitami M, Inoue Y, Atsumi S, Yoshizawa Y, Sato R. Analysis of the region for receptor binding and triggering of oligomerization on Bacillus thuringiensis Cry1Aa toxin. Febs J. 2009;276:5949–59. doi: 10.1111/j.1742-4658.2009.07275.x. [DOI] [PubMed] [Google Scholar]
- [36].Perez C, Munoz-Garay C, Portugal LC, Sanchez J, Gill SS, Soberon M, et al. Bacillus thuringiensis ssp. israelensis Cyt1Aa enhances activity of Cry11Aa toxin by facilitating the formation of a pre-pore oligomeric structure. Cell Microbiol. 2007;9:2931–7. doi: 10.1111/j.1462-5822.2007.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rodríguez-Almazán C, Reyes EZ, Zúñiga-Navarrete F, Muñoz-Garay C, Gómez I, Evans AM, et al. Cadherin binding is not a limiting step for Bacillus thuringiensis subsp. israelensis Cry4Ba toxicity to Aedes aegypti larvae. Biochem J. 2012;443:711–7. doi: 10.1042/BJ20111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang R, Hua G, Urbauer JL, Adang MJ. Synergistic and inhibitory effects of aminopeptidase peptides on Bacillus thuringiensis Cry11Ba toxicity in the mosquito Anopheles gambiae. Biochemistry. 2010;49:8512–9. doi: 10.1021/bi1009908. [DOI] [PubMed] [Google Scholar]
- [40].Zhang X, Candas M, Griko NB, Rose-Young L, Bulla LA., Jr. Cytotoxicity of Bacillus thuringiensis Cry1Ab toxin depends on specific binding of the toxin to the cadherin receptor BT-R1 expressed in insect cells. Cell Death Differ. 2005;12:1407–16. doi: 10.1038/sj.cdd.4401675. [DOI] [PubMed] [Google Scholar]
- [41].Zhang X, Candas M, Griko NB, Taussig R, Bulla LAJ. A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc Natl Acad Sci USA. 2006;103:9897–902. doi: 10.1073/pnas.0604017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.