Abstract
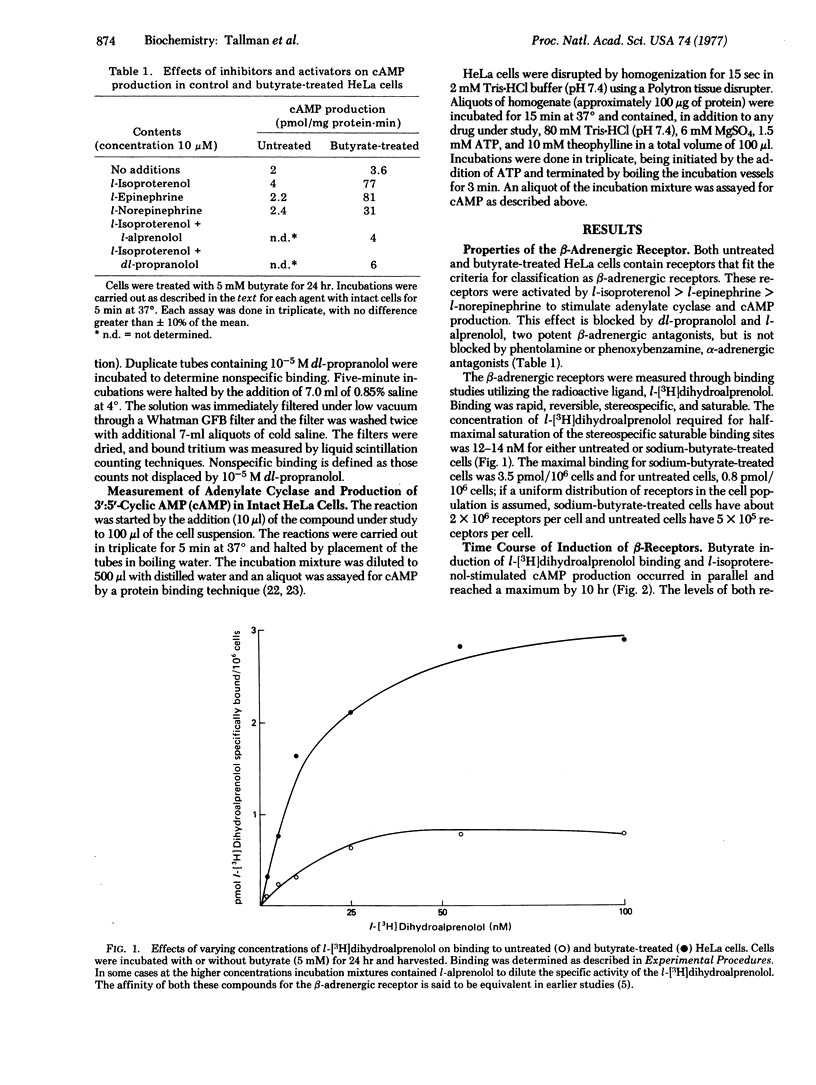
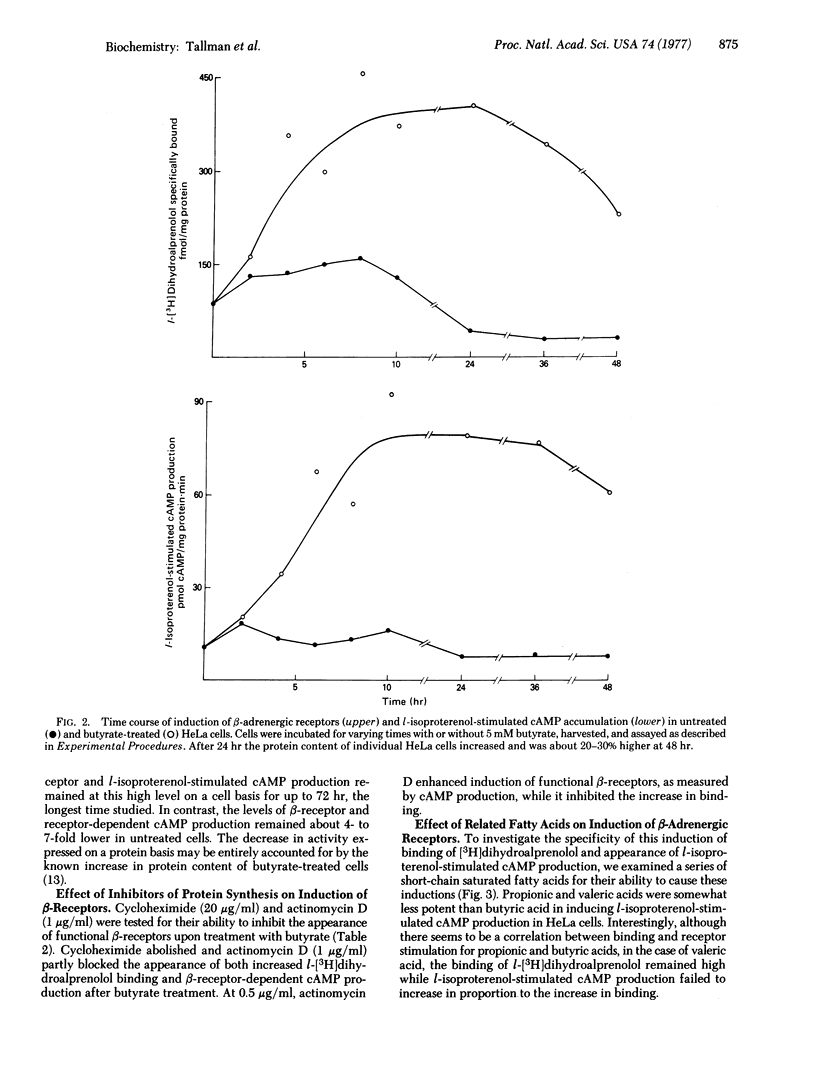
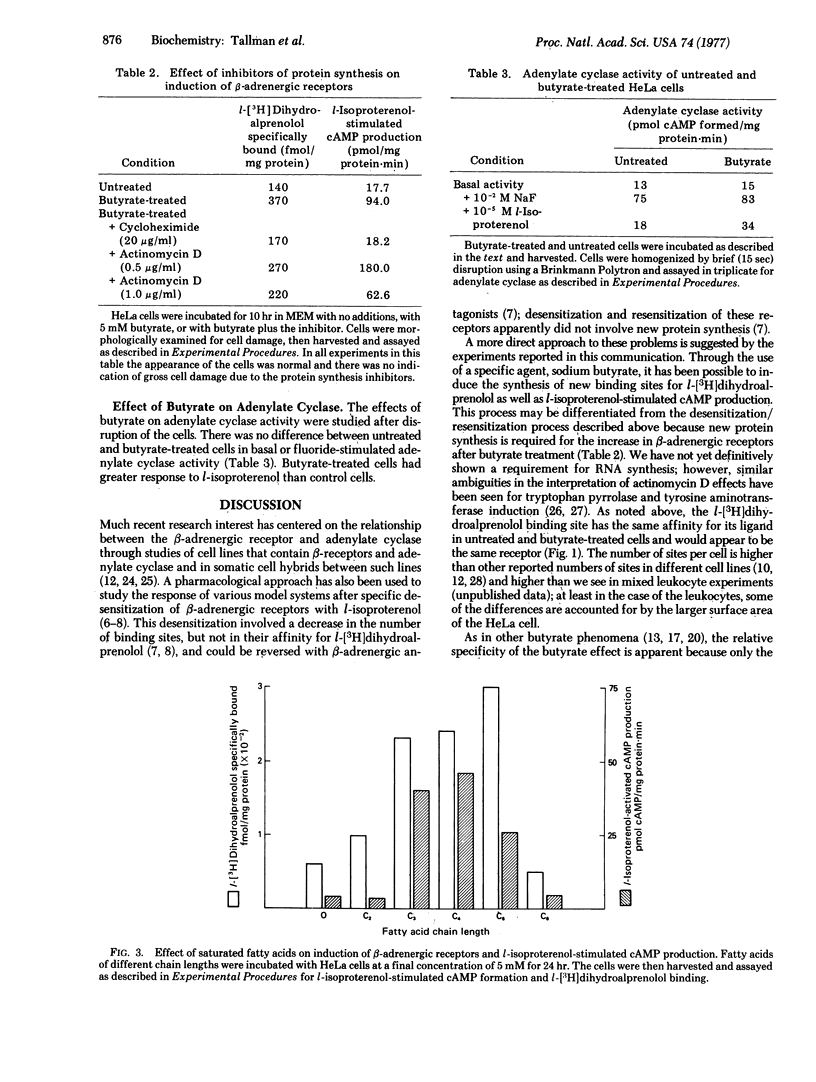
HeLa cells contain beta-adrenergic receptors that are characterized by specific binding of I[3H]dihydroalprenolol, increased 3':5'-cyclic AMP production in intact cells after incubation with l-isoproterenol, and increased adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] activity in the presence of l-isoproterenol. After cells were cultured with butyrate, the number of beta-adrenergic receptors, cyclic AMP production in intact cells, and adenylate cyclase activation by l-isoproterenol were increased severalfold over those of untreated cells. The increase involved the induction of synthesis of new receptor molecules with identical affinities for l-[3H]-dihydroalprenolol; all three processes were blocked by cycloheximide and actinomycin D. This induction was relatively specific for butyric acid and only the closely related short-chain fatty acids, propionic and valeric acids, were capable of partially inducing the same effect. In contrast to induction of beta-adrenergic binding sites, there was no increase in basal or fluoride-activated adenylate cyclase activity, indicating that the beta-adrenergic receptor and adenylate cyclase and different molecules that may be controlled separately.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurbach G. D., Fedak S. A., Woodard C. J., Palmer J. S., Hauser D., Troxler F. Beta-adrenergic receptor: stereospecific interaction of iodinated beta-blocking agent with high affinity site. Science. 1974 Dec 27;186(4170):1223–1224. doi: 10.1126/science.186.4170.1223. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. M., Fedak S. A., Woodard C. J., Aurbach G. D. Beta-Adrenergic receptor interactions. Direct comparison of receptor interaction and biological activity. J Biol Chem. 1976 Mar 10;251(5):1239–1246. [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Simmons J. L., Brady R. O., Freese E. Induction of glycolipid biosynthesis by sodium butyrate in HeLa cells. Biochem Biophys Res Commun. 1974 Jul 10;59(1):292–299. doi: 10.1016/s0006-291x(74)80205-6. [DOI] [PubMed] [Google Scholar]
- GARREN L. D., HOWELL R. R., TOMKINS G. M., CROCCO R. M. A PARADOXICAL EFFECT OF ACTINOMYCIN D: THE MECHANISM OF REGULATION OF ENZYME SYNTHESIS BY HYDROCORTISONE. Proc Natl Acad Sci U S A. 1964 Oct;52:1121–1129. doi: 10.1073/pnas.52.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M. K., Cox R. P. Production of human chorionic gonadotropin in HeLa cell cultures. Nature. 1976 Feb 5;259(5542):416–417. doi: 10.1038/259416a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G., Minna J. D. Expression of genes for metabolism of cyclic adenosine 3':5'-monophosphate in somatic cells. I. Responses to catecholamines in parental and hybrid cells. J Biol Chem. 1973 Oct 10;248(19):6610–6617. [PubMed] [Google Scholar]
- Ginsburg E., Salomon D., Sreevalsan T., Freese E. Growth inhibition and morphological changes caused by lipophilic acids in mammalian cells. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2457–2461. doi: 10.1073/pnas.70.8.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M. J., Price G. H., Bazzell K. L. A study of adenosine 3':5'-cyclic monophosphate, sodium butyrate and cortisol as inducers of HeLa alkaline phosphatase. Arch Biochem Biophys. 1974 Oct;164(2):619–623. doi: 10.1016/0003-9861(74)90073-3. [DOI] [PubMed] [Google Scholar]
- Henneberry R. C., Fishman P. H., Freese E. Morphological changes in cultured mammalian cells: prevention by the calcium ionophore A23187. Cell. 1975 May;5(1):1–9. doi: 10.1016/0092-8674(75)90085-9. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Zatz M., Romero J. A., Axelrod J. Rapid changes in rat pineal beta-adrenergic receptor: alterations in l-(3H)alprenolol binding and adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3735–3739. doi: 10.1073/pnas.72.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J. The beta-adrenergic receptor. Life Sci. 1976 Mar 1;18(5):461–472. doi: 10.1016/0024-3205(76)90323-4. [DOI] [PubMed] [Google Scholar]
- Maguire M. E., Wiklund R. A., Anderson H. J., Gilman A. G. Binding of (125I)iodohydroxybenzylpindolol to putative beta-adrenergic receptors of rat glioma cells and other cell clones. J Biol Chem. 1976 Mar 10;251(5):1221–1231. [PubMed] [Google Scholar]
- Makman M. H. Properties of adenylate cyclase of lymphoid cells. Proc Natl Acad Sci U S A. 1971 May;68(5):885–889. doi: 10.1073/pnas.68.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minna J. D., Gilman A. G. Expression of genes for metabolism of cyclic adenosine 3':5'-monophosphate in somatic cells. II. Effects of prostaglandin E1 and theophylline on parental and hybrid cells. J Biol Chem. 1973 Oct 10;248(19):6618–6625. [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Regulation of adenylate cyclase coupled beta-adrenergic receptors by beta-adrenergic catecholamines. Endocrinology. 1976 Aug;99(2):347–357. doi: 10.1210/endo-99-2-347. [DOI] [PubMed] [Google Scholar]
- Schneider F. H. Effects of sodium butyrate on mouse neuroblastoma cells in culture. Biochem Pharmacol. 1976 Oct 15;25(20):2309–2317. doi: 10.1016/0006-2952(76)90015-0. [DOI] [PubMed] [Google Scholar]
- Simmons J. L., Fishman P. H., Freese E., Brady R. O. Morphological alterations and ganglioside sialyltransferase activity induced by small fatty acids in HeLa cells. J Cell Biol. 1975 Aug;66(2):414–424. doi: 10.1083/jcb.66.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. C., Tallman J. F., Post R. M., van Kammen D. P., Jimerson D. C., Brown G. L. An examination of baseline and drug-induced levels of cyclic nucleotides in the cerebrospinal fluid of control and psychiatric patients. Life Sci. 1976 Jul 1;19(1):131–136. doi: 10.1016/0024-3205(76)90383-0. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Atlas D., Levitzki A. Inter-relations between beta-adrenergic receptors, adenylate cyclase and calcium. N Engl J Med. 1975 Feb 20;292(8):409–414. doi: 10.1056/NEJM197502202920809. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Levinson B. B., Tomkins G. M. "Superinduction" of tyrosine aminotransferase by actinomycin D: a reevaluation. Cell. 1975 May;5(1):29–35. doi: 10.1016/0092-8674(75)90088-4. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Lefkowitz R. J. Identification of beta-adrenergic receptors in human lymphocytes by (-) (3H) alprenolol binding. J Clin Invest. 1976 Jan;57(1):149–155. doi: 10.1172/JCI108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. A. Morphology and growth rate changes in Chinese hamster cells cultured in presence of sodium butyrate. Exp Cell Res. 1973 Apr;78(2):456–460. doi: 10.1016/0014-4827(73)90091-8. [DOI] [PubMed] [Google Scholar]


