Treating human immunodeficiency virus (HIV)-infected cancer patients simultaneously with antiretrovirals and chemotherapy is complicated. Little has been published on this topic. Clinicians should individualize decisions regarding HIV treatment according to clinical and laboratory findings, chemotherapy plan, comorbidities, potential toxic effects, and patient preference.
Keywords: HIV, AIDS, cancer, antiretrovirals
Abstract
The optimal antiretroviral therapy (ART) regimen for human immunodeficiency virus (HIV)–infected patients with cancer remains unknown, as clinical trials are lacking and published data are insufficient to guide recommendations. When concomitant use of chemotherapy and ART is anticipated, overlap of toxic effects and drug–drug interactions between chemotherapy and ART may alter the optimal choice of ART. Prospective studies are urgently needed to further define the toxic effects of combined chemotherapy and ART in HIV-positive cancer patients. Such studies should aid the development of guidelines for treatment of this population. For now, clinicians should individualize decisions regarding treatment of HIV according to clinical and laboratory findings, cancer treatment plan (chemotherapy, radiotherapy, or surgery), liver or renal disease, potential adverse drug effects (eg, rash, gastrointestinal intolerance, bone marrow suppression), and patient preference. This review focuses on what infectious disease specialists need to know to select the most appropriate ART regimens for patients receiving chemotherapy.
Antiretroviral therapy (ART) has led to a dramatic improvement in the outcome of human immunodeficiency virus (HIV)–infected patients [1]. However, declines in overall mortality and aging of HIV-infected cohorts have increased the overall cancer incidence among patients with HIV [2]. Furthermore, people with HIV have a higher risk for several AIDS-defining malignancies (ADMs; including Kaposi sarcoma, non-Hodgkin lymphoma [NHL], and invasive cervical cancer [3]) and non-AIDS-defining malignancies (NADMs). Cancer now accounts for approximately 33% of all HIV-related deaths [4, 5].
Several studies suggest a significant decline in the rates of ADMs and increase in the rates of NADMs (eg, cancers of the head and neck, lung, kidney, liver, gastrointestinal tract, anus, and skin [squamous cell/basal cell carcinoma, melanoma], Hodgkin lymphoma, and leukemia) [2, 6–8]. NADMs now account for more morbidity and mortality than ADMs [2, 9].
Large cohort studies have reported a consistent link between low CD4 cell counts (<350−500 cells/µL) and higher risk of ADMs and/or NADMs, suggesting that initiating ART to suppress HIV replication and maintain CD4 counts >350–500 cells/µL reduces the overall incidence of ADMs and may also reduce the incidence of NADMs [2]. The use of ART plus chemotherapy in HIV-infected cancer patients has been demonstrated to reduce morbidity associated with opportunistic infections and improve overall survival in patients with ADMs [10, 11].
In light of these findings, initiation or optimization of ART is now recommended for cancer patients infected with HIV [12], and treatment with concurrent ART and chemotherapy is increasingly common [13]. However, concomitant administration of ART and anticancer therapy is complicated, and very little has been published on this topic. Unfortunately, patients with HIV are excluded from studies of cancer drug development, and the optimal ART for HIV-infected cancer patients is unknown. Challenges in the diagnosis and management of HIV infection in patients with cancer are listed in Table 1. In this review, we focus on the practical aspects of managing HIV infection in patients with cancer receiving chemotherapy.
Table 1.
Challenges in the Management of HIV Infection in Patients With Cancer
|
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
STUDIES OF CONCOMITANT USE OF DIFFERENT TYPES OF ART AND CHEMOTHERAPY
Some studies have shown that even intensive chemotherapy protocols are feasible in HIV-infected patients, and the outcome of HIV-infected patients with Burkitt lymphoma, diffuse large B-cell lymphoma, and Hodgkin lymphoma is similar to that of HIV-negative patients receiving the same chemotherapy regimens [14]. Unfortunately, most studies of concomitant ART and chemotherapy have focused on oncologic aspects, ignoring details on type of ART and virologic outcome [15–18]. However, a few previous studies of concomitant therapy provide details that can inform selection of ART.
In a study of 80 HIV-infected patients with lung cancer, 44 patients (55%) were receiving ART before and 12 (15%) were started on ART after cancer diagnosis. The most common regimens were nucleoside reverse transcriptase inhibitors (NRTIs) in combination with a nonnucleoside reverse transcriptase inhibitor (NNRTI) and a protease inhibitor (PI) (n = 24), NRTI/PI/PI (n = 22), and NRTI/NRTI (n = 20). ART use did not affect overall survival. However, cancer-specific survival was significantly better for patients who had higher CD4 counts (≥200 cells/mL), which are an indirect effect of ART. Unfortunately, information on ART efficacy, safety, and tolerability was not provided [19].
Another study analyzed 2 groups of patients with NHL: 35 patients treated with ART and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (group 1) and 26 patients with CD20 cell–positive NHL treated with ART plus rituximab, cyclophosphamide, doxorubicin, and etoposide (group 2) [20]. ART regimens included NRTIs, NNRTIs, and PIs, with most patients receiving a PI-based regimen (group 1: 95%; group 2: 72%). Most patients maintained a virological response during chemotherapy (group 1: 84%; group 2: 68%).
Burkitt and Burkitt-like lymphomas account for 25%–40% of HIV-associated NHLs. Only 40%–50% of patients with these lymphomas achieve complete remission, and median survival time is <1 year. In a series of 13 patients with Burkitt lymphoma treated with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, 6 of 7 who received ART during chemotherapy remained alive and in complete remission at a median of 29 months, 1 of 2 patients who started ART late after chemotherapy was alive and in complete remission at 33 months, and the 4 patients who did not receive ART had died [21].
In a retrospective study of 34 HIV-infected patients with diffuse large B-cell lymphoma treated with ART and CHOP between 2002 and 2010 at 3 academic hospitals in Canada [22], 22 patients (65%) received PI-based and 12 (35%) received non-PI-based ART. Most patients on PIs received ritonavir (18 [82%]); patients not receiving PIs received raltegravir (6 [50%]) or efavirenz (6 [50%]). CHOP had similar efficacy and toxicity in the 2 ART groups. Unfortunately, no analysis of patients treated with raltegravir vs efavirenz was performed, and HIV type 1 (HIV-1) RNA was consistently measured in only 10 of the 34 patients, precluding extensive analyses of ART efficacy during chemotherapy [22].
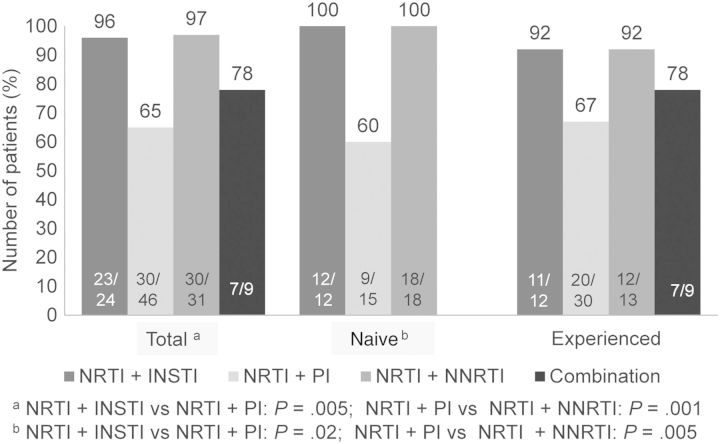
In the largest series to date analyzing different ARTs in HIV-infected patients with cancer [23], we found that PI-based regimens had the least favorable impact (Figure 1). We also found that NNRTIs and integrase strand transfer inhibitor (INSTI)–based treatments had similar efficacy.
Figure 1.
Efficacy at 6 months of antiretroviral therapy–based treatment of HIV infection in cancer patients. Abbreviations: HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
The studies described above suggest that the concomitant use of ART and chemotherapy is tolerable in most cases, is not associated with life-threatening toxic effects, and produces response and disease-free survival rates similar to those observed in patients with cancer without HIV infection [14, 24, 25].
APPROACH TO ART-NAIVE HIV-POSITIVE PATIENTS WITH CANCER
US Department of Health and Human Services (DHHS) guidelines recommend initiation of ART for all HIV-1–infected individuals to reduce the risk of disease progression. The evidence supporting this recommendation is strongest for people with pretreatment CD4 count ≤500 cells/µL [26, 27]. The DHHS guidelines do not specifically discuss ART-naive patients with newly diagnosed cancer, but the same rationale for initiating ART—to reduce the risk of HIV progression—seems to apply to such patients. In the past, the risk of cumulative toxic effects and the potential for complex and serious drug–drug interactions (DDIs) were used to justify postponement or interruption of ART during chemotherapy [28]. Today, however, the availability of >20 approved antiretrovirals permits development of tolerable and easy-to-take regimens that minimize the potential for DDIs and improve compliance with ART during chemotherapy.
Recommended Regimens
For the general population, the preferred ART regimens for ART-naive patients are 2 NRTIs in combination with an NNRTI, a PI (preferably boosted with ritonavir), or an INSTI. Similar regimens can be used in HIV-infected cancer patients, but regimens should be individualized according to cancer treatment plan (chemotherapy, radiotherapy, or surgery), liver or renal disease, potential adverse drug effects (eg, rash, gastrointestinal intolerance, bone marrow suppression, and mitochondrial dysfunction), potential for DDIs with other medications, and patient preference.
Most experienced clinicians lean toward INSTI-based regimens for patients with HIV infection and cancer given the concerns about drug interactions and tolerability with PIs and NNRTIs.
Overcoming Barriers to Initiation of ART
There are barriers to initiating and maintaining ART in patients after a new diagnosis of cancer. A large proportion of patients diagnosed with cancer and HIV simultaneously may be unwilling to initiate treatment for both conditions simultaneously. Treatment usually requires seeing 2 different specialists, and they may not communicate or coordinate care appropriately. Without adequate follow-up by an infectious diseases specialist, adherence with ART may decline, and viral load, genotype, and CD4 count results and side effects of ARTs may not be followed in a timely manner. The period of waiting for staging, pathology, molecular testing results, or insurance approvals may provide time to start ART prior to chemotherapy. Under ideal conditions, 1–2 weeks is sufficient time to initiate ART and monitor the early-onset side effects prior to chemotherapy.
Several conditions increase the urgency of ART, including AIDS-defining conditions, lower CD4 counts (eg, <200 cells/µL), and rapidly declining CD4 counts (eg, >100 cells/µL decrease per year) [2]. The presence of ADMs or NADMS for which chemotherapy is expected to cause a drop in the CD4 count also favors rapid initiation of ART. Patients with CD4 counts ≤500 cells/µL or opportunistic infections experience significantly lower rates of progression to AIDS and death when ART is initiated early, likely because improvement in immune response is critical to prevent clinical progression [29, 30].
Among patients with gastrointestinal intolerance or trouble swallowing pills (eg, patients with cancer of the oral cavity or esophagus, radiation-induced esophagitis, or gastrointestinal graft-vs-host disease [GVHD]), an attempt should be made to use ART agents available in liquid preparations or tablets that can be crushed or dissolved (eg, abacavir, emtricitabine, lamivudine, zidovudine, fosamprenavir, darunavir, tipranavir, and nevirapine). The use of agents with injectable formulations such as enfuvirtide (subcutaneous) or zidovudine (intravenous) can be considered in selected cases.
APPROACH TO ART-EXPERIENCED HIV-POSITIVE PATIENTS WITH CANCER
There is no consensus on the optimal time to change therapy for virologic failure in HIV-infected patients with cancer [2]. Expert advice should be sought in the assessment and management of HIV in ART-experienced cancer patients as several factors associated with virologic failure are commonly encountered in cancer patients, including comorbidities, incomplete medication adherence, drug side effects, difficulty with taking medication (eg, trouble swallowing pills), suboptimal pharmacokinetics (eg, variable absorption in patients with gastrointestinal GVHD), and DDIs. The patient's treatment history and past and current resistance test results should be used to identify at least 2, and preferably 3, fully active agents to combine with an optimized background ART [2].
A stable ART regimen can be modified before chemotherapy to reduce toxicity, improve adherence and tolerability, and avoid DDIs. In our series [23], physicians changed the initial ART regimen in anticipation of potential interactions with chemotherapeutic or antifungal agents (eg, voriconazole) in 11 of 154 patients (7%). Recommendations for alternative ART should balance benefits and risks for patients.
ART interruptions should be avoided because of increased risk of death, AIDS, and serious non-AIDS morbidity associated with untreated HIV infection [30]. However, ART may be interrupted perioperatively or when anticancer drugs have clinically significant DDIs with ART and no alternative anticancer drugs are available. In such cases, ART must be reinitiated as soon as possible. Providers should be mindful that when an ART regimen contains drugs with differing half-lives, stopping all drugs simultaneously may result in functional monotherapy with the drug with the longest half-life (eg, efavirenz), increasing the risk of selection of resistant mutations [2].
In patients with a poor prognosis due to cancer (eg, patients with incurable malignancy) and higher CD4 counts, it may be reasonable to forego ART; such patients are unlikely to have symptoms of HIV infection and are unlikely to have their survival prolonged by ART.
USE OF SPECIFIC ART AGENTS IN HIV-INFECTED PATIENTS WITH CANCER
Nucleoside Reverse Transcriptase Inhibitors
Concomitant use of NRTIs with immunosuppressive agents may be limited by additive toxic effects. Tenofovir may lead to renal dysfunction, particularly in patients receiving other nephrotoxic drugs. Renal function should be monitored over time and the dose adjusted in the case of renal impairment. In our series, tenofovir-based combinations were used in 68% of patients and were not associated with substantial renal toxic effects [23].
In patients to be treated with abacavir-lamivudine fixed-dose combination, baseline screening for HLA-B*5701 should be performed to reduce the risk of hypersensitivity reaction to abacavir. Zidovudine commonly causes nausea, anemia, and myelosuppression [31], which can be potentiated by chemotherapy. Therefore, zidovudine should be reserved for cancer patients unable to receive abacavir or tenofovir.
Protease Inhibitors
The combination of PIs and specific chemotherapy drugs can cause significant nonhematologic [23] and hematologic toxic effects [32], which underscores the need for caution in prescribing these combinations and regular monitoring of patients who receive them. In our series, the rate of nonhematologic side effects was 35% in patients receiving PIs vs 14% in those receiving NNRTIs and 3% in those receiving INSTIs (P = .001). These side effects were attributed to either the ART regimen or overlapping toxic effects between ART and chemotherapy [23].
In one study, 46 patients with AIDS-related NHL treated with chemotherapy and concomitant ART received 190 cycles of cyclophosphamide, doxorubicin, and etoposide. Grade 3 or 4 infections requiring hospitalization were noted in 48% of cycles with PIs and 25% of cycles with PI-sparing ART (P = .002). Grade 4 neutropenia was observed in 54% of cycles with PIs and 38% with PI-sparing ART (P = .05). Despite the increased toxicity, there were no differences in response rate, disease-free survival, or overall survival between the 2 ART groups [32].
Nonnucleoside Reverse Transcriptase Inhibitors
Efavirenz use is limited by DDIs and prolonged half-life. Limited experience exists with treating cancer patients with newer NNRTIs, such as etravirine, which has unpredictable DDIs with immunosuppressants such as cyclosporine, tacrolimus, sirolimus, and mycophenolate mofetil [33]. Rilpivirine, another second-generation NNRTI, is primarily metabolized by CYP3A but does not induce the P450 system and theoretically should not affect immunosuppressant drug levels [33].
Integrase Strand Transfer Inhibitors
Recent guidelines state that raltegravir-based regimens may be considered in malignancy because of their favorable drug interaction profile [30]. Raltegravir undergoes glucuronidation by UGT1A1 and has a lower potential for DDIs than do PIs and NNRTIs [2, 13]. However, it requires twice-daily dosing [2].
Elvitegravir is a CYP3A4 substrate; therefore, DDIs are expected with several drugs commonly used in oncology. Additionally, elvitegravir requires boosting with cobicistat, a potent CYP3A4 inhibitor that may result in DDIs with other concomitant medications [33].
Dolutegravir, the most recently approved INSTI, has a favorable drug interaction profile and is metabolized primarily by uridine 5′-diphosphate-glucuronosyltransferase, with CYP3A4 playing a minor role [2, 34]. Further studies with this INSTI and chemotherapy are needed. Mild increases in the serum creatinine level have been observed with dolutegravir, likely caused by a mechanism similar to that described for trimethoprim [35]. Of all the INSTIs, dolutegravir has the shortest duration of follow-up and the most limited postmarketing experience. Continued safety monitoring is warranted in the postmarketing setting, especially in cancer patients receiving multiple medications with side effects that can overlap with those of dolutegravir. Unlike raltegravir, dolutegravir can be given once per day [36].
Attachment Inhibitors and Additional Agents
Maraviroc is the only approved CCR5 attachment inhibitor. Maraviroc has a theoretical advantage in HIV-infected patients undergoing hematopoietic stem cell transplant (HSCT) as the CCR5 receptor itself appears to play a role in the pathogenesis of GVHD. When maraviroc was added to a conventional GVHD prophylaxis regimen after allogeneic HSCT, a lower incidence of liver and gastrointestinal GVHD was observed [37]. However, the use of maraviroc requires receptor tropism screening [35], and maraviroc is a substrate of the CYP3A enzyme and ABCB1 transporter, and therefore susceptible to many drug interactions [13].
The entry inhibitor enfuvirtide also is used successfully in salvage regimens but is poorly tolerated because of injection site reactions [30].
MONITORING CANCER PATIENTS RECEIVING ART
In HIV-infected cancer patients, as in other HIV-infected patients, CD4 count, HIV-1 RNA level, and ART adherence should be monitored [30]. In HIV-infected cancer patients, CD4 count should be interpreted with caution as an indicator of immunologic response to ART as CD4 counts can be affected by malignancies or their treatment. In a series of 20 patients with lymphoma treated with concomitant ART and chemotherapy, patients experienced a >50% reduction in CD4 counts during the first 3 months of chemotherapy. These counts returned to pretreatment levels within 1 month after chemotherapy was completed [38].
In cancer patients with HIV infection, as in the general population of HIV-infected patients, suppression of plasma HIV-1 RNA level to <50 copies/mL is expected to occur by 24 weeks with effective therapy, regardless of prior treatment experience [30]. No significant change in the HIV-1 RNA load is observed as a result of chemotherapy [38], but frequent monitoring of viral load and ART adherence may be necessary during chemotherapy. We monitor our patients once a month in the first 3 months after induction chemotherapy or HSCT, and at 3-month intervals thereafter.
CHALLENGES IN THE SELECTION OF CHEMOTHERAPY FOR PATIENTS RECEIVING ART
Treating cancer in patients with HIV/AIDS receiving ART is complicated because of the poor clinical understanding of DDIs between antineoplastic agents and ART and the narrow therapeutic index of anticancer agents [7, 11, 13].
Drug–Drug Interactions
A major concern with the use of many ARTs is the potential for DDIs mediated by drug-metabolizing enzymes or transporters that lead to altered drug exposure [39].
For NRTIs, the potential for DDIs is minimal because these agents are not eliminated by the CYP450 system and do not induce or inhibit CYP450 enzymes [11]; however, NRTIs may be involved in transporter-mediated interactions as renal clearance is their major route of elimination [39]. In contrast, for PIs, NNRTIs, and chemokine receptor antagonists, the potential for DDIs is high because these agents are extensively metabolized by and induce or inhibit the CYP450 system [13], which mediates the metabolism of more than one-half of all drugs that undergo hepatic metabolism [40]. To varying degrees, all PIs inhibit CYP3A4 [7]. Ritonavir is the most potent CYP3A4 inhibitor [33] and a strong inhibitor of CYP2C8, CYP2D6, and ABCB1 and a weak inducer of CYP2B6, CYP2C9, CYP3A4, and ABCB1 [40, 41]. Efavirenz is a mixed inducer and inhibitor of CYP3A [42]. The CCR5 antagonist maraviroc has the potential for DDIs as it is a substrate of CYP3A and ABCB1, but maraviroc does not alter metabolism or transport and is unlikely to induce enzyme-mediated interactions [39]. Enfuvirtide undergoes hydrolysis; to date, no DDIs have been reported with this agent [39].
Many anticancer agents are metabolized by CYP450 and therefore have high potential for DDIs with CYP450-metabolized ART. Anthracyclines, antimetabolite agents, antitumor antibiotics, and platinums undergo non-CYP450 routes of elimination and are unlikely to be altered by ART [13]. On the other hand, DDIs can be anticipated with other classes of anticancer agents, including alkylating agents, corticosteroids, epipodophyllotoxins, taxanes, tyrosine-kinase inhibitors, and vinca alkaloids [13]. Drugs commonly used in cancer and HSCT patients and known to inhibit CYP3A4 include voriconazole and clarithromycin [7].
Recently, several preclinical studies have been published on the use of newer chemotherapy agents primarily metabolized by CYP3A concomitantly with PIs or NRTIs. In a study using primary cultures of human hepatocytes, the CYP3A4 inhibitor ritonavir inhibited the metabolism of erlotinib, a tyrosine kinase inhibitor primarily metabolized by CYP3A, by 4.2-fold, whereas efavirenz decreased the exposure of erlotinib by 3-fold [42], suggesting that the clinically used dose of erlotinib (150 mg daily) may have to be substantially reduced (to 25 mg every other day) when coadministered with ritonavir or increased (to 300 mg daily) when coadministered with efavirenz to achieve the desired drug exposure [42].
In a study in mice, efavirenz and dexamethasone, CYP3A4 inducers, did not have a significant effect on exposure (area under the curve [AUC]) of docetaxel, an agent metabolized primarily by CYP3A4. However, ritonavir resulted in a 6.9-fold increase in the AUC of docetaxel [41]. On the basis of these results, it could be anticipated that standard docetaxel dosing would not be tolerable in patients receiving ritonavir-based ART. The authors of the study recommended proceeding with caution with docetaxel administration in patients receiving ritonavir-based ART until further clinical studies are performed [41]. This study and the aforementioned in vitro study of ritonavir plus erlotinib and efavirenz plus erlotinib suggest the need for confirmatory phase 1 dose-finding trials of newer chemotherapy agents in combination with ART.
To better understand potential DDIs and chemotherapy tolerability, the AIDS Malignancy Consortium, a National Cancer Institute–sponsored cooperative group, launched in 2009 a series of prospective clinical studies of new targeted chemotherapy agents in HIV-infected patients with refractory cancers receiving ART [8]. The goal of these studies, which are ongoing, is to identify tolerable dosing regimens that can be applied to complex patient populations [39]. HIV-infected patients were originally stratified into 3 groups based on ART: (1) NNRTI based, (2) non-ritonavir PI based, and (3) ritonavir PI based [43]. Inclusion of some INSTI-based regimens should be considered in future studies analyzing the safety of concomitant use of chemotherapeutic agents and ART. The results of the first of this series of trials were recently published. The chemotherapy agent studied was sunitinib, a multitargeted tyrosine kinase inhibitor approved for the treatment of renal cell carcinoma, gastrointestinal stromal tumors, and pancreatic neuroendocrine tumors that is metabolized by CYP3A4 to produce the primary active metabolite N-desethyl sunitinib. In a modified phase 1 study, patients were stratified into 2 treatment arms according to whether or not their ART was based on ritonavir. Patients receiving non-ritonavir-based ART received sunitinib at the standard dose (50 mg/day). Patients receiving ritonavir-based ART were treated with sunitinib according to a dose escalation design (from 25 mg/day to 50 mg/day). Efavirenz resulted in increased exposure of N-desethyl sunitinib, whereas ritonavir caused decreased exposure of the metabolite. Patients receiving non-ritonavir-based ART tolerated standard dosing of sunitinib; however, patients receiving ritonavir-based ART experienced toxic effects at the 37.5-mg/day sunitinib dose level that were similar to or worse than the toxic effects experienced by patients in the non-ritonavir-based ART group at the 50-mg/day sunitinib dose level, including higher rates of grade 3 neutropenia. Therefore, dose reductions of sunitinib to 37.5 mg/day may be warranted in patients receiving ritonavir. On the other hand, no significant effect of treatment with sunitinib on CD4 count or HIV load parameters was observed [40]. This and future studies will help us to better understand potential DDIs at the level of CYP3A4 and to provide recommendations on the use of targeted anticancer agents in the care of HIV-positive patients receiving ART.
In our series [23], several ARTs were administered with several cancer therapies, including cytotoxic drugs, taxanes, vinca alkaloids, topoisomerase inhibitors, alkylating agents, antimetabolites, antitumor antibiotics, targeted therapy, and immunosuppressants. No significant drug-associated toxic effects or HIV disease progression was observed. Only 1 patient, who was receiving PI-based ART and cyclosporine, developed clinically relevant DDIs (those necessitating adjustment of doses or discontinuation of coadministered agents). However, our results should be interpreted with caution as a multidisciplinary team (infectious diseases specialists, oncologists, and pharmacists) reviewed every ART regimen before its initiation or continuation to prevent clinically relevant DDIs.
The clinical importance of complex drug interactions in HIV-infected cancer patients receiving ARTs and chemotherapy should not be considered trivial, and patients receiving concomitant chemotherapy and ART regimens should be closely monitored. In a study of 2 patients with HIV-associated Kaposi sarcoma who received paclitaxel along with PI-based ART, life-threatening toxic effects developed in both patients [44].
Enhanced and Overlapping Toxic Effects
When ART and antineoplastic agents are combined, enhanced and overlapping toxic effects are possible, even when the oncologic therapy consists of newer targeted therapies [7, 8, 11, 13, 39].
PI-based ART appears to significantly potentiate the myelotoxicity of chemotherapy [45]. Clinicians contemplating taxane-based chemotherapy, for example, should carefully monitor patients for adverse events and, depending on their patient's ART regimen, consider reducing the taxane dose [44].
In one study, 92 of 93 HIV-positive patients with Hodgkin lymphoma received ART concomitantly with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD), and only 1 patient died of treatment-related toxic effects [14]. There was no difference in overall survival and event-free survival between HIV-positive and HIV-negative patients, suggesting that concomitant treatment with ART neither increases the rate of fatal toxic effects in patients treated with ABVD nor jeopardizes their outcome [14]. However, the numbers of patients receiving specific types of ART were not reported.
Because of the potential for arrhythmias and sudden death, combinations of agents that can prolong the QT interval should be avoided. QT prolongation has been associated not only with PIs, such as atazanavir, ritonavir-boosted lopinavir, and saquinavir, but also with newer anticancer agents, including the tyrosine kinase inhibitors (eg, lapatinib and nilotinib) [39].
Prophylaxis Against Opportunistic Infections
The guidelines for prophylaxis against opportunistic infections in patients with HIV take into account risk, serologic testing results, and history of exposure, as well as the status of the immune system, particularly as reflected by the CD4 count, the receipt of and duration of ART, and the response to ART [12]. The guidelines for prevention of infections in patients with cancer are centered on the degree and duration of neutropenia, a key risk factor for infection, but also take into account immunodeficiency associated with malignancy (eg, hypogammaglobulinemia associated with chronic lymphocytic leukemia), disruption of mucosal barriers, use of lymphotoxic agents (eg, high-dose corticosteroids, fludarabine, and alemtuzumab), and HSCT [46, 47]. Both sets of guidelines need to be considered to prevent opportunistic infections in HIV-infected patients with cancer.
Our preference as infectious diseases consultants is to start by administering the recommended prophylaxis against opportunistic infections in patients with HIV. Once the treatment plan for cancer is determined, we add any additional agents recommended for prophylaxis against infections in patients treated with the particular chemotherapy regimen or HSCT. We reevaluate recommendations on a regular basis and adjust as needed, always in coordination with the oncology team. HIV-related prophylaxis may have to be modified as the patient's CD4 count decreases with some chemotherapy regimens or increases after completion of therapy. Use of lymphotoxic agents such as fludarabine or alemtuzumab may lead to significant CD4 count depletion and an increased risk of opportunistic infections such as cytomegalovirus infection, other herpesvirus reactivations, mycobacterial infection, and invasive fungal infection [48, 49]. Specific recommendations for cytomegalovirus prophylaxis and use of mold-active antifungals will usually be included as part of the chemotherapy protocol.
Trimethoprim/sulfamethoxazole may need to be replaced with another prophylactic drug or combination when patients have prolonged myelosuppression or weak recovery of cell counts after chemotherapy or in the preengraftment period after HSCT. Pentamidine, dapsone, and atovaquone are the commonly used substitutes. Prophylaxis against infection during chemotherapy may include drugs that interact with ART. Examples include the mold-active triazoles voriconazole and posaconazole. Efavirenz should not be coadministered with either voriconazole or posaconazole because it decreases the triazole AUC; ritonavir should be avoided with posaconazole [12]. We prefer to avoid efavirenz given that it may decrease the serum concentration of triazoles, particularly because this effect may last for several weeks after efavirenz is discontinued.
CONCLUSIONS
In HIV-infected patients receiving chemotherapy for cancer or HSCT, most ART regimens can be safely implemented to suppress viral replication. Overlapping toxic effects and DDIs between chemotherapy and ARTs may alter the ART choice. Recommendations for diagnosis and management of HIV infection in patients with cancer are summarized in Table 2. Unmet clinical and research needs in the management of HIV infection in patients with cancer and cancer in patients with HIV infection are presented in Table 3. New research may elucidate the potential interactions between ART and new anticancer agents and permit establishment of guidelines for treatment of HIV in patients with specific cancers. HIV-positive cancer patients should not be excluded from studies on cancer drug development and ART.
Table 2.
Recommendations for Diagnosis and Management of HIV Infection in Patients With Cancer
|
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; HSCT, hematopoietic stem cell transplant.
Table 3.
Unmet Clinical and Research Needs Related to Management of HIV Infection in Patients With Cancer and Management of Cancer in Patients With HIV Infection
| Clinical |
| Routine HIV screening in cancer centers. |
| Systematic and aggressive cancer screening of HIV-infected patients. |
| Improvement of cancer prevention capabilities and access to cancer treatment for HIV-infected patients. |
| Early detection and treatment of coinfections caused by carcinogenic viruses (eg, hepatitis B virus, hepatitis C virus, and human papillomavirus) in cancer patients with HIV. |
| Research |
| Inclusion of HIV-infected cancer patients in clinical trials of both cancer drugs and ART. |
| Characterization of drug interactions between ARTs and chemotherapy agents, including newer agents. |
| Well-designed studies of antimicrobial prophylaxis. |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
Notes
Acknowledgments. We thank Stephanie Deming for editorial assistance.
Author contributions. H. A. T. and V. E. M. designed and wrote the article.
Potential conflicts of interest. H. A. T. has been a consultant to Gilead Sciences, Merck & Co, Novartis, Astellas, Pfizer, Genentech, Theravance, and Vertex Pharmaceuticals; and has received research funding from Merck & Co, and Vertex Pharmaceuticals. V. E. M. reports no potential conflicts.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–33. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 7 November 2013.
- 3.Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years—United States, 2008. MMWR Recomm Rep. 2008;57:1–12. [PubMed] [Google Scholar]
- 4.Bonnet F, Burty C, Lewden C, et al. Changes in cancer mortality among HIV-infected patients: the Mortalite 2005 Survey. Clin Infect Dis. 2009;48:633–9. doi: 10.1086/596766. [DOI] [PubMed] [Google Scholar]
- 5.Monforte A, Abrams D, Pradier C, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS. 2008;22:2143–53. doi: 10.1097/QAD.0b013e3283112b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powles T, Robinson D, Stebbing J, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol. 2009;27:884–90. doi: 10.1200/JCO.2008.19.6626. [DOI] [PubMed] [Google Scholar]
- 7.Deeken JF, Pantanowitz L, Dezube BJ. Targeted therapies to treat non-AIDS-defining cancers in patients with HIV on HAART therapy: treatment considerations and research outlook. Curr Opin Oncol. 2009;21:445–54. doi: 10.1097/CCO.0b013e32832f3e04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deeken JF, Tjen ALA, Rudek MA, et al. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis. 2012;55:1228–35. doi: 10.1093/cid/cis613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsuyasu RT. Non-AIDS-defining malignancies in HIV. Top HIV Med. 2008;16:117–21. [PubMed] [Google Scholar]
- 10.Antinori A, Cingolani A, Alba L, et al. Better response to chemotherapy and prolonged survival in AIDS-related lymphomas responding to highly active antiretroviral therapy. AIDS. 2001;15:1483–91. doi: 10.1097/00002030-200108170-00005. [DOI] [PubMed] [Google Scholar]
- 11.Mounier N, Katlama C, Costagliola D, Chichmanian RM, Spano JP. Drug interactions between antineoplastic and antiretroviral therapies: implications and management for clinical practice. Crit Rev Oncol Hematol. 2009;72:10–20. doi: 10.1016/j.critrevonc.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. quiz CE1-4. [PubMed] [Google Scholar]
- 13.Rudek MA, Flexner C, Ambinder RF. Use of antineoplastic agents in patients with cancer who have HIV/AIDS. Lancet Oncol. 2011;12:905–12. doi: 10.1016/S1470-2045(11)70056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montoto S, Shaw K, Okosun J, et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol. 2012;30:4111–6. doi: 10.1200/JCO.2011.41.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berretta M, Di Benedetto F, Dal Maso L, et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma in HIV-positive patients. Anticancer Drugs. 2013;24:212–8. doi: 10.1097/CAD.0b013e32835c032f. [DOI] [PubMed] [Google Scholar]
- 16.Levine AM, Noy A, Lee JY, et al. Pegylated liposomal doxorubicin, rituximab, cyclophosphamide, vincristine, and prednisone in AIDS-related lymphoma: AIDS Malignancy Consortium Study 047. J Clin Oncol. 2013;31:58–64. doi: 10.1200/JCO.2012.42.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo JJ, Echenique IA. Rituximab in combination with chemotherapy versus chemotherapy alone in HIV-associated non-Hodgkin lymphoma: a pooled analysis of 15 prospective studies. Am J Hematol. 2012;87:330–3. doi: 10.1002/ajh.22275. [DOI] [PubMed] [Google Scholar]
- 18.Blazy A, Hennequin C, Gornet JM, et al. Anal carcinomas in HIV-positive patients: high-dose chemoradiotherapy is feasible in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2005;48:1176–81. doi: 10.1007/s10350-004-0910-7. [DOI] [PubMed] [Google Scholar]
- 19.Pakkala S, Chen Z, Rimland D, et al. Human immunodeficiency virus-associated lung cancer in the era of highly active antiretroviral therapy. Cancer. 2012;118:164–72. doi: 10.1002/cncr.26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonelli C, Zanussi S, Cinelli R, et al. Impact of concomitant antiblastic chemotherapy and highly active antiretroviral therapy on human immunodeficiency virus (HIV) viremia and genotyping in HIV-infected patients with non-Hodgkin lymphoma. Clin Infect Dis. 2003;37:820–7. doi: 10.1086/377204. [DOI] [PubMed] [Google Scholar]
- 21.Cortes J, Thomas D, Rios A, et al. Hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone and highly active antiretroviral therapy for patients with acquired immunodeficiency syndrome-related Burkitt lymphoma/leukemia. Cancer. 2002;94:1492–9. doi: 10.1002/cncr.10365. [DOI] [PubMed] [Google Scholar]
- 22.Wong AY, Marcotte S, Laroche M, et al. Safety and efficacy of CHOP for treatment of diffuse large B-cell lymphoma with different combination antiretroviral therapy regimens: SCULPT study. Antivir Ther. 2013;18:699–707. doi: 10.3851/IMP2572. [DOI] [PubMed] [Google Scholar]
- 23.Torres HA, Rallapalli V, Saxena A, et al. Efficacy and safety of antiretrovirals in HIV-infected patients with cancer [Epub ahead of print] Clin Microbiol Infect. 2014 doi: 10.1111/1469-0691.12589. doi:10.1111/1469-0691.12589. [DOI] [PubMed] [Google Scholar]
- 24.Ratner L, Lee J, Tang S, et al. Chemotherapy for human immunodeficiency virus-associated non-Hodgkin's lymphoma in combination with highly active antiretroviral therapy. J Clin Oncol. 2001;19:2171–8. doi: 10.1200/JCO.2001.19.8.2171. [DOI] [PubMed] [Google Scholar]
- 25.Vaccher E, Spina M, di Gennaro G, et al. Concomitant cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy plus highly active antiretroviral therapy in patients with human immunodeficiency virus-related, non-Hodgkin lymphoma. Cancer. 2001;91:155–63. doi: 10.1002/1097-0142(20010101)91:1<155::aid-cncr20>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–44. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann C, Chow KU, Wolf E, et al. Strong impact of highly active antiretroviral therapy on survival in patients with human immunodeficiency virus-associated Hodgkin's disease. Br J Haematol. 2004;125:455–62. doi: 10.1111/j.1365-2141.2004.04934.x. [DOI] [PubMed] [Google Scholar]
- 29.Zolopa A, Andersen J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society–USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 31.Margolis AM, Heverling H, Pham PA, Stolbach A. A review of the toxicity of HIV medications. J Med Toxicol. 2014;10:26–39. doi: 10.1007/s13181-013-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bower M, Powles T, Stebbing J, Thirlwell C. Potential antiretroviral drug interactions with cyclophosphamide, doxorubicin, and etoposide. J Clin Oncol. 2005;23:1328–9. doi: 10.1200/JCO.2005.05.128. author reply 9–30. [DOI] [PubMed] [Google Scholar]
- 33.Primeggia J, Timpone JG, Jr, Kumar PN. Pharmacologic issues of antiretroviral agents and immunosuppressive regimens in HIV-infected solid organ transplant recipients. Infect Dis Clin North Am. 2013;27:473–86. doi: 10.1016/j.idc.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Reese MJ, Savina PM, Generaux GT, et al. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos. 2013;41:353–61. doi: 10.1124/dmd.112.048918. [DOI] [PubMed] [Google Scholar]
- 35.Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369:1807–18. doi: 10.1056/NEJMoa1215541. [DOI] [PubMed] [Google Scholar]
- 36.Arribas JR, Eron J. Advances in antiretroviral therapy. Curr Opin HIV AIDS. 2013;8:341–9. doi: 10.1097/COH.0b013e328361fabd. [DOI] [PubMed] [Google Scholar]
- 37.Reshef R, Luger SM, Hexner EO, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367:135–45. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powles T, Imami N, Nelson M, Gazzard BG, Bower M. Effects of combination chemotherapy and highly active antiretroviral therapy on immune parameters in HIV-1 associated lymphoma. AIDS. 2002;16:531–6. doi: 10.1097/00002030-200203080-00003. [DOI] [PubMed] [Google Scholar]
- 39.Beumer JH, Venkataramanan R, Rudek MA. Pharmacotherapy in cancer patients with HIV/AIDS. Clin Pharmacol Ther. 2014;95:370–2. doi: 10.1038/clpt.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudek MA, Moore PC, Mitsuyasu RT, et al. A phase 1/pharmacokinetic study of sunitinib in combination with highly active antiretroviral therapy in human immunodeficiency virus-positive patients with cancer. AIDS Malignancy Consortium trial AMC 061; 2014. [published onle ahead of print 28 January 2014] Cancer doi:10.1002/cncr.28554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudek MA, Chang CY, Steadman K, Johnson MD, Desai N, Deeken JF. Combination antiretroviral therapy (cART) component ritonavir significantly alters docetaxel exposure. Cancer Chemother Pharmacol. 2014;73:729–36. doi: 10.1007/s00280-014-2399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillai VC, Venkataramanan R, Parise RA, et al. Ritonavir and efavirenz significantly alter the metabolism of erlotinib—an observation in primary cultures of human hepatocytes that is relevant to HIV patients with cancer. Drug Metab Dispos. 2013;41:1843–51. doi: 10.1124/dmd.113.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deeken JF, Mitsuyasu RT, Little RF. Treating HIV+ patients for non-AIDS-defining cancers (NADCs) in the era of targeted chemotherapy: an AIDS malignancy consortium study of sunitinib in patients on ART. J Clin Oncol. 2010;28(suppl 15):TPS161. [Google Scholar]
- 44.Bundow D, Aboulafia DM. Potential drug interaction with paclitaxel and highly active antiretroviral therapy in two patients with AIDS-associated Kaposi sarcoma. Am J Clin Oncol. 2004;27:81–4. doi: 10.1097/01.coc.0000045921.91037.c8. [DOI] [PubMed] [Google Scholar]
- 45.Bower M, McCall-Peat N, Ryan N, et al. Protease inhibitors potentiate chemotherapy-induced neutropenia. Blood. 2004;104:2943–6. doi: 10.1182/blood-2004-05-1747. [DOI] [PubMed] [Google Scholar]
- 46.Segal BH, Freifeld AG, Baden LR, et al. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw. 2008;6:122–74. doi: 10.6004/jnccn.2008.0013. [DOI] [PubMed] [Google Scholar]
- 47.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Q, Tan DC, Samuel M, Chan ES, Linn YC. Fludarabine in comparison to alkylator-based regimen as induction therapy for chronic lymphocytic leukemia: a systematic review and meta-analysis. Leuk Lymphoma. 2004;45:2239–45. doi: 10.1080/10428190412331283260. [DOI] [PubMed] [Google Scholar]
- 49.Fraser G, Smith CA, Imrie K, Meyer R. Alemtuzumab in chronic lymphocytic leukemia. Curr Oncol. 2007;14:96–109. doi: 10.3747/co.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]