We demonstrate autosomal-recessive CARD9 deficiency in a patient with relapsing Candida albicans meningoencephalitis. The novel, hypomorphic mutation impaired granulocyte-macrophage colony-stimulating factor (GM-CSF) but not Th17 responses. Adjunctive GM-CSF therapy resulted in clinical remission, suggesting that a CARD9/GM-CSF axis contributes to susceptibility to candidiasis.
Keywords: CARD9, spontaneous central nervous system candidiasis, Candida, GM-CSF, Th17
Abstract
We demonstrate autosomal-recessive Caspase Recruitment Domain-containing protein 9 (CARD9) deficiency in a patient with relapsing C. albicans meningoencephalitis. We identified a novel, hypomorphic mutation with intact Th17 responses, but impaired GM-CSF responses. We report complete clinical remission with adjunctive GM-CSF therapy, suggesting that a CARD9/GM-CSF axis contributes to susceptibility to candidiasis.
Caspase Recruitment Domain-containing protein 9 (CARD9) is an adaptor molecule that mediates intracellular signaling downstream of innate pattern recognition receptors that are involved in antifungal immunity. CARD9 deficiency is a recently described autosomal-recessive primary immunodeficiency marked by susceptibility to candidiasis. Although mucocutaneous disease may occur, the salient feature is the involvement of the central nervous system (CNS), brain parenchyma and/or meninges, in the absence of trauma, chemotherapy, or systemic disease, that is, spontaneous CNS candidiasis (sCNSc). The seminal report identified a homozygous null CARD9 mutation (p.Q295X) in an Iranian pedigree [1]. Sequenced family members suffered from mucocutaneous disease; notably, 3 others had died from invasive Candida infection of the brain. The null allele was associated with severely reduced circulating CD4+ CD45RO+ interleukin 17 (IL-17+) interferon-gamma (IFN-γ−) Th17 cells ex vivo, as well as abolished Dectin-1–mediated tumor necrosis factor-alpha (TNF-α) responses when transfected into a murine cell line. Recently, Drewniak et al described a girl with relapsing candidal meningoencephalitis who was compound heterozygous for null CARD9 alleles (p.G72S, p.R37P) [2]. Neutrophils from this patient were defective in intracellular killing of Candida yeast forms in low-serum conditions. Most recently, Lanternier et al [3] described a syndrome of deep dermatophytosis associated with recessive CARD9 deficiency, expanding the spectrum of fungal pathogens that may cause disease in this condition. Thus, CARD9 deficiency presents with severe invasive fungal infections, notably sCNSc, that may be recurrent or fatal. Its optimal treatment remains to be defined.
CASE AND RESULTS
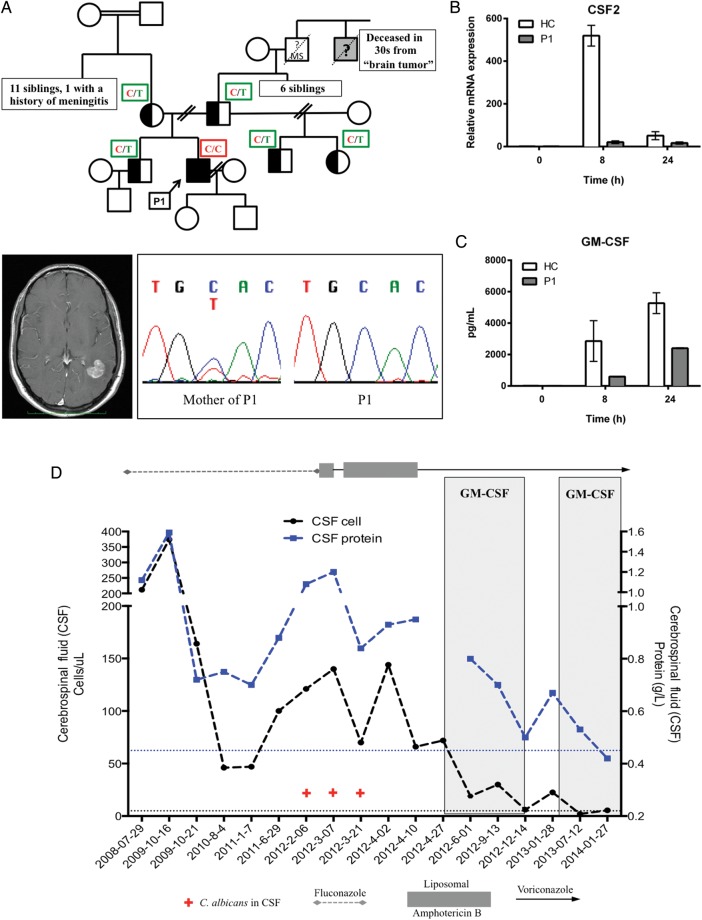
Here, we report a 41-year-old French Canadian male patient (P1) with relapsing Candida albicans meningoencephalitis over an 11-year period, despite appropriate antifungal therapy (Figure 1A). There was no quantitative deficiency in neutrophils, monocytes, or lymphocytes (CD3+T including CD4+ and CD8+ subsets; CD19+ B; CD3−,16/56+ natural killer cells). Although defects in the neutrophil oxidative burst or the IL-12/IL-23/IFN-γ axis have been identified in idiosyncratic, disseminated mycoses, no such defect was identified in this patient. In accordance with the standards of the McGill University Health Centre Research Ethics Board and the Helsinki Declaration 2008, further investigation as to the cause of this susceptibility to fungal infection was performed. Whole exome sequencing identified a novel, homozygous c.T271C variant in the caspase recruitment domain (CARD) of CARD9 and excluded mutations in genes known to cause other primary immunodeficiencies, especially those related to fungal infections. This missense variant replaces the evolutionarily conserved tyrosine at residue 91 with histidine (p.Y91H). The variant was not seen in our internal exome sequencing database, 1000 genome, dbSNP and EVS, and was predicted to be maximally damaging by in silico analysis (SIFT; PolyPhen2). Although there was no direct parental consanguinity, the homozygosity of this unique variant is likely explained by the fact that family members from preceding generations originated from the same village in Québec (including grand-parental consanguinity) and founders of each parental lineage derive from the same isolated village in France. Sanger sequencing of immediate pedigree members confirmed that the susceptibility was inherited in an autosomal-recessive manner (Figure 1). The CARD9 p.Y91H allele was expressed at the mRNA level (by reverse-transcription polymerase chain reaction; Supplementary Figure 1A) as well as at the protein level (via Western blot using antibodies directed to either the N- or the C-terminal; Supplementary Figure 1B) in peripheral blood mononuclear cells (PBMCs) and subject-derived lymphoblastoid cell lines (data not shown). This is distinct from previously reported mutations that have resulted in loss of CARD9 expression.
Figure 1.
CARD9 deficiency, spontaneous CNS candidiasis (sCNSc), and impaired granulocyte-macrophage colony-stimulating factor (GM-CSF) responses. A, Patient (P1) presented with cerebral mass. Excision yielded an abscess with Candida albicans. Sequencing revealed a homozygous c.T271C mutation in CARD9 (chromatogram) that segregated with sCNSc as a recessive trait (pedigree); a great uncle had died of a “brain tumor” when in his 30s but could not be sequenced. B, Zymosan-stimulated peripheral blood mononuclear cells showed impaired GM-CSF gene expression by reverse-transcription–quantitative polymerase chain reaction relative to GAPDH (and GUSB, not shown) with (C) decreased GM-CSF production by enzyme-linked immunosorbent assay. D, Serial CSF analyses revealed ongoing inflammation (pleocytosis and elevated protein; reference range in dotted lines) and recurrent isolation of C. albicans (+), despite antifungals. Adjunctive GM-CSF (shaded) resulted in resolution of symptoms and normalization of CSF parameters. Results are mean of 2–3 experiments (± standard error). Abbreviations: MS, multiple sclerosis; HC, healthy control.
Other primary immunodeficiencies marked by impaired or even abolished Th17 responses have not, to date, been reported to develop sCNSc [4]. This suggests that while the IL-17 pathway, possibly along with IL-22, mediates defenses against superficial candidiasis, an alternative or additional process contributes to susceptibility to invasive disease. In support of this, our patient demonstrated circulating Th17 levels, assessed ex vivo by the method of Renner et al [5], that were comparable to those of healthy controls (Supplementary Figure 2A). Further, PBMCs stimulated with the fungal cell wall agonist, zymosan, secreted IL-17 at levels comparable to those of healthy controls (Supplementary Figure 2B). Despite these intact Th17 responses, P1 continued to have microbiologically documented meningoencephalitic relapses on antifungal therapy (ie, fluconazole 800 mg daily), including a relapse that required multiple antifungal agents to sterilize his cerebrospinal fluid (CSF; Figure 1D). In each relapse, the isolated C. albicans demonstrated in vitro susceptibility to all antifungal agents. From zymosan-stimulated PBMC, P1 produced significantly less granulocyte-macrophage colony-stimulating factor (GM-CSF) compared with healthy controls; this was confirmed by impaired induction of its corresponding gene, CSF2 (Figure 1B and 1C). Induction of other proinflammatory cytokine genes (eg, IL-1β, IL-6) was intact (Supplementary Figure 3). Thus, we hypothesized that the impaired GM-CSF responses in our CARD9-deficient patient contribute to the pathogenesis of sCNSc.
After obtaining informed consent, subcutaneous recombinant human GM-CSF (sargramostim; 500 µg daily) was initiated for the patient (Figure 1D). Subjective improvement was noted within 6 days. Objectively, serial lumbar punctures demonstrated sustained sterility of CSF and, for the first time in more than a decade, near normalization of CSF pleocytosis, protein, and glucose concentration. Relapse of symptoms with worsening CSF parameters upon discontinuation of GM-CSF and disappearance of symptoms and renormalization of parameters upon reinstitution of therapy support a cause-and-effect therapeutic relationship. Attempts to decrease adjunctive GM-CSF to every other day were also accompanied by recurrence of symptoms. Reduction of the GM-CSF dose to 250 µg daily was tolerated and associated with sustained improvement of CSF parameters. The patient has been on adjunctive GM-CSF therapy for 18 months with no adverse events. Overlapping this period, antifungal therapy with voriconazole was not changed, with serum voriconazole levels ranging from 0.22 to 1.53 µg/mL (proposed therapeutic range for mycoses is 1.5–5.5 µg/mL) [6], suggesting that the improvement in symptoms and CSF parameters are not likely attributable to the antifungal effects of the azole alone.
DISCUSSION
CARD9 deficiency predisposes to sCNSc. The fungal portal of entry is most likely at an epithelial surface (eg, skin, gastrointestinal), and access to the CNS is likely hematogenous. The molecular and cellular bases by which CARD9 deficiency permits fungal invasion and dissemination, particularly constrained to the CNS, remain to be defined. Given that CARD9 is expressed in myeloid cells [7], epithelial fungal invasion and asymptomatic dissemination might involve intracellular uptake of Candida by monocyte-derived cells at a portal of entry or regional lymphatic organ. Impaired CARD9 function may result in defective antifungal clearance and, thus, latently infected cells. Eventually, these cells putatively access the CNS and establish disease via a “Trojan horse” model [8]. Further studies are necessary to define the mechanisms by which CARD9 regulates human immunity to fungi.
CONCLUSIONS
Although therapy with antifungal agents that penetrate into the CNS is clearly necessary for management, the cases presented by Drewniak et al [2] and our group demonstrate that the infection may relapse, despite in vitro susceptibility to these agents; those reported by Glocker et al [1] suggest that the infection is potentially fatal. Although hematopoietic stem cell transplantation has been suggested as the only definite option for treating CARD9 deficiency [2], this procedure is associated with significant morbidity and mortality. Importantly, the ability of stem cell transplantation to cure fungal disease in CARD9 deficiency remains untested. To our knowledge, we are the first to demonstrate complete clinical remission with adjunctive GM-CSF therapy in a patient with hypomorphic CARD9 deficiency (ie, the mutation preserves expression while impairing, but not abolishing, function). Other patients with hypomorphic CARD9 deficiency and perhaps those with amorphic mutations (complete loss of expression and function) may benefit similarly. It further suggests that a CARD9/GM-CSF axis contributes to the sCNSc phenotype.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Nancy Hamel for technical advice.
Financial support. This work was supported by start-up funds from McGill University Health Centre, Department of Medicine/Research Institute, and grants from CSL Behring Canada, Astellas Canada, and La Fondation du Grand Défi Pierre Lavoie to D. C. V.
Potential conflicts of interest. D. C. V. has received speaker honoraria and unrestricted educational grants from CSL Behring Canada and has received research funding from Astellas Canada. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Glocker EO, Hennigs A, Nabavi M, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drewniak A, Gazendam RP, Tool AT, et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013;121:2385–92. doi: 10.1182/blood-2012-08-450551. [DOI] [PubMed] [Google Scholar]
- 3.Lanternier F, Pathan S, Vincent QB, et al. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med. 2013;369:1704–14. doi: 10.1056/NEJMoa1208487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12:616–22. doi: 10.1097/ACI.0b013e328358cc0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renner ED, Rylaarsdam S, Anover-Sombke S, et al. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;122:181–7. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pascual A, Csajka C, Buclin T, et al. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin Infect Dis. 2012;55:381–90. doi: 10.1093/cid/cis437. [DOI] [PubMed] [Google Scholar]
- 7.Bertin J, Guo Y, Wang L, et al. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-kappa B. J Biol Chem. 2000;275:41082–6. doi: 10.1074/jbc.C000726200. [DOI] [PubMed] [Google Scholar]
- 8.Kim KS. Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Micro. 2008;6:625–34. doi: 10.1038/nrmicro1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.