Although very similar in chemical and microbiological properties, colistin and polymyxin B differ substantially when administered in their parenteral formulations. Overall, polymyxin B has superior clinical pharmacological properties, but both should be made available for specific types of patients and infections.
Keywords: colistin, polymyxin B, differing clinical pharmacological behaviors, therapeutic implications
Abstract
Colistin and polymyxin B have indistinguishable microbiological activity in vitro, but they differ in the form administered parenterally to patients. Polymyxin B is administered directly as the active antibiotic, whereas colistin is administered as the inactive prodrug, colistin methanesulfonate (CMS). CMS must be converted to colistin in vivo, but this occurs slowly and incompletely. Here we summarize the key differences between parenteral CMS/colistin and polymyxin B, and highlight the clinical implications. We put forth the view that overall polymyxin B has superior clinical pharmacological properties compared with CMS/colistin. We propose that in countries such as the United States where parenteral products of both colistin and polymyxin B are available, prospective studies should be conducted to formally examine their relative efficacy and safety in various types of infections and patients. In the meantime, where clinicians have access to both polymyxins, they should carefully consider the relative merits of each in a given circumstance.
With increasing antibiotic resistance among important gram-negative bacteria and the dry drug development pipeline for new antibiotics, there is resurgence in the clinical use of colistin (polymyxin E) and polymyxin B [1–5]. Until recently, the use of these old polymyxin antibiotics as “last-line” therapy has had a flimsy pharmacological basis because they entered into clinical use in the 1950s, before the introduction of modern drug development processes [2, 5]. However, there has been a recent substantial increase in the understanding of the pharmacology of colistin and polymyxin B [2–6].
Perhaps not surprisingly, in view of their very similar chemical structures and in vitro antibacterial activities, colistin and polymyxin B are often regarded as being equivalent. However, such superficial comparisons conceal very important differences that manifest only when these polymyxins are used clinically, administered to patients by way of their long-established parenteral formulations. In this article, we review the clinically relevant pharmacological characteristics of colistin and polymyxin B and pose the question: Are they like “peas in a pod” or “chalk and cheese”?
CHEMISTRY AND ANTIBACTERIAL ACTIVITY OF THE POLYMYXINS
Colistin and polymyxin B differ by just 1 amino acid in the peptide ring (Figure 1, upper panel) [3]. The polymyxins are produced by fermentation and, as a result, they are mixtures that may contain approximately 30 components. The European Pharmacopoeia has set limits for some of the components of colistin and polymyxin B [7, 8], whereas the US Pharmacopeia has no such limits [9, 10]. There are 2 major components for colistin (colistin A and B) and for polymyxin B (polymyxin B1 and B2), the difference arising from the length of the fatty acyl chain (Figure 1) [3].
Figure 1.
Structures of colistin A and B and polymyxin B1 and B2 (upper panel). In polymyxin B, D-Phe (phenylalanine) replaces the D-Leu (leucine, *). Structures of colistin methanesulfonate A and B are shown (lower panel). Abbreviations: fatty acid: 6-methyloctanoic acid for colistin A and polymyxin B1, and 6-methylheptanoic acid for colistin B and polymyxin B2; Thr, threonine; Dab, α,γ-diaminobutyric acid. α and γ indicate the respective -NH2 involved in the peptide linkage.
The chemistry of the polymyxins is critical to their antibacterial activity. The primary amines of the α,γ-diaminobutyric acid (Dab) residues are ionized at physiological pH and thus the polymyxin molecules carry a net-positive charge, a critical property for their interaction with negatively charged phosphate groups of the lipid A of lipopolysaccharide (LPS). In addition, polymyxins possess hydrophobic regions, the most recognizable being the fatty acyl chain, and these domains are able to interact with the corresponding regions of LPS [3]. As a result of these electrostatic and hydrophobic interactions, the bacterial outer membrane is disrupted [3, 11]. Although this permeabilizing effect on the outer membrane led to the proposing of the “self-promoted” uptake of polymyxins [12], the ultimate mechanism of bacterial killing is still unknown. In view of the very similar chemical structures of colistin and polymyxin B, it is not surprising that they have essentially identical antibacterial activities against gram-negative bacteria [13]. The most common way in which gram-negative bacteria become resistant to these antibiotics is through chemical modification or loss of the initial polymyxin target, LPS [3, 14–16], and cross-resistance exists between the 2 polymyxins.
Whereas there are many similarities in the chemistry of colistin and polymyxin B, there is a very important difference in regard to the chemical nature of what is administered parenterally to patients. Polymyxin B is administered as its sulfate salt. This means that the active antibacterial entity is directly administered to patients. In sharp contrast, colistin is administered parenterally in the form of the sodium salt of colistin methanesulfonate (CMS), also known as colistimethate (Figure 1, lower panel). CMS is produced by chemical modification of colistin, resulting in the masking of the primary amines of the Dab residues with methanesulfonate moieties that are negatively charged at physiological pH. As a consequence, CMS itself lacks antibacterial activity; it is an inactive prodrug and requires conversion to colistin after its administration [17, 18].
Although CMS is usually depicted as a penta-methanesulfonated compound (Figure 1), it is important to recognize its chemical complexity [19]. Because CMS is produced by chemical modification of colistin molecules that contain 5 primary amines, in theory there are 32 (ie, 25) possible compounds that may result (ie, colistin itself, the fully penta-methanesulfonated version, and 30 partially methanesulfonated derivatives) [19]. Given the very substantial complexity involved, it is perhaps not unexpected that neither the US Pharmacopeia nor the European Pharmacopoeia has set limits for the numerous potential components of CMS [7, 9]. It is evident that there is brand-to-brand or even batch-to-batch variability in the composition of CMS formulated as the parenteral product and that this can impact the rate and extent of formation of colistin in vivo [20].
WHICH POLYMYXIN IS ADMINISTERED PARENTERALLY TO PATIENTS IN VARIOUS PARTS OF THE WORLD?
It is unfortunate that in some countries (eg, Japan, South Africa), clinicians do not have access to a parenteral product of either polymyxin. Some areas of the world (eg, Europe, Australia) have only the parenteral formulation of colistin (as CMS), whereas in other areas (eg, United States, Brazil, Malaysia, and Singapore), clinicians can use either the colistin or the polymyxin B parenteral formulation. The pattern of use within such countries may not be uniformly distributed. For example, in the United States, polymyxin B appears to carry favor in New York State, whereas CMS is more widely used in other parts of the country.
DIFFERING DISPOSITIONS OF COLISTIMETHATE AND POLYMYXIN B
As discussed above, colistin is administered as its inactive prodrug, colistimethate (ie, CMS), which requires conversion in vivo to colistin, whereas polymyxin B is administered directly. To understand how this different formulation approach impacts their clinical use (discussed in the following section) requires knowledge of their respective pharmacokinetics.
The overall pharmacokinetic handling of CMS and the colistin formed from it in the body is complex (Figure 2) [2]. The prodrug, CMS, is predominantly cleared by renal excretion [21]. One of the nonrenal clearance pathways for CMS is conversion to colistin, a necessary step to achieve antibacterial activity [2]. Studies conducted over the last decade have revealed that the extent of conversion of CMS to colistin is low, especially in renally competent subjects [21–23]. This arises because, under such circumstances, the renal clearance of CMS is much more efficient than the conversion clearance of CMS to colistin (Figure 2). It can be estimated that in patients with normal kidney function, no more than approximately 20%–25% of a dose of CMS is converted to colistin. In other words, to generate in vivo an amount of colistin sufficient to attain the required plasma concentration of this antibacterial entity, it is necessary to administer about 4–5 times the amount of CMS. This contrasts with the desirable property for a prodrug where complete conversion to the active entity occurs to minimize the xenobiotic burden on body systems (eg, liver, kidneys). A relatively large degree of interindividual variability exists in the conversion of CMS to colistin [22]. Contributing factors toward the variability are the existence and relative magnitudes of competing disposition pathways for CMS (Figure 2) and batch-to-batch variability in the complex composition of CMS that affects the rate and extent of in vivo conversion [20]. Although colistin circulating in blood is excreted in urine to only a very minor extent, urinary concentrations of colistin after administration of CMS can be relatively high. This is the result of conversion within the urinary tract from CMS that is extensively renally excreted (Figure 2) [21, 23, 24].
Figure 2.
Overview of the pharmacokinetic pathways for colistin methanesulfonate (CMS, also known as colistimethate) and colistin (left panel) and polymyxin B (right panel). The thickness of the arrows indicates the relative magnitude of the respective clearance pathways when kidney function is normal. CMS includes fully and all partially methanesulfonated derivatives of colistin. After administration of CMS, extensive renal excretion of the prodrug occurs with some of the excreted CMS converting to colistin within the urinary tract.
A prodrug strategy was not pursued for polymyxin B when it was undergoing development in the 1950s. Thus, in contrast to the complex overall disposition of CMS and formed colistin, polymyxin B is administered in its active antibacterial form, and the disposition is simpler (Figure 2) [5, 25–27]. As occurs for colistin formed from CMS [24], polymyxin B is subject to very extensive renal tubular reabsorption after filtration at the glomerulus. Consequently, polymyxin B is eliminated mainly by nonrenal clearance mechanism(s), and urinary concentrations are relatively low [25–27].
CLINICAL IMPLICATIONS OF THE DIFFERENCES BETWEEN THE 2 POLYMYXINS
The differences described above in the chemical forms administered and overall dispositions have significant implications for the clinical use of CMS/colistin and polymyxin B.
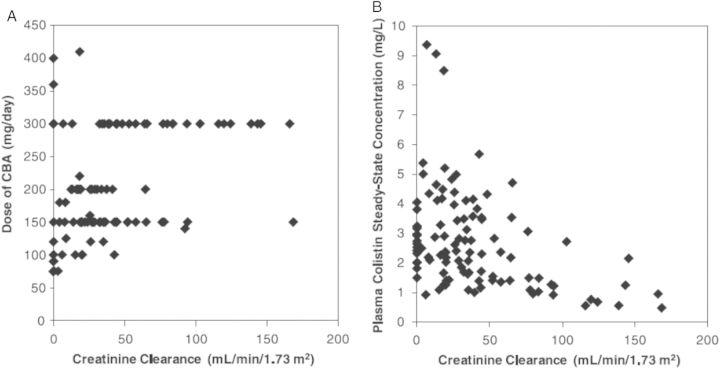
First, the differences impact the ability to relatively rapidly and reliably achieve a desired plasma concentration of the active polymyxin. In critically ill patients commenced on an intravenous CMS dosage regimen, plasma concentrations of formed colistin rise slowly (Figure 3) [28]. Even with a loading dose of CMS at the initiation of therapy, it may take several hours to achieve plasma colistin concentrations that may be effective. This is highly undesirable because delayed initiation of appropriate antibiotic therapy is associated with increased mortality in critically ill patients [29, 30], and low colistin concentrations have been associated with the amplification of colistin-resistant subpopulations [31–33]. Not only the rate but also the extent of conversion of CMS to colistin is low, especially in patients with normal renal function [22, 23]. In patients with creatinine clearance above approximately 80 mL/minute, it is not possible to reliably achieve steady-state plasma concentrations of formed colistin that are likely to be effective [22]. Indeed, at the upper limit of the approved daily dose of CMS (300 mg of colistin base activity), the majority of patients with creatinine clearance >80 mL/minute achieved plasma colistin concentrations <2 mg/L (Figure 4) [22]. This arises because the apparent clearance of formed colistin increases with creatinine clearance due to decreased fractional conversion of CMS to colistin (Figure 2). Unfortunately, in such patients it is not possible to simply increase the daily dose of CMS to compensate for the low conversion of CMS to colistin [22], due to nephrotoxicity, which is the major dose-limiting adverse effect [34]. Therefore, with CMS there are significant limitations in regard to the ability to attain (via a loading dose) and, in patients with normal kidney function, to maintain (via daily maintenance doses) effective plasma colistin concentrations. In contrast, because polymyxin B is not administered as a prodrug, it is possible to use an intravenous loading dose to relatively rapidly achieve a desired plasma polymyxin B concentration that may then be maintained with a suitable daily dosage regimen [27].
Figure 3.
Observed plasma concentrations of formed colistin in 10 individual critically ill patients after administration of the first dose of colistin methanesulfonate in a regimen. Reproduced with permission from the American Society for Microbiology (Mohamed et al [28]).
Figure 4.
Relationship of physician-selected daily dose of colistin base activity (CBA) (A) and the resultant steady-state plasma concentration of formed colistin (B) with creatinine clearance in 105 critically ill patients. A daily dose of 300 mg CBA is the currently approved upper limit dose. Reproduced with permission from the American Society for Microbiology (Garonzik et al [22]).
Second, the differences in parenteral formulations and overall dispositions of CMS/colistin vs polymyxin B impact the need to undertake dosage adjustments in patients with renal impairment. Although renal excretion is only a very minor component of the clearance of formed colistin, a decrease in CMS daily maintenance dose may be required for patients with renal impairment [22]. This arises because as kidney function declines, leading to decreased renal clearance of CMS, a greater fraction of each dose of CMS is available for conversion to colistin (Figure 2). It follows that in patients with diminished kidney function, it is possible to administer submaximal daily doses of CMS and achieve plasma colistin concentrations higher than those in renally competent patients receiving the current upper limit daily dose of CMS (Figure 4) [22]. In contrast, in keeping with the fact that polymyxin B is predominantly nonrenally cleared, its clearance is not related to creatinine clearance (Figure 5) and, therefore, dosage adjustment in patients with diminished kidney function is not required on pharmacokinetic grounds [26, 27, 35].
Figure 5.
Polymyxin B clearance vs calculated creatinine clearance in individual critically ill patients. Open circles represent patients not receiving any form of renal replacement therapy. The filled symbols are for 2 patients receiving continuous venovenous hemodialysis. From Sandri et al [27].
Third, there is substantially greater interindividual variability in the pharmacokinetics of CMS/colistin compared with polymyxin B [22, 27]. At a given creatinine clearance and daily dose of CMS, there is up to a 10-fold range in the steady-state plasma colistin concentration achieved across patients (Figure 4) [22]. As noted above, this is almost certainly the result of the inefficiency of CMS as a prodrug and the potential for batch-to-batch variability in the composition of the material administered [20], thereby impacting the fractional conversion to colistin at a given creatinine clearance. On the other hand, the total body clearance of polymyxin B (and hence daily dosage needed to maintain a given steady-state plasma concentration) is subject to remarkably low interpatient variability (3.3-fold) across a wide range of creatinine clearance values (Figure 5) [27].
Fourth, CMS/colistin and polymyxin B differ in regard to the concentrations of the active antibacterial that can be achieved in urine. CMS is extensively excreted into urine and then partially converted to colistin within the urinary tract as a consequence of the chemical instability of CMS in an aqueous environment (Figure 2) [21, 23]. Thus, urinary concentrations of colistin exceed those that can be achieved with polymyxin B which, like colistin itself, is only excreted into urine to a minor extent (Figure 2) [25, 27].
Fifth, both CMS/colistin and polymyxin B are potentially nephrotoxic. There are numerous literature reports, many of which are relatively small, noncomparative case series, on the nephrotoxicity rate of either CMS/colistin or polymyxin B. The rates vary widely, and it is impossible to use such studies to gauge the relative nephrotoxicity of the 2 polymyxins. A comparative study involving 41 patients who received either one of the polymyxins found similar renal toxicity between the 2 drugs [36]. Two recent comparative studies involving larger numbers of patients reported that nephrotoxicity rates were lower with polymyxin B than CMS/colistin [37, 38]. A possible explanation is that the kidneys are exposed to a substantial load of colistin (via intrarenal conversion from CMS and partially methanesulfonated derivatives) in the process of forming colistin that circulates more generally in the body.
Finally, because both polymyxins are concentration-dependent antibiotics [31, 33, 39, 40] with a narrow therapeutic window and because nephrotoxicity is a dose-limiting adverse effect [22, 34], when available, therapeutic drug monitoring (TDM) should be undertaken to assist in optimizing therapy for individual patients. The case for TDM is even stronger for CMS/colistin than for polymyxin B because of the greater interindividual variability discussed above [22, 27]. Microbiological assays are not appropriate, more so for colistin because such assays are not able to differentiate between the colistin present in a plasma sample at the time of its collection and that formed from ongoing conversion from CMS during the assay [17]. Even with specific analytical methods (eg, high-performance liquid chromatography, liquid chromatography–tandem mass spectrometry), TDM for colistin is difficult because stringent procedures must be implemented to prevent ongoing conversion of CMS to colistin during the sample collection, storage, and transportation to the TDM laboratory [22, 23, 28]. These difficulties do not occur with polymyxin B, as it is not administered as a prodrug.
CONCLUSIONS
The different formulation approach applied to the parenteral products of colistin and polymyxin B converts them from being “peas in a pod” in vitro to being more like “chalk and cheese” in regard to their behavior in patients. Polymyxin B would appear to have superior clinical pharmacological characteristics for infections where it is important to rapidly and reliably attain and maintain plasma concentrations that are likely to be efficacious, across a wide range of renal function. An exception may be the treatment of urinary tract infections where CMS/colistin may be the polymyxin of choice. Because of smaller interindividual variability and lack of impact of renal function on drug clearance, initial dose selection and titration are simpler and more predictable for polymyxin B. TDM for polymyxin B lacks the substantial difficulties that exist for colistin. Thus, it is important to not regard these 2 parenteral polymyxins as “peas in a pod.”
Because cross-resistance exists, it is essential that the dosage regimens of both polymyxins are optimized according to the patient characteristics that influence their respective pharmacokinetics. In countries where both are already available, clinicians should carefully consider the relative merits of each in a given circumstance, and prospective studies should be conducted to formally examine their relative efficacy and safety in various types of infections and patients. It is highly desirable that clinicians around the world have the option of choosing between parenteral products of CMS and polymyxin B. Because polymyxins are likely to remain an important part of our therapeutic armamentarium against gram-negative pathogens for some time, it is essential that they are used optimally to preserve their activity as a class for as long as possible.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases (NIAID) or the National Institutes of Health (NIH).
Financial support. The authors are supported by the NIAID, NIH (award number R01AI098771). T. V. is a National Health and Medical Research Council (NHMRC) Industry Career Development Award Fellow, and J. L. is an NHMRC Senior Research Fellow.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev. 2008;21:449–65. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 3.Velkov T, Roberts KD, Nation RL, Thompson PE, Li J. Pharmacology of polymyxins: new insights into an ‘old’ class of antibiotics. Future Microbiol. 2013;8:711–24. doi: 10.2217/fmb.13.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couet W, Gregoire N, Marchand S, Mimoz O. Colistin pharmacokinetics: the fog is lifting. Clin Microbiol Infect. 2012;18:30–9. doi: 10.1111/j.1469-0691.2011.03667.x. [DOI] [PubMed] [Google Scholar]
- 5.Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother. 2007;60:1206–15. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- 6.Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis. 2009;22:535–43. doi: 10.1097/QCO.0b013e328332e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Pharmacopoeia 8.0 2014. Strasbourg: Directorate for the Quality of Medicines and HealthCare of the Council of Europe; 2014. Monographs for colistimethate sodium and colistin sulfate; pp. 1960–2. [Google Scholar]
- 8.European Pharmacopoeia 8.0 2014. Strasbourg: Directorate for the Quality of Medicines and HealthCare of the Council of Europe; 2014. Monograph for polymyxin B sulfate; pp. 3055–6. [Google Scholar]
- 9.United States Pharmacopoeia 36 2013. Rockville, MD: The United States Pharmacopeial Convention; 2013. Monographs for colistimethate sodium and colistin sulfate; pp. 3098–100. [Google Scholar]
- 10.United States Pharmacopoeia 36 2013. Rockville, MD: The United States Pharmacopeial Convention; 2013. Monograph for polymyxin B sulfate; pp. 4826–7. [Google Scholar]
- 11.Velkov T, Thompson PE, Nation RL, Li J. Structure-activity relationships of polymyxin antibiotics. J Med Chem. 2010;53:1898–916. doi: 10.1021/jm900999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock RE. Peptide antibiotics. Lancet. 1997;349:418–22. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 13.Gales AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin B against a worldwide collection of gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006–09) J Antimicrob Chemother. 2011;66:2070–4. doi: 10.1093/jac/dkr239. [DOI] [PubMed] [Google Scholar]
- 14.Moskowitz SM, Ernst RK, Miller SI. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J Bacteriol. 2004;186:575–9. doi: 10.1128/JB.186.2.575-579.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beceiro A, Llobet E, Aranda J, et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother. 2011;55:3370–9. doi: 10.1128/AAC.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moffatt JH, Harper M, Harrison P, et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother. 2010;54:4971–7. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergen PJ, Li J, Rayner CR, Nation RL. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:1953–8. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett M, Bushby SR, Wilkinson S. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmacol. 1964;23:552–74. doi: 10.1111/j.1476-5381.1964.tb01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K. Stability of colistin and colistin methanesulfonate in aqueous media and plasma studied by high-performance liquid chromatography. Antimicrob Agents Chemother. 2003;47:1364–70. doi: 10.1128/AAC.47.4.1364-1370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He H, Li JC, Nation RL, et al. Pharmacokinetics of four different brands of colistimethate and formed colistin in rats. J Antimicrob Chemother. 2013;68:2311–7. doi: 10.1093/jac/dkt207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother. 2004;53:837–40. doi: 10.1093/jac/dkh167. [DOI] [PubMed] [Google Scholar]
- 22.Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55:3284–94. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couet W, Gregoire N, Gobin P, et al. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther. 2011;89:875–9. doi: 10.1038/clpt.2011.48. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother. 2003;47:1766–70. doi: 10.1128/AAC.47.5.1766-1770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelraouf K, He J, Ledesma KR, Hu M, Tam VH. Pharmacokinetics and renal disposition of polymyxin B in an animal model. Antimicrob Agents Chemother. 2012;56:5724–7. doi: 10.1128/AAC.01333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zavascki AP, Goldani LZ, Cao GY, et al. Pharmacokinetics of intravenous polymyxin B in critically-ill patients. Clin Infect Dis. 2008;47:1298–304. doi: 10.1086/592577. [DOI] [PubMed] [Google Scholar]
- 27.Sandri AM, Landersdorfer CB, Jacob J, et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis. 2013;57:524–31. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 28.Mohamed AF, Karaiskos I, Plachouras D, et al. Application of a loading dose of colistin methanesulphonate (CMS) in critically ill patients: population pharmacokinetics, protein binding and prediction of bacterial kill. Antimicrob Agents Chemother. 2012;56:4241–9. doi: 10.1128/AAC.06426-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 30.Luna CM, Aruj P, Niederman MS, et al. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur Respir J. 2006;27:158–64. doi: 10.1183/09031936.06.00049105. [DOI] [PubMed] [Google Scholar]
- 31.Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J Antimicrob Chemother. 2008;61:636–42. doi: 10.1093/jac/dkm511. [DOI] [PubMed] [Google Scholar]
- 32.Poudyal A, Howden BP, Bell JM, et al. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2008;62:1311–8. doi: 10.1093/jac/dkn425. [DOI] [PubMed] [Google Scholar]
- 33.Tan CH, Li J, Nation RL. Activity of colistin against heteroresistant Acinetobacter baumannii and emergence of resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2007;51:3413–5. doi: 10.1128/AAC.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartzell JD, Neff R, Ake J, et al. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis. 2009;48:1724–8. doi: 10.1086/599225. [DOI] [PubMed] [Google Scholar]
- 35.Kwa AL, Abdelraouf K, Low JG, Tam VH. Pharmacokinetics of polymyxin B in a patient with renal insufficiency: a case report. Clin Infect Dis. 2011;52:1280–1. doi: 10.1093/cid/cir137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira MS, Prado GV, Costa SF, Grinbaum RS, Levin AS. Polymyxin B and colistimethate are comparable as to efficacy and renal toxicity. Diagn Microbiol Infect Dis. 2009;65:431–4. doi: 10.1016/j.diagmicrobio.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 37.Akajagbor DS, Wilson SL, Shere-Wolfe KD, Dakum P, Charurat ME, Gilliam BL. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin Infect Dis. 2013;57:1300–3. doi: 10.1093/cid/cit453. [DOI] [PubMed] [Google Scholar]
- 38.Phe K, Lee Y, McDaneld PM, et al. Comparison of nephrotoxicity rates associated with colistimethate versus polymyxin B therapy: in vitro assessment and a multicenter cohort study. Antimicrob Agents Chemother. 2014;58:2740–6. doi: 10.1128/AAC.02476-13. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Turnidge J, Milne R, Nation RL, Coulthard K. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 2001;45:781–5. doi: 10.1128/AAC.45.3.781-785.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tam VH, Schilling AN, Vo G, et al. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:3624–30. doi: 10.1128/AAC.49.9.3624-3630.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]