Abstract
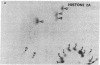
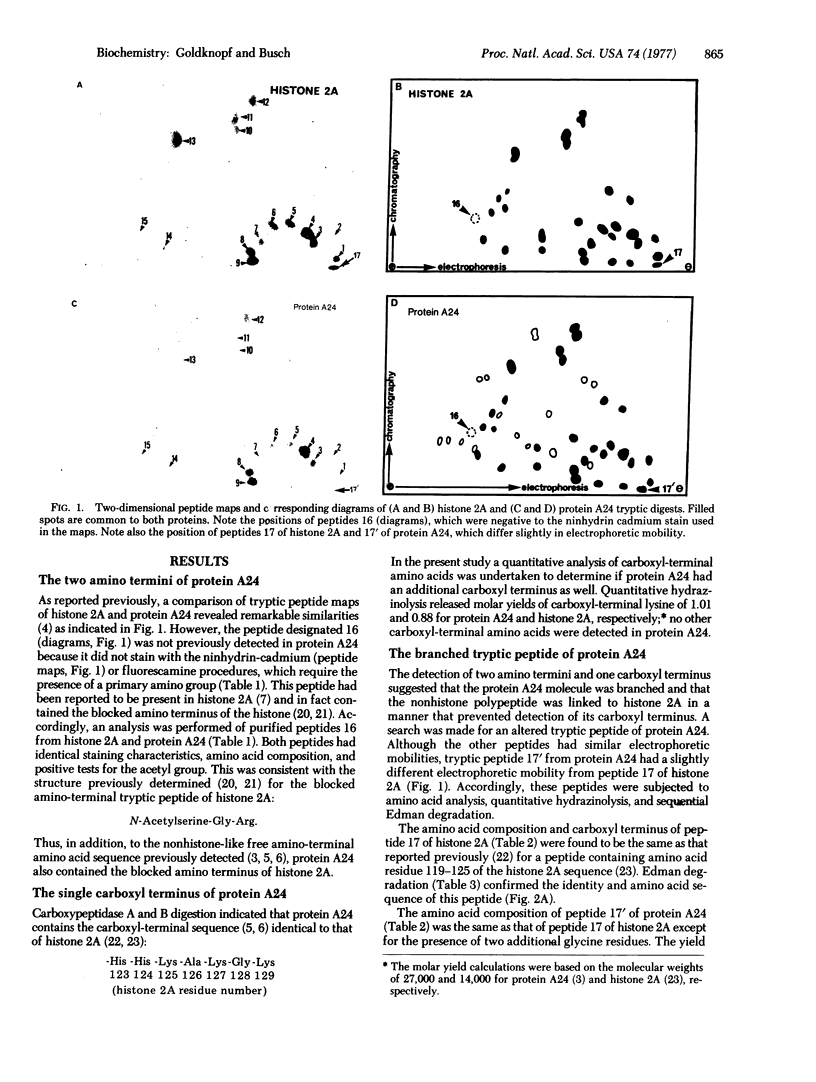
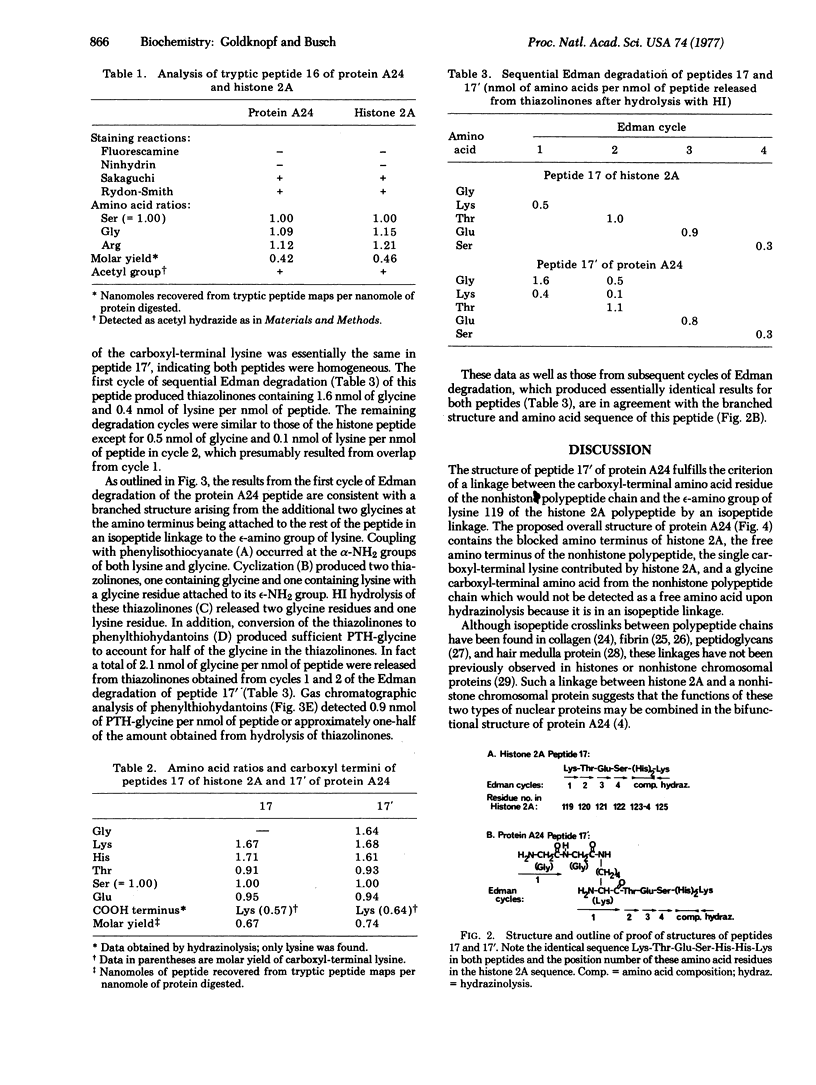
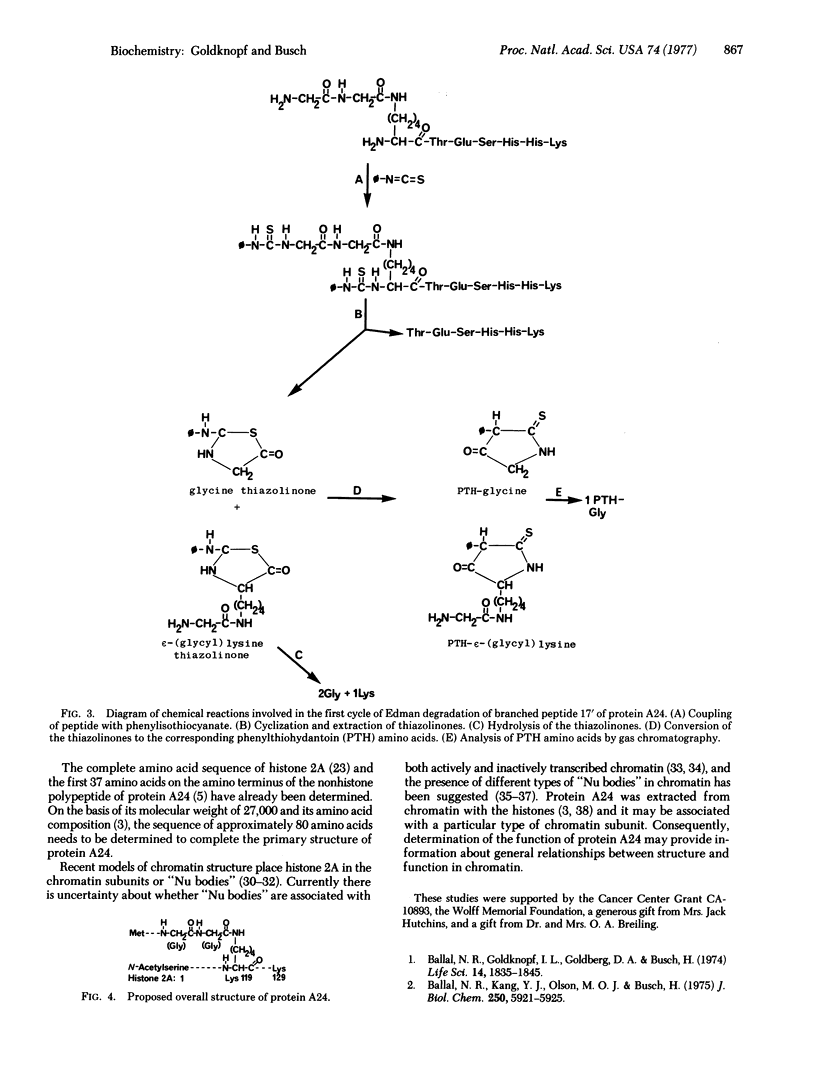
Chromosomal protein A24 has a unique structure inasmuch as it contains histone 2A and a nonhistone polypeptide the sequence of which has been partially determined. Comparative analysis of the ninhydrin-insensitive amino-terminal tryptic peptides of protein A24 and histone 2A and a quantitative analysis of their carboxyl-terminal amino acid indicated that protein A24 has two amino termini and one carboxyl terminus. The amino acid sequence analysis of tryptic peptide 17' of protein A24: (see text) showed it contains tryptic peptide 17 of histone 2A, Lys-Thr-Glu-Ser-His-His-Lys. Lysine 119, the amino terminus of this peptide, which is derived from the histone 2A portion of protein A24, is linked by an isopeptide bond to the carboxyl group of a glycine residue. Accordingly, the branched structure of protein A24 proposed is: (see text).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREAE W. A. The estimation of maleic hydrazide by paper chromatography. Can J Biochem Physiol. 1958 Jan;36(1):71–74. [PubMed] [Google Scholar]
- Ballal N. R., Goldknopf I. L., Goldberg D. A., Busch H. The dynamic state of liver nucleolar proteins as reflected by their changes during administration of thioacetamide. Life Sci. 1974 May 16;14(10):1835–1845. doi: 10.1016/0024-3205(74)90401-9. [DOI] [PubMed] [Google Scholar]
- Ballal N. R., Kang Y. J., Olson M. O., Busch H. Changes in nucleolar proteins and their phosphorylation patterns during liver regeneration. J Biol Chem. 1975 Aug 10;250(15):5921–5925. [PubMed] [Google Scholar]
- Dezélée P., Shockman G. D. Studies of the formation of peptide cross-links in the cell wall peptidoglycan of Streptococcus faecalis. J Biol Chem. 1975 Sep 10;250(17):6806–6816. [PubMed] [Google Scholar]
- Dopheide T. A., Moore S., Stein W. H. The carboxyl-terminal sequence of porcine pepsin. J Biol Chem. 1967 Apr 25;242(8):1833–1837. [PubMed] [Google Scholar]
- Elgin S. C., Weintraub H. Chromosomal proteins and chromatin structure. Annu Rev Biochem. 1975;44:725–774. doi: 10.1146/annurev.bi.44.070175.003453. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. Remarkable similarities of peptide fingerprints of histone 2A and nonhistone chromosomal protein A24. Biochem Biophys Res Commun. 1975 Aug 4;65(3):951–960. doi: 10.1016/s0006-291x(75)80478-5. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Taylor C. W., Baum R. M., Yeoman L. C., Olson M. O., Prestayko A. W., Busch H. Isolation and characterization of protein A24, a "histone-like" non-histone chromosomal protein. J Biol Chem. 1975 Sep 25;250(18):7182–7187. [PubMed] [Google Scholar]
- Harding H. W., Rogers G. E. Isolation of peptides containing citrulline and the cross-link, epsilon-(gamma-glutamyl)lysine, from hair medulla protein. Biochim Biophys Acta. 1976 Mar 18;427(1):315–324. doi: 10.1016/0005-2795(76)90307-x. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Thomas J. O. Chromatin structure; oligomers of the histones. Science. 1974 May 24;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Lacy E., Axel R. Analysis of DNA of isolated chromatin subunits. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3978–3982. doi: 10.1073/pnas.72.10.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matacić S., Loewy A. G. The identification of isopeptide crosslinks in insoluble fibrin. Biochem Biophys Res Commun. 1968 Feb 26;30(4):356–362. doi: 10.1016/0006-291x(68)90750-x. [DOI] [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Reaction of peptides with fluorescamine on paper after chromatography or electrophoresis. Anal Biochem. 1975 May 12;65(1-2):281–292. doi: 10.1016/0003-2697(75)90511-4. [DOI] [PubMed] [Google Scholar]
- NARITA K. Isolation of acetylpeptide from enzymic digests of TMV-protein. Biochim Biophys Acta. 1958 Apr;28(1):184–191. doi: 10.1016/0006-3002(58)90445-1. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E. Spheroid chromatin units (v bodies). Science. 1974 Jan 25;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Olson M. O., Goldknopf I. L., Guetzow K. A., James G. T., Hawkins T. C., Mays-Rothberg C. J., Busch H. The NH2- and COOH-terminal amino acid sequence of nuclear protein A24. J Biol Chem. 1976 Oct 10;251(19):5901–5903. [PubMed] [Google Scholar]
- Olson M. O., Jordan J., Busch H. The amino terminal sequence of calf thymus histone 3. Biochem Biophys Res Commun. 1972 Jan 14;46(1):50–55. doi: 10.1016/0006-291x(72)90628-6. [DOI] [PubMed] [Google Scholar]
- Olson M. O., Sugano N., Yeoman L. C., Johnson B. R., Jordan J., Taylor C. W., Starbuck W. C., Busch H. Homology of the amino terminal sequences of the AL and GAR calf thymus histones. Physiol Chem Phys. 1972;4(1):10–16. [PubMed] [Google Scholar]
- Orrick L. R., Olson M. O., Busch H. Comparison of nucleolar proteins of normal rat liver and Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1973 May;70(5):1316–1320. doi: 10.1073/pnas.70.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J., Malcolm S. A class of chromatin particles associated with nonhistone proteins. Biochemistry. 1976 Aug 10;15(16):3510–3515. doi: 10.1021/bi00661a018. [DOI] [PubMed] [Google Scholar]
- Phillips D. M. N-Terminal acetyl-peptides from two calf thymus histones. Biochem J. 1968 Mar;107(2):135–138. doi: 10.1042/bj1070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano J. J., Finlayson J. S., Peyton M. P. [Cross-link in fibrin polymerized by factor 13: epsilon-(gamma-glutamyl)lysine]. Science. 1968 May 24;160(3830):892–893. doi: 10.1126/science.160.3830.892. [DOI] [PubMed] [Google Scholar]
- Schlesinger D. H., Goldstein G., Niall H. D. The complete amino acid sequence of ubiquitin, an adenylate cyclase stimulating polypeptide probably universal in living cells. Biochemistry. 1975 May 20;14(10):2214–2218. doi: 10.1021/bi00681a026. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Starbuck W. C., Mauritzen C. M., Taylor C. W., Saroja I. S., Busch H. A large scale procedure for isolation of the glycine-rich, arginine-rich histone and the arginine-rich, lysine-rich histone in a highly purified form. J Biol Chem. 1968 Apr 25;243(8):2038–2047. [PubMed] [Google Scholar]
- Sugano N., Olson M. O., Yeoman L. C., Johnson B. R., Taylor C. W., Starbuck W. C., Busch H. Amino acid sequence of the COOH-terminal portion of the arginine-lysine-rich histone of calf thymus. J Biol Chem. 1972 Jun 10;247(11):3589–3591. [PubMed] [Google Scholar]
- Tien Kuo M., Sahasrabuddhe C. G., Saunders G. F. Presence of messenger specifying sequences in the DNA of chromatin subunits. Proc Natl Acad Sci U S A. 1976 May;73(5):1572–1575. doi: 10.1073/pnas.73.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Yeoman L. C., Olson M. O., Sugano N., Jordan J. J., Taylor D. W., Starbuck W. C., Busch H. Amino acid sequence of the center of the arginine-lysine-rich histone from calf thymus. The total sequence. J Biol Chem. 1972 Oct 10;247(19):6018–6023. [PubMed] [Google Scholar]
- Yue R. H., Palmieri R. H., Olson O. E., Kuby S. A. Studies on adenosine triphosphate transphophorylases. V. Studies on the polypeptide chains of the crystalline adenosine triphosphate-creatine transphosphorylase from rabbit skeletal muscle. Biochemistry. 1967 Oct;6(10):3204–3227. doi: 10.1021/bi00862a031. [DOI] [PubMed] [Google Scholar]